Abstract
Viscoelastic testing (VET) in liver transplantation (LT) has been used since its origin, in combination with standard laboratory testing (SLT). There are only a few, small, randomized controlled trials that demonstrated a reduction in transfusion rates using VET to guide coagulation management. Retrospective analyses contrasting VET to SLT have demonstrated mixed results, with a recent concern for overtreatment and the increase in postoperative thrombotic events. An oversight of many studies evaluating VET in LT is a single protocol that does not address the different phases of surgery, in addition to pre- and postoperative management. Furthermore, the coagulation spectrum of patients entering and exiting the operating room is diverse, as these patients can have varying anatomic and physiologic risk factors for thrombosis. A single transfusion strategy for all is short sighted. VET in combination with SLT creates the opportunity for personalized resuscitation in surgery which can address the many challenges in LT where patients are at a paradoxical risk for both life-threatening bleeding and clotting. With emerging data on the role of rebalanced coagulation in cirrhosis and hypercoagulability following LT, there are numerous potential roles in VET management of LT that have been unaddressed.
Keywords: liver transplantation, viscoelastic testing, transfusion
Understanding the dynamic changes in coagulation during liver transplantation (LT) is essential to optimize outcomes.1 Standard laboratory tests (SLTs) paired with viscoelastic testing (VET) have been utilized in LT for decades to aid in the treatment of coagulopathy during surgery.2 The dynamic changes in coagulation range from preoperative coagulopathy from underling liver disease to hyperfibrinolysis after removal of the liver often associated with rebound hypercoagulability.3 Mismanagement puts the patient at risk of exsanguination or pathologic thrombosis.
With the improvements in surgical technique and patient selection, LT can be a transfusion-free operation.4 Minimizing blood product use improves both resource utilization5 and graft survival.6 Numerous reviews have been written on coagulation and transfusion management during LT7–9; however, there is no consensus on the type or timing of coagulation assays or transfusion triggers. The purpose of this review is to explore different modalities to monitor coagulation during LT. The importance of personalized hemostatic resuscitation in LT has been proposed,10 but has not been fully explored with regard to coagulation assessment and management.
Coagulation Changes in End-Stage Liver Disease
Patients with cirrhosis have traditionally been thought of as intrinsically anticoagulated due to the reduction in liver-dependent procoagulant factors and lower platelet count. This concept has been challenged by evidence that suggests that disturbances in procoagulant and anticoagulant processes are “rebalanced” leading to preserved hemostasis.11 Thrombocytopenia due to splenic sequestration and impaired production is compensated by increased levels of von Willebrand factor (VWF) and decreases in the metalloproteinase, ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13).12,13 Procoagulants such as proteins C and S are decreased in concentration due to impaired hepatic synthetic function and represent a potential for increased risk of thrombosis due to impaired regulation of coagulation factors Vand VIII.14 This theory of “rebalanced” hemostasis is supported by the presence of near-normal VET in cirrhotic patients.15 Destabilizing events such as infection and procedural or physiologic stressors can disrupt this precarious balance leading to either hemorrhage or deleterious clotting events.
SLT versus VET in Liver Disease
SLT utilized during the perioperative period of LT includes international normalized ratio (INR), activated partial thromboplastin time (aPTT), complete blood count, comprehensive metabolic panel, Clauss fibrinogen, and arterial blood gas sampling. SLT is limited because it does not give a comprehensive overview of a patient’s coagulation status. Evidence for this stems from the cell-based model of hemostasis.16 In this model, tissue factor–bearing cells help initiate thrombin generation, which in turn is amplified by the cell surface of the platelet and leads to clot propagation. Functional coagulation SLT (INR, aPTT, fibrinogen) partitions the plasma from the cellular components, whereas VET as a whole blood assay assesses these cellular contributors in addition to proteases in generating a clot and its subsequent degradation.
SLT in patients with end-stage liver disease is undertaken routinely, as the model for end-stage liver disease (MELD) score is weighted by the patient’s INR level.17 INR is a measure of the extrinsic and common pathways of the clotting cascade, although mainly affected by abnormalities in factor VII.18 Preoperative INR values have been shown to be a poor predictor of both periprocedural bleeding in cirrhotic patients19 and of transfusion requirements during LT.20,21 Clot strength and stability are measured by VET with either thromboelastography (TEG) maximum amplitude (MA) (TEG 5000 and TEG 6s Thrombelastograph Hemostasis Analyzer; Haemonetics Corporation, Boston MA) or rotational thromboelastography (ROTEM) maximum clot firmness MCF (ROTEM Delta and ROTEM Sigma; Werfen, Warrington, UK) and can reflect derangements in the fibrinogen level as well as the platelet count and function.22 Randomized controlled trials in cirrhotic patients have demonstrated major reductions in transfusion requirements for the management of periprocedural coagulopathy23 and bleeding.24 These data suggest that pretransplant VET provides a better assessment of bleeding risk than SLT due to the rebalance of hemostasis that is captured by whole blood coagulation assessment rather than partitioned assays. Clinical advantages and disadvantages of SLT and VET are listed in Table 1.
Table 1.
Methods for assessing bleeding and coagulation in liver transplant
| SLT | |||||
|---|---|---|---|---|---|
| Indication | What does it test? | Specimen type | Time to resultsa | Interventions | |
| ABG | Graft function and bleeding | Blood gas concentrations and pH | Arterial whole blood | 10–20 min | RBC, bicarbonate |
| Clauss fibrinogen | Coagulation | Fibrinogen concentration | Plasma | 30–45 min | Cryoprecipitate or fibrinogen |
| CBC | Coagulation and bleeding | Platelet count | Whole blood | 30–60 min | Platelet and RBC |
| INR | Coagulation and graft function | Speed of clotting | Plasma | 40–60 min | Plasma, PCC |
| aPTT | Coagulation | Speed of clotting | Plasma | 40–60 min | Plasma, protamine |
| CMP | Graft injury and graft function | Blood chemistry concentrations; liver and kidney function | Plasma | 60–90 min | Graft dysfunction |
| VET | |||||
| Indication | What does it test? | Specimen type | Time to resultsb | Interventions | |
| TEG 5000 | Coagulation | Speed of clotting, platelet function, clot strength, and fibrinolysis | Whole blood | First results 5–10 min Last results 60–90 min |
Plasma, PCC, protamine, cryoprecipitate, fibrinogen platelets, antifibrinolytic |
| TEG 6s | Coagulation | Speed of clotting, platelet function, clot strength, and fibrinolysis | Whole blood | First results 5–10 min Last results 60–90 min |
Plasma, PCC, protamine, cryoprecipitate, fibrinogen platelets, antifibrinolytic |
| ROTEM Delta | Coagulation | Clot formation, clot firmness, and clot fibrinolysis | Whole blood | First results 5–10 min Last results 60–90 min |
Plasma, PCC, protamine cryoprecipitate, fibrinogen, platelets, antifibrinolytic |
| ROTEM Sigma | Coagulation | Clot formation, clot firmness, and clot fibrinolysis | Whole blood | First results 5–10 min Last results 60–90 min |
Plasma, PCC, protamine cryoprecipitate, fibrinogen, platelets, antifibrinolytic |
| HemoSonics Quantra | Coagulation | Speed of clotting and clot strength | Whole blood | First results 5–10 min Last results 60–90 min |
Plasma, PCC, protamine, cryoprecipitate, fibrinogen, platelets, antifibrinolytic |
Abbreviations: ABG, arterial blood glass; CBC, complete blood count; CMP, complete metabolic panel; INR, international normalization of prothrombin time; PCC, prothrombin complex concentrate; aPTT, activated partial thromboplastin time; RBC, red blood cell; ROTEM, rotational thrombelastography; SLT, standard laboratory testing; TEG, thrombelastography; VET, viscoelastic testing.
Time to results for SLT are estimated based on clinical experience.
Time to results for VET list “first results” which refers to the initial results containing information for clotting time and “last results” which provides data on the lysis parameters.
Evidence for Benefits of VET Use in LT
Numerous studies suggest the benefit in using VET-guided resuscitation practices in reducing blood utilization in LT.2,23,25–29 This was first demonstrated in 1985 when Kang and colleagues,2 contrasting a historical cohort pre- and post-VET implementation, showed a 30% reduction in transfused blood products. Nonrandomized studies have since found not only a reduction in blood utilization but extra benefits such as a reduction in the rates of acute kidney injury (AKI), fewer reoperations for hemorrhage, reduced early graft dysfunction, and a lesser need for postoperative vasopressor support.30–33 There remains large heterogeneity in VET studies during LT regarding transfusion triggers, timing of assays, and patient population (Table 2).
Table 2.
VET studies in liver transplant describing parameters for hemostatic interventions
| Study | Study type | N | MELD (median) | Device | Reagents and/or test | Assay inflection points for interventions: | |||
|---|---|---|---|---|---|---|---|---|---|
| Plasma | Cryoprecipitate/Fibrinogen | Platelets | Antifibrinolytic | ||||||
| Trzebicki et al38 | Observational | 39 | 17 | ROTEM Delta | EXTEM, FIBTEM | Clotting time with EXTEM > 90 min | MCF with FIBTEM < 10 | MCF with EXTEM < 50 | Maximum lysis > 15% |
| Wang et al26 | Randomized controlled trial | 28 | 11 | TEG 5000 | Kaolin | Reaction time > 10 min | Angle <45° | Maximum amplitude < 50 mm | None reported |
| Roullet et al39 | Observational | 60 | 19 | ROTEM Delta | EXTEM, FIBTEM | Not reported | Amplitude at 10 min with EXTEM < 26 mm; amplitude at 10 min with EXTEM ≥ 29 mm and with FIBTEM ≤ 8 mm | Amplitude at 10 min with EXTEM < 26 mm and with FIBTEM > 8 mm | “Hyperfibrinolysis present” with EXTEM or FIBTEM |
| De Pietri et al23 | Observational | 386 | 20 | TEG 5000 | Native, FF | Reaction time > 40 min and maximum amplitude with FF > 7 mm | Maximum amplitude < 30 mm and maximum amplitude with FF < 7 mm | Maximum amplitude < 30mm and maximum amplitude with FF > 7 mm | “Fibrinolysis present” |
| Smart et al28 | Observational | 68 | 28 | ROTEM Delta | EXTEM, FIBTEM | Clotting time with EXTEM > 90 min | MCF with FIBTEM < 10 | MCF with EXTEM < 50 | Maximum lysis with EXTEM > 15% |
| Zamper et al27 | Propensity matched | 237 | 22 | ROTEM Delta | INTEM, HEPTEM, EXTEM, FIBTEM | Clotting time with INTEM > 240 min and clotting time with HEPTEM ≥ clotting time with INTEM | Amplitude at 5 min with EXTEM < 25 mm and amplitude at 10 min with FIBTEM < 10 mm | Amplitude at 5 min with EXTEM < 25 mm and amplitude at 10 min with FIBTEM > 10 mm | Clot lysis index at 30 min with EXTEM < 50% after reperfusion |
| Bonnet et al25 | Randomized controlled trial | 81 | 16 | ROTEM Delta | EXTEM, APTEM | Clotting time with EXTEM > 110 min | Amplitude at 10 min with FIBTEM < 10 mm | MCF with EXTEM < 40 mm or amplitude at 10 min < 35 mm and amplitude at 10 min with FIBTEM > 8 mm or MCF > 8 mm | Hyperfibrinolysis with EXTEM or maximum lysis < 15% and with APTEM < 10% clotting time or clot formation time > 15% MCF |
| Scarlatescu et al29 | Propensity matched | 188 | 19 | ROTEM Delta | INTEM, HEPTEM, EXTEM, FIBTEM | Clotting time with INTEM > 280 min and clotting time with HEPTEM > 280 min | Amplitude at 10 min EXTEM < 25 mm and amplitude at 10 min FIBTEM < 8 mm | Amplitude at 10 min with EXTEM < 25 mm and amplitude at 10 min with FIBTEM > 8 mm | Clot lysis index at 60 min EXTEM < 85% in pre-anhepatic phase or clot lysis index at 30 min with EXTEM < 50% anhepatic phase or later |
| Gaspari et al40 | Propensity matched | 226 | 20 | TEG 5000 | Kaolin | Not reported | Angle < 55° | Maximum amplitude < 30 mm | Not reported |
Abbreviations: FF, functional fibrinogen; MELD, Model for End-Stage Liver Disease score; N, population number; ROTEM, rotational thrombelastography; TEG, thrombelastography; VET, viscoelastic testing.
Two randomized control trials have evaluated the use of VET-guided transfusion25,26 identifying a significant reduction in fresh frozen plasma (FFP) but not in other blood products. Overall, the literature suggests that employing VETs in a protocol-based approach during LT can decrease the blood product utilization and may have a positive impact on early postoperative outcomes.34
Limitations of VET in LT
VET is susceptible to considerable technical variation during blood collection and processing. TEG and ROTEM are classified as moderately complex tests by CLIA (Clinical Laboratory Improvement Amendments). Routine training and user proficiency are vital for consistent production and accurate interpretation of results. Older VET technology requires time-intensive quality control and quality assurance practices. Both the newer TEG 6S and ROTEM Sigma systems employ a more fully automated, cartridge-based system with a less cumbersome quality-control and maintenance process, thus decreasing operator variability.35,36
VET may be costlier when compared with SLT; however, this may be offset by a reduction in overall care costs.30 There is also evidence to show that agreement between ROTEM and TEG values is fair to moderate at best and that resuscitation practices may differ depending on which assay is used.37 Finally, VET evaluates coagulation in a static environment and does not consider changes in blood flow or the contribution from endothelial cells,12 and standard VET assays cannot reliably evaluate coagulopathy-related inherited bleeding disorders,15 the effects of direct oral anticoagulants, or platelet inhibitors.36
Not all studies have demonstrated a significant reduction in blood loss with VET.38–40 A propensity-score study comparing blood product requirements in patients undergoing LT using VET or SLT identified no difference in blood product utilization.40 More recently, a VET-based algorithm identified an increased rate for postoperative thrombotic complications associated with the greater use of cryoprecipitate transfusions.41 These data support that ongoing work is needed in the use of VET to guide transfusion management in LT.
Preoperative Coagulation Management
The etiology of liver failure varies widely and can influence preoperative coagulation status. For example, nonalcoholic liver disease patients tend to have normal range VET results with abnormal SLT results, while alcoholic liver disease demonstrates decompensation in both forms of testing.42 Cholestatic liver disease is associated with VET-detected hypercoagulability but with an elevated INR.43 Primary liver cancer has been linked with hypercoagulability related to high fibrinogen levels.44 The combination of hepatocellular carcinoma and increased fibrin clot strength measured by VET is also associated with pretransplant portal vein thrombosis in cirrhotics.45
Fig. 1 represents the range of potential preoperative coagulopathy traits based on LT organ recipients. The timing and indication for coagulation assessment is markedly diverse between patients. Living donor liver recipients can have SLT and VET undertaken the week before their scheduled operation to identify potential coagulopathy. They are at increased risk of hepatic artery thrombosis (HAT),46 largely attributed to technical challenges in small diameter arterial anastomoses but also when preoperative VET demonstrates increased clot strength.47 Other investigators have shown that a low level of fibrinolysis preoperatively is associated with an increased risk of postoperative thrombotic complications.48 These VET preoperative parameters can help guide transfusion strategies during the operation and into recovery, with a tolerance for a more permissive hypocoagulable resuscitation.
Fig. 1.

Spectrum of preoperative coagulopathy of liver transplantation. VET, viscoelastic testing; HCC, hepatocellular carcinoma; PSC, primary sclerosing cholangitis; PBC, primary biliary cirrhosis. Fig. 1 represents the spectrum of patients who can receive organ donation for transplantation. Those patients with acute liver decompensation are the sickest group of recipients and their coagulation system is not intact. VET testing in cirrhotic patients bleeding and at risk of bleeding have demonstrated major reductions in transfusion volumes over SLT, which is critical in this pretransplant population as they often have concurrent renal failure. However, transfusion triggers based on VET do not need to be corrected to a normal range. Repeated routine testing to manage coagulopathy is essential as liver function rarely recovers, and failure to address clotting derangements can result in complication making them ineligible for transplant. On the opposite end of the spectrum are living donors who come from home and are seen the week before transplant with time for coagulation assessment. These patients tend to have well-compensated liver disease and are more at risk of thrombotic complications than cadaveric organs due to small diameter vascular reconstructions during surgery. Most liver transplant patients fall in between these two spectrums and come from home after being called about an organ offer. Some of these patients may have increased risk for thrombosis based on their indication for liver transplant (cancer or inflammatory condition) and VET testing can help risk stratify them for postoperative thrombotic risk and guide a more permissive hypocoagulable strategy during surgery. Other patients coming from home may have harbor unappreciated advanced coagulopathy and have increased risk of massive transfusion. Identification of this cohort can allow the blood bank to prepare for anticipated large blood loss.
On the other end of the spectrum are LT recipients with acute-on-chronic decompensated liver failure. These patients can be profoundly coagulopathic and failure to address coagulopathy can result in uncontrolled nonsurgical bleeding and death before transplant. There are currently no studies identifying the optimal transfusion thresholds using VET for decompensated liver failure patients awaiting transplant. However, several randomized control trials of cirrhotic patients comparing VET to SLT to guide coagulopathy management demonstrated a reduction in transfusions and a trend toward improvement in survival in the VET groups (Table 3).23,24,49
Table 3.
Randomized control trials comparing VET to SLT to guide coagulopathy management
| Study | Patient population | Objective | VET assay | Inflection point |
|---|---|---|---|---|
| De Pietri et al23 | Cirrhotic | Reduce prophylactic blood for procedure | Native TEG | R > 40 min = plasma |
| MA < 20 mm = platelet | ||||
| Rout et al49 | Cirrhotic | Reduce blood product in variceal bleeding | Kaolin TEG | R > 15 min = plasma |
| MA <30 min = platelet | ||||
| Kumar et al24 | Cirrhotic | Reduce blood product in nonvariceal bleeding | Kaolin TEG | R > 10 min = plasma |
| Angle < 45° = fibrinogen | ||||
| MA < 55 mm = platelet | ||||
| Zahr Eldeen et al47 | Liver transplant | Risk stratify for HAT | Kaolin TEG | MA > 65 mm = postoperative risk |
| Nicolau-Raducu et al48 | Liver transplant | Risk stratify for thrombotic event | Kaolin TEG | LY30 <0.9% = postoperative risk |
| Pustavoitau et al50 | Liver transplant | Evaluate risk of intraoperative massive transfusion | Kaolin TEG | R > 6 min = risk for massive transfusion |
| Lawson et al51 | Liver transplant | Evaluate risk of intraoperative massive transfusion | Native TEG | MA < 47 mm = risk for massive transfusion |
| Steib et al52 | Liver transplant | Evaluate risk of intraoperative hyperfibrinolysis | Kaolin TEG | MA < 35 mm = risk for hyperfibrinolysis |
Abbreviations: HAT, hepatic artery thrombosis; MA, maximum amplitude; R, reaction time; SLT, standard laboratory testing; TEG, thrombelastography; VET, viscoelastic testing.
Predictors for massive transfusion during LT have relied on both VET and SLT.50 VET variables that are associated with intraoperative bleeding include a prolonged R time50 and a reduced MA.51 Preoperative VET can also predict hyperfibrinolysis.52 SLTvariables include platelet count,53 hemoglobin,50 and prolonged INR.53 Additional clinical factors including prior operations, MELD score, recipient age, and graft ischemia time can also increase the risk of massive transfusion.54 These laboratory tests in the context of the patient’s clinical status not only help guide early resuscitation in the operating room but can help the blood bank prepare for massive transfusion protocols as needed.
Intraoperative Coagulation Assessment
Native hepatectomy (pre-anhepatic), anhepatic, and reperfusion (neohepatic) represent the three unique phases of LT. Differences in coagulopathy are based on the patient’s underlying pathophysiology of liver disease2 and are influenced by donor graft quality.55 Despite these well-known phases, standardized language to define bleeding diathesis has not been established in LT. As a result, the timing of coagulation assessment and transfusion practices are widely variable within institutions and errors in under- or over-correcting coagulopathy can result in excessive bleeding or unnecessary clotting. Fig. 2 represents a schematic of intraoperative considerations for coagulation assessment and transfusion strategies during the different phases of surgery.
Fig. 2.
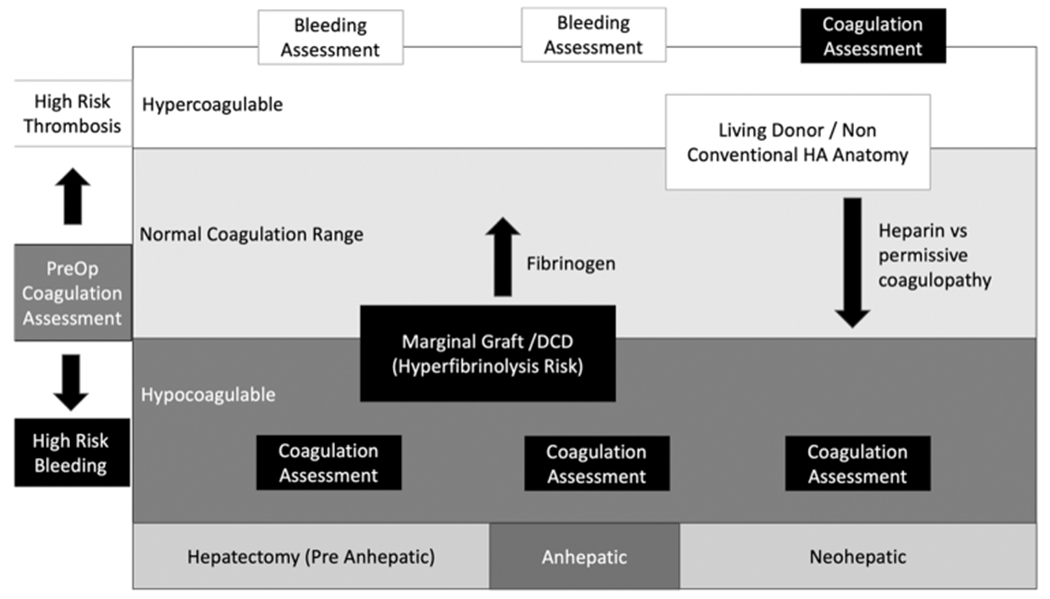
Intraoperative coagulation considerations. VET, viscoelastic testing; DCD, donation after circulatory death; HA, hepatic artery. Fig. 2 represents the dynamic changes in intraoperative coagulation assessment and treatment during the different phases of surgery. Preoperative coagulation assessment can place the patient on their trajectory toward bleeding during surgery or thrombotic complications. In those patients with preoperative hypercoagulable states, routine measurement of coagulation during the pre-anhepatic and anhepatic stage of surgery is not needed. The indication for hemostatic transfusions can be triggered by clinical assessment of bleeding. During the neohepatic phase, large changes in coagulation commonly occur and routine coagulation assessment to identify the patient’s new coagulation status can help guide a transfusion strategy. Hypocoagulable liver transplant patients on the other hand are at increased risk of major blood loss from the start of surgery. Routine coagulation assessment throughout the duration of surgery can be helpful to stay on top of coagulopathic bleeding. This is particularly relevant in LT recipients of marginal organs or DCD grafts at high risk of hyperfibrinolysis. Recipients of these grafts can benefit from preemptive fibrinogen transfusions before graft reperfusion, as there will be an anticipated dropped in fibrinogen. After reperfusion, anatomic considerations of the graft can guide resuscitation based on VET results. High-risk patients for HAT can be passively addressed with a tolerance for declining clotting indices appreciated on VET while the graft is reperfusion and transfusion triggers can be adjusted to tolerated a more hypocoagulable level in the absence of ongoing bleeding. Alternatively, this can be achieved actively with an intraoperative heparin infusion if there is a heightened concern.
Pre-Anhepatic
Early clinical assessment after making an incision is the ideal time to communicate with the anesthesia team personnel regarding any coagulopathy as well discuss preoperative interpretation of coagulation testing. Prior rating of clinical coagulopathy has been proposed by the National Institution of Health regarding trauma56 and has been also validated in transplant patients.57 This score ranges from 1 to 5 in which 1 represents normal anticipated bleeding, 3 represents more than expected bleeding necessitating coagulation assessment, and 5 corresponds to profound coagulopathy warranting empiric transfusions and packing. Bleeding scores in LT patients during the native hepatectomy are predominantly less than 3 suggesting that routine coagulation assessment during this phase of surgery is not needed.57
Cell Salvage Utilization during Noncoagulopathic Bleeding
Autologous blood salvage techniques have been used intraoperatively to scavenge blood from the surgical field for use as autologous transfusion. There has been concern that blood salvage can cause a dilution of procoagulant factors and platelets58 but, in reality, is associated with reduced allogeneic transfusions during LT.20,59 Little is known about the impact cell salvage may have on VET or the use of VET to guide resuscitation with autologous transfusion during LT. In a prospective study evaluating aneurysm surgery, VET parameters never reached a preset threshold for intervention.60 In a smaller study in cardiac surgery, patients with large-volume autologous blood transfusions had a paradoxical procoagulant effect when evaluated by VET.61 More research is needed in this area to explore the use of VET in liver transplants where blood salvage is used, as cirrhotic patients have markedly different coagulation profiles compared with patients with vascular disease.
Bleeding Management during Pre-Anhepatic
In our institution, we aim to keep the intraoperative hemoglobin concentration between 8 and 10 g/dL as demonstrated on serial arterial blood gas analyses. Hemoglobin assessment paired with base deficit trends allows close monitoring of the patient’s resuscitation status throughout the case. This assay has the advantage of quick turnaround time but has limited utilization in monitoring for coagulopathy. Prior to the return of autologous blood, we will match red blood cell (RBC) transfusion 1:1 with FFP unless hypercoagulability is demonstrated on preoperative TEG by a shortened R time. In this case, we may refrain from giving FFP until R time has normalized. We will consider preemptive transfusion of cryoprecipitate or fibrinogen concentrates if preoperative fibrinogen levels are less than 100 mg/dL or there is a severely decreased angle on TEG, or in patients who exhibit moderate hypofibrinogenemia (<200 mg/dL) and there is a concern for ongoing bleeding preoperatively. Preemptive platelet transfusion is similarly approached by the severity of thrombocytopenia, a reduction of the MA on the TEG and clinical evidence of bleeding. While we are more conservative with cryoprecipitate and platelets prior to the neohepatic phase of surgery, VET and SLT can help guide transfusions when there is evidence of clinical coagulopathy.
Anhepatic
This relatively short 30-minute phase of the operation represents a unique coagulation progression in which fibrinolysis is anticipated to be activated. This hyperfibrinolytic phase can cause a rapid drop in fibrinogen levels and associated bleeding.62 Hyperfibrinolysis can be demonstrated by a “tear drop” shape VET waveform detected by TEG and ROTEM.62,63 This is thought to be due to a decrease in hepatic clearance of endogenous tissue plasminogen activator (t-PA) during the anhepatic phase and is punctuated by a dramatic increase in levels immediately following reperfusion of the donor graft. Measurements with conventional coagulation assays such as fibrin degradation products are nonspecific for detecting changes in fibrinolytic activity2 and the turnaround time of a euglobulin lysis assay is clinically prohibitive. VET holds an advantage over SLT in this regard. VET testing has identified that 60% of transplant recipients develop a hyperfibrinolytic trait.62,64 Hyperfibrinolysis is usually a self-limiting process and resolves within 30 minutes of reperfusion without additional treatment, although this is dependent on donor liver quality.62,63,65
Role for Fibrinogen First Resuscitation
Correction of preexisting hypofibrinogenemia decreases intraoperative blood loss during surgery.66 The threshold for transfusion and the preferred testing modality remains under debate as preemptive transfusion of fibrinogen did not appear to reduce intraoperative transfusions in a recent study.67 This is not surprising as there is a wide range of coagulopathy that can present at the time of LT, and only a portion of patients experience hyperfibrinolysis during LT.62,64 Therefore, VET can be a useful tool to guide resuscitation if hyperfibrinolysis is anticipated to exacerbate any existing hypofibrinogenemia during the anhepatic phase. This will manifest on VET as a low angle and clot strength with elevated lysis. Fibrinogen replacement is the initial intervention as it will improve both clot parameters with self-resolution of fibrinolysis after graft reperfusion.68 Preoperative clot strength (MA < 35 mm) has been reported to have 100% predictability of hyperfibrinolysis in surgery.52 VET-guided treatment of hypofibrinogenemia before the neohepatic phase may help prevent the overtransfusion of platelets which is associated with acute lung injury and worse patient outcomes.69
Preemptive Antifibrinolytics
An alternative approach is to preemptively utilize antifibrinolytics during the anhepatic phase of surgery. Initiation of antifibrinolytic therapy should be done with careful consideration of clinical microvascular bleeding and the presence of hyperfibrinolysis on VET.63,70 A systematic review and meta-analysis evaluating antifibrinolytics identified a reduction in blood loss and transfusion requirements but no increase in the risk of thromboembolism in LT.71 The presence of hyperfibrinolysis on VET earlier in the dissection phase of liver transplant may warrant more aggressive intervention with antifibrinolytics9; however, international guidelines do not recommend their routine use.72 There are no trials addressing VET-guided versus empiric treatment of antifibrinolytics.
Bleeding Management during Anhepatic
In those patients with active bleeding and concern for clinical coagulopathy, empiric cryoprecipitate or fibrinogen concentrate is transfused followed by VET testing. We utilize exogenous t-PA in our donation after circulatory death (DCD) donor organs and expect hyperfibrinolysis on reperfusion. We aim to achieve fibrinogen levels greater than 150 mg/dL before graft reperfusion to act as a buffer for anticipated hyperfibrinolysis.
Neohepatic
Early after reperfusion, clinical bleeding is at its most severe and often warrants empiric transfusion.57 The most common coagulation derangement identified by VET during this time is decreased fibrinogen levels and poor platelet contribution to clot strength. Using a fibrinogen-specific VET assay can help differentiate clot strength reduction related to either low platelet counts or low fibrinogen levels.73 While fibrinogen is the likely culprit during the anhepatic phase, post-reperfusion platelet function is often markedly reduced and can remained suppressed for the duration of the operation.74 Ensuring both platelet and fibrinogen contributions to clot strength is important to regain hemostasis. This can also be exacerbated by post-reperfusion syndrome which is associated with a higher frequency of major blood loss.75 As previously stated, coagulation changes may be transient and begin to self-correct within 30 minutes of the graft being reperfused. Persistent coagulopathy may be impaired by lack of clearance of t-PA,62 metabolic metabolites altering coagulation,76 and heparinoids (endogenous and from donor).77 Routine VET and SLT during this time frame are essential in successful resuscitation and a surrogate of graft function.
Adjuncts to Uncontrolled Bleeding and Coagulopathy
In the setting of profound coagulopathy and continued massive bleeding, additional adjuncts can be utilized. Lower quality grafts, often from older donors, have an associated longer time to clot initiation and decreased clot strength when measured by VET.55 VET-detected persistent coagulopathy (native TEG MA < 45 mm) is an indicator for a return to the operating room after index operation.78 Patients with persistently low clot strength after hepatic artery reperfusion with ongoing bleeding can benefit from packing and allowing the anesthesia team to catch up on resuscitation. However, if packing and VET-guided resuscitation are failing, additional hemostatic agents can be considered. Prothrombin complex concentrates (PCCs) are infrequently used but can be considered in patients with persistently elevated INR or clotting time on VET despite aggressive resuscitation. Protamine can be administered when large differences exist in heparinase VET compared with nonheparinase VET in the setting of continued coagulopathy.79 Similarly, antifibrinolytics can be used if fibrinolysis is persistent.80 Reversal of coagulation using these parameters has been reported in case series with lethal rebound hypercoagulability81,82; so, we avoid routine use.
Transitioning from Hypocoagulable to Hypercoagulable
As bleeding begins to slow and the new liver starts to produce coagulation factors, hypercoagulability is often observed. SLT unfortunately gives little information regarding this transition to hypercoagulability. Hypercoagulability is an important driver of morbidity and mortality related to perioperative thromboembolism and can be identified using VET.83 Hypercoagulable tracing on VET has been found to be associated with the development of intracardiac thrombosis despite SLT demonstrating hypocoagulability.84 A recent propensity score matching study evaluating the rates of major thromboembolic complications (pulmonary thromboembolism (PTE), HAT, or ischemic cerebral vascular accident) following liver transplant before and after ROTEM found that both the use of cryoprecipitate and the incidence of major thrombotic complications were increased when ROTEM was used.20 This study highlights the importance of balancing hemostasis with coagulopathy and the threshold of cryoprecipitate transfusions based on VET may need to be tailored to the specific clinical scenario.
Fibrinolysis shutdown occurs almost universally in LT patients by 2 hours post-reperfusion.64 The timing is also associated with graft dysfunction, as failure to correct hyperfibrinolysis by 30 minutes of reperfusion is associated with a high rate of early allograft dysfunction. Fibrinolysis shutdown has been identified by numerous investigators in liver transplant and presumed to be associated with postoperative thrombotic complications.85–87
Bleeding Management Neohepatic
In our institution, we send a routine VET, INR, complete metabolic panel (CMP), and arterial blood gas 30 minutes following reperfusion to assess the patient’s coagulation and physiologic status. If there is clinical coagulopathy with massive bleeding, empiric platelet and cryoprecipitate fractions are transfused. VET is ordered every 30 minutes with arterial blood gas to monitor progress on coagulopathy management and to show evidence of graft recovery. If pH remains critically low, bicarbonate can be used to assist with coagulation by getting the systemic level to a more optimal level for enzymatic activity. After the hepatic artery has been reanastomosed, it is common for the surgical team to pack the surgical field and allow the anesthesia team to address the ongoing coagulopathy. In cases of severe coagulopathy with slow graft function (often associated with high transaminase levels), this can occur several times, and if not corrected we will stage the operation, with temporary abdominal closure and return to the operating room in 24 hours. At the other end of the spectrum are patients with minimal bleeding and hypercoagulable VET tracings. In these patients with increased risk of thrombosis, we will start a heparin infusion at 300 units per hour and draw a PTT, with a targeted range of 40 to 60 for the remainder of the operation.
Transition from the Operating Room to Recovery Room
Obtaining a dry surgical field prior to closing the abdomen does not always offset the benefits of extubation of the patient and cessation of general anesthesia. Decreasing vasoactive blood pressure medications88 and reducing the central venous pressure89 can help with graft recovery as they improve portal blood inflow and hepatic outflow. There are additional benefits to early extubation in the operating room beyond improving graft function, and this practice is becoming more common following LT.90 However, with the fast-track approach to the transition from the operating room to recovery room, new challenges in estimating the patient’s coagulation status have emerged. During this time frame, a well-orchestrated team approach to the management of bleeding is essential, as this period can be an hour or more of care. Failure to continue resuscitative efforts can lead to profound hypotension, prolonged graft dysfunction, and an exacerbation of the existing coagulopathy. Conversely, unnecessary transfusions may exacerbate postoperative hypercoagulability and increase risk factors for other postoperative thrombotic complications and contribute to fluid overload. In the absence of clinical bleeding, transfusions based on abnormally low laboratory values should be treated with cautioned, and can most frequently be observed. With improvement in liver function overtime and hemostasis, coagulopathy can be anticipated to self-resolve, and associated laboratory abnormalities are likely to remain stable to improve without intervention.
Consideration of Early Allograft Dysfunction
The coagulation assessment of patients having early extubation and transfer to the recovery room needs to be tailored to several factors. The first is a consideration of the graft quality and function. Early resolution of hyperfibrinolysis following reperfusion,64 clearance of base deficit,91 and bile acid production92 are supportive of good graft function. In LT recipients who have apparent early allograft dysfunction, obtaining coagulation assays at the time of fascial closure will allow for actionable items at arrival to the recovery room. An additional strategy is empiric red blood cell transfusions. Thrombocytopenia is a near universal occurrence following LT, and platelet counts can remain critically low for weeks following surgery.93 Targeting hemoglobin levels above 10 in the early postoperative period is advantageous, as it provides a buffer for ongoing bleeding, but also optimizes platelet function through margination.94 In patients with unstable hemoglobin trends, additional units of red blood cell transfusions can be given during transfer to help with coagulation efforts. The coagulation advantages of margination are dependent on flow conditions and are not captured in either SLT or VET.
Risk Factors for Hepatic Artery Thrombosis
The next consideration for correction of coagulation is based on recipient risk factors for HAT. Known risk factors for early thrombosis include living donor graft,46 hepatic artery reconstruction of nonconventional anatomy,95 hepatic artery blood flow less than 100mL/minute,96 prior abdominal operation,97 preoperative hypercoagulability,47 and low fibrinolysis activity during surgery.98 These are all consistent with Virchow triad of stasis (low flow), hypercoagulability, and endothelial damage (arterial reconstruction). Historically HAT was perceived to be attributed to technical complications, but coagulation is emerging as potentially modifiable risk factor.99 Patients with concern for increased risk of HAT in the early postoperative benefit by repeated utilization of VET, as this modality of coagulation assessment can identify when the patient is transitioning from a hypo- to a hypercoagulable state. Obtaining VET in these patients prior to transfer can provide invaluable information in the recovery room regarding the decision in transfusing hemostatic blood products, while also considering initiating heparin or antiplatelet therapy in patients who are hemodynamically stable.
Early Postoperative Resuscitation
During the first few hours of postoperative resuscitation, VET offers functional assessment of whole blood coagulation that can be supported by SLT. Randomized controlled trials have demonstrated reduced transfusions in LT were not specifically targeted to postoperative coagulation management.25,26 Bleeding management algorithms in LT and other surgical specialties focus heavily on intraoperative resuscitation.100 Postoperative VET-guided management of LT patients has received little attention101 and represents a key gap in the literature. The concept of “threading the needle” to avoid thrombotic and bleeding complications using VET has been recently demonstrated in COVID-19 patients, which stratifies patients based on clinical risk factors.102 Fig. 3 represents the spectrum of resuscitation strategies that may be utilized in the early postoperative period after the patient has left the operating room.
Fig. 3.

Spectrum of postoperative coagulopathy and bleeding in liver transplantation. CVP, central venous pressure; HAT, hepatic artery thrombosis. Fig. 3 represents the spectrum of postoperative bleeding and coagulopathy liver transplant recipients can manifest. Patients with massive bleeding often coincide with graft dysfunction. This requires empiric transfusions and coagulation assessment serves as a retrospective marker for progress in correcting coagulopathy. As bleeding slows and coagulation assessment results return, the more hemostatic blood products (fibrinogen and platelets) can be given first as indicated by VET. Some liver transplant recipients begin to demonstrate evidence of fluid overload during this phase causing increased CVP that can be exacerbated by declining renal function which can exacerbate graft dysfunction. A more judicious approach to goal-based resuscitation can be utilized at this time with scheduled coagulation assessment until clinical bleeding has resolved. Ultimately these transition into hemodynamic stability and coagulation assessment can be spaced out to several hours and treatment can be based on evidence of bleeding. On the opposite end of the spectrum are patients who come from the operating room with minimal bleeding. These patients can be treated with limited coagulation assessment and transfusions limited to evidence of bleeding. In this patient population, there also may be individuals who are increased risk of thrombotic complications. They can be managed by scheduling coagulation assessment with a restrictive transfusion strategy with consideration of heparin infusion orantiplatelet in the postoperative setting. Most liver transplant patients come out of the operating room between these two extremes and their coagulation assessment and transfusion triggers are guided on where they fall on the spectrum of active bleeding control with the consideration of thrombosis. The most challenging patients to manage are those who emerge with massive bleeding and graft dysfunction which leads to fluid overload with renal impairment and subsequent increased risk for postoperative thrombotic complications. The shifting of transfusion strategies in this population requires a careful vigilant team approach as mismanagement can lead to massive bleeding or thrombosis.
Massive Bleeding
In massively bleeding transplant patients with early graft dysfunction, resuscitation is often based on empiric transfusion with frequent laboratory assessment serving as a marker of successful resuscitation rather than guiding blood products. The time to results of VET and SLT will never be available to determine the current coagulation status. Falling behind in the correction of coagulation may warrant the unguided infusion of blood products. With massive resuscitation, the patient’s intravascular volume status becomes critical. Graft congestion due to increased venous pressure will impair recovery of the liver89 and it is not uncommon for renal function to decline following LT.103 Patients undergoing massive transfusion have large volume shifts and when the kidneys cannot regulate intravascular volume, a downward cycle of volume overload, graft dysfunction, acidosis, and coagulopathy can occur. Unlike the management of major bleeding in general and trauma cases where empiric 1:1 red blood cell to plasma resuscitation is used when volume is needed,104 utilization of fibrinogen or cryoprecipitate can reduce the volume transfused with an equal or greater hemostatic impact. Randomized controlled trials using VET in LT have repeatedly demonstrated that less plasma is transfused, and more fibrinogen-based products are given to address bleeding.25,26
Mechanical causes of graft dysfunction can manifest in persistent coagulopathy, such as intra-abdominal hypertension from undrained hematoma and volume overload. It is common to have patients return for a second operation following LT105 particularly if they had intraoperative coagulopathy78 and underwent a massive transfusion during surgery.106 Second operations rarely find major surgical bleeding and often simply remove hematomas. Local hematomas appear to drive ongoing local coagulopathy that is not identified by systemic coagulation assessment including VET. The pathophysiology remains unclear but in neurosurgery, the presence of chronic clots has been shown to cause ongoing bleeding through local activation of fibrinolysis.107 Patients who have large hematomas with evidence of multisystem organ failure (persistent pressor requirements, acidosis, low urine output) are often best managed with early reexploration than by attempted continued hemostatic blood product resuscitation.
Transitioning from Massive Transfusion to Goal Directed
A common confounder in SLT versus VET during the early postoperative period when fibrinogen has not been repleted is a prolonged INR with a normal clot or reaction time. INR is impacted by fibrinogen levels108 which can represent an artificially elevated INR despite adequate prothrombin activity. R time also represents one of the first TEG-based measurements to identify hypercoagulability despite prolonged INR.109 Both prolonged INR and FIBTEM are associated with postoperative bleeding.110 Low fibrinogen and prolonged INR with normal clot initiation time can be treated with fibrinogen to reduce extra transfusion volumes. This strategy to guide resuscitation in the postoperative period becomes increasingly more important when central venous pressure levels begin to rise and renal function declines. Balancing these risks of volume overload against hemostatic blood product usage warrant continued investigation, as the threshold for fibrinogen-rich products likely needs to be lower in patients in the postoperative setting than in the operating room.
When clinical bleeding has resolved, the frequency of VET can shift from routine to as directed when concerns for rebleeding reoccur. It is common for patients to have critically low platelet counts that can decline for the first 5 days after surgery.93 While the patient may not be bleeding from their surgery, critically low platelet counts can cause catastrophic intracranial bleeds, which often occur in the setting of postoperative hypertension.93 These declining platelet counts after surgery are inversely associated with high transaminases after graft reperfusion. Platelets may have a role in repairing injured liver grafts. Their migration and binding to damaged liver supports why platelet counts decline in living donor recipient and other liver transplant recipients with graft injury. following reperfusion.111 There are no VET assays guidelines for managing thrombocytopenia in the postoperative setting and anecdotally, most centers will transfuse to keep platelet counts above 20,000. In our experience, we have seen delayed coagulopathy occurring in patients having been successfully resuscitated that re-bleed when coagulopathy returned within 12 hours after their operation because of unaddressed falling platelet counts. VET to monitor patients with evidence of early allograft dysfunction based on first transaminase levels may have a potential role in guiding transfusions in this cohort.112
Post-Resuscitation Coagulation Management and Hypercoagulability
Postoperative hypercoagulable states after LT are increasingly observed. Platelet hyperactivity has been identified as occurring within a week of surgery and persisting for at least 4 weeks.113 This may be further exacerbated by the presence of circulating tissue factor expressing monocytes.114 Thrombin generation assays support these observations, where liver transplant recipients have increased thrombin generation capacity despite abnormal elevation in SLT indices.115 These subtle changes in coagulation parameters could be masked with VET activators such as tissue factor.
Despite a paucity of data, VET may still have a role in managing patients in the postoperative setting. LT recipients with thrombocytopenia may need posttransplant invasive procedures and VET can be utilized to guide preprocedure transfusion as utilized in other cirrhotic patients.116 Postoperative VET may also help guide the decision to use antiplatelet medications or heparin in the postoperative setting. As previously mentioned, preoperative increased clot strength (Kaolin TEG MA >65)47 or impaired fibrinolysis (Kaolin TEG LY30 <0.9%)48 may indicate the need to consider some form of anticoagulation strategy since both elevated clot strength and lack of fibrinolysis have been associated with thrombotic complications outside of transplant.117–120
Postoperative management of coagulation following LT remains a large gap in the literature. Heparin-based prophylaxis has questionable efficacy post-LT due to antithrombin deficiency having a high prevalence posttransplant.85 Furthermore, the coagulation system may have a role in liver repair following transplant, particularly in patients who receive a split donor graft or living donor.121 Finding the balance between the risks of coagulopathy and thrombosis will be critical in refining liver transplant management to optimize outcomes.
Conclusion
VET represents a unique opportunity to personalize medicine in LT that occurs at the time of receiving an organ offer. Randomized controlled trials have demonstrated reduced transfusion requirements using VET compared with SLT in both the operating room and managing bleeding pretransplant for patients with cirrhosis. The generalizability of these results remains controversial because of the mixed results in observational studies. A major limitation of VET research in LT is a failure to differentiate treatment strategy based on the patient’s underlying liver disease, graft quality, and anatomic risk for thrombosis. Furthermore, guidelines on postoperative utilization of VET in LT is lacking, and there are opportunities to not only reduce the risk of bleeding and thrombotic complications but also improve graft regeneration. Regardless, favorable outcomes after LT require technical acumen, sound judgment, and attention to detail.
Funding
This research was supported in part by the American Society of Transplant Surgeons Veloxis Fellowship Grant, National Heart Lung and Blood Institute Grant R00-HL151887.
Conflict of Interest
H.B.M. reports grants from Haemonetics, other from Instrument laboratories, and other from HemoSonics, outside the submitted work.
References
- 1.Groth CG, Pechet L, Starzl TE. Coagulation during and after orthotopic transplantation of the human liver. Arch Surg 1969;98(01):31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg 1985;64(09):888–896 [PMC free article] [PubMed] [Google Scholar]
- 3.Von Kaulla KN, Kaye H, von Kaulla E, Marchioro TL, Starzl TE. Changes in blood coagulation. Arch Surg 1966;92(01):71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabbour N, Gagandeep S, Mateo R, et al. Live donor liver transplantation without blood products: strategies developed for Jehovah’s Witnesses offer broad application. Ann Surg 2004;240(02):350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozier Y, Tsou MY. Changing trends in transfusion practice in liver transplantation. Curr Opin Organ Transplant 2008;13(03): 304–309 [DOI] [PubMed] [Google Scholar]
- 6.de Boer MT, Christensen MC, Asmussen M, et al. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth Analg 2008;106(01):32–44, table of contents [DOI] [PubMed] [Google Scholar]
- 7.Bezinover D, Dirkmann D, Findlay J, et al. Perioperative coagulation management in liver transplant recipients. Transplantation 2018;102(04):578–592 [DOI] [PubMed] [Google Scholar]
- 8.Clevenger B, Mallett SV. Transfusion and coagulation management in liver transplantation. World J Gastroenterol 2014;20(20):6146–6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Görlinger K [Coagulation management during liver transplantation]. Hamostaseologie 2006;26(3, Suppl 1):S64–S76 [PubMed] [Google Scholar]
- 10.Bababekov YJ, Nydam TL, Pomposelli JJ, Moore HB. Goal-directed management of coagulation: the right treatment, the right patient, the right time. Transplantation 2018;102(06):e303–e304 [DOI] [PubMed] [Google Scholar]
- 11.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365(02):147–156 [DOI] [PubMed] [Google Scholar]
- 12.Lisman T, Bongers TN, Adelmeijer J, et al. Elevated levels of von Willebrand factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 2006;44(01):53–61 [DOI] [PubMed] [Google Scholar]
- 13.Takaya H, Yoshiji H, Kawaratani H, et al. Decreased activity of plasma ADAMTS13 are related to enhanced cytokinemia and endotoxemia in patients with acute liver failure. Biomed Rep 2017;7(03):277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripodi A, Primignani M, Lemma L, Chantarangkul V, Mannucci PM. Evidence that low protein C contributes to the procoagulant imbalance in cirrhosis. J Hepatol 2013;59(02):265–270 [DOI] [PubMed] [Google Scholar]
- 15.Srivastava A Diagnosis of haemophilia and other inherited bleeding disorders - Is a new paradigm needed? Haemophilia 2021;27(Suppl 3):14–20 [DOI] [PubMed] [Google Scholar]
- 16.Hoffman M, Monroe DM III. A cell-based model of hemostasis. Thromb Haemost 2001;85(06):958–965 [PubMed] [Google Scholar]
- 17.Ivanics T, Leonard-Murali S, Mouzaihem H, et al. Extreme hyponatremia as a risk factor for early mortality after liver transplantation in the MELD-sodium era. Transpl Int 2021;34 (12):2856–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hum J, Amador D, Shatzel JJ, et al. Thromboelastography better reflects hemostatic abnormalities in cirrhotics compared with the international normalized ratio. J Clin Gastroenterol 2020;54(08):741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segal JB, Dzik WHTransfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion 2005;45 (09):1413–1425 [DOI] [PubMed] [Google Scholar]
- 20.Massicotte L, Thibeault L, Beaulieu D, Roy JD, Roy A. Evaluation of cell salvage autotransfusion utility during liver transplantation. HPB (Oxford) 2007;9(01):52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cywinski JB, Alster JM, Miller C, Vogt DP, Parker BM. Prediction of intraoperative transfusion requirements during orthotopic liver transplantation and the influence on postoperative patient survival. Anesth Analg 2014;118(02):428–437 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt AE, Israel AK, Refaai MA. The utility of thromboelastography to guide blood product transfusion. Am J Clin Pathol 2019;152(04):407–422 [DOI] [PubMed] [Google Scholar]
- 23.De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology 2016;63(02):566–573 [DOI] [PubMed] [Google Scholar]
- 24.Kumar M, Ahmad J, Maiwall R, et al. Thromboelastography-guided blood component use in patients with cirrhosis with nonvariceal bleeding: a randomized controlled trial. Hepatology 2020;71(01):235–246 [DOI] [PubMed] [Google Scholar]
- 25.Bonnet A, Gilquin N, Steer N, et al. The use of a thromboelastometry-based algorithm reduces the need for blood product transfusion during orthotopic liver transplantation: a randomised controlled study. Eur J Anaesthesiol 2019;36(11):825–833 [DOI] [PubMed] [Google Scholar]
- 26.Wang SC, Shieh JF, Chang KY, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc 2010;42(07):2590–2593 [DOI] [PubMed] [Google Scholar]
- 27.Zamper RPC, Amorim TC, Queiroz VNF, et al. Association between viscoelastic tests-guided therapy with synthetic factor concentrates and allogenic blood transfusion in liver transplantation: a before-after study. BMC Anesthesiol 2018;18(01):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smart L, Mumtaz K, Scharpf D, et al. Rotational thromboelastometry or conventional coagulation tests in liver transplantation: comparing blood loss, transfusions, and cost. Ann Hepatol 2017;16(06):916–923 [DOI] [PubMed] [Google Scholar]
- 29.Scarlatescu E, Kietaibl SA, Tomescu DR. The effect of a viscoelastic-guided bleeding algorithm implementation on blood products use in adult liver transplant patients. A propensity score-matched before-after study. Transfus Apheresis Sci 2022;61(02):103322. [DOI] [PubMed] [Google Scholar]
- 30.Leon-Justel A, Noval-Padillo JA, Alvarez-Rios AI, et al. Point-of-care haemostasis monitoring during liver transplantation reduces transfusion requirements and improves patient outcome. Clin Chim Acta 2015;446:277–283 [DOI] [PubMed] [Google Scholar]
- 31.Alamo JM, León A, Mellado P, et al. Is “intra-operating room” thromboelastometry useful in liver transplantation? A case-control study in 303 patients. Transplant Proc 2013;45(10):3637–3639 [DOI] [PubMed] [Google Scholar]
- 32.Nascimento JCR, Neto EBL, da Silva EL, et al. Analysis of the hemostatic therapy in liver transplantation guided by rotational thromboelastometry or conventional laboratory tests. Eur J Gastroenterol Hepatol 2020;32(11):1452–1457 [DOI] [PubMed] [Google Scholar]
- 33.Leon-Justel A, Alvarez-Rios AI, Noval-Padillo JA, et al. Point-of-care haemostasis monitoring during liver transplantation is cost effective. Clin Chem Lab Med 2019;57(06):883–890 [DOI] [PubMed] [Google Scholar]
- 34.Gurusamy KS, Pissanou T, Pikhart H, Vaughan J, Burroughs AK, Davidson BR. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev 2011;(12):CD009052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenk B, Görlinger K, Treml B, et al. A comparison of the new ROTEM® sigma with its predecessor, the ROTEMdelta. Anaesthesia 2019;74(03):348–356 [DOI] [PubMed] [Google Scholar]
- 36.Gurbel PA, Bliden KP, Tantry US, et al. First report of the point-of-care TEG: a technical validation study of the TEG-6S system. Platelets 2016;27(07):642–649 [DOI] [PubMed] [Google Scholar]
- 37.Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. J Cardiothorac Vasc Anesth 2006;20(04):548–553 [DOI] [PubMed] [Google Scholar]
- 38.Trzebicki J, Flakiewicz E, Kosieradzki M, et al. The use of thromboelastometry in the assessment of hemostasis during orthotopic liver transplantation reduces the demand for blood products. Ann Transplant 2010;15(03):19–24 [PubMed] [Google Scholar]
- 39.Roullet S, Freyburger G, Cruc M, et al. Management of bleeding and transfusion during liver transplantation before and after the introduction of a rotational thromboelastometry-based algorithm. Liver Transpl 2015;21(02):169–179 [DOI] [PubMed] [Google Scholar]
- 40.Gaspari R, Teofili L, Aceto P, et al. Thromboelastography does not reduce transfusion requirements in liver transplantation: a propensity score-matched study. J Clin Anesth 2021;69:110154. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen-Buckley C, Gao W, Agopian V, Wray C, Steadman RH, Xia VW. Major thromboembolic complications in liver transplantation: the role of rotational thromboelastometry and cryoprecipitate transfusion. Transplantation 2021;105(08):1771–1777 [DOI] [PubMed] [Google Scholar]
- 42.Seeßle J, Löhr J, Kirchner M, Michaelis J, Merle U. Rotational thrombelastometry (ROTEM) improves hemostasis assessment compared to conventional coagulation test in ACLF and non-ACLF patients. BMC Gastroenterol 2020;20(01):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Ari Z, Panagou M, Patch D, et al. Hypercoagulability in patients with primary biliary cirrhosis and primary sclerosing cholangitis evaluated by thrombelastography. J Hepatol 1997;26(3):554–559 [DOI] [PubMed] [Google Scholar]
- 44.Huang G, Jiang H, Lin Y, et al. Prognostic value of plasma fibrinogen in hepatocellular carcinoma: a meta-analysis. Cancer Manag Res 2018;10:5027–5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanetto A, Senzolo M, Vitale A, et al. Thromboelastometry hypercoagulable profiles and portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma. Dig Liver Dis 2017;49(4):440–445 [DOI] [PubMed] [Google Scholar]
- 46.Park GC, Moon DB, Kang SH, et al. Overcoming hepatic artery thrombosis after living donor liver transplantations: an experience from Asan Medical Center. Ann Transplant 2019;24:588–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zahr Eldeen F, Roll GR, Derosas C, et al. Preoperative thromboelastography as a sensitive tool predicting those at risk of developing early hepatic artery thrombosis after adult liver transplantation. Transplantation 2016;100(11):2382–2390 [DOI] [PubMed] [Google Scholar]
- 48.Nicolau-Raducu R, Beduschi T, Vianna R, et al. Fibrinolysis shutdown is associated with thrombotic and hemorrhagic complications and poorer outcomes after liver transplantation. Liver Transpl 2019;25(03):380–387 [DOI] [PubMed] [Google Scholar]
- 49.Rout G, Shalimar Gunjan D, et al. Thromboelastography-guided blood product transfusion in cirrhosis patients with variceal bleeding: a randomized controlled trial. J Clin Gastroenterol 2020;54(03):255–262 [DOI] [PubMed] [Google Scholar]
- 50.Pustavoitau A, Rizkalla NA, Perlstein B, et al. Validation of predictive models identifying patients at risk for massive transfusion during liver transplantation and their potential impact on blood bank resource utilization. Transfusion 2020;60(11):2565–2580 [DOI] [PubMed] [Google Scholar]
- 51.Lawson PJ, Moore HB, Moore EE, et al. Preoperative thrombelastography maximum amplitude predicts massive transfusion in liver transplantation. J Surg Res 2017;220:171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steib A, Gengenwin N, Freys G, Boudjema K, Levy S, Otteni JC. Predictive factors of hyperfibrinolytic activity during liver transplantation in cirrhotic patients. Br J Anaesth 1994;73(05):645–648 [DOI] [PubMed] [Google Scholar]
- 53.McCluskey SA, Karkouti K, Wijeysundera DN, et al. Derivation of a risk index for the prediction of massive blood transfusion in liver transplantation. Liver Transpl 2006;12(11):1584–1593 [DOI] [PubMed] [Google Scholar]
- 54.Eghbal MH, Samadi K, Khosravi MB, et al. The impact of preoperative variables on intraoperative blood loss and transfusion requirements during orthotopic liver transplant. Exp Clin Transplant 2019;17(04):507–512 [DOI] [PubMed] [Google Scholar]
- 55.Tomescu D, Popescu M, Jipa L, et al. The impact of donor liver graft quality on postoperative outcome in liver transplant recipients. A single centre experience. Rom J Anaesth Intensive Care 2016;23(01):19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neal MD, Moore HB, Moore EE, et al. ; TACTIC Investigators. Clinical assessment of trauma-induced coagulopathy and its contribution to postinjury mortality: a TACTIC proposal. J Trauma Acute Care Surg 2015;79(03):490–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulick AC, Moore HB, Walker CB, et al. A clinical coagulopathy score concurrent with viscoelastic testing defines opportunities to improve hemostatic resuscitation and enhance blood product utilization during liver transplantation. Am J Surg 2020;220(06):1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hendriks HG, van der Meer J, Klompmaker IJ, et al. Blood loss in orthotopic liver transplantation: a retrospective analysis of transfusion requirements and the effects of autotransfusion of cell saver blood in 164 consecutive patients. Blood Coagul Fibrinolysis 2000;11(Suppl 1):S87–S93 [DOI] [PubMed] [Google Scholar]
- 59.Kirnap M, Tezcaner T, Ayvazoğlu Soy HE, et al. Efficacy of cell saver use in living-donor liver transplant. Exp Clin Transplant 2015;13(Suppl 1):315–317 [DOI] [PubMed] [Google Scholar]
- 60.Stoneham MD, Von Kier S, Harvey L, Murphy M. Effects of a targeted blood management programme on allogeneic blood transfusion in abdominal aortic aneurysm surgery. Transfus Med 2018;28(04):290–297 [DOI] [PubMed] [Google Scholar]
- 61.Henderson RA, Judd M, Strauss ER, et al. Hematologic evaluation of intraoperative autologous blood collection and allogeneic transfusion in cardiac surgery. Transfusion 2021;61(03):788–798 [DOI] [PubMed] [Google Scholar]
- 62.Porte RJ, Bontempo FA, Knot EA, Lewis JH, Kang YG, Starzl TE. Systemic effects of tissue plasminogen activator-associated fibrinolysis and its relation to thrombin generation in orthotopic liver transplantation. Transplantation 1989;47(06):978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poon KS, Chen CC, Thorat A, et al. Fibrinolysis after reperfusion of liver graft. Acta Anaesthesiol Taiwan 2015;53(01):41–43 [DOI] [PubMed] [Google Scholar]
- 64.Moore HB, Yaffe H, Pomposelli JJ, et al. Detection of early allograft dysfunction at 30 min of reperfusion in liver transplantation: an intraoperative diagnostic tool with real time assessment of graft function. Am J Surg 2020;220(06):1518–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim EH, Ko JS, Gwak MS, Lee SK, Kim GS. Incidence and clinical significance of hyperfibrinolysis during living donor liver transplantation. Blood Coagul Fibrinolysis 2018;29(03):322–326 [DOI] [PubMed] [Google Scholar]
- 66.Sabate A, Gutierrez R, Beltran J, et al. Impact of preemptive fibrinogen concentrate on transfusion requirements in liver transplantation: a multicenter, randomized, double-blind, placebo-controlled trial. Am J Transplant 2016;16(08):2421–2429 [DOI] [PubMed] [Google Scholar]
- 67.Sabate A, Dalmau A. Fibrinogen: a clinical update on liver transplantation. Transplant Proc 2015;47(10):2925–2928 [DOI] [PubMed] [Google Scholar]
- 68.Lang T, Johanning K, Metzler H, et al. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Analg 2009;108(03):751–758 [DOI] [PubMed] [Google Scholar]
- 69.Pereboom IT, de Boer MT, Haagsma EB, Hendriks HG, Lisman T, Porte RJ. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg 2009;108(04):1083–1091 [DOI] [PubMed] [Google Scholar]
- 70.Schofield N, Sugavanam A, Thompson K, Mallett SV. No increase in blood transfusions during liver transplantation since the withdrawal of aprotinin. Liver Transpl 2014;20(05):584–590 [DOI] [PubMed] [Google Scholar]
- 71.Molenaar IQ, Warnaar N, Groen H, Tenvergert EM, Slooff MJ, Porte RJ. Efficacy and safety of antifibrinolytic drugs in liver transplantation: a systematic review and meta-analysis. Am J Transplant 2007;7(01):185–194 [DOI] [PubMed] [Google Scholar]
- 72.Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol 2017;34(06):332–395 [DOI] [PubMed] [Google Scholar]
- 73.Jeong SM, Song JG, Seo H, Choi JH, Jang DM, Hwang GS. Quantification of both platelet count and fibrinogen concentration using maximal clot firmness of thromboelastometry during liver transplantation. Transplant Proc 2015;47(06):1890–1895 [DOI] [PubMed] [Google Scholar]
- 74.Soliman M, Hartmann M. Impedance aggregometry reveals increased platelet aggregation during liver transplantation. J Clin Med 2019;8(11):E1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sahmeddini MA, Tehran SG, Khosravi MB,et al. Risk factors of the post-reperfusion syndrome during orthotopic liver transplantation: a clinical observational study. BMC Anesthesiol 2022;22(01):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moore HB, D’Alessandro A, Moore EE, et al. Increase in postreperfusion sensitivity to tissue plasminogen activator-mediated fibrinolysis during liver transplantation is associated with abnormal metabolic changes and increased blood product utilisation. Blood Transfus 2019;17(04):312–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kettner SC, Gonano C, Seebach F, et al. Endogenous heparin-like substances significantly impair coagulation in patients undergoing orthotopic liver transplantation. Anesth Analg 1998;86(04):691–695 [DOI] [PubMed] [Google Scholar]
- 78.Moore HB, Bababekov YJ, Pomposelli JJ, et al. The vexing triad of obesity, alcohol, and coagulopathy predicts the need for multiple operations in liver transplantation. Am J Surg 2022;224(1, Pt A):69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bayly PJ, Thick M. Reversal of post-reperfusion coagulopathy by protamine sulphate in orthotopic liver transplantation. Br J Anaesth 1994;73(06):840–842 [DOI] [PubMed] [Google Scholar]
- 80.Bechstein WO, Riess H, Blumhardt G, et al. Aprotinin in orthotopic liver transplantation. Semin Thromb Hemost 1993;19(03):262–267 [DOI] [PubMed] [Google Scholar]
- 81.Raveh Y, Shatz V, Lindsay M, Nicolau-Raducu R. Disseminated intravascular coagulation during liver transplantation unleashed by protamine. J Clin Anesth 2019;57:117–118 [DOI] [PubMed] [Google Scholar]
- 82.Gologorsky E, De Wolf AM, Scott V, Aggarwal S, Dishart M, Kang Y. Intracardiac thrombus formation and pulmonary thromboembolism immediately after graft reperfusion in 7 patients undergoing liver transplantation. Liver Transpl 2001;7(09):783–789 [DOI] [PubMed] [Google Scholar]
- 83.Kamel Y, Hassanin A, Ahmed AR, et al. Perioperative thromboelastometry for adult living donor liver transplant recipients with a tendency to hypercoagulability: a prospective observational cohort study. Transfus Med Hemother 2018;45(06):404–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xia VW, Ho JK, Nourmand H, Wray C, Busuttil RW, Steadman RH. Incidental intracardiac thromboemboli during liver transplantation: incidence, risk factors, and management. Liver Transpl 2010;16(12):1421–1427 [DOI] [PubMed] [Google Scholar]
- 85.Arshad F, Lisman T, Porte RJ. Hypercoagulability as a contributor to thrombotic complications in the liver transplant recipient. Liver Int 2013;33(06):820–827 [DOI] [PubMed] [Google Scholar]
- 86.Porte RJ. Coagulation and fibrinolysis in orthotopic liver transplantation: current views and insights. Semin Thromb Hemost 1993;19(03):191–196 [DOI] [PubMed] [Google Scholar]
- 87.Lisman T, Hernandez-Gea V, Magnusson M, et al. The concept of rebalanced hemostasis in patients with liver disease: communication from the ISTH SSC working group on hemostatic management of patients with liver disease. J Thromb Haemost 2021;19(4):1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bown LS, Ricksten SE, Houltz E, et al. Vasopressin-induced changes in splanchnic blood flow and hepatic and portal venous pressures in liver resection. Acta Anaesthesiol Scand 2016;60(5):607–615 [DOI] [PubMed] [Google Scholar]
- 89.Appukuttan M, Kumar S, Bharathy KGS, Pandey VK, Pamecha V. Impact of functional hepatic venous outflow obstruction on perioperative outcome after living-donor liver transplant. Exp Clin Transplant 2019;17(01):64–73 [DOI] [PubMed] [Google Scholar]
- 90.Tinguely P, Badenoch A, Krzanicki D, et al. ; ERAS4OLT.org Working Group. The role of early extubation on short-term outcomes after liver transplantation - a systematic review, meta-analysis and expert recommendations. Clin Transplant 2022:e14642 doi: 10.1111/ctr.14642 [DOI] [PubMed] [Google Scholar]
- 91.Kim S, DeMaria S Jr, Li J, et al. Persistent acidosis after reperfusion - a prognostic indicator of increased 30-day and in-hospital postoperative mortality in liver transplant recipients. ClinTransplant 2019;33(03):e13473. [DOI] [PubMed] [Google Scholar]
- 92.Azer SA, Hasanato R. Use of bile acids as potential markers of liver dysfunction in humans: a systematic review. Medicine (Baltimore) 2021;100(41):e27464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCaughan GW, Herkes R, Powers B, et al. Thrombocytopenia post liver transplantation. Correlations with pre-operative platelet count, blood transfusion requirements, allograft function and outcome. J Hepatol 1992;16(1-2):16–22 [DOI] [PubMed] [Google Scholar]
- 94.Fitzgibbon S, Spann AP, Qi QM, Shaqfeh ESG. In vitro measurement of particle margination in the microchannel flow: effect of varying hematocrit. Biophys J 2015;108(10):2601–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warner P, Fusai G, Glantzounis GK, et al. Risk factors associated with early hepatic artery thrombosis after orthotopic liver transplantation - univariable and multivariable analysis. Transpl Int 2011;24(04):401−408 [DOI] [PubMed] [Google Scholar]
- 96.Marín-Gómez LM, Bernal-Bellido C, Alamo-Martínez JM, et al. Intraoperative hepatic artery blood flow predicts early hepatic artery thrombosis after liver transplantation. Transplant Proc 2012;44(07):2078–2081 [DOI] [PubMed] [Google Scholar]
- 97.Mourad MM, Liossis C, Gunson BK, et al. Etiology and management of hepatic artery thrombosis after adult liver transplantation. Liver Transpl 2014;20(06):713–723 [DOI] [PubMed] [Google Scholar]
- 98.Moore HB, Bababekov YJ, Pomposelli JJ, et al. During the anhepatic phase of liver transplantation, does low fibrinolysis activity predict hepatic artery thrombosis rates? Am J Transplant 2021;21(S4):774 [Google Scholar]
- 99.Pomposelli JJ. Hepatic artery thrombosis after liver transplant: Not a surgical problem? Transplantation 2016;100(11):2251. [DOI] [PubMed] [Google Scholar]
- 100.Görlinger K, Perez-Ferrer A, Dirkmann D, et al. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J Anesthesiol 2019;72(04):297–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hawkins RB, Raymond SL, Hartjes T, et al. Review: the perioperative use of thromboelastography for liver transplant patients. Transplant Proc 2018;50(10):3552–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stillson JE, Bunch CM, Gillespie L, et al. Thromboelastography-guided management of anticoagulated COVID-19 patients to prevent hemorrhage. Semin Thromb Hemost 2021;47(04):442–446 [DOI] [PubMed] [Google Scholar]
- 103.Weber ML, Ibrahim HN, Lake JR. Renal dysfunction in liver transplant recipients: evaluation of the critical issues. Liver Transpl 2012;18(11):1290–1301 [DOI] [PubMed] [Google Scholar]
- 104.Holcomb JB, Tilley BC, Baraniuk S, et al. ; PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313(05):471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.DiNorcia J, Lee MK, Harlander-Locke M, et al. Reoperative complications after primary orthotopic liver transplantation: a contemporary single-center experience in the post-model for end-stage liver disease era. J Am Coll Surg 2014;219(05):993–1000 [DOI] [PubMed] [Google Scholar]
- 106.DiNorcia J, Lee MK, Harlander-Locke MP, et al. Damage control as a strategy to manage postreperfusion hemodynamic instability and coagulopathy in liver transplant. JAMA Surg 2015;150(11):1066–1072 [DOI] [PubMed] [Google Scholar]
- 107.Fujisawa H, Ito H, Saito K, Ikeda K, Nitta H, Yamashita J. Immunohistochemical localization of tissue-type plasminogen activator in the lining wall of chronic subdural hematoma. Surg Neurol 1991;35(06):441–445 [DOI] [PubMed] [Google Scholar]
- 108.Miesbach W, Schenk J, Alesci S, Lindhoff-Last E. Comparison of the fibrinogen Clauss assay and the fibrinogen PT derived method in patients with dysfibrinogenemia. Thromb Res 2010;126(06):e428–e433 [DOI] [PubMed] [Google Scholar]
- 109.Krzanicki D, Sugavanam A, Mallett S. Intraoperative hypercoagulability during liver transplantation as demonstrated by thromboelastography. Liver Transpl 2013;19(08):852–861 [DOI] [PubMed] [Google Scholar]
- 110.Dötsch TM, Dirkmann D, Bezinover D, et al. Assessment of standard laboratory tests and rotational thromboelastometry for the prediction of postoperative bleeding in liver transplantation. Br J Anaesth 2017;119(03):402–410 [DOI] [PubMed] [Google Scholar]
- 111.Pereboom IT, Lisman T, Porte RJ. Platelets in liver transplantation: friend or foe? Liver Transpl 2008;14(07):923–931 [DOI] [PubMed] [Google Scholar]
- 112.Han S, Park HW, Song JH, et al. Association between intraoperative platelet transfusion and early graft regeneration in living donor liver transplantation. Ann Surg 2016;264(06):1065–1072 [DOI] [PubMed] [Google Scholar]
- 113.Eyraud D, Suner L, Dupont A, et al. Evolution of platelet functions in cirrhotic patients undergoing liver transplantation: a prospective exploration over a month. PLoS One 2018;13(08):e0200364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Esch JS, Jurk K, Knoefel WT, et al. Platelet activation and increased tissue factor expression on monocytes in reperfusion injury following orthotopic liver transplantation. Platelets 2010;21(05):348–359 [DOI] [PubMed] [Google Scholar]
- 115.Lisman T, Bakhtiari K, Pereboom IT, Hendriks HG, Meijers JC, Porte RJ. Normal to increased thrombin generation in patients undergoing liver transplantation despite prolonged conventional coagulation tests. J Hepatol 2010;52(03):355–361 [DOI] [PubMed] [Google Scholar]
- 116.Tangcheewinsirikul N, Moonla C, Uaprasert N, Pittayanon R, Rojnuckarin P. Viscoelastometric versus standard coagulation tests to guide periprocedural transfusion in adults with cirrhosis: a meta-analysis of randomized controlled trials. Vox Sang 2022;117(04):553–561 [DOI] [PubMed] [Google Scholar]
- 117.Coleman JR, Kay AB, Moore EE, et al. It’s sooner than you think: blunt solid organ injury patients are already hypercoagulable upon hospital admission - results of a bi-institutional, prospective study. Am J Surg 2019;218(06):1065–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nelson JT, Coleman JR, Carmichael H, et al. High rate of fibrinolytic shutdown and venous thromboembolism in patients with severe pelvic fracture. J Surg Res 2020;246:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsantes AG, Papadopoulos DV, Trikoupis IG, et al. Rotational thromboelastometry findings are associated with symptomatic venous thromboembolic complications after hip fracture surgery. Clin Orthop Relat Res 2021;479(11):2457–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hincker A, Feit J, Sladen RN, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care 2014;18(05):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liang C, Takahashi K, Furuya K, Ohkohchi N, Oda T. Dualistic role of platelets in living donor liver transplantation: Are they harmful? World J Gastroenterol 2022;28(09):897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]


