Abstract
Low fibrinolytic activity has been associated with pathologic thrombosis and multiple-organ failure. Low fibrinolytic activity has two commonly associated terms, hypofibrinolysis and fibrinolysis shutdown. Hypofibrinolysis is a chronic state of lack of ability to generate an appropriate fibrinolytic response when anticipated. Fibrinolysis shutdown is the shutdown of fibrinolysis after systemic activation of the fibrinolytic system. There has been interchanging of these terms to describe critically ill patients in multiple settings. This is problematic in understanding the pathophysiology of disease processes related to these conditions. There is also a lack of research on the cellular mediators of these processes. The purpose of this article is to review the on and off mechanisms of fibrinolysis in the context of low fibrinolytic states to define the importance in differentiating hypofibrinolysis from fibrinolysis shutdown. In many clinical scenarios, the etiology of a low fibrinolytic state cannot be determined due to ambiguity if a preceding fibrinolytic activation event occurred. In this scenario, the term “low fibrinolytic activity” or “fibrinolysis resistance” is a more appropriate descriptor, rather than using assumptive of hypofibrinolysis and fibrinolysis shutdown, particularly in the acute setting of infection, injury, and surgery.
Keywords: fibrinolysis shutdown, hypofibrinolysis, plasminogen activators
Endogenous and Exogenous Activation of the Fibrinolytic System
Fibrinolysis is a counterbalance to clot formation. Since the 1940s, exogenous fibrinolytic activators have been administered in the clinical setting to treat pathologic clotting in patients.1 Subsequently lytic therapy has become instrumental in improving outcomes in myocardial infarction,2 pulmonary embolism,3 and stroke.4 However, excessive fibrinolysis can result in uncontrolled hemorrhage and death.5,6 This also includes the use of exogenous fibrinolytic activators as therapeutics in which lethal bleeding posttreatment can occur.7,8
Plasminogen activation occurs with tissue plasminogen activator (tPA) entering the blood stream from vascular endothelium9 and peripheral sympathetic nerves innervating the adventitia of blood vessels.10 The dual source of plasminogen activators allows the activation of fibrinolysis even when one has been eliminated, but at a submaximal response.11 While tPA was originally identified to be present in multiple tissue types,12 high-concentration tPA-rich cells are present in precapillary arterioles.10 There are also organ-specific regions of high plasminogen activator content, including the brain, heart, lung, and kidney, while other organs such as the liver have minimal activator present.13
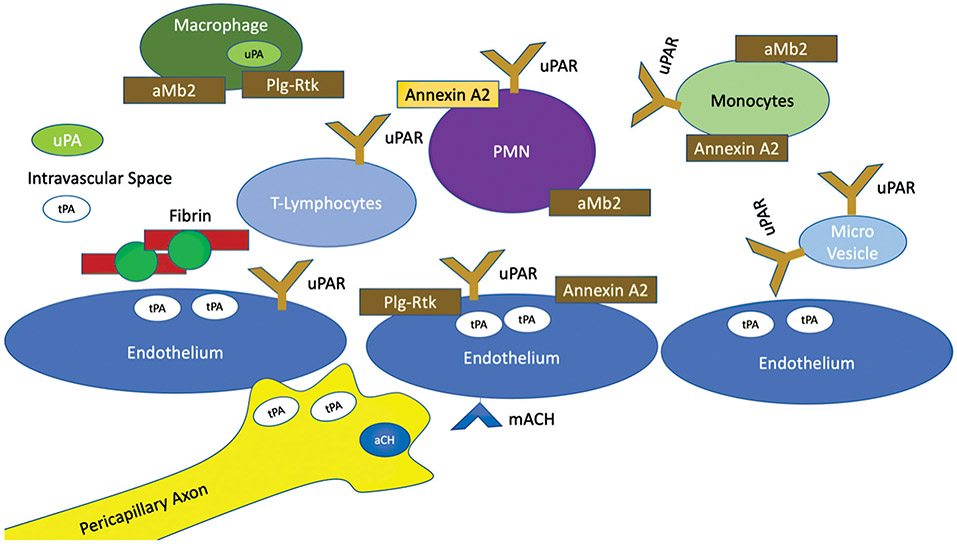
Urokinase as a plasminogen activator has a uniquely different role in tissue regeneration and predominantly circulates as a proenzyme.14 Urokinase does not appear to play a critical role in acute activation of fibrinolysis in the setting of shock. Previous studies have identified tPA15-17 rather than urokinase as the predominantly elevated plasminogen activator in systemic circulation. However, urokinase at the local clot degradation level cannot be excluded as an important factor in fibrinolysis activity. Fibrinolysis initiated with tPA caters to enhanced fibrin degradation with subsequent urokinase administration due to additional lysine-binding sites created by proteolytic cleavage.18 This is relevant, as not only circulating cells such as macrophages express urokinase19 but localization of these cells to fibrin is predominantly tPA dependent.20 This component of cellular-mediated fibrinolysis has not received as much attention in coagulation-based research. Urokinase has been predominantly studied in the context of it binding to its receptors21 and downstream effects of tissue remodeling in its association with tumor biology22,23 and angiogenesis.24 There are also several cell lines applicable to intravascular coagulation in which the urokinase receptor can be upregulated including endothelial cells,25 microparticles released from endothelial cells,26 monocytes, neutrophils, and T lymphocytes.27 This is further complicated by localization of plasminogen, which is also expressed on a large number of circulating cells and endothelium that can modulate local fibrinolytic activity28 (Fig. 1). Mouse knockout models of plasminogen activators demonstrate that a deficiency in one plasminogen activator is tolerated, but the double knock out is associated with extravascular fibrin deposition and decreased survival.29 The recurring theme of overlapping mechanisms of plasminogen activation reflects the biological importance of the fibrinolytic system.
Fig. 1.
Cellular mediators of fibrinolysis activation. This figure demonstrates a schematic on the numerous potential cellular regulators of fibrinolysis. Basal levels of plasminogen activators (tPA and uPA) are present in the systemic circulation. There are additional cellular factors that can release tPA and uPA based on stimuli, in addition to neurons that can directly release tPA and also release neurotransmitters for local tPA release. Many circulating cells also have receptors for plasminogen and uPA which further help localize plasminogen activators in proximity to plasminogen. With local fibrin production, additional binding of plasminogen can occur which also enables tPA binding, enabling high concentrations of cellular and non–cellular-mediated fibrinolysis. tPA, tissue plasminogen activator; uPAR, urokinase-type plasminogen activator receptor.
Inhibitors of Fibrinolysis
Circulating inhibitors of fibrinolysis were appreciated to be in high abundance in circulation early in the understanding of the fibrinolytic system.30 The numerous circulating inhibitors of the system have been extensively studied from a biochemical perspective.31 Plasminogen activator inhibitor 1 (PAI-1) has recently been proposed as the primary regulator of fibrinolysis, as it can be rapidly upregulated in multiple clinical settings and shutdown fibrinolysis.32 PAI-1 mechanism works by directly binding to plasminogen activators, preventing plasmin activation.33 It has been known for decades that tPA activity stimulated by surgery, trauma, or myocardial infarct is quickly shutdown from a systemic inhibitor.34 However, the short half-life of tPA in circulation is largely attributed to the liver’s ability to rapidly clear this protease.35 The dual importance of tPA clearance and circulating plasma inhibition is apparent in liver transplant, when peak levels occur during the anhepatic phase of surgery and early reperfusion when limited tPA clearance is achieved.36
Beyond direct inhibition of activators, fibrinolysis is largely mediated by fibrin itself. The formation of plasmin in the presence of tPA is highly dependent of the colocalization of these proteins on the surface of fibrin, forming a tertiary complex.37 Thrombin activatable fibrinolysis inhibitor (TAFI) effectively attenuates this process through cleavage of the lysine domains on fibrin, where plasminogen binding occurs.38 Factor XIII also has a role in fibrinolysis regulation. While this fibrin cross-linking protein has some influence in making fibrin resistant to degradation by modifying its structure, the primary mechanism is through cross-linking α2-antiplasmin (A2AP) into clots.39 A2AP is considered the primary inhibitor of the fibrinolytic system.40 While A2AP is a downstream inhibitor after plasmin generation, the rapid neutralization of plasmin is one of the fastest occurring biological reactions in the body.41 High circulating levels of A2AP and dependence of fibrin for effective plasmin generation are designed for tight regional specificity of fibrinolysis.42 Overall, the impact of circulating inhibitors, fibrin clot structure, and integrated inhibitors into the clot have a synergistic effect on clot degradation regulation.43
Cellular mediators of fibrinolysis have received less attention than proteases. Platelets have a critical role in fibrinolysis regulation. The utilization of thrombelastography to quantify fibrinolysis activity in whole blood in the 1960s implicated platelets in down regulating plasmin activity, as large differences in activation were appreciated in plasma versus whole blood.44 This has been appreciated in trauma patients, in which inhibition of the ADP pathway of platelet-mediated clot strength was associated with increase susceptibility to tPA-mediated fibrinolysis.45 Platelets are known to form a hierarchical clot structure around a fibrin core.46 The structure of these platelet aggregates forms a dense central region around fibrin, creating a physical barrier to other cells and creates a micro environment that is prothrombotic.47
Platelets contain prestored proteins such as PAI-148 and TAFI49 that functionally inhibit fibrinolysis. There are also additional proteins stored within platelets that can bind tPA and plasminogen that may have a regulatory role in fibrinolysis as well.50 The same principle of hierarchical plasminogen and tPA binding also occurs in which only a thin rim of peripheral fibrin is actively degraded.51 The active zone of fibrinolysis is tPA and plasminogen rich, while a zone of prefibrinolysis has increased plasminogen, but is lacking tPA. Therefore, access to the fibrin surface of tPA in combination with the local fibrin clot surface is critical in determining the rate of clot degradation. This is also impacted by the fibrin–platelet interaction, in which platelet contraction to condense the clot can enhance fibrinolysis resistance, particularly in the presence of factor XIII, via tight localization of A2AP.52 Platelets also release microparticles which bind to fibrin strands and can promote fibrinolysis resistance.53 Local PAI-1 release at the site of injury can also occur with direct thrombin release from the extracellular matrix and thrombin-related increased expression of PAI-1 from smooth muscle.54
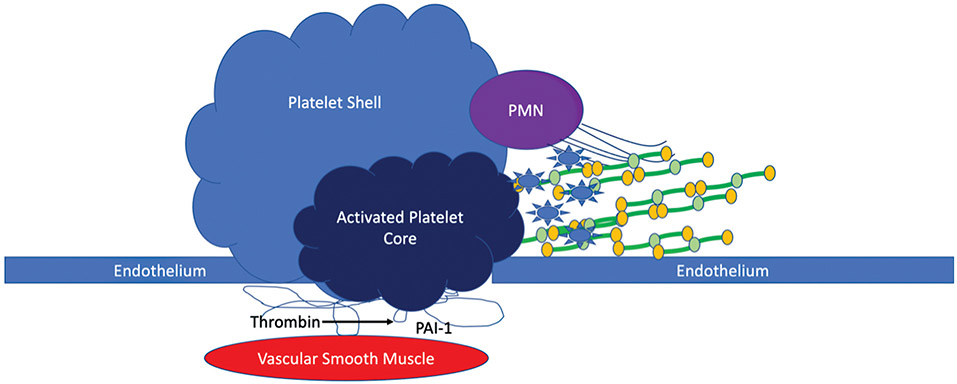
Additional cellular mediators of fibrinolysis inhibition include neutrophil extracellular traps (NETs).55 The combination of both histones and DNA reduces clot susceptibility to fibrinolysis. It has been implicated that damage-associated proteins driving sterile inflammation can prime neutrophils for NETs formation.56 Recent work in stroke has suggested that cell-free DNA from NETs is a culprit in unsuccessful lytic therapy.57 Neutrophil adhesion to fibrin is mediated by MAC-1 which is a shared ligand with other leukocytes.58 Cell-to-cell interactions in the region of fibrin represent a challenging but important future area of research to determine their role in fibrinolysis, particularly as these cellular interactions can be modulated by flow.59 Just as the cell model of hemostasis in thrombin generation has been transformative in understanding clot formation,60 understanding the cellular mediators of fibrinolysis is just as important to address. Tying complex interactions together remains a challenge, as there are no current ways to assess this interaction in a single assay and has been completely clinically neglected. Fig. 2 represents a schematic of the interactions between the potential local negative cellular regulators of fibrinolysis.
Fig. 2.
Cellular mediators of fibrinolysis inhibition. This figure represents a schematic of the potential cellular mediators of decreasing fibrinolytic activity. Local tissue injury exposes platelet activators for aggregation. Based on the level of activation of platelet activity, these platelets form an internal core of tightly bound platelets and release antifibrinolytic proteins, microparticles, and thrombin. This platelet core is buffered by a platelet shell that also incorporates neutrophils that can become activated and release neutrophil extracellular traps, creating a more fibrinolytic resistant clot. The platelet core thrombin release also cleaves PAI-1 from the extracellular matrix, in addition to stimulating PAI-1 expression in underlying vascular smooth muscle cells. PAI-1, plasminogen activator inhibitor 1.
Turning Fibrinolysis On and Off
Despite a clear biological role of fibrinolytic activators, the regulation of their release is poorly understood.61 Early research on the activation of fibrinolysis found a broad range of conditions that would activate fibrinolysis, including emotions, exercise, surgery, and sudden death, with evidence suggesting that a local neurogenic mechanism drove this process.62 This is supported by subsequent work demonstrating that epinephrine,63 DDAVP,64 serotonin,65,66 histamine,67 bradykinin,68 and acetylcholine69 can impact the release of plasminogen activators from blood vessels.
Pathologic activation of fibrinolysis has been implicated as the culprit for terminal pathology in sepsis resulting in disseminated intravascular coagulation (DIC).70 DIC has also been proposed to be a driver of mortality in trauma.71 However, others have postulated a lack of fibrinolysis as the culprit in mortality following these clinical settings.72 It has been hypothesized that this is due to a disbalance between prothrombosis, with lack of anticoagulation, and impaired fibrinolysis activity.73 Observational literature has identified that fibrinolysis has a predictable pattern of activation following injury with subsequent inhibition,74 which was appreciated in a similar time frame of inhibition of fibrinolysis following exogenous administration.1 The timing of fibrinolysis activation with subsequent inhibition has previously been reviewed in the context of traumatic injury75 and demonstrated in animal models of infection.76 This same concept of fibrinolytic activation with subsequent shutdown has also gained attention in severe COVID-19 infections.77,78 There remains a large gap in the literature in understanding pathologic versus physiologic activation, in addition to how system inactivation is returned to normal homeostasis.
Fibrinolysis Shutdown: A Two-Step Process
Fibrinolysis shutdown was first proposed by Innes and Sevitt in 1964 in the setting of trauma,74 but rebound fibrinolysis resistance from a systemic inhibitor had been observed 30 years prior in the setting of streptococcal infection.79 Fibrinolysis activity in the study of Innes and Sevitt74 was quantified by the time it took citrated whole blood to dissolve, in addition to the timing of whole blood clots to dissolve in streptokinase. This reversal of fibrinolytic activation has been proposed to be an acute phase response.34 An evaluation of circulating protease changes in fibrinolysis shutdown demonstrates that PAI-1 complex with tPA increase by 200-fold at 7 hours after fibrinolytic activation, with PAI-1 levels remaining increased by nearly 100-fold at 21 hours.80 A more recent analysis evaluating trauma patients who were hyperfibrinolytic on emergency department arrival based on viscoelastic testing demonstrated that PAI-1 levels are increased at 2 hours following injury with viscoelastic testing demonstrating no fibrinolytic activity in the same time frame.81 While additional regulators of fibrinolysis inhibition can become markedly depleted following systemic fibrinolytic activation, their recovery returns to normal ranges within 24 hours without excessive production.80
Trauma patients with evidence of fibrinolytic activation but subsequent shutdown based on viscoelastic testing have depletion of fibrinolytic inhibitors,82 increases in plasmin antiplasmin complex,83 and elevated D-dimer levels.84 This cohort of patients, despite having a low fibrinolysis activity, also harbor additional coagulation derangements, including an elevated international normalized ratio (INR), and platelet dysfunction and are at risk of bleeding, but do not bleed as much as hyperfibrinolytic patients.82-84 Ex vivo assessment of the impact on tranexamic acid in this patient population identifies that there is no benefit in fibrin clot strength with the administration of tranexamic acid.85 In this study, only patients who are sensitive to exogenous tPA and have increase fibrinolysis activity (low LY30) demonstrated gain in fibrin clot strength with exogenous antifibrinolytics. Patients with low fibrinolytic activity, or who lacked sensitivity to tPA, had no increase in clot strength with antifibrinolytics. These data support that the clinical benefit of antifibrinolytics in increase fibrin clot strength is in the setting of increased fibrinolytic activity with concurrent loss of systemic inhibitors. After a patient has endogenously shut down the fibrinolytic system, the impact of antifibrinolytics in improving coagulation parameters remains unclear. This is likely why the benefit of antifibrinolytics in trauma are appreciated only when given early after injury in severely hypotensive patients.86
Our group has unpublished data demonstrating this early transition to fibrinolysis shutdown in liver transplantation, when viscoelastic testing demonstrates minimal fibrinolytic activity, but there is a heightened sensitivity to exogenous tPA (Fig. 3). This discrepancy in minimal systemic fibrinolytic activity, yet high sensitivity for tPA-mediated fibrinolysis, suggests that the first component of shutting down fibrinolysis is clearance of plasminogen activators. The most likely sources of clearance of plasminogen activator are the multiple liver receptors.35 Delayed tPA clearance in a subcohort of liver transplantation has been appreciated for decades,36 and recently it has been reported that this delayed fibrinolysis shutdown is associated with increased rate of early allograft dysfunction.87 Other investigators have identified that hepatic blood flow is directly proportional to tPA clearance,88 indicative that both flow and receptor binding play a role in clearance. This is further supported by increased levels of tPA following myocardial infarct with decreased cardiac output reducing blood flow to the liver89 and the ability to saturate tPA receptors resulting in impaired clearance.35
Fig. 3.
Differential timing of the fibrinolysis shutdown in liver transplant. This figure represents previously unpublished data in liver transplant in which serial viscoelastic tests demonstrate the shutdown of fibrinolysis activity followed by the increase of fibrinolysis inhibitors. This two-step process is highlighted by the top panel in with LY30 is abruptly decreased at 30 minutes of reperfusion of the liver. In the lower panel, the addition of exogenous tPA to the viscoelastic test demonstrates that the systemic blood is still sensitive to plasminogen activators. Resistance to fibrinolysis activators is not appreciated until 2 hours after reperfusion. These data support that clearance of fibrinolytic activators occurs as the first step of fibrinolysis shutdown, followed by upregulation of fibrinolytic inhibitors hours later. tPA, tissue plasminogen activator; POD, postoperative day.
The liver clears plasminogen activators predominantly through endothelial cells and hepatocytes, and resident macrophages.35,90 The two predominant receptors that clear tPA in the liver are a mannose-binding receptor and low-density lipoprotein receptor–related protein (LRP).91,92 TPA clearance is predominantly through hepatocytes and endothelial cells,35 while urokinase clearance appears to have a differential pattern of clearance in which Kupffer cells and hepatocytes clear the majority of this plasminogen activator.90 Clearance of tPA complexed with PAI-1 is from hepatocytes from the LRP receptor, which is distinct from free tPA clearance.93 Urokinase complexed with PAI-1 can occur in a similar way, but is also cleared by the urokinase receptor, which is expressed in more ubiquitously than the LRP receptor.94
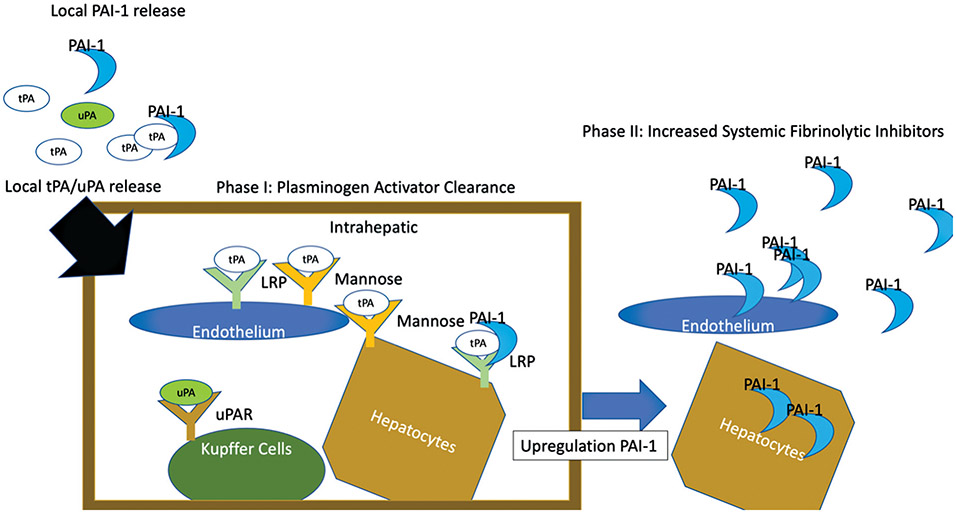
Receptor interactions with plasminogen activators not only clear proteases but also result in downstream cell signaling. tPA has been demonstrated to induce an anti-inflammatory effect on macrophages by binding to the LRP receptor.95 Urokinase receptors have been demonstrated to have a diverse role of biological functions through subsequent cell signaling by modulating cellular interaction with the extracellular matrix, cell survival, and tissue remodeling.21 The role in these signaling pathways in driving subsequent shutdown of fibrinolysis by increasing transcription of fibrinolytic inhibitors via these pathways is not reported. But alternative agonist of hepatocytes and endothelial cells such as thrombin,96 tumor necrosis factor-α,97 interleukin-6, and lipopolysaccharide have been demonstrated to increase PAI-1 production. Within 2 hours of liver reperfusion in transplant, most patients have low fibrinolysis activity and tPA resistance (Fig. 3). This timing is comparable to what has been observed in trauma, in which 50% of patients have tPA resistance up to 2 hours after injury, and 75% of patients demonstrate tPA resistance up to 4 hours, which are associated with increased PAI-1 levels.81 Fibrinolysis shutdown likely represents an acute phase response to controlling hemostasis and activating an innate immune response to prevent the development of infection. The phases of fibrinolysis shutdown are depicted in Fig. 4.
Fig. 4.
Schematic of the two phases of fibrinolysis shutdown. This figure represents a schematic of the proposed mechanism of fibrinolysis shutdown. Plasminogen activators released in circulating blood are initially controlled by local PAI-1 levels. Activators surpass the local PAI-1 binding and enter the liver. The numerous receptors of cells in the liver that have tPA avidity bind the plasminogen activators. This is the phase I of fibrinolysis shutdown. Following this, the response to tPA from local liver cells increases PAI-1 synthesis, resulting in increased release of PAI-1 up to an hour after activator clearance. Additional peripheral endothelial cells also play a role in increase PAI-1 production from cytokine release. This second phase of shutdown is hallmarked by resistance to fibrinolytic activators. PAI-1, plasminogen activator inhibitor 1; tPA, tissue plasminogen activator; uPA, urokinase-type plasminogen activator.
Resolution of fibrinolysis shutdown may play a role in transitioning from hemorrhage and infection control to anti-inflammatory and tissue regeneration. This is supported by work demonstrating plasmin generation attenuating inflammation in macrophages.98 Neutrophils may have a role in the regulation of fibrinolytic shutdown states.99 There is a paucity of data that describe recovery from fibrinolysis shutdown in the literature. It remains an important ongoing area of research to identify if interventions to promote early resolution can improve outcomes. Currently, recovery from fibrinolysis shutdown is limited to observational studies in trauma, in which persistent fibrinolysis shutdown is associated with increased mortality, if patients remain in a low fibrinolytic state for days after admission to the intensive care unit.100,101 Similar data in sepsis have also been reported, in which prolonged tPA resistance is associated with poor outcomes in the intensive care unit.102
Lack of Fibrinolysis Activation—Hypofibrinolysis
Hypofibrinolysis reflects a lack of activation of fibrinolysis when anticipated. Hypofibrinolysis is often confused with fibrinolysis shutdown, as a single measurement of low fibrinolytic activity can occur in both settings. To clearly differentiate the two conditions, a known event of anticipated fibrinolysis activation needs to occur. Liver transplant, as previously mentioned, is one of the most predictable ways to generate a fibrinolytic response via proxy of removal of the liver, and results in increased circulating levels of fibrinolytic activators from impaired clearance and should induce a systemic fibrinolytic response. When a lack of activation occurs, hypofibrinolysis can be identified. This occurs in roughly 40% of liver transplant recipients,36 and is associated with early allograft dysfunction.87 Recently, hypofibrinolysis in the setting of acute liver failure in cirrhosis patients has also been associated with increased 30-day mortality.103
Testing for hypofibrinolysis does not require a liver transplant but is logistically challenging. Assessment of hypofibrinolysis in an individual not undergoing a procedure requires an intervention to stimulate plasminogen activator release. Upper extremity venous occlusion is historically the most common methods of stimulating plasminogen activator release, as forearm appears to have a highest concentration of tPA releasing cells.104 Assessment of fibrinolysis in this setting has been historically through the euglobulin lysis assay, which fractionates whole blood eliminating cellular components and plasma proteins important for fibrinolysis inhibition.105 This lack of fibrinolysis activation is found to occur in 30% of patients with a history of deep venous thrombosis (DVT).106 This can be attributable to either elevated levels of PAI-1 or lack of release of tPA.107 In a study of 100 patients with a history of DVT, 10% had defective tPA release and 20% had elevated inhibitor levels, suggesting that the most common cause of hypofibrinolysis is due to elevated inhibitors.108 Viscoelastic testing to quantify hypofibrinolysis using venous occlusion has not been previously reported.
Impaired vascular release to tPA occurs in the setting of hypertension,109 smoking,110 obesity,111 and renal disease112 and may also be hereditarily acquired.113 The precise mechanism remains unclear. The muscarinic receptor has been identified to play a role in tPA release in the setting of sympathetic activation in the human forearm.114 Regional differences of this tPA release have been described in animal models in the setting of sympathetic activation115 and it remains unclear in humans how the local distribution of sympathetic neurons can impact tPA release. Muscarinic receptors on the endothelium, through G-coupled signal transduction, have previously been described to control the local vascular tone,116 and could have a dual role in tPA release. The bradykinin 2 receptor has also been proposed to be a regulator of tPA release from endothelial cells.117 This receptor is also known to be activated in specific shear conditions of flow over the surface of the receptor in addition to bradykinin.118
Increased inhibitors of fibrinolysis activation are also appreciated in a wide spectrum of pathology. Diabetes,119 obesity,120 inflammatory bowel disease,121 and specific genetic polymorphisms122 are associated with high PAI-1 levels and risk of thrombosis. The source of PAI-1 can come from the adipose tissue,121 hepatocytes,123 endothelium,124 and smooth muscle cells.125 Circulating cells can also increase PAI-1 expression for local release including platelets126 and macrophages/monocytes.127 Stimulation for increased PAI-1 expression has been proposed to come from cytokines, elevated blood glucose, insulin, thrombin, and angiostatin II.128 The multiple mechanisms for hypofibrinolysis are displayed in Fig. 5.
Fig. 5.
Schematic of hypofibrinolysis. Hypofibrinolysis occurs from either lack of release of plasminogen activators or increase basal expression of inhibitors, or a combination of both. This figure represents the multiple steps at which tPA release can be impaired from the endothelial cells and local neurons. Increased release of PAI-1 can occur in multiple cell lines, often due to cytokines and other stimuli. PAI-1, plasminogen activator inhibitor 1; tPA, tissue plasminogen activator.
With the projected increased rate of risk factors associated with hypofibrinolysis anticipated to continue to increase over the next 5 years,129 the prevalence of hypofibrinolysis is likely to continue to increase overtime as well. Hypofibrinolysis represents an underlying disease process that predisposes individuals to adverse outcomes. This is distinctly different from fibrinolysis shutdown, in which there is activation of fibrinolysis with subsequent inhibition. Patients who are in theory at the greatest risk of adverse events are those patients who are anticipated to have a fibrinolytic response from some sort of insult but fail to generate a response.
Conclusion and Future Direction
Fibrinolysis shutdown and hypofibrinolysis are not interchangeable terms. These are important terms to describe different mechanisms of low fibrinolytic states that have been neglected in more contemporary literature. Hypofibrinolysis is a chronic condition in which an individual has an impaired ability to mount an appropriate fibrinolytic response when anticipated due to either lack of plasminogen activator release or high levels of baseline inhibitors. Fibrinolysis shutdown is the process of shutting down fibrinolysis after prior activation, and its duration appears to be associated with pathology. There is a paucity of data that differentiate these two distinct causes of low fibrinolytic activity in hospitalized patients. Furthermore, there has been a lack of research on the cellular mediators of fibrinolysis and their roles in these processes. Using plasma circulating factors likely neglects critical portions of fibrinolysis that are otherwise unrecognized unless performed in whole blood assays. Whole blood assays are also limited in that they neglect endothelial contribution to fibrinolysis regulation in addition to other vascular and organ-specific factors. There is a large amount of research needed in this field, which is undoubtedly becoming increasingly important to address with the continued obesity epidemic that will likely increase the prevalence of hypofibrinolysis which should not be confused with fibrinolysis shutdown.
Ultimately, understanding which patients have hypofibrinolysis rather than fibrinolysis shutdown provides greater insight into the pathophysiology of these distinct processes. This is important, as it will enable the identification of what is pathologic versus physiologic low fibrinolysis and provide a more effective modality of personalized medicine to treat an underlying cause. Before this can occur, the scientific community needs to begin to appropriately label these terms when used in clinical studies, and, if there is ambiguity, describe the patient cohort as low fibrinolytic activity or resistance based on what measurement is being performed.
Funding
This research is supported in part by the American Society of Transplant Surgeons Veloxis Fellowship Grant and National Heart Lung and Blood Institute Grant R00-HL151887.
Footnotes
Conflict of Interest
H.B.M. has patents pending in fibrinolysis diagnostics and has received research support from Haemonetics and Instrumentation Laboratories.
References
- 1.Tillett WS, Sherry S. The effect in patients of streptococcal fibrinolysin (streptokinase) and streptococcal desoxyribonuclease on fibrinous, purulent, and sanguinous pleural exudations. J Clin Invest 1949;28(01):173–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins R, Peto R, Baigent C, Sleight P. Aspirin, heparin, and fibrinolytic therapy in suspected acute myocardial infarction. N Engl J Med 1997;336(12):847–860 [DOI] [PubMed] [Google Scholar]
- 3.Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA 2014;311(07):717–728 [DOI] [PubMed] [Google Scholar]
- 4.Wechsler LR. Intravenous thrombolytic therapy for acute ischemic stroke. N Engl J Med 2011;364(22):2138–2146 [DOI] [PubMed] [Google Scholar]
- 5.Von Kaulla KN, Kaye H, von Kaulla E, Marchioro TL, Starzl TE. Changes in blood coagulation. Arch Surg 1966;92(01):71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attar S, Boyd D, Layne E, McLaughlin J, Mansberger AR, Cowley RA. Alterations in coagulation and fibrinolytic mechanisms in acute trauma. J Trauma 1969;9(11):939–965 [DOI] [PubMed] [Google Scholar]
- 7.Lijnen HR, Collen D. Fibrinolytic agents: mechanisms of activity and pharmacology. Thromb Haemost 1995;74(01):387–390 [PubMed] [Google Scholar]
- 8.Stein PD, Hull RD, Raskob G. Risks for major bleeding from thrombolytic therapy in patients with acute pulmonary embolism. Consideration of noninvasive management. Ann Intern Med 1994;121(05):313–317 [DOI] [PubMed] [Google Scholar]
- 9.van Hinsbergh VW, Kooistra T, Emeis JJ, Koolwijk P. Regulation of plasminogen activator production by endothelial cells: role in fibrinolysis and local proteolysis. Int J Radiat Biol 1991;60(1-2):261–272 [DOI] [PubMed] [Google Scholar]
- 10.O’Rourke J, Jiang X, Hao Z, Cone RE, Hand AR. Distribution of sympathetic tissue plasminogen activator (tPA) to a distant microvasculature. J Neurosci Res 2005;79(06):727–733 [DOI] [PubMed] [Google Scholar]
- 11.Peng T, Jiang X, Wang Y, et al. Sympathectomy decreases and adrenergic stimulation increases the release of tissue plasminogen activator (t-PA) from blood vessels: functional evidence for a neurologic regulation of plasmin production within vessel walls and other tissue matrices. J Neurosci Res 1999;57(05):680–692 [PubMed] [Google Scholar]
- 12.Astrup T, Permin PM. Fibrinolysis in the animal organism. Nature 1947;159(4046):681. [DOI] [PubMed] [Google Scholar]
- 13.Astrup T. Fibrinolysis in the organism. Blood 1956;11(09):781–806 [PubMed] [Google Scholar]
- 14.Fuhrman B. The urokinase system in the pathogenesis of atherosclerosis. Atherosclerosis 2012;222(01):8–14 [DOI] [PubMed] [Google Scholar]
- 15.Cohen MJ, Call M, Nelson M, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg 2012;255(02):379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson PI, Haase N, Perner A, Ostrowski SR. Association between sympathoadrenal activation, fibrinolysis, and endothelial damage in septic patients: a prospective study. J Crit Care 2014;29(03):327–333 [DOI] [PubMed] [Google Scholar]
- 17.Chapman MP, Moore EE, Moore HB, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg 2016;80(01):16–23, discussion 23–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannell R, Li S, Gurewich V. Fibrin-specific and effective clot lysis requires both plasminogen activators and for them to be in a sequential rather than simultaneous combination. J Thromb Thrombolysis 2017;44(02):210–215 [DOI] [PubMed] [Google Scholar]
- 19.Falcone DJ, McCaffrey TA, Haimovitz-Friedman A, Garcia M. Transforming growth factor-beta 1 stimulates macrophage urokinase expression and release of matrix-bound basic fibroblast growth factor. J Cell Physiol 1993;155(03):595–605 [DOI] [PubMed] [Google Scholar]
- 20.Cook AD, Vlahos R, Massa CM, et al. The effect of tissue type-plasminogen activator deletion and associated fibrin(ogen) deposition on macrophage localization in peritoneal inflammation. Thromb Haemost 2006;95(04):659–667 [PubMed] [Google Scholar]
- 21.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 2010;11(01):23–36 [DOI] [PubMed] [Google Scholar]
- 22.Mahmood N, Mihalcioiu C, Rabbani SA. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front Oncol 2018;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwaan HC, Lindholm PF. Fibrin and fibrinolysis in cancer. Semin Thromb Hemost 2019;45(04):413–422 [DOI] [PubMed] [Google Scholar]
- 24.Ismail AA, Shaker BT, Bajou K. The plasminogen-activator plasmin system in physiological and pathophysiological angiogenesis. Int J Mol Sci 2021;23(01):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breuss JM, Uhrin P. VEGF-initiated angiogenesis and the uPA/uPAR system. Cell Adhes Migr 2012;6(06):535–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacroix R, Sabatier F, Mialhe A, et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood 2007;110(07):2432–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plesner T, Behrendt N, Ploug M. Structure, function and expression on blood and bone marrow cells of the urokinase-type plasminogen activator receptor, uPAR. Stem Cells 1997;15(06):398–408 [DOI] [PubMed] [Google Scholar]
- 28.Plow EF, Doeuvre L, Das R. So many plasminogen receptors: why? J Biomed Biotechnol 2012;2012:141806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmeliet P, Schoonjans L, Kieckens L, et al. Physiological consequences of loss of plasminogen activator gene function in mice. Nature 1994;368(6470):419–424 [DOI] [PubMed] [Google Scholar]
- 30.Astrup T. The biological significance of fibrinolysis. Lancet 1956;271(6942):565–568 [DOI] [PubMed] [Google Scholar]
- 31.Rijken DC, Sakharov DV. Basic principles in thrombolysis: regulatory role of plasminogen. Thromb Res 2001;103(Suppl 1):S41–S49 [DOI] [PubMed] [Google Scholar]
- 32.Urano T, Suzuki Y, Iwaki T, Sano H, Honkura N, Castellino FJ. Recognition of plasminogen activator inhibitor type 1 as the primary regulator of fibrinolysis. Curr Drug Targets 2019;20(16):1695–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reilly TM, Mousa SA, Seetharam R, Racanelli AL. Recombinant plasminogen activator inhibitor type 1: a review of structural, functional, and biological aspects. Blood Coagul Fibrinolysis 1994;5(01):73–81 [PubMed] [Google Scholar]
- 34.Kluft C, Verheijen JH, Jie AF, et al. The postoperative fibrinolytic shutdown: a rapidly reverting acute phase pattern for the fast-acting inhibitor of tissue-type plasminogen activator after trauma. Scand J Clin Lab Invest 1985;45(07):605–610 [DOI] [PubMed] [Google Scholar]
- 35.Otter M, Kuiper J, van Berkel TJ, Rijken DC. Mechanisms of tissue-type plasminogen activator (tPA) clearance by the liver. Ann N Y Acad Sci 1992;667:431–442 [DOI] [PubMed] [Google Scholar]
- 36.Porte RJ, Bontempo FA, Knot EA, Lewis JH, Kang YG, Starzl TE. Systemic effects of tissue plasminogen activator-associated fibrinolysis and its relation to thrombin generation in orthotopic liver transplantation. Transplantation 1989;47(06):978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem 1982;257(06):2912–2919 [PubMed] [Google Scholar]
- 38.Bouma BN, Mosnier LO. Thrombin activatable fibrinolysis inhibitor (TAFI)-how does thrombin regulate fibrinolysis? Ann Med 2006;38(06):378–388 [DOI] [PubMed] [Google Scholar]
- 39.Rijken DC, Uitte de Willige S. Inhibition of fibrinolysis by coagulation factor XIII. BioMed Res Int 2017;2017:1209676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee KN, Lee CS, Tae WC, Jackson KW, Christiansen VJ, McKee PA. Crosslinking of alpha 2-antiplasmin to fibrin. Ann N Y Acad Sci 2001;936:335–339 [DOI] [PubMed] [Google Scholar]
- 41.Collen D. Natural inhibitors of fibrinolysis. J Clin Pathol Suppl (R Coll Pathol) 1980;14:24–30 [PMC free article] [PubMed] [Google Scholar]
- 42.Booth NA. Fibrinolysis and thrombosis. Best Pract Res Clin Haematol 1999;12(03):423–433 [DOI] [PubMed] [Google Scholar]
- 43.Mutch NJ, Thomas L, Moore NR, Lisiak KM, Booth NA. TAFIa, PAI-1 and alpha-antiplasmin: complementary roles in regulating lysis of thrombi and plasma clots. J Thromb Haemost 2007;5(04):812–817 [DOI] [PubMed] [Google Scholar]
- 44.Egeblad K, Astrup T. Effects of human plasma on thrombelastographic patterns produced by fibrinolytic agents in fibrin clots. Br J Haematol 1967;13(01):10–13 [DOI] [PubMed] [Google Scholar]
- 45.Moore HB, Moore EE, Chapman MP, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost 2015;13(10):1878–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brass LF, Diamond SL, Stalker TJ. Platelets and hemostasis: a new perspective on an old subject. Blood Adv 2016;1(01):5–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brass LF, Stalker TJ. Minding the gaps – and the junctions, too. Circulation 2012;125(20):2414–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huebner BR, Moore EE, Moore HB, et al. Thrombin provokes degranulation of platelet α-granules leading to the release of active plasminogen activator inhibitor-1 (PAI-1). Shock 2018;50(06):671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mosnier LO, Buijtenhuijs P, Marx PF, Meijers JC, Bouma BN. Identification of thrombin activatable fibrinolysis inhibitor (TAFI) in human platelets. Blood 2003;101(12):4844–4846 [DOI] [PubMed] [Google Scholar]
- 50.Moore HB, Moore EE, Gonzalez E, et al. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock 2015;43(01):39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakharov DV, Nagelkerke JF, Rijken DC. Rearrangements of the fibrin network and spatial distribution of fibrinolytic components during plasma clot lysis. Study with confocal microscopy. J Biol Chem 1996;271(04):2133–2138 [DOI] [PubMed] [Google Scholar]
- 52.Mutch NJ, Koikkalainen JS, Fraser SR, et al. Model thrombi formed under flow reveal the role of factor XIII-mediated cross-linking in resistance to fibrinolysis. J Thromb Haemost 2010;8(09): 2017–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zubairova LD, Nabiullina RM, Nagaswami C, et al. Circulating microparticles alter formation, structure, and properties of fibrin clots. Sci Rep 2015;5:17611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cockell KA, Ren S, Sun J, Angel A, Shen GX. Effect of thrombin on release of plasminogen activator inhibitor-1 from cultured primate arterial smooth muscle cells. Thromb Res 1995;77(02):119–131 [DOI] [PubMed] [Google Scholar]
- 55.Longstaff C, Varjú I, Sótonyi P, et al. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem 2013;288(10):6946–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gould TJ, Lysov Z, Liaw PC. Extracellular DNA and histones: double-edged swords in immunothrombosis. J Thromb Haemost 2015;13(Suppl 1):S82–S91 [DOI] [PubMed] [Google Scholar]
- 57.Xie F, Tan Q, Yu A, et al. The role of cell-free DNA in fibrinolysis for intraventricular hemorrhage. J Neurosurg 2021; doi: 10.3171/2020.7.JNS201429 [DOI] [PubMed] [Google Scholar]
- 58.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost 2005;3(08):1894–1904 [DOI] [PubMed] [Google Scholar]
- 59.Mangin PH, Neeves KB, Lam WA, et al. ; Subcommittee on Biorheology. In vitro flow-based assay: from simple toward more sophisticated models for mimicking hemostasis and thrombosis. J Thromb Haemost 2021;19(02):582–587 [DOI] [PubMed] [Google Scholar]
- 60.Hoffman M, Monroe DM III. A cell-based model of hemostasis. Thromb Haemost 2001;85(06):958–965 [PubMed] [Google Scholar]
- 61.Kwaan HC. Fibrinolysis – a perspective. Prog Cardiovasc Dis 1979;21(05):397–403 [DOI] [PubMed] [Google Scholar]
- 62.Sherry S, Lindemeyer RI, Fletcher AP, Alkjaersig N. Studies on enhanced fibrinolytic activity in man. J Clin Invest 1959;38(05):810–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kjaeldgaard A, Kjaeldgaard M. In vitro stimulation of plasminogen activator release from vein walls by adrenaline. J Clin Pathol 1986;39(11):1241–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keber D, Stegnar M, Kluft C. Different tissue plasminogen activator release in the arm and leg during venous occlusion is equalized after DDAVP infusion. Thromb Haemost 1990;63(01): 72–75 [PubMed] [Google Scholar]
- 65.Urano T, Malyszko J, Takada Y, Takada A. Close relationships between serotonergic and fibrinolytic systems revealed by a monoamine oxidase inhibitor treatment in rats. Haemostasis 1995;25(06):277–282 [DOI] [PubMed] [Google Scholar]
- 66.Kwaan HC, Lo R, McFadzean AJ. The production of plasma fibrinolytic activity in vivo by serotonin (5-hydroxytryptamine) creatinine sulphate. Clin Sci 1957;16(02):255–259 [PubMed] [Google Scholar]
- 67.Holemans R, Langdell RD. Histamine-induced increase in fibrinolytic activity. Proc Soc Exp Biol Med 1964;115:584–587 [DOI] [PubMed] [Google Scholar]
- 68.Brown NJ, Gainer JV, Murphey LJ, Vaughan DE. Bradykinin stimulates tissue plasminogen activator release from human forearm vasculature through B(2) receptor-dependent, NO synthase-independent, and cyclooxygenase-independent pathway. Circulation 2000;102(18):2190–2196 [DOI] [PubMed] [Google Scholar]
- 69.Andreotti L, Nuzzaci G, Paneraia. [Fibrinolytic effect of acetylcholine administered by intra-arterial route. Preliminary note]. Boll Soc Ital Biol Sper 1961;37:1273–1274 [PubMed] [Google Scholar]
- 70.Vervloet MG, Thijs LG, Hack CE. Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin Thromb Hemost 1998;24(01):33–44 [DOI] [PubMed] [Google Scholar]
- 71.Gando S. Disseminated intravascular coagulation in trauma patients. Semin Thromb Hemost 2001;27(06):585–592 [DOI] [PubMed] [Google Scholar]
- 72.Hardaway RM Trauma, sepsis, and disseminated intravascular coagulation. J Intensive Care Med 1995;10(03):145–152 [DOI] [PubMed] [Google Scholar]
- 73.Levi M, van der Poll T. A short contemporary history of disseminated intravascular coagulation. Semin Thromb Hemost 2014;40(08):874–880 [DOI] [PubMed] [Google Scholar]
- 74.Innes D, Sevitt S. Coagulation and fibrinolysis in injured patients. J Clin Pathol 1964;17:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore HB, Moore EE. Temporal changes in fibrinolysis following injury. Semin Thromb Hemost 2020;46(02):189–198 [DOI] [PubMed] [Google Scholar]
- 76.Biemond BJ, Levi M, Ten Cate H, et al. Plasminogen activator and plasminogen activator inhibitor I release during experimental endotoxaemia in chimpanzees: effect of interventions in the cytokine and coagulation cascades. Clin Sci (Lond) 1995;88(05):587–594 [DOI] [PubMed] [Google Scholar]
- 77.Kwaan HC, Lindholm PF. The central role of fibrinolytic response in COVID-19 - a hematologist’s perspective. Int J Mol Sci 2021;22(03):1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meizoso JP, Moore HB, Moore EE. Fibrinolysis shutdown in COVID-19: clinical manifestations, molecular mechanisms, and therapeutic implications. J Am Coll Surg 2021;232(06):995–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tillett WS, Edwards LB, Garner RL. Fibrinolytic activity of hemolytic streptococci. The development of resistance to fibrinolysis following acute hemolytic streptococcus infections. J Clin Invest 1934;13(01):47–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bennett B, Croll A, Ferguson K, Booth NA. Complexing of tissue plasminogen activator with PAI-1, alpha 2-macroglobulin, and C1-inhibitor: studies in patients with defibrination and a fibrinolytic state after electroshock or complicated labor. Blood 1990;75(03):671–676 [PubMed] [Google Scholar]
- 81.Moore HB, Moore ME, Gonzalez E, Huebner BJ, et al. Reperfusion shutdown: delayed onset of fibrinolysis resistance after resuscitation from hemorrhagic shock is associated with increased circulating levels of plasminogen activator inhibitor-1 and post-injury complications. Blood 2016;128:206 [Google Scholar]
- 82.Moore HB, Moore EE, Huebner BR, et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg 2017;83(06):1014–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cardenas JC, Wade CE, Cotton BA, et al. ; PROPPR Study Group. TEG lysis shutdown represents coagulopathy in bleeding trauma patients: analysis of the PROPPR cohort. Shock 2019;51(03):273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gall LS, Vulliamy P, Gillespie S, et al. The S100A10 pathway mediates an occult hyperfibrinolytic subtype in trauma patients. Ann Surg 2018;229:92–101 [DOI] [PubMed] [Google Scholar]
- 85.Moore HB, Moore EE, Chapman MP, et al. Does tranexamic acid improve clot strength in severely injured patients who have elevated fibrin degradation products and low fibrinolytic activity, measured by thrombelastography? J Am Coll Surg 2019;229(01):92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shakur H, Roberts I, Bautista R, et al. ; CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23–32 [DOI] [PubMed] [Google Scholar]
- 87.Moore HB, Yaffe H, Pomposelli JJ, et al. Detection of early allograft dysfunction at 30 min of reperfusion in liver transplantation: an intraoperative diagnostic tool with real time assessment of graft function. Am J Surg 2020;220(06):1518–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Boer A, Kluft C, Kroon JM, et al. Liver blood flow as a major determinant of the clearance of recombinant human tissue-type plasminogen activator. Thromb Haemost 1992;67(01):83–87 [PubMed] [Google Scholar]
- 89.van Griensven JM, Koster RW, Burggraaf J, et al. Effects of liver blood flow on the pharmacokinetics of tissue-type plasminogen activator (alteplase) during thrombolysis in patients with acute myocardial infarction. Clin Pharmacol Ther 1998;63(01):39–47 [DOI] [PubMed] [Google Scholar]
- 90.van der Kaaden ME, Rijken DC, Kruijt JK, van Berkel TJ, Kuiper J. The role of the low-density lipoprotein receptor-related protein (LRP) in the plasma clearance and liver uptake of recombinant single-chain urokinase-type plasminogen activator in rats. Thromb Haemost 1997;77(04):710–717 [PubMed] [Google Scholar]
- 91.Biessen EA, van Teijlingen M, Vietsch H, et al. Antagonists of the mannose receptor and the LDL receptor-related protein dramatically delay the clearance of tissue plasminogen activator. Circulation 1997;95(01):46–52 [DOI] [PubMed] [Google Scholar]
- 92.Narita M, Bu G, Herz J, Schwartz AL. Two receptor systems are involved in the plasma clearance of tissue-type plasminogen activator (t-PA) in vivo. J Clin Invest 1995;96(02):1164–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuiper J, Otter M, Voorschuur AH, van Zonneveld AJ, Rijken DC, van Berkel TJ. Characterization of the interaction of a complex of tissue-type plasminogen activator and plasminogen activator inhibitor type 1 with rat liver cells. Thromb Haemost 1995;74(05):1298–1304 [PubMed] [Google Scholar]
- 94.Andreasen PA, Sottrup-Jensen L, Kjøller L, et al. Receptor-mediated endocytosis of plasminogen activators and activator/inhibitor complexes. FEBS Lett 1994;338(03):239–245 [DOI] [PubMed] [Google Scholar]
- 95.Mantuano E, Azmoon P, Brifault C, et al. Tissue-type plasminogen activator regulates macrophage activation and innate immunity. Blood 2017;130(11):1364–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huebner BR, Moore EE, Moore HB, et al. Thrombin stimulates increased plasminogen activator inhibitor-1 release from liver compared to lung endothelium. J Surg Res 2018;225:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fearns C, Loskutoff DJ. Induction of plasminogen activator inhibitor 1 gene expression in murine liver by lipopolysaccharide. Cellular localization and role of endogenous tumor necrosis factor-alpha. Am J Pathol 1997;150(02):579–590 [PMC free article] [PubMed] [Google Scholar]
- 98.Sugimoto MA, Ribeiro ALC, Costa BRC, et al. Plasmin and plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via annexin A1. Blood 2017;129(21):2896–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barrett CD, Moore HB, Banerjee A, Silliman CC, Moore EE, Yaffe MB. Human neutrophil elastase mediates fibrinolysis shutdown through competitive degradation of plasminogen and generation of angiostatin. J Trauma Acute Care Surg 2017;83(06):1053–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roberts DJ, Kalkwarf KJ, Moore HB, et al. Time course and outcomes associated with transient versus persistent fibrinolytic phenotypes after injury: a nested, prospective, multicenter cohort study. J Trauma Acute Care Surg 2019;86(02):206–213 [DOI] [PubMed] [Google Scholar]
- 101.Meizoso JP Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. J Am Coll Surg 2017;224(04):575–582 [DOI] [PubMed] [Google Scholar]
- 102.Schmitt FCF, Manolov V, Morgenstern J, et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care 2019;9(01):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blasi A, Patel VC, Adelmeijer J, et al. Mixed fibrinolytic phenotypes in decompensated cirrhosis and acute-on-chronic liver failure with hypofibrinolysis in those with complications and poor survival. Hepatology 2020;71(04):1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keber D, Blinc A, Fettich J. Increase of tissue plasminogen activator in limbs during venous occlusion: a simple haemodynamic model. Thromb Haemost 1990;64(03):433–437 [PubMed] [Google Scholar]
- 105.Katz J, Lurie A, Becker D, Metz J. The euglobulin lysis time test: an ineffectual monitor of the therapeutic inhibition of fibrinolysis. J Clin Pathol 1970;23(06):529–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Conard J, Veuillet-Duval A, Horellou MH, Samama M. [Study of coagulation and fibrinolysis in 131 cases of recurrent deep vein thrombosis]. Nouv Rev Fr Hematol (1978) 1982;24(03):205–209 [PubMed] [Google Scholar]
- 107.Nguyen G, Horellou MH, Kruithof EK, Conard J, Samama MM. Residual plasminogen activator inhibitor activity after venous stasis as a criterion for hypofibrinolysis: a study in 83 patients with confirmed deep vein thrombosis. Blood 1988;72(02):601–605 [PubMed] [Google Scholar]
- 108.Nilsson IM, Ljungnér H, Tengborn L. Two different mechanisms in patients with venous thrombosis and defective fibrinolysis: low concentration of plasminogen activator or increased concentration of plasminogen activator inhibitor. Br Med J (Clin Res Ed) 1985;290(6480):1453–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hrafnkelsdóttir T, Ottosson P, Gudnason T, Samuelsson O, Jern S. Impaired endothelial release of tissue-type plasminogen activator in patients with chronic kidney disease and hypertension. Hypertension 2004;44(03):300–304 [DOI] [PubMed] [Google Scholar]
- 110.Newby DE, Wright RA, Labinjoh C, et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation 1999;99(11):1411–1415 [DOI] [PubMed] [Google Scholar]
- 111.Van Guilder GP, Hoetzer GL, Smith DT, et al. Endothelial t-PA release is impaired in overweight and obese adults but can be improved with regular aerobic exercise. Am J Physiol Endocrinol Metab 2005;289(05):E807–E813 [DOI] [PubMed] [Google Scholar]
- 112.Brommer EJ, Verheijen JH, Chang GT, Rijken DC. Masking of fibrinolytic response to stimulation by an inhibitor of tissue-type plasminogen activator in plasma. Thromb Haemost 1984;52(02):154–156 [PubMed] [Google Scholar]
- 113.Petäjä J, Rasi V, Vahtera E, Myllylä G Familial clustering of defective release of t-PA. Br J Haematol 1991;79(02):291–295 [DOI] [PubMed] [Google Scholar]
- 114.Jern C, Selin L, Tengborn L, Jern S. Sympathoadrenal activation and muscarinic receptor stimulation induce acute release of tissue-type plasminogen activator but not von Willebrand factor across the human forearm. Thromb Haemost 1997;78(02):887–891 [PubMed] [Google Scholar]
- 115.Jern C, Seeman-Lodding H, Biber B, Winsö O, Jern S. An experimental multiple-organ model for the study of regional net release/uptake rates of tissue-type plasminogen activator in the intact pig. Thromb Haemost 1997;78(03):1150–1156 [PubMed] [Google Scholar]
- 116.Saternos HC, Almarghalani DA, Gibson HM, et al. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol Genomics 2018;50(01):1–9 [DOI] [PubMed] [Google Scholar]
- 117.Gryglewski RJ, Uracz W, Chłopicki S, Marcinkiewicz E. Bradykinin as a major endogenous regulator of endothelial function. Pediatr Pathol Mol Med 2002;21(03):279–290 [DOI] [PubMed] [Google Scholar]
- 118.Hu Y, Chen M, Wang M, Li X. Flow-mediated vasodilation through mechanosensitive G protein-coupled receptors in endothelial cells. Trends Cardiovasc Med 2022;32(02):61–70 [DOI] [PubMed] [Google Scholar]
- 119.Juhan-Vague I, Alessi MC, Morange PE. Hypofibrinolysis and increased PAI-1 are linked to atherothrombosis via insulin resistance and obesity. Ann Med 2000;32(Suppl 1):78–84 [PubMed] [Google Scholar]
- 120.Solá E, Vayá A, España F, et al. Plasminogen activator inhibitor-1 levels in severe and morbid obesity. Effect of weight loss and influence of 4G/5G polymorphism. Thromb Res 2008;122(03):320–327 [DOI] [PubMed] [Google Scholar]
- 121.Vrij AA, Rijken J, van Wersch JW, Stockbrügger RW. Coagulation and fibrinolysis in inflammatory bowel disease and in giant cell arteritis. Pathophysiol Haemost Thromb 2003;33(02):75–83 [DOI] [PubMed] [Google Scholar]
- 122.Tsantes AE, Nikolopoulos GK, Bagos PG, Bonovas S, Kopterides P, Vaiopoulos G. The effect of the plasminogen activator inhibitor-1 4G/5G polymorphism on the thrombotic risk. Thromb Res 2008;122(06):736–742 [DOI] [PubMed] [Google Scholar]
- 123.Zheng Z, Nakamura K, Gershbaum S, et al. Interacting hepatic PAI-1/tPA gene regulatory pathways influence impaired fibrinolysis severity in obesity. J Clin Invest 2020;130(08):4348–4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Preidl RHM, Reuss S, Neukam FW, Kesting M, Wehrhan F. Endothelial inflammatory and thrombogenic expression changes in microvascular anastomoses - an immunohistochemical analysis. J Craniomaxillofac Surg 2021;49(05):422–429 [DOI] [PubMed] [Google Scholar]
- 125.Lupu F, Bergonzelli GE, Heim DA, et al. Localization and production of plasminogen activator inhibitor-1 in human healthy and atherosclerotic arteries. Arterioscler Thromb 1993;13(07):1090–1100 [DOI] [PubMed] [Google Scholar]
- 126.Whyte CS, Mitchell JL, Mutch NJ. Platelet-mediated modulation of fibrinolysis. Semin Thromb Hemost 2017;43(02):115–128 [DOI] [PubMed] [Google Scholar]
- 127.Uchida Y, Takeshita K, Yamamoto K, et al. Stress augments insulin resistance and prothrombotic state: role of visceral adipose-derived monocyte chemoattractant protein-1. Diabetes 2012;61(06):1552–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morrow GB, Whyte CS, Mutch NJ. A serpin with a finger in many PAIs: PAI-1’s central function in thromboinflammation and cardiovascular disease. Front Cardiovasc Med 2021;8:653655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mohebi R, Chen C, Ibrahim NE, et al. Cardiovascular disease projections in the United States based on the 2020 census estimates. J Am Coll Cardiol 2022;80(06):565–578 [DOI] [PMC free article] [PubMed] [Google Scholar]