Abstract
Goldenhar Syndrome is a rare congenital disorder characterized by hemifacial microsomia. Although select mutations have been mapped for this disorder, the genetic etiologies in the majority of cases remain unknown. A recent clinical report of a Goldenhar Syndrome patient identified a homozygous missense mutation in FRK , a gene associated with various types of cancer. In this work, we precisely modeled the disease-associated missense mutation in the C. elegans FRK ortholog src-2 , using CRISPR/Cas9 gene editing, and investigated the physiological role of this mutation and the src-2 gene. In addition, we generated a conserved variant in src-1 ( FYN ortholog) to assess the functional redundancy of the conserved variant. The putative pathogenic variants src-1 (Val190Ile) or src-2 (Val170Ile) caused only subtle phenotypes, suggesting that these mutations alone are not sufficient to explain the facial deformities observed in the Goldenhar Syndrome patient. However, the src-2 (Val170Ile) mutant exhibited reduced brood size and moderately enhanced embryonic developmental phenotypes, including epidermal and neuronal patterning defects, in the src-1 (RNAi) condition, indicating that the src-2 (Val170Ile) locus could play a supportive role during developmental processes. Overall, however, these studies showed that src-1 /FYN is essential for regulating embryogenesis and morphogenesis, while src-2 /FRK is largely dispensable for normal embryonic development, suggesting FYN , not FRK , is the dominant non-receptor protein kinase during embryonic development in C. elegans .
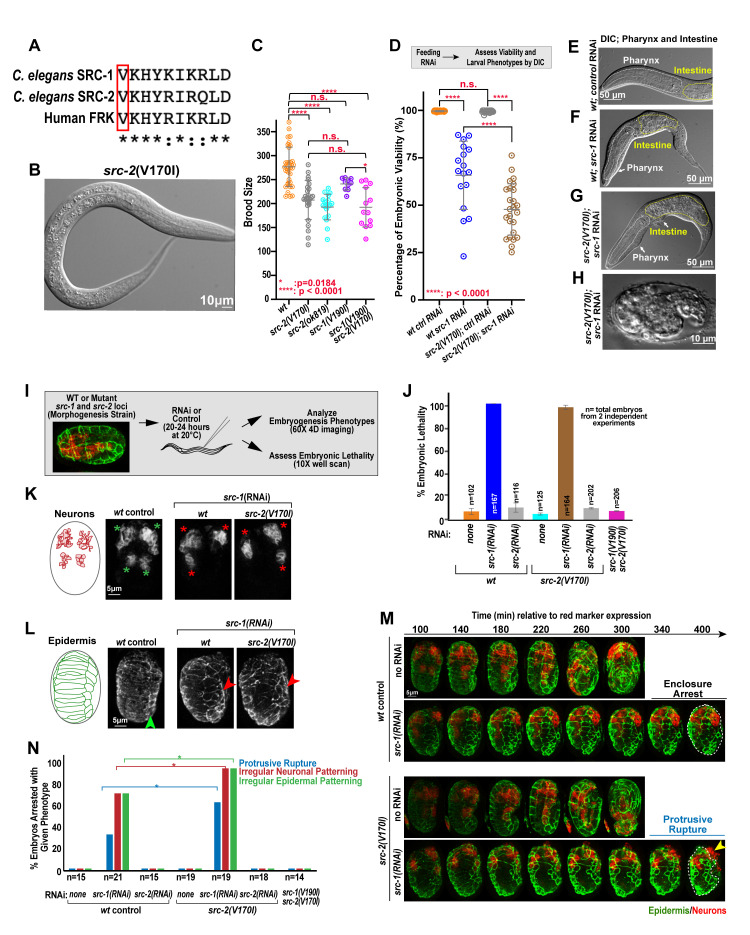
Figure 1. src-2 (Val170Ile ) mutant exhibits reduced brood size and enhances embryonic morphogenesis defects in src-1 (RNAi) conditions .
(A) Sequence alignment of the conserved valine residue of C. elegans SRC-1 , SRC-2 , and human FRK. (B) Representative DIC images of src-2 (Val170Ile) mutant. (C) Quantification of brood size of wild type and missense mutants of src-1 and src-2 . (D) Percent embryonic viability after feeding control or src-1 (RNAi) in wild-type, and missense mutants of src-1 and src-2 . (E-H) Representative DIC images of wild type and defective larvae and embryos in missense mutants or following src-1 (RNAi) . (I) Schematic outlining the 4D embryonic developmental imaging regime. (J) Plot highlights embryonic lethality data captured by 10x whole well-scan, following overnight imaging regime. (K) Schematic and images show initial patterning of neuronal precursors in wild-type control embryos and after src-1 (RNAi) in control and src-2 (Val170Ile) mutant embryos. Green asterisks highlight patterning of neurons into four quadrants (expected), and red asterisks highlight abnormal neuronal number and/or position. (L) Images show epidermal patterning from the dorsal surface in wild-type control embryos, and after src-1 (RNAi ) in control and src-2 (Val170Ile) mutant embryos. Green arrow points to uniform row of seam cells along the lateral surface in control embryo, whereas red arrow indicates disorganized population of seam cells. (M) Images depict time series during enclosure and elongation (controls) and arrest at enclosure or protrusive rupture (yellow arrow) at enclosure in control or src-1 (RNAi) embryos (respectively). Epidermis shown in green, and neurons shown in red. Time is relative to initial red marker expression. (N) Graph quantifies the relative frequency of embryonic phenotypes observed in all tested conditions. Asterisks indicate P<0.05 by one tailed chi-square test in (M). P-values: * = 0.0184 (C); **** <0.0001 (C-D) (one-way ANOVA test). Asterisks indicate P<0.05 by one tailed chi-square test (N). Scale bars are indicated in each graphic.
Description
Fyn-related Src family tyrosine kinase (FRK) belongs to the family of non-receptor protein kinases, serving as a signaling intermediary during G1 and S phase to negatively regulate cell proliferation (Hua et al., 2014; Lee et al., 1994). Dysfunction of FRK gene has been linked to various types of cancers, including breast and brain cancers (Goel and Lukong, 2016). In a recent clinical report, a homozygous missense mutation of the FRK gene, NM_002031.3 (FRK): c.484G>A (p.Val162Ile) ( https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV001162776.2 ), was identified in a Goldenhar Syndrome patient presenting with hemifacial microsomia (HFM). HFM is a common birth defect and although several chromosomal abnormalities and causal gene variants have been identified, genetic etiologies in the majority of cases remain unknown. The phenotypic features associated with this disease suggest that defects may arise from abnormities in cranial neural crest cells (CNCC) (Wang et al., 2020), which are an ectoderm-derived set of cells that migrate to the pharyngeal arches and facilitate craniofacial development (Bhatt et al., 2013; Siismets and Hatch, 2020). To investigate the physiological effect of this putative pathogenic variant, we precisely modeled the Val170Ile variant in the C. elegans FRK ortholog src-2 , using CRISPR/Cas9 gene editing. Since a sequence alignment indicated that src-1 (FYN ortholog) is the paralog of src-2 in C. elegans , we also generated the conserved variant Val190Ile in src-1 to characterize the functional redundancy of the variant ( Fig. 1A ).
Homozygous animals carrying src-1 (Val190Ile) mutant alone, src-2 (Val170Ile ) mutant alone, and src-1 (Val190Ile); src-2 (Val170Ile) double mutant fail to exhibit obvious morphological defects in hatched animals ( Fig. 1B ). However, animals carrying the src-2 (Val170Ile) mutation have reduced brood sizes, similar to those of a null src-2 mutant, src-2 ( ok819 ) , which bears a full-length deletion of the src-2 gene ( Fig. 1C ). The src-1 (Val190Ile) variant alone does not cause a significant reduction of brood size compared to wild type ( Fig. 1C ). Additionally, the src-1 (Val190Ile) src-2 (Val170Ile) double mutant shows a similar brood size when compared to the src-2 (Val170Ile) mutant alone, suggesting that the src-1 (Val190Ile) variant does not have functional redundancy with the src-2 (Val170Ile) variant ( Fig. 1C ) with respect to its role regulating brood size.
While the src-2 (Val170Ile ) mutant alone does not result in morphological or embryonic lethality defects ( Fig. 1B ), depletion of src-1 , by feeding RNAi, in either wildtype or src-2 (Val170Ile) is associated with a variety of tissue morphogenesis defects, including abnormal pharyngeal, intestinal, and epidermal morphogenesis ( Fig. 1D -H ); larval morphogenesis defects and embryonic lethality phenotypes are enhanced when src-1 (RNAi) is combined with src-2 (Val170Ile ) mutant condition ( Fig. 1D -H ). These findings are consistent with prior work suggesting src-1 is the predominant FRK/SRC during embryonic development in C. elegans ; lethality studies have shown essential roles for src-1 , but not src-2 during embryogenesis (Bei et al., 2002; Hirose et al., 2003) and single-cell sequencing data has shown that src-1 is widely expressed across all tissues during development, while src-2 expression is restricted to developing pharynx and adjacent regions (Packer et al., 2019).
To further assess the nature of the embryonic defects when FRK gene expression is disrupted, we employed a previously described automated 4D imaging regime, which enables collection of semi-high throughput embryogenesis data and allows for profiling of embryonic developmental phenotypes following perturbation (Wang et al., 2019) ( Fig. 1I -N). For this experiment, we used a morphogenesis reporter strain (Wang et al., 2019) that reports specifically on ectodermally derived tissue, since the HFM defect in humans likely arises from mis-patterning of this tissue during development. Specifically, this strain expresses a GFP-tagged epithelial junction marker in the epidermis (P dlg-1 Δ7 :: DLG-1 ::GFP) and a mCherry-tagged plasma membrane marker in a subset of the developing neurons (P cnd-1 ::mCherry::PH). In this strain, or in the same strain background carrying the src-2 (Val170Ile) or src-1 (Val190Ile) ; src-2 (Val170Ile) double mutation, created by CRISPR gene editing, we depleted src-1 and src-2 by injection RNAi ( Fig. 1J -N ). Consistent with our previous results, the src-2 (Val170Ile) mutant alone and the src-1 (V190I) src-2 (Val170Ile) double mutant embryos do not exhibit significant defects or embryonic lethality in this strain background ( Fig. 1J ), suggesting that the patient isolated mutation is not sufficient to trigger developmental defects in C. elegans . Next, we performed src-1 (RNAi) , by injection, in wild type or mutant src-2 (Val170Ile) conditions to examine if these mutations can enhance src-1 (RNAi) phenotypes. We found that src-1 (RNAi) alone or in combination with the src-2 (Val170Ile) mutation results in penetrant embryonic lethality, whereas control and src-2 (RNAi) depletion conditions do not ( Fig 1J ).
To understand the nature of the embryonic lethality in src-1 (RNAi) and determine if enhancement is observed when the src-2 missense mutation is combined with this condition, we analyzed the high-resolution 4D embryonic development data; this revealed that most embryos arrest at or near comma stage in src-1 (RNAi) depleted embryos in control or the src-2 (Val170Ile) strain (62% and 74% respectively) ( Fig. 1N ). We did not observe gross abnormalities in the proliferation of cells, prior to arrest, in the morphogenesis reporter strain in any background. Similarly, the same perturbation conditions were tested using a germ-layer reporter strain, which marks endoderm, ectoderm, and mesoderm cells (Wang et al., 2019). We found the nuclear counts for each germ layer at the comma stage to be largely comparable to controls. However, despite largely normal nuclear counts, the arrested embryos under src-1 (RNAi) conditions frequently exhibit enclosure defects, disrupted germ-layer organization, and irregular neuronal and hypodermal patterning ( Fig. 1K -N ), suggesting that tissue mis-patterning may underpin the observed enclosure defects. While both src-1 (RNAi) in control embryos and in the src-2 (Val170Ile) strain tended to result in defects during epidermal enclosure, src-1 (RNAi) in combination with src-2 (Val170Ile) was more likely to result in embryos that undergo a dramatic protrusive rupture event, whereby the enclosing epidermal tissue retracts and releases or partially releases neuronal tissue (33% vs 63% respectively) ( Fig. 1M, N ). A similar uptick in prevalence was observed in irregular neuronal and epidermal patterning in src-1 (RNAi) in the src-2 (Val170Ile) strain, relative to src-1 (RNAi) alone ( Fig. 1K ). During development, neuronal precursors are established in four quadrants in control embryos, with precise symmetry along the anterior-posterior axis. However, in src-1 (RNAi) control embryos and in the src-1 (RNAi) , src-2 (Val170Ile) strain condition, abnormal patterning phenotypes were observed in 71% and 95% of the imaged embryos (respectively); both conditions frequently exhibit three asymmetrical loci instead of the expected 4 symmetrical loci (compare red to green asterisks, Fig. 1K, N ). Similarly, epidermal patterning in control embryos is characterized by an organized strip of dorsal hypodermal cells and a single row of seam cells (arrows) that stretch along each of the lateral surfaces during enclosure. In contrast, in src-1 (RNAi) and in the src-1 (RNAi) , src-2 (Val170Ile) condition, a disorganized epidermis is observed, with ill-defined dorsal hypodermis and seam cells (71% and 95%, Fig. 1L, N ). The co-occurrence of neuronal and epidermal asymmetrical patterning defects in these conditions may indicate a general disruption of ectoderm-derived tissues or may suggest a feedback mechanism between neuronal and epidermal tissue morphogenic processes in the src-1 (RNAi) condition. Altogether, developmental phenotypes, including severe neuronal patterning, epidermal patterning, and protrusive rupture phenotypes were enhanced in the src-1 (RNAi) src-2 (Val170Ile) combined condition compared to src-1 (RNAi) controls alone by 24%, 24%, and 30%, respectively (P= 0.0263, P= 0.0263, and P= 0.0296 by one-tailed chi-square test) ( Fig. 1N ). The src-2 (Val170Ile) allele modestly enhances the src-1 (RNAi) phenotype suggesting that SRC-2 has a role in supporting the function of its paralog SRC-1 during embryonic development. Alternatively, we cannot rule out the possibility that the brood size effects in src-2 (Val170Ile) may synergize with src-1 (RNAi) in unexpected ways to manifest in developmental defects, due to altered loading kinetics of the injected RNA into forming embryos.
Our studies indicate that src-1 /FYN is an essential gene in regulating embryogenesis and morphogenesis, whereas src-2 /FRK is largely dispensable for normal embryonic development, consistent with prior work (Bei et al., 2002) . This suggests that FYN, not FRK, is the dominant non-receptor protein kinase during development in C. elegans . We find that the developmental defects observed in the src-1 (RNAi) condition are largely due to asymmetric mis-patterning of ectodermal tissues, including both the epidermis and neurons. The putative pathogenic variants src-1 (Val190Ile) or src-2 (Val170Ile) caused only subtle phenotypes, which suggests that these mutations are not sufficient to explain the facial deformities observed in the Goldenhar Syndrome patient. However, we cannot rule out the possibility that the human FYN/FRK could have acquired new molecular interactions that are only required in human facial tissues that are specifically disrupted by the mutation. Nevertheless, the src-2 (Val170Ile) mutant exhibits reduced brood size and moderately enhanced embryonic developmental phenotypes in the src-1 (RNAi) condition. Thus, we cannot rule out that the src-2 (Val170Ile) locus could play a supportive role during developmental processes.
Methods
4D imaging was conducted as previously described (Wang, Ochoa, Khaliullin 2019). Briefly, L4 stage animals (strains used OD1719 , OD1689 , AG605 , AG606 , and AG607 ) were either not injected or were injected with dsRNA, manufactured in house, directed at src-1 (oligos: TAATACGACTCACTATAGGGCAATGTGATCATCCGAATC, AATTAACCCTCACTAAAGGGATACGGAACCTGTCCCTTT) or src-2 (oligos: TAATACGACTCACTATAGGCGCTGTGAAAAAGCTAAAGG, AATTAACCCTCACTAAAGGTGAGCAGGATTCCAAAACTC) 20-22 hours prior to imaging. The injected dsRNA were used in the panel (J-M). Embryos were dissected from gravid adults in ice cold M9 + 0.1mg/ml tetramisole hydrochloride and transferred to 384-well glass bottom plate, which was kept on ice until wells were loaded and ready to be imaged. Embryos were imaged at 18°C by 2 micron steps, every 20 minutes for approximately 10 hours on a CV1000 confocal scanner box, equipped with a microlens-enhanced dual Nipkow spinning disk (Yokogawa Electric Corporation) and a 60×1.35NA U-PlanApo objective. Imaging was performed in a temperature-controlled room and data collection was done using CellVoyager software (Yokogawa Electric Corporation). Following overnight imaging, a whole well brightfield scan was performed at 10× to capture embryonic lethality and larval phenotypes. For more details on image acquisition see Wang, Ochoa, Khaliullin 2019. Individual embryos were cropped from the broader field using a custom embryo cropping algorithm (10, 11) and developmental arrest points and phenotypic defects were manually scored. Embryonic lethality was assessed by scoring unhatched embryos from 10×post-imaging whole well scans as previously described (10, 11). The feeding constructs for RNAi targeting src-2 was selected from the Ahringer library, while the src-1 (RNAi ) feeding construct was kindly gifted by Dr. Erin Cram. The targeting insert of the src-1 (RNAi) feeding plasmid were amplified by two primers: F400 5' aggctagcgagagtgaagaatggtacg 3', and R1449 5' agagacagcattggcatacggtagcc (Cram et al., 2006). The src-1 (RNAi) feeding construct was used in the panel (D, F-H). RNAi bacteria were cultured until log phase was reached, and then spread onto MYOB plates supplemented with 1mM IPTG and 25 μg/ml carbenicillin. The seeded plates were incubated overnight before conducting the brood size assay. To knock down the expression of the target genes src-1 and src-2 , mid-L4 hermaphrodites were transferred onto the IPTG-induced RNAi plates. Animals were then grown on RNAi plates at 20°C for 60 hours for brood size and other assays. All DIC images were acquired using a spinning disk confocal system equipped with a Photometrics Prime 95B EMCCD camera, a Yokagawa CSU-X1 confocal scanner unit, and a Nikon 60X 1.2 NA water objective.
Reagents
N2 : Bristol (wild type)
AG538 : src-2 ( av233 ) [Val170Ile] I
AG605 : src-2 ( av233 ) [Val170Ile] I ltSi249 [pOD1274/pSW098; P dlg-1 delta7:: dlg-1 -GFP:: unc-54 -3'UTR; cb-unc-119 (+)]I; ltSi511 [pOD2983/pSW207;P cnd-1 ::mCherry-PH:: unc-54 _3'UTR; cb-unc-119 (+)]II
AG606 : src-2 ( av233 ) [Val170Ile] I; stIs10389 [ pha-4 ::TGF(3E3)::GFP::TY1::3xFLAG inserted into fosmid WRM0617dE06 as C-terminal protein fusion]; ltSi539 [pOD1519/pSW224; P dlg-1 delta7::mCherry:: his-72 :: unc-54 _3’UTR; P cnd-1 ::mCherry:: his-72 :: unc-54 _3’UTR; cb-unc-119 (+)]II; ltSi507 [pOD1492/pSW201; Phlh1::GFP:: his-72 ::tbb-2_3’UTR, Phlh-1::mCherry:: his-72 ::tbb-2_3’UTR; cb-unc-119 (+)]IV
AG757 : src-1 ( av298 ) [Val190Ile] I
AG625 : src-1 ( av298 ) [Val190Ile] I src-2 ( av233 ) [Val170Ile] I
AG607 : src-1 ( av298 ) [Val190Ile] I src-2 ( av233 ) [Val170Ile] I; ltSi249 [pOD1274/pSW098; P dlg-1 delta7:: dlg-1 -GFP:: unc-54 -3'UTR; cb-unc-119 (+)]I; ltSi511 [pOD2983/pSW207;P cnd-1 ::mCherry-PH:: unc-54 _3'UTR; cb-unc-119 (+)]II
OD1689 : ltSi249 [pOD1274/pSW098; Pdlg1delta7:: dlg-1 -GFP:: unc-54 -3'UTR; cb-unc-119 (+)]I; ltSi511 [pOD2983/pSW207;P cnd-1 ::mCherry-PH:: unc-54 _3'UTR; cb-unc-119 (+)]II
OD1719 : stIs10389 [ pha-4 ::TGF(3E3)::GFP::TY1::3xFLAG inserted into fosmid WRM0617dE06 as C-terminal protein fusion]; ltSi539 [pOD1519/pSW224; P dlg-1 delta7::mCherry:: his-72 :: unc-54 _3’UTR; P cnd-1 ::mCherry:: his-72 :: unc-54 _3’UTR; cb-unc-119 (+)]II; ltSi507 [pOD1492/pSW201; Phlh-1::GFP:: his-72 ::tbb-2_3’UTR, Phlh-1::mCherry:: his-72 ::tbb-2_3’UTR; cb-unc-119 (+)]IV
Acknowledgments
Acknowledgments
We thank the Caenorhabditis Genetics Center, which is funded by National Institutes of Health Office of Research Infrastructure Programs (P40OD010440), for providing the strains mentioned above in this study.
Funding Statement
This work was supported by Intramural Research Program of National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (XB, CT, and AG). This work was supported by a grant from the NIH to R.G. and K.O. (GM147265). K.O. received salary from the Ludwig Institute for Cancer Research.
References
- Bei, Y., Hogan, J., Berkowitz, L.A., Soto, M., Rocheleau, C.E., Pang, K.M., Collins, J., and Mello, C.C. (2002). SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev Cell 3 , 113-125.Bhatt, S., Diaz, R., and Trainor, P.A. (2013). Signals and switches in Mammalian neural crest cell differentiation. Cold Spring Harb Perspect Biol 5 .Cram J. E., Shang H., Schwarzbauer J.E. (2006). A systematic RNA interference screen reveals a cell migration gene network in C. elegans. J Cell Sci. 119, 4811-8. Goel, R.K., and Lukong, K.E. (2016). Understanding the cellular roles of Fyn-related kinase (FRK): implications in cancer biology. Cancer Metastasis Rev 35 , 179-199.Hirose, T., Koga, M., Ohshima, Y., and Okada, M. (2003). Distinct roles of the Src family kinases, SRC-1 and KIN-22, that are negatively regulated by CSK-1 in C-elegans. Febs Lett 534 , 133-138.Hua, L., Zhu, M., Song, X., Wang, J., Fang, Z., Zhang, C., Shi, Q., Zhan, W., Wang, L., Meng, Q. , et al. (2014). FRK suppresses the proliferation of human glioma cells by inhibiting cyclin D1 nuclear accumulation. J Neurooncol 119 , 49-58.Khaliullin, R.N., Hendel, J.M., Gerson-Gurwitz, A., Wang, S., Ochoa, S.D., Zhao, Z., Desai, A., Oegema, K., and Green, R.A. (2019). A Semi-high-throughput Imaging Method and Data Visualization Toolkit to Analyze C. elegans Embryonic Development. J Vis Exp.Lee, J., Wang, Z., Luoh, S.M., Wood, W.I., and Scadden, D.T. (1994). Cloning of FRK, a novel human intracellular SRC-like tyrosine kinase-encoding gene. Gene 138 , 247-251.Packer, J.S., Zhu, Q., Huynh, C., Sivaramakrishnan, P., Preston, E., Dueck, H., Stefanik, D., Tan, K., Trapnell, C., Kim, J. , et al. (2019). A lineage-resolved molecular atlas of C. elegans embryogenesis at single-cell resolution. Science 365 .Siismets, E.M., and Hatch, N.E. (2020). Cranial Neural Crest Cells and Their Role in the Pathogenesis of Craniofacial Anomalies and Coronal Craniosynostosis. J Dev Biol 8 .Wang, S., Ochoa, S.D., Khaliullin, R.N., Gerson-Gurwitz, A., Hendel, J.M., Zhao, Z., Biggs, R., Chisholm, A.D., Desai, A., Oegema, K. , et al. (2019). A high-content imaging approach to profile C. elegans embryonic development. Development 146 .Wang, Y., Ping, L., Luan, X., Chen, Y., Fan, X., Li, L., Liu, Y., Wang, P., Zhang, S., Zhang, B. , et al. (2020). A Mutation in VWA1, Encoding von Willebrand Factor A Domain-Containing Protein 1, Is Associated With Hemifacial Microsomia. Front Cell Dev Biol 8 , 571004.