Abstract
Purpose
Although remimazolam is a popular novel anesthetic, there is a lack of data in the literature about current and future trends. Therefore, the aim of this study was to explore emerging trends and potential hotspots of remimazolam research over the past 15 years through bibliometric methods.
Methods
Relevant articles on remimazolam published from 2007 to 2022 and propofol from 1997 to 2001 were retrieved from the Web of Science Core Collection database. Data were collected using Microsoft Excel and graphs were generated with the Bibliometrix package in R software. Visual bibliometric maps were created using VOS viewer and CiteSpace software.
Results
In total, 184 articles were included for analysis. Remimazolam-related research tended to increase, especially from 2020 to 2022. China produced the most publications (327), whereas the USA dominated in quality (h-index = 16). Among institutions, PAION Deutschland GmbH produced the most articles (Np = 21). Similar to initial research and development of propofol, the hotspots of remimazolam research have extended beyond pharmacokinetics and pharmacodynamics to adverse reactions, clinical scenarios, specific populations, and compatible regimens, as confirmed by high numbers of common references and keywords.
Conclusion
Remimazolam research has developed rapidly over the past two years. Remimazolam can achieve faster onset and recovery, and more stable hemodynamics than midazolam or propofol, enabling gradual piloting of applications from endoscopy and general anesthesia to sedation of critical care patients; foreseeing specific population (patients with hepatic or renal impairment and reduced cardiovascular reserve, the elderly, and children) through compatible anesthetics regimens to more optimal and safe. Future studies of remimazolam are likely to include adverse reactions, effects on different organ systems, and identification of monitoring indicators.
Keywords: remimazolam, CNS 7056, bibliometric analysis, applications, specific population, research trends
Introduction
Remimazolam is a novel ultra-short-acting benzodiazepine derivative that acts on γ-aminobutyric acid receptors. As compared to midazolam, remimazolam is more rapidly hydrolyzed into an inactive metabolite (CNS 7054), has a shorter half-life and faster onset, offset by prolonged infusion, and reversal by flumazenil.1 As compared to propofol, remimazolam has superior clinical safety and is less likely to cause hypotension, cardiorespiratory depression, and pain upon injection.1,2
Bibliometrics are mathematical and statistical methods for qualitative and quantitative analyses of publications in various fields to estimate the contributions and collaborations of different countries/regions, institutions, journals, and authors, as well as cluster and burst analyses of keywords and references to identify research hotspots, evaluate academic productivity, and assess directions in clinical research.3,4
Although remimazolam is a popular novel anesthetic, there is a lack of data in the literature about current and future trends. Therefore, the aims of this study were to provide quantitative and visual information on the current use of remimazolam globally, summarize and compare directions of present research, and explore emerging trends.
Materials and Methods
Data Source and Extraction
Relevant articles published from 2007–01-01 to 2022–10-20 were retrieved from the Web of Science Core Collection (WoSCC) database using the keywords “remimazolam” OR “CNS 7056” OR “remimazolam besylate” OR “remimazolam tosilate” OR “remimazolam CNS 7056” OR “CNA-7056” OR “CNS 7056B” OR “CNS 7056BS” OR “GW502056” OR “ONO-2745” OR “HR 7056”. The search was limited to original research and review articles written in English. In addition, two of the authors (Wu and Hu) independently extracted basic information, which included the titles of the articles, abstracts, keywords, names of the authors, institutions, countries/regions, references, journal titles, and years of publication. Discrepancies were resolved by consensus. CiteSpace software (http://cluster.cis.drexel.edu/~cchen/citespace/)5 was used to visualize and analyze trends and patterns among the retrieved data.
Data Analysis and Visualization
Microsoft Excel 2021 (https://www.microsoftstore.com.cn/) was used to categorize the data and the “bibliometrix” package in R (https://cran.r-project.org/web/packages/bibliometrix/)6 was used to generate graphs. The total number of publications (Np), total number of citations (Tc), Hirsch index (h-index), and impact factor (IF) were determined.
VOS viewer 1.6.18 (https://www.vosviewer.com/)7 was used to construct bibliometric maps of clustering, overlay, and density views of items based on differences in distances, sizes, and densities between nodes. The size of the nodes indicates the Np or the frequency of co-occurrence of various items, such as authors, institutions, and keywords,7 where the larger the node, the greater the frequency or research intensity. Nodes of the different cluster are assigned different colors. The total link strength (TLS) is defined as the sum of the link strength of a node with every other node. In this study, the VOS viewer was used for analyses of co-occurrence among (i) authors, (ii) countries/regions, (iii) institutions, (iv) keywords, and (v) keyword clusters.
CiteSpace 6.1.R3 provides visual knowledge maps consisting of nodes and links. A link between nodes indicates a co-occurrence relationship and the thickness of the link indicates the degree of connection.8 To identify the most prominent network structure, all time slices were set to 1 and the “Pathfinder” and “Trim slice network” options were selected. In this study, CiteSpace was used to detect strong citation bursts and the associated timelines.
Data Compared to Propofol
Trends in propofol research over a 15-year period (from 1986, when the drug was first available on the market, to 2001) were determined for reference and comparison with the trends and potential hotspots of remimazolam research. In total, 2402 articles published in English from 1986 to 2001 were retrieved from the WOSCC database.
Results and Analysis
Annual Publications and Trends
In total, 235 articles were initially retrieved from the WOSCC database. Of these, 184 articles published in English from 2007 to 2022 were included for analysis. By April 2023, The total number of citations was 2745. After exclusion of self-citation= 1494, articles remained with an average number of citations of 14.92 and an average h-index of 29.
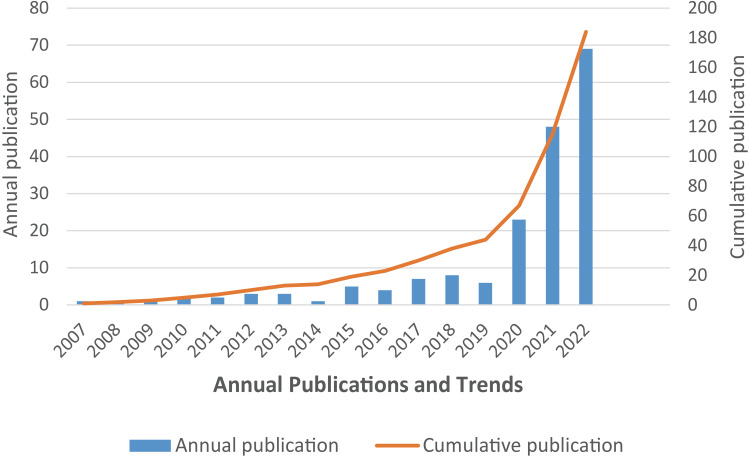
The annual and cumulative Np values were calculated. As shown in Figure 1, the Np related to remimazolam tended to increase from 2007 to 2022. Further classification into two stages revealed that there were only slight increases in the Np from 2007 to 2019, whereas the Np had exponentially increased from 2020 to 2022. The cumulative Np notably increased from the first stage (2007–2019) to the second stage (2020–2022) of 134, especially the annual Np in 2022 of 64, indicating that remimazolam is attracting increased interest by researchers worldwide.
Figure 1.
Trends in the Np annually and cumulatively.
Publications by Countries/Regions
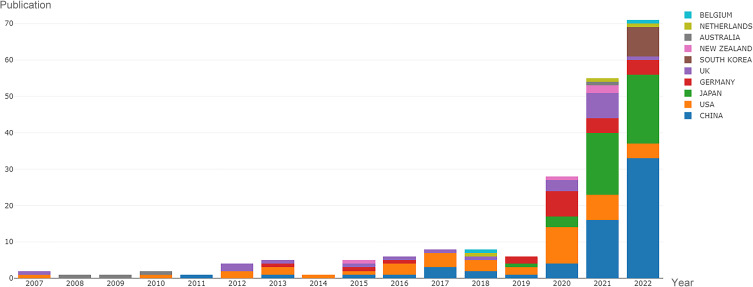
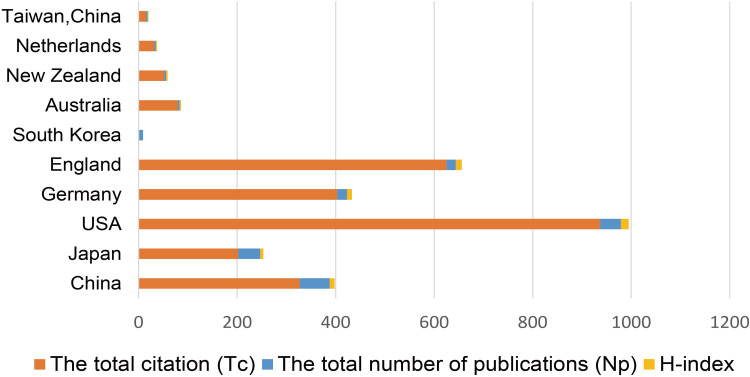
The Np of remimazolam-related articles published from 2007 to 2022 by countries/regions are shown in Figure 2. During this period, authors in China published the most articles (Np = 61, 33.15%), followed by Japan (Np = 45, 24.26%) and the USA (Np = 42, 22.83%). The top 10 countries/regions with the highest total Np, h-index, and Tc values are shown in Figure 3. Although China had the highest Np, the USA had the highest academic productivity and scholarly contributions in the field of remimazolam research.9
Figure 2.
Remimazolam-related articles published from 2007 to 2022 by countries/regions.
Figure 3.
Top 10 countries/regions with the most remimazolam-related articles published from 2007 to 2022.
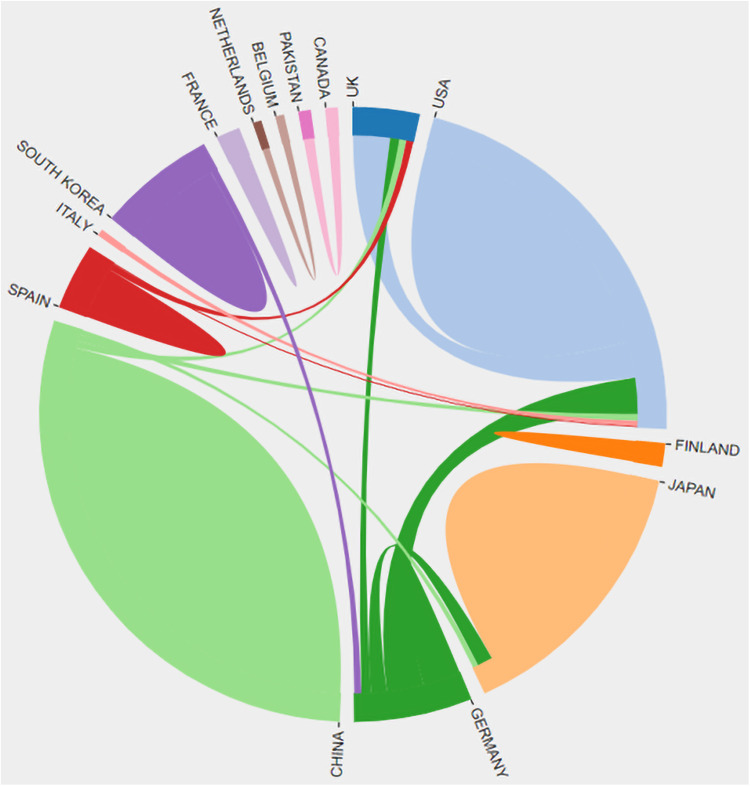
As shown by the co-occurrence network among 12 countries/regions presented in Figure 4, clusters were formed by China and Japan as well as countries in the European Union and the USA. Although the publication frequencies were relatively low, South Korea, Spain, and Italy established cooperation networks with other countries.
Figure 4.
Co-occurrence network among 14 countries/regions.
Analysis of Institutions
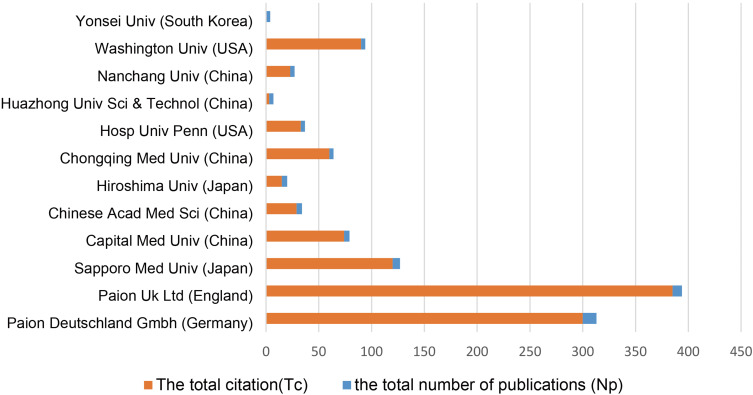
In total, 335 different institutions published remimazolam-related articles from 2007 to 2022. Institutions with ≥ 4 remimazolam-related articles are shown in Figure 5. The top three institutions were PAION Deutschland GmbH (Np = 13), PAION UK Ltd. (Np = 9), and Sapporo Medical University (Np = 7). Interestingly, most of the institutions in the network are located in China (Capital Medical University, Chinese Academy of Medical Sciences, Chongqing Medical University, Huazhong University of Science and Technology, and Nanchang University). Although the Np of these institutions was 4–5, there is strong interest and enthusiasm for remimazolam-related research in China. The Tc values of PAION UK Ltd. and PAION Deutschland GmbH were 385 and 300, respectively, demonstrating the impact this biopharmaceutical company in actively promoting remimazolam research.
Figure 5.
Institutions with ≥ 4 remimazolam-related articles published from 2007 to 2022.
Analysis of Journals
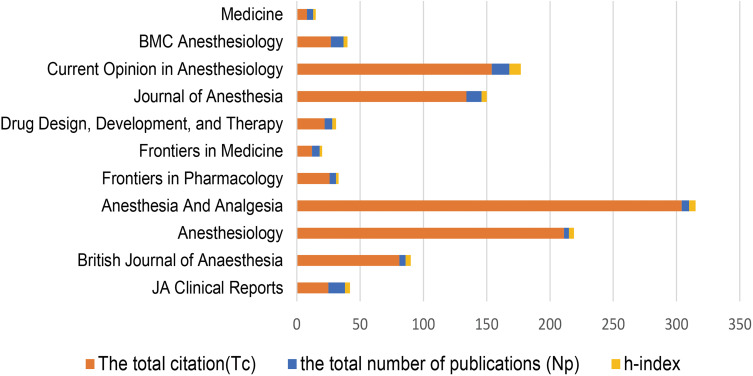
The top three journals with the most publications of remimazolam-related articles were Current Opinion in Anesthesiology (Np = 14), JA Clinical Reports (Np = 13), and BMC Anesthesiology (Np = 12) (Figure 6).
Figure 6.
Top journals with the most remimazolam-related articles.
The 10 most active journals (ranked by Np, IF, JCR quartile, Tc, h-index, and TLS) are listed in Table 1. Among them, Current Opinion in Anesthesiology had the highest h-index and Tc value (9 and 154, respectively), although the IF was only 2.733.
Table 1.
Parameters of the Top Journals with the Most Remimazolam-Related Articles
| Journal | JCR | IF | Np | Tc | h-Index | TLS |
|---|---|---|---|---|---|---|
| Current Opinion in Anesthesiology | Q3 | 2.733 | 14 | 154 | 9 | 69 |
| JA Clinical Reports | / | / | 13 | 25 | 4 | 55 |
| Journal of Anesthesia | Q3 | 2.931 | 12 | 134 | 4 | 120 |
| BMC Anesthesiology | Q3 | 2.376 | 10 | 27 | 3 | 84 |
| Anesthesia And Analgesia | Q1 | 6.627 | 6 | 304 | 5 | 147 |
| Drug Design, Development, and Therapy | Q2 | 4.319 | 6 | 22 | 3 | 57 |
| Frontiers in Medicine | Q1 | 5.058 | 6 | 12 | 2 | 51 |
| British Journal of Anaesthesia | Q1 | 11.72 | 5 | 81 | 4 | 55 |
| Frontiers in Pharmacology | Q1 | 5.988 | 5 | 26 | 2 | 77 |
| Medicine | Q3 | 1.817 | 5 | 8 | 2 | 20 |
| Anesthesiology | Q1 | 9.198 | 4 | 211 | 4 | 112 |
The journals with the highest IF values were the British Journal of Anaesthesia (11.719), followed by Anesthesiology (9.198), Anesthesia and Analgesia (6.627). Each of these three journals are classified as the top publications in the field of anesthesiology or pharmacology (2019 Journal Citation Reports). Anesthesia and Analgesia had the highest Tc (304) from 2007 to 2022.
Journals with the most publications of remimazolam-related articles studies were in the fields of anesthesia and analgesia, clinical translation and pharmacology, and gastroenterology.
Analysis of Authors and Co-Authors
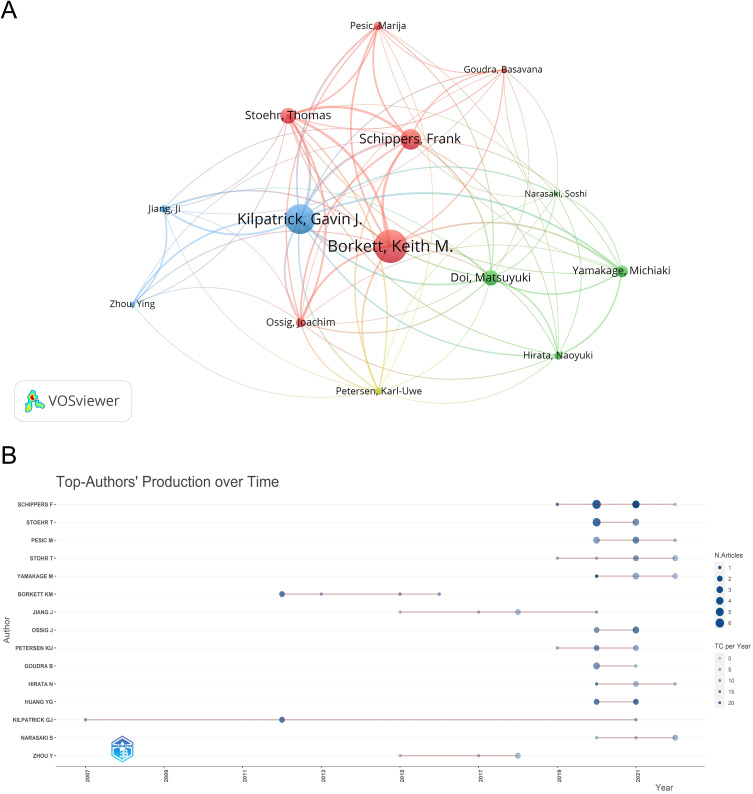
A co-occurrence network of the top 14 authors in terms of citations is shown in Figure 7A. Among them, the top three authors were Keith M. Borkett (Tc = 331), Gavin J. Kilpatrick (Tc = 287), and Frank Schippers (Tc = 173). Notably, Borkett and Schippers are both associated with PAION Deutschland GmbH.
Figure 7.
(A) A co-occurrence network of the top 14 authors in terms of citations. (B) A time graph of remimazolam-related articles by author.
A time graph of remimazolam-related articles by author is shown in Figure 7B, where the name of the author is shown as the vertical coordinate and the year of publication as the horizontal coordinate. The larger the circle, the more publications, and the darker the color, the greater average annual Tc. Gavin J. Kilpatrick is considered a pioneer in remimazolam-related research, while Thomas Stohr, Michiaki Yamakage, and Soshi Narasaki are considered especially enthusiastic authors. Among these authors, Schippers has the highest h-index of 8, in addition to leading Np and Tc values, indicating authority in the field of remimazolam research.
Top Keywords and Keyword Co-Occurrence Clusters
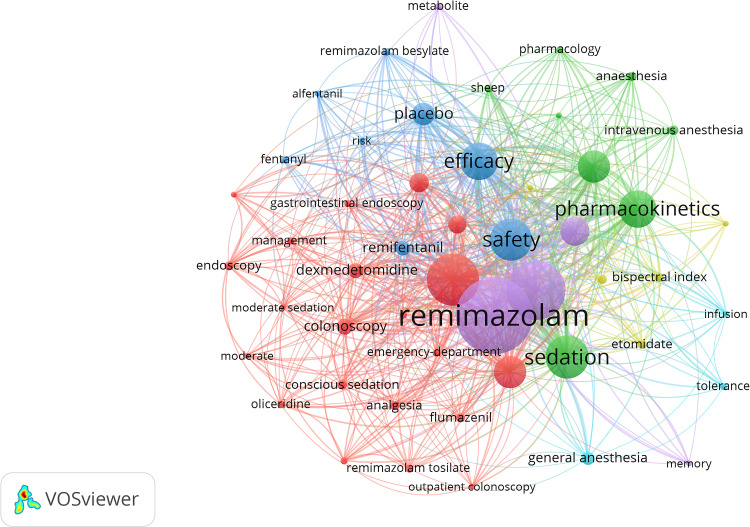
The top 50 occurring keywords were divided into six clusters (Figure 8), where cluster 1 (red) is focused on the areas of endoscopic sedation examination, procedural sedation, and hypotension due to propofol, cluster 2 (green) mainly includes pharmacokinetics and pharmacodynamics, cluster 3 (blue) is mainly related to the use of fentanyl analogs and the safety and efficacy of anesthesia, cluster 4 (yellow) focuses on the depth of anesthesia and the use of induction drugs, cluster 5 (purple) describes the metabolic effects of benzodiazepines, and cluster 6 (cyan) concerns the indications and tolerability of general anesthesia.
Figure 8.
Co-occurrence clusters of the top 50 keywords related to remimazolam.
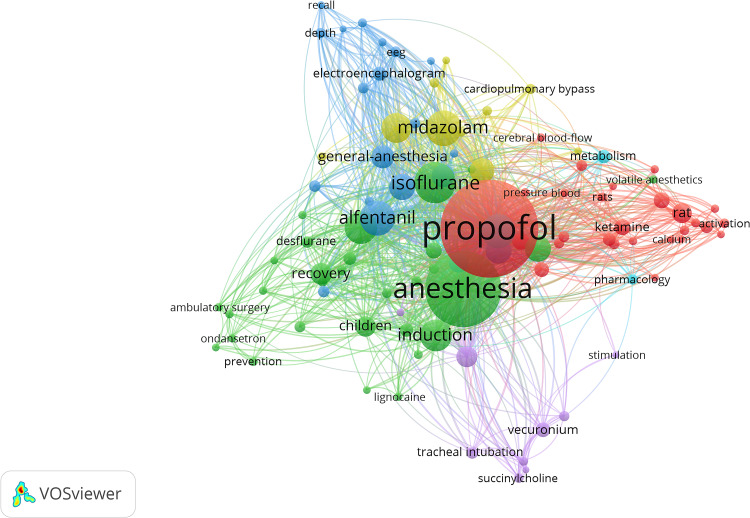
A literature search of propofol was conducted in articles published from 1997 to 2001. Of 6869 keywords, 99 were selected and divided into six clusters. As shown in Figure 9, the keywords in cluster 1 (red) focus on the mechanisms and pathways of propofol through animal experiments and comparisons with other anesthetics, cluster 2 (green) mainly includes the management and medication of short and pediatric surgery, cluster 3 (blue) is mainly related to the use of fentanyl analogs and monitoring of the nervous system during anesthesia, cluster 4 (yellow) is focused on complications associated with benzodiazepine and organ system, cluster 5 (purple) describes mechanical ventilation with the use of muscle relaxants, and cluster 6 (cyan) is focused on pharmacokinetics and pharmacodynamics.
Figure 9.
Co-occurrence clusters of the top 99 keywords related to propofol.
The top 20 keywords with the highest occurrences in the literature related to remimazolam and propofol with the use of TLS as an impact indicator are listed in Table 2 and Table 3, respectively. “Remimazolam” and the alternative chemical forms “remimazolam besylate” and “remimazolam tosilate” were the most frequently occurring keywords (610 + 42 + 37 = 689 occurrences). “Midazolam” (549) was the second most frequent, as the molecular structure coincides with that of remimazolam.
Table 2.
The Top 20 Keywords Related to Remimazolam
| Keyword | TLS | Occurrence | Average Year of Publication | Average Number of Citations |
|---|---|---|---|---|
| Remimazolam | 610 | 130 | 2020 | 8.0462 |
| Midazolam | 549 | 92 | 2020 | 9.7935 |
| Propofol | 483 | 77 | 2020 | 12.9351 |
| Sedation | 354 | 59 | 2020 | 13.9153 |
| Safety | 395 | 57 | 2020 | 8.0351 |
| Efficacy | 339 | 49 | 2020 | 9.7347 |
| Pharmacokinetics | 324 | 49 | 2019 | 12.4082 |
| Pharmacodynamics | 295 | 40 | 2019 | 14.025 |
| Anesthesia | 220 | 39 | 2020 | 8.1026 |
| Benzodiazepines | 199 | 34 | 2019 | 11.3235 |
| Placebo | 192 | 24 | 2021 | 10.5417 |
| Procedural Sedation | 123 | 20 | 2019 | 18.55 |
| Double-Blind | 113 | 18 | 2020 | 12.1111 |
| Colonoscopy | 127 | 15 | 2021 | 8.2667 |
| Remifentanil | 104 | 14 | 2021 | 4.9286 |
| Dexmedetomidine | 80 | 14 | 2020 | 4.6429 |
| General Anesthesia | 35 | 11 | 2021 | 12.7273 |
| Conscious Sedation | 54 | 8 | 2021 | 3.125 |
| Intravenous Anesthesia | 34 | 8 | 2016 | 16.625 |
| Flumazenil | 33 | 7 | 2022 | 1.2857 |
Table 3.
The Top the Top 20 Keywords Related to Propofol
| Keyword | TLS | Occurrence | Average Number of Citations |
|---|---|---|---|
| Propofol | 3690 | 1042 | 40.7582 |
| Anesthesia | 2654 | 736 | 36.5761 |
| Isoflurane | 1389 | 314 | 42.7293 |
| Midazolam | 1127 | 257 | 63.0661 |
| Alfentanil | 1268 | 254 | 49.9291 |
| Pharmacokinetics | 1017 | 248 | 43.7621 |
| Surgery | 918 | 228 | 41.3596 |
| Induction | 960 | 222 | 31.1171 |
| Sedation | 936 | 203 | 57.8079 |
| Humans | 697 | 168 | 40.1964 |
| Nitrous-Oxide | 753 | 167 | 39.4012 |
| Halothane | 703 | 166 | 36.3072 |
| Infusion | 648 | 157 | 38.8726 |
| General-Anesthesia | 566 | 142 | 44.2606 |
| Fentanyl | 683 | 138 | 50.971 |
| Recovery | 597 | 130 | 33.5462 |
| Thiopentone | 521 | 126 | 28.1905 |
| Children | 459 | 121 | 43.314 |
| Pharmacodynamics | 550 | 116 | 49.0603 |
| Rat | 384 | 115 | 42.3304 |
Among the studies related to propofol, the keywords with the highest occurrences were “propofol” (1042), followed by “isoflurane” (314), “midazolam” (257), and “alfentanil” (254).
Co-Cited References with Cluster Analysis and Strongest Citation Burst
The co-cited reference analyses provide the authoritative direction of remimazolam research. Graphs, cluster networks, and burst graphs of co-citations were generated by CiteSpace of 184 and 2402 references cited in studies of remimazolam (Figure 10) and propofol (Figure 11), respectively.
Figure 10.
(A) Co-citation graph of remimazolam-related articles. (B) Cluster network graph of the remimazolam-related articles. (C) Top 10 references with the strongest citation bursts.
Figure 11.
Cluster network graph of references in propofol-related articles.
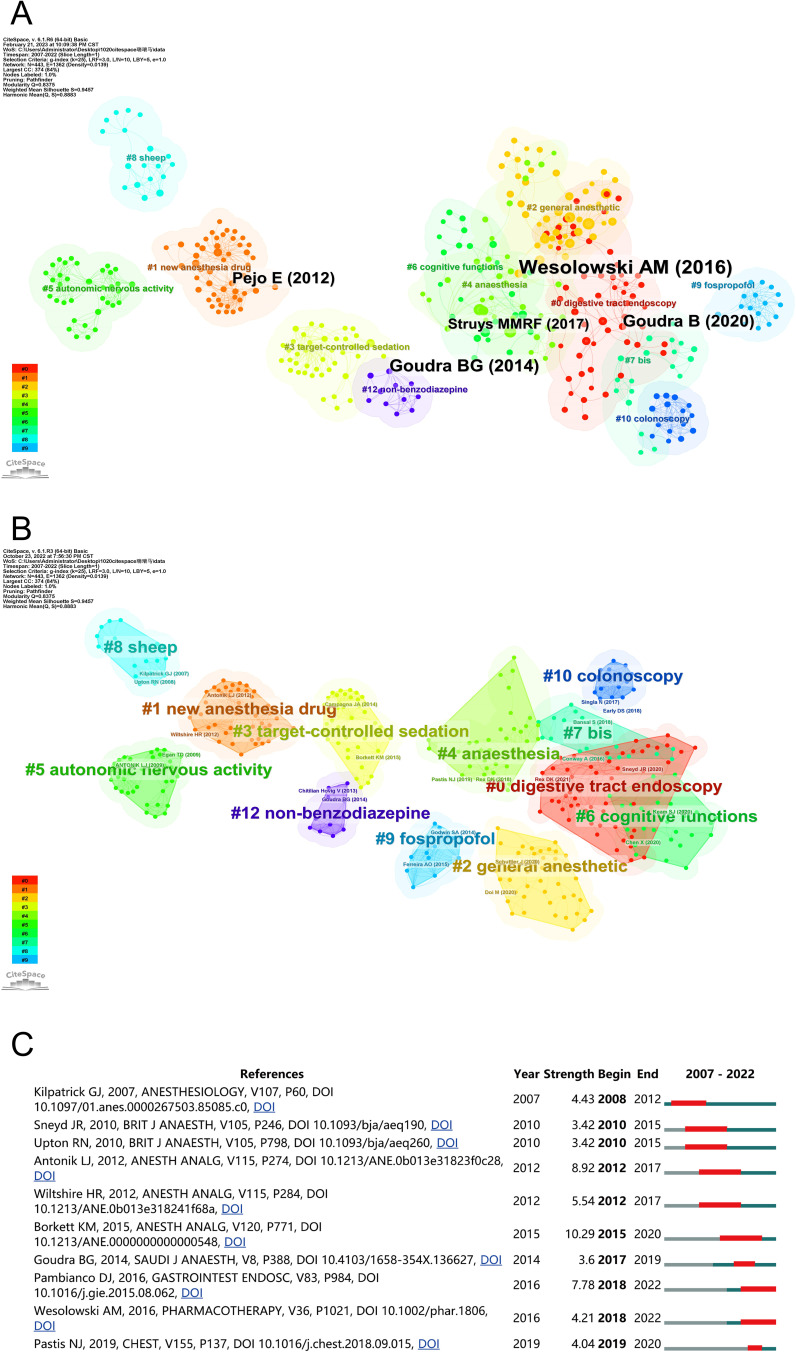
A graph of co-citations in remimazolam-related articles (Figure 10A) contained 443 nodes and 1362 links. The references with the highest centeredness (critical nodes) were Wesolowski AM (2016), Goudra BG (2014), Goudra B (2020), Pejo E (2012), and Struys MMRF (2017).
A cluster network graph of remimazolam-related articles was divided into 10 main clusters (Figure 10B). This study focused on four categories: category 1, which consisted of 0#gastrointestinal endoscopy and 10#colonoscopy; category 2, which comprised 1#novel anesthetic drugs and 9#propofol; category 3, which included 2#general anesthesia and 3#goal-controlled sedation; and category 4, which was composed of 5#autonomic nervous activity, 6#cognitive function, and 7#bis. Top 10 references with the strongest citation bursts are presented in the remimazolam network shown in Figure 10C. Articles not yet published are indicated by light blue lines, whereas published articles are indicated by dark blue lines, and the burst period is indicated by a red line. The use of “STRENGTH” refers to burst intensity.
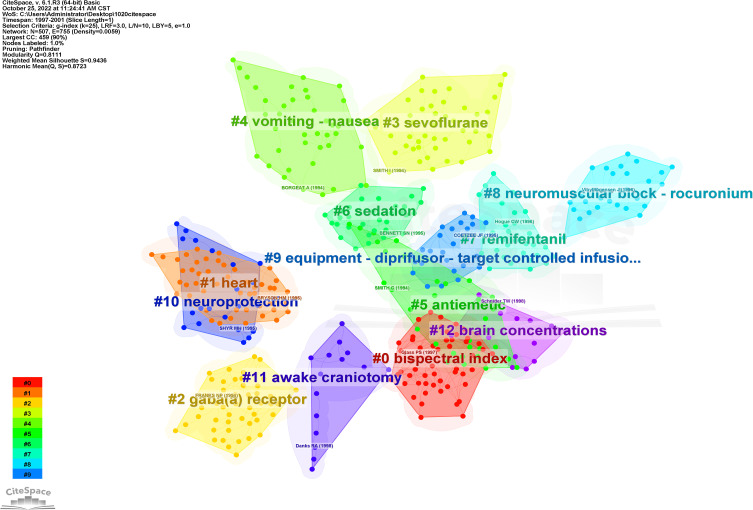
The cluster network of references in propofol-related articles (Figure 11) were divided into 12 main clusters. The propofol cluster labels are most similar to remimazolam cluster labels included #0 bis, #2 gaba(a) receptor, #6 sedation, and#9 equipment-diprifusor-target controlled infusion. The different labels were broadly divided into three categories: propofol with different anesthetics or myorelaxants (#3 sevoflurane, #7 remifentanil, and #8 neuro muscular blockade-rocuronium); adverse reactions of propofol (#4 nausea-vomiting and #5 antiemetic); and the effect and monitoring of propofol on different organ systems (#1 cardiac, #10 neuroprotection, #11 awake craniotomy, and #12 brain concentrations).
Keywords with the Strongest Citation Burst and Timeline
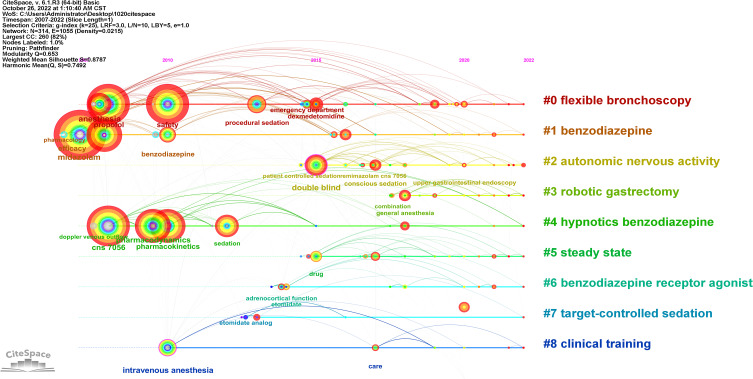
The timeline graph presented in Figure 12 reflects changes to remimazolam research over time. The horizontal changes from cool to warm represent the years 2007–2022 and each circle of the tree represents a node keyword, where the larger the node, the greater the co-citation frequency. The node color from cool to warm represents the years 2007–2022. The pink outer circle of the node indicates high centrality. Each horizontal axis represents the connection and development of trends among keywords within the cluster. The right vertical axis shows the different clustering labels.
Figure 12.
Timeline graph of keywords.
The keywords related to remimazolam were divided into eight clusters. Earlier studies mostly focused on pharmacology, remimazolam-related mechanisms, and comparisons of remimazolam to midazolam (see #1 Benzodiazepine). Later studies focused on the pharmacodynamics and pharmacokinetics of remimazolam used for procedural sedation (see #0 Analgesics and Sedation, #2 Procedural sedation, #4 Efficacy and safety). Then, the direction of remimazolam-related research shifted to adverse reactions, addressing the efficacy and safety of remimazolam compared to propofol, dexmedetomidine, and etomidate in adrenal cortical function after prolonged infusion (see #0 Analgesics and Sedation, #6 New formulations, #7 Target-controlled sedation). Subsequently, the research focus shifted to the clinical application of remimazolam in bronchoscopy, hysteroscopy, conscious sedation, patient-controlled sedation, general anesthesia, and nonoperating room anesthesia (see 2# Procedural sedation, 3 # General anesthesia-hemodynamic stability, 5# Steady state, 8# Clinical training). Finally, the latest research has shifted to special uses of remimazolam and the dosage or regimen of target populations receiving care in the intensive care unit (ICU), emergency department and special populations.
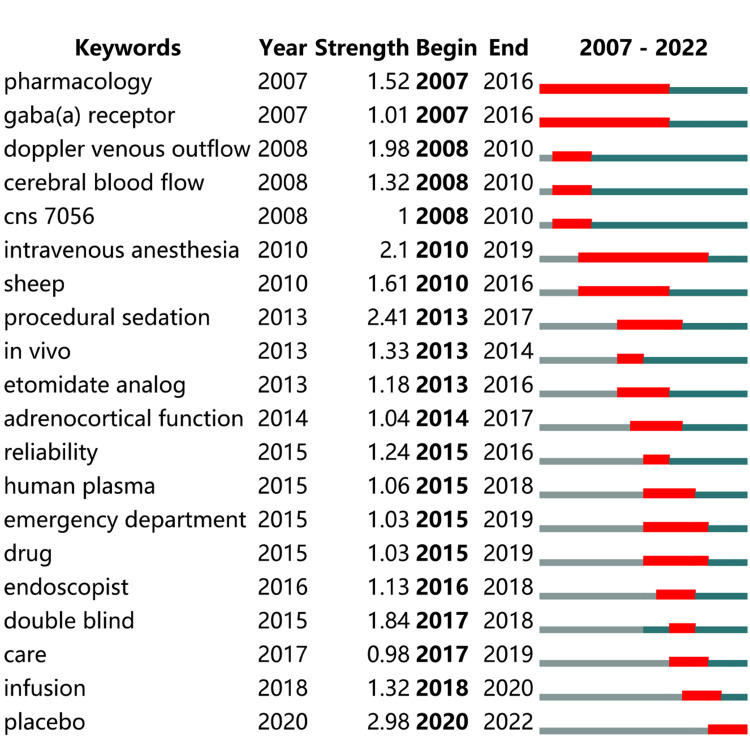
Next, keywords with the strongest citation bursts were identified. As shown in Figure 13, “intravenous anesthesia”, as the infusion mode of remimazolam, has the longest burst duration, followed by “pharmacology” and “gaba(a) receptor”. In terms of burst strength, “placebo use” and “procedural sedation” were ranked first and second, indicating the importance of both in clinical applications.
Figure 13.
Keywords with the strongest citation bursts.
Discussion
Visual bibliometric analysis of remimazolam-related articles published from 2007 to 2022 show that the greatest proportion of articles originated in China, while articles from the USA dominated in quality. Among institutions, PAION Deutschland GmbH was the largest contributor. Analysis of the most co-cited references and keywords demonstrated that, similar to initial studies and development of propofol, the hotspots of remimazolam research ranged from pharmacokinetics and pharmacodynamics to adverse reactions, clinical scenarios, specific populations, and compatible regimens. As the first bibliometric analysis of the global status, hotspots, and directions of remimazolam research, this study provides relevant references and new insights.
Overview of the Results
In total, 184 original research and review articles related to remimazolam (Tc = 1957) were included for analysis. The frequencies of annual and cumulative publications tended to increase, especially from 2020 to 2022, which is probably related to the approval of remimazolam for procedural sedation in China (December 2019), along with approval from the United States Food and Drug Administration (July 2020) and the European Medicines Agency (March 2021). In addition, remimazolam was approved for general anesthesia in Japan (January 2020) and South Korea (January 2021).
The top three countries with the highest Np of remimazolam-related articles were China, the USA, and Japan. In terms of publication quality, the USA ranked first (h-index = 16, Tc = 937), while China ranked third in productivity (h-index = 10) and fourth in citation impact (Tc = 327). In regard to institutions, PAION UK Ltd., PAION Deutschland GmbH, and Sapporo Medical University produced the most articles, while Washington University (Tc = 90), Harvard Medical School (Tc = 78), and the Hospital of the University of Pennsylvania (Tc = 33) had the highest inter-institutional collaboration, frequency of citations, and academic productivity. Based on the above, collaborative communication among multiple institutions and countries is essential for continuation of in-depth and innovation research of remimazolam.
Clusters and Bursts of Co-Citations
Cluster and burst analyses identified the 10 most co-cited reference. Burst analysis clarified hotspots in remimazolam research from 2007 to 2022. Initial research explored the pharmacokinetics and pharmacodynamics of remimazolam in animal experiments with rodents and sheep.1,10 Over the past five years, research has focused on the pharmacological characteristics of remimazolam as compared to other sedatives, such as midazolam and propofol. Other studies investigated the clinical efficacy and safety of remimazolam for use in endoscopy and procedural sedation, as well as potential use in clinical practice for general anesthesia.11,12 Similar to research of propofol, future studies of remimazolam are likely to include adverse reactions, effects on different organ systems, and identification of monitoring indicators.
Comparative Analyses of Benzodiazepines and Remimazolam Based on Keywords
Midazolam is metabolized in the liver via cytochrome P450 enzymes,12 whereas remimazolam is hydrolyzed into the inactive metabolite CNS7054 by tissue carboxylesterase and is rapidly cleared from the body after prolonged or high-dose injection.13 Additionally, remimazolam circumvents the risk of rebound sedation with midazolam after flumazenil antagonism.12 Remimazolam is reported to achieve good sedation without any adverse events both for digestive endoscopy14,15 and bronchoscopy,16 while the onset, recovery, and discharge times of remimazolam are superior to midazolam, especially for postoperative neuropsychiatric recovery. Moreover, the safety of remimazolam for procedural sedation is similar for high- and low-risk patients.15 A recent meta-analysis concluded that remimazolam at 0.10–0.15 mg/kg provides approximately equivalent sedation as midazolam at 0.075 mg/kg.17
Comparative Analyses of Non-Benzodiazepines and Remimazolam Based on Keywords
The sedation success rate of remimazolam was 97.34% for gastroscopy and 96.91% for colonoscopy, which were not inferior to those of propofol,18 although the incidences of adverse events of propofol, such as pain at the injection site, hypotension, respiratory depression, and decreased SpO2, were significantly lower, demonstrating that remimazolam is a safer alternative.19 In addition, Hysteroscopic studies reported a 100% success rate for sedation using remimazolam,20 which can be combined with remifentanil without a neuromuscular blocking agent for general intravenous anesthesia.21 For maintenance of general anesthesia, Remimazolam was non-inferior to propofol.22,23 Pharmacokinetic/pharmacodynamic modeling showed that remimazolam for induction (6 mg/kg/h), maintenance (1 mg/kg/h), and post-surgical sedation (0.25 mg/kg/h) was optimal, regardless of ASA class or subject of sensitivity.24
A Phase I study of ICU patients showed that remimazolam at 0.125–0.150 mg/kg/h was rapid and safe for mild to moderate short-term sedation with hemodynamic stability.25 Remimazolam is also safe and effective for long-term sedation (>24 h) of ICU patients. However, these two studies had small sample sizes, thus, the findings should be interpreted with caution.26
Recent research of remimazolam vs propofol has shifted from pre- and intraoperative application to postoperative recovery. A previous study based on a standard patient-reported outcome measure (15-item Quality of Recovery [QoR]) reported that recovery from general intravenous anesthesia with remimazolam was not inferior to propofol, while pain intensity and the requirement for analgesics were decreased.27 Similar results were obtained with the 40-item QoR. In contrast, a recent retrospective study found higher incidences of nausea and vomiting after anesthesia with remimazolam than propofol.28 Moreover, a recent randomized controlled trial reported a significantly lower first-day QoR score for remimazolam vs propofol, which was attributed to physical comfort and emotional state.29 However, further studies are needed to assess the impact of remimazolam on postoperative recovery, especially postoperative nausea and vomiting.28
As an inevitable new trend, compatible regimens with remimazolam and esketamine can maintain hemodynamics and significantly lower the incidences of adverse reactions and complications during gastroscopy,30 endoscopic retrograde cholangiopancreatography,31 and induction of general anesthesia. In addition, remimazolam combined with alfentanil is reportedly a safer and more effective alternative to propofol for procedural sedation with fewer adverse events.32,33 Hence, future studies are warranted to investigate the safety of remimazolam combined with different sedatives and analgesics, especially in terms of respiratory and circulatory complications.
Advantages of Remimazolam for Specific Populations
For critical care patients with hepatic or renal impairment and reduced cardiovascular reserve, remimazolam is not metabolized independently of the liver and kidneys and has excellent hemodynamic and circulatory stability.1,13 Thus, remimazolam might be beneficial for critical care patients. A recent study found that plasma clearance of remimazolam was comparable in terms of renal impairment, as no unexpected remimazolam-related adverse events were identified in patients with renal or hepatic impairment.34 In addition, a pilot study reported the use of remimazolam as an induction agent with timely infusion of vasopressors for elderly patients with severe aortic stenosis,35 which was later confirmed in a randomized controlled trial.36 A retrospective analysis found that remimazolam is relatively safe for aortic valve replacement surgery.37 However, due to the limited number of studies, this conclusion is questionable.
In elderly patients, remimazolam as an intravenous anesthetic can achieve faster neuropsychiatric recovery than midazolam.14,16 In another study, there was no significant difference in postoperative cognitive function between the low-dose remimazolam and propofol groups.38 However, further studies are needed to assess the effects of remimazolam on postoperative cognitive function (eg, memory, attention, executive function, and postoperative delirium). Notably, the bispectral index (BIS) is a good indicator of propofol sedation depth, but is insensitive to benzodiazepines.39 Therefore, surgeons should not solely rely on the BIS for monitoring of brain function, but also consider the beta ratio of electroencephalography as an alternative.40,41
Several case reports have reported the safety of remimazolam for general anesthesia in pediatric patients with compromised health.42–44 Remimazolam at 0.2 mg/kg can prevent delirium in children under sevoflurane anesthesia.45 However, further clinical trials are needed to determine whether remimazolam is advantageous for pediatric patients during examinations and surgery.
Conclusion
In conclusion, as compared to traditional reviews, a variety of bibliometric tools (CiteSpace, VOS viewer, Bibliometrix package in R) were applied in this study to more objectively and multi-dimensionally assess the use of remimazolam. Inevitably, there were some limitations to this study that should be addressed. First, the study was limited by the specific search strategy settings of CiteSpace software and the selection of articles solely from the WOSCC database for visual analysis, while ignoring other search engines, such as the Web of Science and Scopus. Since remimazolam research is an emerging hotspot in the field of anesthesiology, the number of studies continues to rapidly increase. Therefore, relevant studies were likely omitted. Second, the study was limited to original research and review articles published in English, thus excluding potentially important articles published in other languages. Therefore, future studies should overcome this limitation by broadening the scope of the literature search. As compared to midazolam, remimazolam can achieve faster onset and recovery and as compared to propofol, remimazolam can better maintain hemodynamics and lower the incidence of adverse events, although effect on QoR remains controversial. Current scenarios for the application of remimazolam are gradually going beyond endoscopy and general anesthesia to sedation of ICU patients. In the near future, remimazolam will be likely applied to specific populations, such as patients with hepatic or renal impairment and reduced cardiovascular reserve, the elderly, and children. Lastly, remimazolam can be safely combined with compatible anesthetic regimens.
Funding Statement
This work was supported by National Natural Science Foundation of China (grant no. 81772131).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a Novel Ultra–short-acting benzodiazepine. Anesthesiology. 2007;107(1):60–66. doi: 10.1097/01.anes.0000267503.85085.c0 [DOI] [PubMed] [Google Scholar]
- 2.Keam SJ. Remimazolam: first Approval. Drugs. 2020;80(6):625–633. doi: 10.1007/s40265-020-01299-8 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Jin Q, Fang H, et al. Analytic network process: academic insights and perspectives analysis. J Clean Prod. 2019;235:1276–1294. doi: 10.1016/j.jclepro.2019.07.016 [DOI] [Google Scholar]
- 4.Ninkov A, Frank JR, Maggio LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ. 2022;11(3):173–176. doi: 10.1007/s40037-021-00695-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724–728. [PMC free article] [PubMed] [Google Scholar]
- 6.Aria M, Cuccurullo C. bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. 2017;11(4):959–975. doi: 10.1016/j.joi.2017.08.007 [DOI] [Google Scholar]
- 7.van Eck NJ, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C. Predictive effects of structural variation on citation counts. J Am Soc Inf Sci. 2012;63(3):431–449. doi: 10.1002/asi.21694 [DOI] [Google Scholar]
- 9.Jones T, Huggett S, Kamalski J. Finding a way through the scientific literature: indexes and measures. World Neurosurg. 2011;76(1):36–38. doi: 10.1016/j.wneu.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 10.Upton RN, Somogyi AA, Martinez AM, Colvill J, Grant C. Pharmacokinetics and pharmacodynamics of the short-acting sedative CNS 7056 in sheep. Br J Anaesth. 2010;105(6):798–809. doi: 10.1093/bja/aeq260 [DOI] [PubMed] [Google Scholar]
- 11.Pambianco DJ, Borkett KM, Riff DS, et al. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016;83(5):984–992. doi: 10.1016/j.gie.2015.08.062 [DOI] [PubMed] [Google Scholar]
- 12.Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36(9):1021–1027. doi: 10.1002/phar.1806 [DOI] [PubMed] [Google Scholar]
- 13.Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115(2):274–283. doi: 10.1213/ANE.0b013e31823f0c28 [DOI] [PubMed] [Google Scholar]
- 14.Rex DK, Bhandari R, Desta T, et al. A Phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–437.e6. doi: 10.1016/j.gie.2018.04.2351 [DOI] [PubMed] [Google Scholar]
- 15.Rex DK, Bhandari R, Lorch DG, Meyers M, Schippers F, Bernstein D. Safety and efficacy of remimazolam in high risk colonoscopy: a randomized trial. Dig Liver Dis. 2021;53(1):94–101. doi: 10.1016/j.dld.2020.10.039 [DOI] [PubMed] [Google Scholar]
- 16.Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155(1):137–146. doi: 10.1016/j.chest.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 17.Dou D, Feng Y, Jiang L. Efficiency and safety of remimazolam and midazolam in digestive endoscopic sedation: systematic review and meta-analysis. Dig Endosc. 2022;34(3):653. doi: 10.1111/den.14219 [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Zhao X, Yin P, Bao X, Tang H, Kang X. Profile of remimazolam in anesthesiology: a narrative review of clinical research progress. Drug Des Devel Ther. 2022;16:3431–3444. doi: 10.2147/DDDT.S375957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Wang J, Xu X, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. 2020;12(8):4594–4603. [PMC free article] [PubMed] [Google Scholar]
- 20.Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021;12:690875. doi: 10.3389/fphar.2021.690875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park I, Cho M, Nam SW, Hwang JW, Do SH, Na HS. Total intravenous anesthesia induced and maintained by a combination of remimazolam and remifentanil without a neuromuscular blocking agent: a prospective, observational pilot study. BMC Anesthesiol. 2022;22(1):237. doi: 10.1186/s12871-022-01779-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34(4):491–501. doi: 10.1007/s00540-020-02776-w [DOI] [PubMed] [Google Scholar]
- 23.Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–553. doi: 10.1007/s00540-020-02788-6 [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Leonowens C, Ivaturi VD, et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66:109899. doi: 10.1016/j.jclinane.2020.109899 [DOI] [PubMed] [Google Scholar]
- 25.Tang Y, Yang X, Shu H, et al. Remimazolam besylate for sedation of postoperative patients in intensive care units: a Phase I, open label, dose-finding study. Chin Med J. 2022;135(17):2134–2136. doi: 10.1097/CM9.0000000000002243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Y, Yang X, Yu Y, et al. Remimazolam besylate versus propofol for long-term sedation during invasive mechanical ventilation: a pilot study. Crit Care. 2022;26(1):279. doi: 10.1186/s13054-022-04168-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JY, Lee HS, Kim JY, et al. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: a randomized non-inferiority trial. J Clin Anesth. 2022;82:110955. doi: 10.1016/j.jclinane.2022.110955 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki Y, Kawashima S, Makino H, Doi M, Nakajima Y. A comparison of remimazolam and propofol for postoperative nausea and vomiting: a propensity score-matched, observational, single-center cohort study. Korean J Anesthesiol. 2022;76:143–151. doi: 10.4097/kja.22441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao Y, Guo J, Yuan J, Zhao E, Yang J. Quality of recovery after general anesthesia with remimazolam in patients’ undergoing urologic surgery: a randomized controlled trial comparing remimazolam with propofol. DDDT. 2022;16:1199–1209. doi: 10.2147/DDDT.S359496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu C, Ren J, Guo X, Qian J. Effects of remimazolam combined with esketamine anesthesia on circulatory and respiratory function during painless gastroenteroscopy. Contrast Media Mol Imaging. 2022;2022:1079099. doi: 10.1155/2022/1079099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi X, Xu W, Li A. The clinical application of remimazolam benzenesulfonate combined with esketamine intravenous anesthesia in endoscopic retrograde cholangiopancreatography. Biomed Res Int. 2022;2022:5628687. doi: 10.1155/2022/5628687 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Xu C, He L, Ren J, et al. Efficacy and safety of remimazolam besylate combined with alfentanil in painless gastroscopy: a randomized, single-blind, parallel controlled study. Contrast Media Mol Imaging. 2022;2022:7102293. doi: 10.1155/2022/7102293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong SA, Guo Y, Liu SS, et al. A randomized, controlled clinical trial comparing remimazolam to propofol when combined with alfentanil for sedation during ERCP procedures. J Clin Anesth. 2023;86:111077. doi: 10.1016/j.jclinane.2023.111077 [DOI] [PubMed] [Google Scholar]
- 34.Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127(3):415–423. doi: 10.1016/j.bja.2021.05.027 [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi T, Sento Y, Kamimura Y, Tsuji T, Kako E, Sobue K. Remimazolam for induction of anesthesia in elderly patients with severe aortic stenosis: a prospective, observational pilot study. BMC Anesthesiol. 2021;21(1):306. doi: 10.1186/s12871-021-01530-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T, Lai T, Chen J, et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021;9(5):e00851. doi: 10.1002/prp2.851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyoshi H, Watanabe T, Kido K, et al. Remimazolam requires less vasopressor support during induction and maintenance of general anesthesia in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement: a retrospective analysis from a single center. Biomed Res Int. 2022;2022:6386606. doi: 10.1155/2022/6386606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan Y, Ouyang W, Tang Y, Fang N, Fang C, Quan C. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. J Gastroenterol Hepatol. 2022;37(3):576–583. doi: 10.1111/jgh.15761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibrahim AE, Taraday JK, Kharasch ED. Bispectral index monitoring during sedation with sevoflurane, midazolam, and propofol. Anesthesiology. 2001;95(5):1151–1159. doi: 10.1097/00000542-200111000-00019 [DOI] [PubMed] [Google Scholar]
- 40.Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–651. doi: 10.1097/ALN.0000000000003103 [DOI] [PubMed] [Google Scholar]
- 41.Eisenried A, Schuettler J, Lerch M, Ihmsen H, Jeleazcov C. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers part II. Pharmacodynamics of electroencephalogram effects. Anesthesiology. 2020;132(4):652–666. doi: 10.1097/ALN.0000000000003102 [DOI] [PubMed] [Google Scholar]
- 42.Horikoshi Y, Kuratani N, Tateno K, et al. Anesthetic management with remimazolam for a pediatric patient with Duchenne muscular dystrophy. Medicine. 2021;100(49):e28209. doi: 10.1097/MD.0000000000028209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petkus H, Willer BL, Tobias JD. Remimazolam in a pediatric patient with a suspected family history of malignant hyperthermia. J Med Cases. 2022;13(8):386–390. doi: 10.14740/jmc3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamadori Y, Yamagami Y, Matsumoto Y, et al. General anesthesia with remimazolam for a pediatric patient with MELAS and recurrent epilepsy: a case report. JA Clin Rep. 2022;8(1):75. doi: 10.1186/s40981-022-00564-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, Lin C, Chen S, Huang Y, Cheng Q, Yao Y. Remimazolam for the prevention of emergence delirium in children following tonsillectomy and adenoidectomy under sevoflurane anesthesia: a randomized controlled study. Drug Des Devel Ther. 2022;16:3413–3420. doi: 10.2147/DDDT.S381611 [DOI] [PMC free article] [PubMed] [Google Scholar]