Abstract
The presence of a systemic right ventricle (sRV) with biventricular physiology (biV) is associated with increased patient morbidity and mortality. To date, no pharmacologic therapy for heart failure has been proven effective for patients with systolic dysfunction of the sRV-biV. We designed a randomized, double-blind, placebo-controlled crossover trial to compare sacubitril/valsartan treatment to placebo in adults (aged ≥ 18 years) with moderate-to-severe sRV-biV dysfunction and New York Heart Association functional class II to III symptoms. Two primary efficacy endpoints are assessed in the trial: exercise capacity (submaximal exercise duration) and neurohormonal activation (N-terminal prohormone brain natriuretic peptide). Secondary objectives include assessing a change in the Kansas City Cardiomyopathy Questionnaire score and evaluating the safety and tolerance of sacubitril/valsartan. A 6-week open run-in phase identifies the maximum tolerated dose of sacubitril/valsartan, up to 97 mg/103 mg twice daily. After a 2-week washout period, patients are randomized 1:1 to sacubitril/valsartan treatment vs placebo for a 24-week phase, followed by another 2-week washout period and subsequent crossover to the alternative treatment arm for an additional 24-week phase. Data to assess primary and secondary endpoints are collected at baseline and at the end of each phase. A total of 48 patients is required to provide > 80% power to detect a 30% difference in distance walked and in N-terminal prohormone brain natriuretic peptide levels with sacubitril/valsartan treatment vs placebo, each with a 2-sided P-value of 0.025. In summary, the Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor vs Placebo in Patients With Congenital Systemic Right Ventricular Heart Failure Trial (PARACYS-RV) should determine the role of sacubitril/valsartan in treating heart failure in patients with sRV-biV and carries the potential to alter management of this patient population.
Résumé
La présence d’un ventricule droit systémique (VDs) avec physiologie biventriculaire (PbiV) est associée à une morbidité et une mortalité accrues chez les patients. À ce jour, aucune pharmacothérapie de l’insuffisance cardiaque ne s’est révélée efficace chez les patients atteints d’une dysfonction systolique du VDs-PbiV. Nous avons conçu un essai croisé, à répartition aléatoire et à double insu, contrôlé par placebo pour comparer la bithérapie sacubitril-valsartan au placebo chez les adultes (≥ 18 ans) ayant une dysfonction modérée ou sévère du VDs-PbiV et des symptômes de la classe fonctionnelle II à III de la New York Heart Association. Deux paramètres d’évaluation principaux de l’efficacité sont définis pour l’essai : tolérance à l’effort (durée d’effort sous-maximal) et activation neurohormonale (propeptide natriurétique de type B N-Terminal [NT-proBNP]). La mesure d’une variation du score au questionnaire sur la cardiomyopathie de Kansas City de même que l’évaluation de l’innocuité et de la tolérance de la bithérapie sacubitril-valsartan sont des objectifs secondaires. Une phase préparatoire de six semaines en mode ouvert permet d’établir la dose maximale tolérée de sacubitril-valsartan, jusqu’à concurrence de 97 mg/103 mg deux fois par jour. Après une période de repos thérapeutique de deux semaines, les patients sont affectés au hasard, dans un rapport 1:1, à la bithérapie sacubitril-valsartan ou au placebo pendant une phase de traitement de 24 semaines, suivie d’une autre période de repos thérapeutique de deux semaines et d’un passage subséquent à l’autre groupe de traitement pendant une phase additionnelle de 24 semaines. Les données sur les paramètres d’évaluation principaux et secondaires sont recueillies au début de l’essai et à la fin de chaque phase. Il faut un total de 48 patients afin d’obtenir une puissance supérieure à 80 % pour détecter une différence de 30 % entre la bithérapie sacubitril-valsartan et le placebo quant à la distance parcourue à la marche et aux taux de NT-proBNP, la valeur p bilatérale étant de 0,025 pour les deux valeurs. En résumé, l’essai PARACYS-RV (Prospective Comparison ofAngiotensinReceptor-Neprilysin Inhibitor vs Placebo in Patients WithCongenital SystemicRightVentricular Heart Failure) doit déterminer le rôle de la bithérapie sacubitril-valsartan dans le traitement de l’insuffisance cardiaque chez les patients ayant un VDs-PbiV et pourrait modifier la prise en charge de cette population de patients.
A systemic right ventricle (sRV) associated with biventricular physiology (biV) occurs in the context of complete transposition of the great arteries with an atrial switch operation 1,2 and congenitally corrected transposition of the great arteries (ccTGA).3 Despite the typical quiescent clinical course during childhood, many patients will develop complications in adulthood. An sRV-biV that is subjected to systemic pressures is associated with severe morbidity and a shortened lifespan. After the third decade of life, the sRV often begins to fail, as manifested by progressively impaired exercise capacity, heart failure (HF), systemic atrioventricular valve regurgitation, and pulmonary hypertension.4, 5, 6, 7, 8, 9, 10 The prevalence of HF reaches ∼60% by age 40 years.11 Moreover, a substantial number of patients develop end-stage HF resulting in premature death. To date, no pharmacologic therapy for HF has been proven effective in patients with systolic dysfunction of the sRV-biV.4,12,13
In clinical trials of patients with an sRV-biV, renin angiotensin aldosterone system (RAAS) inhibitors did not result in an increased ejection fraction or 6-minute walk test distance, nor in a reduction in N-terminal prohormone of brain natriuretic peptide (NT-pro-BNP) levels.14,15 However, decreases in the sRV end-diastolic and end-systolic volumes were observed by cardiac magnetic resonance imaging.15 Post-trial follow-up of a 3-year placebo-controlled trial of valsartan in 87 symptomatic patients with an sRV-biV continued to demonstrate a reduction in adverse events (ie, arrhythmias, worsening HF, and tricuspid valve surgery) at 8.3 years. A meta-analysis of studies assessing medical therapy for sRV-biV dysfunction did not identify a single effective treatment for HF associated with an sRV-biV, including angiotensin-converting enzyme inhibitors (ACEis) and angiotensin II receptor blockers (ARBs).16 Therefore, no specific medical therapy for HF is currently endorsed by clinical practice guidelines for the treatment of systolic dysfunction of sRV-biV.17, 18, 19
Recent case reports support the safety and promising effect of the combination of sacubitril and valsartan in patients with an sRV-biV.20, 21, 22 An open-label single-centre study comparing 18 patients with sRV-biV failure (ejection fraction < 35%), at baseline and after 6 months of sacubritril/valsartan medication, showed encouraging results. A significant decrease in NT-proBNP levels was observed, along with improvements in echocardiographic parameters of sRV-biV function (fractional area change, global longitudinal strain), 6-minute walk test distance, and quality of life assessed by the Netherlands Organization for Applied Scientific Research/Academic Hospital Leiden Adult Quality of Life Questionnaire.23 No major adverse events, including renal dysfunction, were reported.23 Similarly, a recent prospective open-label study of 50 patients (35% ccTGA) with a sRV-biV reported an improvement in New York Heart Association (NYHA) functional class, 6-minute walk test distance, and quality of life after 1 year of treatment with sacubitril/valsartan, compared to baseline.24 A reduction in NT-pro-BNP levels was observed 1 month after initiation of sacubitril/valsartan, followed by a return to the baseline value at 1 year. Echocardiographic parameters of systolic function and dilatation of the sRV-RV improved after 1 year of sacubitril/valsartan treatment, compared to baseline.
A randomized clinical trial was deemed necessary to confirm the benefits of sacubitril/valsartan treatment in patients with sRV-biV systolic dysfunction. The Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor vs Placebo in Patients With Congenital Systemic Right Ventricular Heart Failure Trial (PARACYS-RV) is a randomized, double-blind, placebo-controlled, crossover trial designed for this purpose.
Trial Design and Methods
Study population
Inclusion criteria are as follows: (i) age ≥ 18 years, with clinical follow-up at the Montreal Heart Institute Adult Congenital Heart Centre; (ii) presence of an sRV-biV; (iii) moderate to severe sRV-biV dysfunction by transthoracic echocardiography or an sRV ejection fraction < 40% by magnetic resonance imaging; (iv) NYHA functional class II or III symptoms or peak exercise capacity < 80% of predicted on a previous standard treadmill exercise stress test; (v) ability to provide informed consent to the study; (vi) access to a telephone and/or an internet connection for teleconference calls; (vii) a mailing address to receive the study drugs; and (viii) capacity to perform self-measurements of blood pressure using a blood pressure monitor provided. Exclusion criteria are listed Table 1.
Table 1.
Exclusion criteria
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ACEi, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; Qp/Qs, ratio of pulmonary blood flow to systemic blood flow; SGOT, serum glutamic-oxaloacetic transaminase; SGPT, serum glutamic pyruvic transferase.
Study design
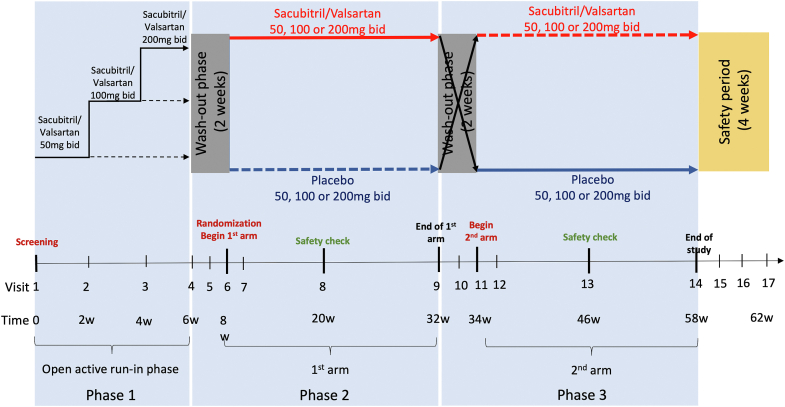
The study consists of 3 phases. An overview of the study timeline is shown in Figure 1. The first phase includes the screening visit with baseline measurements (Table 2), followed by an open active run-in phase of 6 weeks to identify the maximum tolerated dose of sacubitril/valsartan and ensure the safety of patients under the active medication. The second phase begins with a 2-week washout period. Patients are then randomized in a 1:1 ratio to the sequence of active therapy in the first 24-week arm of the trial (phase 2), followed by the corresponding placebo in the second 24-week arm (phase 3), or vice versa. Primary, secondary, and exploratory endpoints are measured at the end of each 24-week treatment period (Table 2). A 2-week washout period is incorporated prior to crossover to the alternative treatment arm in the third phase. Safety monitoring is evaluated at the halfway time point for each treatment arm. The rationale for the crossover design is addressed in the Discussion.
Figure 1.
Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor vs Placebo in Patients With Congenital Systemic Right Ventricular Heart Failure Trial (PARACYS-RV) study schema. This figure provides an overview of the study design. Phase 1 includes the screening visit and the open active run-in phase of 6 weeks. Phase 2 begins by a 2-week washout period; then, patients are randomized in the first arm of 24 weeks of active therapy or the corresponding placebo. Phase 3 is the mirror of the second phase; each patient receives the active therapy or the corresponding placebo, depending on phase 2 after a 2-week washout period. Safety monitoring is evaluated at a halfway time point for each treatment arm. A safety period of 4 weeks ends the study. bid, twice daily.
Table 2.
Endpoints collected during the Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor vs Placebo in Patients With Congenital Systemic Right Ventricular Heart Failure Trial (PARACYS-RV)
| Endpoints | Description | Visits of endpoints collection |
|---|---|---|
| Primary efficacy | ||
| Exercise capacity and neurohormonal activation |
|
At screening (V1) and at the end of each treatment arm (V9 and V14) |
| Secondary efficacy | ||
| Quality of life | Kansas City Cardiomyopathy Questionnaire | At screening (V1) and at the end of each treatment arm (V9 and V14) |
| Secondary safety | ||
| Safety of medication | Electrolytes (serum potassium level), renal function (creatinine, eGFR, urea), blood pressure Adverse clinical events occurred during each treatment arm: cough, postural symptoms, angioedema. |
At screening (V1), at the halfway time point for each treatment arm (V8 and V13) and at the end of each treatment arm (V9 and V14). From screening (V1) until end of the follow-up (run in phase, 2 arms phases, and safety period) |
| Exploratory | ||
| NYHA functional class | NYHA class | At screening (V1) and at the end of each treatment arm (V9 and V14) |
| Occurrence of clinical events | Heart failure, hospitalizations, arrhythmias (supraventricular and ventricular), mortality | From screening (V1) until end of the follow-up (run in phase, 2 arms phases, and safety period) |
| Myocardial injury biomarker | High-sensitivity Troponin T level | At screening (V1) and at the end of each treatment arm (V9 and V14) |
| sRv-biV size and function | TAPSe, S’wave, fractional area change, global longitudinal strain, end diastolic area, end systolic area by transthoracic echocardiography | At screening (V1) and at the end of each treatment arm (V9 and V14) |
| Exercise capacity complementary parameters | Anaerobic threshold, functional capacity METs, heart rate response, blood pressure response, oxygen saturation during exercise, respiratory exchange ratio VE/VCO2 slope | At screening (V1) and at the end of each treatment arm (V9 and V14) |
| Metabolic profile | Lipidomic analysis | At screening (V1) and at the end of each treatment arm (V9 and V14) |
eGFR, estimated glomerular filtration rate; MET, metabolic equivalent; NT-proBNP, N-terminal prohormone brain natriuretic peptide; NYHA, New York Heart Association; sRv-biV, systemic right ventricle with biventricular physiology; TAPSe, tricuspid annular plane systolic excursion; V, visit; VE/VCO2 slope, ventilation/carbon dioxide production slope.
Screening (visit 1)
At the screening visit, patient eligibility is assessed according to inclusion/exclusion criteria. Transthoracic echocardiography performed within 6 months is deemed acceptable to assess eligibility, barring a major intercurrent event (eg, new onset of arrhythmia, electrophysiological intervention, HF deterioration with hospitalization, cardiac surgery). A blood pressure monitor to perform home self-measurements is provided. Following successful screening, the patient is enrolled in a run-in phase. Patients already treated by sacubitril/valsartan are excluded, as are those receiving an ACEi or an ARB if replacement by another medication is considered contraindicated. Otherwise, the ACEi or ARB is discontinued at the beginning of the run-in phase. For subjects previously on an ACEi, a 48-hour washout period is factored in prior to the run-in phase. Beta-blockers or calcium blockers are introduced or increased to control blood pressure if needed after interruption of ACEi or ARB treatment. Other therapies, including aldosterone, are continued. Doses of diuretics are titrated according to clinical circumstances throughout the trial.
Active run-in phase (visits 2, 3, and 4)
The first dose of sacubitril/valsartan is based on Canadian HF management guidelines.25 Sacubitril/valsartan is then increased every 2 weeks until the maximum tolerated dose or a dose of 200 mg twice a day is reached. Ten days after initiation and after every change in dose, laboratory tests, self-measurements of blood pressure, and a nurse evaluation by phone are performed to verify safety criteria (Table 3). The dose is uptitrated consequent to this evaluation by an adult congenital heart disease physician investigator, if all safety criteria are met.
Table 3.
Safety criteria
|
|
|
|
| Postural symptoms: physical symptoms that occur or worsen when changing positions, such as standing up from a seated or lying position (ie, fainting, dizziness, lightheadedness, blurred vision, weakness, fatigue, nausea, palpitations, and headache). |
| Adverse events include any unfavorable and unintended sign, including an abnormal laboratory finding, symptom, or disease that occurs during the study, whether or not it is considered to be related to the study drug. |
| Serious adverse events are defined by any adverse event fulfilling at least one of the following criteria: fatal, life-threatening, requiring in-patient hospitalization, or prolongation of existing hospitalization, resulting in persistent or significant disability or incapacity or medically significant, or requires intervention to prevent at least one of the outcomes listed above. |
Washout period (visits 5, 6 10, and 11)
A 2-week washout period during which no study drug is administered, prior to initiating each treatment phase of the trial, allows each subject’s condition to return to the baseline state. One week into the washout period, the patient is evaluated by phone by the nurse to ensure the absence of HF symptoms (visits 5 and 10). At the end of the 2-week period (visits 6 and 11), the patient is assessed during a conference call by a physician, and blood tests are performed.
Double-blind 1:1 randomization to sacubitril/valsartan vs placebo (visit 6)
A randomization sequence list was performed at the beginning of the study by the statistical department. Patients are randomized according to a computer-generated randomization sequence, with 1:1 distribution using randomly permuted blocks of 4 and 6. Hospital pharmacists, who are blinded to patients’ characteristics and baseline data, act as third parties and have access to the randomization sequence list. The randomization sequence remains in the possession of the pharmacy until all data are collected and analyzed. The randomization code is kept strictly confidential. Hospital pharmacists do not have any contact with the patients. Patients and their nurses and physicians are blinded to the sequence assignment. For the first treatment phase of the trial, each patient is randomized to active therapy (50, 100, or 200 mg twice a day of sacubitril/valsartan, based on the run-in phase) or the corresponding placebo (matching tablets for the 50, 100, or 200 mg of sacubitril/valsartan), with crossover to the other treatment arm in the second phase. Upon reintroduction of sacubitril/valsartan after 2 weeks of washout during phases 2 and 3, the patient is instructed to take half the dose determined during the run-in phase (or matching placebo) for 1 week. The dose is then increased to the full amount and maintained for 24 weeks. If the dose determined by the run-in phase is 50 mg twice a day, it does not change throughout (ie, no initial half-dose is administered). A telephonic evaluation is conducted at the end of the first week to evaluate tolerance to the medication (visit 7).
End of the study (visit 14)
The end of the study for an individual subject will correspond to the last visit performed in the context of the trial. For subjects who complete the second treatment phase following crossover, per the protocol, the end of the study will occur at visit 14. For subjects who prematurely discontinue the study drug, the date of the end of the study will be documented accordingly. For all subjects, the reason for discontinuing the study, and (if applicable) the decider (physician, subject), will be noted.
Safety monitoring
Patients are assessed at each visit for potential side effects. Safety checks with evaluation of safety criteria (Table 3) will be performed at the halfway point of both phases of the trial (visits 8 and 13). In case of mild symptomatic hypotension or postural symptoms, the study drug can be down-titrated to a lower dose at the investigator’s discretion. In the presence of any serious adverse event, the study will be terminated. Occurrence of angioedema is monitored. At any time during the study, unscheduled site visits may be performed, as necessary, at the discretion of the investigator. The following are collected throughout the study: all nonserious adverse events (AEs); all serious adverse events (SAEs); reports of drug exposure during pregnancy or lactation; all reports of misuse and abuse of the study drug; other medication errors and uses outside of what is foreseen in the protocol (irrespective of whether a clinical event occurred); all reports of overdose, medication error, or occupational exposure (irrespective of whether a clinical event occurred); and all reports of unusual lack of efficacy of study drugs.
Safety period (visits 15, 16, and 17)
A 4-week safety period will occur after the end of the second treatment phase, with assessment of secondary safety endpoints. Remote evaluation of HF symptoms will be performed at 1, 2, and 4 weeks (visits 15, 16, and 17) after the end of the study with safety criteria (Table 3) evaluated at 2 weeks (visit 16).
Study objectives
Primary objectives
The purpose of this study is to assess the efficacy of sacubitril/valsartan treatment, compared with placebo, in improving exercise capacity and neurohormonal activation in adults with moderate-to-severe sRV-biV dysfunction and NYHA functional class II/III symptoms. The trial will assess the following 2 primary endpoints (each at an alpha of 0.025): (i) change in submaximal total exercise duration between baseline (visit 1) and the end of each treatment arm (visits 9 and 14); and (ii) change in NT-proBNP levels between baseline (visit 1) and the end of each treatment arm (visits 9 and 14).
Secondary objectives
The secondary efficacy objective is to test whether sacubitril/valsartan compared to placebo is superior in improving the Kansas City Cardiomyopathy Questionnaire score. The secondary safety objective is to evaluate the tolerance and safety of sacubitril/valsartan treatment in patients with an sRV-biV, by assessing the safety criteria (Table 3). Adverse clinical events of symptomatic postural hypotension will be investigated, including fainting, dizziness, lightheadedness, blurred vision, weakness, fatigue, nausea, palpitations, and headache upon standing.
Exploratory objectives
The exploratory objectives are to evaluate the change in NYHA functional class, the impact on adverse clinical events (hospitalizations for HF, symptomatic and clinically significant supraventricular, and ventricular arrhythmia), mortality, impact on myocardial injury (high-sensitivity troponin-T level), change in sRV-biV function and size (tricuspid annular plane systolic excursion, S’wave, fractional area change, global longitudinal strain, end diastolic area, end systolic area, and tricuspid regurgitation) and complementary parameters of exercise capacity, and the impact on the metabolic profile.
Study management and committees
PARACYS-RV is an investigator-initiated trial conducted by the Montreal Heart Institute and funded by Novartis. Safety will be monitored throughout the trial by a Data and Safety Monitoring Board (DSMB) composed of 3 independent members. Although the trial is not assessing outcomes such as mortality or major morbidity, for which use of a DSMB is advised, US Food and Drug Administration guidelines recommend that DSMBs be used for trials that involve high-risk populations. Patients with complex congenital heart disease and sRV-biV failure are deemed to fall into this “high-risk” category. Given the brief duration of the trial, the DSMB will be required to formally meet once when half of the study subjects are recruited, to ensure their safety. Another meeting could be required at the steering committee’s discretion.
Statistical aspects
Sample size and power calculations
The sample size was selected to demonstrate a treatment effect on at least one of 2 primary endpoints, while limiting the overall type I error rate to 0.05. For exercise duration, based on best available evidence, the placebo-treated arm is anticipated to have an average time walked of 9.3 minutes with a within-patient standard deviation (ie, standard deviation of the difference between sacubitril/valsartan and placebo) of 4.1 minutes.14 With the crossover design, a total of 24 patients would provide 80% power to detect a 30% difference in the distance walked, between those receiving sacubitril/valsartan treatment vs placebo, with a 2-sided P-value of 0.025. For the NT-proBNP endpoint, the anticipated mean value is 998 pg/mL in the placebo arm (value obtained from preliminary results of an sRV-biV cohort followed in the Montreal Heart Institute). The expected between-patient standard deviation is believed to be between 500 and 700 pg/mL. Although no data are available on within-patient variation, we conservatively assumed a within-patient standard deviation of 600 pg/mL. With these assumptions, a total of 41 patients would provide 80% power to detect a 30% difference in NT-proBNP levels between those receiving sacubitril/valsartan treatment vs placebo, with a 2-sided P-value of 0.025. Factoring in a 10% attrition rate, a sample size of 48 patients (24 per randomized sequence) will be required. Descriptive statistics will be provided for all study variables, overall and by treatment arm.
Statistical analyses
Prior to any analysis, the assumptions (eg, normality) underlying planned models will be verified with data transformation (eg, logarithmic transformation), performed as needed. The 2 co-primary endpoints will be analyzed by use of analysis of variance models for repeated measures that will account for assigned treatment arm and treatment phase (first 24 weeks vs second 24 weeks). The treatment effect will be tested at the 0.025 significance level for each co-primary endpoint. For illustrative purposes, the carry-over effect will be tested by adding to the above models a term for the sequence of treatment (sacubitril/valsartan followed by placebo, vs placebo followed by sacubitril/valsartan). Analyses of secondary endpoints will use similar methods. Adverse events occurring during each phase of the study will be presented per treatment arm using descriptive statistics. All statistical testing will be 2-sided and will be conducted using a 0.05 significance level, except for the 2 co-primary endpoints. Statistical analysis will be performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Approval and registration
The protocol was approved by the ethics committee/institutional review board affiliated with the Montreal Heart Institute and by Health Canada. Enrollment in the PARACYS-RV trial began on March 15, 2022. The estimated enrollment was evaluated at 2 years. The study is conducted in accordance with Good Clinical Practice, Declaration of Helsinki 2002. The trial has been registered on Clinicaltrials.gov, NCT05117736.
Discussion
We hypothesize that the combination of sacubitril and valsartan carries the potential to alter the sRV-biV’s adaptive stress pathways and improve prognosis. The adaptive pathways to stress in the RV differ from those in the left ventricle, with RAAS activation not being the primary mechanism implicated.26,27 The RV is more susceptible to oxidative stress, due to the absence of activation of antioxidant enzymes resulting in increased activation of cell death pathways and RV fibrosis.27 The metabolic adaptation process for the RV is marked by an acute and permanent shift of fatty acid metabolism to glycolysis.27 Overactivation of growth factors, including fibroblast growth factors and their receptors, leads to early maladaptive hypertrophy.26 The sRV-biV is more susceptible to functional ischemia by virtue of an inefficient coronary circulation, relying on a single coronary artery to irrigate a hypertrophied sRV-biV with a reduced angiogenic response, even in the absence of coronary atherosclerosis.26 ACEis or ARBs, by reducing RAAS activation, lead to a reduction in overall activation of vasoconstrictor neurohormones, which in turn attenuate overactivation of growth factors, reduce hemodynamic stress, and improve cardiac metabolism. Sacubitril/valsartan, by inhibiting the metabolism of natriuretic peptides, and several other vasodilatory peptides, would be expected to enhance any beneficial effects of RAAS blockade and have particularly beneficial effects on coronary circulation and cardiac metabolism. The combination of the 2 molecules could theoretically have a substantial effect on mechanisms to prevent cell death and maladaptive lipidic metabolism that exceed what could be achieved by use of an ACEi or ARB alone. Previous cases reports and the 2 recent open-label trials reported promising results of sacubitril/valsartan use, with good tolerance in patients with sRV-biV. A double-blinded controlled randomized clinical trial is required to definitively demonstrate the benefits of sacubitril/valsartan treatment in patients with sRV-biV dysfunction.
Several features of the design of PARACYS-RV merit discussion. A crossover design is particularly advantageous for the study of small and heterogeneous patient populations. This design provides the major advantage of reducing variability in outcome measures resulting from extraneous confounders, as each patient serves as his or her own control. By reducing variability measurements, precision and efficiency are enhanced, and smaller sample sizes are required. A crossover design also provides the opportunity for each research subject to receive active therapy, which could be attractive for subjects and critical to enhancing feasibility in the context of a limited population.
Patients recruited into PARACYS-RV are symptomatic, with NYHA functional class II or III symptoms. Prior trials of RAAS inhibitors in patients with an sRV-biV comprised a majority of asymptomatic (NYHA I) patients.14,15,28 Asymptomatic patients have normal levels of angiotensin-II, indicating minimal RAAS activation, potentially contributing to the lack of effect of RAAS inhibitors.14 These observations prompted the decision to limit recruitment to symptomatic patients. The choice of placebo as the comparator (as opposed to an ACEi or ARB) was made owing to the absence of clinical trials demonstrating efficacy with RAAS inhibitors in patients with sRV-biV, combined with the fact that these therapies are not currently recommended by evidence-based management guidelines for this patient population.17, 18, 19
Washout periods in which no therapy is administered prior to initiating the first and the second treatments arms of the trial were deemed necessary to allow each subject’s condition to return to the state closest to baseline. A 2-week duration was judged by the executive committee to be sufficient, as the medication is eliminated within 3 days (5 half-lives). To mitigate against the risk of worsening HF symptoms during this period, follow-up remote visits at 1 week and at the end of the washout period were planned. Moreover, additional remote visits were incorporated 1 week after the beginning of each treatment phase (increase from half of the dose to a full dose of sacubitril/valsartan or matched placebo).
The 2 primary endpoints comparing a change from screening vs at the end of the treatment phase, that is, submaximal exercise duration and NT-proBNP level, were chosen to capture HF associated with sRV-biV dysfunction. The submaximal cardiopulmonary exercise test protocol is well established and has been used previously as a primary endpoint for clinical trials by our research team.29, 30, 31, 32 The submaximal treadmill test will be performed using a constant-load protocol at an intensity corresponding to 75% of the peak VO2max (maximal oxygen consumption) determined by the maximal cardiopulmonary exercise test performed previously. After a 2-minute warm-up, the slope and speed of the treadmill will be programmed to predetermined settings corresponding to 75% of the most recent peak VO2max. In the open-label single-centre study comparing 18 patients with sRV-biV failure (ejection fraction < 35%), at baseline and after 6 months of sacubritril/valsartan medication, no change in VO2max was observed, but the submaximal parameter—that is, the 6-minute-walk test distance—improved significantly.23 A more accurate submaximal parameter, such as submaximal exercise duration, is therefore believed to be advantageous. Submaximal exercise duration has excellent sensitivity in assessing therapeutic interventions (superior to VO2max and 6-minute-walk test distance) and reflects daily physical activities. Daily physical activities are defined by the activities normally undertaken in daily living, such as eating, bathing, dressing, grooming, working, homemaking, housecleaning, transportation, walking, and shopping. In contrast to the 6-minute-walk test, which is also a submaximal test, the submaximal exercise protocol in PARACYS-RV benefits from measuring cardiopulmonary exercise parameters to allow for a more comprehensive assessment of contractile reserve. Cardiopulmonary exercise testing is a powerful tool to gain insights into both cardiac and pulmonary efficiencies and functional reserve.
NT-proBNP level is currently the most sensitive neurohormonal marker for HF that correlates with disease severity and prognosis. A reduction in NT-proBNP level predicts clinical improvement and is indicative of therapeutic effectiveness. In the Prospective Comparison of ARNi With ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial, the NT-proBNP level fell acutely after initiation of sacubitril/valsartan treatment and continued to drop over time until the end of the trial. Zandstra et al. showed a significant decrease in NT-proBNP levels in 18 patients with sRV-biV after 6 months of sacubritril/valsartan therapy, compared to baseline.23 In contrast, in Fusco et al.’s study of 50 patients with sRV-biV, the significant drop in NT-proBNP level 1 month after initiation was followed by a subsequent return to the baseline value after 3 months.24
Conclusion
PARACYS-RV is the first double-blind randomized trial to assess the efficacy of sacubitril/valsartan in the treatment of HF in patients with systolic dysfunction of a morphologic right ventricle in the systemic position. HF is a prevalent issue in this relatively young and unique patient population, for whom no accepted, evidenced-based, guideline-directed medical therapy for this indication is currently available. Given this context, the trial carries the potential to alter clinical management and improve outcomes in this patient population.
Disclosures
Other than the source of funding, the authors have no conflicts of interest to disclose.
Acknowledgments
Ethics Statement
The protocol was approved by the ethics committee/institutional review board affiliated with the Montreal Heart Institute and by Health Canada. The study is conducted in accordance with good clinical practice, per the Declaration of Helsinki 2002.
Patient Consent
All participants provided written informed consent.
Funding Sources
The trial is funded by an investigator-initiated grant from Novartis. The sponsor (Novartis) provided feedback on the study design, and will provide feedback on the results before publication, but has no role in data collection, analysis, interpretation, writing of papers, or decisions to submit manuscripts for publication. M.A.C. is supported by a clinician research scholarship by the Fonds de recherche du Québec—Santé (FRQS). P.K. is supported by the André Chagnon research chair in electrophysiology and congenital heart disease. The other authors have no funding sources to declare.
Footnotes
See page 543 for disclosure information.
References
- 1.Senning A. Surgical correction of transposition of the great vessels. Surgery. 1959;45:966–980. [PubMed] [Google Scholar]
- 2.Mustard W.T. Successful two-stage correction of transposition of the great vessels. Surgery. 1964;55:469–472. [PubMed] [Google Scholar]
- 3.Reller M.D., Strickland M.J., Riehle-Colarusso T., Mahle W.T., Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr. 2008;153:807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davlouros P.A., Niwa K., Webb G., Gatzoulis M.A. The right ventricle in congenital heart disease. Heart. 2006;92(suppl 1):i27–i38. doi: 10.1136/hrt.2005.077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipczynska M., Szymanski P., Kumor M., Klisiewicz A., Hoffman P. Collagen turnover biomarkers and systemic right ventricle remodeling in adults with previous atrial switch procedure for transposition of the great arteries. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avila P., Chaix M.A., Mondesert B., Khairy P. Sudden cardiac death in adult congenital heart disease. Card Electrophysiol Clin. 2017;9:225–234. doi: 10.1016/j.ccep.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Filippov A.A., Del Nido P.J., Vasilyev N.V. Management of systemic right ventricular failure in patients with congenitally corrected transposition of the great arteries. Circulation. 2016;134:1293–1302. doi: 10.1161/CIRCULATIONAHA.116.022106. [DOI] [PubMed] [Google Scholar]
- 8.Vejlstrup N., Sorensen K., Mattsson E., et al. Long-term outcome of Mustard/Senning correction for transposition of the great arteries in Sweden and Denmark. Circulation. 2015;132:633–638. doi: 10.1161/CIRCULATIONAHA.114.010770. [DOI] [PubMed] [Google Scholar]
- 9.De Leon L.E., Mery C.M., Verm R.A., et al. Mid-term outcomes in patients with congenitally corrected transposition of the great arteries: a single center experience. J Am Coll Surg. 2017;224:707–715. doi: 10.1016/j.jamcollsurg.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Chaix M.A., Dore A., Mercier L.A., et al. Late onset postcapillary pulmonary hypertension in patients with transposition of the great arteries and Mustard or Senning baffles. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norozi K., Wessel A., Alpers V., et al. Incidence and risk distribution of heart failure in adolescents and adults with congenital heart disease after cardiac surgery. Am J Cardiol. 2006;97:1238–1243. doi: 10.1016/j.amjcard.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 12.Brida M., Diller G.P., Gatzoulis M.A. Systemic right ventricle in adults with congenital heart disease: anatomic and phenotypic spectrum and current approach to management. Circulation. 2018;137:508–518. doi: 10.1161/CIRCULATIONAHA.117.031544. [DOI] [PubMed] [Google Scholar]
- 13.Alonso-Gonzalez R., Dimopoulos K., Ho S., Oliver J.M., Gatzoulis M.A. The right heart and pulmonary circulation (IX). The right heart in adults with congenital heart disease. Rev Esp Cardiol. 2010;63:1070–1086. doi: 10.1016/s1885-5857(10)70211-5. [DOI] [PubMed] [Google Scholar]
- 14.Dore A., Houde C., Chan K.L., et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005;112:2411–2416. doi: 10.1161/CIRCULATIONAHA.105.543470. [DOI] [PubMed] [Google Scholar]
- 15.van der Bom T., Winter M.M., Bouma B.J., et al. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation. 2013;127:322–330. doi: 10.1161/CIRCULATIONAHA.112.135392. [DOI] [PubMed] [Google Scholar]
- 16.Zaragoza-Macias E., Zaidi A.N., Dendukuri N., Marelli A. Medical therapy for systemic right ventricles: a systematic review (Part 1) for the 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:1564–1578. doi: 10.1016/j.jacc.2018.08.1030. [DOI] [PubMed] [Google Scholar]
- 17.Marelli A., Beauchesne L., Colman J., et al. Canadian Cardiovascular Society 2022 guidelines for cardiovascular interventions in adults with congenital heart disease. Can J Cardiol. 2022;38:862–896. doi: 10.1016/j.cjca.2022.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner H., De Backer J., Babu-Narayan S.V., et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. doi: 10.1093/eurheartj/ehaa554. [DOI] [PubMed] [Google Scholar]
- 19.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e81–e192. doi: 10.1016/j.jacc.2018.08.1029. [DOI] [PubMed] [Google Scholar]
- 20.Lluri G., Lin J., Reardon L., et al. Early experience with sacubitril/valsartan in adult patients with congenital heart disease. World J Pediatr Congenit Heart Surg. 2019;10:292–295. doi: 10.1177/2150135119825599. [DOI] [PubMed] [Google Scholar]
- 21.Appadurai V., Thoreau J., Malpas T., Nicolae M. Sacubitril/valsartan in adult congenital heart disease patients with chronic heart failure—a single centre case series and call for an international registry. Heart Lung Circ. 2020;29:137–141. doi: 10.1016/j.hlc.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Maurer S.J., Pujol Salvador C., Schiele S., et al. Sacubitril/valsartan for heart failure in adults with complex congenital heart disease. Int J Cardiol. 2020;300:137–140. doi: 10.1016/j.ijcard.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Zandstra T.E., Nederend M., Jongbloed M.R.M., et al. Sacubitril/valsartan in the treatment of systemic right ventricular failure. Heart. 2021;107:1725–1730. doi: 10.1136/heartjnl-2020-318074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fusco F., Scognamiglio G., Merola A., et al. Safety and efficacy of sacubitril/valsartan in patients with a failing systemic right ventricle: a prospective single-center study. Circ Heart Fail. 2022;16 doi: 10.1161/CIRCHEARTFAILURE.122.009848. [DOI] [PubMed] [Google Scholar]
- 25.Ezekowitz J.A., O'Meara E., McDonald M.A., et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33:1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman B.D., Desai M., Reddy S., et al. Genomic profiling of left and right ventricular hypertrophy in congenital heart disease. J Card Fail. 2008;14:760–767. doi: 10.1016/j.cardfail.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Reddy S., Bernstein D. Molecular mechanisms of right ventricular failure. Circulation. 2015;132:1734–1742. doi: 10.1161/CIRCULATIONAHA.114.012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therrien J., Provost Y., Harrison J., et al. Effect of angiotensin receptor blockade on systemic right ventricular function and size: a small, randomized, placebo-controlled study. Int J Cardiol. 2008;129:187–192. doi: 10.1016/j.ijcard.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 29.Thibault B., Harel F., Ducharme A., et al. Cardiac resynchronization therapy in patients with heart failure and a QRS complex < 120 milliseconds: the Evaluation of Resynchronization Therapy for Heart Failure (LESSER-EARTH) trial. Circulation. 2013;127:873–881. doi: 10.1161/CIRCULATIONAHA.112.001239. [DOI] [PubMed] [Google Scholar]
- 30.Blanchet M., Ducharme A., Racine N., et al. Effects of cold exposure on submaximal exercise performance and adrenergic activation in patients with congestive heart failure and the effects of beta-adrenergic blockade (carvedilol or metoprolol) Am J Cardiol. 2003;92:548–553. doi: 10.1016/s0002-9149(03)00723-9. [DOI] [PubMed] [Google Scholar]
- 31.Blanchet M., Sheppard R., Racine N., et al. Effects of angiotensin-converting enzyme inhibitor plus irbesartan on maximal and submaximal exercise capacity and neurohumoral activation in patients with congestive heart failure. Am Heart J. 2005;149:938.e1–938.e7. doi: 10.1016/j.ahj.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Thibault B., Ducharme A., Harel F., et al. Left ventricular versus simultaneous biventricular pacing in patients with heart failure and a QRS complex > / = 120 milliseconds. Circulation. 2011;124:2874–2881. doi: 10.1161/CIRCULATIONAHA.111.032904. [DOI] [PubMed] [Google Scholar]