Highlights
-
•
Non-coding RNA research in cancer is expected to advance rapidly in the coming years, focusing on developing targeted therapies, exploring liquid biopsy diagnostics, and utilizing bioinformatic tools for analysis.
-
•
Non-coding RNAs are more sensitive and specific than traditional cancer markers and can be easily detected in blood, urine, and other bodily fluids, making them a promising tool for early cancer detection.
-
•
The review addresses the challenges associated with non-coding RNA-based diagnostics and therapeutics in cancer, aiming to optimize their efficacy, specificity, and delivery methods.
Keywords: Biomarkers, Liquid biopsy, Diagnostics, Therapy, Anti-miRNA oligonucleotides
Abstract
Non-coding RNAs (ncRNAs) have emerged as key regulators of gene expression, with growing evidence implicating their involvement in cancer development and progression. The potential of ncRNAs as diagnostic and prognostic biomarkers for cancer is promising, with emphasis on their use in liquid biopsy and tissue-based diagnostics. In a nutshell, the review comprehensively summarizes the diverse classes of ncRNAs implicated in cancer, including microRNAs, long non-coding RNAs, and circular RNAs, and their functions and mechanisms of action. Furthermore, we describe the potential therapeutic applications of ncRNAs, including anti-miRNA oligonucleotides, siRNAs, and other RNA-based therapeutics in cancer treatment. However, significant challenges remain in developing effective ncRNA-based diagnostics and therapeutics, including the lack of specificity, limited understanding of mechanisms, and delivery challenges. This review also covers the current state-of-the-art non-coding RNA research technologies and bioinformatic analysis tools. Lastly, we outline future research directions in non-coding RNA research in cancer, including developing novel biomarkers, therapeutic targets, and modalities. In summary, this review provides a comprehensive understanding of non-coding RNAs in cancer and their potential clinical applications, highlighting both the opportunities and challenges in this rapidly evolving field.
Graphical abstract
Various Non-coding RNAs in Cancer Research and Therapy
Navigating the complex world of non-coding RNAs: an introduction
Non-coding RNAs (ncRNAs) are molecules that do not code for proteins yet serve vital functions in regulating gene expression. They are split into two groups: short ncRNAs and long ncRNAs. Short ncRNAs include microRNAs (miRNAs), small interfering RNAs (siRNAs), and PIWI-interacting RNAs (piRNAs). Long ncRNAs include long intergenic ncRNAs (lincRNAs), circular RNAs (circRNAs), and others. Non-coding RNAs have been found to play vital roles in many biological processes, including development, differentiation, and disease, including cancer [16].
In cancer, non-coding RNAs have been found to have both oncogenic and tumor-suppressive roles. Many ncRNAs are dysregulated in cancer, and they can affect cancer development and progression by regulating cellular processes such as cell proliferation, apoptosis, invasion, and metastasis. For example, some miRNAs can target oncogenes, while others can target tumor suppressor genes. Similarly, some lincRNAs can promote cancer progression by regulating key signaling pathways, while others can act as tumor suppressors by inhibiting tumor growth and metastasis [22].
Recent studies have highlighted the critical roles of ncRNAs in cancer development and progression. For instance, a study by [13] showed that the expression of a specific lncRNA HOTAIR was upregulated in breast cancer cells and associated with poor clinical outcomes. Another study by [100] demonstrated that the upregulation of miR-181a-5p was associated with poor prognosis in gastric cancer patients. Moreover, a study by [65] revealed that circRNA_0084043 promoted the proliferation and invasion of pancreatic cancer cells by targeting the miR-153–3p/FOXM1 pathway.
Moreover, non-coding RNAs are crucial regulators of gene expression, and their dysregulation can contribute to cancer development and progression. Additional research is required to understand the complex roles of ncRNAs in cancer and to develop new ncRNA-based diagnostic and therapeutic approaches.
Deciphering the diversity of non-coding RNAs in cancer
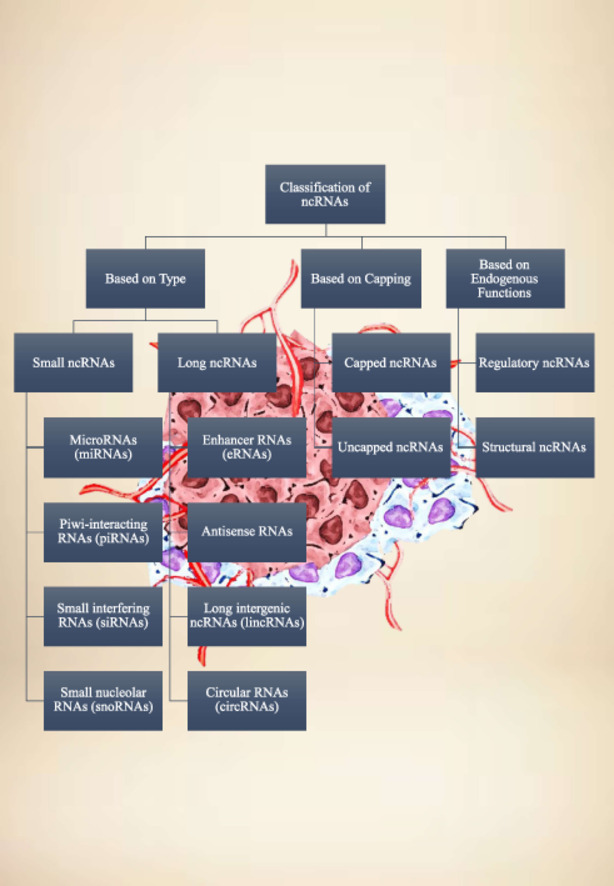
Over the past decade, studies have implicated numerous ncRNAs in cancer development and progression. Classification of non-coding RNAs (ncRNAs) can be based on different criteria, including their type, capping, and endogenous functions (Fig. 1). Long non-coding RNAs (lncRNAs), miRNAs, and circular RNAs (circRNAs) are pivotal players in the intricate landscape of gene expression regulation. These non-coding RNAs exert their regulatory functions through diverse mechanisms. Their functions and mechanisms of action in cancer are explored in this section.
Fig. 1.
Classification of ncRNAs based on their type, capping and endogenous functions.
Long non-coding RNAs (lncRNAs)
LncRNAs are RNA molecules of more than 200 nucleotides that do not code for proteins. Like miRNAs, lncRNAs can act as oncogenes or tumor suppressors in cancer, depending on their target genes. LncRNAs participate in numerous biological activities, such as transcriptional control, chromatin remodeling, and RNA splicing [19].
Recent studies have identified several lncRNAs that play crucial roles in cancer. For example, the lncRNA HOTAIR is overexpressed in many cancers and promotes tumor growth and metastasis by recruiting chromatin-modifying enzymes to target genes [66]. Another study discovered that the lncRNA LINC00511 is involved in the proliferation and migration of gastric cancer cells by targeting the tumor suppressor miR-765 [82]. In addition, A. Yang et al. [101] have shown that lncRNA UCA1 promotes the invasion and metastasis of bladder cancer cells by sponging miR-145 and activating the PI3K/AKT pathway. These studies highlight the potential of lncRNAs as therapeutic targets for cancer treatment.
lncRNA-mediated gene regulation
-
•
Transcriptional regulation: Certain lncRNAs exhibit interactions with DNA regulatory elements, such as promoters or enhancers, facilitating the recruitment of chromatin-modifying complexes. By modulating chromatin structure, DNA methylation, or histone modifications, they can effectively activate or repress gene transcription [81].
-
•
Post-transcriptional regulation: lncRNAs govern mRNA stability and translation by directly binding to target messenger RNAs (mRNAs). Acting as "decoys," they sequester microRNAs (miRNAs), impeding their binding to target mRNAs and thereby influencing mRNA stability. Alternatively, lncRNAs can engage in interactions with RNA-binding proteins, thereby influencing mRNA processing, localization, or translation [19].
-
•
Epigenetic regulation: Certain lncRNAs guide chromatin modifications to specific genomic loci. They serve as molecular scaffolds for the assembly of protein complexes involved in histone modifications, DNA methylation, or nucleosome remodeling, thereby playing a role in shaping the epigenetic landscape [93].
MicroRNAs (miRNAs)
miRNAs are short (usually 18–25 nucleotides) ncRNAs that regulate gene expression by aiming mRNAs for degradation or translational repression. In cancer, miRNAs can act as oncogenes (promote tumor growth) or tumor suppressors (inhibit tumor growth), depending on their target genes. For example, miR-21 is overexpressed in many cancers and promotes tumor growth by targeting tumor suppressor genes, while miR-34a is downregulated in many cancers and functions as a tumor suppressor [2,29,64].
Recent studies have shown that miRNAs also regulate cancer stem cells (CSCs), a subpopulation of cells within tumors that drive tumor growth and resistance to therapy. For example, Khan et al. [45] showed that miR-21 promotes CSC self-renewal in breast cancer by targeting the tumor suppressor PTEN, while miR-200c inhibits CSC self-renewal and tumor growth in pancreatic cancer by targeting the stem cell marker CD133 [42].
Another study found that miR-21 promotes the development of ovarian cancer by inhibiting the expression of the tumor suppressor PTEN, leading to the activation of the PI3K/AKT pathway [63]. In addition, Liu and Tang [57] have demonstrated that miR-34a hinders the growth and metastasis of lung cancer by targeting the oncogenic transcription factor SNAIL. These studies highlight the potential of miRNAs as therapeutic targets for cancer treatment.
miRNA mediated-gene regulation
The binding of the miRNA to the target mRNA can lead to the regulation of gene expression through several mechanisms. The most common mechanism is the inhibition of translation, where the miRNA-RISC complex prevents the binding of ribosomes to the mRNA, thereby preventing protein synthesis. Additionally, miRNAs can induce mRNA degradation by promoting deadenylation, decapping, and exonucleolytic decay. They can also influence mRNA stability by protecting target mRNAs from degradation or by promoting their stabilization [90].
Circular RNAs (circRNAs)
CircRNAs are a type of ncRNA that form closed loops by covalently linking their 3′ and 5′ ends. CircRNAs, like lncRNAs, circRNAs can act as oncogenes or tumor suppressors in cancer [91]. The circRNA circPVT1 is upregulated in many cancers and promotes tumor growth and metastasis by regulating target gene expression [111]. Conversely, the circRNA circHIPK3 is downregulated in many cancers and acts as a tumor suppressor by inhibiting cell proliferation and promoting apoptosis [9].
Another study found that the circRNA circ_0067934 promotes the progression of pancreatic cancer by sponging miR-1324 and activating the Wnt/β-catenin signaling pathway [113]. In addition, the circRNA circ_0008450 promotes the spread and invasion of gastric cancer cells by sponging miR-422a and activating the expression of the oncogene SOX4 [72]. These studies highlight the potential of circRNAs as diagnostic and prognostic markers and emphasize therapeutic targets for cancer treatment.
circRNA-mediated gene regulation
-
•
miRNA sponging: circRNAs function as competitive endogenous RNAs (ceRNAs) by harboring binding sites for miRNAs. Through sequestration, circRNAs effectively inhibit the binding of miRNAs to their target mRNAs, resulting in enhanced stability and expression of the target mRNAs [15].
-
•
Interaction with RNA-binding proteins: circRNAs can interact with RNA-binding proteins (RBPs) and modulate their activity. This interaction can influence the stability, localization, or function of RBPs, consequently exerting an impact on gene expression [35].
-
•
Translational regulation: Some circRNAs have been discovered to associate with ribosomes, raising the possibility of translation into small peptides. This translational capacity of circRNAs adds another layer of complexity to the regulatory landscape, potentially influencing protein synthesis and contributing to regulatory processes [50].
lncRNAs, miRNAs, and circRNAs exhibit distinct tissue-specific and developmentally regulated expression patterns. They have been implicated in various biological processes, including cellular differentiation, development, immune response, and disease pathogenesis. Nonetheless, the precise mechanisms underlying lncRNA, miRNAs, and circRNA-mediated gene regulation remain active areas of investigation, necessitating further research to unravel their full significance in cellular processes.
PiwiRNAs and their functional implications in cancer development and progression
PiwiRNAs, also known as PIWI-interacting RNAs, are small non-coding RNA molecules that play crucial roles in gene regulation and genome stability. They are primarily expressed in the germline cells of animals and function in the silencing of transposable elements (TEs) and epigenetic regulation [83].
PiwiRNAs function primarily by suppressing transposable elements or transposons, which are mobile genetic elements that can disrupt genomic stability. Through the formation of ribonucleoprotein complexes known as piRNA-induced silencing complexes (piRISCs), mature piwiRNAs associate with Piwi proteins and recognize complementary sequences on transposon transcripts or transposon DNA within the nucleus. The Piwi protein, along with other associated proteins, induces various silencing mechanisms, such as DNA methylation, histone modifications, and inhibition of transcription or translation [39].
PiwiRNAs, traditionally recognized for their significance in germline development and genome stability, have also emerged as potential players in cancer biology. Studies indicate that dysregulation of PiwiRNAs and the associated Piwi pathway may contribute to oncogenesis and tumor progression. Key roles of PiwiRNAs in oncology include:
Tumor initiation and progression: PiwiRNAs exhibit abnormal expression patterns in various cancer types. Piwi proteins, such as PIWIL1 and PIWIL2, are upregulated in cancer cells and have been associated with enhanced tumor growth, invasion, metastasis, cancer stemness, epithelial-mesenchymal transition (EMT), and chemotherapy resistance [69].
Epigenetic regulation: PiwiRNAs are implicated in the modulation of epigenetic modifications, including DNA methylation and histone modifications. Altered DNA methylation patterns and histone marks are common features of cancer cells. PiwiRNAs, along with Piwi proteins, can influence these epigenetic marks, potentially leading to dysregulated gene expression and tumor development [104].
Transposable element regulation: PiwiRNAs play a critical role in the suppression of transposable elements (TEs), which are repetitive DNA sequences capable of disrupting genomic integrity and contributing to cancer. Dysregulation of the Piwi pathway can activate TEs, leading to genomic instability and increased susceptibility to oncogenic transformation.
Interactions with non-coding RNAs: PiwiRNAs interact with other non-coding RNAs, such as long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs), which are known to be involved in cancer. Through these interactions, PiwiRNAs can modulate the expression and function of non-coding RNAs, thereby influencing various oncogenic pathways [96].
Diagnostic and prognostic biomarkers: PiwiRNAs hold promise as diagnostic and prognostic biomarkers in cancer. Aberrant expression profiles of PiwiRNAs in tumor tissues and body fluids, such as blood and urine, have shown associations with cancer diagnosis, staging, and prediction of patient outcomes [27].
Coding potential of ncRNAs and miR-mRNA interactions
The coding potential of certain non-coding RNAs (ncRNAs) has gained growing recognition. Although traditionally categorized as non-coding due to their presumed lack of protein-coding capacity, recent investigations have uncovered that specific ncRNAs can engage in translation processes, giving rise to peptides or small proteins that exert regulatory functions in diverse biological processes [61]. This revelation disrupts the established classification of ncRNAs and emphasizes the intricate nature of gene expression regulation.
MicroRNAs (miRNAs) are pivotal regulators of gene expression at the post-transcriptional level, exerting their influence by intricately binding to messenger RNA (mRNA) molecules and modulating their stability and translational efficiency. These interactions exhibit remarkable specificity, relying on precise complementary base pairing between the miRNA and its target mRNA. The process of miRNA-mediated gene regulation initiates with the biogenesis of miRNAs. Within the nucleus, the primary transcript, known as pri-miRNA, undergoes meticulous processing orchestrated by the Drosha enzyme, leading to the generation of a precursor miRNA (pre-miRNA). Subsequently, the pre-miRNA embarks on a journey to the cytoplasm, where it encounters the Dicer enzyme, an essential player in the final stages of processing. This culminates in the production of a mature miRNA duplex. Of the miRNA duplex, one strand, aptly named the guide strand, is selectively incorporated into the RNA-induced silencing complex (RISC), while the other, termed the passenger strand, generally faces degradation. The guide strand of the miRNA then engages in a delicate dance with the target mRNA, forging a binding interaction characterized by partial complementarity. Predominantly, the binding event occurs within the 3′ untranslated region (UTR) of the target mRNA. Here, the miRNA showcases its remarkable acumen, identifying specific target sites known as miRNA response elements (MREs) nestled within the 3′ UTR. Notably, the binding between the miRNA and the target mRNA exhibits imperfections, encompassing partial complementarity [56].
The interaction between the miRNA and the mRNA elicits gene regulation through two principal mechanisms: mRNA degradation and translational repression. In the case of mRNA degradation, the binding of the miRNA to the target mRNA recruits proteins that orchestrate mRNA decay, culminating in diminished levels of the target mRNA. Conversely, translational repression ensues when the miRNA binding hampers the translation process, impeding the synthesis of the protein encoded by the target mRNA [90].
So, miRNA-mRNA interactions epitomize a pivotal mechanism governing post-transcriptional gene regulation. By delicately modulating mRNA stability and translation, miRNAs meticulously fine-tune gene expression. The intricate and specific interactions between miRNAs and their target mRNAs weave a complex tapestry of regulatory networks that govern a myriad of biological processes.
Non-coding RNAs in liquid biopsy and tissue-based diagnostics
ncRNAs are involved in a broad range of cellular processes, including development, differentiation, and disease. Recent studies have demonstrated that ncRNAs can serve as diagnostic and prognostic biomarkers for cancer.
One application of ncRNAs in cancer diagnosis is through liquid biopsy. Liquid biopsy is a minimally invasive technique that involves the analysis of biomolecules in the blood, for example circulating tumor cells (CTCs), cell-free DNA (cfDNA), and extracellular vesicles (EVs). ncRNAs are stable in blood and detected in these circulating biomolecules. For example, the expression levels of miRNAs in EVs from blood samples are related to cancer progression and therapy response. Additionally, long non-coding RNAs (lncRNAs) are present in cfDNA and can distinguish between different cancer types [7,37,74,109].
Another application of ncRNAs in cancer diagnosis is through tissue-based diagnostics. Tissue-based diagnostics involve the analysis of tissue samples obtained through biopsy or surgery. ncRNAs are differentially expressed in cancer tissue compared to normal tissue, making them useful diagnostic markers. For example, the expression levels of miR-21 in breast cancer and miR-155 in lymphoma, and lncRNAs are dysregulated in various cancer types, such as MALAT1 in lung cancer and HOTAIR in breast cancer [21,78,88,95].
In addition to their diagnostic potential, ncRNAs have prognostic value in cancer. Prognostic biomarkers predict the likelihood of disease progression and patient outcome. For example, the expression levels of miR-21 and miR-155 are associated with poor prognosis in breast cancer and lymphoma, respectively. Similarly, lncRNAs such as HOTAIR and HULC are persistent with poor prognosis in various cancer types [11,36,58,70]. ncRNAs have the potential to be robust diagnostic and prognostic biomarkers for cancer. Their stability in circulating biomolecules and differential expression in cancer tissue make them attractive targets for liquid biopsy and tissue-based diagnostics. Future research in this field will likely uncover even more ncRNAs with diagnostic and prognostic potential.
Targeting anti-miRNA oligonucleotides, siRNAs, and RNA-Based therapeutics
Anti-miRNA oligonucleotides and siRNAs have emerged as promising RNA-based therapeutics for cancer treatment. These therapeutics function by modulating the expression of target genes involved in cancer cell growth and survival [10]. In a study by [86], the scientists showed that miR-195–5p enhances the sensitivity of hepatocellular carcinoma cells to sorafenib by suppressing the Wnt/β-catenin signaling pathway. The study suggests that miR-195–5p could be a potential therapeutic target in treating hepatocellular carcinoma.
In another study, [24] described a novel RNA aptamer that targets survivin and inhibits proliferation while promoting apoptosis of human hepatocellular carcinoma cells. The study highlights the potential of RNA-based therapeutics in developing targeted cancer therapies.
These examples demonstrate the potential of anti-miRNA oligonucleotides, siRNAs, and other RNA-based therapeutics in cancer treatment and suggest that these approaches may provide a promising avenue for developing targeted cancer therapies.
Unraveling the complexity of non-coding RNAs in drug resistance
Non-coding RNAs (ncRNAs) can act as oncogenes or tumor suppressors and regulate genes involved in drug metabolism, apoptosis, and DNA damage repair [6]. This section discusses ncRNA's role in drug resistance, including its impact on chemotherapy and targeted therapy response.
In a study by [3,85], the authors found that miR-21 overexpression was associated with resistance to cisplatin-based chemotherapy in non-small cell lung cancer (NSCLC). The study suggests targeting miR-21 may be a potential strategy for overcoming chemotherapy resistance in NSCLC.
Similarly, other ncRNAs have been found to play a role in resistance to targeted therapies. For example, in studies by [77,103], the authors demonstrated that long non-coding RNA (lncRNA) TTN-AS1 promotes resistance to cetuximab in colorectal cancer cells by upregulating the PI3K/Akt pathway. The study suggests that targeting TTN-AS1 may be a potential strategy for overcoming resistance to cetuximab in colorectal cancer.
In addition, studies showed that circRNA- hsa_circ_0002130 was upregulated in gefitinib-resistant NSCLC cells and promoted resistance by regulating the expression of epidermal growth factor receptor (EGFR) and downstream signaling pathways. The study suggests that targeting hsa_circ_0002130 may be a potential strategy for overcoming resistance to EGFR-targeted therapies in NSCLC [59,67].
These examples demonstrate that ncRNAs regulate drug resistance and their impact on chemotherapy and targeted therapy response. Targeting these ncRNAs may provide a promising strategy for overcoming drug resistance and improving treatment outcomes for cancer patients. The involvement of ncRNAs in drug resistance can be attributed to several mechanisms, including:
-
1
Drug Efflux: Certain ncRNAs, such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), have been shown to regulate the expression of drug efflux pumps, such as the ATP-binding cassette (ABC) transporters. By upregulating these transporters, ncRNAs facilitate the efflux of anticancer drugs from cancer cells, reducing their intracellular concentration and rendering the cells resistant to treatment.
-
2
Epithelial-Mesenchymal Transition (EMT): ncRNAs are involved in the induction of EMT, a process characterized by the loss of epithelial traits and the acquisition of mesenchymal properties by cancer cells. During EMT, cancer cells become more invasive and resistant to therapy. Several ncRNAs, including miRNAs and lncRNAs, regulate the expression of EMT-associated genes, leading to the activation of EMT signaling pathways and the promotion of drug resistance.
-
3
Inhibition of Apoptosis: Apoptosis, or programmed cell death, is a crucial mechanism for eliminating cancer cells. However, ncRNAs can interfere with the apoptotic pathway, thereby promoting drug resistance. For instance, ncRNAs can modulate the expression of anti-apoptotic proteins (e.g., Bcl-2) or inhibit the expression of pro-apoptotic proteins (e.g., Bax), leading to reduced sensitivity of cancer cells to chemotherapy-induced cell death.
-
4
Activation of Survival Signaling Pathways: ncRNAs can activate various survival signaling pathways that promote cell survival and confer resistance to anticancer drugs. For example, certain miRNAs can downregulate tumor suppressor genes, such as PTEN, leading to the activation of pro-survival pathways like PI3K/AKT/mTOR. This activation enhances cell survival and diminishes the effectiveness of cancer treatments.
-
5
Regulation of DNA Repair: ncRNAs can influence the DNA damage response and repair processes, which are critical determinants of drug sensitivity. By modulating the expression of DNA repair genes, ncRNAs can affect the efficiency of DNA damage repair, allowing cancer cells to evade the cytotoxic effects of chemotherapy or radiation.
RNA aptamer therapy in cancer
Aptamer-siRNA conjugates have emerged as a promising therapeutic modality in the field of molecular medicine due to their unique combination of aptamer targeting and siRNA-mediated gene silencing. These conjugates offer several therapeutic benefits that make them attractive for the treatment of various diseases. One key advantage of aptamer-siRNA conjugates is their ability to enable targeted delivery of siRNA molecules. Aptamers, which are single-stranded DNA or RNA molecules, possess high affinity and specificity for target molecules, such as cell surface receptors or disease-associated proteins. By conjugating siRNA to aptamers, the therapeutic payload can be directed specifically to the desired cells or tissues, minimizing off-target effects and improving the efficiency of siRNA delivery [48].
The gene silencing potential of siRNA is another major benefit of aptamer-siRNA conjugates. siRNA molecules are designed to bind to complementary messenger RNA (mRNA) sequences, leading to the degradation of the targeted mRNA and subsequent silencing of the corresponding gene. By delivering siRNA through aptamer conjugation, the therapeutic effect can be precisely directed to specific cell types or disease-associated proteins, allowing for the modulation of gene expression with high specificity. Aptamer-siRNA conjugates also offer enhanced stability and improved pharmacokinetics compared to naked siRNA molecules. The conjugation of siRNA to aptamers provides protection against degradation by nucleases, thereby increasing their stability in biological environments. Additionally, aptamers can improve the circulation time of siRNA in the bloodstream, prolonging its exposure to target cells. These enhanced stability and pharmacokinetic properties expand the therapeutic window and enhance the efficacy of aptamer-siRNA conjugates [112].
Another advantage of these conjugates is their reduced immunogenicity. Aptamers are generally less immunogenic compared to other nucleic acid-based therapeutics. This lower immunogenicity reduces the risk of immune responses and improves the safety profile of aptamer-siRNA conjugates. It also allows for repeated administration, if needed, for chronic or long-term treatments. Furthermore, the combination of aptamers and siRNA in a single conjugate provides synergistic effects. The aptamer moiety enables targeted delivery and specific binding to disease-associated targets, while the siRNA component facilitates gene silencing. This synergistic action enhances the therapeutic efficacy of aptamer-siRNA conjugates and has the potential to improve patient outcomes [46].
Overcoming the challenges of non-coding RNA-based diagnostics and therapeutics in cancer
Non-coding RNAs (ncRNAs) are pivotal in gene expression regulation and cellular processes, but their dysregulation is implicated in cancer development and progression. Despite their potential as diagnostic and therapeutic targets, studying ncRNAs in the context of cancer poses numerous challenges. Functional characterization is a primary obstacle as the roles and interactions of ncRNAs within cellular pathways are intricate and not yet fully understood. Unraveling the functional significance of specific ncRNAs and their involvement in cancer initiation, progression, and metastasis remains a significant challenge.
Cancer's high heterogeneity and contextual dependency further complicate the study of ncRNAs. Expression and activity of ncRNAs vary depending on cancer type, stage, and individual patient characteristics, making it difficult to establish consistent associations between specific ncRNAs and cancer phenotypes. Moreover, ncRNA impacts on cancer biology can be context-dependent, adding another layer of complexity to their investigation and clinical application. Identifying and validating ncRNAs as diagnostic or prognostic biomarkers in cancer is a complex process. Large-scale profiling studies, including transcriptomic analyses of patient samples, are necessary to discover potential ncRNA biomarkers. However, validating candidate biomarkers in independent cohorts and establishing standardized detection methods pose challenges due to technical variability, inter-patient heterogeneity, and the need for rigorous statistical analyses.
Exploiting the therapeutic potential of ncRNAs in cancer encounters hurdles in delivery and targeting. Efficient and specific delivery systems are required to transport ncRNA-based therapeutics to tumor cells while minimizing off-target effects and systemic toxicity. Moreover, the intricate intracellular machinery involved in ncRNA regulation presents challenges in achieving effective and sustained therapeutic outcomes. Regulatory considerations are vital for the development and clinical translation of ncRNA-based therapies. Adherence to regulatory guidelines and safety assessments is crucial due to the unique properties of ncRNAs, such as their potential for off-target effects or unintended interactions with host cellular processes. Comprehensive preclinical evaluation and regulatory oversight are necessary in this regard. Addressing these challenges necessitates multidisciplinary collaborations, advanced technologies for ncRNA characterization, and robust clinical studies. Despite the complexities involved, understanding and harnessing the potential of ncRNAs in cancer hold significant promise for improved diagnostics, prognostics, and therapeutic interventions in the future.While there are many promising applications of ncRNAs in cancer, significant challenges like lack of specificity, limited understanding of mechanisms, and delivery challenges remain in developing effective ncRNA-based diagnostics and therapeutics [14].
Lack of specificity
Numerous forms of ncRNAs are dysregulated in cancer, and the role that these ncRNAs play in the initiation and progression of cancer has been the subject of a significant amount of research. However, one of the considerable challenges in the study of ncRNAs in cancer is the lack of specificity in their functions, which makes it challenging to identify their precise role in cancer biology and develop targeted therapies. Several recent studies have highlighted the lack of specificity in the case of ncRNAs in cancer. The study also highlighted the need for more research to identify the precise molecular mechanisms underlying the functions of ncRNAs in cancer [49].
Another study focuses on the role of a specific ncRNA called SNHG15 in cancer. The study found that SNHG15 was upregulated in several types of cancer and promoted cancer cell proliferation and migration. However, the study also noted that SNHG15 was not specific to cancer cells and expressed in normal cells, indicating that its role in cancer was unclear [97].
Several approaches have been proposed to overcome the challenge of lack of specificity in the case of ncRNAs in cancer. These include using advanced technologies such as single-cell sequencing and spatial transcriptomics to study the expression and function of ncRNAs at a more specific level, and the development of targeted therapies that modulate the function of dysregulated ncRNAs in cancer cells [20].
Mechanistic understanding
In addition to the challenge of lack of specificity, another major challenge in the study of ncRNAs in cancer is the limited understanding of the mechanisms underlying their functions. While many ncRNAs have been implicated in cancer development and progression, the precise molecular mechanisms through which they exert their effects are often not well understood.
A recent study investigated the role of a specific ncRNA called miR-195 in ovarian cancer. The study found that miR-195 was downregulated in ovarian cancer cells and inhibited cancer cell proliferation, migration, and invasion. However, the study was not able to fully elucidate the molecular mechanisms underlying these effects, highlighting the need for further research to better understand the functions of ncRNAs in cancer [28].
Similar studies investigated the role of a specific type of ncRNA called circular RNA (circRNA) in gastric cancer. The study found that a circRNA called circPVT1 was upregulated in gastric cancer cells and promoted cancer cell proliferation and invasion. However, the study was not able to fully elucidate the molecular mechanisms through which circPVT1 exerted its effects, instigating the genesis of further research to better understand the functions of circRNAs in cancer [23,71].
To address the challenge of limited understanding of mechanisms in the study of ncRNAs in cancer, researchers are using a variety of approaches. These include the use of advanced technologies such as CRISPR/Cas9 genome editing and RNA sequencing to identify the target genes and pathways regulated by ncRNAs, as well as the development of animal models and in vitro cell culture systems to study the functions of ncRNAs in a more controlled environment [52,102,107]
Delivery
There is a significant challenge in the study of ncRNAs in cancer, as delivering ncRNAs to target tissues or cells is necessary for their successful use in cancer therapy. One of the primary challenges associated with ncRNA delivery is their instability and susceptibility to degradation by nucleases in the bloodstream. Additionally, once the ncRNAs reach the target tissue, they need to penetrate the cell membrane and enter the cytoplasm or nucleus to exert their therapeutic effects [60].
Several strategies have been developed to address the challenge of ncRNA delivery:
-
•
One approach is to use nanoparticles, such as liposomes or nanoparticles made of biodegradable polymers, to encapsulate and protect the ncRNAs from degradation. This approach has been used successfully in preclinical studies to deliver a variety of ncRNAs, including miRNAs and siRNAs, to cancer cells in vitro and in vivo. Researchers are also targeting polyethyleneimine-modified liposomes to provide a tumor suppressor miRNA called miR-34a to prostate cancer cells in vitro and a mouse model, resulting in reduced tumor growth and metastasis [[1], [54], [79]].
-
•
Another approach to ncRNA delivery is to use viral vectors, such as lentiviruses or adeno-associated viruses (AAVs), to deliver the ncRNAs to the target cells. Viral vectors effectively deliver ncRNAs to cells, but they have limitations, including the potential for immune responses and the risk of insertional mutagenesis [94].
-
•
Exosome-based delivery of ncRNAs is a promising approach that has gained attention recently. Exosomes are small vesicles pinched off by cells that can be isolated and modified to carry ncRNAs. Exosomes are effective at delivering miRNAs and siRNAs to target cells and they have the advantage of being non-immunogenic and biocompatible. For example, a study published in Nature Communications in 2019 used exosomes to deliver a miRNA called miR-193a to lung cancer cells in vitro and a mouse model, resulting in reduced tumor growth and increased survival [8,51,53].
Clinical translation
Despite the progress made in ncRNA delivery, significant challenges remain that need to be overcome to make ncRNA-based cancer therapies a reality. Further research is required to optimize the delivery strategies, to ensure that the ncRNAs are explicitly delivered to the target cells and tissues, and to minimize the potential for off-target effects. Several other challenges need to be addressed in the study of ncRNAs in cancer like
-
•
Tumor heterogeneity: Tumors are highly heterogeneous, and ncRNA expression can vary greatly between different regions of the tumor. This heterogeneity can make it problematic to target ncRNAs to specific tumor regions and affect the effectiveness of ncRNA-based therapies. A recent study published in Nature Communications (2018) used single-cell RNA sequencing to analyze the heterogeneity of ncRNA expression in breast cancer and identified subpopulations of cells sensitive to ncRNA-based therapies. This study highlights the importance of considering tumor heterogeneity in developing ncRNA-based cancer therapies [17,34,55,92].
-
•
Off-target effects: ncRNAs can have unintended consequences on non-target genes and pathways, leading to off-target results that can harm the patient. To minimize the potential for off-target effects, design ncRNAs specific to their target and carefully evaluate their safety and efficacy in preclinical studies. Recent studies published in Nature Communications used CRISPR-Cas9 screening to identify the off-target effects of a miRNA called miR-155 and found that it regulates a broad range of genes and pathways, highlighting the need for careful evaluation of the safety of ncRNA-based therapies [44,89].
-
•
Immunogenicity: Some delivery systems, such as viral vectors, can trigger immune responses that can limit their effectiveness and cause adverse effects in patients. To minimize the potential for immune responses, one has to design delivery systems that are non-immunogenic or that can be modified to reduce their immunogenicity. Recent studies show the development of a non-immunogenic delivery system based on cationic lipids that could deliver miRNAs to breast cancer cells in vitro and a mouse model without triggering an immune response [33,105].
-
•
Pharmacokinetics: The pharmacokinetics of ncRNAs can vary greatly depending on the delivery system used, which can affect their distribution, metabolism, and excretion. Understanding the pharmacokinetics of ncRNAs is vital for optimizing their delivery and dosing in clinical trials. Various studies target on developed a fluorescence-based method for tracking the pharmacokinetics of a miRNA delivery system based on nanoparticles in vivo, allowing for real-time monitoring of the distribution and clearance of the nanoparticles [98,108].
Exploring non-coding RNA research technologies and bioinformatic analysis tools for cancer: opportunities and limitations
The study of ncRNAs has expanded rapidly in recent years, and various technologies have been developed to investigate their roles and mechanisms of action. Table 1 addresses the most commonly used ncRNA research technologies.
Table 1.
Summarized description of some of the commonly used research technologies for studying non-coding RNAs.
| Technology | Description | Refs. |
|---|---|---|
| RNA sequencing (RNA-seq) | High-throughput sequencing technique to measure the abundance and diversity of all RNAs in a sample, including ncRNAs. | [5] |
| Northern blotting | Classical method for detecting and quantifying RNA molecules based on their size and sequence. | [31] |
| RNA fluorescence in situ hybridization (RNA-FISH) | Technique for visualizing RNA molecules in fixed cells or tissues using fluorescently labeled probes. | [76] |
| CRISPR/Cas9-mediated knockdown or editing | Genome editing tool to manipulate ncRNA expression or function. | ([32]; Li et al., 2020b) |
| RNA pull-down | Technique for identifying protein partners of specific ncRNAs by capturing them with biotinylated RNA probes. | [30,40] |
| RNA immunoprecipitation (RIP) | Technique for identifying RNA molecules that interact with specific proteins in cells or tissues using antibodies. | [4] |
| Small RNA sequencing | High-throughput sequencing technique to measure the abundance and diversity of small RNAs, including miRNAs and siRNAs. | [18] |
| Ribosome profiling | Technique for measuring the translation efficiency of mRNAs by sequencing the ribosome-protected fragments of RNA. | [38] |
| RNA interference (RNAi) | Technique for knocking down gene expression using siRNAs or shRNAs. | [26,87] |
| Chromatin isolation by RNA purification (ChIRP) | Technique for identifying genomic loci that are bound by specific ncRNAs, by purifying the chromatin associated with the RNA. | [12] |
As the field continues to evolve, we must develop new and more sophisticated methods to understand better ncRNA's complex roles and mechanisms in health and disease. In complementation with these methods, bioinformatics analysis tools are essential for studying the complex functions and interactions of ncRNA molecules. These are a few of the many technologies that are available for studying ncRNAs. A few examples of bioinformatics tools used in ncRNA research are entailed in Table 2.
Table 2.
Few bioinformatics analysis tools essential for studying the complex functions and interactions of non-coding RNA (ncRNA) molecules.
| Tool | Description | Examples | Refs. |
|---|---|---|---|
| Rfam | RNA family database for both coding and non-coding RNAs | ncRNA families | [43] |
| miRBase | MicroRNA database for sequences, structures, targets, and functions | microRNAs | [47] |
| RNAfold | Software for predicting RNA secondary structures based on thermodynamic principles | lncRNAs, circRNAs | [62] |
| Cytoscape | Software for visualizing and analyzing complex networks | ncRNA-mRNA interactions, regulatory pathways, signaling pathways | [80] |
| CLIP-seq analysis tools | Bioinformatic tools for analyzing CLIP-seq data for RNA-protein interactions | CIMS, PIPE-CLIP, Piranha | [25,68,106] |
| RNAcentral | A unified database for all types of RNA sequences, structures, and annotations | all types of RNAs | [84] |
| lncRNAdb | A database of annotated lncRNAs with information on sequences, structures, and functions | lncRNAs | [75] |
| NPInter | A database of non-coding RNA-protein interactions with experimental evidence | ncRNA-protein interactions | [110] |
| miRNApath | A web-based tool for predicting miRNA-target interactions and functional pathways | miRNA-target interactions, functional pathways | 10.18129/B9.bioc.miRNApath |
| MINTbase | A database of molecular interactions for non-coding RNAs, including miRNAs, lncRNAs, and circRNAs | ncRNA interactions | [73] |
| LncRNA2Target | A web-based tool for predicting lncRNA-mRNA interactions and functional roles | lncRNA-mRNA interactions, functional roles | [41] |
| deepBase | A database of deep-sequencing data for ncRNAs, including miRNAs, lncRNAs, and circRNAs, with annotations and analyses | ncRNA deep-sequencing data | [99] |
Charting the future directions of non-coding RNA research in cancer
Few future research directions in ncRNA research in cancer, including the development of novel biomarkers, therapeutic targets, and therapeutic modalities, can be
-
a
Identification and validation of ncRNA biomarkers: Identifying specific ncRNA biomarkers that can be used for early recognition, diagnosis, and prognosis of cancer is an active area of research. For example, miRNAs have been identified as potential biomarkers for breast, colorectal, and lung cancer, among others. In addition, other ncRNAs, such as lncRNAs and circRNAs, are also being investigated as potential biomarkers. Researchers are working to validate these biomarkers in large patient cohorts to ensure their clinical utility.
-
b
Understanding the role of ncRNAs in cancer progression: Many ncRNAs have been found to play essential roles in cancer progression, such as promoting tumor growth, invasion, and metastasis targeting to treat cancer.
-
c
Development of therapeutic strategies targeting ncRNAs: ncRNAs are potential targets for cancer therapy, and several methods are being explored. One approach uses antisense oligonucleotides (ASOs) or small interfering RNAs (siRNAs) to target and degrade specific ncRNAs. Another way is to use small molecules or natural compounds that can inhibit the function of particular ncRNAs. In addition, some researchers are exploring CRISPR-Cas9 gene editing to modify ncRNA expression in cancer cells.
-
d
Combination therapy with ncRNA-targeting agents: Researchers are exploring the potential of combining ncRNA-targeting agents with other cancer therapies, such as chemotherapy, radiation therapy, or immunotherapy. Combination therapy may help to overcome resistance to existing cancer treatments and improve patient outcomes.
Conclusion
Current understanding of ncRNAs in cancer suggests that they are involved in several key aspects of tumorigenesis, including cell proliferation, apoptosis, migration, and invasion. Different types of ncRNAs, such as microRNAs, long non-coding RNAs, and circular RNAs, are differentially expressed in other cancer types, and some have been identified as potential biomarkers for cancer diagnosis and prognosis.
Potential clinical applications of ncRNAs in cancer include their use as diagnostic and prognostic markers and targets for cancer therapy. For example, preclinical studies have shown promise in targeting specific ncRNAs with small molecule inhibitors or RNA-based therapies, such as antisense oligonucleotides. In addition, ncRNA-based immunotherapies are also being developed to stimulate the immune system to recognize and eliminate cancer cells. Furthermore, the implications of ncRNAs in drug resistance have highlighted the need for extensive research to identify new targets and modalities for cancer treatment. Bioinformatics analysis tools have enabled researchers to understand the complexity of ncRNAs in cancer better and explore emerging biomarkers and therapeutic targets.
However, there are several challenges in this rapidly evolving field. One major challenge is the identification and validation of specific ncRNA biomarkers, as well as the development of robust and reliable methods for their detection. Another challenge is the delivery of ncRNA-targeting agents to the cancer cells, as they need to be efficiently delivered to the target site without causing off-target effects. Moreover, preclinical and clinical studies must thoroughly evaluate the potential off-target effects of ncRNA-targeting agents and their long-term safety.
Overall, the study of ncRNAs in cancer represents a promising area of research, with potential applications in cancer diagnosis, prognosis, and therapy. The use of ncRNAs in cancer care has the potential to be a game-changer in the ongoing struggle against the disease.
Authors contribution
Author's contribution: KRU and HJC supervised the project. AG, RJ, and KP analyzed and interpreted the data for the tabulations. KSM wrote the manuscript with support from KP, KVK, AG, NK, AK. All authors offered timely feedback and facilitated in the manuscript preparation
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Kalyan Ram Uppaluri, Email: kalyan@khdreamlife.com.
Sri Manjari K, Email: manjari@khdreamlife.com.
References
- 1.Ashrafizadeh M., Hushmandi K., Rahmani Moghadam E., Zarrin V., Hosseinzadeh Kashani S., Bokaie S., Najafi M., Tavakol S., Mohammadinejad R., Nabavi N., Hsieh C.L., Zarepour A., Zare E.N., Zarrabi A., Makvandi P. Progress in delivery of siRNA-based therapeutics employing nano-vehicles for treatment of prostate cancer. Bioengineering. 2020;7:91. doi: 10.3390/bioengineering7030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009 doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bica-Pop C., Cojocneanu-Petric R., Magdo L., Raduly L., Gulei D., Berindan-Neagoe I. Overview upon miR-21 in lung cancer: focus on NSCLC. Cell. Mol. Life Sci. 2018;75:3539–3551. doi: 10.1007/s00018-018-2877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bierhoff, H., 2018. Analysis of lncRNA-protein interactions by RNA-protein pull-down assays and RNA immunoprecipitation (RIP). pp. 241–250. doi: 10.1007/978-1-4939-7371-2_17. [DOI] [PubMed]
- 5.Cabili M.N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A., Rinn J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen B., Dragomir M.P., Yang C., Li Q., Horst D., Calin G.A. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct. Target Ther. 2022;7:121. doi: 10.1038/s41392-022-00975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B., Zhang R.N., Fan X., Wang Jue, Xu C., An B., Wang Q., Wang Jing, Leung E.L.H., Sui X., Wu Q. Clinical diagnostic value of long non-coding RNAs in Colorectal Cancer: a systematic review and meta-analysis. J. Cancer. 2020 doi: 10.7150/jca.46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q., Li Y., Liu Y., Xu W., Zhu X. Exosomal non-coding RNAs-mediated crosstalk in the tumor microenvironment. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.646864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Mao R., Su W., Yang X., Geng Q., Guo C., Wang Z., Wang J., Kresty L.A., Beer D.G., Chang A.C., Chen G. Circular RNA circHIPK3 modulates autophagy via MIR124-3p-STAT3-PRKAA/AMPKα signaling in STK11 mutant lung cancer. Autophagy. 2020;16 doi: 10.1080/15548627.2019.1634945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., Gao D.Y., Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv. Drug. Deliv. Rev. 2015 doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu H.S., Somvanshi S., Patel E., Chen T.W., et al. Pan-cancer analysis of lncrna regulation supports their targeting of cancer genes in each tumor context. Cell Rep. 2018;23 doi: 10.1016/j.celrep.2018.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu C., Quinn J., Chang H.Y. Chromatin Isolation by RNA Purification (ChIRP) J. Vis. Exp. 2012 doi: 10.3791/3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng S., Wang J., Zhang L., Li J., Jin Y. Lncrna hotair promotes cancer stem-like cells properties by sponging mir-34a to activate the jak2/stat3 pathway in pancreatic ductal adenocarcinoma. Onco Targets Ther. 2021;14 doi: 10.2147/OTT.S286666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.di Leva G., Croce C.M. miRNA profiling of cancer. Curr. Opin. Genet. Dev. 2013;23:3–11. doi: 10.1016/j.gde.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebert M.S., Sharp P.A. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–2050. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011 doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 17.Fang T., Lv H., Lv G., Li T., Wang C., Han Q., Yu L., Su B., Guo L., Huang S., Cao D., Tang L., Tang S., Wu M., Yang W., Wang H. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018;9:191. doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishman A., Light D., Lamm A.T. QsRNA-seq: a method for high-throughput profiling and quantifying small RNAs. Genome Biol. 2018;19:113. doi: 10.1186/s13059-018-1495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao N., Li Yueheng, Li J., Gao Z., Yang Z., Li Yong, Liu H., Fan T. Long non-coding RNAs: the regulatory mechanisms, research strategies, and future directions in cancers. Front. Oncol. 2020 doi: 10.3389/fonc.2020.598817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gawronski K.A.B., Kim J. Single cell transcriptomics of noncoding <scp>RNAs</scp>and their cell-specificity. WIREs RNA. 2017;8 doi: 10.1002/wrna.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge X., Chen Y., Liao X., Liu D., Li F., Ruan H., Jia W. Overexpression of long noncoding RNA PCAT-1 is a novel biomarker of poor prognosis in patients with colorectal cancer. Med. Oncol. 2013;30 doi: 10.1007/s12032-013-0588-6. [DOI] [PubMed] [Google Scholar]
- 22.Gebert L.F.R., MacRae I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019 doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghafouri-Fard S., Khoshbakht T., Taheri M., Jamali E. A concise review on the role of CircPVT1 in tumorigenesis, drug sensitivity, and cancer prognosis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.762960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gholikhani T., Kumar S., Valizadeh H., Mahdinloo S., Adibkia K., Zakeri-Milani P., Barzegar-Jalali M., Jimenez B. Advances in aptamers-based applications in breast cancer: drug delivery, therapeutics, and diagnostics. Int. J. Mol. Sci. 2022;23:14475. doi: 10.3390/ijms232214475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafner M., Katsantoni M., Köster T., Marks J., Mukherjee J., Staiger D., Ule J., Zavolan M. CLIP and complementary methods. Nat. Rev. Methods Primers. 2021;1:20. doi: 10.1038/s43586-021-00018-1. [DOI] [Google Scholar]
- 26.Han, H., 2018. RNA interference to knock down gene expression. pp. 293–302. doi: 10.1007/978-1-4939-7471-9_16. [DOI] [PMC free article] [PubMed]
- 27.Hanusek K., Poletajew S., Kryst P., Piekiełko-Witkowska A., Bogusławska J. piRNAs and PIWI proteins as diagnostic and prognostic markers of genitourinary cancers. Biomolecules. 2022;12:186. doi: 10.3390/biom12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hao X., Jia Q., Yuan J., Shi X., Guo H., Gao J., Guo Y. MicroRNA 195 suppresses cell proliferation, migration and invasion in epithelial ovarian carcinoma via inhibition of the CDC42/CCND1 pathway. Int. J. Mol. Med. 2020 doi: 10.3892/ijmm.2020.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes J., Peruzzi P.P., Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol. Med. 2014 doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Hirose T., Virnicchi G., Tanigawa A., Naganuma T., Li R., Kimura H., Yokoi T., Nakagawa S., Bénard M., Fox A.H., Pierron G. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol. Biol. Cell. 2014;25:169–183. doi: 10.1091/mbc.e13-09-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosoda K., Matsuura T., Kita H., Ichihashi N., Tsukada K., Urabe I., Yomo T. A novel sequence-specific RNA quantification method using nicking endonuclease, dual-labeled fluorescent DNA probe, and conformation-interchangeable oligo-DNA. RNA. 2008;14:584–592. doi: 10.1261/rna.761708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseini S.A., Salehifard Jouneghani A., Ghatrehsamani M., Yaghoobi H., Elahian F., Mirzaei S.A. CRISPR/Cas9 as precision and high-throughput genetic engineering tools in gastrointestinal cancer research and therapy. Int. J. Biol. Macromol. 2022;223:732–754. doi: 10.1016/j.ijbiomac.2022.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu W., Wang T., Yang Y., Zheng S. Tumor heterogeneity uncovered by dynamic expression of long noncoding RNA at single-cell resolution. Cancer Genet. 2015;208:581–586. doi: 10.1016/j.cancergen.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Huang A., Zheng H., Wu Z., Chen M., Huang Y. Circular RNA-protein interactions: functions, mechanisms, and identification. Theranostics. 2020;10:3503–3517. doi: 10.7150/thno.42174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang L., Liao L.M., Liu A.W., Wu J.B., Cheng X.L., Lin J.X., Zheng M. Overexpression of long noncoding RNA HOTAIR predicts a poor prognosis in patients with cervical cancer. Arch. Gynecol. Obstet. 2014;290 doi: 10.1007/s00404-014-3236-2. [DOI] [PubMed] [Google Scholar]
- 37.Huang X., Yuan T., Tschannen M., Sun Z., Jacob H., Du M., Liang Meihua, Dittmar R.L., Liu Y., Liang Mingyu, Kohli M., Thibodeau S.N., Boardman L., Wang L. Characterization of human plasma-derived exosomal RNAs by deep sequencing. Bmc Genomics [Electronic Resource] 2013;14 doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingolia N.T. Ribosome Footprint Profiling of Translation throughout the Genome. Cell. 2016;165:22–33. doi: 10.1016/j.cell.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasaki Y.W., Siomi M.C., Siomi H. PIWI-interacting RNA: its biogenesis and functions. Annu. Rev. Biochem. 2015;84:405–433. doi: 10.1146/annurev-biochem-060614-034258. [DOI] [PubMed] [Google Scholar]
- 40.Jazurek M., Ciesiolka A., Starega-Roslan J., Bilinska K., Krzyzosiak W.J. Identifying proteins that bind to specific RNAs - focus on simple repeat expansion diseases. Nucleic. Acids. Res. 2016;gkw803 doi: 10.1093/nar/gkw803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Q., Wang J., Wu X., Ma R., Zhang T., Jin S., Han Z., Tan R., Peng J., Liu G., Li Y., Wang Y. LncRNA2Target: a database for differentially expressed genes after lncRNA knockdown or overexpression. Nucleic. Acids. Res. 2015;43:D193–D196. doi: 10.1093/nar/gku1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiao X., Qian X., Wu L., Li B., Wang Y., Kong X., Xiong L. microRNA: the impact on cancer stemness and therapeutic resistance. Cells. 2019;9:8. doi: 10.3390/cells9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalvari I., Nawrocki E.P., Ontiveros-Palacios N., Argasinska J., Lamkiewicz K., Marz M., Griffiths-Jones S., Toffano-Nioche C., Gautheret D., Weinberg Z., Rivas E., Eddy S.R., Finn R.D., Bateman A., Petrov A.I. Rfam 14: expanded coverage of metagenomic, viral and microRNA families. Nucleic. Acids. Res. 2021;49:D192–D200. doi: 10.1093/nar/gkaa1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katti A., Diaz B.J., Caragine C.M., Sanjana N.E., Dow L.E. CRISPR in cancer biology and therapy. Nat. Rev. Cancer. 2022;22:259–279. doi: 10.1038/s41568-022-00441-w. [DOI] [PubMed] [Google Scholar]
- 45.Khan A.Q., Ahmed E.I., Elareer N.R., Junejo K., Steinhoff M., Uddin S. Role of miRNA-regulated cancer stem cells in the pathogenesis of human malignancies. Cells. 2019 doi: 10.3390/cells8080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kovacevic K.D., Gilbert J.C., Jilma B. Pharmacokinetics, pharmacodynamics and safety of aptamers. Adv. Drug. Deliv. Rev. 2018;134:36–50. doi: 10.1016/j.addr.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Kozomara A., Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic. Acids. Res. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kruspe S., Giangrande P. Aptamer-siRNA Chimeras: discovery, progress, and future prospects. Biomedicines. 2017;5:45. doi: 10.3390/biomedicines5030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le P., Romano G., Nana-Sinkam P., Acunzo M. Non-coding rnas in cancer diagnosis and therapy: focus on lung cancer. Cancers. 2021 doi: 10.3390/cancers13061372. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei M., Zheng G., Ning Q., Zheng J., Dong D. Translation and functional roles of circular RNAs in human cancer. Mol. Cancer. 2020;19:30. doi: 10.1186/s12943-020-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li C., Ni Y.Q., Xu H., Xiang Q.Y., Zhao Y., Zhan J.K., He J.Y., Li S., Liu Y.S. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct. Target Ther. 2021 doi: 10.1038/s41392-021-00779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H., Yang Y., Hong W., Huang M., Wu M., Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct. Target Ther. 2020;5:1. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W., Wang X., Li C., Chen T., Yang Q. Exosomal non-coding RNAs: emerging roles in bilateral communication between cancer cells and macrophages. Mol. Ther. 2022;30:1036–1053. doi: 10.1016/j.ymthe.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W., Wang Y., Liu R., Kasinski A.L., Shen H., Slack F.J., Tang D.G. MicroRNA-34a: potent tumor suppressor, cancer stem cell inhibitor, and potential anticancer therapeutic. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.640587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang Y.L., Zhang Y., Tan X.R., Qiao H., Liu S.R., Tang L.L., Mao Y.P., Chen L., Li W.F., Zhou G.Q., Zhao Y., Li J.Y., Li Q., Huang S.Y., Gong S., Zheng Z.Q., Li Z.X., Sun Y., Jiang W., Ma J., Li Y.Q., Liu N. A lncRNA signature associated with tumor immune heterogeneity predicts distant metastasis in locoregionally advanced nasopharyngeal carcinoma. Nat. Commun. 2022;13:2996. doi: 10.1038/s41467-022-30709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ling H., Fabbri M., Calin G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu C., Tang D.G. MicroRNA regulation of cancer stem cells. Cancer Res. 2011;71:5950–5954. doi: 10.1158/0008-5472.CAN-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu M., Jia J., Wang X., Liu Y., Wang C., Fan R. Long non-coding RNA HOTAIR promotes cervical cancer progression through regulating BCL2 via targeting miR-143-3p. Cancer Biol. Ther. 2018;19 doi: 10.1080/15384047.2018.1423921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y., Ao X., Yu W., Zhang Y., Wang J. Biogenesis, functions, and clinical implications of circular RNAs in non-small cell lung cancer. Mol. Ther. Nucleic Acids. 2022;27:50–72. doi: 10.1016/j.omtn.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu, Y., Wang, J., 2016. Therapeutic potentials of noncoding RNAs: targeted delivery of ncRNAs in cancer cells. pp. 429–458. doi: 10.1007/978-981-10-1498-7_16. [DOI] [PubMed]
- 61.López-Urrutia E., Bustamante Montes L.P., Ladrón de Guevara Cervantes D., Pérez-Plasencia C., Campos-Parra A.D. Crosstalk between long non-coding RNAs, Micro-RNAs and mRNAs: deciphering molecular mechanisms of master regulators in cancer. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lorenz R., Bernhart S.H., Höner zu Siederdissen C., Tafer H., Flamm C., Stadler P.F., Hofacker I.L. ViennaRNA package 2.0. Algorithms for Mol. Biol. 2011;6:26. doi: 10.1186/1748-7188-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lou Y., Yang X., Wang F., Cui Z., Huang Y. MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int. J. Mol. Med. 2010;26 doi: 10.3892/ijmm_00000530. [DOI] [PubMed] [Google Scholar]
- 64.Lu J., Getz G., Miska E.A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B.L., Mak R.H., Ferrando A.A., Downing J.R., Jacks T., Horvitz H.R., Golub T.R. MicroRNA expression profiles classify human cancers. Nature. 2005;435 doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 65.Luan W., Shi Y., Zhou Z., Xia Y., Wang J. Corrigendum to circRNA_0084043 promote malignant melanoma progression via miR-153-3p/Snail axis. Biochem. Biophys. Res. Commun. 2022;587 doi: 10.1016/j.bbrc.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Lv N., Shen S., Chen Q., Tong J. Long noncoding RNAs: glycolysis regulators in gynaecologic cancers. Cancer Cell Int. 2023;23:4. doi: 10.1186/s12935-023-02849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma J., Qi G., Li L. <p>A Novel Serum Exosomes-Based Biomarker hsa_circ_0002130 Facilitates Osimertinib-Resistance in Non-Small Cell Lung Cancer by Sponging miR-498</p>. Oncol. Targets Ther. Volume. 2020;13:5293–5307. doi: 10.2147/OTT.S243214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maragkakis M., Alexiou P., Nakaya T., Mourelatos Z. CLIPSeqTools—A novel bioinformatics CLIP-seq analysis suite. RNA. 2016;22:1–9. doi: 10.1261/rna.052167.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mokarram P., Niknam M., Sadeghdoust M., Aligolighasemabadi F., Siri M., Dastghaib S., Brim H., Ashktorab H. PIWI interacting RNAs perspectives: a new avenues in future cancer investigations. Bioengineered. 2021;12:10401–10419. doi: 10.1080/21655979.2021.1997078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ni Z., Wang X., Zhang T., Li L., Li J. Comprehensive analysis of differential expression profiles reveals potential biomarkers associated with the cell cycle and regulated by p53 in human small cell lung cancer. Exp. Ther. Med. 2018;15 doi: 10.3892/etm.2018.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palcau A.C., Canu V., Donzelli S., Strano S., Pulito C., Blandino G. CircPVT1: a pivotal circular node intersecting Long Non-Coding-PVT1 and c-MYC oncogenic signals. Mol. Cancer. 2022;21:33. doi: 10.1186/s12943-022-01514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng Z., Fang S., Jiang M., Zhao X., Zhou C., Gong Z. Circular RNAs: regulatory functions in respiratory tract cancers. Clin. Chim. Acta. 2020 doi: 10.1016/j.cca.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 73.Pliatsika V., Loher P., Magee R., Telonis A.G., Londin E., Shigematsu M., Kirino Y., Rigoutsos I. MINTbase v2.0: a comprehensive database for tRNA-derived fragments that includes nuclear and mitochondrial fragments from all The Cancer Genome Atlas projects. Nucleic. Acids. Res. 2018;46:D152–D159. doi: 10.1093/nar/gkx1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013 doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quek X.C., Thomson D.W., Maag J.L.V., Bartonicek N., Signal B., Clark M.B., Gloss B.S., Dinger M.E. lncRNAdb v2.0: expanding the reference database for functional long noncoding RNAs. Nucleic. Acids. Res. 2015;43:D168–D173. doi: 10.1093/nar/gku988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raj A., Rinn J.L. Illuminating Genomic Dark Matter with RNA Imaging. Cold Spring Harb. Perspect. Biol. 2019;11 doi: 10.1101/cshperspect.a032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sánchez-Marín D., Trujano-Camacho S., Pérez-Plasencia C., de León D.C., Campos-Parra A.D. LncRNAs driving feedback loops to boost drug resistance: sinuous pathways in cancer. Cancer Lett. 2022;543 doi: 10.1016/j.canlet.2022.215763. [DOI] [PubMed] [Google Scholar]
- 78.Schmidt L.H., Spieker T., Koschmieder S., Humberg J., Jungen D., Bulk E., Hascher A., Wittmer D., Marra A., Hillejan L., Wiebe K., Berdel W.E., Wiewrodt R., Muller-Tidow C. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J. Thorac. Oncol. 2011;6 doi: 10.1097/JTO.0b013e3182307eac. [DOI] [PubMed] [Google Scholar]
- 79.Setten R.L., Rossi J.J., Han S. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 80.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Statello L., Guo C.J., Chen L.L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun C.B., Wang H.Y., Han X.Q., Liu Y.N., Wang M.C., Zhang H.X., Gu Y.F., Leng X.G. LINC00511 promotes gastric cancer cell growth by acting as a ceRNA. World. J. Gastrointest. Oncol. 2020;12 doi: 10.4251/WJGO.V12.I4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suzuki R., Honda S., Kirino Y. PIWI expression and function in Cancer. Front Genet. 2012;3 doi: 10.3389/fgene.2012.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sweeney B.A., Petrov A.I., Burkov B., Finn R.D., Bateman A., Szymanski M., Karlowski W.M., Gorodkin J., Seemann S.E., Cannone J.J., Gutell R.R., Fey P., Basu S., Kay S., Cochrane G., Billis K., Emmert D., Marygold S.J., Huntley R.P., Lovering R.C., Frankish A., Chan P.P., Lowe T.M., Bruford E., Seal R., Vandesompele J., Volders P.J., Paraskevopoulou M., Ma L., Zhang Z., Griffiths-Jones S., Bujnicki J.M., Boccaletto P., Blake J.A., Bult C.J., Chen R., Zhao Y., Wood V., Rutherford K., Rivas E., Cole J., Laulederkind S.J.F., Shimoyama M., Gillespie M.E., Orlic-Milacic M., Kalvari I., Nawrocki E., Engel S.R., Cherry J.M., Team S., Berardini T.Z., Hatzigeorgiou A., Karagkouni D., Howe K., Davis P., Dinger M., He S., Yoshihama M., Kenmochi N., Stadler P.F., Williams K.P. RNAcentral: a hub of information for non-coding RNA sequences. Nucleic. Acids. Res. 2019;47:D221–D229. doi: 10.1093/nar/gky1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szejniuk W.M., Robles A.I., McCulloch T., Falkmer U.G.I., Røe O.D. Epigenetic predictive biomarkers for response or outcome to platinum-based chemotherapy in non-small cell lung cancer, current state-of-art. Pharmacogenomics J. 2019;19:5–14. doi: 10.1038/s41397-018-0029-1. [DOI] [PubMed] [Google Scholar]
- 86.Tabnak P., Ghasemi Y., Natami M., Khorram R., Ebrahimnezhad M. Role of m6A modification in dysregulation of Wnt/β-catenin pathway in cancer. Biomed. Pharmacother. 2023;157 doi: 10.1016/j.biopha.2022.114023. [DOI] [PubMed] [Google Scholar]
- 87.Taxman, D.J., Moore, C.B., Guthrie, E.H., Huang, M.T.-H., 2010. Short Hairpin RNA (shRNA): design, delivery, and assessment of gene knockdown. pp. 139–156. doi: 10.1007/978-1-60761-657-3_10. [DOI] [PMC free article] [PubMed]
- 88.Thorns C., Schurmann C., Gebauer N., Wallaschofski H., Kümpers C., Bernard V., Feller A.C., Keck T., Habermann J.K., Begum N., Lehnert H., Brabant G. Global MicroRNA profiling of pancreatic neuroendocrine Neoplasias. Anticancer Res. 2014;34 [PubMed] [Google Scholar]
- 89.Tycko J., Wainberg M., Marinov G.K., Ursu O., Hess G.T., Ego B.K., Aradhana, Li A., Truong A., Trevino A.E., Spees K., Yao D., Kaplow I.M., Greenside P.G., Morgens D.W., Phanstiel D.H., Snyder M.P., Bintu L., Greenleaf W.J., Kundaje A., Bassik M.C. Mitigation of off-target toxicity in CRISPR-Cas9 screens for essential non-coding elements. Nat. Commun. 2019;10:4063. doi: 10.1038/s41467-019-11955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valinezhad Orang A., Safaralizadeh R., Kazemzadeh-Bavili M. Mechanisms of miRNA-mediated gene regulation from common downregulation to mRNA-specific upregulation. Int. J. Genomics. 2014;2014:1–15. doi: 10.1155/2014/970607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verduci L., Strano S., Yarden Y., Blandino G. The circRNA–microRNA code: emerging implications for cancer diagnosis and treatment. Mol. Oncol. 2019 doi: 10.1002/1878-0261.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vishnubalaji R., Elango R., Alajez N.M. LncRNA-based classification of triple negative breast cancer revealed inherent tumor heterogeneity and vulnerabilities. Noncoding RNA. 2022;8:44. doi: 10.3390/ncrna8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang C., Wang L., Ding Y., Lu X., Zhang G., Yang J., Zheng H., Wang H., Jiang Y., Xu L. LncRNA structural characteristics in epigenetic regulation. Int. J. Mol. Sci. 2017;18:2659. doi: 10.3390/ijms18122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang S., Liang K., Hu Q., Li P., Song J., Yang Y., Yao J., Mangala L.S., Li C., Yang W., Park P.K., Hawke D.H., Zhou J., Zhou Y., Xia W., Hung M.C., Marks J.R., Gallick G.E., Lopez-Berestein G., Flores E.R., Sood A.K., Huang S., Yu D., Yang L., Lin C. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J. Clin. Invest. 2017;127 doi: 10.1172/JCI91553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weng W., Li H., Goel A. Piwi-interacting RNAs (piRNAs) and cancer: emerging biological concepts and potential clinical implications. Biochim. Biophys. Acta (BBA) - Rev. Cancer. 2019;1871:160–169. doi: 10.1016/j.bbcan.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu D.M., Wang S., Wen X., Han X.R., Wang Y.J., Shen M., Fan S.H., Zhang Z.F., Shan Q., Li M.Q., Hu B., Lu J., Chen G.Q., Zheng Y.L. LncRNA SNHG15 acts as a ceRNA to regulate YAP1-Hippo signaling pathway by sponging miR-200a-3p in papillary thyroid carcinoma. Cell Death. Dis. 2018;9 doi: 10.1038/s41419-018-0975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu W., Hu Q., Wang M., Shao S., Zhao X., Bai H., Huang J., Tang G., Liang T. A PEGylated megamer-based microRNA delivery system activatable by stepwise microenvironment stimulation. Chem. Commun. 2019;55:9363–9366. doi: 10.1039/C9CC03846A. [DOI] [PubMed] [Google Scholar]
- 99.Xie F., Liu S., Wang J., Xuan J., Zhang X., Qu L., Zheng L., Yang J. deepBase v3.0: expression atlas and interactive analysis of ncRNAs from thousands of deep-sequencing data. Nucleic. Acids. Res. 2021;49:D877–D883. doi: 10.1093/nar/gkaa1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu G., Yang Y., Yang J., Xiao L., Wang X., Qin L., Gao J., Xuan R., Wu X., Chen Z., Sun R., Song G. Screening and identification of miR-181a-5p in oral squamous cell carcinoma and functional verification in vivo and in vitro. BMC Cancer. 2023;23:162. doi: 10.1186/s12885-023-10600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang A., Liu X., Liu P., Feng Y., Liu H., Gao S., Huo L., Han X., Wang J., Kong W. LncRNA UCA1 promotes development of gastric cancer via the miR-145/MYO6 axis. Cell. Mol. Biol. Lett. 2021;26 doi: 10.1186/s11658-021-00275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang J., Meng X., Pan J., Jiang N., Zhou C., Wu Z., Gong Z. CRISPR/Cas9-mediated noncoding RNA editing in human cancers. RNA Biol. 2018;15:35–43. doi: 10.1080/15476286.2017.1391443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang Y., Yan X., Li X., Ma Y., Goel A. Long non-coding RNAs in colorectal cancer: novel oncogenic mechanisms and promising clinical applications. Cancer Lett. 2021 doi: 10.1016/j.canlet.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao J., Xie M., Ma X., Song J., Wang Y., Xue X. PIWI-interacting RNAs in cancer: biogenesis, function, and clinical significance. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.965684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yonezawa S., Koide H., Asai T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug. Deliv. Rev. 2020;154–155:64–78. doi: 10.1016/j.addr.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zarnegar B.J., Flynn R.A., Shen Y., Do B.T., Chang H.Y., Khavari P.A. irCLIP platform for efficient characterization of protein–RNA interactions. Nat. Methods. 2016;13:489–492. doi: 10.1038/nmeth.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang H., Qin C., An C., Zheng X., Wen S., Chen W., Liu X., Lv Z., Yang P., Xu W., Gao W., Wu Y. Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer. Mol. Cancer. 2021;20:126. doi: 10.1186/s12943-021-01431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L., Yang X., Lv Y., Xin X., Qin C., Han X., Yang L., He W., Yin L. Cytosolic co-delivery of miRNA-34a and docetaxel with core-shell nanocarriers via caveolae-mediated pathway for the treatment of metastatic breast cancer. Sci. Rep. 2017;7:46186. doi: 10.1038/srep46186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang W., Xia W., Lv Z., Ni C., Xin Y., Yang L. Liquid biopsy for cancer: circulating tumor cells, circulating free DNA or exosomes? Cell. Physiol. Biochem. 2017;41 doi: 10.1159/000458736. [DOI] [PubMed] [Google Scholar]
- 110.Zheng Y., Luo H., Teng X., Hao X., Yan X., Tang Y., Zhang W., Wang Y., Zhang P., Li Y., Zhao Y., Chen R., He S. NPInter v5.0: ncRNA interaction database in a new era. Nucleic. Acids. Res. 2023;51:D232–D239. doi: 10.1093/nar/gkac1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng Z., Chen Z., Zhong Q., Zhu D., Xie Y., Shangguan W., Xie W. CircPVT1 promotes progression in clear cell renal cell carcinoma by sponging miR-145-5p and regulating TBX15 expression. Cancer Sci. 2021;112 doi: 10.1111/cas.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou J., Rossi J.J. Therapeutic potential of aptamer-siRNA conjugates for treatment of HIV-1. BioDrugs. 2012;26:393–400. doi: 10.1007/BF03261896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu Q., Lu G., Luo Z., Gui F., Wu J., Zhang D., Ni Y. CircRNA circ_0067934 promotes tumor growth and metastasis in hepatocellular carcinoma through regulation of miR-1324/FZD5/Wnt/β-catenin axis. Biochem. Biophys. Res. Commun. 2018;497 doi: 10.1016/j.bbrc.2018.02.119. [DOI] [PubMed] [Google Scholar]



