Abstract
Background
Anterior cruciate ligament (ACL) injury is recognized as a risk factor for osteoarthritis (OA) progression. Herein, the function of TAF15 in ACL injury-induced OA was investigated.
Methods
OA cell model and OA mouse model were established by interleukin-1 beta (IL-1β) stimulation and ACL transection administration, respectively. The pathological changes were analyzed by histopathology. The mRNA and protein expressions were assessed using qRT-PCR, Western blot and IHC. Chondrocyte viability and apoptosis were examined by CCK8 assay and TUNEL staining, respectively. The interactions between TAF15, BRD4 and GREM1 were analyzed by RIP or ChIP assay.
Results
TAF15 expression was markedly elevated in OA, and its knockdown suppressed IL-1β-induced chondrocyte apoptosis and ECM degradation in vivo and cartilage pathological changes in vitro. TAF15 promoted BRD4 mRNA stability, and TAF15 silencing's repression on chondrocyte apoptosis and ECM degradation induced by IL-1β was abrogated following BRD4 overexpression. BRD4 promoted GREM1 expression by directly binding with GREM1. In addition, the GREM1/NF-κB pathway functioned as the downstream pathway of BRD4 in promoting OA progression.
Conclusion
TAF15 upregulation facilitated chondrocyte apoptosis and ECM degradation during OA development by acting on the BRD4/GREM1/NF-κB axis, which provided a theoretical basis for the development of novel therapies for OA.
Keywords: Osteoarthritis, Anterior cruciate ligament injury, TAF15, BRD4, The gremlin-1-NF-κB pathway
1. Introduction
Osteoarthritis (OA) is a common degenerative joint disease, affecting 70% of people worldwide [1]. The main phenotype of OA includes the degeneration and inflammation of multiple articular cartilages including cartilage, synovium, meniscus, and ligaments [2], among which anterior cruciate ligament (ACL) is the most important joint. As widely described, traumatic or degenerative fractures-induced ACL injury is an important risk factor affecting OA development [3]. Despite increasing efforts that have been devoted to uncovering the pathological processes of ACL injury-induced OA, the molecular mechanisms remain unclear. Therefore, there is an unmet medical need to find new therapeutic targets to develop more effective therapeutic strategies for OA.
TATA-box binding protein associated factor 15 (TAF15) functions in maintaining target mRNA stability by regulating mRNA transcription, splicing and trafficking [4]. TAF15 is markedly upregulated in cartilage tissues of OA patients and OA mice [5], revealing the risking role of TAF15 in affecting OA progression. It was also previously described that TAF15 upregulation repressed chondrogenic differentiation during OA progression by maintaining FUT1 mRNA stability [5]. Although the risking role of TAF15 in OA has been explained, more research is needed on the specific molecular mechanism by which TAF15 regulates the progression of ACL injury-induced OA.
Bromodomain-containing 4 (BRD4), a ubiquitously expressed 200 kDa nuclear protein, is a mammalian bromodomain protein which functions in regulating various biological processes by binding to acetylated chromatin [6]. The expression change of BRD4 is one of the factors affecting inflammatory disease progression. As proof, Zhu et al. revealed that BRD4 inhibition attenuated pathological cardiac hypertrophy by inhibiting cardiomyocyte fibrosis and inflammation [7]. In addition, as previously illustrated, BRD4 blockade prevented myocardial ischemia/reperfusion injury by inhibiting inflammation and oxidative stress [8]. Recent studies have emphasized the function of BRD4 in OA. BRD4 was reported to be upregulated in articular cartilage of OA, and its inhibition could attenuate cartilage destruction ACL transection-induced OA mice by inactivating nuclear transcription factor-κB (NF-κB) signaling pathway [9]. Moreover, BRD4 repression alleviated mechanical stress-induced temporomandibular joint OA-like pathological changes and inhibited inflammation as well [10]. In the current research, by using starBase, it was predicted that TAF15 had a potential binding site to BRD4. Therefore, it's speculated that TAF15 achieves its biological role in OA by maintaining BRD4 mRNA stability, which deserves further research.
Gremlin-1 (GREM1), as a member of the bone morphogenetic protein (BMP) antagonist family, functions in regulating organogenesis and tissue differentiation [11]. GREM1 was reported to be upregulated in OA, and it acted as the downstream target of miR-183-5p in facilitating IL-1β-induced apoptosis and extracellular matrix (ECM) degradation in chondrocytes [12]. Chang et al. also identified GREM1 as a key pathogenic factor accelerating excessive mechanical loading-induced accelerates cartilage degeneration; mechanistically, GREM1 contributed to cartilage degeneration by activating NF-κB signaling [13]. Notably, it was illustrated by Lucia et al. that BRD4 promoted GREM1 expression by binding with GREM1 promoter region in glomerulonephritis [14]. Nevertheless, this regulatory relationship has not been verified in OA.
We hypothesized that TAF15 promoted GREM1 expression by maintaining BRD4 mRNA stability, thereby activating the gremlin-1-NF-κB pathway and promoting OA progression, providing a potential therapeutic strategy for OA.
2. Materials and methods
2.1. Clinical sample collection
The cartilage tissues were collected from patients with femoral neck fractures without OA history undergoing total hip replacement surgery (normal, n = 10), patients with ACL injury (ACL-NC, n = 10) undergoing knee arthroscopy and patients with ACL injury and OA (ACL-OA, n = 20) undergoing knee arthroplasty surgery at Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University). This study was passed the review of Ethics Committee of Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University) before enrollment of patients and all participants signed informed consent.
2.2. Animal experiments
20 male C57BL/6 mice (8-week-old) were obtained from SJA Laboratory Animal Co, Ltd (Hunan, China). After acclimation for 1 week, mice in the ACLT group, the ACLT + sh-NC group and the ACLT + sh-TAF15 group received ACL transection (ACLT) via medial parapatellar approach. The mice were anesthetized with 2% isoflurane and temperature was maintained at 37 °C. The ACL was severed near tibia insertion by a sharp scalpel. The sh-TAF15 lentiviral vector and sh-NC were constructed by GenePharma. Lentivirus of sh-TAF15 and sh-NC were injected into the joint cavity of mice in the ACLT + sh-NC group and the ACLT + sh-TAF15 group at 0, 15, 30 and 45 post-surgery. After 6 weeks, mice were sacrificed, and the cartilage tissues were collected. The animal studies were approved by Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University).
2.3. Cell culture and treatment
C28/I2 cells, purchased from ATCC (VA, USA), were cultured in DMEM (Gibco, MD, USA) mixed with 10% FBS (Gibco) at 37 °C with 5% CO2. C28/I2 cells were incubated with 10 ng/mL IL-1β (PeproTech, NJ, USA) for 24 h to establish the OA cell model as previously described [15].
2.4. Cell transfection
The small interfering RNA of TAF15 (si-TAF15), the overexpression plasmid of BRD4 (oe-BRD4), si-BRD4, oe-GREM1 and their negative controls, synthesized by GenePharma (Shanghai, China), were transfected into cells with Lipofectamine™ 3000 (Invitrogen, Carlsbad, CA, USA).
2.5. Cell counting kit-8 (CCK-8) assay
Cells were cultured in 24-well plates (2 × 104 cells/well) for 24 h and incubated with CCK-8 (10 μL, Sangon, Shanghai, China) at 37 °C for 3 h. Absorbance was analyzed at 450 nm.
2.6. RNA degradation assay
Cells were incubated with 5 mg/mL actinomycin D (Sigma–Aldrich, MO, USA) for 0 h, 3 h, 6 h and 9 h. Then RNA was extracted from cells, reverse transcribed to cDNA and analyzed by qRT-PCR.
2.7. Immunohistochemistry (IHC)
The cartilage tissue sections (4 μm in thickness) were prepared. After deparaffinization and antigen retrieval, sections were blocked and incubated with antibodies against MMP13 (Abcam, 1:200, ab219620) and Collagen II (Abcam, 1:200, ab34712) overnight followed by incubation with secondary antibody (Abcam, 1:500, ab150077) for 1 h. The sections were stained with DAB and then counterstained with hematoxylin, dehydrated and mounted. The images were taken using a microscope (Nikon, Tokyo, Japan).
2.8. RNA immunoprecipitation (RIP) assay
Cells were lysed with a complete RIP lysis buffer and incubated with IgG (Abcam, Cambridge, UK, 1:50, ab172730) and TAF15 (Abcam, 1:50, ab134916) antibodies at 4 °C overnight. cDNA was synthesized and used for qRT-PCR assay.
2.9. Chromatin immunoprecipitation (ChIP) assay
Cells were fixed and quenched. DNA was fragmented by sonication. Then cell lysate was incubated with anti-BRD4 (Abcam, 1:100, ab128874) or anti-IgG (Abcam, 1:100, ab172730) at 4 °C overnight. DNA was enriched and subjected to qRT-PCR.
2.10. TdT-mediated dUTP nick-end labeling (TUNEL) staining
Cells were fixed permeabilized. TUNEL staining was performed with the kit obtained from Elabscience (Wuhan, China). The nucleus was stained with DAPI (Sangon). Cartilage tissue sections (4 μm in thickness) were prepared. The sections were subjected to protein kinase K and stained with TUNEL solution for 1 h. Sections were incubated with specific antibodies labeled with horseradish peroxidase for 1 h. Following counterstaining with DAB, sections were stained with hematoxylin and observed under a microscope.
2.11. Hematoxylin-eosin (HE) staining
The cartilage tissue sections (5 μm in thickness) were prepared and dehydrated. The sections were subsequently stained with HE (Sigma–Aldrich). The images were collected by a microscope (Nikon).
2.12. Safranin O staining
Cartilage tissue sections (4 μm in thickness) were prepared. The sections were subsequently deparaffinized by xylene and hydrated by ethanol, stained with 0.1% Safranin-O (Sangon) for 10 min and observed under a microscope (Nikon).
2.13. Osteoarthritis research society international (OARSI) scores
The histological characteristics of the regenerated cartilage tissue were scored using the OARSI scoring system [16]. HE and Safranin O-stained sections were analyzed by three independent doctors blinded to the grouping, and the average scores were calculated.
2.14. Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted with TRIzol (ThermoFisher Scientific. MA. USA). The cDNA was synthesized using the Reverse Transcription Kit (Toyobo, Tokyo, Japan) and subjected to qRT-PCR by using SYBR (ThermoFisher Scientific). GAPDH was used as the reference gene. The data were analyzed with 2−ΔΔCT method. The primers were listed as follows (5′-3′):
TAF15 (F): GATTCTGGAAGTTACGGTCAGTC.
TAF15 (R): AGCTTTGTGATGCTTGTCCATAG.
BRD4 (F): ACCTCCAACCCTAACAAGCC.
BRD4 (R): TTTCCATAGTGTCTTGAGCACC.
GREM1 (F): CGGAGCGCAAATACCTGAAG.
GREM1 (R): GGTTGATGATGGTGCGACTGT.
GAPDH (F): GGAGCGAGATCCCTCCAAAAT.
GAPDH (R): GGCTGTTGTCATACTTCTCATGG.
2.15. Western blot
The proteins were isolated with RIPA, separated and transferred to a PVDF membrane (Millipore). Then, membranes were incubated overnight with antibodies against TAF15 (Abcam, 1:1000, ab134916), Bax (Abcam, 1:1000, ab32503), Bcl-2 (Abcam, 1:1000, ab182858), MMP13 (Abcam, 1:1000, ab39012), ADAMTS5 (Abcam, 1:1000, ab41037), Collagen II (Abcam, 1:1000, ab34712), Aggrecan (Abcam, 1:1000, ab3778), BRD4 (Abcam, 1:1000, ab128874), GREM1 (R&D systems, MN, USA, 1:1000, AF956), p-p65 (Cell Signaling Technology, 1:1000, #3031) and GAPDH (Abcam, 1:10000, ab8245). After washed with PBS-T, membranes were then incubated with secondary antibody (Abcam, 1:10000, ab7090) for 60 min. The membranes were visualized and imaged by GEL imaging system (Bio-Rad, CA, USA).
2.16. Statistical analysis
All our data were obtained from three independent experiments. Statistical data were analyzed by SPSS 19.0 (IBM, Armonk, NY) and expressed as means ± SD. Between-group differences and multi-group comparisons were analyzed by Student's t-test and one-way ANOVA, respectively. The p values less than 0.05 were considered significant.
3. Results
3.1. TAF15 was significantly upregulated in OA cartilage tissue and OA cell model, and its knockdown inhibited IL-1β-induced chondrocyte apoptosis and ECM degradation
The cartilage tissues from patients without OA history (normal), patients with ACL injury (ACL-NC) and patients with ACL injury and OA (ACL-OA) were collected, and it was observed that the levels of TAF15, BRD4 and GREM1were markedly upregulated in the ACL-OA group compared to that in the ACL-NC and normal groups (Fig. 1A). The OA cell model was established by IL-1β stimulation. It was observed that TAF15 expression in human chondrocytes was markedly increased by IL-1β stimulation (Fig. 1B–C). To further investigate the biological role of TAF15 in OA, TAF15 knockdown was induced in IL-1β-treated chondrocytes by transfecting si-TAF15 into cells. si-TAF15 reversed IL-1β-induced high expression of TAF15 (Fig. 1D–E). IL-1β treatment resulted in decreased chondrocyte viability, which was abrogated by TAF15 downregulation (Fig. 1F). In addition, chondrocyte apoptosis was markedly increased by IL-1β stimulation, while this change was reversed by si-TAF15 transfection (Fig. 1G). Consistently, IL-1β treatment led to the increased Bax level and reduced Bcl-2 level, while these changes were abrogated by TAF15 knockdown (Fig. 1H). Moreover, IL-1β stimulation resulted in increased MMP13 and ADAMTS5 levels (key metalloproteinases that degrade cartilage matrix) and reduced Collagen II and Aggrecan levels, whereas these effects were eliminated by TAF15 silencing (Fig. 1I). All these results suggested that TAF15 was highly expressed in OA, and its knockdown repressed chondrocyte apoptosis and ECM degradation during OA progression.
Fig. 1.
TAF15 was significantly upregulated in OA cartilage tissue and OA cell model, and its knockdown inhibited IL-1β-induced chondrocyte apoptosis and ECM degradation. (A) TAF15, BRD4 and GREM1 mRNA levels in cartilage tissues were detected by qRT-PCR (B–C) TAF15 expression in chondrocytes following IL-1β treatment was assessed by qRT-PCR and Western blot. TAF15 knockdown was induced in IL-1β-treated chondrocytes by transfecting si-TAF15 into cells (D–E) qRT-PCR and Western blot were employed to examine TAF15 expression level in chondrocytes (F) CCK8 assay was employed to analyze chondrocyte viability (G) Chondrocyte apoptosis was assessed by TUNEL staining (H–I) Bax, Bcl-2, MMP13, ADAMTS5, Collagen II and Aggrecan protein levels in cells were detected using Western blot. Data were expressed as mean ± SD. All data were obtained from three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.2. TAF15 maintained BRD4 mRNA stability in IL-1β-treated chondrocytes
It has been widely illustrated that TAF15 achieves its biological effects by maintaining target mRNA stability [4]. We subsequently aimed to probe the downstream target of TAF15 in regulating OA development. IL-1β increased BRD4 expression level in human chondrocytes (Fig. 2A–B). Encouragingly, high BRD4 expression in OA cell model was reduced by si-TAF15 transfection (Fig. 2C–D). RIP assay subsequently revealed that TAF15 is directly bound with BRD4 (Fig. 2E). Moreover, as displayed in Fig. 2F, TAF15 knockdown accelerated BRD4 mRNA degradation. Collectively, TAF15 promoted BRD4 expression by maintaining BRD4 mRNA stability in IL-1β-treated chondrocytes.
Fig. 2.
TAF15 maintained BRD4 mRNA stability in IL-1β-treated chondrocytes
(A) BRD4 mRNA level in cartilage tissues was detected by qRT-PCR (B) BRD4 expression level in chondrocytes following IL-1β treatment was assessed by qRT-PCR and Western blot (C–D) BRD4 expression in IL-1β-treated chondrocytes following si-NC or si-TAF15 transfection was determined by qRT-PCR and Western blot (E) RIP assay was carried out to analyze the interaction between TAF15 and BRD4 (F) BRD4 mRNA stability in chondrocytes after TAF15 knockdown was assessed using RNA degradation assay. Data were expressed as mean ± SD. All data were obtained from three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
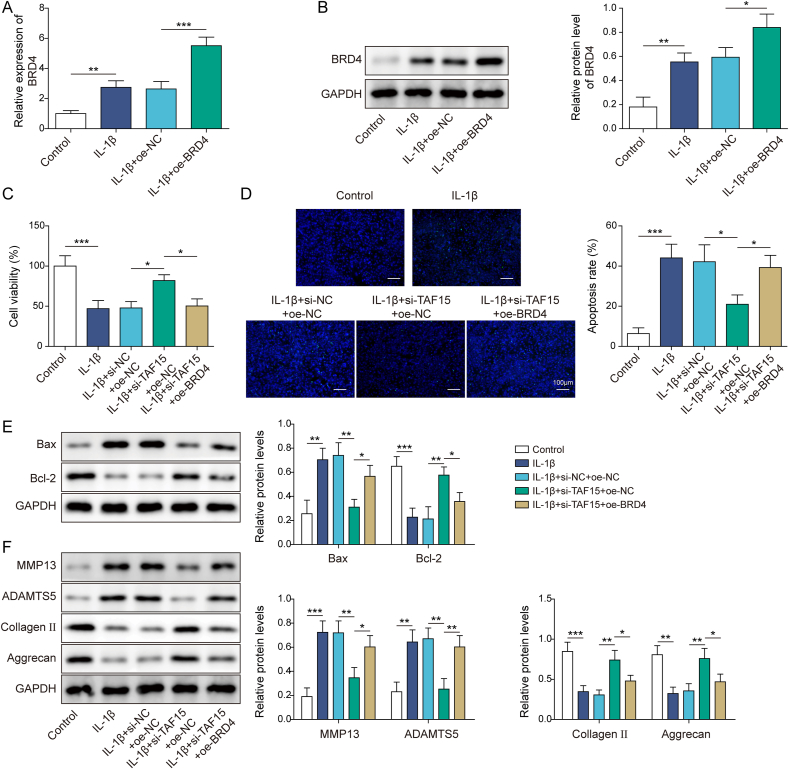
3.3. BRD4 overexpression reversed the inhibitory effect of TAF15 knockdown on IL-1β-induced chondrocyte apoptosis and ECM degradation
To study the role of BRD4 in TAF15-mediated inhibitory effects on IL-1β-induced chondrocyte apoptosis and ECM degradation, both TAF15 knockdown and BRD4 overexpression were induced. IL-1β induced a higher level of BRD4 in chondrocytes, and oe-BRD4 transfection further promoted BRD4 expression level in IL-1β-treated human chondrocytes (Fig. 3A–B), suggesting the transfection was successful. Functional experiments subsequently demonstrated IL-1β stimulation resulted in reduced chondrocyte viability and increased apoptosis, while these changes caused by IL-1β were reversed by TAF15 knockdown, and oe-BRD4 co-transfection eliminated the effects of TAF15 knockdown (Fig. 3C–D). Meanwhile, IL-1β induced higher Bax level and lower Bcl-2 level in chondrocytes, while TAF15 knockdown inhibited Bax level and promoted Bcl-2 level in IL-1β-treated chondrocytes, and oe-BRD4 co-transfection eliminated these effects of TAF15 knockdown (Fig. 3E). Additionally, IL-1β induced higher MMP13 and ADAMTS5 levels and lower Collagen II and Aggrecan levels in chondrocytes, while TAF15 knockdown inhibited MMP13 and ADAMTS5 levels and promoted Collagen II and Aggrecan levels in IL-1β-treated chondrocytes, and oe-BRD4 co-transfection eliminated these effects of TAF15 knockdown (Fig. 3F). Taken together, TAF15 facilitated chondrocyte apoptosis and ECM degradation in OA by promoting BRD4 expression.
Fig. 3.
BRD4 overexpression reversed the inhibitory effect of TAF15 knockdown on IL-1β-induced chondrocyte apoptosis and ECM degradation. (A–B) BRD4 expression level in IL-1β-treated chondrocytes following oe-NC or oe-BRD4 transfection was assessed by qRT-PCR and Western blot. Both TAF15 knockdown and BRD4 overexpression were induced in IL-1β-treated human chondrocytes (C) Chondrocyte viability was examined by CCK8 assay (D) TUNEL staining was conducted to analyze chondrocyte apoptosis (E–F) Western blot was employed to assess Bax, Bcl-2, MMP13, ADAMTS5, Collagen II and Aggrecan protein levels in cells. Data were expressed as mean ± SD. All data were obtained from three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.4. BRD4 promoted GREM1 expression in IL-1β-treated chondrocytes
GREM1 acts as the target of BRD4 in regulating glomerulonephritis progression [14]. Herein, GREM1 expression in human chondrocytes was markedly increased by IL-1β treatment (Fig. 4A–B). We subsequently induced BRD4 knockdown in chondrocytes, and it was found that si-BRD4 transfection markedly reduced BRD4 expression level in IL-1β-treated chondrocytes (Fig. 4C–D). BRD4 knockdown resulted in reduced GREM1 expression in IL-1β-treated chondrocytes, whereas BRD4 overexpression resulted in increased GREM1 expression in IL-1β-treated chondrocytes (Fig. 4E–F). Meanwhile, the result from ChIP assay revealed that BRD4 is directly bound with GREM1(Fig. 4G). In conclusion, BRD4 promoted GREM1 expression in OA by directly binding to BRD4.
Fig. 4.
BRD4 promoted BRD4 expression in IL-1β-treated chondrocytes (A–B) GREM1 mRNA level in cartilage tissues was detected by qRT-PCR and Western blot (C) GREM1 expression level in chondrocytes following IL-1β treatment was determined using qRT-PCR (D) BRD4 expression level in IL-1β-treated chondrocytes following si-NC or si-BRD4 transfection was determined using qRT-PCR and Western blot (E–F) GREM1 expression level in IL-1β-treated chondrocytes following BRD4 knockdown or BRD4 overexpression was determined using qRT-PCR and Western blot (G) RIP assay was carried out to analyze the interaction between BRD4 and GREM1. Data were expressed as mean ± SD. All data were obtained from three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
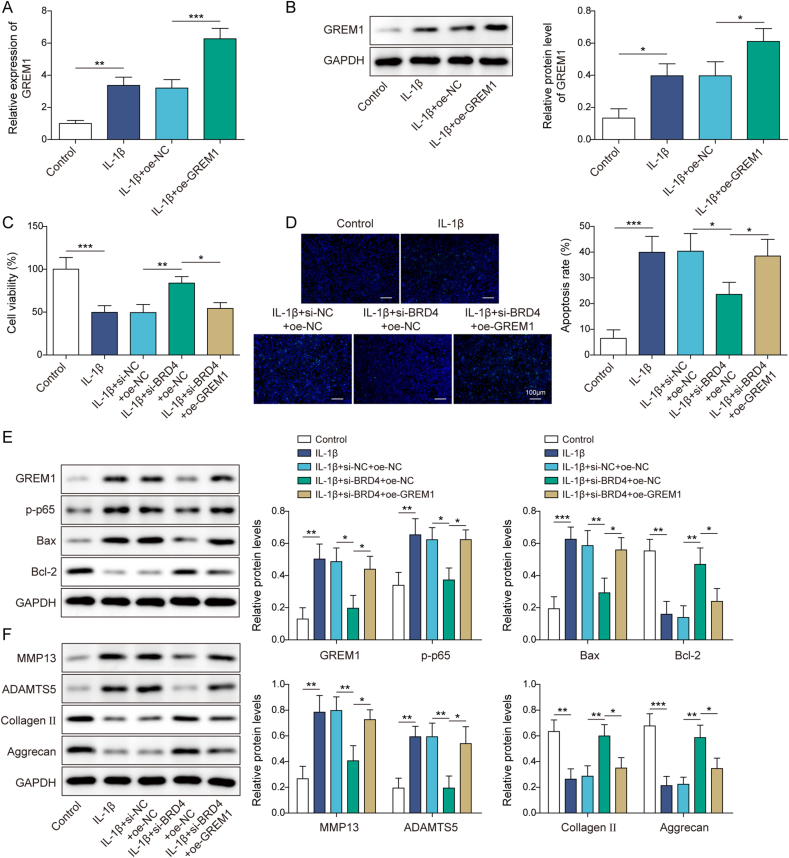
3.5. BRD4 knockdown inhibited IL-1β-induced chondrocyte apoptosis and ECM degradation by suppressing the GREM1/NF-κB pathway
To study the role of BRD4/GREM1 in regulating OA progression, both BRD4 knockdown and GREM1 overexpression were induced in IL-1β-treated human chondrocytes. As demonstrated in Fig. 5A–B, IL-1β increased GREM1 expression in chondrocytes, and GREM1 expression level in IL-1β-treated chondrocytes was further increased by oe-GREM1 transfection, revealing the transfection was successful. It also turned out that BRD4 knockdown ameliorated IL-1β-induced increase in chondrocyte apoptosis and prevented IL-1β-induced decrease in chondrocyte viability, while these effects of BRD4 knockdown were abrogated by GREM1 overexpression (Fig. 5C–D). Additionally, si-BRD4 transfection ameliorated IL-1β-induced increase in GREM1, p-p65 and Bax levels in chondrocytes and prevented IL-1β-induced decrease in Bcl2 level, while these protein changes caused by BRD4 knockdown were reversed by GREM1 upregulation (Fig. 5E). Furthermore, BRD4 knockdown ameliorated IL-1β-induced increase in MMP13 and ADAMTS5 levels in chondrocytes and prevented IL-1β-induced decrease in Collagen II and Aggrecan levels, while these effects of BRD4 knockdown were abrogated by GREM1 overexpression (Fig. 5F). Collectively, the GREM1/NF-κB pathway functioned as the downstream pathway of BRD4 in promoting chondrocyte apoptosis and ECM degradation in OA.
Fig. 5.
BRD4 knockdown inhibited IL-1β-induced chondrocyte apoptosis and ECM degradation by suppressing the GREM1-NF-κB pathway (A–B) GREM1 expression level in IL-1β-treated chondrocytes following oe-NC or oe-GREM1 transfection was examined by qRT-PCR and Western blot. Both BRD4 knockdown and GREM1 overexpression were induced in IL-1β-treated human chondrocytes (C) CCK8 assay was employed to analyze chondrocyte viability (D) Chondrocyte apoptosis was assessed by TUNEL staining (E–F) GREM1, p-p65, Bax, Bcl-2, MMP13, ADAMTS5, Collagen II and Aggrecan protein levels in cells were detected using Western blot. Data were expressed as mean ± SD. All our data were obtained from three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
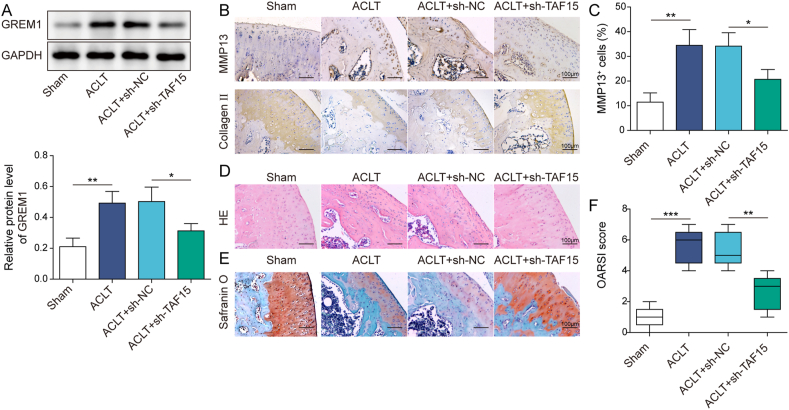
3.6. TAF15 knockdown attenuated OA in ACLT mice
The role of TAF15 in OA was further investigated by using ACLT-induced mouse model. It was first observed that TAF15 protein level in mouse cartilage tissues was markedly increased by ACLT administration, which was reversed by sh-TAF15 adenovirus infection (Fig. 6A). ACLT led to an increase in the number of MMP13-positive cells and downregulation of Collagen II expression in mouse cartilage tissues, whereas these changes were all reversed by TAF15 knockdown (Fig. 6B–C). The knee joint showed severe degeneration, accompanied by severe cartilage damage and decreased number of chondrocytes in ACLT group, while these cartilage pathological changes caused by ACLT were partially reversed by TAF15 silencing (Fig. 6D–E). Further analysis demonstrated that OARSI score was significantly increased by ACLT administration, while ACLT-induced OARSI score upregulation was abrogated by TAF15 knockdown (Fig. 6F). In total, TAF15 downregulation attenuated OA progression in ACLT mice.
Fig. 6.
TAF15 knockdown attenuated OA in ACLT mice. TAF15 was knocked down by adenovirus in ACLT-induced mouse model with knee OA (A) GREM1 protein level in mouse cartilage tissues was determined by Western blot (B–C) The number of MMP13-positive cells and Collagen II level in mouse cartilage tissues were examined using IHC (D–E) HE and Safranin O-stained sections were analyzed by three independent doctors blinded to the grouping, and the average scores were calculated (F) Histology analysis were employed to analyze the cartilage pathological changes. Data were expressed as mean ± SD. n = 5. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
4. Discussion
ACL injury is an important factor that caused post-traumatic OA [17]. Among the factors thought to initiate ACL injury-induced OA include elevated levels of inflammatory cytokines and proteases and increased ECM degradation [18]. All of these processes may trigger catabolic responses in joints that degrade the cartilage matrix and promote apoptosis [19]. There are currently no effective interventions other than total joint replacement surgery to slow the progression of ACL injury-induced OA. In the current study, we discovered an exact molecular mechanism involved in OA pathogenesis that TAF15 activated the GREM1-NF-κB pathway by promoting BRD4 mRNA stability to accelerate OA development.
TAF15 functions in regulating cell proliferation and apoptosis [20]. A previous study revealed that TAF15 regulated BMSC osteogenic differentiation by stabilizing DLX2 [21]. In addition, TAF15 was upregulated in OA [5]. Consistently, it was observed that TAF15 was markedly upregulated in cartilage tissues of ACL-OA patients and ACLT mice as well as in IL-1β-treated human chondrocytes in the present study. It also turned out that TAF15 knockdown suppressed chondrocyte apoptosis and ECM degradation induced by IL-1β. Moreover, TAF15 silencing attenuated OA in ACLT mice. Collectively, all our results suggested that TAF15 contributed to chondrocyte apoptosis and ECM degradation during OA progression.
As an RNA-binding protein, TAP15 plays an important role in mediating mRNA stability by regulating mRNA transcription, RNA splicing and trafficking, and this regulatory mechanism has been elucidated in numerous human malignant diseases [22]. As proof, TAF15 facilitated lung squamous cell carcinoma cell proliferation and migration by stabilizing HMGB3 mRNA [4]. Herein, a novel regulatory mechanism is proposed in OA development whereby TAF15 accelerates OA progression by maintaining BRD4 mRNA stability. BRD4 is essential for inducible inflammatory gene expression in inflammatory diseases. In the study of OA, the significance of BRD4 in inflammation has been emphasized, and BRD4 inhibition alleviates pathological changes in OA [10,23]. In addition, it was previously described that BRD4 recruitment to acetylated chromatin promoted proinflammatory cytokine-induced ECM degradation in chondrocytes during OA development [24]. In the current study, BRD4 expression in human chondrocytes was significantly increased following IL-1β stimulation. Encouragingly, it was found that TAF15 promoted BRD4 expression by directly binding with BRD4 and maintaining BRD4 mRNA stability. In addition, BRD4 upregulation TAF15 knockdown's repression on chondrocyte apoptosis and ECM degradation induced by IL-1β. Collectively, TAF15 promoted chondrocyte apoptosis and ECM degradation during OA progression by promoting BRD4 expression.
In the previous study, GREM1 was identified as a key risk factor promoting cartilage degeneration by excessive mechanical loading, mechanistically, GREM1 activated NF-κB pathway, resulting in subsequent cartilage degeneration [13]. And excessive mechanical loading is regarded as the essence of OA pathogenesis, leading to joint instability, trauma, and joint inflammation [13]. What is more, excessive mechanical loading promoted temporomandibular joint OA by upregulating BRD4 [10]. However, the relationship between TAF15 and mechanical stress remains unclear and needs further exploration. In the present research, IL-1β treatment resulted in elevated GREM1 expression in chondrocytes. As a reason, BRD4 promoted GREM1 expression in IL-1β-treated chondrocytes by directly targeting GREM1. Loss-and gain-of function experiments subsequently demonstrated that the GREM1-NF-κB functioned as the downstream pathway of BRD4 in facilitating chondrocyte apoptosis and ECM degradation induced by IL-1β. Taken together, BRD4 facilitated chondrocyte apoptosis and ECM degradation in OA by activating the GREM1/NF-κB pathway, and we speculated that mechanical loading may accelerate OA pathogenesis through TAF15-BRD4-GREM1axis.
Taken together, our research provided evidence that TAF15 upregulation TAF15 promoted GREM1 expression by maintaining BRD4 mRNA stability, thereby activating the gremlin-1/NF-κB pathway and promoting OA development. Our findings provided a rationale for developing novel therapies for ACL injury-induced OA.
Ethics approval and consent to participate
This study was passed the review of Ethics Committee of Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University) before enrollment of patients and all participants signed informed consent. The animal studies were approved by Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University).
Consent for publication
The informed consent was obtained from study participants.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
The authors express their gratitude for his help for directing our article. This work was supported by grants from Cultivation 530 Project of National Natural Science Foundation of China (General Project): Mechanism and application of NF-κB-Gremlin-1 pathway in osteoarthritis after anterior cruciate ligament injury (2021MSXM10); Hainan Health industry scientific research project: Joint instability and mechanical loading contribute to the mechanism of osteoarthritis through the Gremlin-1 NF-κB pathway (20A200467); Research and Cultivation Fund project of Hainan Medical College: Study on the mechanism and application of Gremlin-1 gene in osteoarthritis after anterior cruciate ligament injury(HYPY2020014).
Authors' contributions
Xiufan Du: Conceptualization; Methodology; Validation; Ruomei Xin: Formal analysis; Investigation; Xiaoyan Chen: Resources; Data Curation; Guangji Wang: Writing - Original Draft; Chunhang Huang: Visualization; Kai Zhou: Supervision; Project administration; Shunli Zhang: Writing - Review & Editing; Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Abbreviations
- (OA)
Osteoarthritis
- (ACL)
Anterior cruciate ligament
- (TAF15)
TATA-box binding protein associated factor 15
- (BMSC)
Bone marrow mesenchymal stem cell
- (NF-κB)
Nuclear transcription factor-κB
- (BMP)
Bone morphogenetic protein
- (GREM1)
Gremlin-1
- (IL-1β)
Interleukin-1 beta
- (ECM)
Extracellular matrix
- (ACLT)
Anterior cruciate ligament transection
- (CCK-8)
Cell counting kit-8
- (IHC)
Immunohistochemistry
- (RIP)
RNA immunoprecipitation
- (ChIP)
Chromatin immunoprecipitation
- (TUNEL)
TdT-mediated dUTP nick-end labeling
- (HE)
Hematoxylin-eosin
- (OARSI)
Osteoarthritis Research Society International
- (qRT-PCR)
Quantitative real-time polymerase chain reaction
References
- 1.Carbone A., Rodeo S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J Orthop Res. 2017;35(3):397–405. doi: 10.1002/jor.23341. [DOI] [PubMed] [Google Scholar]
- 2.Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.P., Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 3.Stein V., Li L., Lo G., Guermazi A., Zhang Y., Kent Kwoh C., et al. Pattern of joint damage in persons with knee osteoarthritis and concomitant ACL tears. Rheumatol Int. 2012;32(5):1197–1208. doi: 10.1007/s00296-010-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren P., Xing L., Hong X., Chang L., Zhang H. LncRNA PITPNA-AS1 boosts the proliferation and migration of lung squamous cell carcinoma cells by recruiting TAF15 to stabilize HMGB3 mRNA. Cancer Med. 2020;9(20):7706–7716. doi: 10.1002/cam4.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J.Y., Cheng M., Ye J.L., Peng C.H., Chen J., Luo B., et al. YY1-induced lncRNA XIST inhibits cartilage differentiation of BMSCs by binding with TAF15 to stabilizing FUT1 expression. Regen Ther. 2022;20:41–50. doi: 10.1016/j.reth.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y., Zhou J., Ye F., Xiong H., Peng L., Zheng Z., et al. BRD4 inhibitor inhibits colorectal cancer growth and metastasis. Int J Mol Sci. 2015;16(1):1928–1948. doi: 10.3390/ijms16011928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu W., Wu R.D., Lv Y.G., Liu Y.M., Huang H., Xu J.Q. BRD4 blockage alleviates pathological cardiac hypertrophy through the suppression of fibrosis and inflammation via reducing ROS generation. Biomed Pharmacother. 2020;121:109368. doi: 10.1016/j.biopha.2019.109368. [DOI] [PubMed] [Google Scholar]
- 8.Wei H., Xue Q., Sun L., Lv J. BRD4 inhibition protects against myocardial ischemia/reperfusion injury by suppressing inflammation and oxidative stress through the PI3K/AKT signaling pathway. J Cardiovasc Pharmacol. 2021;78(6):839–846. doi: 10.1097/FJC.0000000000001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y., Zhu L., Zhang T., Lu H., Wang C., Xue B., et al. BRD4 has dual effects on the HMGB1 and NF-κB signalling pathways and is a potential therapeutic target for osteoarthritis. Biochim Biophys Acta, Mol Basis Dis. 2017;1863(12):3001–3015. doi: 10.1016/j.bbadis.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z., Yang R., Zhang L., Zhu M., Zhang C., Wen J., et al. BRD4 inhibition alleviates mechanical stress-induced TMJ OA-like pathological changes and attenuates TREM1-mediated inflammatory response. Clin Epigenet. 2021;13(1):10. doi: 10.1186/s13148-021-01008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khokha M.K., Hsu D., Brunet L.J., Dionne M.S., Harland R.M. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet. 2003;34(3):303–307. doi: 10.1038/ng1178. [DOI] [PubMed] [Google Scholar]
- 12.Jiang R., Gao H., Cong F., Zhang W., Song T., Yu Z. Circ_DHRS3 positively regulates GREM1 expression by competitively targeting miR-183-5p to modulate IL-1β-administered chondrocyte proliferation, apoptosis and ECM degradation. Int Immunopharm. 2021;91:107293. doi: 10.1016/j.intimp.2020.107293. [DOI] [PubMed] [Google Scholar]
- 13.Chang S.H., Mori D., Kobayashi H., Mori Y., Nakamoto H., Okada K., et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1-NF-κB pathway. Nat Commun. 2019;10(1):1442. doi: 10.1038/s41467-019-09491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tejedor-Santamaria L., Morgado-Pascual J.L., Marquez-Exposito L., Suarez-Alvarez B., Rodrigues-Diez R.R., Tejera-Muñoz A., et al. Epigenetic modulation of gremlin-1/NOTCH pathway in experimental crescentic immune-mediated glomerulonephritis. Pharmaceuticals. 2022;15(2) doi: 10.3390/ph15020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahy N., Farrell E., Ritter T., Ryan A.E., Murphy J.M. Immune modulation to improve tissue engineering outcomes for cartilage repair in the osteoarthritic joint. Tissue Eng B Rev. 2015;21(1):55–66. doi: 10.1089/ten.teb.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobson F., Hinman R.S., Roos E.M., Abbott J.H., Stratford P., Davis A.M., et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(8):1042–1052. doi: 10.1016/j.joca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Ajuied A., Wong F., Smith C., Norris M., Earnshaw P., Back D., et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242–2252. doi: 10.1177/0363546513508376. [DOI] [PubMed] [Google Scholar]
- 18.Haslauer C.M., Elsaid K.A., Fleming B.C., Proffen B.L., Johnson V.M., Murray M.M. Loss of extracellular matrix from articular cartilage is mediated by the synovium and ligament after anterior cruciate ligament injury. Osteoarthritis Cartilage. 2013;21(12):1950–1957. doi: 10.1016/j.joca.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson D.D, Chubinskaya S., Guilak F., Martin J.A., Oegema T.R., Olson S.A., et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballarino M., Jobert L., Dembélé D., de la Grange P., Auboeuf D., Tora L. TAF15 is important for cellular proliferation and regulates the expression of a subset of cell cycle genes through miRNAs. Oncogene. 2013;32(39):4646–4655. doi: 10.1038/onc.2012.490. [DOI] [PubMed] [Google Scholar]
- 21.Zeng X., Dong Q., Liu Q., Tan W.J., Liu X.D. LncRNA HOTTIP facilitates osteogenic differentiation in bone marrow mesenchymal stem cells and induces angiogenesis via interacting with TAF15 to stabilize DLX2. Exp Cell Res. 2022;417(2):113226. doi: 10.1016/j.yexcr.2022.113226. [DOI] [PubMed] [Google Scholar]
- 22.Ruan X., Zheng J., Liu X., Liu Y., Liu L., Ma J., et al. lncRNA LINC00665 stabilized by TAF15 impeded the malignant biological behaviors of glioma cells via STAU1-mediated mRNA degradation. Mol Ther Nucleic Acids. 2020;20:823–840. doi: 10.1016/j.omtn.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukui T., Yik J.H.N., Doyran B., Davis J., Haudenschild A.K., Adamopoulos I.E., et al. Bromodomain-containing-protein-4 and cyclin-dependent-kinase-9 inhibitors interact synergistically in vitro and combined treatment reduces post-traumatic osteoarthritis severity in mice. Osteoarthritis Cartilage. 2021;29(1):68–77. doi: 10.1016/j.joca.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai J., Zhou S., Ge Q., Qin J., Li J., Ju H., et al. Recruitment of Brd3 and Brd4 to acetylated chromatin is essential for proinflammatory cytokine-induced matrix-degrading enzyme expression. J Orthop Surg Res. 2019;14(1):59. doi: 10.1186/s13018-019-1091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.