Highlights
-
•
Cyclovirobuxine inhibits growth of castration-resistant cancer cells.
-
•
Cyclovirbuvine induces apoptosis and cell cycle arrest.
-
•
Cyclovirbuvine promotes the accumulation of DNA damage.
-
•
Cyclovirbuvine controls multiple DNA repiar pathways.
Keywords: Castration-resistant prostate cancer, Cyclovirobuxine, Network pharmacology, DNA repair, Apoptosis
Abstract
Background
Castration-resistant prostate cancer (CRPC) is a deadly malignancy without effective therapeutics. Cyclovirobuxine (CVB) can play an anticancer role by inhibiting mitochondrial function, regulating tumor cell apoptosis, dysregulating autophagy, and other mechanisms. This study aimed to examine the function and mechanism of CVB in CRPC to provide new insights into CRPC treatment.
Methods
The effect of CVB on PC3 and C4-2 cell viability was determined using a CCK8 assay. Core therapeutic targets of CVB in CRPC cells were identified using RNA sequencing, online database, and PPI network analyses. Western blotting, RT–qPCR and molecular docking were performed to evaluate the regulation of core targets by CVB. Utilizing GO and KEGG enrichment analyses, the probable anti-CRPC mechanism of CVB was investigated. Immunofluorescence, flow cytometry and colony formation assays were used to verify the potential phenotypic regulatory role of CVB in CRPC.
Results
CVB inhibited CRPC cell activity in a concentration-dependent manner. Mechanistically, it primarily regulated BRCA1-, POLD1-, BLM-, MSH2-, MSH6- and PCNA-mediated mismatch repair, homologous recombination repair, base excision repair, Fanconi anemia repair, and nucleotide excision repair pathways. Immunofluorescence, Western blot, flow cytometry and colony formation experiments showed that CVB induced DNA damage accumulation, cell apoptosis, and cell cycle arrest and inhibited CRPC cell proliferation.
Conclusion
CVB can induce DNA damage accumulation in CRPC cells by targeting DNA repair pathways and then induce cell apoptosis and cell cycle arrest, eventually leading to inhibition of the long-term proliferation of CRPC cells.
Introduction
Prostate cancer (PCa) is still one of the most important tumors that endangers men's health worldwide. Its morbidity and mortality are the second and fifth highest, respectively, among male cancers [1]. Multiple treatment modalities, including surgery, endocrine therapy (androgen deprivation therapy), chemoradiotherapy and immunotherapy, have somewhat improved the 5-year survival rate of PCa patients. However, almost all patients with advanced PCa develop castration-resistant prostate cancer (CRPC) after endocrine therapy. CRPC seriously affects the survival time and well-being of patients [2,3]. Consequently, it is necessary to find new treatment strategies to prolong the overall survival of CRPC patients and improve their quality of life.
Synthetic lethality is a concept proposed by geneticists nearly a century ago to describe a phenomenon in which defects in any one gene is not lethal in an organism, but a combination of defects in two genes is lethal [4]. The theoretical basis for applying synthetic lethality in tumor therapy is that tumor cells are highly dependent on another repair pathway in an environment of high proliferation, high stress and high metabolism due to the lack of one DNA repair pathway. Under these conditions, targeted inhibition of another repair pathway leads to tumor cell death, i.e., synthetic lethality [4]. Therefore, the application of synthetic lethality is a very effective treatment strategy for tumors with DNA repair pathway defects. Studies have found that the DNA repair pathway is generally impaired in PCa, with an even higher frequency in CRPC patients [5]. Thus, inhibitors of poly ADP-ribose polymerase (PARP) that target DNA repair are being used in the treatment of PCa [6]. A phase II clinical study published in the journal Lancet Oncology showed that talazoparib (PARP inhibitor) also showed long-lasting antitumor activity in patients with advanced metastatic CRPC with DNA repair gene defects [7]. However, inhibition of DNA repair pathways inevitably causes adverse events such as hypertension, liver damage, pneumonia and secondary hematological malignancies [8]. Therefore, it is necessary to find efficient and low-toxicity DNA repair inhibitors to improve the 5-year survival rate of CRPC patients.
Cyclovirobuxine (also called cyclovirobuxine D) is a triterpenoid alkaloid isolated from Buxus sinica as a Chinese medicine that has been used for millennia in traditional Chinese medicine in China to prevent and cure a variety of cardiovascular disorders, including arrhythmia and heart failure [9,10]. Experiments have confirmed that CVB inhibits cell proliferation, migration, and invasion and induces apoptosis and cell cycle arrest in clear cell renal cell carcinoma through the IGFBP3-AKT/STAT3/MAPK-Snail signaling pathway [11]. CVB can also enhance apoptosis induction in lung cancer cells by inducing mitophagy [12]. Additionally, prior research has confirmed that CVB significantly affects the treatment of glioblastoma cells by controlling the ROS-mediated mitochondrial translocation of cofilin [13]. However, its association with CRPC has not been reported to date.
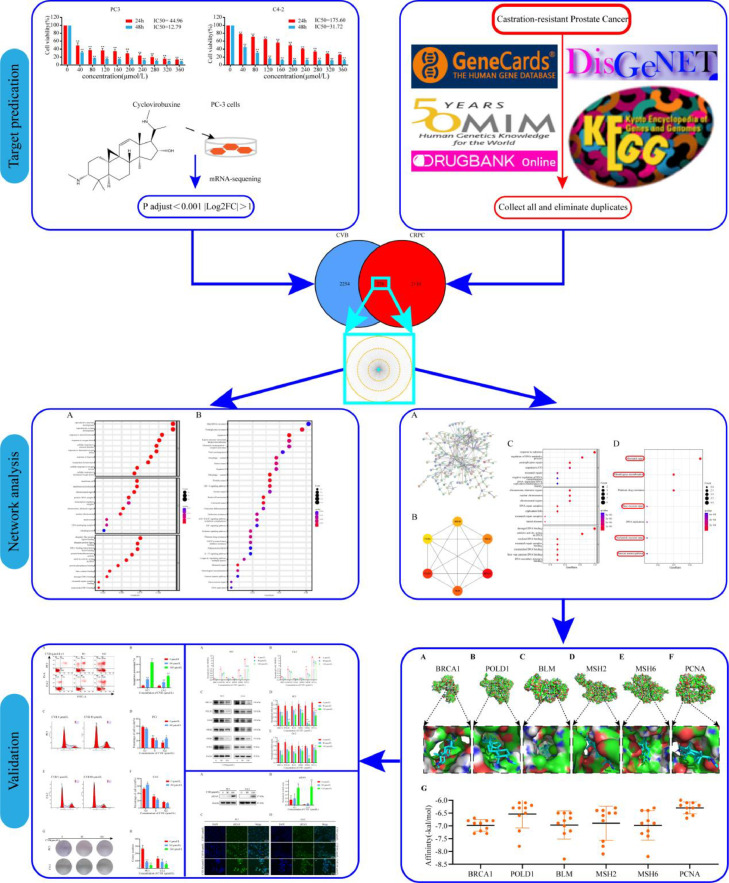
Therefore, in the present study, we used RNA sequencing, systems pharmacology and molecular docking approaches to systematically explore the role of CVB in CRPC and investigate its molecular regulatory mechanism. Fig. 1 shows the study design.
Fig. 1.
Process diagram of the study.
Methods and materials
Cell culture
Human CRPC cell lines, including PC-3 and C4-2, were obtained from the State Key Laboratory of Tumor Biology, Department of Biochemistry and Molecular Biology, Air Force Military Medical University (Xi'an, China). Figs. S1 and S2 show the STR Authentication of PC3 and C4-2 cells. We used cell morphology and STR Authentication to confirm the PC3 and C4-2 cell lines before the experiment. Our cell line was obtained by passage of primary cells 5 times. We employed the same generation of cells for experimental confirmation in further investigations. Cells were grown in DMEM and RPMI 1640 medium (both obtained from Gibco, Invitrogen, USA) containing 10% fetal bovine serum (FBS), 100 U of 1000 × penicillin G, and 100 μg of streptomycin (from Yeasen, Shanghai, China) per ml in a humidified environment of 5% CO2 and 37°C. Five milligrams of CVB (purchased from Yuanye (Shanghai) Co., Ltd.; purity >98% as described by the supplier) was completely dissolved in 100 μL of methanol (purity 98%) and stored at 4°C.
CCK8 assay
PC3 and C4-2 cells were separately grown in a 96-well plate seeded at a density of 4 × 103. Cells in the exponential growth phase were treated with different concentrations (0, 40, 80, 120, 160, 200, 240, 280, 320 and 360 μmol/L) of CVB and incubated for two different time intervals of 24 h and 48 h. Subsequently, cell viability was evaluated using a CCK8 Kit (Shanghai Diyi Biological Co., Ltd.). After 3 h, the OD values of each group at 450 nm were measured using a microplate reader. The percentage of viable cells was calculated in comparison with that of untreated cells. Three independent experiments were conducted, each with three technical replicates.
RNA sequencing (RNA-seq)
Based on the results of the CCK8 assay, the more sensitive PC-3 cell line was selected for mRNA sequencing. Cells (3 × 105) were seeded in 6 cm culture dishes. After preliminary experiments, the PC3 cell line was incubated with 80 μmol/L CVB for 12 h, and the inhibition rate reached 30%-40%. This procedure can produce the required number of cells for mRNA sequencing (Fig. S2). Therefore, when the cells were 90% confluent, the culture medium was replaced with culture medium containing 80 μmol/L CVB. After 12 h of culture, mRNA of the adherent cells was isolated with TRIzol. Triplicate dishes were established for the control and CVB groups. The RNA samples were sequenced at Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) using paired-end sequencing on the Illumina NovaSeq 6000 PE150 platform, and the data were examined online using the Majorbio Cloud Platform (www.majorbio.com). In this study, RNA-seq was used to predict the putative targets of CVB in CRPC. Differential genes with |Log2FC| greater than 1 and an adjusted p (padjust) value of less than 0.001 were considered potential targets of CVB.
Collection and prediction of CRPC-related targets
After RNA-seq analysis, the target hits associated with CRPC were searched in the DisGeNET (Disease Specificity Index >0) (https://www.disgenet.org/, accessed on 13 August 2022), GeneCards (Relevance score >1) (https://www.genecards.org/, accessed on 13 August 2022), OMIM (https://omim.org/, accessed on 13 August 2022), DrugBank (https://go.drugbank.com/, accessed on 13 August 2022) and KEGG (http://db.idrblab.net/ttd/, accessed on 13 August 2022) databases using the keyword "castration-resistant prostate cancer". All targets from the five databases were collected, and duplicates were deleted. These targets were intersected with the potential targets of CVB to obtain CRPC-related therapeutic targets, and further analysis was performed.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of the therapeutic targets of CVB
We used the R program (version 4.1.3) and its backend database org.Hs.eg.db to collect potential target gene IDs (entrezIDs). Disease Ontology Semantic and Enrichment (DOSE) analysis, clusterProfiler, and pathview packages (Bioconductor) were used to perform GO functional enrichment and KEGG pathway enrichment analyses of the putative CVB targets in the treatment of CRPC. A p value of 0.05 and a q value of 0.05 were set as the cutoff criteria for enrichment in the GO and KEGG analyses. Biological process (BP), cellular component (CC), and molecular function (MF) were the three components in GO analysis. Bubble plots were utilized to display the top 10 and top 7 enriched terms, and each group was sorted according to significance. For KEGG analysis, the top 30 and top 7 pathways are shown on bubble plots.
Establishment of the protein‒protein interaction (PPI) network, identification of the key targets, and GO and KEGG pathway enrichment analyses of the key targets of CVB
The overlapping targets were uploaded to the STRING database (https://www.string-db.org, accessed on August 31, 2022) to establish a PPI network. To construct the PPI network, the minimum required interaction score was set to "highest confidence (0.900)", and unconnected nodes were hidden. Protein species were also set to be of human origin. These targets were entered into Cytoscape 3.9.1 using data in TSV format [14]. The top six targets acquired using cytoHubba's MCC algorithm were considered to represent the main targets of CVB against CRPC [15].
Reverse transcription quantitative polymerase chain reaction (RT–qPCR) using isolated RNA
Total RNA was extracted with TRIzol solution in accordance with the manufacturer's instructions. A Nanodrop 2000 (Thermo Scientific) was used to measure the amount of RNA. For reverse transcription to produce complementary DNA (cDNA), 1 μg RNA and 4 μl of DNAse with RNase-free water form a 20 μl reaction system with the following steps: 37°C for 30 minutes, 85°C for 15 seconds and storage at 4°C. In a thermal cycler, 10 μl of qPCR enzyme, 0.8 μl of forward primer, 0.8 μl of reverse primer, and 6.4 μl of RNA-free enzyme water were combined to create a 20 μl RT reaction system. The reaction conditions were as follows: 95°C for 3 min; 95°C for 10 s; and 54 cycles at 56°C for 15 s, 72°C for 15 s, 65°C for 5 s and 95°C for 5 s. Melting curve analysis was then performed from 60°C to 95°C. Triplicate samples were used for RT-qPCR (Bio-Rad IQ5). In accordance with our prior configuration, we normalized targeted mRNA expression data using the most trustworthy reference genes, 18S RNA and β-actin, respectively. Following normalization, the 2−ΔΔCt technique was used to compute and express the relative quantities of the targeted mRNA. The primer pairs and their nucleotide sequences used for quantitative gene expression analysis are shown in Table S1. Three independent experiments were conducted with three technical replicates each.
Western blotting
Radioimmunoprecipitation assay (RIPA) lysis buffer (Solarbio, Cat# R0020) containing 1% PMSF was used to extract total protein from cells, and the target protein range was mapped based on the marker positions after sodium dodecyl sulfate‒polyacrylamide gel electrophoresis (SDS‒PAGE). Then, the isolated proteins were transferred to a nitrocellulose membrane. Skim milk powder (5%) in TBS-Tween 20 (TBST) buffer was used to block the membrane for 1 h at room temperature. Then, the membrane was incubated with primary antibodies against β-actin (1:2000, mouse mAb, A5441) obtained from Sigma‒Aldrich, Merck KGaA (Darmstadt, Germany), and POLD1 (1:2000, rabbit mAb, 15646-1-AP) purchased from Proteintech (Wuhan, China) and antibodies against BRCA1 (1:1000, rabbit mAb HA500015), MSH2 (1:2000, rabbit mAb, EM1801-05), PCNA (1:2000, rabbit mAb, ET1605-38), MSH6 (1:2000, rabbit mAb, ET1604-39) from Hangzhou, China), BLM (1:2000, rabbit mAb, CST # 2742S), and histone H2AX (phospho-Ser139) (1:2000, mouse mAb, M63324S) purchased from Abmart overnight at 4°C on a shaker. The membrane was washed with TBST before incubation with the horseradish peroxidase (HRP)-labeled secondary antibody (1:2000; CW0103S, goat anti-rabbit or CW0102, goat anti-mouse), which was acquired from CWBIO (Beijing, China). Finally, an improved chemiluminescence technique was used to visualize the protein bands (Cat# MI00607A, Mishubio). Three independent experiments were conducted with three technical replicates each.
Molecular docking
The SDF format file for CVB was downloaded from PubChem. (https://pubchem.ncbi.nlm.nih.gov/, accessed on September 10, 2022) and converted to mol2 format using ChemBio3D Ultra software. The molecular structures of the core targets in the PPI network were obtained from the Protein Data Bank (PDB) database (http://www.rcsb.org, accessed on September 10, 2022). Molecular docking of CVB and the important targets was performed using AutoDockTools 1.5.6. The ligand and receptor protein engaged in a binding event, as evidenced by the binding energy of -4.25 kcal/mol. The pairs exhibited strong binding activity, as indicated by the binding energy of -5.0 kcal/mol. The ligand and receptor exhibited substantial complexation activity, as shown by the binding energy of -7.0 kcal/mol [16]. The evaluation metric was the binding energy, and PyMOL was utilized to visualize the results.
Immunofluorescence assay
CRPC cells (1 × 105 cells per well) were plated on a 24-well plate and grown to approximately 80% confluence. Different concentrations of CVB (0, 80, and 160 μmol/L) were added for 24 h. Triton X-100 (0.5%) was used to permeabilize cell membranes at room temperature. The samples were blocked with 1% bovine serum albumin (1 h) and incubated overnight with a primary antibody against γH2AX (histone H2AX (phospho-Ser139)) at 4°C. The next day, the samples were incubated with a fluorescein (Cy3)-conjugated secondary antibody (1 h). CRPC cell nuclei were stained with DAPI (1:10000) containing antiquenching solution, and the samples were then examined and imaged using a fluorescence microscope. Three independent experiments were conducted with three technical replicates each.
Apoptosis assay
PC3 and C4-2 cells were treated with CVB (0, 80, and 160 μmol/L) for 24 h, and adherent and floating CRPC cells were washed with PBS and harvested with trypsin. These washed cells were then collected by centrifugation and resuspended in staining buffer containing 5 µl of annexin V and 10 µl of propidium iodide (PI) (200 µg/ml) (BD Pharmingen, Franklin Lakes, NJ, USA). After the cells were incubated at room temperature for 5 min in the dark, the proportion of cells at different apoptosis stages was quantified by flow cytometry. Three independent experiments were conducted with three technical replicates each.
Cell cycle assay
In brief, PC3 and C4-2 cells were harvested after CVB (80 μmol/L) treatment for 12 h in a 6 cm dish, washed twice in cold PBS and fixed overnight with 70% ethanol. The next day, the fixed cells were washed with PBS again and were then stained with 1 ml of PI/RNase A (BD Pharmingen, Franklin Lakes, NJ, USA) at 37°C in a darkroom for 30 min. A flow cytometer (BD Beckman Coulter, USA) was used to determine the cell cycle distribution. Three independent experiments were conducted with three technical replicates each.
Plate colony formation assay
PC-3 and C4-2 cells (300 cells per well) were seeded into 6 cm dishes. After incubation for 48 h (to allow complete adherence to the wall), the cells were treated with CVB (0, 80, and 160 μmol/L) for 3 h. Then, the normal complete culture medium was replaced. After culture for 10 days, 4% paraformaldehyde was used to fix the cells, and 0.5% crystal violet was then used to stain them for 20 min at room temperature. Cell colonies were counted using ImageJ 6.0. Three independent experiments were conducted with three technical replicates each.
Statistical analysis
For statistical analysis, GraphPad Prism 8.01 and IBM SPSS 22.0 were utilized. The mean ± standard deviation (mean±SD) values are used to present the results. One-way analysis of variance was used to compare more than two independent sets of data. A t test was used for pairwise comparisons between multiple groups. *P < 0.05 and **P < 0.01 were considered statistically significant.
Results
CVB inhibits the growth of CRPC cells in vitro
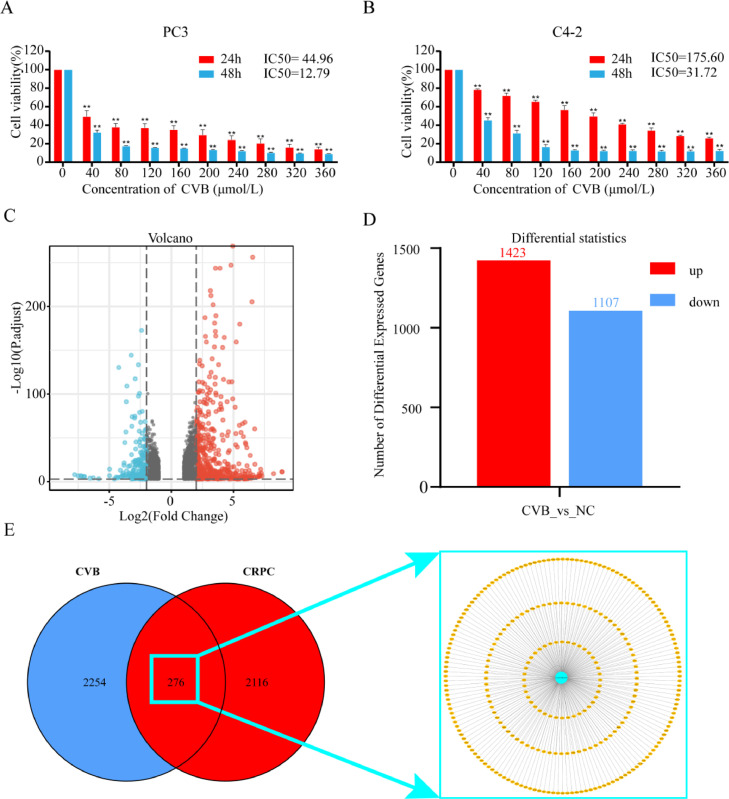
According to the CCK8 assay data, the viability of PC3 and C4-2 cells substantially decreased with increasing CVB concentration compared to that of control cells (*P<0.01 and **P<0.001, respectively). The half-maximal inhibitory concentration (IC50) values of CVB in the PC3 and C4-2 cell lines for proliferation in vitro were 44.94 μmol/L and 175.6 μmol/L, respectively. The IC50 values at 48 h were 12.79 μmol/L and 31.72 μmol/L, respectively. Thus, CVB inhibited the growth of PC3 and C4-2 cells in a concentration-dependent manner (Fig. 2A and B). CVB demonstrated a more potent inhibitory impact in the PC3 cell line.
Fig. 2.
Impact of CVB on CRPC cell viability and therapeutic targets of CVB. (A, B) After exposure to CVB (0-360 μmol/L) for 24 and 48 h, the viability of PC3 and C4-2 cells was determined using a CCK8 assay. *P < 0.01, **P < 0.001 vs. the control group. (C, D) Volcano plot showing differentially expressed genes in PC3 cells following CVB treatment and the numbers of up- and downregulated genes compared to the control group, as determined using RNA-seq analysis and filtering criteria of P adjust < 0.001 and |log2FC| > 1. (E) Venn diagram showing the overlapping treatment targets in CRPC collected from five databases and differentially expressed genes from mRNAseq and shown with the use of Cytoscape 3.9.1 software.
Associated CVB targets in the management of CRPC
After screening the RNA-seq results using the above method, 2530 potential CVB targets were obtained (Fig. 2C and D; Table S2). After collecting all targets and eliminating duplicates from the GeneCards (1703), DisGeNET (683), OMIM (498), DrugBank (9), and KEGG databases (3), 2392 CRPC-related targets were obtained (Table S3). Using a Venn diagram, the 2530 predicted targets of CVB were intersected, resulting in 276 overlapping targets, which were the possible active targets of CVB in CRPC (Fig. 2E; Table S4).
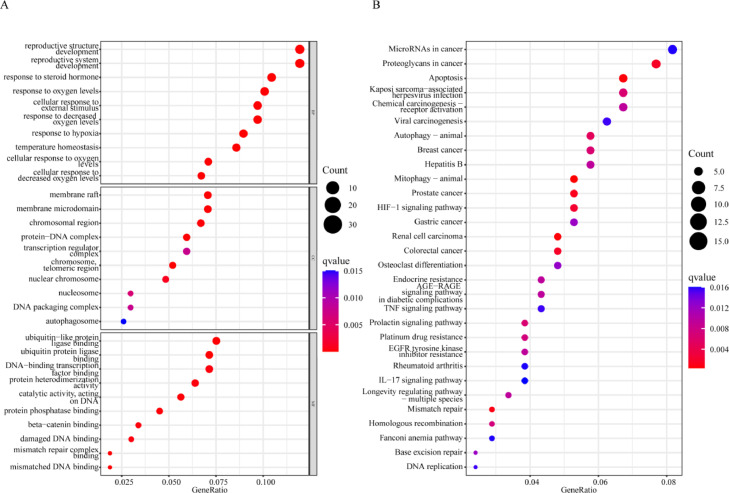
GO term and KEGG pathway enrichment analyses of the CRPC-related therapeutic targets of CVB
To explore the mechanisms of CVB in the treatment of CRPC, the biological functions and signaling pathways enriched with all 276 anti-CRPC targets of CVB were analyzed using an R script. In addition, 1535 GO terms were identified, among which 1434 were related to BPs, including temperature homeostasis, reproductive structure development, reproductive system development, response to steroid hormone, response to oxygen levels, cellular response to external stimulus, response to decreased oxygen levels, cellular response to oxygen levels, response to hypoxia, and cellular response to decreased oxygen levels. A total of 77 GO terms were related to MFs, including ubiquitin-like protein ligase binding, ubiquitin protein ligase binding, mismatch repair complex binding, mismatched DNA binding, catalytic activity, acting on DNA, protein phosphatase binding, beta-catenin binding, DNA-binding transcription factor binding, damaged DNA binding, and protein heterodimerization activity. In addition, 24 CC terms, including protein‒DNA complex chromosome, telomeric region, membrane raft, membrane microdomain, chromosomal region nuclear chromosome, nucleosome, transcription regulator complex, DNA packaging complex, and autophagosome, were enriched (Fig. 3A; Table S5). In addition, in the KEGG pathway analysis, a total of 54 KEGG pathways related to all core targets were identified (q-value<0.05). The top ten enriched pathways were mitophagy–animal, renal cell carcinoma, apoptosis, mismatch repair, PCa, colorectal cancer, proteoglycans in cancer, HIF-1 signaling pathway, autophagy–animal and breast cancer (Fig. 3B; Table S6).
Fig. 3.
Potential mechanism of CVB in the treatment of CRPC. (A) Biological functional enrichment analysis of the CRPC-related therapeutic targets of CVB. The top 10 BP, CC, and MF terms are shown by significance on a bubble plot. (B) KEGG pathway enrichment analysis of the CRPC-related targets of CVB. The top 30 pathways are shown based on significance on a bubble plot.
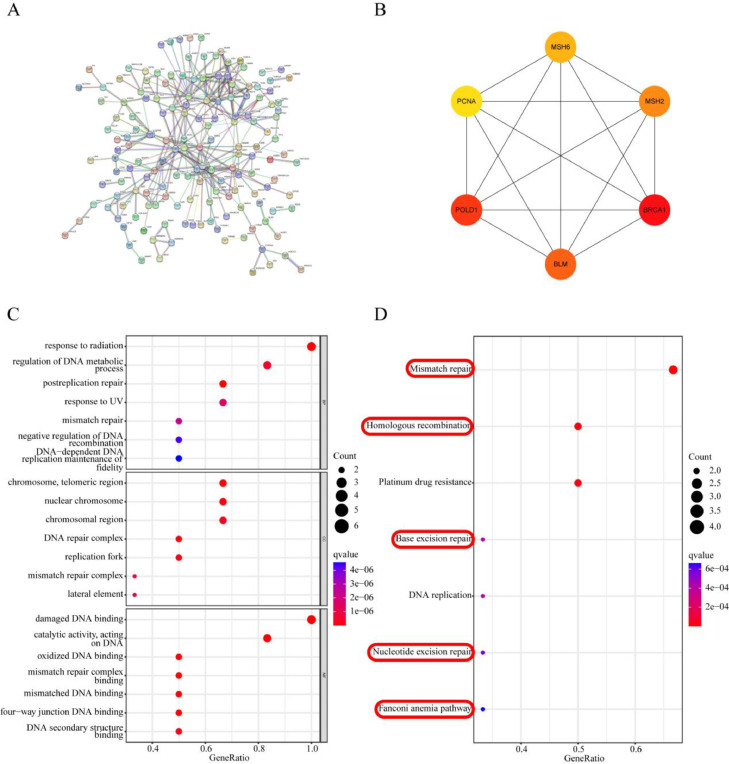
PPI network analysis and identification of core targets
The PPI network contained 267 nodes and 314 edges, meaning that 267 targets might interact with one another to produce a total of 314 interactions (Fig. 4A). Through the MCC algorithm in cytoHubba, we obtained a core target network composed of BRCA1, POLD1, BLM, MSH2, MSH6 and PCNA. The network was visualized by Cytoscape (Fig. 4B, Table S7).
Fig. 4.
Identification of fundamental targets and mechanisms. (A) Construction of the PPI network of CVB in the treatment of CRPC using the STRING database. (B) Top 6 core targets according to the MCC algorithm. (C) Biofunctional enrichment analysis of the core targets. The top 7 BP, CC, and MF terms are presented according to significance. (D) KEGG pathway enrichment analysis of the core targets, with the top 7 pathways presented by significance.
Regulation of DNA repair is the key function of CVB in the treatment of CRPC
To further clarify the key function of CVB in the treatment of CRPC, the GO terms enriched with the key targets were determined using an R package and filtering criteria of p value=0.05 and q value=0.05. CVB was involved in MFs such as damaged DNA binding, catalytic activity, acting on DNA, oxidized DNA binding, mismatch repair complex binding, mismatched DNA binding, four-way junction DNA binding, DNA secondary structure binding, ATP-dependent activity, acting on DNA ADP binding, and single-stranded DNA binding through CCs such as chromosome, telomeric region, DNA repair complex, nuclear chromosome, replication fork, chromosomal region, mismatch repair complex, lateral element, replisome, and nuclear replication fork and synaptonemal complex to affect BPs such as postreplication repair, response to radiation, regulation of DNA metabolic process, response to UV, mismatch repair, negative regulation of DNA recombination, DNA-dependent DNA replication maintenance of fidelity, DNA recombination, response to light stimulus, and mitotic DNA damage checkpoint signaling (Fig. 4C; Table S8). In addition, in the KEGG pathway analysis, a total of 8 KEGG pathways related to all core targets (q-value<0.05) were identified. These pathways included mismatch repair, homologous recombination, platinum drug resistance, DNA replication, nucleotide excision repair, base excision repair, and the Fanconi anemia pathway (Fig. 4D; Table S9). Therefore, inhibition of DNA repair was considered to be the key function of CVB in the treatment of CRPC.
Molecular docking
Molecular docking of CVB with the top six anti-CRPC core targets (BRCA1, POLD1, BLM, MSH2, MSH6 and PCNA) was performed. The findings are shown in Fig. 5A-F and Table S10, which show the docked complexes of CVB and the anti-CRPC core targets. Fig. 5G shows the scatter plot of the binding energy distribution of 10 different conformations of the core proteins in complex with CVB. CVB had a high affinity for all anti-CRPC core targets. However, CVB had a higher binding affinity for amino acids GLY-1803, ARG-1074, GLY-694 and GLY-692, ASN-139, and ARG-772 in the top 5 core targets (BRCA1, POLD1, BLM, MSH2 and MSH6, respectively) and had a binding energy of ≤−7.3 kcal/mol. On the other hand, CVB had a modest binding affinity for MET-40 in PCNA, with a binding energy of −6.7 kcal/mol.
Fig. 5.
Demonstration of molecular docking between CVB and the core targets. The details of the core targets ((A-F) BRCA1, POLD1, BLM, MSH2, MSH6 and PCNA) in complex with CVB are shown. (G) The top 10 binding energy distributions of the core targets’ various conformations in complex with CVB are shown in a scatter plot.
Experimental confirmation of the key targets regulated by CVB
The PPI network data revealed possible major targets of CVB in CRPC treatment. First, the mRNA levels of these important targets (BRCA1, POLD1, BLM, MSH2, MSH6, and PCNA) were measured by RT–qPCR in PC3 and C4-2 cells. We performed RT-qPCR tests with 18S RNA and β-actin as standardized internal references using the same batch of samples to prevent the inaccuracy of the results brought on by a single internal reference. Fig. 6(A, B) and (Fig. S3) demonstrate that after 12 h of treatment, the mRNA levels of the core targets were significantly downregulated in the CVB-treated groups of both PC-3 and C4-2 cells (**P<0.01). Thus, CVB inhibited the mRNA expression of BRCA1, POLD1, BLM, MSH2, MSH6 and PCNA. Due to the lag between changes in mRNA expression and changes in protein expression, Western blot analysis was then used to examine the protein expression of key targets in CRPC cells following incubation with CVB for 24 h (Fig. 6C, D and E). The results of triplicate experiments demonstrated that CVB might, in a concentration- and time-dependent manner, downregulate the protein expression of essential targets in CRPC cells. In summary, CVB inhibits the mismatch repair (MMR), homologous recombination repair (HRR), nucleotide excision repair (NER), base excision repair (BER) and Fanconi anemia repair (FA) pathways in CRPC cells by targeting related repair factors.
Fig. 6.
CVB inhibits the transcription and translation of its core targets. (A, B) mRNA expression of the core targets (BRCA1, POLD1, BLM, MSH2, MSH6 and PCNA) after CVB treatment (0, 80, and 160 μmol/L; 12 h). *P < 0.05, **P < 0.01 vs. the control group. The expression of the indicated factors was examined by RT‒qPCR. 18S RNA was used as the loading control. (C, D and E) The protein levels of the core targets (BRCA1, POLD1, BLM, MSH2, MSH6 and PCNA) were decreased after CVB treatment (0, 80, and 160 μmol/L; 24 h). The expression of the indicated factors was examined by Western blotting. β-actin was used as the loading control. *P < 0.05, **P < 0.01 vs. the control group.
CVB promotes DNA damage accumulation in CRPC cells
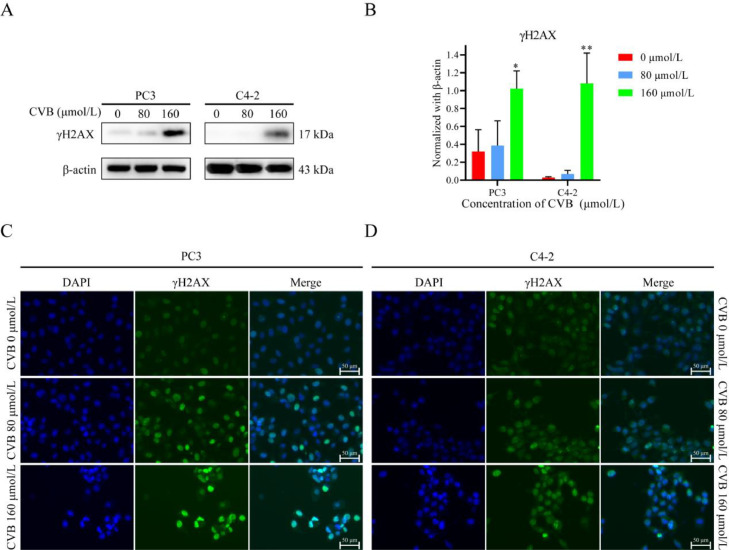
DNA repair and DNA damage signaling pathways are critical for the maintenance of genomic stability [17]. DNA repair procedures that are precise and prompt prevent DNA damage, restore genomic integrity, and ensure regular cellular activity [17]. When DNA single/double-strand breaks occur, histone H2AX is rapidly phosphorylated, forming γH2AX. Because the phosphorylation of H2AX at Ser 139 is abundant and rapid and has a good correlation with each double-strand break, γH2AX is considered a molecular marker of DNA damage repair [18]. We previously found that CVB can target a variety of DNA repair-related proteins to inhibit various DNA repair pathways. In CRPC cells with high proliferation and high replication pressure, the frequency of DNA damage is significantly increased. CVB blocks DNA damage repair in CRPC cells by inhibiting multiple DNA repair pathways, which might leads to the accumulation of DNA damage in CRPC cells. Therefore, we evaluated the changes in the level of γH2AX using Western blotting and immunofluorescence staining. CVB increased the protein level of γH2AX in a concentration-dependent manner (Fig. 7A, B). In addition, immunofluorescence staining showed that the fluorescence intensity of γH2AX gradually increased with increasing CVB concentration (Fig. 7C, D). Therefore, CVB downregulated DNA repair in CRPC cells and then promoted DNA damage in CRPC cells to inhibit the growth activity of PC3 and C4-2 cells.
Fig. 7.
CVB promotes the accumulation of DNA damage in CRPC cells. (A, B) The protein level of γH2AX after CVB treatment (0-160 μmol/L, 24 h). The data are shown as the means ± SD (n = 3); **P < 0.01. vs. the control group. (C) Immunofluorescence assay (scale bar: 50 μm) of γH2AX after CVB treatment (0-160 μmol/L, 24 h).
CVB induces apoptosis, and cell cycle arrest and inhibits the proliferation of CRPC cells
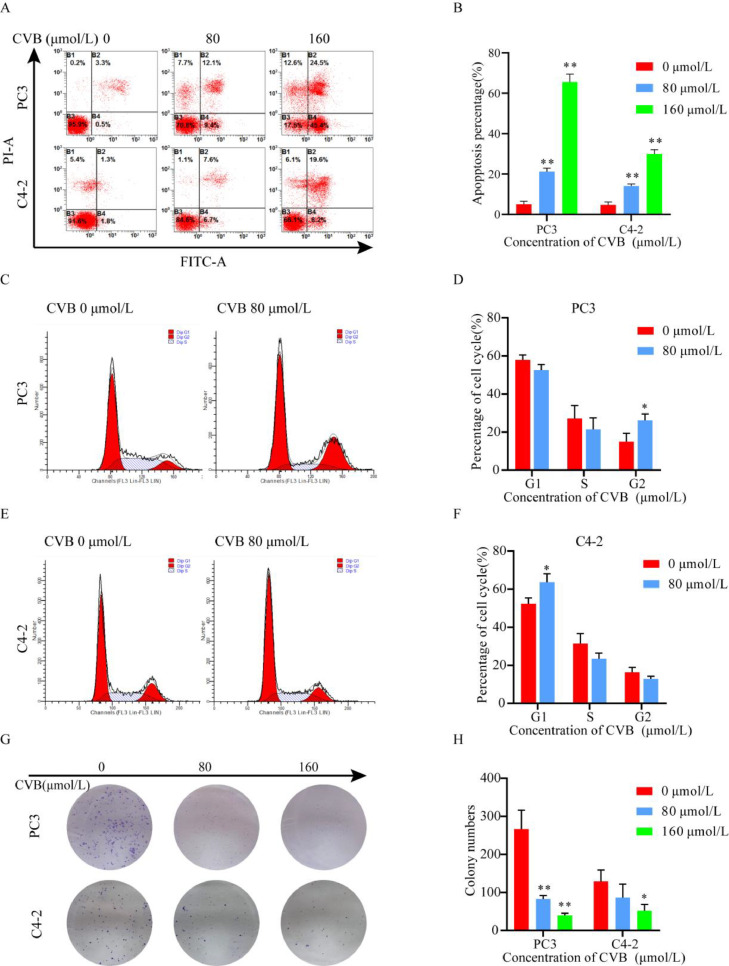
Rapid and precise repair of large amounts of daily DNA damage preserves genomic stability and protects cells against apoptosis, senescence and even malignant transformation [19]. In present study, flow cytometric analysis showed that CVB induced apoptosis in CRPC cells in a concentration-dependent manner (Fig. 8A and B). Interestingly, in the cell cycle assay, CVB arrested PC3 cells in G2/M phase and arrested C4-2 cells in G0/G1 phase (Fig. 8C, D, E and F). We believe that this effect may be related to the characteristics of the two cell lines. However, both cell lines undoubtedly exit the cell cycle permanently. CVB inhibited the long-term proliferation of PC3 and C4-2 cells in a concentration-dependent manner (Fig. 8G and H). These results suggested that CVB has excellent medicinal potential in the treatment of CRPC.
Fig. 8.
CVB induces apoptosis, and cell cycle arrest and inhibits the proliferation of PC3 and C4-2 cells. (A, B) Apoptosis assay of CRPC cells after treatment with CVB at different concentrations (0-160 μmol/L, 24 h). The data are presented as the mean ± SD (n = 3); *P < 0.01, **P < 0.001. vs. the control group. (C, D, E and F) Cell cycle assay of PC3 and C4-2 cells after treatment with CVB (80 μmol/L, 24 h). The data are presented as the mean ± SD (n = 3); *P < 0.05. vs. the control group. (G, H) A colony formation assay was used to confirm the growth inhibitory effect of CVB (0-160 μmol/L, 3 h). The data are presented as the mean ± SD (n = 3); *P < 0.05.vs. the control group.
Discussion
Natural alkaloids have good antitumor activity and have been increasingly evaluated in preclinical studies in recent years. In general, natural alkaloids can exert antitumor effects by regulating reactive oxygen species, inducing apoptosis and cell cycle arrest in tumor cells, and regulating autophagy and epithelial-mesenchymal transition [20], [21], [22], [23]. CVB is a natural triterpenoid alkaloid and exerts noticeable antitumor effects on several types of tumors, such as clear cell renal cell carcinoma and lung cancer [11,12]. However, the function of CVB in CRPC is unclear. In the present study, for the first time, we revealed that CVB can promote senescence, apoptosis, and cell cycle arrest and inhibit the proliferation of CRPC cells.
CVB can inhibit diverse tumor activities. Consistent with previous studies, CVB inhibited the activity of PC3 and C4-2 cells in a concentration-dependent manner. To systematically explore its mechanism of action, we selected the CVB-sensitive cell line PC3 for RNA-seq, identified differentially expressed genes, and screened them with strict criteria. In addition, we searched five databases to obtain CRPC treatment-related targets. After intersecting the differentially expressed genes and CRPC treatment-related targets, we identified 276 genes as the main anti-CRPC targets of CVB CRPC. Subsequently, we performed KEGG and GO enrichment analyses to explore the main functions of this target set and found that CVB may play an anti-CRPC role by regulating mitophagy in animals, apoptosis, mismatch repair, proteoglycans in cancer, the HIF-1 signaling pathway, autophagy in animals and other pathways.
Furthermore, to determine the function with a major role in the antitumor effects of CVB, we first constructed a PPI network with these 276 genes and then used the MMC algorithm in cytoHubba in Cytoscape software to determine the core targets. Eventually, BRCA1, POLD1, BLM, MSH2, MSH6 and PCNA were identified as the key targets in the network. Functional enrichment analysis of the key targets indicated that the MMR, HRR, NER, BER, and FA repair pathways, among others, are linked to DNA repair pathways.
Faithful DNA repair is vital for maintaining cell and tissue homeostasis, as evident by the observation that loss-of-function mutations in DNA repair factors cause genetic disorders and increased susceptibility to cancer [24]. At least nine separate pathways—MMR, BER, NER, translesion synthesis (TLS), HR, nonhomologous end joining (NHEJ), alternative end joining (alt-EJ), FA repair, and O6-methylguanine DNA methyltransferase (MGMT)—are used in mammalian cells to repair a variety of different genotoxic lesions [25,26]. More than 450 distinct effectors that are involved in the repair of DNA single-strand and double-strand breaks and single nucleotide abnormalities control these DNA repair pathways. These repair routes can typically function in lieu of one another and are not redundant. However, a failure in one or more DNA repair pathways is present in the majority of cancers, increasing genomic instability and encouraging carcinogenesis. This flaw also restricts the growth of tumors during the tumor development process [27]. Tumor cells depend on their remaining DNA repair ability to repair damage brought on by replication and genotoxic stress to withstand the pressure of fast multiplication. When some DNA repair pathways are inhibited, tumor cells will suffer fatal damage, that is, synthetic lethality [28].
The term synthetic lethality, which was initially used in the 1920s, describes the phenomenon in which the co-occurrence of two viable gene mutations results in cell death [29,30]. By blocking special DNA repair pathways with a particular chemical agent, a foundation for synthetic lethality can be established in tumors with DNA repair deficiencies [31], [32], [33]. Previous research has demonstrated that CRPC is more likely than PCa to acquire inactivating mutations in the HR and NER pathways [34]. Therefore, the mode of action of CVB—targeting multiple DNA repair pathways—poses a serious threat to the survival of CRPC cells. Furthermore, since almost all patients with advanced PCa develop CRPC after endocrine therapy, we analyzed mRNA data for PCa in The Cancer Genome Atlas (TCGA) and found that BRCA1, POLD1, BLM, MSH2, MSH6 and PCNA were associated with higher T stage and with lymph node metastasis (Table S11). Therefore, these genes are potential therapeutic targets in CRPC.
In seeking to further understand the mechanism by which CVB controls these repair pathways, we found by molecular docking that CVB has a strong ability to bind BRCA1, POLD1, BLM, MSH2, MSH6 and PCNA. Western blotting and RT–qPCR showed that CVB significantly downregulated both the protein and mRNA expression of these core targets. Therefore, we believe that CVB targets BRCA1-, POLD1-, BLM-, MSH2-, MSH6- and PCNA-mediated MMR, HR, NER, BER and FA repair pathways to decrease the activity of CRPC cells. Interestingly, We found that PC3 was more sensitive to CVB than C4-2 cell line at 24 h and 48 h by CCK8 assay. However, in the detection of RT-qPCR and Western blot, there was no significant difference in the effect of CVB on the expression of core targets in PC3 and C4-2 cell lines. This is indeed an interesting finding. We believe that this phenomenon is due to the different speeds of cell proliferation. In our study, we observed that the proliferation rate of PC3 was significantly higher than that of C4-2, which was confirmed by colony formation. At 24 h, PC3 cells were in a rapid proliferation phase, which led to a high dependence on the DNA repair pathway. At this time, PC3 was more sensitive to CVB, and its IC50 was only 44.96 μmol/L. Since C4-2 has not yet entered the rapid proliferation stage, although CVB inhibits various DNA repair pathways in C4-2 cell lines, C4-2 has not yet been significantly dependent on DNA repair pathways. Therefore, it was less affected by CVB at 24 h. At 48 h, since C4-2 entered the rapid proliferation stage, it was dependent on the DNA repair pathway. Therefore, the C4-2 cell line became sensitive to CVB, and the IC50 of C4-2 at 48 hours confirmed this, with a value of only 31.72 μmol/L. Therefore, the difference in sensitivity to CVB is mainly determined by the time it enters the rapid proliferation stage. This may explain that targeting multiple DNA repair pathways is a common mechanism for CVB treatment of PC3 and C4-2. Therefore, our study shows that CVB can target a variety of DNA repair pathways to inhibit CRPC cells proliferation .
Inhibition of multiple DNA repair pathways leads to the accumulation of DNA damage in CRPC cells and eventually to severe DNA double-strand breaks. Subsequently, we evaluated the expression of DNA damage marker protein γH2AX and confirmed this observation by Western blotting and immunofluorescence staining. Therefore, CVB induces DNA damage accumulation in CRPC cells by regulating multiple DNA repair pathways.
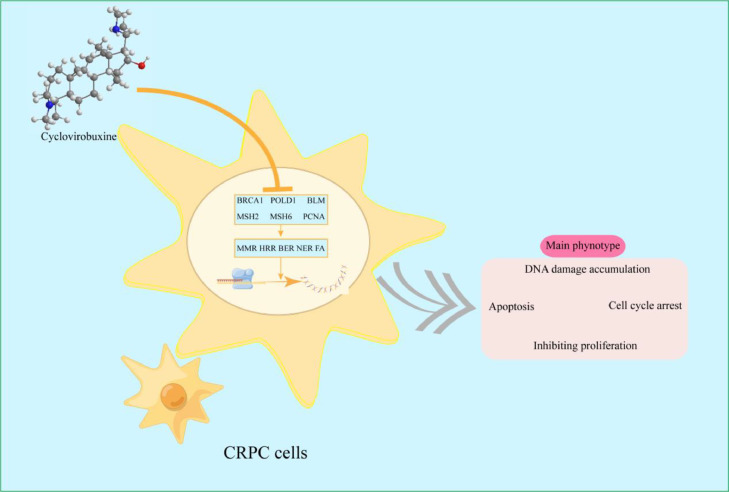
Numerous studies have shown that the accumulation of DNA damage promotes cell apoptosis and cycle arrest [35,36]. In our research,CVB promoted apoptosis in PC3 and C4-2 cells in a concentration-dependent manner. We noticed that the expression of the DNA damage marker protein γH2AX did not significantly change when CVB was 80 μmol/L, while the results of flow cytometry revealed that CRPC cells experienced significant apoptosis at this concentration, indicating that CVB may not only affect CRPC cells' ability to DNA damage repair, but also regulate other apoptosis-related signaling pathways. Notably, CVB arrested PC3 cells in the G2/M phase while blocking C4-2 cells in the G0/G1 phase, which may be related to the selectivity of various cell lines. To prevent drawing incorrect findings as a result of cell line specificity, it is essential to use as many cell lines as feasible while examining the impact of tumor intervention strategies. However, both cell lines undoubtedly exit the cell cycle permanently because we found through a colony formation assay that CVB inhibited the long-term proliferation of PC3 and C4-2 cells. Therefore, by targeting multiple DNA repair pathways, CVB leads to the accumulation of DNA damage in CRPC cells, which in turn induces apoptosis, and cell cycle arrest and ultimately inhibits the growth of CRPC cells. In short, these data suggest that CVB may have an anticancer impact via a complex signal network, which may have benefits over chemotherapy medicines with a single target (Fig. 9).
Fig. 9.
Mechanism through which CVB inhibits CRPC cell growth.
Last, and most crucially, on the one hand, the mechanism of CVB, which has a special DNA repair inhibitory function, suggests that it may be coupled with radiotherapy, chemotherapy, and immunotherapy to increase the therapeutic efficacy of antitumor treatment. On the other hand, some preclinical investigations have demonstrated that inhibition of tumor DNA repair mechanisms that are resistant to chemoradiotherapy can restore their therapeutic sensitivity [37], [38], [39]. This shows that CVB offers a fresh approach to the treatment of malignancies resistant to chemoradiotherapy. These results suggest that CVB has great potential in the treatment of CRPC.
However, there are certain restrictions as well. First, Despite the fact that our research showed that the IC50 values for the PC3 and C4-2 cell lines at 48 hours were only 12.79 μmol/L and 31.72 μmol/L, respectively. To assess the drug's safety, effectiveness, and dose-response relationship in humans, however, pharmacokinetic and toxicological studies are first required.. Second, the direct control of CVB on key targets must be further investigated. This will be the direction of our follow-up research. As a result, our research offers a novel strategy to treat CRPC and a theoretical foundation for the clinical transformation of CVB.
Conclusion
This work identified a novelty and therapeutic potential targeted drug for CRPC, CVB. We demonstrated for the first time that CVB exerts obvious inhibitory effects on cell proliferation and intensively facilitates DNA damage, apoptosis and cell cycle arrest in CRPC cells. We further indicated that CVB exerts these effects principally by inhibiting the MMR, HR, BER, NER and FA pathways mediated by BRCA1, POLD1, BLM, MSH2, MSH6 and PCNA. We initially proposed a novel antitumor mechanism of CVB and established a theoretical framework for its clinical use.
CRediT authorship contribution statement
Conceptualization,methodology and validation, H.B.J. and K.G. Writing original draft and data curationg, S.Y.Z. and G.J.W. Writing reviewing & editng Y.H.Q., C.Z., X.S.L J.H.G and Q.J.S. Visualization, S.Y. Z. Funding acquisition, K.G. and H.B.J. All authors have read and approved the final manuscript.
Consent for publication
We have obtained consents to publish this paper from all the participants of this study.
Funding
This work was supported by General Research Project of Xi 'an Health Commission [grant number 2022yb14].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank yaodong Chen and mingwei Zhu for their advice and timely help regarding experimental design and data management and analysis, respectively.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101708.
Contributor Information
Guojun Wu, Email: wuguojun@med.nwu.edu.cn.
Hanbing Jiang, Email: jianghanbing123@fmmu.edu.cn.
Appendix. Supplementary materials
Data availability
All data generated or analyzed during this study are included in this published article. Additional datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Gillessen S., Attard G., Beer T.M., Omlin A. Management of patients with advanced prostate cancer: report of the advanced prostate cancer consensus conference 2019. Eur. Urol. 2020;77:508–547. doi: 10.1016/j.eururo.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Cornford P., Bellmunt J., Bolla M., Mottet N. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and Castration-Resistant prostate cancer. Eur. Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Topatana W., Juengpanich S., Li S., Cai X. Advances in synthetic lethality for cancer therapy: Cellular mechanism and clinical translation. J. Hematol. Oncol. 2020:13. doi: 10.1186/s13045-020-00956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashworth A., Lord C.J., Reis-Filho J.S. Genetic interactions in cancer progression and treatment. Cell. 2011;145:30–38. doi: 10.1016/j.cell.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Teyssonneau D., Margot H., Cabart M., Roubaud G. Prostate cancer and PARP inhibitors: progress and challenges. J. Hematol. Oncol. 2021;14:51. doi: 10.1186/s13045-021-01061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bono J.S., Mehra N., Scagliotti G.V., Fizazi ...K. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): An open-label, phase 2 trial. Lancet Oncol. 2021;22:1250–1264. doi: 10.1016/S1470-2045(21)00376-4. [DOI] [PubMed] [Google Scholar]
- 8.Martorana F., Da S.L., Sessa C., Colombo I. Everything comes with a price: the toxicity profile of DNA-damage response targeting agents. Cancer. (Basel) 2022:14. doi: 10.3390/cancers14040953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei J.B., Lai Q., Shumyak S.P., Xu L.F., Zhang C.X., Ling J.J., Yu Y. An LC/MS quantitative and microdialysis method for cyclovirobuxine D pharmacokinetics in rat plasma and brain: The pharmacokinetic comparison of three different drug delivery routes. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015;1002:185–193. doi: 10.1016/j.jchromb.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Ke Z., Hou X., Jia X.B. Design and optimization of self-nanoemulsifying drug delivery systems for improved bioavailability of cyclovirobuxine D. Drug. Des. Devel. Ther. 2016;10:2049–2060. doi: 10.2147/DDDT.S106356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y., Lv H., Li X., Wang Z. Cyclovirobuxine inhibits the progression of clear cell renal cell carcinoma by suppressing the IGFBP3-AKT/STAT3/MAPK-Snail signalling pathway. Int. J. Biol. Sci. 2021;17:3522–3537. doi: 10.7150/ijbs.62114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng C., Zou T., Qu J., Chen X., Zhang S., Lin Z. Cyclovirobuxine d Induced-Mitophagy through the p65/BNIP3/LC3 axis potentiates its Apoptosis-Inducing effects in lung cancer cells. Int. J. Mol. Sci. 2021:22. doi: 10.3390/ijms22115820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Fu R., Duan D., Chen J. Cyclovirobuxine d induces apoptosis and mitochondrial damage in glioblastoma cells through ROS-Mediated mitochondrial translocation of cofilin. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.656184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin A., Ochagavia M.E., Rabasa L.C., Miranda J., Fernandez-De-Cossio J., Bringas R. BisoGenet: A new tool for gene network building, visualization and analysis. BMC Bioinformat. 2010;11:91. doi: 10.1186/1471-2105-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chin C.H., Chen S.H., Wu H.H., Ho C.W., Ko M.T., Lin C.Y. CytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014;8(4) doi: 10.1186/1752-0509-8-S4-S11. SupplS11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsin K.Y., Ghosh S., Kitano H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS One. 2013;8:e83922. doi: 10.1371/journal.pone.0083922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins J.L., Lan L., Zou L. DNA repair defects in cancer and therapeutic opportunities. Gene. Dev. 2022;36:278–293. doi: 10.1101/gad.349431.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonner W.M., Redon C.E., Dickey J.S., Nakamura A.J., Sedelnikova O.A., Solier S., Pommier Y. GammaH2AX and cancer. Nat. Rev. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rayess H., Wang M.B., Srivatsan E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer. 2012;130:1715–1725. doi: 10.1002/ijc.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu M., Niu J., Jiang J., Dong T., Chen Y., Yang X., Liu P. Chelerythrine inhibits the progression of glioblastoma by suppressing the TGFB1-ERK1/2/Smad2/3-Snail/ZEB1 signaling pathway. Life Sci. 2022;293 doi: 10.1016/j.lfs.2022.120358. [DOI] [PubMed] [Google Scholar]
- 21.Li Q., Huang H., He Z., Sun Y., Tang Y., Shang X., Wang C. Regulatory effects of antitumor agent matrine on FOXO and PI3K-AKT pathway in castration-resistant prostate cancer cells. Sci. China Life Sci. 2018;61:550–558. doi: 10.1007/s11427-016-9050-6. [DOI] [PubMed] [Google Scholar]
- 22.Tang Z.H., Cao W.X., Wang Z.Y., Lu J.H., Liu B., Chen X., Lu J.J. Induction of reactive oxygen species-stimulated distinctive autophagy by chelerythrine in non-small cell lung cancer cells. Redox Biol. 2017;12:367–376. doi: 10.1016/j.redox.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B., Wang X., Deng J., Wang F. P53-dependent upregulation of miR-16-2 by sanguinarine induces cell cycle arrest and apoptosis in hepatocellular carcinoma. Cancer Lett. 2019;459:50–58. doi: 10.1016/j.canlet.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Carusillo A., Mussolino C. DNA damage: From threat to treatment. Cells. 2020:9. doi: 10.3390/cells9071665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S., Li X., Gao F., de Groot J.F., Koul D., Yung W. PARP-mediated PARylation of MGMT is critical to promote repair of temozolomide-induced O6-methylguanine DNA damage in glioblastoma. Neuro Oncol. 2021;23:920–931. doi: 10.1093/neuonc/noab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pilie P.G., Tang C., Mills G.B., Yap T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019;16:81–104. doi: 10.1038/s41571-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdak-Rothkamm S., Rothkamm K. DNA damage repair deficiency and synthetic lethality for cancer treatment. Trend. Mol. Med. 2021;27:91–92. doi: 10.1016/j.molmed.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Dobzhansky T. Genetics of natural populations; Recombination and variability in populations of Drosophila pseudoobscura. Genetics. 1946;31:269–290. doi: 10.1093/genetics/31.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucchesi J.C. Synthetic lethality and semi-lethality among functionally related mutants of Drosophila melanfgaster. Genetics. 1968;59:37–44. doi: 10.1093/genetics/59.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.T D. Genetics of natural populations; Recombination and variability in populations of Drosophila pseudoobscura. Genetics. 1946;31:269–290. doi: 10.1093/genetics/31.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker D.J. The origins of the developmental origins theory. J. Intern. Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 34.Klinakis A., Karagiannis D., Rampias T. Targeting DNA repair in cancer: Current state and novel approaches. Cell. Mol. Life Sci. 2020;77:677–703. doi: 10.1007/s00018-019-03299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian H., Gao Z., Li H., Zheng J. DNA damage response–a double-edged sword in cancer prevention and cancer therapy. Cancer Lett. 2015;358:8–16. doi: 10.1016/j.canlet.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 36.Kaina B. DNA damage-triggered apoptosis: Critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem. Pharmacol. 2003;66:1547–1554. doi: 10.1016/s0006-2952(03)00510-0. [DOI] [PubMed] [Google Scholar]
- 37.Llaguno-Munive M., Romero-Pina M., Serrano-Bello J., Garcia-Lopez P. Mifepristone overcomes tumor resistance to temozolomide associated with DNA damage repair and apoptosis in an orthotopic model of glioblastoma. Cancer. (Basel) 2018:11. doi: 10.3390/cancers11010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ni M., Li J., Zhao H., Wu X. BRD4 inhibition sensitizes cervical cancer to radiotherapy by attenuating DNA repair. Oncogene. 2021;40:2711–2724. doi: 10.1038/s41388-021-01735-3. [DOI] [PubMed] [Google Scholar]
- 39.Suo D., Wang Z., Li L., Li Y. HOXC10 upregulation confers resistance to chemoradiotherapy in ESCC tumor cells and predicts poor prognosis. Oncogene. 2020;39:5441–5454. doi: 10.1038/s41388-020-1375-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Additional datasets analyzed during the current study are available from the corresponding author on reasonable request.