Abstract
Background
Risk stratification is fundamental in the management of pulmonary arterial hypertension (PAH). Pulmonary artery pulsatility index (PAPi), defined as pulmonary arterial pulse pressure divided by right atrial pressure (RAP), is a hemodynamic index shown to predict acute right ventricular (RV) dysfunction in several settings. Our objective was to test the prognostic utility of PAPi in a diverse multicentre cohort of patients with PAH.
Methods
A multicentre retrospective cohort study of consecutive adult patients with a new diagnosis of PAH on right heart catheterization between January 2016 and December 2020 was undertaken across 4 major centres in Canada. Hemodynamic data, clinical data, and outcomes were collected. The association of PAPi and other hemodynamic variables with mortality was assessed by receiver-operating characteristic curves and Cox proportional hazards modeling.
Results
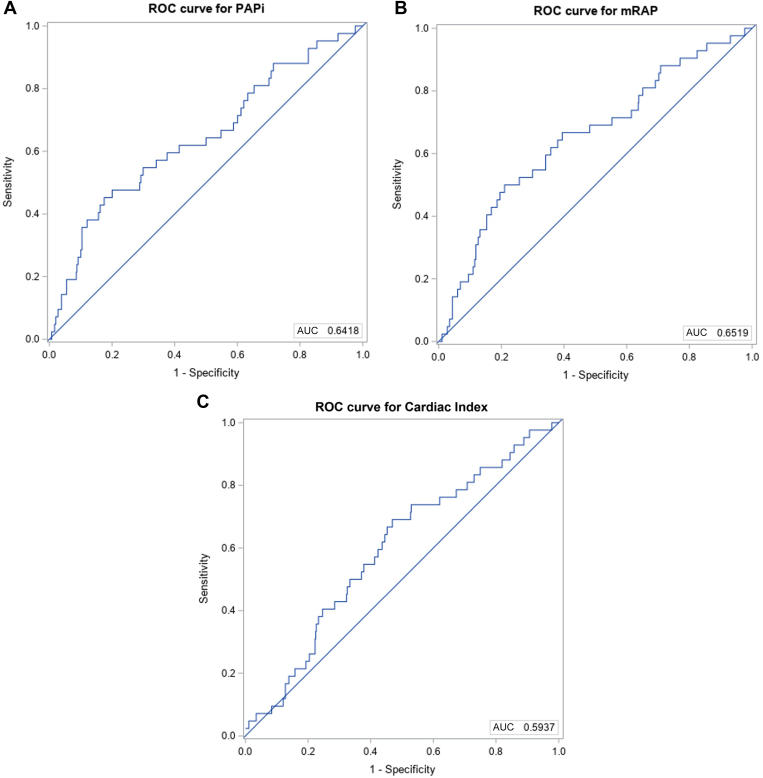
We identified 590 patients with a mean age of 61.4 ± 15.5 years, with 66.3% being female. A low PAPi (defined as < 5.3) was associated with higher mortality at 1 year: 10.2% vs 5.2% (P = 0.02). In a multivariable model including age, sex, body mass index, and functional class, a low PAPi was associated with mortality at 1 year (area under the curveof 0.64 (95% confidence interval 0.55-0.74). However, high RAP (> 8 mm Hg) was similarly predictive of mortality, with an area under the curve of 0.65.
Conclusion
PAPi was associated with mortality in a large incident PAH cohort. However, the discriminative value of PAPi was not higher than that of RAP alone.
Résumé
Contexte
La stratification des risques est fondamentale dans la prise en charge de l’hypertension artérielle pulmonaire (HTAP). L’indice de pulsatilité des artères pulmonaires (iPAP), défini comme la pression différentielle dans les artères pulmonaires divisée par la pression auriculaire droite (PAD), est un indice hémodynamique qui s’est révélé prédictif d’une dysfonction ventriculaire droite (VD) aiguë dans plusieurs situations. Notre objectif était d’évaluer l’utilité pronostique de l’iPAP dans une cohorte multicentrique diversifiée de patients atteints d’HTAP.
Méthodologie
Une étude de cohorte multicentrique rétrospective de patients adultes consécutifs atteints d’une HTAP nouvellement diagnostiquée par cathétérisme cardiaque droit entre janvier 2016 et décembre 2020 a été effectuée dans quatre grands centres au Canada. Les données hémodynamiques, les données cliniques et les résultats ont été recueillis. La corrélation de l’iPAP et d’autres va-riables hémodynamiques avec la mortalité a été évaluée par les courbes caractéristiques opérationnelles du receveur et des modèles à risques proportionnels de Cox.
Résultats
Nous avons recensé 590 patients dont l’âge moyen était de 61,4 ± 15,5 ans; la proportion de femmes était de 66,3 %. Un faible iPAP (défini comme une valeur < 5,3) a été associé à une hausse de la mortalité à 1 an : 10,2 % contre 5,2 % (p= 0,02). Dans un modèle multivarié comprenant l’âge, le sexe, l’indice de masse corporelle et la classe fonctionnelle, un faible iPAP a été associé à la mortalité à 1 an (aire sous la courbe de 0,64 [intervalle de confiance à 95 %; de 0,55 à 0,74]). Cependant, une PAD élevée (> 8 mmHg) a aussi été un facteur prédictif de mortalité, l’aire sous la courbe étant de 0,65.
Conclusions
L’iPAP a été associé à la mortalité dans une vaste cohorte de patients atteints d’une HTAP. Toutefois, la valeur discriminante de l’iPAP n’a pas été supérieure à celle de la PAD seule.
Pulmonary arterial hypertension (PAH) is a progressive chronic disease characterized by pulmonary vascular remodeling, eventually culminating in right ventricular (RV) failure and death if untreated.1 Despite advances in treatment, PAH continues to be associated with high levels of morbidity and mortality.2,3 The most recent guidelines for the diagnosis and treatment of PAH propose a risk stratification tool based on multiple variables, including hemodynamic parameters, such as mean right atrial pressure (mRAP), cardiac index, and mixed venous oxygen saturation (SvO2).4,5 A comprehensive assessment is recommended, as no single variable provides sufficient diagnostic and prognostic information. Additional markers may be beneficial for further refining the process of assessment of prognosis, which is fundamental, as it guides therapeutic decisions, including listing for transplantation.
RV function and RV-pulmonary arterial (PA) coupling are strongly associated with prognosis in PAH.6 RV-PA coupling, defined by a ratio of end-systolic to arterial elastances, has been shown to detect developing RV failure.7 Additionally, this parameter has been shown to be an independent predictor of survival in patients with PAH.8 The pulmonary artery pulsatility index (PAPi), defined as the ratio of the PA pulse pressure to the mRAP, has been proposed as an easily derivable marker of RV-PA coupling. Since its initial description in patients with acute inferior wall myocardial infarction, it has not yet received widespread evaluation in a large multicentre study of patients with PAH.9 The objectives of this study were to investigate the association between PAPi and mortality in patients with PAH, and to determine whether it provides superior prognostic value compared to other hemodynamic variables from right heart catheterization (RHC).
Methods
Study design and population
This retrospective, multi-centre cohort study was conducted across 4 pulmonary hypertension expert referral centres participating in the Canadian Pulmonary Hypertension Registry (CPHR) in Canada (Vancouver, Calgary, Ottawa, Hamilton). The study was coordinated through the University of British Columbia (H21-03574), and institutional ethics board approval was obtained at each participating centre. Eligible patients were identified through a structured chart review by study authors (N.B., A.A., J.S., J.W., N.H., L.M.). All incident patients with a clinical diagnosis of PAH confirmed on RHC between January 1, 2016 and December 31, 2020 were included. In this study, the hemodynamic criteria for PAH proposed by the 6th World Symposium on Pulmonary Hypertension (PH) were used (mean pulmonary arterial pressure [mPAP] > 20 mm Hg; a pulmonary arterial wedge pressure ≤ 15 mm Hg; and a pulmonary vascular resistance > 3 WU). Patients with other causes of PH, including those with group 2 (PH due to left heart disease), group 3 (PH due to lung disease and/or hypoxia), group 4 (PH due to pulmonary artery obstructions), or group 5 (PH with unclear and/or multifactorial mechanisms) were excluded. Patients with missing hemodynamic data were excluded from the analysis. Patient demographics, including age, sex, body mass index (BMI), and etiology of PH as determined by the treating specialist, were obtained from electronic medical records or clinical files. Hemodynamic variables were extracted from baseline RHCs. The primary outcome was all-cause mortality at 1 year following the index RHC, as determined from clinic records. We chose to analyze survival at 1 year, as longer-term survival was expected to be more strongly influenced by response to PAH therapy, as opposed to baseline hemodynamic parameters.
Hemodynamic variables
RHCs were performed by experienced operators, according to routine clinical care protocol. During RHC, the zero line was set at the mid-thoracic height. Measurements were obtained from hemodynamic tracing according to current guidelines. Variables measured during the RHC included, but were not limited to, the mRAP; systolic (s) pulmonary arterial pressure (PAP), diastolic (d)PAP, and mean (m)PAP; pulmonary arterial wedge pressure (PAWP); and SvO2. Cardiac output and index were determined by either thermodilution or the Fick method, at the discretion of the operator. However, the thermodilution method was preferred in the absence of shunting. PAPi was calculated as pulmonary artery pulse pressure (sPAP – dPAP) divided by mRAP. PAPi was compared to other parameters shown to have prognostic importance in PAH, including the RV stroke work index (RVSWi), effective PA elastance (PAE), and PA capacitance (PAC).
PAPi was analyzed as a continuous variable, and also by dichotomizing it into a binary variable. We used our data to find the cutoff value associated with the optimal c-statistic, and we also investigated the use of cutoff values published previously.10 As mRAP and cardiac index already have well known reference ranges, we dichotomized these values accordingly. A high mRAP was defined as one > 8 mm Hg, and a low cardiac index was defined as one ≤ 2.4 L/min per m2.11
Statistical analysis
Categorical variables were summarized as numbers and percentages, whereas continuous variables were summarized as mean values ± standard deviations. Comparisons between groups were done using the χ2 test or the Kruskal-Wallis test, as appropriate.
To assess the association between each hemodynamic parameter and 1-year mortality, we constructed a multivariate logistic regression model and its corresponding area under the curve (AUC) curve; each model was adjusted for age, BMI, sex, and advanced functional class.
For each hemodynamic parameter, we also produced a Cox proportional hazards model adjusted for the same covariates as above, to also assess the association between the hemodynamic parameter and 1-year mortality via hazards ratios. These last regressions were performed as a sensitivity analysis to confirm the findings from our logistic regressions, despite Cox models being a time-to-event analysis.
Statistical significance was determined as a P-value < 0.01.
Results
We identified 590 incident patients with PAH from the participating centres. Table 1 demonstrates the baseline characteristics for the total cohort, and for patients who had survived at 1 year vs those who did not. The mean age was 61.4 ± 15.5 years, and 66.3% were female. The majority (51.5%) had idiopathic PAH. Only 2 patients underwent a lung transplant over the time course of the study. Table 2 shows all hemodynamic variables tested, analyzed as continuous variables. Neither PAPi nor any other of the variables tested was significantly different for survivors vs nonsurvivors (P > 0.01 for each).
Table 1.
Baseline characteristics, of total cohort, and based on survival status at 1 year
| Characteristic | 1-year mortality |
Total (N = 590) | P | |
|---|---|---|---|---|
| 0 (N = 547) | 1 (N = 43) | |||
| Age, y | 61.2 (15.44) | 64.4 (16.19) | 61.4 (15.51) | 0.1548 |
| Female | 365 (66.7) | 26 (60.5) | 391 (66.3) | 0.4030 |
| PAH subtype | ||||
| iPAH | 274 (51.3) | 23 (53.5) | 297 (51.5) | |
| Heritable | 3 (0.6) | 0 (0.0) | 3 (0.5) | |
| Drug and toxins | 20 (3.7) | 0 (0.0) | 20 (3.5) | |
| CTD | 187 (35.0) | 8 (18.6) | 195 (33.8) | |
| CHD | 30 (5.6) | 3 (7.0) | 33 (5.7) | |
| Portal hypertension | 11 (2.1) | 8 (18.6) | 19 (3.3) | |
| HIV | 4 (0.7) | 0 (0.0) | 4 (0.7) | |
| Low-risk, functional class (WHO FC 1, 2) | 169 (30.9) | 11 (25.6) | 180 (30.5) | 0.4662 |
| Height, cm, mean (SD) | 164.8 (9.81) | 165.0 (11.48) | 164.8 (9.92) | 0.9452 |
| Weight, Kg, mean (SD) | 79.2 (22.33) | 75.8 (20.53) | 79.0 (22.20) | 0.3044 |
| BMI, kg/m2, mean (SD) | 29.1 (7.73) | 27.8 (6.70) | 29.0 (7.66) | 0.3646 |
| Lung transplant | 2 (0.4) | 0 (0.0) | 2 (0.3) | 0.6912 |
Values are n (%), unless otherwise indicated.
BMI, body mass index; CHD, congenital heart disease; CTD, connective tissue disease; HIV, human immunodeficiency virus; iPAH, idiopathic PAH; PAH, pulmonary arterial hypertension; SD, standard deviation; WHO FC, World Health Organization functional class.
Table 2.
Hemodynamic parameters, for total cohort, and based on survival status at 1 year
| Parameter | Total (N = 590) | Death at 1 year |
P | |
|---|---|---|---|---|
| No (N = 547) | Yes (N = 43) | |||
| PAWP, mm Hg | ||||
| 10.2 (4.75) | 10.2 (4.76) | 10.3 (4.76) | 0.6744 | |
| 10.0 (7.0, 13.0) | 10.0 (7.0, 13.0) | 10.0 (6.0, 14.0) | ||
| PVR, WU | ||||
| 9.4 (5.50) | 9.3 (5.53) | 10.3 (5.10) | 0.0911 | |
| 8.1 (5.4, 12.2) | 8.0 (5.3, 12.2) | 8.7 (6.6, 12.7) | ||
| SvO2, % | ||||
| 61.7 (9.46) | 62.1 (9.39) | 57.3 (9.29) | 0.0014 | |
| 62.8 (56.0, 68.5) | 63.0 (56.3, 68.9) | 58.0 (53.2, 65.0) | ||
| mPAP, mm Hg | ||||
| 44.8 (11.91) | 44.5 (12.06) | 48.3 (9.27) | 0.0189 | |
| 45.0 (36.0, 52.0) | 45.0 (35.0, 52.0) | 47.0 (43.0, 55.0) | ||
| mRAP, mm Hg | ||||
| 8.3 (5.16) | 8.2 (5.07) | 9.7 (6.09) | 0.0605 | |
| 7.0 (5.0, 10.0) | 7.0 (5.0, 10.0) | 9.0 (6.0, 12.0) | ||
| sPAP, mm Hg | ||||
| 73.2 (19.26) | 72.9 (19.63) | 77.0 (13.39) | 0.0899 | |
| 73.0 (60.0, 85.0) | 73.0 (59.0, 85.0) | 80.0 (67.0, 85.0) | ||
| Stroke volume, mL | ||||
| 57.9 (21.94) | 58.3 (22.10) | 53.1 (19.27) | 0.2188 | |
| 54.8 (41.7, 69.8) | 55.1 (42.7, 70.0) | 50.0 (38.6, 65.2) | ||
| Stroke volume index, mL/m2 | ||||
| 30.7 (10.51) | 30.9 (10.58) | 28.7 (9.40) | 0.2515 | |
| 29.6 (23.0, 36.9) | 29.7 (23.0, 37.1) | 26.5 (22.1, 34.8) | ||
| Cardiac index, L/min per m2 | ||||
| 2.3 (0.74) | 2.3 (0.74) | 2.2 (0.64) | 0.8963 | |
| 2.1 (1.8, 2.7) | 2.1 (1.8, 2.7) | 2.1 (1.7, 2.7) | ||
| PAPi | ||||
| 8.7 (8.63) | 8.6 (8.41) | 8.8 (11.22) | 0.1095 | |
| 6.1 (4.2, 9.7) | 6.2 (4.2, 9.7) | 4.7 (3.4, 9.8) | ||
| RV stroke work index, mmHg.mL/m2 | ||||
| 151.7 (122.87) | 149.2 (120.94) | 184.3 (143.46) | 0.0584 | |
| 120.7 (70.3, 200.4) | 119.2 (69.6, 197.9) | 156.3 (110.1, 221.2) | ||
| Effective PA elastance, mmHg/mL | ||||
| 0.9 (0.51) | 0.9 (0.51) | 1.1 (0.51) | 0.0222 | |
| 0.8 (0.5, 1.2) | 0.8 (0.5, 1.2) | 1.0 (0.7, 1.3) | ||
| PA capacitance, mL/mmHg | ||||
| 1.6 (3.26) | 1.6 (3.38) | 1.2 (0.65) | 0.0907 | |
| 1.2 (0.9, 1.8) | 1.2 (0.9, 1.8) | 1.0 (0.8, 1.5) | ||
Values are mean (standard deviation) followed by median (interquartile range). P-values are from Kruskal-Wallis test. PAPi is defined as pulmonary arterial pulse pressure divided by right atrial pressure.
mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; PA, pulmonary artery; PAPi, pulmonary artery pulsatility index; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance; RV, right ventricular; sPAP, systolic pulmonary arterial pressure; SVO2, venous oxygen saturation.
As the purpose of this work was to evaluate PAPi specifically, we evaluated whether dichotomizing PAPI into low PAPi vs high PAPi gave a stronger association with mortality. We investigated the ideal value of PAPi—that is, the one that would perform best in a multivariate model. The ideal cutoff value for our data was 5.1, giving an AUC of 0.66. However, as this value was very close to the cuttoff value of 5.3 used in previous work,10 we decided to use that same cutoff, to maintain consistency in the literature.
Table 3 shows the characteristics for low PAPi and high PAPi, respectively. At 1 year, 43 of the patients (7%) had died. Table 4 shows the number of deaths for low and high PAPi, cardiac index, and mRAP. In the univariate analysis, a PAPi of < 5.3 was seen in 58% of patients who died at 1 year, compared to 40% of surviving patients (P = 0.02). Similarly, an mRAP > 8 mm Hg was seen in 65% of patients who died and 47% of patients who survived (P = 0.03). Neither baseline functional class nor cardiac index > 2.4 L/min per m2 were associated with mortality at 1 year, nor was age as a continuous variable.
Table 3.
Characteristics of patients with high vs low PAPi
| Characteristic | Low PAPi (N = 246) | High PAPi (N = 345) | P |
|---|---|---|---|
| Age, y, mean (SD) | 60.8 (16.34) | 61.9 (14.87) | 0.6326 |
| Female | 161 (65.4) | 231 (67.0) | 0.7019 |
| Etiology of PAH | 0.8547 | ||
| iPAH | 129 (52.4) | 168 (48.7) | |
| Heritable | 1 (0.4) | 2 (0.6) | |
| Drugs and toxins | 11 (4.5) | 9 (2.6) | |
| CTD | 78 (31.7) | 118 (34.2) | |
| CHD | 12 (4.9) | 21 (6.1) | |
| Portal hypertension | 7 (2.8) | 12 (3.5) | |
| HIV | 1 (0.4) | 3 (0.9) | |
| Low-risk functional class (1, 2) | 67 (27.2) | 114 (33.0) | 0.1311 |
| Height, cm, mean (SD) | 164.7 (9.95) | 164.8 (9.91) | 0.8999 |
| Weight, Kg, mean (SD) | 83.9 (25.19) | 75.5 (19.10) | 0.0003 |
| BMI, kg/m2, mean (SD) | 30.9 (8.89) | 27.7 (6.37) | < 0.0001 |
| Lung transplant | 2 (0.8) | 0 (0.0) | 0.0934 |
Values are n (%), unless otherwise indicated. PAPi is defined as pulmonary arterial pulse pressure divided by right atrial pressure.
BMI, body mass index; CHD, congenital heart disease; CTD, connective tissue disease; HIV, human immunodeficiency virus; iPAH, idiopathic PAH; PAH, pulmonary arterial hypertension; PAPi, pulmonary artery pulsatility index; SD, standard deviation.
Table 4.
Hemodynamic parameters by 1-year mortality
| Parameter | Death at 1 year |
Total n | P | |
|---|---|---|---|---|
| No (N = 548) | Yes (N = 43) | |||
| PAPi | ||||
| < 5.3 | 221 (89.8) | 25 (10.2) | 246 | 0.02 |
| ≥ 5.3 | 327 (94.8) | 18 (5.2) | 345 | |
| mRAP, mm Hg | ||||
| < 8 | 292 (95.1) | 15 (4.9) | 307 | 0.02 |
| ≥ 8 | 256 (90.1) | 28 (9.9) | 284 | |
| Cardiac index, L/min per m2 | ||||
| < 2.4 | 350 (92.6) | 28 (7.4) | 378 | 0.87 |
| ≥ 2.4 | 198 (93.0) | 15 (7) | 213 | |
Values are n (%), unless otherwise indicated.
P values are from χ2 test. PAPi is defined as pulmonary arterial pulse pressure divided by right atrial pressure.
mRAP, mean right atrial pressure; PAPi, pulmonary artery pulsatility index.
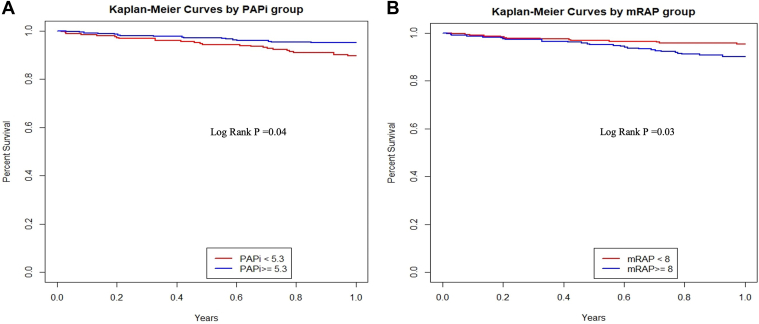
We constructed receiver operator curves to investigate the ability of PAPi and other parameters to discriminate 1-year mortality. In a multivariate model including age, sex, BMI, and functional class, PAPi was associated with 1-year mortality with an AUC of 0.64 (95% confidence interval [CI] 0.55-0.73; Table 5; Fig. 1). Similarly, mRAP > 8 mm Hg was also able to predict mortality with a similar AUC of 0.65 (95% CI 0.56-0.74). A cardiac index of < 2.4 was not predictive of mortality. Kaplan-Meier curves were created for high vs low PAPi and mRAP values (Fig. 2). As shown, survival curves for PAPi < 5.3 and mRAP > 8 up to 1 year postcatheterization were similar. Table 3 shows the adjusted estimates for the logistic regression and Cox proportional hazards models for each of the 3 hemodynamic variables evaluated.
Table 5.
Logistic and Cox proportional hazards model adjusted estimates for hemodynamic parameters
| Hemodynamic parameter | Logistic regression |
Cox PH |
|||
|---|---|---|---|---|---|
| Adjusted estimates OR (95% CI) |
P | AUC (95% CI) | Adjusted estimates HR (95% CI) |
P | |
| PAPi < 5.3 | 2.4 (1.2, 4.6) | 0.009 | 0.64 (0.55, 0.73) | 2.5 (1.3, 4.6) | 0.006 |
| mRAP, ≥8, mm Hg | 2.5 (1.3, 4.8) | 0.009 | 0.65 (0.56, 0.74) | 2.56 (1.32, 4.98) | 0.006 |
| Cardiac index, ≥ 2.4, L/min per m2 | 1.1 (0.6, 2.2) | 0.793 | 0.59 (0.51, 0.68) | 0.99 (0.51, 1.92) | 0.971 |
PAPi is defined as pulmonary arterial pulse pressure divided by right atrial pressure.
AUC, area under curve; CI, confidence interval; Cox PH, Cox proportional hazards; HR, hazard ratio; mRAP, mean right atrial pressure; PAPi, pulmonary artery pulsatility index.
Figure 1.
Receiver operating curve (ROC) for (A) PAPi (pulmonary artery pulsatility index), (B) mean right atrial pressure (mRAP), and (C) cardiac index. PAPi is defined as pulmonary arterial pulse pressure divided by right atrial pressure. AUC, area under the curve.
Figure 2.
Kaplan-Meier curves for (A) pulmonary artery pulsatility index (PAPi) and (B) mean right atrial pressure (mRAP).
We performed a subgroup analysis, stratifying for sex. The results for PAPi are shown in Table 6. Given the low number of events seen for both female and male patients, the CIs are wide, and P was > 0.01 for both. The model appeared to perform better for male patients than for female patients, with a higher AUC (0.73 vs 0.66). However, this difference was related not to PAPi but to the other parameters in the model.
Table 6.
Logistic and Cox PH model adjusted estimates for PAPi, stratified by sex
| PAPi < 5.3 | Logistic regression |
Cox PH |
|||
|---|---|---|---|---|---|
| Adjusted estimates OR (95% CI) |
P | AUC (95% CI) | Adjusted estimates HR (95% CI) |
P | |
| Total cohort | 2.4 (1.2, 4.6) | 0.009 | 0.64 (0.55, 0.73) | 2.5 (1.3, 4.6) | 0.006 |
| Female patients | 1.79 (0.79, 4.06) | 0.16 | 0.66 (0.54, 0.77) | 1.9 (0.9, 4.3) | 0.11 |
| Male patients | 3.85 (1.26 ,11.78) | 0.018 | 0.73 (0.61 ,0.85) | 3.5 (1.2 ,9.9) | 0.02 |
PAPi is defined as pulmonary arterial pulse pressure divided by right atrial pressure.
AUC, area under curve; CI, confidence interval; Cox PH, Cox proportional hazards; HR, hazard ratio; OR, odds ratio; PAPi, pulmonary artery pulsatility index.
As shown in Table 7, we did notice an association between BMI and both mRAP and PAPi. As BMI increased, we saw a strong association with increasing mRAP, and a decrease in the median PAPi. The association with BMI appeared stronger with mRAP, compared to PAPi. However, the mean PAPi still remained in the low-risk range for the highest BMI category.
Table 7.
Mean RAP and PAPI stratified by body mass index
| Parameter | BMI (kg/m2) |
Total (N = 590) | P | |||
|---|---|---|---|---|---|---|
| < 18.5 (N = 20) | 18.5-25 (N = 181) | 25-30 (N = 180) | > 30 (N = 209) | |||
| mRAP, mm Hg | < 0.0001 | |||||
| 6.2 (3.11) | 7.2 (4.74) | 8.2 (4.98) | 9.4 (5.57) | 8.3 (5.16) | ||
| 6 (3.5, 7.5) | 6 (4, 10) | 7.0 (5.0, 1.0) | 9 (6, 12) | 7 (5, 10) | ||
| PAPi | 0.0014 | |||||
| 8.3 (4.16) | 10.0 (10.22) | 8.0 (6.67) | 8.1 (8.86) | 8.7 (8.63) | ||
| 7.0 (5.7, 10.1) | 6.7 (4.6, 11.2) | 5.8 (4.2, 9.4) | 5.3 (3.5, 8.8) | 6.1 (4.2, 9.7) | ||
Values are mean (standard deviation), followed by median (interquartile range). P values are from Kruskal-Wallis test. PAPi is defined as pulmonary arterial pulse pressure divided by right atrial pressure.
BMI, body mass index; mRAP, mean right atrial pressure; PAPi, pulmonary artery pulsatility index.
Discussion
In this large, multicentre Canadian study of 590 patients with incident PAH, we found that PAPi was not associated with survival when analyzed as a continuous variable. However, when low vs high PAPi, with a cutoff of 5.3, was analyzed, low PAPi was associated with a higher 1-year mortality. The ability of PAPi to discriminate 1-year mortality was no different than that of RAP alone.
This study is the largest to evaluate the clinical significance and prognostic utility of PAPi in a PAH population. The PAPi is a novel hemodynamic parameter of RV-PA coupling that has recently been found to be useful in predicting outcomes in a variety of states influenced by RV function. This parameter predicts RV dysfunction in patients with acute inferior wall myocardial infarction or after left ventricular assist device implantation.9,12,13 A more recent study has reported PAPi to be a significant predictor of mortality and hospitalization in individuals with advanced heart failure.14 Additionally, a lower PAPi was associated with a decreased left ventricular ejection fraction, more severe tricuspid regurgitation, inferior vena cava dilatation, and lower cardiac index. In PAH, the literature on PAPi is limited to small and predominantly single-centre studies. Lim et al. investigated the association between PAPi and survival in a population of 102 patients with PAH from Singapore.10 These patients were recruited over a large time range, from 2003 to 2016, during which therapeutic options and use of combination therapy changed significantly. Another study by Mazimba and colleagues evaluated the association between PAPi and survival in the historical National Institutes of Health (NIH) primary PH database, finding that baseline PAPi was predictive of survival.15 However, a point to note is that the study cohort from this database predates the development of modern PAH therapy and is therefore of limited relevance in the context of modern care of PAH. We limited our population to the modern era of combination therapy use (after the publication of the Ambrisentan and Tadalafil in Patients With Pulmonary Arterial Hypertension (AMBITION) trial and the 2015 European Society of Cardiology/European Respiratory Society guidelines,5,16 making these results more applicable to modern practice.
In our study, PAPi was associated with 1-year mortality only when it was dichotomized. Moreover, many other prognostic parameters were not associated with mortality at 1 year. The primary reason for this discrepancy is likely low statistical power, as only 7% mortality was noted in 1 year. When PAPi was dichotomized, it was associated with mortality, but the association was not stronger than that with mPAP alone. Similar findings were seen in the Lim et al. study.10 We chose a shorter-term follow up of 1 year in our study, understanding that response to PAH therapy would likely be a greater predictor of survival over the long term compared to baseline hemodynamic data. Our analysis adjusted for age, sex, BMI, and functional class at baseline, and produced an AUC for PAPi similar to that seen by Lim and colleagues.10 Interestingly, neither our study nor the Lim et al. study10 showed an association between baseline cardiac index and mortality, despite the fact that it is an established prognostic variable from older studies.17,18 Data from the French PAH registry in the modern era also found that baseline hemodynamic variables were not associated with long-term survival, whereas follow-up hemodynamic variables were.19,20 Together, these data support the concept that prognosis is governed not so much by baseline hemodynamics as by response to therapy. We strongly suspect that differential changes in hemodynamics with initiation of PAH therapy also explain the limited prognostic utility of baseline PAPi and the other hemodynamic parameters tested. However, once decompensation and right-sided heart failure with elevated RA pressures has developed, these may be a signal of worse survival that persists despite initiation of therapy.
This study has some limitations to acknowledge. The design was retrospective and observational, based on registry data, which has inherent limitations and bias. Additionally, lack of follow-up RHC is a limitation, as it was not routinely done in each centre, making it impossible to evaluate associations between survival and follow-up PAPi or change in PAPi after initial therapy. We were not powered to investigate other outcomes of interest, such as lung transplantation or hospital admission for PAH. We did not employ a competing risks model for lung transplantation and mortality, but the number of patients transplanted within 1 year was so low that omitting this model is justifiable. The major strength of our study is its large multicentre nature, with well phenotyped patients who were diagnosed and treated in PAH expert centres. An additional strength is that inclusion of patients was limited to those diagnosed in the modern era of initial combination therapy.
Conclusions
As in other studies, we found that the association between PAPi and survival was modest, and not superior to RA pressure alone. Our findings cast further doubt on whether PAPi is a useful measure in PAH and on the long-term prognostic utility of baseline hemodynamic measures in the era of effective early combination therapy.
Acknowledgments
Ethics Statement
The study was coordinated through the University of British Columbia (H21-03574) and institutional ethics board approval was obtained at each participating centre.
Patient Consent
The authors confirm that patient consent is not applicable to this article. This is a retrospective case report using de-identified data; therefore the IRB did not require consent from the patient.
Funding Sources
The Canadian Pulmonary Hypertension registry was supported by grants from Janssen, United Therapeutics, and Bayer Pharmaceuticals.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 552 for disclosure information.
References
- 1.Parikh V., Bhardwaj A., Nair A. Pharmacotherapy for pulmonary arterial hypertension. J Thorac Dis. 2019;11(suppl 14):S1767–S1781. doi: 10.21037/jtd.2019.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucly A., Weatherald J., Savale L., et al. External validation of a refined four-stratum risk assessment score from the French pulmonary hypertension registry. Eur Respir J. 2022;59 doi: 10.1183/13993003.02419-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zelt J.G.E., Sugarman J., Weatherald J., et al. Mortality trends in pulmonary arterial hypertension in Canada: a temporal analysis of survival per ESC/ERS guideline era. Eur Respir J. 2022;59 doi: 10.1183/13993003.01552-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galie N., Channick R.N., Frantz R.P., et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53 doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galie N., Humbert M., Vachiery J.L., et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 6.Sachdev A., Villarraga H.R., Frantz R.P., et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest. 2011;139:1299–1309. doi: 10.1378/chest.10-2015. [DOI] [PubMed] [Google Scholar]
- 7.Tello K., Dalmer A., Axmann J., et al. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.118.005512. [DOI] [PubMed] [Google Scholar]
- 8.Nie L., Li J., Zhang S., et al. Correlation between right ventricular-pulmonary artery coupling and the prognosis of patients with pulmonary arterial hypertension. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000017369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korabathina R., Heffernan K.S., Paruchuri V., et al. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv. 2012;80:593–600. doi: 10.1002/ccd.23309. [DOI] [PubMed] [Google Scholar]
- 10.Lim Y., Low T.T., Chan S.P., et al. Does pulmonary artery pulsatility index predict mortality in pulmonary arterial hypertension? ESC Heart Fail. 2021;8:3835–3844. doi: 10.1002/ehf2.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 12.Kang G., Ha R., Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. 2016;35:67–73. doi: 10.1016/j.healun.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Morine K.J., Kiernan M.S., Pham D.T., et al. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail. 2016;22:110–116. doi: 10.1016/j.cardfail.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Kochav S.M., Flores R.J., Truby L.K., Topkara V.K. Prognostic impact of pulmonary artery pulsatility index (PAPi) in patients with advanced heart failure: insights from the ESCAPE trial. J Card Fail. 2018;24:453–459. doi: 10.1016/j.cardfail.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Mazimba S., Welch T.S., Mwansa H., et al. Haemodynamically derived pulmonary artery pulsatility index predicts mortality in pulmonary arterial hypertension. Heart Lung Circ. 2019;28:752–760. doi: 10.1016/j.hlc.2018.04.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galie N., Barbera J.A., Frost A.E., et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373:834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin V.V., Shillington A., Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477–1482. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 18.Sitbon O., Humbert M., Nunes H., et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 19.Weatherald J., Boucly A., Chemla D., et al. Prognostic value of follow-up hemodynamic variables after initial management in pulmonary arterial hypertension. Circulation. 2018;137:693–704. doi: 10.1161/CIRCULATIONAHA.117.029254. [DOI] [PubMed] [Google Scholar]
- 20.Weatherald J., Boucly A., Launay D., et al. Haemodynamics and serial risk assessment in systemic sclerosis associated pulmonary arterial hypertension. Eur Respir J. 2018;52 doi: 10.1183/13993003.00678-2018. [DOI] [PubMed] [Google Scholar]