Highlights
-
•
Currently available dopamine agonists treat motor symptoms of Parkinson’s disease but may cause nonmotor adverse events.
-
•
Some adverse events associated with dopamine agonists have been linked to selective activation of D2/D3 dopamine receptors.
-
•
Selective targeting of D1/D5 dopamine receptors may provide robust motor benefit with reduced risk of adverse events.
-
•
Specific therapeutic classifications reflecting the functional selectivity of selective dopamine agonists may be needed.
Keywords: Parkinson’s disease, Dopamine agonists, D1 receptors, D2 receptors, Partial agonism, Dopamine receptor selectivity, Direct pathway, Parkinson’s disease treatment
Abstract
Dopamine agonists (DAs) have demonstrated efficacy for the treatment of Parkinson’s disease (PD) but are limited by adverse effects (AEs). DAs can vary considerably in their receptor subtype selectivity and affinity, chemical composition, receptor occupancy, and intrinsic activity on the receptor. Most currently approved DAs for PD treatment primarily target D2/D3 (D2-like) dopamine receptors. However, selective activation of D1/D5 (D1-like) dopamine receptors may enable robust activation of motor function while avoiding AEs related to D2/D3 receptor agonism. Full D1/D5 receptor-selective agonists have been explored in small, early-phase clinical studies, and although their efficacy for motor symptoms was robust, challenges with pharmacokinetics, bioavailability, cardiovascular AEs, and dyskinesia rates similar to levodopa prevented clinical advancement. Generally, repeated dopaminergic stimulation with full DAs is associated with frontostriatal dysfunction and sensitization that may induce plastic changes in the motor system, and neuroadaptations that produce long-term motor and nonmotor complications, respectively. Recent preclinical and clinical studies suggest that a D1/D5 receptor-selective partial agonist may hold promise for providing sustained, predictable, and robust motor control, while reducing risk for motor complications (e.g., levodopa-induced dyskinesia) and nonmotor AEs (e.g., impulse control disorders and excessive daytime sleepiness). Clinical trials are ongoing to evaluate this hypothesis. The potential emerging availability of novel dopamine receptor agonists with selective dopamine receptor pharmacology suggests that the older terminology “dopamine agonist” may need revision to distinguish older-generation D2/D3–selective agonists from D1/D5-selective agonists with distinct efficacy and tolerability characteristics.
1. Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with increasing worldwide prevalence, and its incidence steadily increases with age [1], [2]. The Global Burden of Disease Study estimates that the number of PD cases will double from approximately 6 million in 2015 to more than 12 million in 2040, becoming a leading source of disability [3]. In 2017, more than 1 million individuals in the United States were estimated to be affected by PD [4], with numbers expected to increase [5]. Motor symptoms are cardinal features of PD, and motor function progressively worsens with prolonged disease duration, leading to disability and reduced quality of life [6], [7]. Treatment to replenish striatal dopaminergic deficiency with levodopa typically results in robust clinical motor symptom improvement [7]. However, patients with PD commonly experience levodopa-related motor complications, including motor fluctuations and dyskinesia, that emerge and progress over time [7].
The cornerstone of current medical approaches for PD emerged in the 1960s, when reduced dopamine levels were observed in the striatum of patients with PD; subsequently, levodopa, a dopamine precursor, was found to improve PD motor symptoms by acting as an exogeneous source of dopamine [8], [9], [10]. Dopamine receptor agonism emerged as another effective therapeutic approach for PD in the early 1970s [8]. Unlike levodopa, which is converted to dopamine in monoaminergic neurons and released to activate all dopamine receptors, dopamine agonists (DAs) may have substantial and variable specificity for subsets of dopamine receptor subtypes [8], [11], [12]. Bromocriptine, apomorphine, and pergolide were the earliest DAs found to be efficacious in PD, followed by others such as ropinirole, pramipexole, and rotigotine [8], [13], [14], [15]. Current DAs were historically split into 2 groups based on their chemical structures: first-generation ergoline agonists (ergot-like derivatives; eg, bromocriptine, cabergoline, lisuride, pergolide) and later non–ergoline-derived agonists (eg, pramipexole, ropinirole, rotigotine, apomorphine) [11], [16], [17]. Safety concerns of ergoline DAs were associated with peritoneal, pulmonary, and cardiac or valvular fibrosis, owing to their off-target activation of certain serotonergic and adrenergic receptors; these compounds, along with the chemical moiety-based classification, are no longer routinely used [17], [18].
Dopamine receptor agonists are broadly subdivided into two groups based on their affinity for two families of dopamine receptors coupled to G proteins: D1-like (D1 and D5 receptor subtypes) and D2-like (D2, D3, and D4 receptor subtypes) [19]. D1-like and D2-like receptors differ in their recruitment of downstream signaling pathways; D1-like receptors are coupled to Gs/olf proteins and have a stimulatory effect on adenylate cyclase, whereas D2-like receptors are coupled to Gi/o receptors and inhibit adenylate cyclase activity [20]. D2-like receptors may also modulate ion channel activity via Gβγ [20].
Currently available oral and transdermal DAs tend to be more selective for D2/D3 receptors relative to D1/D5-like receptors, with most being exclusively D2/D3–selective DAs [17]. However, the relative specificity of these agents at clinical concentrations varies. Apomorphine, for example, preferentially binds to D2/D3/D4 receptors, but is less selective and is reported to have modest but functionally meaningful engagement of D1/D5 receptors at therapeutic concentrations [17], [21], [22], [23], [24].
When compared with levodopa treatment, currently approved D2- and D3-selective DAs are associated with a lower risk of dyskinesias in the initial 3–5 years of PD treatment, but they also have overall less efficacy than levodopa and lead to higher incidences of other AEs at efficacious doses [18]. Some AEs (eg, psychosis, impulse control disorders [ICDs], and excessive daytime sleepiness [EDS]) associated with the use of DAs may be related to the selective activation of D2 and/or D3 receptors in the mesolimbic pathway, where these subtypes regulate neurocircuitry related to reward, punishment, and behavioral sensitization [17], [25], [26], [27].
The implications of the dopamine receptor pharmacology profile of a specific DA on its clinical efficacy and tolerability are not fully understood, and there are few large direct comparison studies conducted to date. Currently available oral and transdermal DAs are often considered generally equivalent to each other by many clinicians; however, molecules that directly engage and activate dopamine receptors can vary considerably in chemical composition (eg, ergoline versus non-ergoline), receptor subtype selectivity, and intrinsic activity on the receptor (ie, full agonism with maximal stimulation versus partial agonism with submaximal activation) [17], [28]. In this review, we discuss evidence to date that suggests how a D1 and D5 dopamine receptor–selective (D1-like) partial agonist may have a distinct clinical efficacy and safety profile compared with that of currently approved D2- and D3-selective (D2-like) DAs.
2. Can activation of D1-Like receptors versus D2-Like receptors drive differences in the clinical efficacy profiles of DAs?
The basal ganglia of the brain are modeled with two functional circuits (direct and indirect pathways) that help translate cortical inputs into specific “go” (facilitation) and “stop” (suppression) signals from the thalamus to the broader motor system [29], [30]. This model posits that the go and stop signals are propagated through direct and indirect pathways, respectively, which project to the globus pallidus pars interna (GPi), the primary output of basal ganglia [30]. GPi output exerts an inhibitory effect on thalamocortical neurons that promote movement [29]. Dopamine facilitates movement by stimulating the direct (go) pathway and inhibiting the indirect (stop) pathway [31]. Dopaminergic activation of direct pathway medium spiny neurons inhibits the GPi by releasing GABA, thereby promoting movement, whereas activation of indirect pathway medium spiny neurons inhibits GPi indirectly via the globus pallidus externa and subthalamic nucleus to promote movement [29], [32]. Ultimately, integration between the direct and indirect pathways produces coordinated motor output and fine temporal patterning of neural activity [29]. Of note, while all dopamine receptors are expressed in striatum, D1 receptors and D2 receptors are most abundantly expressed on striatal neurons [33]. Striatal D1 and D2 receptors are localized to distinct neuronal populations, with D1 receptors expressed on direct pathway neurons and D2 receptors expressed in the indirect pathway [26]. Specific or differential functional involvement of D3, D4, and D5 receptors in the striatal motor circuitry is less known, particularly due to relatively lower expression and nonselectivity of available tools [33], [34].
Most approved DAs primarily target D2/D3 (D2-like) dopamine receptors [21], [22], [24], [25]. Bromocriptine, one of the major earlier generation ergot-derived DAs, primarily activates D2/D3/D4 receptors [17], [35], [36]. Among other major ergoline DAs, pergolide activates D2/D3/D4 receptors but may also have some affinity for D1/D5 receptors [17], [24], [37], [38], [39]. Second-generation, nonergoline DAs (Table 1) such as pramipexole, ropinirole, and rotigotine, also primarily activate D2/D3/D4 (D2-like) dopamine receptors [17], [23], [24], [40]. Apomorphine has been proposed to act as a less-specific DA that activates all dopamine receptor subtypes, including D1/D5 receptors, although its affinity for D1 receptors is reported to be lower than that for other dopamine receptor subtypes [21], [22], [23]. However, apomorphine’s affinity for D1/D5 receptors is still considerably higher compared to other D2/D3 DAs such as pramipexole and ropinirole [24]. Among DAs, apomorphine has clinical efficacy in PD that is most comparable to that of levodopa, possibly because it has a moderate-to-high affinity for most dopamine receptor subtypes, including D1/D5 receptors, unlike most other approved D2- and D3-selective DAs [22]. However, apomorphine has limited oral bioavailability, and only parenteral, subcutaneous, or sublingual formulations of apomorphine have been investigated [21], [22].
Table 1.
Current and Notable Clinical-Stage Nonergoline DAs in PD and Their Dopamine Receptor Subtype Selectivity.
| Name of DAa | Clinical statusb | Dopamine receptor selectivityc |
|---|---|---|
| Pramipexole | Approved in the EU and United States [101], [102] | D3 > D4 > D2≫D1,D5 [24] |
| Ropinirole | Approved in the EU and United States [103], [104] | D3 > D2 ∼ D4 > D1,D5 [24] |
| Rotigotine | Approved in the EU and United States [105], [106] | D3≫D4 ∼ D5 ∼ D2 > D1 [40] |
| Apomorphine | Approved in the United States [21] | D4 > D5 > D3 > D2 > D1 [24] |
| Piribedil | Approved in the EU [107] | D2 ∼ D3 ∼ D4≫D1,D5 [24] |
| Tavapadon | Investigational, phase 3 [108], [109], [110] | D1, D5 selective [50] |
| KDT3594 | Investigational, phase 2 [111] | D2 selective [112] |
| Lu AF28996 | Investigational, phase 1 [113] | D1, D2 selective [114] |
This table does not distinguish agents by type of formulation (e.g., sublingual, extended release, transdermal, subcutaneous, oral, infusion, polymer conjugate, etc.).
As of November 2022.
For approved DAs, dopamine receptor selectivity is presented based on binding affinities (Ki) as reported in the literature [24], [40]. For investigational DAs where Ki values were not reported, selectivity is presented based on descriptions in reported literature. DA, dopamine agonist; PD, Parkinson’s disease.
Given the sparsity of D1/D5–selective DAs in clinical development to date (Table 2), limited clinical data are available to directly assess potential differences in efficacy between selective activation of D1/D5 and D2/D3 receptors. However, available evidence suggests that D1/D5 receptor selective agonism may potentially provide robust motor control. The D1/D5–selective DA ABT-431 and its active metabolite A-86929 were shown to improve disability score and motor function in preclinical PD models, including in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) primate models [41], [42], [43]. Intravenous ABT-431 in a placebo-controlled study that included 14 patients and assessed peak percent change from baseline in motor subsection of the Unified Parkinson's Disease Rating Scale (UPDRS) score demonstrated a clinically and statistically significant difference relative to placebo in favor of ABT-431 at doses of 10–40 mg [44]. Similarly, ABT-431 demonstrated antiparkinsonian benefit comparable to levodopa for up to 6 h after therapeutic administration in another pilot randomized clinical study that included 20 patients and used the motor assessment of the UPDRS score as an efficacy endpoint [45]. A separate D1/D5 receptor–selective DA, dihydrexidine, was investigated in a small pilot study, although the sample size was too small to make reliable conclusions regarding motor control [46], [47]. In an early-phase trial where 13 participants with PD were treated with the D1/D5 receptor–selective partial agonist PF–06412562, participants exhibited clinically meaningful motor improvement from baseline when compared with placebo-treated participants, as assessed by mean Movement Disorder Society (MDS)–UPDRS Part III motor score [48]. In a preclinical primate study in which another selective D1/D5 DA, tavapadon, was compared with levodopa, tavapadon promoted comparable maximal locomotor activity with a longer activity time [49]. Additionally, the disability-free time recorded for tavapadon was approximately 3-fold longer (350 versus 120 min) than that for levodopa. In a phase 2 randomized study of tavapadon in 57 patients with early-stage PD, tavapadon-treated participants had a significantly greater improvement in mean MDS-UPDRS Part III score at 15 weeks compared with placebo-treated participants, although enrollment in this study was terminated early due to its linkage to a concurrent phase 2 trial of tavapadon in advanced PD that failed to meet a prespecified interim efficacy threshold [50]. In that phase 2 trial, adults with motor fluctuations due to PD were treated with once-daily tavapadon (15 mg) or placebo for 15 weeks [51]. After 10 weeks of treatment, tavapadon-treated participants (n = 41) reported a least squares mean of 1.66 fewer daily OFF hours compared with baseline, whereas participants who received placebo reported 0.97 fewer off hours compared with baseline [51].
Table 2.
D1-selective DAs with available clinical evidence in PD.
| Name of DA | Dopamine receptor selectivity | Current clinical statusa | Evidence of motor control |
|---|---|---|---|
| ABT-431 | D1/D5 full [43], [45] | Discontinued after pilot clinical study [45] | Clinically and statistically significant difference relative to placebo in motor subsection of the UPDRS score in pilot study in advanced PD [45] |
| Dihydrexidine | D1/D5 full [46], [47] | Discontinued after pilot clinical study [46] | 3 of 4 patients did not have motor improvement; 1 patient had motor response similar to levodopa, as assessed by UPDRS motor score immediately after dosing in pilot study in mild/moderate PD [46] |
| PF-06412562 | D1/D5 partial [48] | Discontinued after phase 1 study [115] | Clinically meaningful motor improvement relative to placebo, as assessed by LSM MDS–UPDRS Part III motor score in phase 1 study in all patients with PD [48] |
| Tavapadon | D1/D5 partial | Phase 3, ongoing [108], [109], [110] | Significantly greater improvement in mean MDS-UPDRS Part III score at 15 weeks relative to placebo in phase 2 study in early-stage PD [50] |
As of July 2022. DA, dopamine agonist; LSM, least-squares mean; MDS, Movement Disorder Society; PD, Parkinson’s disease; UPDRS, Unified Parkinson's Disease Rating Scale.
3. How does preferential activation of D2-Like dopamine receptors affect the safety and tolerability profiles of DAs?
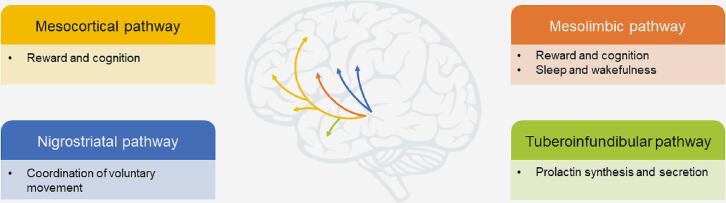
The selective activation of dopamine receptor subtypes also has implications for the safety and tolerability profile of DAs. The 4 major dopaminergic pathways in the brain are the nigrostriatal, mesolimbic, mesocortical, and tuberoinfundibular pathways (Fig. 1) [52]. Although DAs are targeted to address decreased dopaminergic activity in the nigrostriatal pathway on the basis of its involvement in the coordination of movement and PD pathology as described above, DAs may also activate other dopaminergic pathways (e.g., tuberoinfundibular, mesocortical, and mesolimbic pathways) [52]. Such activation can affect endocrine function and cause behavioral and psychiatric AEs [17], [52], [53]. Importantly, while D1 and D2 receptors both show robust expression within the striatum, expression patterns in extrastriatal areas are more variable (Table 3) [20], [54], [55]. Although most data supporting direct comparisons of dopamine receptor subtype expression have been generated in preclinical models (e.g., rats and nonhuman primates), the overall patterns of receptor expression are often found to be translatable to humans, with the potential exception of differences in fine expression patterns within the cortex [56], [57]. In addition to dense staining of the basal ganglia and nucleus accumbens (the primary targets of the nigrostriatal and mesolimbic dopamine pathways, respectively), D1 receptors are also found in the cortex, hippocampus, olfactory bulb, and hypothalamus [55], [58], [59]. D5 receptors show a similar expression pattern, although with generally sparser expression, and have also been identified in the thalamus and cerebellum (areas with little to no D1 receptor expression) [20], [60]. Conversely, D2 receptors show limited cortical expression and are highly expressed in the hypothalamus, thalamus, and hindbrain regions [56], [59]. D3 and D4 receptors are somewhat unique in that they are widely distributed throughout the brain, with dense expression throughout limbic regions [57], [61]. In particular, D3 receptors show limited striatal expression, but are highly expressed in the nucleus accumbens and associated structures [55], [57].
Fig. 1.
Four major dopaminergic pathways in the brain and their key physiological functions. Arrows in the brain denote dopaminergic pathways and the color of the arrows are aligned with the color of the box denoting the corresponding dopaminergic pathway.
Table 3.
Dopamine Receptor Expressiona Throughout Brain Regions of Interest.
| Receptor | Dorsal striatum | Ventral striatum | Cortex | Hippocampus | Thalamus | Hypothalamus | Cerebellum | Hindbrain |
|---|---|---|---|---|---|---|---|---|
| D1[20], [55], [56], [58] | +++ | +++ | ++ | ++ | + | + | – | + |
| D2[20], [54], [55], [56], [59] | +++ | +++ | + | + | +/- | ++ | – | +++ |
| D3[20], [57] | + | +++ | +/- | + | + | + | + | + |
| D4[61] | +++ | ++ | + | +++ | ++ | ++ | + | + |
| D5[20], [54], [60], [116] | ++ | + | +++ | +++ | + | + | + | + |
Comparisons of expression are relative to expression of each receptor across other regions, and do not necessarily indicate relative expression of receptors in comparison to each other.
Selective expression of dopamine receptors within these regions likely underscores distinct functional roles for each receptor subtype. D1 receptors are involved in locomotor activity, reward, learning, memory, and renal functions [62]. D2/D3 receptors are associated with locomotion, learning, memory, cognition, impulse control, sleep, and regulation of food intake [62]. Relatedly, some of the key physiologic functions associated with D4 receptors are cognition, impulse control, attention, and sleep [62]. Finally, D5 receptors have been linked to cognition, attention, decision-making, and motor learning [62]. Neurodegeneration of dopamine circuitry in the parkinsonian brain does not impact each dopamine pathway equally, but instead leads to selective degeneration of the nigrostriatal pathway, particularly in early stages of the disease [63]. Dopamine agonists, however, enhance dopamine signaling throughout the brain, including pathways that may not be degenerated [63]. Therefore, the overactivation of extrastriatal dopamine receptors may be responsible for many of the side effects associated with DAs [17], [63]. Some of these side effects, including nausea, vomiting, dizziness, and orthostatic hypotension, are associated with short-term exposure [18], [64]. Other side effects, such as EDS and other sleep disturbances, cognitive issues, psychosis, ICDs, and peripheral edema, may be associated with long-term treatment [18], [52]. AEs such as nausea and EDS are also associated with other dopaminergic replacement therapies such as levodopa [17], [65]. A subset of these events are also nonmotor symptoms in PD that are exacerbated by DAs [17]. As the disease progresses, patients become more prone to some of these events, including hallucinations [66]. Repeated pulsatile stimulation of dopamine receptors may also potentiate receptor signaling, leading to a further imbalance in dopamine pathways and increased risk of AEs [67], [68].
Select AEs associated with current DAs (eg, ICDs, EDS, and psychosis) may therefore be related to overactivation of D2/D3 receptors in the central nervous system, as well as activation of dopamine receptors in peripheral tissues (eg, edema) [17], [18], [25], [69], [70]. Studies suggest that some of these AEs occur with D2- and D3-selective DAs in particular, most likely due to off-target activation of dopamine receptors outside the nigrostriatal motor pathways, including the mesolimbic pathway, which plays a role in reward, punishment, and behavioral sensitization [17], [25], [26], [52], [71], [72]. Preclinical evidence suggests that D2/D3 receptors in the nucleus accumbens are associated with impulsivity and reward motivation [25], [73]. Animal models also indicate that D3 dopamine receptors are associated with addiction and reinstatement of drug-seeking behavior [74]. D3 receptors in brain regions associated with ICDs are upregulated following chronic administration of D2/D3 DAs, again highlighting the potential role of D3 receptors in the development of these AEs [25]. Some of these preclinical findings are further corroborated by observations in humans. For example, clinical evidence indicates that humans exhibiting ICDs such as gambling have an association of ventral striatal D2/D3 receptors with temporal discounting when administered dopaminergic ligands and assessed by positron emission tomography [25], [75]. Furthermore, D3 dopamine receptors are also found to be upregulated in individuals with cocaine addiction, highlighting a broad role in reward-related behaviors [76]. The risk of ICDs following repeated overstimulation of D2/D3 receptors in the mesolimbic dopamine pathway may be compounded by the pathophysiology of PD, leading to increased risk of ICDs in those with more advanced disease [77], [78]. Relative preservation of the mesolimbic pathway in comparison to the degeneration of the nigrostriatal pathway leads to an imbalance in dopamine signaling and potential impairments in decision-making [63], [79], [80]. Development of ICDs in individuals with PD is associated with increased mesolimbic dopamine release during gambling tasks or in response to rewarding stimuli [81]. Further activation of D2/D3 receptors in mesolimbic pathways associated with impulsivity may then potentiate the risk of ICDs in vulnerable patients [77].
Similar interactions between DAs and the pathophysiology of PDs have been linked to the incidence of hallucinations and other cognitive AEs [68]. In further support of the association between D2 receptors and psychosis, D2 dopamine receptor antagonism is the established approach to treating patients with schizophrenia [19], [82]. In a large, open-label, randomized trial, 12% (76/632) of patients with early-stage PD receiving D2/D3–selective DAs reported psychiatric events such as psychosis, confusion, and depression [83]. DAs have also been associated with hallucinations in studies of patients with advanced PD [84], [85], suggesting that psychiatric AEs linked to D2/D3–selective DAs may increase with PD progression, motor symptom severity, and increasing dosage and polypharmacy [27], [68]. Understanding the underlying cause of AEs associated with currently available DAs may be further complicated by off-target effects on nondopaminergic receptor systems. Some D2/D3–selective DAs also have affinity for serotonergic, adrenergic, and histaminergic receptors [17], [22], [23], [24]. These agents may have agonistic or antagonistic properties at nondopaminergic receptors, or both [22], [24]. Notably, serotonergic and noradrenergic circuitries have been implicated in regulation of behaviors commonly impacted by DAs, including somnolence, depression, and psychosis [27], [68]. Activation of serotonin receptors may also be linked to motor AEs, including dyskinesia [86], underscoring the relevance of off-target activation of other receptor classes to the AE profiles of commonly used DAs for the treatment of PD.
Due to the safety profile of D2/D3 DAs, their use may not be recommended in specific populations. Recently published guidelines from the American Academy of Neurology suggest that, due to an increased risk for AEs, D2/D3 DAs should generally not be prescribed to patients aged >70 years [78]. Elderly patients may have reduced renal or hepatic function, leading to altered drug metabolism and increasing toxicity, potentially at doses that are too low to provide sufficient clinical benefit [87], [88]. Psychiatric AEs such as hallucinations or psychosis are observed at higher frequencies in aged populations [88]. In a retrospective chart review of patients aged ≥80 years who were prescribed a D2/D3 DA for treatment of PD, only 46% remained on a D2/D3 DA at least six months after initiating treatment, largely due to the reduced tolerability of D2/D3 DAs in this population [88]. Similarly, the risk of AEs associated with D2/D3 DAs changes with PD progression, with increased incidence of nonmotor AEs (e.g., ICDs, hallucinations), even in younger patients with more advanced disease [78], [88]. D2/D3 DAs are therefore less likely to be used as a monotherapy in older people or in those with advanced PD [89]. However, D2/D3 DAs may be preferred for initial treatment of PD in those aged <60 years at diagnosis due to potential for reduced risk of troublesome dyskinesias associated with long-term treatment with levodopa [78]. This approach has been driven by observations that people with early-stage PD treated with D2/D3 DAs have reduced incidence of motor complications (e.g., wearing-OFF phenomena, dyskinesias) [89], [90]. Still, the relatively small treatment difference in dyskinesia combined with the overall superior efficacy of levodopa suggests that delaying treatment with levodopa in favor of D2/D3 DAs in early PD may not provide substantial long-term benefits [87]. Novel approaches that balance risk of motor (e.g., dyskinesias) versus nonmotor (e.g., ICDs, hallucinations) AEs across disease stages are needed.
Limited data are currently available from advanced-phase clinical studies to directly evaluate potential differences in safety and tolerability profiles between D1/D5 (D1-like) receptor–selective agonists and D2/D3 (D2-like) receptor–selective agonists in patients with PD. However, retrospective analyses and comparative open-label studies have suggested that apomorphine, which has a somewhat distinctive dopamine receptor pharmacology [24] compared with most other DAs, is associated with fewer AEs potentially related to D2/D3 receptor-selective activation; it may improve some nonmotor symptoms, such as sleep, mood disturbances, and constipation, and is associated with lower rates of ICDs than oral DAs such as pramipexole and ropinirole, which more selectively activate D2/D3 receptors than apomorphine, which also activates D4 and D1/D5 receptors [22], [91], [92]. Apomorphine is suggested to be relatively well tolerated in patients with psychosis and hallucinations, and small, open-label clinical studies have observed fewer hallucinations in patients with neuropsychiatric symptoms following apomorphine treatment [92], [93], [94]. Rotigotine, another less-selective DA with affinity for D1 receptors in addition to D2/D3/D4 receptors, is also associated with lower incidence of ICDs, further suggesting that these AEs may be specifically linked to the selective activation of D2/D3 receptors [40], [91].
The clinical profile of newer, selective D1/D5 DAs is emerging. In a study of 13 adults with PD, acute administration of PF-06412562, the selective D1/D5 DA, resulted in a tolerable safety profile without causing substantial changes in cardiovascular parameters, such as the safety events observed previously with the selective D1/D5 DA ABT-431 [45], [48]. Similarly, no notable abnormalities in laboratory or electrocardiogram parameters were observed across early-phase studies of the selective D1/D5 DA tavapadon, with nausea and headache being the most-frequent AEs [50], [95]. In a phase 2 randomized study of 47 patients with early-stage PD, no apparent differences were noted between tavapadon-treated and placebo-treated cohorts in ICDs, as assessed by the Questionnaire for Impulsive Compulsive Disorders in Parkinson’s Disease Rating Scale, or in sleepiness, as assessed by the Epworth Sleepiness Scale [50]. Tavapadon was also evaluated in a phase 2 randomized study of patients with advanced PD, although as previously mentioned, this study was terminated prior to completion [50], [96]. This study was not terminated because of safety concerns, and there were no reports of suicidality or changes from baseline in incidence of ICDs in the 24 participants who completed 15 weeks of treatment with tavapadon [96].
4. What evidence has been generated for the use of partial versus full DAs in PD?
DAs differ not only in their regional selectivity and differential affinity for specific receptors, but also in the degree to which they activate downstream receptor signaling; agonists that induce downstream activation comparable to the endogenous ligand are considered full agonists, whereas partial agonists display reduced recruitment of downstream signaling cascades [97], [98]. Partial DAs have already proven therapeutically useful for the treatment of neuropsychiatric disorders, most notably schizophrenia [98]. However, most DAs currently approved for PD, such as pramipexole and ropinirole, are full DAs with a high affinity for D2/D3 (D2-like) receptors [25]. Similar to other approved DAs, apomorphine has also been reported as a full agonist of dopamine receptors [99], [100]. Long-term treatment with full agonists for D2/D3 receptors may lead to upregulation and hyperactivation of D2/D3 receptors, which may be a relevant mechanism for troublesome nonmotor side effects such as ICDs [25]. Similar to approved DAs, the full agonist profile of previously investigated selective D1/D5 (D1-like) DAs may have driven the safety profiles observed with those agents in clinical studies, including side effects. When full D1/D5 receptor-selective agonists such as ABT-431 and dihydrexidine were explored in small, early-phase clinical studies, they demonstrated robust ability to alleviate motor symptoms [45], [46]. Nevertheless, cardiovascular AEs such as cardiac ischemia and hypotension, and dyskinesias comparable to those associated with levodopa, made them ill-suited for clinical advancement [45], [46], [95]. Because of these limitations, the overall promise of selective D1/D5 receptor activation in PD has not yet been well investigated.
As summarized above, some evidence suggests that selective D1/D5 agonism may provide motor control while ameliorating some of the AEs associated with D2/D3 agonism. Selective D1/D5 partial agonists have also been explored to avoid the side effects associated with selective D1/D5 full agonists. Preclinical evidence from a study in a nonhuman primate model of PD suggests that maximal efficacy of tavapadon, a selective D1/D5 DA with partial agonism properties, can be reached at a brain exposure corresponding to a calculated D1 receptor occupancy of just under 50% [49]. The maximal dose of tavapadon in this preclinical study was associated with mild dyskinesia, whereas the dose of levodopa with a magnitude of motor benefit similar to tavapadon led to severe dyskinesia. The authors suggested that when treating patients for whom repeated levodopa treatment had already led to dyskinesias, selective partial D1/D5 receptor activation with tavapadon may lead to modest dyskinesia that may not worsen with dose escalation [49]. Although published clinical studies of tavapadon have all been of 15 weeks duration or shorter, it is encouraging that AEs such as ICDs and EDS, which are associated with D2/D3 receptor–selective full agonists, have not been observed with tavapadon [50], [95].
5. Summary
Collectively, the emerging research described in this review suggests that selective D1/D5 (D1-like) agonism may potentially enable robust motor control comparable to that observed with nonselective DAs (eg, levodopa). Additionally, the potential association between specific AEs that are mechanistically and clinically associated with current D2/D3 (D2-like) receptor–preferring DAs suggests that more selective agonism of D1/D5 receptors could be explored with the aim of reducing the incidence of these AEs. Finally, partial dopamine agonism may potentially ameliorate long-term motor and nonmotor complications.
On the basis of available evidence on early initiation of dopaminergic treatment of motor symptoms, a recent American Academy of Neurology evidence-based review concluded that initial treatment with levodopa had greater motor benefit than currently available D2/D3–selective DAs and monoamine oxidase B inhibitors, and was associated with fewer dopaminergic AEs (e.g., EDS, nausea, edema, ICDs, and hallucinations). The review also concluded that initial treatment of PD with levodopa has a higher likelihood of inducing dyskinesia than treatment with currently used DAs for up to 5 years of follow-up [78]. Initial treatment with levodopa was recommended as the preferential dopaminergic therapy for early-stage PD, except in patients younger than 60 years who are at high risk for dyskinesia [78].
Notably, levodopa has superior efficacy to approved D2/D3 receptor–selective DAs (eg, oral bromocriptine, ropinirole, pramipexole) [83]. Although the reason levodopa is more efficacious than these currently used DAs is not firmly established, one potential explanation is that as a dopamine precursor [10], levodopa administration ultimately engages both direct and indirect motor pathways, whereas selective targeting of predominantly D2/D3 dopamine receptor subtypes results in less-robust motor activation. The American Academy of Neurology guidelines recommend against the use of currently approved D2-like DAs in early-stage PD for patients with a high risk of medication-related AEs, including patients with a history of ICDs and EDS [78]. It is also recommended that clinicians inform patients and caregivers of side effects that are linked to existing D2-like selective agonists, including ICDs, EDS, sudden-onset sleep, postural hypotension, and hallucinations [78]. These recommendations are based on evidence regarding the association of current-generation DAs with a greater risk of side effects such as EDS and ICDs, and on data suggesting that DAs may exacerbate postural hypotension [17], [78].
The term dopamine agonist has been broadly used to refer to all available DAs, which may reflect the historical viewpoint that all DAs are the same or comparable, regardless of their dopamine receptor subtype selectivity. These perceptions may be partially attributed to similarities across approved DAs in dopamine receptor pharmacology, which ultimately drives efficacy and safety profiles. Although additional supporting evidence from ongoing clinical trials is needed, the research described in this review suggests that selective partial agonism of D1/D5 receptors may be an important new tool to provide motor control while minimizing motor complications associated with levodopa, and may reduce the occurrence of some mechanistically related nonmotor AEs (e.g., psychosis, ICDs, EDS) associated with currently approved D2/D3 receptor–selective DAs. If ongoing clinical trials support these initial observations, the general therapeutic classification of dopamine agonist may need revision to reflect the emerging view that not all DAs are functionally equivalent. As new DAs with clinically differentiated dopamine receptor pharmacology become available, more specific dopamine agonist therapeutic classifications (e.g., D1-selective partial DA) that better reflect the clinical efficacy and tolerability of distinct receptor pharmacology will be needed.
In conclusion, there remains a major unmet need to identify novel medications that provide robust (levodopa-like) efficacy without increasing risk of dyskinesia and motor fluctuations, and without the neuropsychiatric adverse effects attributable to D2/D3 receptor activation. Research described in this review suggests that D1/D5 receptor–selective partial DAs may help address this unmet need. Additional data from ongoing, placebo-controlled, randomized clinical trials are needed to further substantiate this approach.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Sridhar Duvvuri reports financial support was provided by Cerevel Therapeutics Inc. Sridhar Duvvuri reports a relationship with Cerevel Therapeutics Inc that includes: employment. Stuart Isaacson has received honoraria for CME for, was a consultant for, received research grants from, and/or served as a promotional speaker on behalf of AbbVie, Acadia Pharmaceuticals Inc., Acorda Therapeutics, Inc., Adamas Pharmaceuticals, Inc., Addex Therapeutics, AFFiRiS AG, Alexza Pharmaceuticals, Allergan, Amarantus BioScience, Amneal Pharmaceuticals LLC, Aptinyx Inc., Bial, Benevolent, Biogen, Biovie, Britannia Pharmaceuticals Ltd, Cala Health, Cerecor Inc., Cerevel Therapeutics, Eli Lilly, Enterin Inc., GE Healthcare, Global Kinetics Pty Ltd, Impax Laboratories, Ipsen, Jazz Pharmaceuticals, Kyowa Kirin, Lundbeck, Merz Pharmaceuticals, Michael J. Fox Foundation, Mitsubishi Tanabe, Neuralys Inc, Neurocrine Biosciences, Inc., Neuroderm, Novartis, Parkinson Study Group, Pharma Two B Ltd., Praxis, Revance, Roche, Sage, Sanofi, Scion, Scion Neurostim, Stoparkinson, Sunovion Pharmaceuticals Inc., Sun Pharma, Supernus Pharmaceuticals, Inc., Theravance Biopharma, Transposon, and UCB. Robert Hauser has received consulting fees from AbbVie, Acadia, Acorda, Adamas, Alterity, Amneal, Aptinyx, Britannia, Cerevance, Curium Pharma, Enterin, Inhibikase, Jazz, KeiferRx, Kyowa Kirin, Lundbeck A/S, Merck, Merz, Neurocrine Biosciences, Novus, Pharma Two B, Pharmather, Revance Therapeutics, Roche, Sage Therapeutics, Scion NeuroStim, Sio Gene Therapies, Sunovion, Supernus, Tolmar, US WorldMeds, and Vivifi Biotech, and has received speaker fees from AbbVie, Acorda, Adamas, Amneal, Kyowa Kirin, Neurocrine Biosciences, and Sunovion. He holds stock in Axial Biotherapeutics and Inhibikase. His institution, University of South Florida, received research fees from AbbVie, Axovant Sciences, Biogen, Bukwang Pharmaceuticals, Cavion, Centogene, Cerevance, Cerevel Therapeutics, Cynapsus Therapeutics, Enterin, F. Hoffmann-La Roche, Genentech, Global Kinetics Corporation, Impax Specialty Pharma, Intec Pharma, Integrative Research Laboratories Sweden AB, Jazz Pharmaceuticals, MJFF, Neuraly, NeuroDerm, Neurocrine Biosciences, Northwestern University, Pfizer, Pharma Two B, Revance Therapeutics, Sanofi US Services, Sun Pharma Advanced Research Company, Sunovion Pharmaceuticals, and UCB Biopharma SPRL. Rajesh Pahwah serves as a consultant for Abbott, AbbVie, ACADIA, Acorda, Amneal, Artemida, Britannia, CalaHealth, Global Kinetics, Impel, Insightec, Jazz, Neuropharma, Kyowa, Neurocrine, PhotoPharmics, Sage, Scineuro, Sunovion, Supernus and XWPharma. He receives research support from Abbott, AbbVie, Addex, Biogen, Biohaven, Boston Scientific, EIP, Global Kinetics, Impax, Intec, Lilly, Neuroderm, Neuraly, Parkinson’s Foundation, Pharma 2B, Prilenia, Roche, Sage, SIS, Sun Pharma, Sunovion, Theranexus, Theravance, and Voyager. David Gray is a former employee of Cerevel Therapeutics and may hold stock and/or stock options in the company.
Acknowledgments
Development of this manuscript was supported by Cerevel Therapeutics. Medical writing and editorial assistance were provided under the direction of the authors by Katie Yoest, PhD, and Emilia Raszkiewicz, ELS, of MedThink SciCom, with funding from Cerevel Therapeutics.
References
- 1.Cacabelos R. Parkinson's disease: from pathogenesis to pharmacogenomics. Int. J. Mol. Sci. 2017;18(3):551. doi: 10.3390/ijms18030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.G.B.D. Neurology Collaborators, 2016, Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016, Lancet Neurol. 17 (11) (2018) 939–953. [DOI] [PMC free article] [PubMed]
- 3.Dorsey E.R., Sherer T., Okun M.S., Bloem B.R. The emerging evidence of the Parkinson pandemic. J. Parkinsons Dis. 2018;8(S1):S3–S8. doi: 10.3233/jpd-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Lewin Group, Inc. Economic Burden and Future Impact of Parkinson's Disease. Final Report. Updated 5 July 2019. Accessed 6 February 2023. https://www.michaeljfox.org/sites/default/files/media/document/2019%20Parkinson%27s%20Economic%20Burden%20Study%20-%20FINAL.pdf.
- 5.Rossi A., Berger K., Chen H., Leslie D., Mailman R.B., Huang X. Projection of the prevalence of Parkinson's disease in the coming decades: Revisited. Mov. Disord. 2018;33(1):156–159. doi: 10.1002/mds.27063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeWitt P.A., Chaudhuri K.R. Unmet needs in Parkinson disease: motor and non-motor. Parkinsonism Relat. Disord. 2020;80(Suppl 1):S7–S12. doi: 10.1016/j.parkreldis.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Sveinbjornsdottir S. The clinical symptoms of Parkinson's disease. J. Neurochem. 2016;139(Suppl 1):318–324. doi: 10.1111/jnc.13691. [DOI] [PubMed] [Google Scholar]
- 8.Li S., Le W. Milestones of Parkinson's disease research: 200 years of history and beyond. Neurosci. Bull. 2017;33(5):598–602. doi: 10.1007/s12264-017-0178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goedert M., Spillantini M.G., Del Tredici K., Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013;9(1):13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 10.Ellis J.M., Fell M.J. Current approaches to the treatment of Parkinson’s disease. Bioorganic Med. Chem. Lett. 2017;27(18):4247–4255. doi: 10.1016/j.bmcl.2017.07.075. [DOI] [PubMed] [Google Scholar]
- 11.Reichmann H., Bilsing A., Ehret R., Greulich W., Schulz J.B., Schwartz A., Rascol O. Ergoline and non-ergoline derivatives in the treatment of Parkinson's disease. J. Neurol. 2006;253(S4):iv36–iv38. doi: 10.1007/s00415-006-4009-z. [DOI] [PubMed] [Google Scholar]
- 12.Albin R.L., Leventhal D.K. The missing, the short, and the long: levodopa responses and dopamine actions. Ann. Neurol. 2017;82(1):4–19. doi: 10.1002/ana.24961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonuccelli U., Colzi A., Del Dotto P. Pergolide in the treatment of patients with early and advanced Parkinson's disease. Clin. Neuropharmacol. 2002;25(1):1–10. doi: 10.1097/00002826-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 14.McDonald C., Gordon G., Hand A., Walker R.W., Fisher J.M. 200 years of Parkinson's disease: what have we learnt from James Parkinson? Age Ageing. 2018;47(2):209–214. doi: 10.1093/ageing/afx196. [DOI] [PubMed] [Google Scholar]
- 15.Smith Y., Wichmann T., Factor S.A., DeLong M.R. Parkinson's disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacology. 2012;37:213–246. doi: 10.1038/npp.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oertel W., Schulz J.B. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J. Neurochem. 2016;139(Suppl 1):325–337. doi: 10.1111/jnc.13750. [DOI] [PubMed] [Google Scholar]
- 17.Borovac J.A. Side effects of a dopamine agonist therapy for Parkinson's disease: a mini-review of clinical pharmacology. Yale J. Biol. Med. 2016;89:37–47. [PMC free article] [PubMed] [Google Scholar]
- 18.de Bie R.M.A., Clarke C.E., Espay A.J., Fox S.H., Lang A.E. Initiation of pharmacological therapy in Parkinson's disease: when, why, and how. Lancet Neurol. 2020;19(5):452–461. doi: 10.1016/S1474-4422(20)30036-3. [DOI] [PubMed] [Google Scholar]
- 19.J.C. Martel, S. Gatti McArthur, Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front. Pharmacol. 11 (2020 Jul 14) 1003. eCollection 2020. 10.3389/fphar.2020.01003. [DOI] [PMC free article] [PubMed]
- 20.A. Ledonne, N.B. Mercuri, Current concepts on the physiopathological relevance of dopaminergic receptors, Front. Cell. Neurosci. 11 (2017 Feb 8) 27. eCollection 2017. 10.3389/fncel.2017.00027. [DOI] [PMC free article] [PubMed]
- 21.Apokyn. Product Information. Last revised March 2017. Accessed 21 February 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021264s014lbl.pdf.
- 22.Carbone F., Djamshidian A., Seppi K., Poewe W. Apomorphine for Parkinson's disease: efficacy and safety of current and new formulations. CNS Drugs. 2019;33:905–918. doi: 10.1007/s40263-019-00661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenner P., Katzenschlager R. Apomorphine – pharmacological properties and clinical trials in Parkinson's disease. Parkinsonism Relat. Disord. 2016;33(Suppl 1):S13–S21. doi: 10.1016/j.parkreldis.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Millan M.J., Maiofiss L., Cussac D., Audinot V., Boutin J.-A., Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J. Pharmacol. Exp. Ther. 2002;303(2):791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- 25.Napier T.C., Persons A.L. Pharmacological insights into impulsive-compulsive spectrum disorders associated with dopaminergic therapy. Eur. J. Neurosci. 2019;50(3):2492–2502. doi: 10.1111/ejn.14177. [DOI] [PubMed] [Google Scholar]
- 26.Soares-Cunha C., Coimbra B., Sousa N., Rodrigues A.J. Reappraising striatal D1- and D2-neurons in reward and aversion. Neurosci. Biobehav. Rev. 2016;68:370–386. doi: 10.1016/j.neubiorev.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Powell A., Ireland C., Lewis S.J.G. Visual hallucinations and the role of medications in Parkinson's disease: triggers, pathophysiology, and management. J. Neuropsychiatry Clin. Neurosci. 2020;32(4):334–343. doi: 10.1176/appi.neuropsych.19110316. [DOI] [PubMed] [Google Scholar]
- 28.Watts V.J., Lawler C.P., Gilmore J.H., Southerland S.B., Nichols D.E., Mailman R.B. Dopamine D1 receptors: efficacy of full (dihydrexidine) vs. partial (SKF38393) agonists in primates vs. rodents. Eur. J. Pharmacol. 1993;242:165–172. doi: 10.1016/0014-2999(93)90076-t. [DOI] [PubMed] [Google Scholar]
- 29.Calabresi P., Picconi B., Tozzi A., Ghiglieri V., Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat. Neurosci. 2014;17(8):1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 30.Dunovan K., Lynch B., Molesworth T., Verstynen T. Competing basal ganglia pathways determine the difference between stopping and deciding not to go. eLife. 2015;4:e08723. doi: 10.7554/eLife.08723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redgrave P., Rodriguez M., Smith Y., Rodriguez-Oroz M.C., Lehericy S., Bergman H., Agid Y., DeLong M.R., Obeso J.A. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat. Rev. Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keeler J.F., Pretsell D.O., Robbins T.W. Functional implications of dopamine D1 vs. D2 receptors: A 'prepare and select' model of the striatal direct vs. indirect pathways. Neuroscience. 2014;282:156–175. doi: 10.1016/j.neuroscience.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Xie K., Martemyanov K.A. Control of striatal signaling by g protein regulators. Front. Neuroanat. 2011;5 doi: 10.3389/fnana.2011.00049. 49, eCollection 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castello J., Cortes M., Malave L., Kottmann A., Sibley D.R., Friedman E., Rebholz H. The Dopamine D5 receptor contributes to activation of cholinergic interneurons during L-DOPA induced dyskinesia. Sci. Rep. 2020;10:2542. doi: 10.1038/s41598-020-59011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cycloset. Product Information. Last revised February 2017. Accessed 13 May 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020866s006s007lbl.pdf.
- 36.Zhuang Y., Xu P., Mao C., Wang L., Krumm B., Zhou X.E., Huang S., Liu H., Cheng X.i., Huang X.-P., Shen D.-D., Xu T., Liu Y.-F., Wang Y., Guo J., Jiang Y.i., Jiang H., Melcher K., Roth B.L., Zhang Y., Zhang C., Xu H.E. Structural insights into the human D1 and D2 dopamine receptor signaling complexes. Cell. 2021;184(4):931–942.e18. doi: 10.1016/j.cell.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perachon S., Schwartz J.-C., Sokoloff P. Functional potencies of new antiparkinsonian drugs at recombinant human dopamine D1, D2 and D3 receptors. Eur. J. Pharmacol. 1999;366(2-3):293–300. doi: 10.1016/s0014-2999(98)00896-6. [DOI] [PubMed] [Google Scholar]
- 38.Ralph-Williams R.J., Lehmann-Masten V., Geyer M.A. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28(1):108–118. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- 39.Permax. Product Information. Accessed 13 May 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/19385slr030,031,035_permax_lbl.pdf.
- 40.Scheller D., Ullmer C., Berkels R., Gwarek M., Lubbert H. The in vitro receptor profile of rotigotine: a new agent for the treatment of Parkinson's disease. Naunyn-Schmiedeb. Arch. 2009;379:73–86. doi: 10.1007/s00210-008-0341-4. [DOI] [PubMed] [Google Scholar]
- 41.Asin K.E., Domino E.F., Nikkel A., Shiosaki K. The selective dopamine D1 receptor agonist A-86929 maintains efficacy with repeated treatment in rodent and primate models of Parkinson's disease. J. Pharmacol. Exp. Ther. 1997;281:454–459. [PubMed] [Google Scholar]
- 42.Shiosaki K., Jenner P., Asin K.E., Britton D.R., Lin C.W., Michaelides M., Smith L., Bianchi B., Didomenico S., Hodges L., Hong Y., Mahan L., Mikusa J., Miller T., Nikkel A., Stashko M., Witte D., Williams M. ABT-431: the diacetyl prodrug of A-86929, a potent and selective dopamine D1 receptor agonist: in vitro characterization and effects in animal models of Parkinson's disease. J. Pharmacol. Exp. Ther. 1996;276:150–160. [PubMed] [Google Scholar]
- 43.Giardina W.J., Williams M. Adrogolide HCl (ABT-431; DAS-431), a prodrug of the dopamine D1 receptor agonist, A-86929: preclinical pharmacology and clinical data. CNS Drug Rev. 2001;7:305–316. doi: 10.1111/j.1527-3458.2001.tb00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rascol O., Blin O., Thalamas C., Descombes S., Soubrouillard C., Azulay P., Fabre N., Viallet F., Lafnitzegger K., Wright S., Carter J.H., Nutt J.G. ABT-431, a D1 receptor agonist prodrug, has efficacy in Parkinson's disease. Ann. Neurol. 1999;45(6):736–741. doi: 10.1002/1531-8249(199906)45:6<736::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 45.Rascol O., Nutt J.G., Blin O., Goetz C.G., Trugman J.M., Soubrouillard C., Carter J.H., Currie L.J., Fabre N., Thalamas C., Giardina W.W., Wright S. Induction by dopamine D1 receptor agonist ABT-431 of dyskinesia similar to levodopa in patients with Parkinson disease. Arch. Neurol. 2001;58:249–254. doi: 10.1001/archneur.58.2.249. [DOI] [PubMed] [Google Scholar]
- 46.Blanchet P.J., Fang J., Gillespie M., Sabounjian L., Locke K.W., Gammans R., Mouradian M.M., Chase T.N. Effects of the full dopamine D1 receptor agonist dihydrexidine in Parkinson's disease. Clin. Neuropharmacol. 1998;21:339–343. [PubMed] [Google Scholar]
- 47.Gorelova N.A., Yang C.R. Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. J. Neurophysiol. 2000;84(1):75–87. doi: 10.1152/jn.2000.84.1.75. [DOI] [PubMed] [Google Scholar]
- 48.Papapetropoulos S., Liu W., Duvvuri S., Thayer K., Gray D.L. Evaluation of D1/D5 partial agonist PF-06412562 in Parkinson's disease following oral administration. Neurodegener. Dis. 2018;18:262–269. doi: 10.1159/000492498. [DOI] [PubMed] [Google Scholar]
- 49.Young D., Popiolek M., Trapa P., Fonseca K.R., Brevard J., Gray D.L., Kozak R. D1 agonist improved movement of parkinsonian nonhuman primates with limited dyskinesia side effects. ACS Chem. Neurosci. 2020;11(4):560–566. doi: 10.1021/acschemneuro.9b00589. [DOI] [PubMed] [Google Scholar]
- 50.Riesenberg R., Werth J., Zhang Y., Duvvuri S., Gray D. PF-06649751 efficacy and safety in early Parkinson’s disease: a randomized, placebo-controlled trial. Ther. Adv. Neurol. Disord. 2020;13 doi: 10.1177/1756286420911296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Institutes of Health, US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT02687542. Accessed 1 December 2022.
- 52.J. Choi, K.A. Horner. Dopamine Agonists. [Updated 2022 Jun 27]. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, Florida. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551686/.
- 53.Athanasoulia-Kaspar A.P., Popp K.H., Stalla G.K. Neuropsychiatric and metabolic aspects of dopaminergic therapy: perspectives from an endocrinologist and a psychiatrist. Endocr. Connect. 2018;7:R88–R94. doi: 10.1530/EC-18-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sung Choi W., Machida C.A., Ronnekleiv O.K. Distribution of dopamine D1, D2, and D5 receptor mRNAs in the monkey brain: ribonuclease protection assay analysis. Brain Res Mol. Brain Res. 1995;31(1–2):86–94. doi: 10.1016/0169-328x(95)00038-t. [DOI] [PubMed] [Google Scholar]
- 55.Richtand N.M., Kelsoe J.R., Segal D.S., Kuczenski R. Regional quantification of D1, D2, and D3 dopamine receptor mRNA in rat brain using a ribonuclease protection assay. Brain Res. Mol. Brain Res. 1995;33(1):97–103. doi: 10.1016/0169-328x(95)00112-6. [DOI] [PubMed] [Google Scholar]
- 56.Hurd Y.L., Suzuki M., Sedvall G.C. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J. Chem. Neuroanat. 2001;22(1-2):127–137. doi: 10.1016/s0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki M., Hurd Y.L., Sokoloff P., Schwartz J.-C., Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res. 1998;779(1-2):58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- 58.Fremeau R.T., Duncan G.E., Fornaretto M.G., Dearry A., Gingrich J.A., Breese G.R., Caron M.G. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc. Natl. Acad. Sci. U.S.A. 1991;88(9):3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiner D.M., Levey A.I., Sunahara R.K., Niznik H.B., O'Dowd B.F., Seeman P., Brann M.R. D1 and D2 dopamine receptor mRNA in rat brain. Proc. Natl. Acad. Sci. U.S.A. 1991;88(5):1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciliax B.J., Nash N., Heilman C., Sunahara R., Hartney A., Tiberi M., Rye D.B., Caron M.G., Niznik H.B., Levey A.I. Dopamine D(5) receptor immunolocalization in rat and monkey brain. Synapse. 2000;37(2):125–145. doi: 10.1002/1098-2396(200008)37:2<125::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 61.Defagot M.C., Malchiodi E.L., Villar M.J., Antonelli M.C. Distribution of D4 dopamine receptor in rat brain with sequence-specific antibodies. Brain Res. Mol. Brain Res. 1997;45(1):1–12. doi: 10.1016/s0169-328x(96)00235-5. [DOI] [PubMed] [Google Scholar]
- 62.Mishra A., Singh S., Shukla S. Physiological and functional basis of dopamine receptors and their role in neurogenesis: possible implication for Parkinson’s disease. J. Exp. Neurosci. 2018;12 doi: 10.1177/1179069518779829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.A. Martini, D. Dal Lago, N.M.J. Edelstyn, J.A. Grange, S. Tamburin, Impulse control disorder in Parkinson's disease: a meta-analysis of cognitive, affective, and motivational correlates, Front. Neurol. 9 (2018 Aug 28) 654. eCollection 2018. 10.3389/fneur.2018.00654. [DOI] [PMC free article] [PubMed]
- 64.Kujawa K., Leurgans S., Raman R., Blasucci L., Goetz C.G. Acute orthostatic hypotension when starting dopamine agonists in Parkinson's disease. Arch. Neurol. 2000;57:1461–1463. doi: 10.1001/archneur.57.10.1461. [DOI] [PubMed] [Google Scholar]
- 65.K.R. Gandhi, A. Saadabadi. Levodopa (L-Dopa) [Updated 2022 May 2]. In: StatPearls [Internet]. Stat Pearls Publishing, Treasure Island, Florida. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482140/.
- 66.Forsaa E.B., Larsen J.P., Wentzel-Larsen T., Goetz C.G., Stebbins G.T., Aarsland D., Alves G. A 12-year population-based study of psychosis in Parkinson disease. Arch. Neurol. 2010;67:996–1001. doi: 10.1001/archneurol.2010.166. [DOI] [PubMed] [Google Scholar]
- 67.P.J. Garcia-Ruiz. Impulse control disorders and dopamine-related creativity: pathogenesis and mechanism, short review, and hypothesis, Front. Neurol. 9 (2018 Dec 6) 1041. eCollection 2018. 10.3389/fneur.2018.01041. [DOI] [PMC free article] [PubMed]
- 68.Voon V., Fernagut P.O., Wickens J., Baunez C., Rodriguez M., Pavon N., Juncos J.L., Obeso J.A., Bezard E. Chronic dopaminergic stimulation in Parkinson's disease: from dyskinesias to impulse control disorder. Lancet Neurol. 2009;8:1140–1149. doi: 10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- 69.Dzirasa K., Ribeiro S., Costa R., Santos L.M., Lin S.-C., Grosmark A., Sotnikova T.D., Gainetdinov R.R., Caron M.G., Nicolelis M.A.L. Dopaminergic control of sleep-wake states. J. Neurosci. 2006;26(41):10577–10589. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biglan K.M., Holloway R.G., McDermott M.P., Richard I.H. C-PDI Parkinson Study Group, Risk factors for somnolence, edema, and hallucinations in early Parkinson disease. Neurology. 2007;69(2):187–195. doi: 10.1212/01.wnl.0000265593.34438.00. [DOI] [PubMed] [Google Scholar]
- 71.Stoker T.B., Barker R.A. Recent developments in the treatment of Parkinson's Disease. F1000Res. 2020;9:862. doi: 10.12688/f1000research.25634.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.I. Zahoor, A. Shafi, E. Haq. Pharmacological treatment of Parkinson's disease, in: T.B. Stoker, J.C. Greenland (Eds.), Parkinson's Disease: Pathogenesis and Clinical Aspects, Codon Publishing, Brisbane, Australia; 2018.
- 73.Moreno M., Economidou D., Mar A.C., López-Granero C., Caprioli D., Theobald D.E., Fernando A., Newman A.H., Robbins T.W., Dalley J.W. Divergent effects of D(2)/(3) receptor activation in the nucleus accumbens core and shell on impulsivity and locomotor activity in high and low impulsive rats. Psychopharmacology (Berl). 2013;228(1):19–30. doi: 10.1007/s00213-013-3010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heidbreder C.A., Gardner E.L., Xi Z.-X., Thanos P.K., Mugnaini M., Hagan J.J., Ashby C.R. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res. Rev. 2005;49(1):77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joutsa J., Voon V., Johansson J., Niemelä S., Bergman J., Kaasinen V. Dopaminergic function and intertemporal choice. Transl. Psychiatry. 2015;5(1):e491. doi: 10.1038/tp.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Payer D.E., Behzadi A., Kish S.J., Houle S., Wilson A.A., Rusjan P.M., Tong J., Selby P., George T.P., McCluskey T., Boileau I. Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [11C]-+-PHNO. Neuropsychopharmacology. 2014;39:311–318. doi: 10.1038/npp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.R. De Micco, A. Russo, G. Tedeschi, A. Tessitore, Impulse control behaviors in Parkinson's disease: drugs or disease? contribution from imaging studies, Front. Neurol. 9 (2018 Oct 25) 893. eCollection 2018. 10.3389/fneur.2018.00893. [DOI] [PMC free article] [PubMed]
- 78.Pringsheim T., Day G.S., Smith D.B., et al. Dopaminergic therapy for motor symptoms in early Parkinson Disease Practice Guideline Summary: a report of the AAN guideline subcommittee. Neurology. 2021;97:942–957. doi: 10.1212/WNL.0000000000012868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.R.S. Eisinger, A. Ramirez-Zamora, S. Carbunaru, B. Ptak, Z. Peng-Chen, M.S. Okun, A. Gunduz, Medications, deep brain stimulation, and other factors influencing impulse control disorders in Parkinson's disease, Front. Neurol. 10 (2019) 86. eCollection 2019. 10.3389/fneur.2019.00086. [DOI] [PMC free article] [PubMed]
- 80.E.M. Gatto, V. Aldinio, Impulse control disorders in Parkinson's Disease. a brief and comprehensive review, Front. Neurol. 10 (2019) 351. eCollection 2019. 10.3389/fneur.2019.00351. [DOI] [PMC free article] [PubMed]
- 81.A. Martini, D. Dal Lago, N.M.J. Edelstyn, M. Salgarello, F. Lugoboni, S. Tamburin, Dopaminergic neurotransmission in patients with Parkinson's disease and impulse control disorders: a systematic review and meta-analysis of PET and SPECT studies, Front. Neurol. 9 (2018) 1018. eCollection 2018. 10.3389/fneur.2018.01018. [DOI] [PMC free article] [PubMed]
- 82.Seeman P. Dopamine D2 receptors as treatment targets in schizophrenia. Clin. Schizophr. Relat. Psychoses. 2010;4(1):56–73. [PubMed] [Google Scholar]
- 83.PDMC Group, Gray R., Ives N., Rick C., Patel S., Gray A., Jenkinson C., McIntosh E., Wheatley K., Williams A., Clarke C.E. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson's disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 2014;384:1196–1205. doi: 10.1016/S0140-6736(14)60683-8. [DOI] [PubMed] [Google Scholar]
- 84.Hauser R.A., Schapira A.H., Barone P., Mizuno Y., Rascol O., Busse M., Debieuvre C., Fraessdorf M., Poewe W., Pramipexole E.R. Studies Group, Long-term safety and sustained efficacy of extended-release pramipexole in early and advanced Parkinson's disease. Eur. J. Neurol. 2014;21:736–743. doi: 10.1111/ene.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.LeWitt P.A., Boroojerdi B., Surmann E., Poewe W., Group S.P.S. Rotigotine transdermal system for long-term treatment of patients with advanced Parkinson's disease: results of two open-label extension studies. CLEOPATRA-PD and PREFER. J. Neural. Transm. (Vienna) 2013;120:1069–1081. doi: 10.1007/s00702-012-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bastide M.F., Meissner W.G., Picconi B., Fasano S., Fernagut P.-O., Feyder M., Francardo V., Alcacer C., Ding Y., Brambilla R., Fisone G., Stoessl A.J., Bourdenx M., Engel M., Navailles S., De Deurwardère P., Ko W.K.D., Simola N., Morelli M., Groc L., Rodriguez M.-C., Gurevich E.V., Quik M., Morari M., Mellone M., Gardoni F., Tronci E., Guehl D., Tison F., Crossman A.R., Jung Kang U., Steece-Collier K., Fox S., Carta M., Angela Cenci M., Bézard E. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson's disease. Prog. Neurobiol. 2015;132:96–168. doi: 10.1016/j.pneurobio.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Latt M.D., Lewis S., Zekry O., Fung V.S.C. Factors to consider in the selection of dopamine agonists for older persons with Parkinson's Disease. Drugs Aging. 2019;36:189–202. doi: 10.1007/s40266-018-0629-0. [DOI] [PubMed] [Google Scholar]
- 88.Shulman L.M., Minagar A., Rabinstein A., Weiner W.J. The use of dopamine agonists in very elderly patients with Parkinson's disease. Mov. Disord. 2000;15(4):664–668. doi: 10.1002/1531-8257(200007)15:4<664::aid-mds1010>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 89.Jankovic J. Parkinson's disease therapy: tailoring choices for early and late disease, young and old patients. Clin. Neuropharmacol. 2000;23(5):252–261. doi: 10.1097/00002826-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 90.Barbosa E.R., Limongi J.C.P., Chien H.F., Barbosa P.M., Torres M.R.C. How I treat Parkinson's disease. Arq. Neuropsiquiatr. 2022;80(5 Suppl 1):94–104. doi: 10.1590/0004-282X-ANP-2022-S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moore T.J., Glenmullen J., Mattison D.R. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern. Med. 2014;174:1930–1933. doi: 10.1001/jamainternmed.2014.5262. [DOI] [PubMed] [Google Scholar]
- 92.Rosa-Grilo M., Qamar M.A., Evans A., Chaudhuri K.R. The efficacy of apomorphine - A non-motor perspective. Parkinsonism Relat. Disord. 2016;33(Suppl 1):S28–S35. doi: 10.1016/j.parkreldis.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 93.Ellis C., Lemmens G., Parkes J.D., Abbott R.J., Pye I.F., Leigh P.N., Chaudhuri K.R. Use of apomorphine in parkinsonian patients with neuropsychiatric complications to oral treatment. Parkinsonism Relat. Disord. 1997;3(2):103–107. doi: 10.1016/s1353-8020(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 94.van Laar T., Postma A.G., Drent M. Continuous subcutaneous infusion of apomorphine can be used safely in patients with Parkinson's disease and pre-existing visual hallucinations. Parkinsonism Relat. Disord. 2010;16(1):71–72. doi: 10.1016/j.parkreldis.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Sohur U.S., Gray D.L., Duvvuri S., Zhang Y., Thayer K., Feng G. Phase 1 Parkinson’s disease studies show the dopamine D1/D5 agonist PF-06649751 is safe and well tolerated. Neurol Ther. 2018;7(2):307–319. doi: 10.1007/s40120-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.National Institutes of Health, US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT02687542. Accessed 6 February 2023.
- 97.Gray D.L., Allen J.A., Mente S., O’Connor R.E., DeMarco G.J., Efremov I., Tierney P., Volfson D., Davoren J., Guilmette E., Salafia M., Kozak R., Ehlers M.D. Impaired beta-arrestin recruitment and reduced desensitization by non-catechol agonists of the D1 dopamine receptor. Nat. Commun. 2018;9:674. doi: 10.1038/s41467-017-02776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mailman R.B., Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr. Pharm. Des. 2010;16:488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Piercey M.F., Hoffmann W.E., Vogelsang G.D., Travis M. Electrophysiological evaluation of a partial agonist of dopamine receptors. J. Pharmacol. Exp. Ther. 1987;243:391–396. [PubMed] [Google Scholar]
- 100.T. Zhao, H. Zondervan-van der Linde, L.A. Severijnen, B.A. Oostra, R. Willemsen, V. Bonifati, Dopaminergic neuronal loss and dopamine-dependent locomotor defects in Fbxo7-deficient zebrafish, PLoS One. 7 (2012) e48911. Epub 2012 Nov 2. 10.1371/journal.pone.0048911. [DOI] [PMC free article] [PubMed]
- 101.Mirapex. Product Information. Last revised May 2018. Accessed 11 May 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020667s036lbl.pdf.
- 102.European Public Assessment Report Summary for the Public: Pramipexole Teva. European Medicines Agency. Accessed 3 November 2022. https://www.ema.europa.eu/en/documents/overview/pramipexole-teva-epar-summary-public_en.pdf.
- 103.Requip. Approval Package. Accessed 25 July 2022. Center for Drug Evaluation and Research. https://www.accessdata.fda.gov/drugsatfda_docs/nda/97/020658ap_Requip_apltr.pdf.
- 104.List of nationally authorised medicinal products. Active substance: ropinirole. European Medicines Agency. Accessed 3 November 2022. https://www.ema.europa.eu/en/documents/psusa/ropinirole-list-nationally-authorised-medicinal-products-psusa/00002661201907_en.pdf.
- 105.Neupro. Product Information. Last revised April 2012. Accessed 11 May 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021829s001lbl.pdf.
- 106.European Public Assessment Report Summary for the Public: Neupro (rotigotine). European Medicines Agency. Accessed 3 November 2022. https://www.ema.europa.eu/en/documents/overview/neupro-epar-summary-public_en.pdf.
- 107.List of nationally authorised medicinal products. Active substance: piribedil. European Medicines Agency. Accessed 25 July 2022. https://www.ema.europa.eu/en/documents/psusa/piribedil-list-nationally-authorised-medicinal-products-psusa/00002436/202003_en.pdf.
- 108.National Institutes of Health, US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04201093. Accessed 20 May 2022.
- 109.National Institutes of Health, US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04223193. Accessed 20 May 2022.
- 110.National Institutes of Health, US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04542499. Accessed 20 May 2022.
- 111.National Institutes of Health, US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03845387. Accessed 26 July 2022.
- 112.Mao Q.i., Qin W.-Z., Zhang A., Ye N. Recent advances in dopaminergic strategies for the treatment of Parkinson’s disease. Acta Pharmacol. Sin. 2020;41(4):471–482. doi: 10.1038/s41401-020-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.National Institutes of Health, US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04291859. Accessed 26 July 2022.
- 114.K. McFarthing, G. Rafaloff, M.A.S. Baptista, R.K. Wyse, S.R.W. Stott, Parkinson's disease drug therapies in the clinical trial pipeline: 2021 update, J. Parkinsons. Dis. 11 (2021) 891–903. [DOI] [PMC free article] [PubMed]
- 115.National Institutes of Health, US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT02006290. Accessed July 26, 2022.
- 116.Bergson C., Mrzljak L., Smiley J.F., Pappy M., Levenson R., Goldman-Rakic P.S. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J. Neurosci. 1995;15(12):7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]