Highlights
-
•
Huntington’s disease incurs greater costs in Israel compared with patients without.
-
•
Low medication coverage suggests Huntington’s disease is undertreated in Israel.
-
•
Prevalence of Huntington’s disease in Israel is comparable with Western populations.
Keywords: Huntington’s disease, Medication use, Economic, Costs
Abstract
Introduction
Data on Huntington’s disease (HD) epidemiology, treatment patterns, and economic burden in Israel are scarce.
Methods
Annual prevalence and incidence of HD (ICD-9-CM 333.4) were assessed in the Israel-based Maccabi Healthcare Services (MHS) database 2016–2018. Adherence (medication possession rate [MPR], proportion of disease covered) were assessed for adult people with HD (PwHD) 2013–2018. Healthcare resources utilization (HCRU) and costs related to inpatient and outpatient visits and all medications in 2018 were assessed for PwHD, who were randomly matched to MHS members without HD (1:3) by birth-year and sex.
Results
Overall, 164 patients had at least one HD diagnosis. Annual prevalence and incidence were 4.45 and 0.24/100,000, respectively. A total of 67.0% of adult patients (n = 106) were taking tetrabenazine (median MPR and proportion of disease covered, 74.3% and 30.2%, respectively), 65.1% benzodiazepines (75.8% and 32.3%), and 11.3% amantadine (79.2% and 6.0%). Over a 1-year follow-up, PwHD (n = 81) had significantly more neurologist, psychiatrist, physiotherapist, and speech therapist visits (P < 0.05 for each) and more hospitalization days (P < 0.0001) compared with matched controls (n = 243). Total healthcare and medication costs per patient (US dollars) were significantly higher for PwHD than controls ($7,343 vs. $3,625; P < 0.001).
Discussion/Conclusion
PwHD have greater annual HCRU and medical costs than MHS members without HD in Israel. Among those who have taken medications, adherence was lower than 80% (both MPR and proportion of disease covered), which may translate into suboptimal symptom relief and quality of life.
1. Introduction
Huntington’s disease (HD), an incurable, inherited neurodegenerative disease comprising a triad of movement, cognitive, and psychiatric disorders, along with metabolic disturbances, profoundly affects patients’ quality of life, from the psychological impact of parental diagnosis, through progressive decline in functional abilities, to possible requirement for 24-hour care in the end stages [1], [2], [3], [4]. Two drugs, tetrabenazine and deutetrabenazine, are approved by the US Food and Drug Administration (since 2008 and 2017, respectively) and the Ministry of Health of Israel (since 2004 and 2021, respectively) to treat chorea associated with HD [5], [6], but they do not alter the long-term course of the disease.
HD prevalence varies by geographic region, being generally lower in Asian populations and highest in North America [7], with substantial medical costs and informal care worldwide [8], [9], [10], [11]. Understanding the burden of HD in individual communities is important to provide appropriate healthcare facilities and resources for patient care [7]. However, in Israel, the burden of HD has not yet been characterized. The current study identified the epidemiology of HD and healthcare resource utilization (HCRU), including the marginal economic burden of HD in the Israeli population.
2. Methods
2.1. Study design and data source
We retrospectively analyzed data from the Maccabi Health Services (MHS) database, a nationwide health plan (payer-provider) representing 26% of the population in Israel with a >99% annual retention rate. Data are automatically collected and include comprehensive medical data: diagnoses, laboratory data from a single central laboratory, full pharmacy prescription and purchase data, consultations and hospitalization and extensive demographic data. The study was approved by the Institutional Ethics Committee of Maccabi Health Services (study number 0111-19-MHS) which granted a waiver of consent due to the anonymity of the analyses.
2.2. Study population
All people with HD diagnosis according to the International Classification of Disease, ninth Revision, Clinical Modification (ICD-9-CM) code 333.4 who were diagnosed by (1) a neurologist (2) hospital-discharge report, (3) medicine pre-authorization, (4) “chronic patient” diagnosis, or (5) ≥ 2 diagnoses of HD by any other healthcare professional, at any time prior to 2019, were identified. No age limits were used for prevalence and incidence analyses. For treatment patterns and economic burden assessment, only people with HD (PwHD) over the age of 18 years who had at least 12 months of MHS continuous enrollment prior to diagnosis, were included. To better reflect current therapeutic regimens, treatment patterns were assessed for PwHD who were alive between 2013 and 2018. In order to have 1 full calendar year of data, healthcare resources utilization and related costs were assessed in PwHD who were alive on January 1, 2018. These patients were randomly matched (1:3) to MHS members without HD by birth-year and sex.
2.3. Outcomes
Prevalence was assessed for each year from 2016 to 2018 and included all living HD patients in the MHS database, regardless of age, out of the total number of MHS insurers who were alive on July 1 of each year. Incidence analyses included the number of newly diagnosed HD patients who had ≥ 1 year of continuous enrollment in the MHS database prior to their first HD diagnosis (to ensure incident cases), regardless of age for each year from 2016 to 2018 out of the total number of MHS insurers who were alive and had ≥ 1 year of continuous data on July 1 of each year. Confidence intervals (CIs) were calculated using binomial distribution. Annual mortality rate included HD patients who died in a specific year of all MHS members alive on July 1 of that year. Median survival time data were also collected. The end of follow-up was defined as the earliest of: (1) date of death, (2) end of data availability in the MHS database, or (3) December 31, 2018.
Treatment patterns were assessed from 2000 to 2018 for the identified population. All medications evaluated were available in Israel since 2000. Adherence to treatment (percentage medication possession rate [MPR]) was defined as the number of days covered by the treatment divided by the total duration (number of days between first and last purchase plus number of days of medication supplied by last purchase). MPR was calculated using medications purchased after the first HD diagnosis. Proportion of disease covered was defined as the number of days covered by the medication divided by the disease duration (from first HD diagnosis to end of follow-up). Permanent discontinuation was defined as a gap of more than 120 days between the last purchase (in addition to the number of days of medication supplied) and the end of follow-up. Temporary discontinuation was defined as a gap between two consecutive purchases greater than the number of days of medication supplied by the last purchase plus a grace period of 120 days.
Time to first purchase of HD medication was recorded for each new HD medication class purchase (see Table S1 in the Appendix for medications). If a patient initiated ≥ 1 HD medication class, the time to each class was counted. The proportion of patients with ≥ 1 purchase of an antipsychotic medication was also recorded (see Table S2 in the Appendix for medications).
The population comorbidities were assessed using MHS highly validated chronic disease registries (i.e., cardiovascular disease [12], diabetes [13], hypertension [14], chronic kidney disease [12], and cancer [15]). The MHS cancer registry is based on the national cancer registry and thus all cancer types were included in this analysis. Socioeconomic status (SES) scores were ranked with 1 (lowest) to 10 and derived for commercial purposes by Points Location Intelligence using geographic information systems and data related to expenditures at retail chains, credits, and housing. This score is highly correlated with SES measured by the Central Bureau of Statistics [16]. SES was categorized into low (1–4), medium (5–6), and high (7–10).
2.4. Statistical analysis
Descriptive statistics are presented as n (%), mean (standard deviation [SD]), or median (interquartile range [IQR]), as appropriate. Median survival time was calculated using Kaplan-Meier survival analysis. Patient characteristics for the HD and non-HD groups were compared with P values calculated from independent t tests for continuous variables and chi-square tests for categorical variables. Significances and estimated means were calculated using generalized linear models with negative binomial with log-link distribution for HCRU and gamma distribution with log-link distribution for cost analysis.
HCRU was categorized as any hospitalization (not including day hospitalization), emergency room (ER) visit, or outpatient visit to a general practitioner, psychiatrist, neurologist, orthopedist, physiotherapist, or speech therapist.
Healthcare costs are presented in US dollars (conversion factor according to purchasing-power parities in 2018 of 1 USD = 3.752 Israeli Shekels [ILS]) and were assessed for any hospitalization plus ER visits, all outpatient visits in the MHS and hospital outpatient clinics, all medications purchased by the patient, and total healthcare and medication costs (sum of hospitalizations, ER visits, outpatient visits, and all medications).
3. Results
3.1. Patient characteristics and disposition
A total of 164 MHS members with ≥ 1 HD diagnosis made by reliable sources were identified from 2000 to 2018 (Fig. S1). Of these, 106 PwHD were alive at some point between 2013 and 2018 and were used to assess treatment patterns. Mean (SD) age at diagnosis was 49.3 (13.6) years and 57.5% were female. Median (95% CI) time from first diagnosis to end of follow-up for PwHD alive from 2013 to 2018 was 8.3 (7.2–9.4) years.
In total, 81 PwHD were eligible for HCRU and cost assessments and were randomly matched, based on birth-year and sex, with 243 MHS members without HD. There were no significant differences in patient characteristics and rates of common comorbidities between groups (Table S3). Mean (SD) age at the beginning of 2018 was 56.4 (13.8) years; 59.3% of patients were female in both groups. Six patients in the HCRU and cost analyses did not have a full calendar year of follow-up due to death (HD, n = 3; non-HD, n = 2) and other reasons (HD, n = 1).
3.2. Prevalence, incidence, and mortality rates
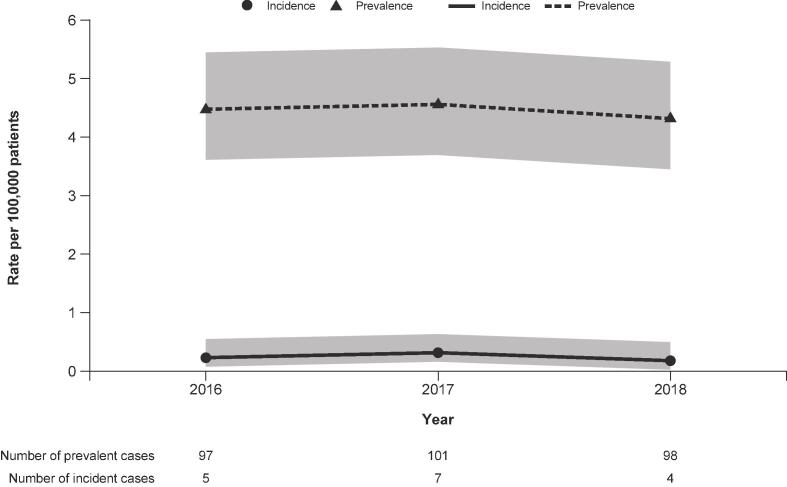
Annual prevalence and incidence over time are shown in Fig. 1. From 2016 to 2018, mean prevalence rate was 4.45 (95% CI 3.71–5.55) per 100,000 and mean incidence rate was 0.24 (95% CI 0.11–0.55) per 100,000 for PwHD. Mean annual mortality rate was 0.18 per 100,000 and median survival time was 13 years post-diagnosis (95% CI 11–15; Fig. S2).
Fig. 1.
Annual prevalence and incidence rates of HD over time. HD, Huntington’s disease. Gray bar indicates 95% confidence interval.
3.3. Medication use
Of the 106 PwHD alive from 2013 to 2018, 71 (67.0%), 69 (65.1%) and 12 (11.3%) were taking tetrabenazine, benzodiazepines, and amantadine, respectively, at one point during the study. Of those who initiated treatment, 58 (81.7%), 55 (79.7%), and 11 (91.7%) patients, respectively, either permanently or temporarily discontinued treatment before end of follow-up. Median (IQR) time from HD diagnosis to treatment initiation was 20.3 (0.7–50.8), 52.9 (8.5–94.9), and 31.5 (0.1–84.0) months, respectively. Median MPR and proportion of disease covered are shown in Table 1. Additionally, 24.5% and 45.3% of patients were using first-generation and second-generation antipsychotics, respectively.
Table 1.
Adherence to dispensed HD medications among PwHD alive from 2013 to 2018 (n = 106).
| Drug* | MPR† (median [IQR]) | Proportion of disease covered (median [IQR])‡ |
|---|---|---|
| Tetrabenazine | 74.3% (52.5%–99.0%) | 30.2% (5.3%–54.0%) |
| Benzodiazepines | 75.8% (33.6%–100.0%) | 32.3% (7.6%–62.8%) |
| Amantadine | 79.2% (53.2%–95.2%) | 6.0% (1.3%–48.0%) |
HD, Huntington's disease; MPR, medication possession rate; IQR, interquartile range.
HD medications in each class are listed in Supplementary Table 1 in the Appendix.
Adherence was based on the MPR, which was defined as the number of days supplied divided by the number of days in the period between the first and last purchases of medication after HD first diagnosis.
Proportion of disease covered was defined as the number of days covered by the medication divided by the disease duration (from HD first diagnosis to end of follow-up).
3.4. HCRU and healthcare costs
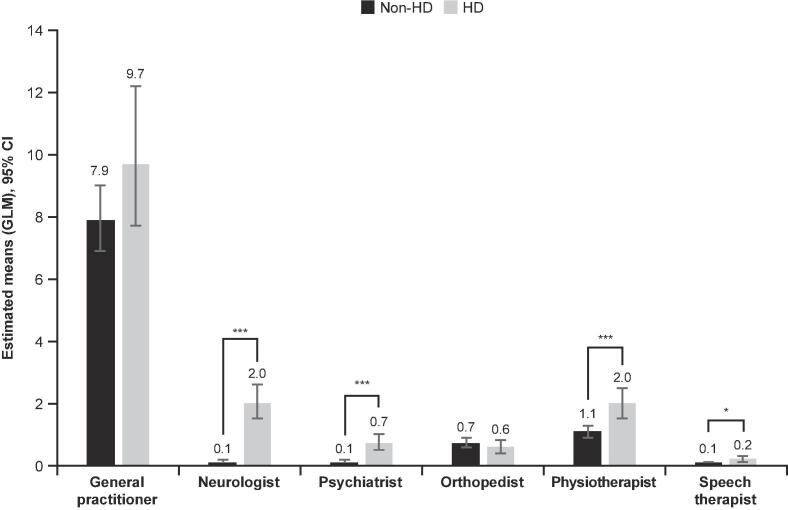
PwHD had significantly more visits to neurologists, psychiatrists, physiotherapists, and speech therapists compared with MHS members without HD over 1-year of follow-up (all P < 0.05; Fig. 2). There were no statistically significant differences between PwHD and those without HD, respectively, in the number of ER visits (mean [95% CI]: 0.38 [0.25–0.58] and 0.26 [0.19–0.34]; P = 0.125) and hospitalizations (0.18 [0.11–0.32] and 0.11 [0.08–0.17]; P = 0.169). However, hospitalized PwHD were admitted for longer durations vs. members without HD (estimated mean [95% CI]: 4.46 [3.51–5.68] vs. 0.90 [0.75–1.10] days, respectively; P < 0.0001).
Fig. 2.
Outpatient visits in 2018. CI, confidence interval; GLM, generalized linear model; HD, Huntington’s disease. *P < 0.05. ***P < 0.0001. Significance and estimated means (95% CI) were calculated using GLMs with negative binomial with log-link distribution.
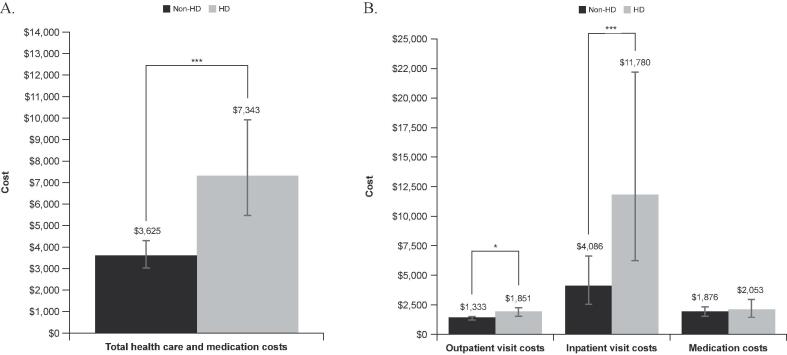
Total healthcare and medication costs for HCRU in 2018 were significantly higher for PwHD vs. members without HD (P < 0.0001; Fig. 3A). Costs for outpatient and inpatient visits were also significantly higher for PwHD vs. those without HD (P < 0.05 and P < 0.001, respectively); medication costs were similar between groups (Fig. 3B).
Fig. 3.
Total healthcare and medication costs† (A) and costs of healthcare visits and medications†† (B) in 2018. HD, Huntington's disease; ER, emergency room. †Includes the costs from all outpatients’ clinics visitations, hospitalizations, ER visits and medication purchased by the patients during 2018. The estimated means (per person) were extracted from the generalized linear model analyses. ††Cost in patients with ≥ 1 event. *P < 0.05. ***P < 0.0001. Significances and estimated means were calculated using generalized linear models with gamma distribution with log link distribution.
4. Discussion/conclusion
Although the epidemiology and natural history of HD have been studied in a few large observational studies [17], [18], [19], [20], real-world data on the burden of HD in Israel are scarce. This retrospective database study found annual prevalence and incidence rates of 4.45 and 0.24 per 100,000 patients, respectively, for the MHS population. Among those who had taken medications, adherence was lower than 80% (both MPR and proportion of disease covered), which suggests HD symptoms may be undertreated in Israel. Lastly, this study found HCRU and healthcare and medication costs for PwHD were significantly higher than for matched patients without HD.
The prevalence and incidence of HD in the current study, based on the MHS population, were comparable to those reported previously in the Western population for patients of all ages (prevalence, 4.45 vs. 5.70 per 100,000 patients; incidence, 0.24 vs. 0.38 per 100,000 patients) [21]. Due to a strong genetic component in HD pathophysiology, variable HD prevalence and incidence rates are expected [22]. A recent systematic review reported an average HD prevalence of 0.4 per 100,000 in Asia and 7.33 per 100,000 in North America [7]. In Middle Eastern or South European populations, prevalence rates range between 2.5 and 5.4 per 100,000 persons [23], [24]. Future real-world studies will help to mitigate uncertainty in the epidemiology of HD in Israel.
At the time of the study, mainly tetrabenazine, benzodiazepines, amantadine, and antipsychotics were used in Israel to treat HD. In our study, patients had cumulative coverage by tetrabenazine, benzodiazepines, and amantadine for a median of 6–32% of the time since their first documented diagnosis. Our analysis does not reflect patients’ management of HD symptoms over a long-term period (e.g., beginning/terminating different treatment regimens, switching medications or temporary discontinuations throughout the treatment period). Additionally, there were many discontinuations in all three medication classes, suggesting there may be efficacy or tolerability issues and a potential treatment gap in some patients.
Of treatment options available in Israel at the time of the study, tetrabenazine was the only medication indicated for chorea associated with HD. Although we cannot confirm whether all PwHD in our study experienced chorea symptoms, the proportion is expected to be very high, as chorea is typically prevalent in ∼ 90% of those with adult-onset HD [3]. If we apply this information on our cohort, we find that 74% (n = 71) of the patient with “assumed chorea” (96 patients, 90% of 106 patients) initiated tetrabenazine, similar to a recent US retrospective chart review, in which ∼ 74% of PwHD with chorea initiated tetrabenazine therapy [25]. In our study, patients were covered by tetrabenazine for only ∼ 30% of disease duration. Possible reasons for this low coverage may include inadequate chorea control, tolerability issues, or prioritization of treatment for other HD symptoms. Chorea often begins early and persists over time following natural disease progression [2]; therefore, we anticipate efficacy or tolerability concerns with available treatment may affect adherence and effective chorea control. This implies there is an unmet need for additional HD treatment options in Israel.
Consistent with previous studies, healthcare costs for HD patients in Israel were higher than costs in people without HD. Estimated costs in this study are within the range of those reported from studies in other parts of the world. In a US analysis of the MarketScan commercial and Medicaid databases, annual costs were $4,947 per person (US dollars) in early stages of HD, increasing to $22,582 in late-stage commercial patients, and $3,257 in early-stage Medicaid patients to $37,495 in late-stage Medicaid patients [11]. In another study, total annual HD costs in Peru were approximately $1.2 million (2015 US dollars), with direct healthcare costs accounting for approximately $85,000 [9].
We found that PwHD had more visits to the neurologist, psychiatrist, physiotherapist, or speech therapist, reflecting their HD symptoms (e.g., chorea, behavioral issues, motor impairment). The HD population also had significantly longer hospital stays, which is consistent with findings from a previous study in pediatric patients [26]. Potential reasons for longer hospital stays include psychosocial and behavioral challenges and metabolic disturbances associated with HD, which may increase the likelihood of discharge to residential care facilities [27].
To our knowledge, this is the first real-world data study comparing HCRU and costs between HD and non-HD populations in Israel. A 2018 study comparing HCRU and costs between pre-diagnosis and post-diagnosis PwHD in the US population found higher HCRU in the 6-month post-HD diagnosis category compared with the 6-month pre-diagnosis group, highlighting the need for efficacious, tolerable, and continuous treatments for people with HD that do not exacerbate psychiatric-associated comorbidities [10]. Other studies have shown that cost increase is directly proportional to increasing disease severity [11], and more than half of this cost is associated with long-term/nursing home care [8]. Despite the possible explanations for greater economic burden in HD described above, PwHD are not consistently treated with medications to address their HD symptoms. The relationship between efficacious and tolerable treatment and HCRU, and whether treatment adherence reduces healthcare costs, warrants further investigation.
One study limitation is the small cohort size, as the Maccabi database represents ∼ 26% of the Israeli population. Additionally, we were unable to differentiate patients with and without chorea, family origin, and number of children. Furthermore, PwHD are sometimes primarily diagnosed with psychiatric disease (e.g., schizophrenia) and the diagnosis may not be changed to HD. Therefore, the actual prevalence and incidence of HD may be higher than reported here.
In conclusion, prevalence and incidence of HD in the Israeli population were consistent with those described in literature. Given the low coverage rate for HD medications in this study, HD symptoms, including chorea, may be undertreated in Israel. PwHD have greater annual HCRU and, consequently, higher medical costs than people without HD in Israel. These real-world data on the epidemiological, clinical, and economic burden of HD may help clinicians and decision-makers better quantify the needs of PwHD in Israel and allocate resources accordingly.
5. Statement of ethics
The study was approved by the Institutional Ethics Committee of Maccabi Health Services (study number 0111-19-MHS). All data were anonymously analyzed, and patient consent was therefore not required. A waiver of consent was granted by the Maccabi Ethics Committee.
Funding sources
This study was funded by Teva Pharmaceutical Industries Ltd., Tel Aviv. The sponsor was involved in the study design, conduct, and analysis, and preparation of the manuscript.
Author contributions
Rinat Ribalov, Ayelet Yaari, Ron Maor, Qais Arow, John Logan, and Tanya Gurevich contributed to the study design and conduct, data interpretation, and drafting of the manuscript. Gabriel Chodick and Yael Barer contributed to the study design and conduct, data analysis and interpretation, and drafting of the manuscript. All authors reviewed and approved the final manuscript for submission.
Data availability statement
Qualified researchers may request access to patient level data and related study documents, including the study protocol and the statistical analysis plan. Requests will be reviewed for scientific merit, product approval status, and conflicts of interest. Patient level data will be de-identified, and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please email USMedInfo@tevapharm.com to make your request.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Yael Barer: Employee of Maccabi Institute for Research & Innovation; Rinat Ribalov: Employee of Teva Pharmaceuticals; Ayelet Yaari: Employee of Teva Pharmaceuticals; Ron Maor: Employee of Teva Pharmaceuticals; Qais Arow: Employee of Teva Pharmaceuticals; John Logan: Employee of Teva Pharmaceuticals at the time of this reasearch; Gabriel Chodick: Employee of Maccabi Institute for Research & Innovation; Tanya Gurevich: Director, Movement Disorders Unit, Israeli National Huntington’s Disease Center, Neurological Institute, Tel-Aviv Sourasky Medical Center, Sackler School of Medicine and Sagol School of Neuroscience, Tel Aviv University. Tanya Gurevich served as a consultant and/or scientific advisor for Teva, Neuroderm, AbbVie, Allergan, Truemed and Medison. She has received research support from Parkinson’s Foundation, Israel Innovation Authority, and BrainBoost Program at the Sagol School of Neuroscience, Tel Aviv University.
Acknowledgments
We thank Melanie Chen, PharmD (Cello Health Communications/MedErgy with funding from Teva Pharmaceuticals), for editorial assistance in the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prdoa.2023.100208.
Contributor Information
Yael Barer, Email: barer_y@mac.org.il.
Rinat Ribalov, Email: Rinat.Ribalov@teva.co.il.
Ayelet Yaari, Email: Ayelet.Yaari@teva.co.il.
Ron Maor, Email: Ron.Maor@teva.co.il.
Qais Arow, Email: Qais.Arow01@teva.co.il.
John Logan, Email: John.Logan@teva.ie.
Gabriel Chodick, Email: hodik_g@mac.org.il.
Tanya Gurevich, Email: tanyag@tlvmc.gov.il.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ross C.A., Reilmann R., Cardoso F., McCusker E.A., Testa C.M., Stout J.C., Leavitt B.R., Pei Z., Landwehrmeyer B., Martinez A., Levey J., Srajer T., Bang J., Tabrizi S.J. Movement Disorder Society Task Force viewpoint: Huntington's disease diagnostic categories. Mov. Disord. Clin. Pract. 2019;6(7):541–546. doi: 10.1002/mdc3.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross C.A., Aylward E.H., Wild E.J., Langbehn D.R., Long J.D., Warner J.H., Scahill R.I., Leavitt B.R., Stout J.C., Paulsen J.S., Reilmann R., Unschuld P.G., Wexler A., Margolis R.L., Tabrizi S.J. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 2014;10(4):204–216. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- 3.Thorley E.M., Iyer R.G., Wicks P., Curran C., Gandhi S.K., Abler V., Anderson K.E., Carlozzi N.E. Understanding how chorea affects health-related quality of life in Huntington disease: an online survey of patients and caregivers in the United States. Patient. 2018;11(5):547–559. doi: 10.1007/s40271-018-0312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McColgan P., Tabrizi S.J. Huntington's disease: a clinical review. Eur. J. Neurol. 2018;25(1):24–34. doi: 10.1111/ene.13413. [DOI] [PubMed] [Google Scholar]
- 5.Xenazine® (tetrabenazine). Prescribing Information, Lundbeck, 2020.
- 6.Austedo® (deutetrabenazine) tablets. Prescribing Information, Teva Neuroscience, Inc. 2023.
- 7.Rawlins M.D., Wexler N.S., Wexler A.R., Tabrizi S.J., Douglas I., Evans S.J., Smeeth L. The prevalence of Huntington's disease. Neuroepidemiology. 2016;46(2):144–153. doi: 10.1159/000443738. [DOI] [PubMed] [Google Scholar]
- 8.Jones C., Busse M., Quinn L., Dawes H., Drew C., Kelson M., Hood K., Rosser A., Edwards R.T. The societal cost of Huntington's disease: are we underestimating the burden? Eur. J. Neurol. 2016;23(10):1588–1590. doi: 10.1111/ene.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva-Paredes G., Urbanos-Garrido R.M., Inca-Martinez M., Rabinowitz D., Cornejo-Olivas M.R. Economic burden of Huntington's disease in Peru. BMC Health Serv. Res. 2019;19(1):1017. doi: 10.1186/s12913-019-4806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung V.W., Iyer R.G., Gandhi S.K., Abler V., Davis B., Irwin D.E., Anderson K.E. Retrospective analysis of healthcare resource use, treatment patterns, and treatment-related events in patients with Huntington’s disease-associated chorea initiated on tetrabenazine. J. Health Econ. Outcomes Res. 2018;6(1):15–24. doi: 10.36469/9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Divino V., Dekoven M., Warner J.H., Giuliano J., Anderson K.E., Langbehn D., Lee W.C. The direct medical costs of Huntington's disease by stage. A retrospective commercial and Medicaid claims data analysis. J. Med. Econ. 2013;16(8):1043–1050. doi: 10.3111/13696998.2013.818545. [DOI] [PubMed] [Google Scholar]
- 12.Coresh J., Turin T.C., Matsushita K., Sang Y., Ballew S.H., Appel L.J., Arima H., Chadban S.J., Cirillo M., Djurdjev O., Green J.A., Heine G.H., Inker L.A., Irie F., Ishani A., Ix J.H., Kovesdy C.P., Marks A., Ohkubo T., Shalev V., Shankar A., Wen C.P., de Jong P.E., Iseki K., Stengel B., Gansevoort R.T., Levey A.S. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. J. Am. Med. Assoc. 2014;311(24):2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chodick G., Heymann A.D., Shalev V., Kookia E. The epidemiology of diabetes in a large Israeli HMO. Eur. J. Epidemiol. 2003;18(12):1143–1146. doi: 10.1023/b:ejep.0000006635.36802.c8. [DOI] [PubMed] [Google Scholar]
- 14.Weitzman D., Chodick G., Shalev V., Grossman C., Grossman E. Prevalence and factors associated with resistant hypertension in a large health maintenance organization in Israel. Hypertension. 2014;64(3):501–507. doi: 10.1161/HYPERTENSIONAHA.114.03718. [DOI] [PubMed] [Google Scholar]
- 15.Y. Fishler, A. Chetrit, M. Barchana, B. Modan, Estimation of completeness of the Cancer Registry in Israel, 2003.
- 16.Israel Central Bureau of Statistics, The 1995 Census of Population and Housing.
- 17.Paulsen J.S., Long J.D., Ross C.A., Harrington D.L., Erwin C.J., Williams J.K., Westervelt H.J., Johnson H.J., Aylward E.H., Zhang Y., Bockholt H.J., Barker R.A. Prediction of manifest Huntington's disease with clinical and imaging measures: a prospective observational study. Lancet Neurol. 2014;13(12):1193–1201. doi: 10.1016/S1474-4422(14)70238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorsey E. Characterization of a large group of individuals with huntington disease and their relatives enrolled in the COHORT study. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0029522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biglan K.M., Shoulson I., Kieburtz K., Oakes D., Kayson E., Shinaman M.A., Zhao H., Romer M., Young A., Hersch S., Penney J., Marder K., Paulsen J., Quaid K., Siemers E., Tanner C., Mallonee W., Suter G., Dubinsky R., Gray C., Nance M., Bundlie S., Radtke D., Kostyk S., Baic C., Caress J., Walker F., Hunt V., O'Neill C., Chouinard S., Factor S., Greenamyre T., Wood-Siverio C., Corey-Bloom J., Song D., Peavy G., Moskowitz C., Wesson M., Samii A., Bird T., Lipe H., Blindauer K., Marshall F., Zimmerman C., Goldstein J., Rosas D., Novak P., Caviness J., Adler C., Duffy A., Wheelock V., Tempkin T., Richman D., Seeberger L., Albin R., Chou K.L., Racette B., Perlmutter J.S., Perlman S., Bordelon Y., Martin W., Wieler M., Leavitt B., Raymond L., Decolongon J., Clarke L., Jankovic J., Hunter C., Hauser R.A., Sanchez-Ramos J., Furtado S., Suchowersky O., Klimek M.L., Guttman M., Sethna R., Feigin A., Cox M., Shannon B., Percy A., Dure L., Harrison M., Johnson W., Higgins D., Molho E., Nickerson C., Evans S., Hobson D., Singer C., Galvez-Jimenez N., Shannon K., Comella C., Ross C., Saint-Hilaire M.H., Testa C., Rosenblatt A., Hogarth P., Weiner W., Como P., Kumar R., Cotto C., Stout J., Brocht A., Watts A., Eberly S., Weaver C., Foroud T., Gusella J., MacDonald M., Myers R., Fahn S., Shults C. Clinical-genetic associations in the Prospective Huntington at Risk Observational Study (PHAROS): implications for clinical trials. JAMA Neurol. 2016;73(1):102–110. doi: 10.1001/jamaneurol.2015.2736. [DOI] [PubMed] [Google Scholar]
- 20.Tabrizi S.J., Scahill R.I., Owen G., Durr A., Leavitt B.R., Roos R.A., Borowsky B., Landwehrmeyer B., Frost C., Johnson H., Craufurd D., Reilmann R., Stout J.C., Langbehn D.R. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12(7):637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- 21.Pringsheim T., Wiltshire K., Day L., Dykeman J., Steeves T., Jette N. The incidence and prevalence of Huntington's disease: a systematic review and meta-analysis. Mov. Disord. 2012;27(9):1083–1091. doi: 10.1002/mds.25075. [DOI] [PubMed] [Google Scholar]
- 22.Bates G.P., Dorsey R., Gusella J.F., Hayden M.R., Kay C., Leavitt B.R., Nance M., Ross C.A., Scahill R.I., Wetzel R., Wild E.J., Tabrizi S.J. Huntington disease. Nat. Rev. Dis. Primers. 2015;1:15005. doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- 23.Panas M., Karadima G., Vassos E., Kalfakis N., Kladi A., Christodoulou K., Vassilopoulos D. Huntington's disease in Greece: the experience of 14 years. Clin. Genet. 2011;80(6):586–590. doi: 10.1111/j.1399-0004.2010.01603.x. [DOI] [PubMed] [Google Scholar]
- 24.Ricco M., Vezzosi L., Balzarini F., Gualerzi G., Ranzieri S. Prevalence of Huntington disease in Italy: a systematic review and meta-analysis. Acta Biomed. 2020;91(3-S):119–127. doi: 10.23750/abm.v91i3-S.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claassen D.O., Iyer R.G., Shah-Manek B., DiBonaventura M., Abler V., Sung V.W. Tetrabenazine treatment patterns and outcomes for chorea associated with Huntington disease: a retrospective chart review. J. Huntingtons Dis. 2018;7(4):345–353. doi: 10.3233/JHD-170286. [DOI] [PubMed] [Google Scholar]
- 26.Mendizabal A., Ngo Vu A.T., Thibault D., Gonzalez-Alegre P., Willis A. Hospitalizations of children with Huntington’s Disease in the United States. Mov. Disord. Clin. Pract. 2017;4(5):682–688. doi: 10.1002/mdc3.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher F., Andrews S., Churchyard A., Mathers S. Home or residential care? The role of behavioral and psychosocial factors in determining discharge outcomes for inpatients with Huntington's Disease. J. Huntingtons Dis. 2012;1(2):187–193. doi: 10.3233/JHD-120022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents, including the study protocol and the statistical analysis plan. Requests will be reviewed for scientific merit, product approval status, and conflicts of interest. Patient level data will be de-identified, and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please email USMedInfo@tevapharm.com to make your request.