Highlights
-
•
Exosomal miRNAs including miR-223-3p from tumor associated macrophages can be delivered into 4T1 cells.
-
•
miR-223-3p promotes pulmonary metastasis of 4T1 cells.
-
•
Cbx5 is a novel target of miR-223-3p.
Keywords: TAMs, Exosomes, miR-223-3p, Cbx5, Metastasis
Abstract
Research about the effect of exosomes derived from tumor associated macrophages (TAM-exos) in the distant organ metastasis of breast cancer is limited. In this study, we found that TAM-exos could promote the migration of 4T1 cells. Through comparing the expression of microRNAs in 4T1 cells, TAM-exos, and exosomes from bone marrow derived macrophages (BMDM-exos) by sequencing, miR-223-3p and miR-379-5p were screened out as two noteworthy differentially expressed microRNAs. Furthermore, miR-223-3p was confirmed to be the reason for the improved migration and metastasis of 4T1 cells. The expression of miR-223-3p was also increased in 4T1 cells isolated from the lung of tumor-bearing mice. Cbx5, which has been reported to be closely related with metastasis of breast cancer, was identified to be the target of miR-223-3p. Based on the information of breast cancer patients from online databases, miR-223-3p had a negative correlation with the overall survival rate of breast cancer patients within a three-year follow-up, while Cbx5 showed an opposite relationship. Taken together, miR-223-3p in TAM-exos can be delivered into 4T1 cells and exosomal miR-223-3p promotes pulmonary metastasis of 4T1 cells by targeting Cbx5.
Introduction
According to the Global Cancer Statistics 2020, breast cancer ranks first for its highest incidence and mortality in female malignancies [1]. Estimated new cases of breast cancer in the United States in 2022 are projected to account for nearly one-third of all new cancer diagnoses [2]. Distant organ metastases are responsible for the incurability with current therapies and have a significant impact on prognosis [3]. The organ tropism of metastatic breast cancer includes bone, lung, liver and brain in sequence according to the occurrence rate in metastatic cases [4]. Pulmonary metastasis has drawn much attention as it is associated with both a relative high morbidity and mortality rate. Investigation of the molecular mechanisms behind the metastasis can offer some new targets and strategies in the future therapies. The process of cancer spread is multi-step and inefficient. Only when the tumor cells have escaped from the primary lesion, succeeded in the intravasation, survived in the circulation, adapted to the new microenvironment after extravasation, can they colonize in the distant organs and form metastatic lesions. This complicated process can't be completed without supports from other cells, such as macrophages.
Macrophages is the major component of tumor infiltrating immune cells, which accounts for about 30-50% of the total. It has been summarized that through expressing cytokines, chemokines, enzymes, exosomes et al, tumor associated macrophages (TAMs) could promote invasiveness and metastasis of tumor cells both in the primary lesions and in the pre-metastatic niches [5]. Exosome is a type of membranous vesicle which could be produced by various kinds of cells. It communicates between cells and delivers messages by encapsulating proteins, lipids and nucleic acids. It is reported that integrins α6β4 and α6β1 on tumor cells exosomes determine lung metastasis while αvβ5 is related to liver metastasis [6]. Exosomes from macrophages have also been found to help cancer cells to spread [7]. Yue et al demonstrated that when the gene progranulin in macrophages is knocked out, the ability of macrophages derived exosomes to accelerate invasion and migration is impaired [8]. However, Xu et al discovered that exosomes from THP-1 induced macrophages could significantly inhibit proliferation and metastasis of breast cancer cells [9]. Treating tumor bearing mice with exosomes from M1-polarized macrophages obviously reduced the tumor burden [10]. These seemingly contradictory results suggest that the contents in the exosomes is of great importance. microRNAs are common cargoes loaded by exosomes that can be transferred into another cell. As reported by Zhang et al, PTEN-targeting miRNAs derived from exosomes of astrocytes initiate brain metastasis outgrowth after being transferred into breast cancer cells [11]. miR-16 and miR-148a contained in the cancer-associated fibroblasts exosomes function as inhibitors of tumor cells migration and proliferation [12]. Our previous data also proved that tumor exosomal miR-183 induced IL-1β, IL-6 and TNF-α secretion from macrophages [13]. Since most of the current research focuses on exosomes secreted by cancer cells, the relationship between exosomes produced by TAMs, especially miRNAs contained within them, and metastasis of breast cancers remains largely unclear.
In this study, we aim to figure out the role of exosomal miRNAs derived from TAMs in the lung metastasis of breast cancer.
Materials and Methods
Stimulation of TAMs and cell culture
For the induction of bone marrow derived macrophages (BMDMs), primary cells were isolated from tibias and femurs of Balb/c mice and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin and 30 ng/ml recombinant murine macrophage colony-stimulating factor (M-CSF). Replacing with fresh medium containing M-CSF of same concentration every third day, BDMDs can be obtained at day 6. If the BMDMs were further cultured with 4T1-conditioned medium for 2 days, TAMs were generated in vitro (Fig. 1A). The murine breast cancer cell line 4T1 was a kindly gift from Prof. R. A. Reisfeld (The Scripps Institute, La Jolla, CA). 4T1 cells and its derivatives, whose construction will be introduced in the following parts, were cultured in RPMI-1640 medium with 10% FBS. Human-derived breast cancer cell line MDA-MB-231 and human acute monocytic leukemia cell THP-1 were purchased from American Type Culture Collection (ATCC) and cultured as guidelines. THP-1 cells were stimulated into macrophages with a final concentration of PMA at 320nM 6h. Then the culture supernatant of MDA-MB-231 was added to induce tumor associated macrophages.
Fig. 1.
TAMs derived exosomes promote tumor cells migration. (A) Preparation of TAMs derived exosomes. (B) TEM images of exosomes. (C) exosomes detected by NTA. (D) exosomes identified by western blot. (E) transwell assays of 4T1 cells co-cultured with exosomes from BMDM (BMDM-exos) or TAMs (TAM-exos). (F) statistics of migrated cells in transwell assays. (G) Wound healing assays of 4T1 cells co-cultured with BMDM-exos or TAM-exos. (H) statistics of wound closure in wound healing assays.
Isolation and characterization of exosomes
Supernatants of around 1 × 108 BMDMs or TAMs cultured for three days were collected. Centrifuging at 1000 g for 10 mins to remove cells and cell debris. Then the supernatants were filtered through 0.22 µm filter (SLGLP033RB, Millipore) to remove larger vesicles, following by ultracentrifuging at 100,000 g for 4 h at 4°C (optima L-100XP, 70Ti, BECKMAN). The pellets were supposed to be exosomes and dissolved in PBS for identification.
Samples at a concentration of 3 × 107 particles/ml were used for nanoparticle tracking analysis (NTA) (Particle Metrix, Meerbusch, Germany). The data was analyzed by ZetaView 8.04.02 SP2.
For transmission electron microscopy (TEM) detection, drop 50 µl exosomes suspension onto a 200-mesh copper grid and incubate at room temperature for 5mins. 1% phosphotungstic acid was used for negative staining. Wash the copper grids with distilled water droplets for twice. When the copper grid dries, it can be examined under a TEM-1400plus transmission electron microscope at 80 KV.
Alix and TSG101 are typical biomarkers often used to identify exosomes [14,15] Their levels in our extracts were detected by western blot. GAPDH was used as a negative control. Antibody against Alix (ab117600, abcam,1:1000 dilution), TSG101 (ab30871, abcam, 1:1000 dilution), GAPDH (ab36233, Multi Sciences, 1:2000 dilution) were used. The final images were recorded by chemiluminescence image analysis system (5200, Tanon).
Construction of 4T1miR-223-3p and 4T1miR-379-5p
The precursor sequence of miR-223 or miR-379 was respectively inserted into lentivirus backbone plasmid LV10N (U6/mcherry&Puro) provided by Shanghai GenePharma Co., Ltd. 4T1 cells were infected by packaged lentivirus with 8 µg/ml polybrene (107689, sigma). 48 hours after infection, 2 mg/ml puromycin was used to screen for the cells with a stable expression of corresponding microRNA.
Animal experiments
Female Balb/c mice were purchased from SiPeiFu (Beijing) Biotechnology Co., Ltd. 2 × 105 breast cancer cells (4T1, 4T1miR-223-3p or 4T1miR-379-5p) resuspended in 100 µl PBS were inoculated into the fourth mammary pad of each mouse. The weight of mouse and volume of tumor were recorded every three days since day6. All the mice were sacrificed at day26. Tumor and lung of each mouse were obtained for further analysis. These experiments were approved by the Animal Ethics Committee of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences.
miRNA sequencing
RNAs in exosomes derived from TAMs or BMDMs were extracted by miRNeasy Mini Kit (217004, Qiagen). For each group, three repetitive samples were prepared and sent to Guangzhou Ribobio Co., Ltd. The miRNA sequencing was conducted by Hiseq 2500 system, and the data was analyzed by Agilent 2200. The miRNA sequencing data has been submitted and can be achieved from BioProject (ID:PRJNA807949. Website: http://www.ncbi.nlm.nih.gov/bioproject/807949).
Quantitative real-time PCR
TaqMan MicroRNA Reverse Transcription Kit (4366596, ThermoFisher) was used for reverse transcription of microRNA. TaqMan Fast Advanced Mix (4444556, ThermoFisher) was used for quantitative real-time PCR (qPCR). TaqMan MicroRNA Assays for miR-223-3p/miR-379-5p/U6 (ID: 002295; 001138; 001973) (4427975, ThermoFisher) included the primers and probes needed for reverse transcription and qPCR.
For the detection of miRNA target genes, TransScript® Uni All-in-One First-Strand cDNA Sysnthesis SuperMix (AU341-02, TransGen) and PerfectStart Green qPCR SuperMix (AQ601-04, TransGen) were used. qPCR primers for each target gene in mice were listed as follows: Rasa1-F: TGTGGTGATTACTACATTGGTGG; Rasa1-R: CGCCTTCTATCTTCTACTGGCTC. Fat1-F: CTACGGAGGAACGTGCATGG; Fat1-R: ATCTTTGCAGTACGGACTAAGC. Mef2c-F: ATCCCGATGCAGACGATTCAG; Mef2c-R: AACAGCACACAATCTTTGCCT. Ankrd17-F: TTGGACCAGGATGATTTGGAGA; Ankrd17-R: GCCGTCGATAACTTGCCTATTC. Rbpj-F: AGTTGCACAGAAGTCTTACGG; Rbpj-R: CCTATTCCAATAAACGCACAGGG. Kpna3-F: TCGGGAACTTCTGCACAGAC; Kpna3-R: ACACCGCTTGTTCACAAACATT. Pds5b-F: GGACTGCTCAAGCTATTGAACC; Pds5b-R: ACCTGGAGGCGTTCTTCATTAT. Mbnl1-F: CAAATGCAGTTAGCCAATGCC; Mbnl1-R: GTAAGGGTTAAAGGCTGCTGAT. Ptbp2-F: ATGGACGGAATTGTCACTGAGG; Ptbp2-R: TGCCACTCATATTAGAGTTGGGG. Cbx5-F: GACAGGCGCATGGTTAAGG; Cbx5-R: CCTGGGCTTATTGTTTTCACCC. Cbfb-F: CCGCGAGTGCGAGATTAAGTA; Cbfb-R: GTTCTGGAAGCGTGTCTGG.
Transwell and wound healing assays
The concentration of 4T1 cells was adjusted to 2 × 106 cells/ml and 100 µl cell suspension was seeded into transwell inserts (3422, Corning). The outer well was filled with complete medium with or without exosomes. After incubation for 12 h, the permeable supports were fixed and stained with 0.1% crystal violet (G1063, Solarbio). Take pictures under a microscope and the analysis was performed with Image J software.
For wound healing assays, 2 × 105 4T1 cells were seeded into 24-well plate. When the confluence of cells was close to 90%, a straight scratch wound was created with a sterile pipette tip. Then the width of the scratch was measured at 0 h, 12 h and 24 h.
Hematoxylin and eosin staining
Paraffin-embedded sections were deparaffinized by immersing the slides into ethanol with gradient concentration. After rehydration, the slides were stained with Harris hematoxylin (BSBA-4097, ZSGB-BIO), differentiated with acid alcohol, blued with ammonia water, and counterstained with eosin (ZLI-9613, ZSGB-BIO) in sequence. Apply a drop of neutral balsam (10004160, Sinopharm) to each slide and cover it with a coverslip. The slides can be observed using a microscope.
Dual-luciferase reporter-system assays
The sequence predicted to be bound by miR-223-3p in the 3’UTR of Cbx5 was cloned into psiCHECK-2 vector (C8021, Promega), locating at the 3’UTR of synthetic renilla luciferase gene. A mutant vector of which the miR-223-3p binding base pairs were mutated was constructed at the same time. After co-transfection of the Cbx5-WT-psiCHECK-2 vector or the Cbx5-mut-psiCHECK-2 vector with miR-223-3p, the firefly luciferase and renilla luciferase activities were detected using a dual-luciferase reporter assay system (E1910, Promega).
Statistical analysis
Data for survival analysis were obtained from the KMplotter database (https://kmplot.com/analysis/). Statistical analysis and plots were drawn with Hiplot (https://hiplot.com.cn/). Correlation analysis of miRNA and target genes was performed using the ENCORI Pan-Cancer Analysis Platform. The rest of statistical analyses were performed using GraphPad Prism 8 software. Quantitative data were analyzed using a two-tailed, unpaired Student's t-test (two groups). The growth curves of tumor between two groups were compared using two-way ANOVA. Data are shown as mean ± sem. p < 0.05 was used as a criterion for statistical significance (*, p < 0.05; **, p < 0.01; ***, p < 0.001)
Results
exosomes derived from TAMs (TAM-exos) promote tumor cells migration
According to our previous data, BMDMs stimulated with 4T1 cells conditional medium shared similar characteristics with TAMs isolated from 4T1 tumor-bearing mice [13]. Exosomes were collected from supernatant of conditional medium induced TAMs in vitro as specified in Fig. 1A. And the extracts were identified by TEM (Fig. 1B), NTA (Fig. 1C) and western blot (Fig. 1D). Then we added the exosomes into 4T1 culture medium. The exosomes derived from BMDMs (BMDM-exos) were used as a control. Transwell assay proved that TAM-exos significantly enhanced the migration ability of breast cancer cells (Fig. 1E-F). The wound healing assay showed the same trend (Fig. 1G-H). Similar tests were repeated in human-derived breast cancer cell line MDA-MB-231. Human acute monocytic leukemia cell line THP-1 was induced into tumor associated macrophage and its exosomes (TAM-exos) were collected. Exosomes from original THP-1 (THP-1-exos) were used as a control. After stimulation with the TAM-exos, the migration and invasion abilities of MDA-MB-231 were also significantly elevated (Fig. S1B and S1C).
miR-223-3p and miR-379-5p are differentially expressed microRNAs in TAM-exos
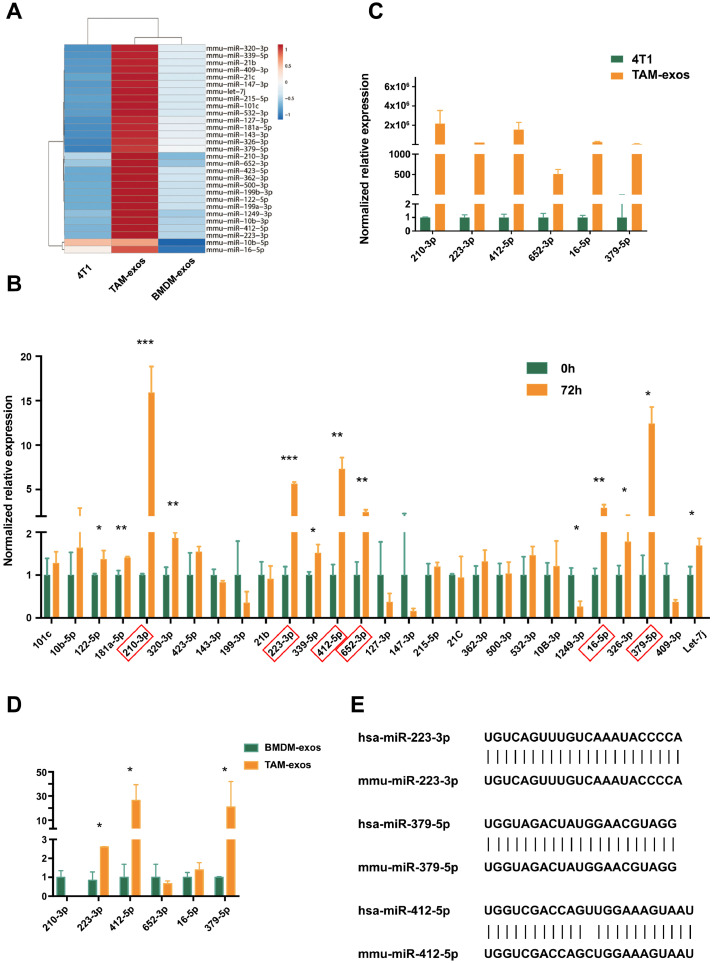
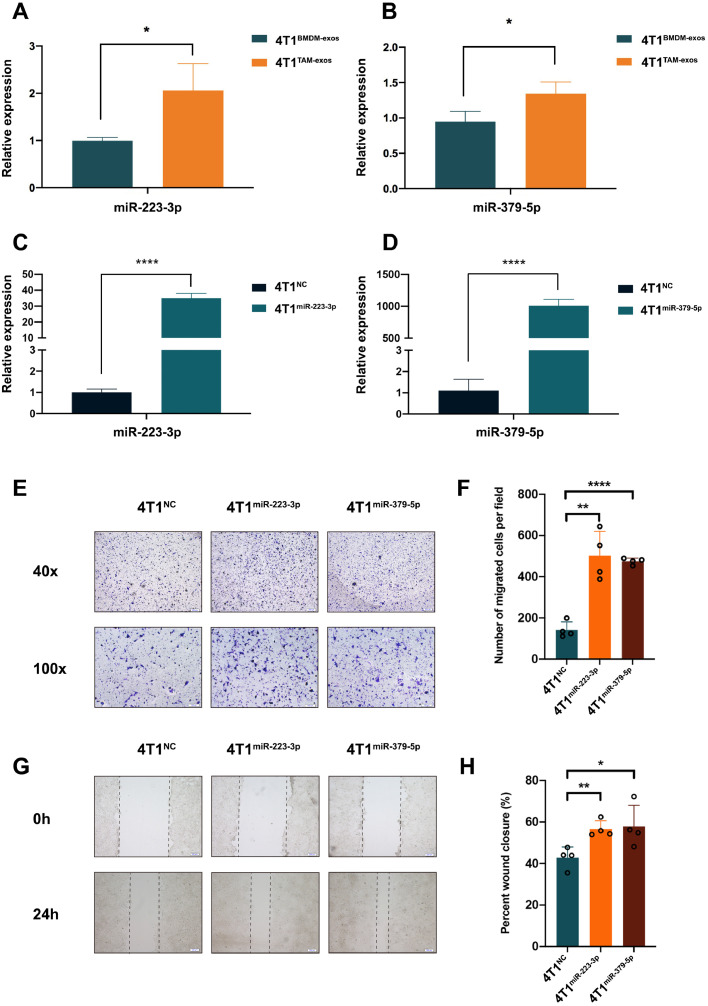
In order to figure out the key microRNAs in the TAM-exos which contributed to the migration of cancer cells, next-generation sequencing was performed. The expression levels of key microRNAs that delivered from TAM-exos to cancer cells were supposed to be higher in TAM-exos than in BMDM-exos. In addition, their constitutive expression in the 4T1 cells should be low, so that the transmitted microRNAs could cause obvious phenotypic changes in recipient cells. Thus, the eligible microRNAs were clustered and listed in Fig. 2A. Then we checked the levels of these microRNAs in 4T1 cells before or after co-culturing with TAMs by qPCR. As shown in Fig. 2B, six microRNAs (miR-210-3p, miR-223-3p, miR-412-5p, miR-652-3p, miR-16-5p, miR-379-5p) increased significantly after the co-culture for 72h and the fold change is greater than two. In the meanwhile, the qPCR results confirmed that the expression levels of the six microRNAs were hundreds of times higher in TAMs derived exosomes than that of 4T1 cells (Fig. 2C). Further comparison between TAM-exos and BMDM-exos narrowed the candidates down to three microRNAs, that is miR-223-3p, miR-412-5p, miR-379-5p (Fig. 2D). Analysis based on the Oncomir database (http://www.oncomir.org/cgi-bin/dbSearch.cgi) showed that the expression level of miR-412-5 was very low, while miR-223-3p and miR-379-5p were highly expressed in breast cancer (Fig. S1E). In addition, an overall survival analysis of miR-412-5p demonstrated that people with high and low expression of miR-412-5p in breast cancer did not have a bias in the overall survival time (Fig. S1F). Since sequences of mature miR-223-3p and miR-379-5p in mice were highly homologous to those in human (Fig. 2E), these two microRNAs were selected for further analysis. When TAM-exos were added into the cultural supernatant, the levels of both miR-223-3p and miR-379-5p (Fig. 3A and B) were significantly elevated in 4T1 cells, comparing to BMDM-exos. This result was confirmed in MDA-MB-231. After stimulating with the THP-1 induced TAM-derived exosomes (TAM-exos), the expression of miR-223-3p in MDA-MB- 231 was significantly higher than that with THP-1-derived exosomes (THP1-exos) (Fig. S1A).
Fig. 2.
Screening for differentially expressed miRNA in TAMs derived exosomes. (A) cluster of differentially expressed miRNAs among 4T1, TAM-exos, BMDM-exos. (B) comparing the expression levels of 28 candidate miRNAs in 4T1 cells before and after co-culture with TAMs for 72h by qPCR. (C) comparing the expression of 6 further screened candidate miRNAs in 4T1 cells with those in TAM-exos. (D) comparing the expression of 6 candidate miRNAs in BMDM-exos with those in TAM-exos. (E) sequence of mature miRNAs in human and mice.
Fig. 3.
The influence of miR-223-3p and miR-379-5p on the migration of 4T1 cells in vitro. (A-B) confirming the expression of miR-223-3p or miR-379-5p in 4T1 cells co-cultured with BMDM-exos or TAM-exos. (C-D) confirming the expression of miR-223-3p or miR-379-5p in 4T1 cells stably overexpressed with miR-223-3p or miR-379-5p. (E) transwell assays of 4T1NC, 4T1miR-223-3p and 4T1miR-379-5p cells. (F) statistics of migrated cells in transwell assays. (G) Wound healing assays of 4T1NC, 4T1miR-223-3p and 4T1miR-379-5p cells. (H) statistics of wound closure in wound healing assays.
miR-223-3p enhance the migration and metastasis of 4T1 cells
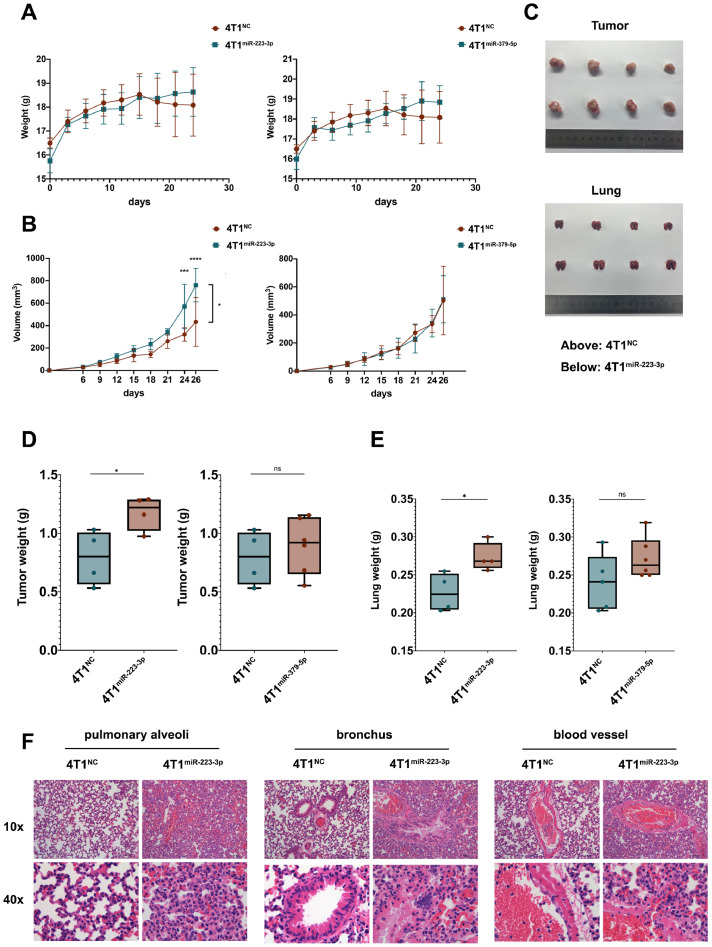
Then 4T1 cells stably over-expressed with miR-223-3p or miR-379-5p (marked as 4T1miR-223-3p or 4T1miR-379-5p) were constructed (Fig. 3C and D). It is proved that both miR-223-3p and miR-379-5p could increase the migration of 4T1 cells (Fig. 3E and F). So were their effects on the motility of 4T1 cells (Fig. 3G and H). When inoculating these breast cancer cells into the mammary fat pad of Balb/c mice, there is a tendency for the 4T1miR-223-3p group or the 4T1miR379-5p group to gain weight at the point of last observation, comparing with the 4T1NC group (Fig. 4A). Tumor volume was measured every three days and the data showed that tumors induced by 4T1miR-223-3p but not 4T1miR-379-5p grew more quickly (Fig. 4B). After the mice were sacrificed, tumors and lungs were removed and photographed (Fig. 4C). Statistical results demonstrated that both the tumors and lungs were heavier in the 4T1miR-223-3p group (Fig. 4D and E). However, no statistically differences exist between the 4T1miR-379-5p and 4T1NC group. Hematoxylin-eosin staining of lung tissues suggested that miR-223-3p could promote pulmonary metastasis of breast cancer 4T1 cells. Representative images near pulmonary alveoli, bronchus and blood vessels were displayed in Fig. 4F. It is worth mentioning that no increase but a decrease in proliferation can be observed in 4T1 cells stably over-expressed with miR-223-3p comparing to 4T1NC cells, indicating that the differences in migration between these two groups were not caused by proliferation (Fig. S1D). There were no more pulmonary metastatic nodules in 4T1miR-379-5p than 4T1NC group (data not shown).
Fig. 4.
miR-223-3p enhance the metastasis of 4T1 cells. (A) weight of mice inoculated with 4T1NC, 4T1miR-223-3p or 4T1miR-379-5p cells. (B) tumor volume. (C) pictures of tumors and lungs from 4T1NC and 4T1miR-223-3p cells inoculated mice. (D) tumor weight. (E) lung weight. (F) hematoxylin and eosin staining of representative images near pulmonary alveoli, bronchus and blood vessel of lung.
miR-223-3p is up-regulated in lung-metastatic 4T1 cells
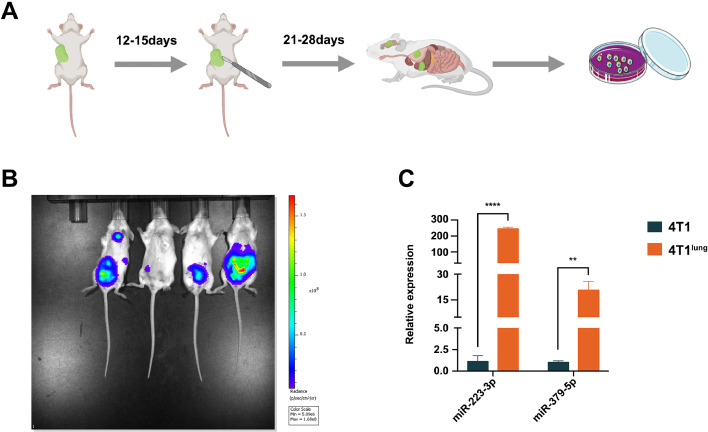
Since it is not easy to obtain the tumor cells at the metastatic nodules of breast cancer patients, metastatic cancer cells were isolated from lungs, livers, brains and kidneys of tumor-bearing mice which were inoculated with 4T1 cells in the mammary fat pad 28 days after the tumor in situ were removed (Fig. 5A). The isolated cancer cells from lung were inoculated in mammary fat pad and isolated from lung again. The screening was repeated for three times. The similar strategy was used in cancer cells isolated from livers, brains and kidneys. It is worth mentioning that in this experiment, a luciferase gene had been transferred into 4T1 cells by lentivirus before inoculation. So when the 4T1lung, 4T1liver, 4T1brain or 4T1kidney was respectively injected into the mammary fat pad of mice, their metastasis can be observed by Xenogen IVIS spectrum imaging system. And the image confirmed that 4T1lung had a high propensity for pulmonary metastasis (Fig. 5B). But the 4T1liver, 4T1brain and 4T1kidney cells failed to metastasize to corresponding organ specifically. Then the expression level of miR-223-3p and miR-379-5p were detected in 4T1 and 4T1lung. As expected, the expression level of both miR-223-3p and miR-379-5p were elevated in 4T1lung (Fig. 5C).
Fig. 5.
Verification of miR-223-3p and miR-379-5p in 4T1lung. (A) strategy of isolating metastatic 4T1lung, 4T1liver, 4T1brain and 4T1kidney from mice inoculated with 4T1 in mammary fat pad. (B) Imaging of mice inoculated with 4T1lung, 4T1liver, 4T1brain and 4T1kidney (from left to right: 4T1lung, 4T1liver, 4T1brain and 4T1kidney). (C) expression of miR-223-3p and miR-379-5p in 4T1 and 4T1lung.
Cbx5 is a target of miR-223-3p
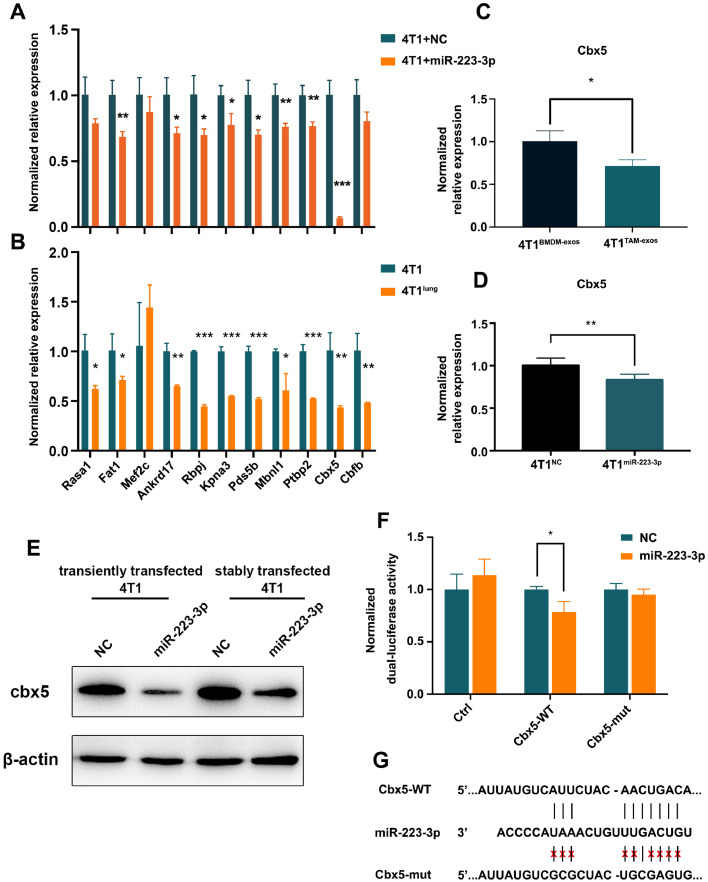
To explain how miR-223-3p functioned as a pulmonary metastasis-promoting factor, its target genes were screened by following rules: 1) the gene was predicted to be a target by no less than two databases among Targetscan, miRDB, PicTar and miRWalk; 2) the gene was down-regulated in lung metastases of breast cancer according to two or more downloaded GEO datasets (GSE54773, GSE63627, GSE110101, GSE56493); 3) it has not been reported as a potential oncogene in breast cancer. Thus, eleven candidate genes (Rasa1, Fat1, Mef2c, Ankrd17, Rbpj, Kpna3, Pds5b, Mbnl1, Ptbp2, Cbx5, Cbfb) were selected. When the mimic of miR-223-3p was transiently transfected into 4T1 cells, the mRNA level of Fat1, Ankrd17, Rbpj, Kpna3, Pds5b, Mbnl1, Ptbp2, Cbx5 were significantly decreased (Fig. 6A). In addition, all the candidate target genes except Mef2c had a lower expression in 4T1lung. As Cbx5 had been widely reported as a down-regulated gene in invasive breast cancer, a suppressor of cell migration and invasion, a positive prognostic factor in breast cancer, and it had not been found to be a target gene of miR-223-3p, we chose it for further confirmation [16], [17], [18], [19]. When co-culturing 4T1 cells with BMDM-exos or TAM-exos, the expression of Cbx5 were significantly reduced in the latter group (Fig. 6C). This reduction reappeared in 4T1 cells stably over-expressed with miR-223-3p (4T1miR-223-3p) (Fig. 6D). In parallel, we also verified the protein expression of Cbx5 in 4T1 cells transiently transfected and stably over-expressed with miR-223-3p (Fig. 6E). Additionally, dual-luciferase report assays demonstrated that miR-223-3p inhibited the luciferase activity, of which 3’UTR was inserted with a fragment of Cbx5 3’UTR sequence (Fig. 6F). However, this inhibition was relieved (Fig. 6F) after the sequence in Cbx5 3’UTR matching with the seed sequence of miR-223-3p was mutated (Fig. 6G).
Fig. 6.
Identifying the target genes of miR-223-3p. (A) mRNA levels of candidate target genes in 4T1 and 4T1 cells transiently transfected with mimic of miR-223-3p. (B) mRNA levels of candidate target genes in 4T1 and 4T1lung cells. (C) expression of Cbx5 in 4T1 cells co-cultured with BMDM-exos or TAM-exos. (D) expression of Cbx5 in 4T1NC and 4T1miR-223-3p cells. (E) Expression of cbx5 by western blot in 4T1 and 4T1miR-223-3p. (F) dual-luciferase report assays. (G) predicted miR-223-3p binding sequence in 3’UTR of Cbx5.
The expression of miR-223-3p is associated with the prognosis of breast cancer and negatively correlated with that of Cbx5
Furthermore, the association between miR-223-3p and overall survival rate of breast cancer patients were analyzed based on GSE40267. The patients with higher expression of miR-223-3p had a relative lower survival rate during 36 months of follow-up (p=0.042) (Fig. 7A). A significant negative correlation existed between the expression of miR-223-3p and Cbx5 based on 1085 breast cancer patients (r=-0.153, p=4.24e-07) (Fig. 7B). In line with previous findings, Cbx5 was strongly associated with good overall survival (p=0.009), recurrence-free survival (p=1.3e-04), distant metastasis-free survival (p=0.0021) of breast cancer patients, lung metastasis-free survival (p=0.035)(Fig. 7C-F).
Fig. 7.
Association between miR-223-3p/Cbx5 and survival rate in breast cancer patients. (A) relationship between miR-223-3p and overall survival rate. (B) correlation of miR-223-3p and Cbx5. (C-F) relationship between Cbx5 and overall survival, recurrence-free survival, metastasis-free survival rate, lung metastasis-free survival rate.
Discussion
It has been summarized that exosomal microRNAs had a close relationship with organotropism in breast cancer metastasis [20]. Our results provided new evidence which came from TAM-exos. According to our data, TAM-exos encapsulating with plenty of differential microRNAs enhanced the capability of tumor cells to metastasize. In particular, exosomal miR-223-3p was confirmed to be delivered into 4T1 cells and function as a tumor-promoting factor. miR-223-3p had been reported to be an oncogene in various cancers including breast cancer by a lot of researches. It could promote proliferation, invasion and migration of breast cancer cells and activate Hippo/Yap signaling pathway [21]. In addition, miR-223-3p promotes epithelial-mesenchymal transition and metastasis through the Wnt/β-catenin signaling pathway or different targets [22,23]. Our study confirmed that the way of mediating metastasis is not through cell proliferation. It was also found to be elevated in serum exosomes of breast cancer patients and FOXO1 was its target gene [24]. Simultaneous inhibition of miR-223-3p in breast cancer patients treated with celastrol significantly enhanced the anti-tumor effects [25]. Gap junction-mediated transportation of miR-223 from bone marrow stromal cells reduced the expression of CXCL12, contributing to the quiescence of breast cancer cells [26]. There was also evidence indicated that miR-223-3p was shuttled into breast cancers by microvesicles produced by IL-4-activated macrophages [27]. Since IL-4 activated macrophages didn't fully represent TAMs, our rounds of selection demonstrated that TAM-exos was an important source of elevated miR-223-3p in breast cancer cells and this miRNA played a notable role in the pulmonary metastasis of breast cancer cells.
In addition, this is the first time to prove that Cbx5 is a target of miR-223-3p. Cbx5 encodes heterochromatin protein 1α (HP1α), which is one of the chromobox proteins that recognizes histone 3 lysine tri-methylation at residues K9 (H3K9me3). H3K9me3 preferentially appears in heterochromatin and represents a common marker of transcriptional silencing [28]. The revolutionary conserved protein HP1α contains a chromo domain in the N-terminus, a chromo shadow domain in the C-terminus and a hinge domain linking them. It combines with methylated histones on the chromatin through chromo domain while interacting with other proteins through chromo shadow domain [29]. Structural analysis showed that a symmetric HP1 dimer could provide a bridge between two H3K9me3 nucleosomes [30]. Several mechanisms about heterochromatin proteins especially HP1α in modulating gene transcription have been discovered. For example, the interaction between HP1α and heterochromatin brings two distant regions on the genomic DNA together, leading to a compact state of the chromatin which represses transcription [31]. Besides the interaction between chromo domain and H3K9me3, the flexible hinge domain is essential for the establishment and maintenance of heterochromatin through recognizing parallel RNA and DNA G-quadruplexes [32]. In addition, phosphorylated or DNA-bound HP1α has the ability to form phase-separated droplets, which could induce DNA strands to compact into puncta [33]. Since only 2% of HP1α is located in gene promoter, transcription repression could just explain a small part of its function mechanisms [34]. It is hypothesized that, HP1α may be involved in the process of epithelial-to-mesenchymal transition, which usually occurs at the initial stages of metastasis, through regulating alternative splicing directly or indirectly [29]. More mechanisms are still under exploration. Other genes predicted to be the targets of miR-223-3p from database have also been found to have some anti-tumor effects, such as FAT1, whose significantly decrease has been reported to be associated with poor prognosis and invasiveness in invasive ductal carcinoma [35]; MBNL1 is demonstrated to be significantly downregulated in breast and gastric cancers in several studies, and MBNL1 can interact directly with ZFP36 to downregulate CENPA, thereby inhibiting the proliferation and stemness of breast cancer cells [36,37]. CBFB, together with its binding partner RUNX1, regulates multiple signaling pathways, and breast cancer cells may evade both translational and transcriptional surveillance simultaneously caused by CBFB downregulation [38]. These targets above also corroborate the role of miR-223-3p in promoting metastasis of cancer cells.
In summary, our data screened out that miR-223-3p was enriched in the exosomes of TAMs. It can be delivered into recipient cells along with the uptake of these exosomes by 4T1 cells, leading to a marked elevation of miR-223-3p. Through targeting Cbx5, miR-223-3p played an important role in promoting pulmonary metastasis of 4T1 cells. These findings suggested that exosomal miR-223-3p could possibly be an indicator of breast cancer metastasis and targeting TAMs exosomal miR-223-3p-Cbx5 axis might be a potential treatment strategy to inhibit breast cancer metastasis.
Funding
This project was supported by the Fundamental Research Funds for the Central Universities (3332022181, 3332020033, to Zhaojun Duan), the National Natural Science Foundation of China (NSFC; 81672914, to Yunping Luo) and the Bilateral Inter-Governmental S&T Cooperation Project from Ministry of Science and Technology of China (2018YFE0114300, to Yunping Luo).
CRediT authorship contribution statement
Ziyuan Wang: Data curation, Formal analysis, Validation. Chen Zhang: Supervision. Jian Guo: Methodology. Wei Wang: Methodology. Qin Si: Investigation. Chong Chen: Investigation. Yunping Luo: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. Zhaojun Duan: Conceptualization, Visualization, Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101715.
Contributor Information
Yunping Luo, Email: ypluo@ibms.pumc.edu.cn.
Zhaojun Duan, Email: duanzhaojun@ibms.pumc.edu.cn.
Appendix. Supplementary materials
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. 2022. [DOI] [PubMed] [Google Scholar]
- 3.Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., Ruddy K., Tsang J., Cardoso F. Breast cancer. Nat. Rev. Dis. Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 4.Liang Y., Zhang H., Song X., Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 2020;60:14–27. doi: 10.1016/j.semcancer.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Duan Z., Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. 2021;6:127. doi: 10.1038/s41392-021-00506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., Singh S., Williams C., Soplop N., Uryu K., Pharmer L., King T., Bojmar L., Davies A.E., Ararso Y., Zhang T., Zhang H., Hernandez J., Weiss J.M., Dumont-Cole V.D., Kramer K., Wexler L.H., Narendran A., Schwartz G.K., Healey J.H., Sandstrom P., Labori K.J., Kure E.H., Grandgenett P.M., Hollingsworth M.A., de Sousa M., Kaur S., Jain M., Mallya K., Batra S.K., Jarnagin W.R., Brady M.S., Fodstad O., Muller V., Pantel K., Minn A.J., Bissell M.J., Garcia B.A., Kang Y., Rajasekhar V.K., Ghajar C.M., Matei I., Peinado H., Bromberg J., Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan J., Sun L., Xu F., Liu L., Hu F., Song D., Hou Z., Wu W., Luo X., Wang J., Yuan X., Hu J., Wang G. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 8.Yue S., Ye X., Zhou T., Gan D., Qian H., Fang W., Yao M., Zhang D., Shi H., Chen T. PGRN(-/-) TAMs-derived exosomes inhibit breast cancer cell invasion and migration and its mechanism exploration. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118687. [DOI] [PubMed] [Google Scholar]
- 9.Yu X., Zhang Q., Zhang X., Han Q., Li H., Mao Y., Wang X., Guo H., Irwin D.M., Niu G., Tan H. Exosomes from macrophages exposed to apoptotic breast cancer cells promote breast cancer proliferation and metastasis. J. Cancer. 2019;10:2892–2906. doi: 10.7150/jca.31241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P., Wang H., Huang Q., Peng C., Yao L., Chen H., Qiu Z., Wu Y., Wang L., Chen W. Exosomes from M1-Polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9:1714–1727. doi: 10.7150/thno.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., Zhang S., Yao J., Lowery F.J., Zhang Q., Huang W.C., Li P., Li M., Wang X., Zhang C., Wang H., Ellis K., Cheerathodi M., McCarty J.H., Palmieri D., Saunus J., Lakhani S., Huang S., Sahin A.A., Aldape K.D., Steeg P.S., Yu D. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H.J., Hao M., Yeo S.K., Guan J.L. FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene. 2020;39:2539–2549. doi: 10.1038/s41388-020-1162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo J., Duan Z., Zhang C., Wang W., He H., Liu Y., Wu P., Wang S., Song M., Chen H., Chen C., Si Q., Xiang R., Luo Y. Mouse 4T1 breast cancer cell-derived exosomes induce proinflammatory cytokine production in macrophages via miR-183. J. Immunol. 2020;205:2916–2925. doi: 10.4049/jimmunol.1901104. [DOI] [PubMed] [Google Scholar]
- 14.Laulagnier K., Javalet C., Hemming F.J., Sadoul R. Purification and analysis of exosomes released by mature cortical neurons following synaptic activation. Methods Mol. Biol. 2017;1545:129–138. doi: 10.1007/978-1-4939-6728-5_9. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q., Poulsen S.B., Murali S.K., Grimm P.R., Su X.T., Delpire E., Welling P.A., Ellison D.H., Fenton R.A. Large-scale proteomic assessment of urinary extracellular vesicles highlights their reliability in reflecting protein changes in the kidney. J. Am. Soc. Nephrol. 2021;32:2195–2209. doi: 10.1681/ASN.2020071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen R., Christensen D.B., Rosborg S., Linnet T.E., Blechingberg J., Nielsen A.L. Analysis of HP1alpha regulation in human breast cancer cells. Mol. Carcinog. 2011;50:601–613. doi: 10.1002/mc.20755. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y.K., Lin H.Y., Chen C.F. Zeng, prognostic values of distinct CBX family members in breast cancer. Oncotarget. 2017;8:92375–92387. doi: 10.18632/oncotarget.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu L., Zhang W., Wang P., Zhang Q., Cong A., Yang X., Sang K. LncRNA SNHG11 aggravates cell proliferation and migration in triple-negative breast cancer via sponging miR-2355-5p and targeting CBX5. Exp. Ther. Med. 2021;22:892. doi: 10.3892/etm.2021.10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirschmann D.A., Lininger R.A., Gardner L.M., Seftor E.A., Odero V.A., Ainsztein A.M., Earnshaw W.C., Wallrath L.L., Hendrix M.J. Down-regulation of HP1Hsalpha expression is associated with the metastatic phenotype in breast cancer. Cancer Res. 2000;60:3359–3363. [PubMed] [Google Scholar]
- 20.Wong G.L., Abu Jalboush S., Lo H.W. Exosomal MicroRNAs and organotropism in breast cancer metastasis. Cancers. 2020:12. doi: 10.3390/cancers12071827. Basel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du T., Wang D., Wan X., Xu J., Xiao Q., Liu B. Regulatory effect of microRNA-223-3p on breast cancer cell processes via the Hippo/Yap signaling pathway. Oncol. Lett. 2021;22:516. doi: 10.3892/ol.2021.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L., Liu P., He C., Xu C. miRNA-223-3p regulates ECT2 to promote proliferation, invasion, and metastasis of gastric cancer through the Wnt/beta-catenin signaling pathway. J. Cancer Res. Clin. Oncol. 2023;149:121–134. doi: 10.1007/s00432-022-04453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Shi S., Wang Y., Zhang X., Liu X., Li J., Li P., Du L., Wang C. miR-223-3p targets FBXW7 to promote epithelial-mesenchymal transition and metastasis in breast cancer. Thorac Cancer. 2022;13:474–482. doi: 10.1111/1759-7714.14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei Y.T., Guo D.W., Hou X.Z., Jiang D.Q. miRNA-223 suppresses FOXO1 and functions as a potential tumor marker in breast cancer. Cell. Mol. Biol. 2017;63:113–118. doi: 10.14715/cmb/2017.63.5.21. Noisy-le-grand. [DOI] [PubMed] [Google Scholar]
- 25.Cao L., Zhang X., Cao F., Wang Y., Shen Y., Yang C., Uzan G., Peng B., Zhang D. Inhibiting inducible miR-223 further reduces viable cells in human cancer cell lines MCF-7 and PC3 treated by celastrol. BMC Cancer. 2015;15:873. doi: 10.1186/s12885-015-1909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim P.K., Bliss S.A., Patel S.A., Taborga M., Dave M.A., Gregory L.A., Greco S.J., Bryan M., Patel P.S., Rameshwar P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 27.Yang M., Chen J., Su F., Yu B., Su F., Lin L., Liu Y., Huang J.D., Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachner M., Jenuwein T. The many faces of histone lysine methylation. Curr. Opin. Cell Biol. 2002;14:286–298. doi: 10.1016/s0955-0674(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 29.Vad-Nielsen J., Nielsen A.L. Beyond the histone tale: HP1alpha deregulation in breast cancer epigenetics. Cancer Biol. Ther. 2015;16:189–200. doi: 10.1080/15384047.2014.1001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machida S., Takizawa Y., Ishimaru M., Sugita Y., Sekine S., Nakayama J.I., Wolf M., Kurumizaka H. Structural basis of heterochromatin formation by human HP1. Mol. Cell. 2018;69:385. doi: 10.1016/j.molcel.2017.12.011. 397 e388. [DOI] [PubMed] [Google Scholar]
- 31.Gao Y., Han M., Shang S., Wang H., Qi L.S. Interrogation of the dynamic properties of higher-order heterochromatin using CRISPR-dCas9. Mol. Cell. 2021;81:4287. doi: 10.1016/j.molcel.2021.07.034. 4299 e4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roach R.J., Garavis M., Gonzalez C., Jameson G.B., Filichev V.V., Hale T.K. Heterochromatin protein 1alpha interacts with parallel RNA and DNA G-quadruplexes. Nucleic. Acids Res. 2020;48:682–693. doi: 10.1093/nar/gkz1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larson A.G., Elnatan D., Keenen M.M., Trnka M.J., Johnston J.B., Burlingame A.L., Agard D.A., Redding S., Narlikar G.J. Liquid droplet formation by HP1alpha suggests a role for phase separation in heterochromatin. Nature. 2017;547:236–240. doi: 10.1038/nature22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeRoy G., Chepelev I., DiMaggio P.A., Blanco M.A., Zee B.M., Zhao K., Garcia B.A. Proteogenomic characterization and mapping of nucleosomes decoded by Brd and HP1 proteins. Genome Biol. 2012;13:R68. doi: 10.1186/gb-2012-13-8-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., Lyu S., Wang S., Shen H., Niu F., Liu X., Liu J., Niu Y. Loss of FAT1 during the progression from DCIS to IDC and predict poor clinical outcome in breast cancer. Exp. Mol. Pathol. 2016;100:177–183. doi: 10.1016/j.yexmp.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Ding Y., Li Y., Duan Y., Wang W., Zheng W., Cheng W., Qi Y., Feng J., Chen Z., Yu T., Hu A., Wang T., Li M., Zhang H., Li Y., Ma F., Guo B. LncRNA MBNL1-AS1 represses proliferation and cancer stem-like properties of breast cancer through MBNL1-AS1/ZFP36/CENPA Axis. J. Oncol. 2022;2022 doi: 10.1155/2022/9999343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su J., Chen D., Ruan Y., Tian Y., Lv K., Zhou X., Ying D., Lu Y. LncRNA MBNL1-AS1 represses gastric cancer progression via the TGF-beta pathway by modulating miR-424-5p/Smad7 axis. Bioengineered. 2022;13:6978–6995. doi: 10.1080/21655979.2022.2037921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malik N., Yan H., Moshkovich N., Palangat M., Yang H., Sanchez V., Cai Z., Peat T.J., Jiang S., Liu C., Lee M., Mock B.A., Yuspa S.H., Larson D., Wakefield L.M., Huang J. The transcription factor CBFB suppresses breast cancer through orchestrating translation and transcription. Nat. Commun. 2019;10:2071. doi: 10.1038/s41467-019-10102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.