Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Blissus insularis (Hemiptera: Heteroptera: Blissidae) for the European Union (EU) territory. B. insularis, known in the US as the southern chinch bug, primarily feeds on St. Augustine grass (Stenotaphrum secundatum, Poaceae, subfamily Panicoideae). This is a lawn grass grown in warm, tropical and subtropical regions of the world and which is widely grown in the southern US and also used in southern EU as a lawn and amenity grass. Adults and nymphs aggregate to feed at the base of the grass. B. insularis occurs in the southern continental US, Hawaii, Guam, Mexico, Central and South America, and across the Caribbean. In the EU, B. insularis was first detected in Portugal in 2019, where following a national survey, it has now been found in 10 municipalities across the central and southern parts of the country. The pathway for entry into Portugal is unknown. B. insularis is not a regulated pest in the EU. It could further enter and spread within the EU via the import and movement of host plants for planting. S. secundatum is vegetatively propagated because seed is largely sterile. Many Poaceae plants for planting are prohibited from entering the EU, other than some ornamental perennial grasses. Whether S. secundatum is considered an ornamental grass within phytosanitary legislation is not clear. Host availability and climate suitability suggest that southern EU regions extending from the Atlantic coast of Portugal through the Mediterranean would be suitable for B. insularis establishment. The introduction of B. insularis to such areas of the EU would likely cause impacts to St. Augustine grass, as already seen in Portugal. Measures to prevent further entry and spread are available. Options to reduce the impact of established populations are also available. B. insularis satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest.
Keywords: pest risk, plant health, plant pest, quarantine, southern chinch bug, St. Augustine grass
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. The finding of the non‐regulated pest Blissus insularis in the Union territory and its observed impact thus far in the outbreak area has been discussed.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to prepare a pest categorisation for Blissus insularis, including a detailed analysis of the host range of the pest and the pathways of its introduction and spread.
1.2. Interpretation of the Terms of Reference
B. insularisis is to be subject to pest categorisation to determine whether it fulfils the criteria of a potential Union quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform EU decision making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/2072. Particular attention should be given to identifying hosts and pathways for introduction and spread. If B. insularis fulfils the criteria to be potentially listed as a Union quarantine pest, risk reduction options will be identified.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on B. insularis was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Papers relevant for the pest categorisation were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online), the CABI databases and scientific literature databases as referred above in Section 2.1.1.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt to TRACES in May 2020.
GenBank was searched to determine whether it contained any nucleotide sequences for B. insularis which could be used as reference material for molecular diagnosis. GenBank® (www.ncbi.nlm.nih.gov/genbank/) is a comprehensive publicly available database that as of August 2019 (release version 227) contained over 6.25 trillion base pairs from over 1.6 billion nucleotide sequences for 450,000 formally described species (Sayers et al., 2020).
2.2. Methodologies
The Panel performed the pest categorisation for B. insularis following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013).
The criteria to be considered when categorising a pest as a potential Union quarantine pest (QP) is given in Regulation (EU) 2016/2031 Article 3 and Annex I, Section 1 of the Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met the Panel uses its best professional judgement (EFSA Scientific Committee, 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
Table 1.
Pest categorisation criteria under evaluation, as derived from Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest (article 3) |
|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed. |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in and spread within, the EU territory? If yes, briefly list the pathways for entry and spread. |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests' introduction have an economic or environmental impact on the EU territory? |
| Available measures (Section 3.6 ) | Are there measures available to prevent pest entry, establishment, spread or impacts? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. |
The Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs, and make a judgement about potential likely impacts in the EU. Whilst the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Article 3 (d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for quarantine pest status. Assessing social impact is outside the remit of the Panel.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and/or to be transmissible?
Yes. The identity of the species is established and Blissus insularis Barber is the accepted name.
Blissus insularis is an insect known in the US as the southern chinch bug. It is in the Order Hemiptera, Suborder Heteroptera and Family Blissidae. In Portuguese it is known as percevejo‐do‐sul‐americano (EPPO, online).
The EPPO code 1 (Griessinger and Roy, 2015; EPPO, 2019) for this species is: BLISIN (EPPO, online).
3.1.2. Biology of the pest
In southern Florida B. insularis is active all year round with multiple overlapping generations. In cooler northern Florida during the winter, adults will shelter around the roots and base of their favoured host, Stenotaphrum secundatum (St. Augustine grass) and will enter a temporary dormant condition during the coldest periods. In Alabama, adults overwinter in leaf litter (Eden and Self, 1960). Adults emerge from grass tussocks and leaf litter in the spring and mate after a few days (Eden and Self, 1960). Females usually lay between 100 and 300 eggs over the course of their adulthood which lasts around 10 weeks (Kerr, 1966) but varies depending on the temperature. Eggs are laid on S. secundatum close to the soil surface (Eden and Self, 1960); between 75% and 85% of eggs hatch (Kerr, 1966). In southern Florida during the summer, eggs hatch after about 8–10 days, whilst in the winter it may take a month before eggs hatch. There are normally five nymphal instars. Nymphs and adults feed on phloem of hosts just above and below the soil surface.
B. insularis prefer host turf in open sunny areas (Vazquez and Buss, 2006; Reinert et al., 2011) and form aggregations consisting of nymphs and adults, both males and females. The chemical ecology of this aggregative behaviour has been studied by Addesso et al. (2012), but it is not clearly understood. Aggregations can reach over 2,000 individuals per 0.1 m2 (Reinert and Kerr, 1973). As the hosts on which they feed die, the aggregations move to adjacent hosts and patches of dead grass will be created (see Section 3.1.5). Aggregations of nymphs and adults can be found at the boundary of patches where they feed.
Of note, adults can either have long or short wings (Reinert and Kerr, 1973; Cherry, 2001). Long‐winged females produce fewer eggs and oviposit at a slower rate than short‐winged females; the proportion of long‐winged females increases as population density increases (Cherry and Wilson, 2003). Only long‐winged adults are known to fly.
In Florida, B. insularis grows well during warm, damp summer months and infestations peak in early July (Kerr, 1966; Woods, 2021). Table 2 lists some important features of the life history strategy of B. insularis at two temperatures.
Table 2.
Important features of the life history strategy of Blissus insularis at two temperatures (Source: Kerr, 1966)
| Life stage | Temperature | |
|---|---|---|
| 21.1°C | 28.3°C | |
| Eggs | ||
|
24.6 | 8.8 |
| Nymphs | ||
|
68.9 | 25.5 |
| Adult female pre‐oviposition period | ||
|
14.2 | 5.6 |
3.1.3. Host range/species affected
St. Augustine grass (S. secundatum) is the host most seriously harmed by B. insularis, although other grasses are fed upon without noticeable damaged being caused (Kerr, 1966; Reinert et al., 2011). Development of B. insularis on grasses other than S. secundatum is generally poor (Leonard, 1966; Slater, 1976; Reinert et al., 2011). A comprehensive study of host plant relationships within the Blissidae (Slater, 1976) lists the grasses which B. insularis:
Feeds and breeds on (S. secundatum, Cynodon dactlyon),
Only feeds on (Panicum repens, P. maximum, P. bartowense), or
Has only been found on (Axonopus compresses, Digitaria decumbens, Eremochla ophiuroides).
Reinert et al. (2011) conducted glasshouse feeding experiments to determine the susceptibility of 24 cultivars of eight grass genera. Three cultivars of S. secundatum (‘Raleigh’, ‘Texas Common’ and ‘Captiva’) were the most susceptible (Appendix A).
Kerr (1966) notes that when B. insularis is reported feeding on other grasses, the grasses are usually close to patches or lawns of S. secundatum where large populations of B. insularis have fed and depleted their favoured host.
Appendix A is a compilation of breeding hosts, feeding plants and the grasses on which B. insularis has been found on, based on reports in the literature. A supplementary table in Appendix A summarises results from Reinert et al. (2011).
3.1.4. Intraspecific diversity
Adults exhibit diversity in wing length, as noted above.
3.1.5. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes. Infestations cause visible symptoms and samples can be collected using conventional ecological field techniques, such as suction sampling and flotation of soil samples. Detailed morphological descriptions of the species are available to allow taxonomic identification. Molecular methods are also available.
Detection
Infestations of B. insularis can be detected following the appearance of symptoms such as yellow or brownish spots in lawns of S. secundatum. An infestation is recognisable as a brown dead patch with a yellow border that expands as the infestation moves outward to infest new plants (Addesso et al., 2012). However, such symptoms are not diagnostic and could also be caused by root rot and other diseases, nematodes, abiotic disorders and grass feeding insect pests. To confirm the presence of B. insularis, specimens should be collected and identified.
Specimens can be collected using the flotation method (Edmund, 1978) whereby samples of soil are placed into containers of water and insect fauna, including B. insularis, floats to the water surface. Specimens can also be collected using suction samplers (Edmund, 1978; MacLeod et al., 1994).
Samples should be taken from discoloured patches of S. secundatum, particularly at the edges of a patch where nymphs and adults may be actively feeding (Woods, 2021).
Identification
Leonard (1968) provides a key to adult Blissus found in the eastern US. Slater (1979) provides a key to world genera of Blissidae, previously known as a subfamily within Lygaeidae (Henry, 1997). Based on detailed descriptions in Slater and Baranowski (1990) and Anderson et al. (2006) the species can be identified taking into account the adults' body size, pubescence, general colour patterns, the colour of the posterior and anterior lobe of the pronotum and mouthpart morphology (Lima et al., 2021).
Molecular methods are available to identify B. insularis. Lima et al. (2021) describe the protocol used to identify B. insularis in Portugal, based on the amplification of the mitochondrial cytochrome C oxidase subunit I gene (COI). There are multiple accessions in Genbank.
Description
Eggs: small (less than 0.2 mm long), oval, with four small projections at one end. Eggs are white when first laid then become amber and finally red before hatching (Wilson, 1929; Eden and Self, 1960).
Nymphs: there are usually five nymphal instars. The first is yellow, approximately 0.8 mm long. The second is reddish with yellow legs ~ 1.4 mm long with a white band across the abdomen. The third and fourth instar are yellowish with a white band and are ~ 1.9–2.1 mm long, the final instar is brown–black with a white band and is ~ 3 mm long (Eden and Self, 1960; Leonard, 1968).
Adults: adults are black, 1 mm wide and vary in length from approximately 3 mm (Leonard, 1968; Lima et al., 2021) to approximately 5 or 6 mm (Wilson, 1929; Eden and Self, 1960). The wings are white with a black spot on the edge of the forewings. The legs and the bases of the antennae are red (Wilson, 1929). Adults exhibit wing polymorphism and have either long wings which reach almost to the end of the abdomen, or short wings which extend only about half the length of the abdomen (Kerr, 1966; Cherry and Wilson, 2003).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
B. insularis is a Neotropical species mainly found across the southern US and in Mexico, Central and South America. It also occurs in Bermuda and the Caribbean as well as in Hawaii and Guam (Leonard, 1966; Slater, 1979; EPPO, online; Figure 1).
Figure 1.

Global distribution of Blissus insularis (Source: EPPO Global Database (EPPO, online) accessed on 9 June 2023)
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed.
Yes, B. insularis is present in the EU; it has been reported from Portugal where it has a restricted distribution. B. insularis is considered to be not widely distributed in the EU.
Lima et al. (2021) report how B. insularis was first detected in Portugal following reports of damage in St. Augustine grass lawns at Seixal in Setubal district south of Lisbon in October 2019. The NPPO of Portugal conducted an official survey and the presence of B. insularis was confirmed in additional locations (EPPO Reporting Service, 2021, 2023). Conspicuous symptoms and numerous B. insularis were observed on S. secundatum lawns in private and public gardens in the following areas:
Área Metropolitana de Lisboa: in the municipalities of Cascais, Sintra, Almada, Seixal, Palmela and Olivais.
Alentejo region: in the municipalities of Alcácer do Sal, Porto Covo and Sines.
Algarve region: in the municipalities of Aljezur, Vila Real de Santo António, Faro and Loulé.
Within the EPPO Global database (EPPO, online), following a national survey the NPPO of Portugal report B. insularis as ‘Present, only in some parts’ (Figure 2) which EPPO then categorises as ‘Present, restricted distribution’.
Figure 2.
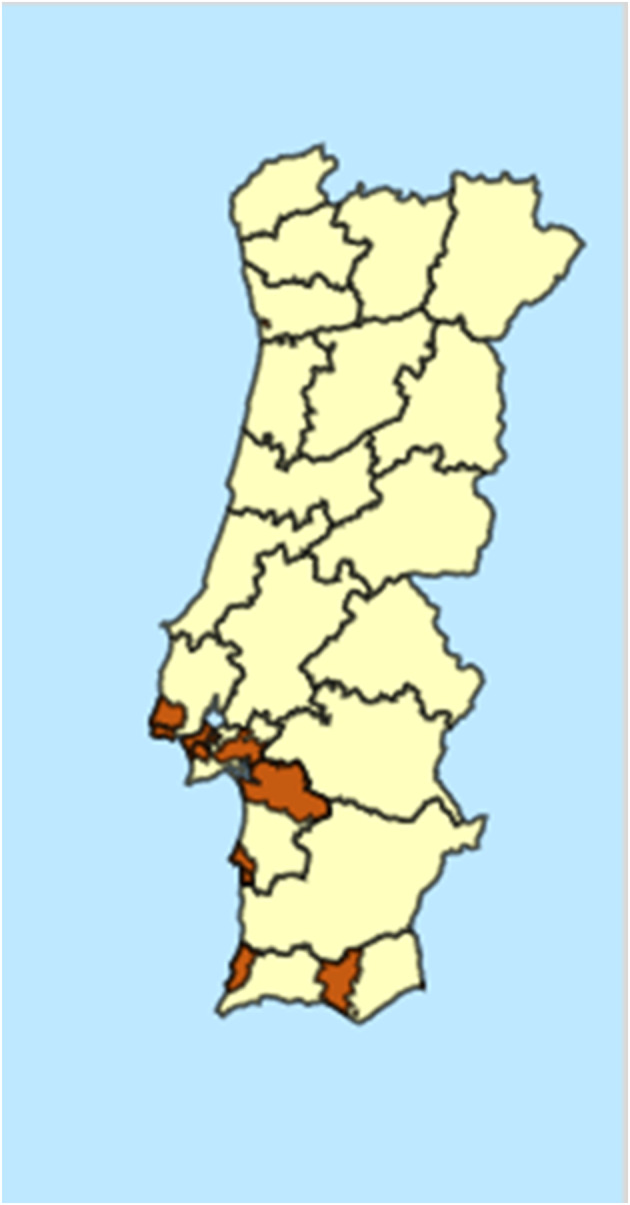
Distribution of Blissus insularis in Portugal following a survey by the NPPO (Dark regions indicate presence)
It is noteworthy that B. insularis was included in the EFSA Plant Health Newsletter – Scientific Literature Monitoring no. 28 in April 2021 (EFSA, 2021) and added to the EPPO Alert List in February 2023 following its introduction and reports of damage in Portugal.
3.3. Regulatory status
3.3.1. Commission implementing Regulation 2019/2072
B. insularis is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072, an implementing act of Regulation (EU) 2016/2031, or in any emergency plant health legislation.
3.3.2. Hosts or species affected that are prohibited from entering the union from third countries
Table 3 lists regulated articles prohibited from entering the EU and relevant to the entry of B. insularis.
Table 3.
List of plants, plant products and other objects that are Blissus insularis hosts whose introduction into the Union from certain third countries is prohibited (Source: Commission Implementing Regulation (EU) 2019/2072, Annex VI)
| List of plants, plant products and other objects whose introduction into the Union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN Code | Third country, group of third countries or specific area of third country | |
| 14. | Plants for planting of the family Poaceae, other than plants of ornamental perennial grasses of the subfamilies Bambusoideae and Panicoideae and of the genera Buchloe, Bouteloua Lag., Calamagrostis, Cortaderia Stapf., Glyceria R. Br., Hakonechloa Mak. ex Honda, Hystrix, Molinia, Phalaris L., Shibataea, Spartina Schreb., Stipa L. and Uniola L., other than seeds |
ex 0602 90 50 ex 0602 90 91 ex 0602 90 99 |
Third countries other than Albania, Algeria, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Egypt, Faeroe Islands, Georgia, Iceland, Israel, Jordan, Lebanon, Libya, Liechtenstein, Moldova, Monaco, Montenegro, Morocco, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐ Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Syria, Tunisia, Türkiye, Ukraine and the United Kingdom |
| 19 | Soil as such consisting in part of solid organic substances |
ex 2530 90 00 ex 3824 99 93 |
Third countries other than Switzerland |
S. secundatum, the major host of B. insularis, is a perennial grass in the subfamily Panicoideae (Appendix A). The subfamilies of permitted specific genera are shown in Appendix C.
S. secundatum is used as a lawn/amenity grass. If such grass is considered ornamental, then it is not prohibited from being introduced into the EU. It is unclear whether S. secundatum (St. Augustine grass) can be considered an ornamental grass.
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways.
Yes, the presence of B. insularis in Portugal indicates that it can enter the EU. The likely potential pathways are host plants for planting, soil and as a hitchhiker (stowaway).
Comment on plants for planting as a pathway.
Most S. secundatum seed is naturally sterile and consequently S. secundatum is mostly propagated vegetatively. Vegetatively propagated S. secundatum shipped as plants for planting would thus provide a pathway for entry and would facilitate establishment.
Rabitsch (2010) reported that most of the alien species of Heteroptera in Europe are from North America with imports of ornamental plants and movement as stowaways with vehicles being the major pathways for introduction. Table 4 lists potential pathways.
Table 4.
Potential pathways for Blissus insularis into the EU 27
| Pathways (e.g. host/intended use/source) | Life stage | Relevant mitigations [e.g. prohibitions (Annex VI), special requirements (Annex VII) or phytosanitary certificates (Annex XI) within Implementing Regulation 2019/2072] |
|---|---|---|
| Host plants for planting e.g. Stenotaphrum secundatum (Poaceae, subfamily Panicoideae) | Eggs, nymphs, adults. |
Annex VI prohibition although ornamental perennial grasses in subfamily Panicoideae are exempt and Annex VII (6.) requirements apply e.g. Official statement that the plants: (a) have been grown in nurseries; (b) are free from plants debris, flowers and fruits; (c) have been inspected and prior to export; (d) are found to be free from symptoms of harmful bacteria, viruses and virus like organisms; and (e) are found to be free from signs or symptoms of harmful nematodes, insects, mites and fungi, or have been subjected to appropriate treatment to eliminate such organisms. |
| Soil | Eggs | Annex VI prohibition |
Most S. secundatum seed is naturally sterile and consequently S. secundatum is mostly propagated vegetatively via creeping stolons and transplanted as plugs or turfs (Anon, 2020; Beaulieu, 2023). It is not known if S. secundatum could be regarded as an ornamental grass and can therefore be imported.
Detailed species‐specific trade statistics for growing plants are generally not collected by customs authorities and so it is not possible to determine from Eurostat the quantity of specific species traded internationally. Nevertheless, aggregate statistics on live outdoor plants are collected (Table 5). If S. secundatum was imported it could, for example, have been recorded within the CN code 0602 9050 (live outdoor plants including their roots).
Table 5.
EU 27 Imports of plants and other objects 2018–2022 based on CN codes in Table 3 (Tonnes) (Source Eurostat DS‐045409 – EU trade since 1988 by HS2‐4‐6 and CN8, https://epp.eurostat.ec.europa.eu/newxtweb/setupdimselection.do)
| CN code | Description | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|
| 0602 90 50 | Live outdoor plants, incl. their roots (excl. bulbs, tubers, tuberous roots, corms, crowns and rhizomes, incl. chicory plants and roots, unrooted cuttings, slips, rhododendrons, azaleas, roses, mushroom spawn, pineapple plants, vegetable and strawberry plants, trees, shrubs and bushes) | 188.8 | 239.1 | 133.9 | 60.7 | 16.1 |
| 0602 90 91 | Indoor flowering plants with buds or flowers (excl. cacti) | 2.6 | 6.1 | 2.4 | 2.4 | 0.7 |
| 0602 90 99 | Live indoor plants and cacti (excl. rooted cuttings, young plants and flowering plants with buds or flowers) | 247.0 | 299.0 | 126.6 | 132.7 | 341.2 |
| 2530 90 00 | Arsenic sulfides, alunite, pozzuolana, earth colours and other mineral substances, n.e.s. | 41,608.3 | 16,988.9 | 13,898.9 | 22,421.4 | 20,059.2 |
| 3824 99 93 | Chemical products or preparations, predominantly composed of organic compounds, n.e.s. (excl. in liquid form at 20°C) | 9,814.2 | 9,461.2 | 8,878.7 | 10,492.8 | 10,986.5 |
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. As at 04.05.2023, there were no records of interception of B. insularis in the Europhyt and TRACES databases.
3.4.2. Establishment
Is the pest able to become established in the EU territory?
Yes, B. insularis is able to establish in the EU. The presence of damaging populations in multiple locations within Portugal, at least since 2019, suggests that for the foreseeable future, populations can perpetuate. Within the EU establishment is most suitable in southern areas, especially around the Mediterranean where St. Augustine grass grows.
Climatic mapping is the principal method for identifying areas that could provide suitable conditions for the establishment of a pest taking key abiotic factors into account (Baker, 2002). Availability of hosts is considered in 3.4.2.1. Climatic factors are considered in 3.4.2.2.
3.4.2.1. EU distribution of main host plants
S. secundatum grows primarily in the seasonally dry tropical biome and is native to south‐east US, eastern and southern South America and tropical Africa but has been introduced as a lawn grass in warm, tropical and subtropical regions of the world (Sauer, 1972), and has also been introduced into the EU. Although it grows best in tropical and sub‐tropical climates with 1,000 to > 2,000 mm annual rainfall, it does grow in regions where annual rainfall is 750 mm or more (Anon, 2020). It also grows in most Mediterranean areas wherever there is a good amount of moisture (Anon, 2020) e.g. 750 mm or more each year, which can be natural or through irrigation. S. secundatum is the least cold‐hardy turf grass species (Li et al., 2010) and is easily damaged by low temperatures; it survives in plant hardiness zones 7–12 (Beaulieu, 2023). S. secundatum is tolerant to sea‐spray and thus is a suitable grass for coastal gardens (Royal Horticultural Society, undated). As a non‐native amenity species of lawn grass, there are few data on the EU distribution of S. secundatum. Nevertheless, within the EU, S. secundatum is found in Austria, France, Greece, Italy, Portugal and Spain (Plants of the world, online, accessed 30 April 2023). In Portugal, S. secundatum is often used as a lawn grass in public and private gardens, mainly in central and southern regions (Lima et al., 2021).
3.4.2.2. Climatic conditions affecting establishment
The global Köppen‐Geiger climate zones (Kottek et al., 2006) describe terrestrial climate in terms of average minimum winter temperatures and summer maxima, amount of precipitation and seasonality (rainfall pattern). B. insularis occurs in a range of climate zones in the Americas, Oceania and more recently in mainland Portugal. Some climatic zones in which B. insularis occurs are also found in the EU (Figure 3). For example, Mediterranean type climates, Csa and Csb, that occupy ~ 15% of all EU 27 five arcmin grid cells (MacLeod and Korycinska, 2019). Climate type Cfa (temperate, humid, sub‐tropical) is found in large parts of the southern and eastern US where B. insularis occurs. Such a climate represents ~ 7% of EU 27 five arcmin grid cells.
Figure 3.
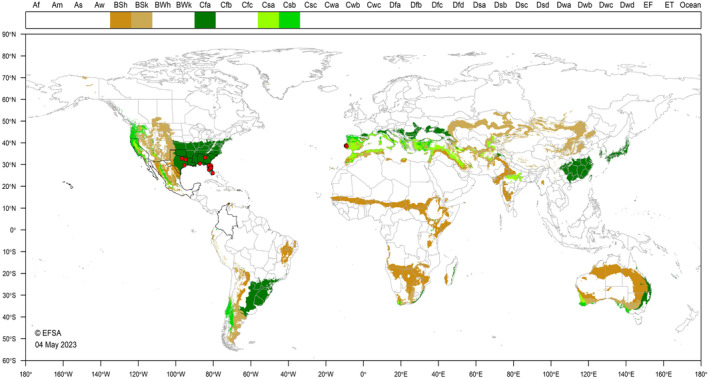
Distribution of selected Köppen –Geiger climate types that occur in the EU and in countries where Blissus insularis has been reported
3.4.3. Spread
Describe how the pest would be able to spread within the EU territory following establishment?
Adults and nymphs can spread by walking and move from lawn to lawn to feed. Long‐winged adults can fly to disperse; the proportion of females with long wings increases with population density although most still simply walk.
Comment on plants for planting as a mechanism of spread.
Infested host grass moved as turf or as plugs (i.e. as plants for planting) would likely be the primary means of spreading B. insularis quickly over long distances.
A population of B. insularis generally remains in the same area throughout the year (Kerr, 1966). However, as the population density increases, a growing proportion of adult females will develop long wings which they may use to disperse (Cherry and Wilson, 2003). Nevertheless, only a small proportion of a population will disperse by flying. Most individuals walk, including those with long wings, and will spread from lawn to lawn to find new food resources (Kerr, 1966; Woods, 2021). Natural spread will therefore occur relatively slowly.
B. insularis occurs in at least 10 regions of Portugal. It is assumed that such distribution is due to natural spread by walking or flying or with infested turfs or plugs used for transplanting St. Augustine grass and not because there were 10 separate introduction events of B. insularis.
3.5. Impacts
Would the pests' introduction have an economic or environmental impact on the EU territory?
Yes. The introduction of B. insularis into Portugal is already having an economic and environmental impact. Impacts will increase if the pest spreads more widely in the EU.
B. insularis is a pest species in the southern US where it mainly feeds on St. Augustine grass (S. secundatum), the grass makes up over half of all commercially produced turfgrass in the southeastern US (Satterthwaite et al., 2009) and it is a commonly used turfgrass species more widely across the southern US (van der Laat et al., 2022). B. insularis is its major insect pest (Crocker, 1993; Cherry, 2001) and is able to build up very large populations, with more than 2,000 insects per 0.1 m2 (Reinert and Kerr, 1973). St. Augustine grass turf is damaged when the density of the pest reaches 800 adults/m2 (Busey and Snyder, 1993 cited in Busey, 1995). Nymphs and adults of B. insularis are phloem feeders; feeding causes gradual yellowing and eventual dead patches of turf (Leonard, 1966). As they feed, they also inject a toxin into the host, the toxin prevents the plant from taking up water properly, causing wilting, chlorosis, stunting and death of damaged plants (Addesso et al., 2012). B. insularis feeds gregariously and large numbers of adults and nymphs aggregate and feed at the base of a plant (Slater, 1976). Once the group of insects has killed one section of grass stolon they move together to the next adjacent stolon (Woods, 2021). Thus, initially damage is limited to small patches, but the patch eventually expands, and entire lawns can be killed over time (Vazquez and Buss, 2006; Reinert et al., 2011).
Damage by B. insularis to lawns of St. Augustine grass allow weeds to establish. The weeds disrupt the visual and aesthetic appeal of a lawn (Rainbolt et al., 2006).
In Florida, US, B. insularis provides major challenges to the production and use of St. Augustine grass (Crocker, 1993), and numerous insecticide applications are used annually to maintain the turf's aesthetic value. Costs are incurred when dead patches are replaced with new turf.
In Portugal, Lima et al. (2021) report symptoms of grass stunting, reddish to yellow feeding marks, wilted and dead plants. All St Augustine grass lawns that were sampled showed damage symptoms with sunny areas suffering most heavily. Photographs of damaged lawns in Portugal are provided in Lima et al. (2021).
3.6. Available measures and their limitations
Are there measures available to prevent pest entry, establishment, spread or impacts such that the risk becomes mitigated?
Yes, see Section 3.3.2 on current measures inhibiting entry. Additional measures are also available to inhibit entry and spread. Control options to mitigate impact are also available.
3.6.1. Identification of potential additional measures
Phytosanitary measures (prohibitions) are currently applied to some host plants for planting (see Section 3.3.2).
Additional potential risk reduction options and supporting measures are shown in Sections 3.6.1.1 and 3.6.1.2.
3.6.1.1. Additional potential risk reduction options
Potential additional control measures are listed in Table 6.
Table 6.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance
| Control measure/Risk reduction option (Blue underline = Zenodo doc, Blue = WIP) | RRO summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Require pest freedom | Source host plants from a pest free area, pest free place of production or pest free production site. | Entry/Spread |
| Growing plants in isolation | The host, especially plugs for transplanting, could be grown vegetatively in insect‐proof greenhouses. | Entry/Spread |
| Managed growing conditions | In the US, pest control is based on a combination of cultural practices (mowing, fertilisation and irrigation) and use of tolerant cultivars (see below) in addition to chemical control with insecticides (see below). | Impact |
| Use of resistant and tolerant plant species/Varieties |
Resistant plants are used to restrict the growth and development of a specified pest and/or the damage they cause when compared to susceptible plant varieties under similar environmental conditions and pest pressure. Use of resistant cultivars of St. Augustine grass has been a major component of managing B. insularis in the US. However, 12 years after the first resistant cultivar was introduced most populations of B. insularis in Florida had overcome the resistance (Nagata and Cherry, 2003). Populations in Texas have also overcome host‐plant resistance (Busey, 1995; Cherry and Nagata, 1997; Rangasamy et al., 2009; Reinert, 2008; Reinert et al., 2011). Nevertheless, investigations into resistance (e.g. Youngs et al., 2014) and breeding of new cultivars for resistance to B. insularis has continued (e.g. Milla‐Lewis et al., 2017) Physical properties of resistant cultivars, such as the amount as shoot lignification and the thickness of cell walls around the vascular bundle may reduce stylet penetration (Rangasamy et al., 2009) |
Impact |
| Roguing |
Roguing is defined as the removal of infested plants and/or uninfested host plants in a delimited area Patches and grass around infested patches should be removed. |
Spread/Impact |
| Chemical treatments on crops including reproductive material | When an outbreak of B. insularis is detected, insecticides are required to kill nymphs and adults to try and protect the plants. Because eggs are often resistant to insecticides, multiple insecticide applications may be required for effective population control. The insecticide active substances acephate, chlorpyrifos and lambda‐cyhalothrin were effective against nymphs and adults (Nagata and Cherry, 1999) but frequent use of insecticides in southern Florida (e.g. 6–12 insecticide applications per year (Vázquez et al., 2010) led to the development of insecticide resistance (Cherry and Nagata 2005, 2007) and active ingredients such as deltamethrin, lambda‐cyhalothrin and imidacloprid are no longer effective in US. Resistance to bifenthrin and permethrin has also been reported (Vázquez et al., 2011). Some of these active substances are no longer authorised in the EU. (a) | Spread/Impact |
| Cleaning and disinfection of facilities, tools and machinery |
The physical and chemical cleaning and disinfection of facilities, tools, machinery, transport means, facilities and other accessories. Infested soil could carry eggs, nymphs and adults although without a host, nymphs and adults will not live long. Soil should be cleaned from tools and machinery. Prior to their export machinery and vehicles which have been operated for landscaping purposes are cleaned and free from soil and plant debris |
Entry/Spread |
| Limits on soil | Minimal amounts of soil should be moved with plants for planting. | Entry/Spread |
| Waste management | If roguing is applied, the removed parts should be destroyed (e.g. deep burial) | Spread |
| Post‐entry quarantine and other restrictions of movement in the importing country | Post‐entry quarantine (PEQ) could be used | Entry/Spread |
3.6.1.2. Additional supporting measures
Potential additional supporting measures are listed in Table 7.
Table 7.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance
| Supporting measure (Blue underline = Zenodo doc, Blue = WIP) | Summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Inspection and trapping |
Inspection is defined as the official visual examination of plants, plant products or other regulated articles to determine if pests are present or to determine compliance with phytosanitary regulations (ISPM 5). The effectiveness of sampling and subsequent inspection to detect pests may be enhanced by including trapping and luring techniques. |
Entry/Spread |
| Laboratory testing | Examination, other than visual, to determine if pests are present using official diagnostic protocols. Diagnostic protocols describe the minimum requirements for reliable diagnosis of regulated pests. | Entry (Linked to inspection) |
| Sampling |
According to ISPM 31, it is usually not feasible to inspect entire consignments, so phytosanitary inspection is performed mainly on samples obtained from a consignment. It is noted that the sampling concepts presented in this standard may also apply to other phytosanitary procedures, notably selection of units for testing. For inspection, testing and/or surveillance purposes the sample may be taken according to a statistically based or a non‐statistical sampling methodology. |
Entry (Linked to inspection) |
| Phytosanitary certificate and plant passport |
An official paper document or its official electronic equivalent, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements (ISPM 5) (a) export certificate (import) (b) plant passport (EU internal trade) |
Entry/Spread |
| Certified and approved premises | Mandatory/voluntary certification/approval of premises is a process including a set of procedures and of actions implemented by producers, conditioners and traders contributing to ensure the phytosanitary compliance of consignments. It can be a part of a larger system maintained by the NPPO in order to guarantee the fulfilment of plant health requirements of plants and plant products intended for trade. Key property of certified or approved premises is the traceability of activities and tasks (and their components) inherent the pursued phytosanitary objective. Traceability aims to provide access to all trustful pieces of information that may help to prove the compliance of consignments with phytosanitary requirements of importing countries. | Entry/Spread |
| Delimitation of buffer zones | ISPM 5 defines a buffer zone as ‘an area surrounding or adjacent to an area officially delimited for phytosanitary purposes in order to minimise the probability of spread of the target pest into or out of the delimited area, and subject to phytosanitary or other control measures, if appropriate’ (ISPM 5). The objectives for delimiting a buffer zone can be to prevent spread from the outbreak area and to maintain a pest free production place (PFPP), site (PFPS) or area (PFA). | Spread |
| Surveillance | Surveillance to guarantee that plants and produce originate from a Pest Free Area could be an option. | Spread |
3.6.1.3. Biological or technical factors limiting the effectiveness of measures
Very small eggs difficult to detect.
Populations build‐up before symptoms are noticed.
Ability to overcome host plant resistance.
Ability to develop insecticide resistance.
Lack of effective natural enemies and biological control agents (Woods, 2021).
3.7. Uncertainty
There are no key uncertainties in this pest categorisation. B. insularis has been introduced into the EU and is not known to be widely distributed. The NPPO of Portugal has conducted a nationwide survey and reported results indicating that B. insularis has restricted distribution.
4. Conclusions
B. insularis satisfies the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. Table 8 provides a summary of the PLH Panel conclusions.
Table 8.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the species is established; Blissus insularis Barber is the accepted name and authority. | None |
| Absence/presence of the pest in the EU (Section 3.2 ) | B. insularis is present in the EU; it has been reported from Portugal where it has a restricted distribution. B. insularis is not considered to be widely distributed in the EU. | None |
| Pest potential for entry, establishment and spread in the EU (Section 3.4 ) | The presence of B. insularis in Portugal indicates that it can enter and establish the EU. Infested host grass moved as turf or as plugs (i.e. as plants for planting) provide a likely pathway. B. insularis has been present since at least 2019 and is now reported in central and southern regions of Portugal, suggesting that it has spread and can perpetuate for the foreseeable future. | None |
| Potential for consequences in the EU (Section 3.5 ) | The introduction of B. insularis into Portugal is having an economic and environmental impact. Lawns of St. Augustine grass (a non‐native species) are being damaged in Portugal. The grass is widely grown around the Mediterranean. Impacts will increase if the pest spreads more widely around the EU. | None |
| Available measures (Section 3.6 ) | Measures are available to inhibit entry and spread. Control options to mitigate impact are also available. | None |
| Conclusion (Section 4 ) | B. insularis satisfies the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. | None |
| Aspects of assessment to focus on/scenarios to address in future if appropriate: | ||
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2021).
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2021).
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2021).
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2021).
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2021).
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment.
- Hitchhiker
An organism sheltering or transported accidentally via inanimate pathways including with machinery, shipping containers and vehicles; such organisms are also known as contaminating pests or stowaways (Toy and Newfield, 2010).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units.
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2021).
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2021).
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2021).
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2021).
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager.
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2021)
Appendix A – Blissus insularis host plants/species affected
1.
All plants fed upon by B. insularis are in the family Poaceae. The table below is a compilation of breeding hosts, feeding plants and the grasses on which B. insularis has been studied or found on, based on reports in the literature and is presented in descending order of apparent suitability.
| Host name | Subfamily | Common name | Comments | References |
|---|---|---|---|---|
| Stenotaphrum secundatum | Panicoideae | St. Augustine grass | Major host, feeds and breeds on | Kerr (1966), Slater (1976), Vittum et al. (1999), Reinert et al. (2011) |
| Zoysia spp. | Chloridoideae | Zoysia grass | Experimental host/some cultivars support feeding and breeding in no‐choice experiment | Reinert et al. (2011) |
| Bouteloua dactyloides (=Buchloë dactyloides) | Chloridoideae | Buffalo grass | Experimental host/one cultivar supported feeding and breeding in no‐choice experiment | Reinert et al. (2011) |
| Cynodon dactylon | Chloridoideae | Bermuda grass | Breeds on; Poor host. | Slater (1976), Reinert et al. (2011) |
| Paspalum notatum | Panicoideae | Bahia grass | Poor host in no‐choice experiment | Sweet (2000), Reinert et al. (2011) |
| Paspalum vaginatum | Panicoideae | Seashore paspalum | Poor host in no‐choice experiment. | Reinert et al. (2011) |
| Lolium arundinaceum (=Festuca arundinacea) | Pooideae | Tall fescue | Poor host | Reinert et al. (2011) |
| Panicum bartowense | Panicoideae | Fall panic grass | Feeds on (but no breeding) | Slater (1976) |
| Panicum maximum | Panicoideae | Guinea grass | Feeds on (but no breeding) | Slater (1976) |
| Panicum repens | Panicoideae | Torpedo grass | Feeds on (but no breeding) | Slater (1976) |
| Eremochla ophiuroides | Panicoideae | Centipede grass |
Found on Poor host in no‐choice experiment |
Slater (1976), Reinert et al. (2011) |
| Axonopus compresses | Panicoideae | American carpet‐grass | Found on | Slater (1976) |
| Digitaria decumbens | Panicoideae | Pangola grass | Found on | Slater (1976) |
The table below summarises results from experiments by Reinert et al. (2011) who placed five adult males and five adult females into rearing cages each containing a single grass cultivar. After 11 weeks the number of nymphs and adults were counted. The greatest population development was on three varieties of S. secundatum. Some cultivars of other grass species also supported population growth but at a much lower level.
Reproduction and development of Blissus insularis on cultivars and genera of turf grasses (Source: Reinert et al., 2011).
| Genera | Common name | Cultivar | Mean number of adults and nymphs after 11 weeks (starting with five males +five females) |
|---|---|---|---|
| Stenotaphrum secundatum | St Augustine grass | Raleigh | 180.8 |
| TX Common | 121.8 | ||
| Captiva | 97.6 | ||
| FX‐10 | 1.3 | ||
| Floratam | 1.1 | ||
| Zoysia spp. | Zoysia grass | Palisades | 19.0 |
| Emerald | 9.8 | ||
| Zorro | 8.0 | ||
| Empire | 5.6 | ||
| Cavalier | 1.1 | ||
| Buchloë dactyloides | Buffalo grass | 609 | 10.0 |
| Prairie | 1.8 | ||
| Festuca arundinacea | Tall fescue | Rebel | 6.0 |
| Paladin | 4.1 | ||
| Cynodon spp. | Bermuda grass | Tifton 10 | 3.1 |
| Tifway | 1.3 | ||
| Texturf 10 | 0.5 | ||
| TifSport | 0.0 | ||
| Common | 0.0 | ||
| Paspalum notatum | Bahia grass | Argintine | 2.0 |
| Pensacola | 0.0 | ||
| Paspalum vaginatum | Seashore paspalum | Seadwarf | 2.0 |
| AZ‐1 | 0.0 | ||
| Eremochloa ophiuroides | Centipede grass | Tifblaire | 0.6 |
Appendix B – Distribution of Blissus insularis
1.
Distribution records based on EPPO Global Database (EPPO, online) (unless marked*).
| Region | Country | Sub‐national (e.g. State) | Status |
|---|---|---|---|
| North America | USA | Alabama, Arizona, Arkansas, California, Florida, Georgia, (Hawaii), Louisiana, Mississippi, New Mexico, North Carolina, Oklahoma, South Carolina, Texas | Present, no details (all states) |
| Mexico* | Present, no details | ||
| Central America & Caribbean | Antigua and Barbuda | Present, no details | |
| Bahamas | Present, no details | ||
| Bermuda | Present, no details | ||
| Cuba | Present, no details | ||
| Dominica | Present, no details | ||
| Dominican Republic* | St Domingo | Present, no details | |
| Grenada | Present, no details | ||
| Haiti | Present, no details | ||
| Jamaica | Present, no details | ||
| Martinique | Present, no details | ||
| Panama | Present, no details | ||
| Puerto Rico | Present, no details | ||
| St Vincent and the Grenadines | Present, no details | ||
| Trinidad and Tobago | Present, no details | ||
| Turks and Caicos Islands | Present, no details | ||
| US Virgin Islands* | St Croix | Present, no details | |
| South America | Colombia | Present, no details | |
| Europe | Portugal | Present, restricted distribution | |
| Oceania | Guam | Present, no details | |
| (USA) | Hawaii | Present, no details |
Appendix C – Table showing the subfamily for each of the named ornamental genera listed in Commission Implementing Regulation (EU) 2019/2072, Annex VI, point 14 exempt from prohibition
1.
Annex VI of Commission Implementing Regulation (EU) 2019/2072, lists the plants, plant products and other objects whose introduction into the Union from certain third countries are prohibited.
Point 14 of Annex VI states that plants for planting of the family Poaceae, other than plants of ornamental perennial grasses of the subfamilies Bambusoideae and Panicoideae and of the genera Buchloe, Bouteloua Lag., Calamagrostis, Cortaderia Stapf., Glyceria R. Br., Hakonechloa Mak. ex Honda, Hystrix, Molinia, Phalaris L., Shibataea, Spartina Schreb., Stipa L. and Uniola L., other than seeds are prohibited from third countries other than those specified in section 14 (see Section 3.3.1).
The table below shows which subfamily each permitted ornamental genera is in.
| Permitted ornamental Genera | Subfamily |
|---|---|
| Buchloe (1) | Chloridoideae |
| Bouteloua | Chloridoideae |
| Calamagrostis | Pooideae |
| Cortaderia | Danthonioideae |
| Glyceria | Pooideae |
| Hakonechloa | Arundinoideae |
| Hystrix (= Elymus) (2) | Pooideae |
| Molinia | Arundinoideae |
| Phalaris | Pooideae |
| Shibataea | Bambusoideae |
| Spartina (= Sporobolus) (3) | Chloridoideae |
| Uniola | Chloridoideae |
Buchloe is a synonym of Bouteloua https://powo.science.kew.org/results?q=Buchloe.
Hystrix is a synonym of Elymus https://powo.science.kew.org/results?q=Hystrix.
Spartina is a synonym of Sporbolus https://powo.science.kew.org/results?q=Spartina.
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio F., Gonthier, P. , Jacques Miret, J. A. , Justesen, A. F. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H ., Van der Werf, W. , Vicent Civera, A. , Yuen, J. … MacLeod, A. , 2023. Pest categorisation of Blissus insularis . EFSA Journal, 21(7), 1–25. 10.2903/j.efsa.2023.8121
Requestor European Commission
Question number EFSA‐Q‐2023‐00394
Panel members Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Declaration of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements EFSA wishes to acknowledge Oresteia Sfyra for her substantial contribution throughout the preparation of the scientific opinion and Alex Gobbi for coordinating the climate suitability exercise.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 27 June 2023
Notes
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed the EPPO code remains the same. This provides a harmonised system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (Griessinger and Roy, 2015; EPPO, 2019)
References
- Addesso KM, Mcauslane HJ and Cherry R, 2012. Aggregation behaviour of the southern chinch bug (Hemiptera: Blissidae). Environmental Entomology, 41(4), 887–895. [Google Scholar]
- Anderson WG, Heng‐moss T, Baxendale FP, Baird M, Sarath G and Higley L, 2006. Chinch bug (Hemiptera: Blissidae) mouthpart morphology. Probing Frequencies and Locations on Resistant and Susceptible Germplasm. Resource document Publications from USDA‐ARS/UNL Faculty, 38 Available online: http://digitalcommons.unl.edu/usdaarsfacpub/38 [DOI] [PubMed]
- Anon , 2020. Tropical Forages. Available online: https://www.tropicalforages.info/text/entities/stenotaphrum_spp.htm
- Baker RHA, 2002. Predicting the limits to the potential distribution of alien crop pests. In: Hallman GJ and Schwalbe CP (eds.). Invasive Arthropods in Agriculture: problems and solutions. Science Publishers Inc, Enfield, USA. pp. 207–241. [Google Scholar]
- Beaulieu D, 2023. How to Grow and Care for St. Augustine Grass. Planting, Care, Similar Grasses, and Disadvantages The Spruce. Last updated on 22nd January 2023. Available online: https://www.thespruce.com/st-augustine-grass-care-guide-5199869
- Busey P, 1995. Field and laboratory resistance of St. Augustinegrass germplasm to the southern chinch bug. HortScience, 30(6), 1253–1255. [Google Scholar]
- Cherry R and Nagata R, 1997. Ovipositional preference and survival of southern chinch bugs (Blissus insularis Barber) on different grasses. International Turfgrass Society Research Journal, 8, 981–986. [Google Scholar]
- Cherry R, 2001. Seasonal wing polymorphism in southern chinch bugs (Hemiptera: Lygaeidae). The Florida entomologist., 84(4), 737–739. [Google Scholar]
- Cherry R and Wilson A, 2003. Morphology and fertility of wing polymorphic adults of southern chinch bugs (Hemiptera: Lygaeidae). Journal of Entomological Science, 38(4), 688–691. [Google Scholar]
- Cherry R and Nagata R, 2005. Development of resistance in southern chinch bugs (Hemiptera: Lygaeidae) to the insecticide bifenthrin. Florida Entomologist, 88(2), 219–221. [Google Scholar]
- Cherry R and Nagata R, 2007. Resistance to two classes of insecticides in southern chinch bugs (Hemiptera: Lygaeidae). Florida Entomologist, 90(3), 431–434. [Google Scholar]
- Crocker RL, 1993. Chemical control of southern chinch bug in St. Augustinegrass. International Turfgrass Society Research Journal, 7, 358–365. [Google Scholar]
- Dellapé PM and Thomas JH, 2023. Lygaeoidea species file Version 5.0/5.0. Available online: http://Lygaeoidea.SpeciesFile.org. [Accessed: 17 February 2023].
- Eden WG and Self RL, 1960. Controlling chinch bugs on St. Augustine grass lawns.
- Edmund STR, 1978. Ecological methods: with particular reference to the study of insect populations. Chapman & Hall, University of Oxford, Oxford, UK. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertesz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtueña Martınez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority , 2021. Plant Health Newsletter: Scientific Literature Monitoring No.28 April 2021. EFSA Supporting Publications 2021;EN‐6580, 28: 14 pp. 10.2903/sp.efsa.2021.EN-6580 [DOI] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed 22 March 2023].
- EPPO (European and Mediterranean Plant Protection Organization) , 2019. EPPO codes. Available online: https://www.eppo.int/RESOURCES/eppo_databases/eppo_codes
- EPPO Reporting Service , 2021. First report of Blissus insularis in Portugal and in Europe. EPPO Reporting Service 2021, 02, Article 055. Available online: https://gd.eppo.int/reporting/article-6994
- EPPO Reporting Service , 2023. Blissus insularis (Hemiptera: Blissidae, southern chinch bug): addition to the EPPO Alert List, EPPO Reporting Service 2023, 02, Article 043. Available online: https://gd.eppo.int/reporting/article-7525
- FAO (Food and Agriculture Organization of the United Nations) , 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) , 2021. International Standards for Phytosanitary Measures. ISPM 5 Glossary of phytosanitary terms. FAO, Rome. Available online: https://www.fao.org/3/mc891e/mc891e.pdf
- Griessinger D and Roy A‐S, 2015. EPPO codes: a brief description. Available online: https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Henry TJ, 1997. Phylogenetic analysis of family groups within the infraorder Pentatomomorpha (Hemiptera: Heteroptera), with emphasis on the Lygaeoidea. Annals of the Entomological Society of America., 90(3), 275–301. 10.1093/aesa/90.3.275 [DOI] [Google Scholar]
- Kerr SH, 1966. Biology of the Lawn Chinch Bug, Blissus insularis. The Florida Entomologist, 49(1), 9–18. [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of the Köppen_Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. 10.1127/0941-2948/2006/0130 [DOI] [Google Scholar]
- Leonard DE, 1968. A revision of the genus Blissus (Heteroptera: Lygaeidae) in eastern North America. Annals of the Entomological Society of America, 61(2), 239–250. [Google Scholar]
- Leonard DE, 1966. Biosystematics of the "Leucopterus Complex" of the genus Blissus. Heteroptera: Lygaeidae. The Connecticut Agricultural Experiment Station. Bulletin 677, March 1966, New Haven.
- Li R, Qu R, Bruneau AH and Livingston DP, 2010. Selection for freezing tolerance in St. Augustinegrass through somaclonal variation and germplasm evaluation. Plant breeding, 129(4), 417–421. [Google Scholar]
- Lima A, Valada T, Caetano MF, Franco JC and Ramos AP, 2021. First record of the lawn chinch bug Blissus insularis barber (Hemiptera: Blissidae) in Europe. Phytoparasitica, 49(4), 539–545. [Google Scholar]
- MacLeod A, Wratten SD and Harwood RW, 1994. The efficiency of a new lightweight suction sampler for sampling aphids and their predators in arable land. Annals of Applied Biology, 124(1), 11–17. [Google Scholar]
- MacLeod A and Korycinska A, 2019. Detailing Köppen–Geiger climate zones at sub‐national to continental scale: a resource for pest risk analysis. EPPO Bulletin, 49(1), 73–82. [Google Scholar]
- Milla‐Lewis SR, Youngs KM, Arrellano C and Cardoza YJ, 2017. Tolerance in St. Augustinegrass Germplasm against Blissus insularis Barber (Hemiptera: blissidae). Crop Science, 57(S1), S–26. [Google Scholar]
- Nagata RT and Cherry RH, 1999. Survival of different life stages of the southern chinch bug (Hemiptera: Lygaeidae) following insecticidal applications. Journal of Entomological Science., 34(1), 126–131. [Google Scholar]
- Nagata R and Cherry R, 2003. New source of southern chinch bug (Hemiptera: Lygaeidae) resistance in a diploid selection of St. Augustinegrass. Journal of Entomological Science., 38(4), 654–659. [Google Scholar]
- Plants of the World , Online. Stenotaphrum secundatum Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:423051-1
- Rabitsch W, 2010. True Bugs (Hemiptera, Heteroptera). Chapter 9.1. In: Roques A et al. (eds.) Alien terrestrial arthropods of Europe. BioRisk, 4(1), 407–433. 10.3897/biorisk.4.44 [DOI] [Google Scholar]
- Rainbolt C, Cherry R, Nagata R and Bittencourt M, 2006. Effect of southern chinch bug (Hemiptera: Lygaeidae) on weed establishment in St. Augustinegrass. Journal of Entomological Science, 41(4), 405–408. [Google Scholar]
- Rangasamy M, Rathinasabapathi B, McAuslane HJ, Cherry RH and Nagata RT, 2009. Role of leaf sheath lignification and anatomy in resistance against southern chinch bug (Hemiptera: Blissidae) in St. Augustinegrass. Journal of Economic Entomology., 102(1), 432–439. [DOI] [PubMed] [Google Scholar]
- Reinert, J.A. , 2008. Resistance to Tropical Sod Webworm in Cultivars of St. Augustinegrass. Turf News, May/June 2008, pp. 62‐643.
- Reinert JA, Chandra A and Engelke MC, 2011. Susceptibility of genera and cultivars of turfgrass to southern chinch bug Blissus insularis (Hemiptera: Blissidae). Florida Entomologist, 94(2), 158–163. [Google Scholar]
- Reinert JA and Kerr SH, 1973. Bionomics and control of lawn chinch bugs. Bulletin of the Entomological Society of America, 19(2), 91–92. [Google Scholar]
- Royal Horticultural Society , Undated. Available online: https://digiuat.rhs.org.uk/lawns/lawns-in-mediterranean-regions
- Sayers EW, Cavanaugh M, Clark K, Ostell J, Pruitt KD and Karsch‐Mizrachi I, 2020. Genbank. Nucleic Acids Research. 48 pp, Database issue. 10.1093/nar/gkz956 [DOI] [PMC free article] [PubMed]
- Satterthwaite LN, Hodges AW, Haydu JJ and Cisa JL, 2009. An Agronomic and Economic Profile of Florida's Sod Industry in 2007 University of Florida, Florida Agricultural Experiment Stations, Florida Cooperative Extension Service. Available online: https://fred.ifas.ufl.edu/pdf/economic-impact-analysis/sod2007.pdf
- Slater JA, 1976. Monocots and Chinch Bugs: A Study of Host Plant Relationships in the Lygaeid Subfamily Blissinae (Hemiptera: Lygaeidae). Biotropica, 8(3), 143–165. [Google Scholar]
- Slater JA, 1979. The systematics, phylogeny, and zoogeography of the Blissinae of the world (Hemiptera, Lygaeidae). Bulletin of the American Museum of Natural History, 165(1), 1–180. [Google Scholar]
- Slater JA and Baranowski RM, 1990. The Lygaeidae of Florida (Hemiptera: Lygaeidae). Arthropods of Florida and neighboring land areas. 14. Florida Dept. Agriculture and Consumer Services, Division of Plant, Gainesville. [Google Scholar]
- Slater JA and O'Donnell J, 1995. A Catalogue of the Lygaeidae of the World (1960–1994). New York Entomological Society, New York. 411 p. [Google Scholar]
- Toy SJ and Newfield MJ, 2010. The accidental introduction of invasive animals as hitchhikers through inanimate pathways: a New Zealand perspective. Revue scientifique et technique (International Office of Epizootics)., 29(1), 123–133. [DOI] [PubMed] [Google Scholar]
- van der Laat R, Dale AG, Arellano C and Milla‐Lewis SR, 2022. Variation in southern chinch bug (Blissus insularis) survival and damage on St. Augustinegrass germplasm. International Turfgrass Society Research Journal, 14(1), 642–651. [Google Scholar]
- Vazquez JC and Buss EA, 2006. Southern chinch bug feeding impact on St. Augustinegrass growth under different irrigation regimes. Applied Turfgrass Science, 3, 1–5. [Google Scholar]
- Vázquez C, Hoy MA, Royalty RN and Buss EA, 2010. A synchronous rearing method for Blissus insularis (Hemiptera: Blissidae). Journal of economic entomology., 103(3), 726–734. [DOI] [PubMed] [Google Scholar]
- Vázquez C, Royalty RN and Buss EA, 2011. Susceptibility of Blissus insularis (Heteroptera: Hemiptera: Blissidae) populations in Florida to bifenthrin and permethrin. Florida Entomologist., 94(3), 571–581. [Google Scholar]
- Vittum PJ, Villani MG and Tashiro H, 1999. Turfgrass insects of the United States and Canada. Cornell University Press. [Google Scholar]
- Wilson RN, 1929. The chinch bug in relation to St. Augustinegrass USDA Circular No. 51. Available online: https://archive.org/details/chinchbuginrela51wils/page/n1/mode/2up
- Woods S, 2021. Southern Chinch Bug, Blissus insularis Barber (Insecta: Hemiptera: Blissidae). University of Florida. EENY‐226 Available online: https://entnemdept.ufl.edu/creatures/orn/turf/southern_chinch_bug.htm
- Youngs KM, Milla‐Lewis SR, Brandenburg RL and Cardoza YJ, 2014. St. Augustinegrass Germplasm Resistant to Blissus insularis (Hemiptera: Blissidae). Journal of Economic Entomology, 107(4), 1688–1694. [DOI] [PubMed] [Google Scholar]


