Key Points
Question
Does mechanical thrombectomy result in better clinical outcomes for pediatric patients with large vessel occlusion stroke?
Findings
In this case-control study, mechanical thrombectomy (n = 26) was associated with superior clinical outcomes on the pediatric modified Rankin Scale score at 3 months compared with matched controls (n = 26) who received medical treatment only. The difference between groups was statistically significant, and these superior outcomes were maintained at final available follow-up.
Meaning
In this study, mechanical thrombectomy resulted in better clinical outcomes than conservative management for acute large vessel occlusion stroke in children and adolescents aged 2 to 18 years.
This case-control study evaluates whether pediatric patients with acute large vessel occlusion (LVO) stroke who undergo thrombectomy have better clinical outcomes than matched patients managed conservatively.
Abstract
Importance
Pediatric large vessel occlusion (LVO) stroke has a poor natural history. However, uptake of mechanical thrombectomy is hindered by a lack of clinical trial data in children. A randomized clinical trial is not feasible due to small sample sizes and absence of equipoise.
Objective
To evaluate whether pediatric patients with acute LVO stroke who undergo thrombectomy have better clinical outcomes than matched patients managed conservatively.
Design, Setting, and Participants
This matched case-control study used pooled stroke registry data from 5 tertiary referral hospitals in Australia and Canada from January 2011 to April 2022. Patients were aged 1 month to younger than 18 years with acute LVO stroke. Pooled data identified 31 thrombectomy patients and 46 control patients. Five patients undergoing thrombectomy with basilar artery occlusion were excluded due to insufficient controls. Using a hierarchal matching system (site of occlusion, age group, side of occlusion, and sex), deidentified consensus matching of patients and controls was undertaken while blinded to clinical outcome. Data were analyzed from July to November 2022.
Exposure
In the case cohort, mechanical thrombectomy was undertaken for management of acute LVO stroke. The control cohort received medical treatment only.
Main Outcomes and Measures
The primary outcome was the functional clinical status 3 months following stroke, measured by the pediatric modified Rankin Scale (mRS). Clinical outcomes were compared between groups using ordinal regression analysis.
Results
Of 52 included patients, 31 (60%) were male, and the mean (SD) age was 10.3 (4.4) years. Matching was achieved for 26 children undergoing thrombectomy with 26 controls. There was no significant difference between groups for site or side of occlusion, age, sex, etiology, thrombolysis status, baseline Alberta Stroke Programme Early CT Score, or time since last seen well to presentation. Patients undergoing thrombectomy had superior clinical outcomes than control patients at 3 months on the pediatric mRS (odds ratio, 3.76; 95% CI, 1.32-10.67; P = .01). These superior outcomes were maintained at final follow-up (odds ratio, 3.65; 95% CI, 1.25-10.68; P = .02).
Conclusions and Relevance
In the absence of a randomized clinical trial, this case-control study demonstrates better clinical outcomes with thrombectomy than medical management alone for pediatric patients aged 2 to 18 years with anterior circulation LVO stroke.
Introduction
Mechanical thrombectomy is the standard of care for adults with large vessel occlusion (LVO) stroke after the publication of 5 positive randomized clinical trials in 2015.1,2,3,4,5 None of these trials included patients younger than 18 years. As a result, mechanical thrombectomy for pediatric LVO stroke remains less accepted, with limited recommendations for its use in pediatric stroke treatment guidelines.6,7
We previously published a population-based study of clinical outcomes in pediatric LVO stroke.8 For patients managed conservatively, unfavorable clinical outcomes occurred in 73.1% at 3 months and 57.7% at final follow-up.9 Therefore, the natural history of pediatric LVO stroke managed conservatively is poor, with most experiencing long-term moderate to severe disability or death.9 These results demonstrate the need for effective reperfusion therapies in children.
More than 400 pediatric patients undergoing mechanical thrombectomy are reported in the literature, including case reports, case series, retrospective single-arm cohort studies,9,10,11 and national inpatient database analyses.8,12 Favorable clinical outcomes in these studies, equivalent to a modified Rankin Scale (mRS) score of 0 to 2, vary from 55.3% to 89.5%.8,9,10,11,12 Adolescents are disproportionately represented in these studies, relative to younger children.8,9,10 Given the meta-analysis rate of favorable outcomes with mechanical thrombectomy in adults at 3 months is 46.0%,13 these observations raise concerns of selection and publication bias.
We reasoned a randomized clinical trial for thrombectomy in pediatric patients with LVO stroke would now be unethical due to loss of equipoise. In addition, such a trial is unlikely to be feasible due to small sample sizes and recruitment challenges, as observed in the abandoned randomized clinical trial for intravenous thrombolysis in pediatric stroke.14 Therefore, we undertook a case-control study matching patients undergoing thrombectomy and control patients for age, site of occlusion, and sex, aiming to provide the next highest level of comparative evidence.
Methods
Study Design
Institutional ethics approval was obtained from each participating center and from the Sydney Children’s Hospital Network Human Research Ethics Committee. Informed consent was waived due to the retrospective design and long period of time covered in the cohort. This was a multicenter retrospective matched case-control study, pooling data from pediatric stroke registries and databases across 5 centers in New South Wales, Australia, and Ontario, Canada, covering the period January 2010 to April 2022. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Registry data from each center were filtered using the following inclusion criteria: (1) age of 1 month to younger than 18 years at initial assessment, (2) acute arterial ischemic stroke diagnosed by a neurologist with supportive imaging findings, and (3) LVO demonstrated on baseline computed tomography angiography, magnetic resonance angiography, or digital subtraction angiography. Neonatal patients with stroke (younger than 29 days) were excluded.
Patients who received mechanical thrombectomy were matched 1:1 with control patients who received medical therapy only (ie, neuroprotective measures with or without antithrombotic therapy with antiplatelets, anticoagulants, or fibrinolytics), using a hierarchal matching system. Patients consented for mechanical thrombectomy who received bridging intravenous thrombolytic and had recanalization on digital subtraction angiography were also included as cases, using an intention-to-treat analysis.
Definition of LVO
The definition of LVO for this study is detailed in the eAppendix in Supplement 1 and has been previously published in our prior study on the natural history of pediatric LVO.9
Matching Process
All pediatric patients with LVO fulfilling the inclusion criteria were deidentified and allocated a study identifier; clinical outcome data were separated, and deidentified data blinded to clinical outcome were pooled across all 5 centers. Patients undergoing mechanical thrombectomy and controls were separated into 2 lists. Matching (1:1) was undertaken at a single teleconference session by a committee of 5 investigators (2 pediatric neurologists [S.C. and E.P.] and 3 pediatric neurointerventionists [K.D.B., P.M., and C.P.F.]) from both countries, using deidentified data while blinded to clinical outcome. Disputes were resolved by majority vote.
Matching was undertaken using a 4-tier hierarchal system with primary matching by location of the most proximal intracranial vascular occlusion (intracranial internal carotid artery [ICA]; proximal to mid M1; M1-2 junction and M2; basilar artery including vertebro-basilar junction), then secondary matching by age group (younger than 2 years, aged 2-6 years, aged 7-11 years, or aged 12-16 years), then by side of occlusion (left, right, or midline), and lastly by sex. Patients that could not be matched by the primary criteria (site of occlusion) were excluded from the study. Age matching was tailored to minimize age differences between patients undergoing thrombectomy and controls. The pooled matched deidentified data set was then linked to clinical outcome data and other imaging data via the study identifier for further analyses.
Outcome Measures
The primary outcome for this study was the functional clinical outcome 3 months after stroke onset, measured using the pediatric mRS score.15 For patients whose pediatric mRS score was not prospectively recorded in the stroke registries, it was obtained by conversion from Pediatric Stroke Outcome Measure (PSOM) scores (eTable in Supplement 1) or retrospectively derived based on prior assessments undertaken by a pediatric neurologist, neurosurgeon, or rehabilitation physician 2 to 4 months after stroke onset. The primary safety outcome was the rate of symptomatic intracranial hemorrhage (sICH), as defined in the protocol for the European Co-operative Acute Stroke Study III.16
The secondary outcomes were final pediatric mRS score available and its timing poststroke, PSOM scores at 3 months and at final available follow-up, etiology, timing of initial vascular imaging (hours since last seen well), baseline Alberta Stroke Programme Early CT Score (ASPECTS; a measure of baseline established infarct on noncontrast computed tomography imaging ranging from 0 to 10, with lower scores indicating greater infarct extent)17 or diffusion-weighted imaging (DWI)–ASPECTS18 on last available imaging study prior to treatment decision-making. In addition, we evaluated timing of clinical presentation, intravenous and intra-arterial thrombolysis status, and selection criteria fulfilment status (other than age) for the Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischaemic Stroke in the Netherlands (MR-CLEAN) trial,1 DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Stroke Undergoing Neurointervention with Trevo (DAWN) trial,19 or American Heart Association class I-IIb recommendations for use of thrombectomy in adults.20 Where available, pediatric National Institute of Health Stroke Scale scores at initial assessment and 24 hours later were recorded, but these were not retrospectively derived. The secondary safety outcomes included decompressive craniectomy status and major access site complications requiring blood transfusion, surgery, or endovascular treatment.
Imaging Analysis
ASPECTS was determined from the last available imaging study prior to treatment decision-making, based on either computed tomography or magnetic resonance imaging (DWI). The presence and location of the most proximal intracranial occlusion were independently assessed by a pediatric neuroradiologist blinded to clinical outcome in each country.
Statistical Analysis
The primary analysis, comparing pediatric mRS scores at 3 months after stroke onset between the case and control groups, was undertaken with ordinal logistic regression to determine the odds ratio (OR) and P value for a better outcome on the pediatric mRS (ie, a lower score on the scale from 0 to 6). Secondary analyses were undertaken using ordinal logistic regression for pediatric mRS and PSOM data, χ2 testing for categorical variables, and the unpaired t test for continuous variables. Complete-case analysis was used to account for missing data. All P values were 2-tailed, and significance was set at P < .05. All statistical analyses were undertaken using the statistical software MiniTab version 19.2020.2.0 (MiniTab).
Results
Initial pooling of stroke registry data yielded 31 potential patients (with 34 thrombectomy procedures) and 46 potential controls. A total of 5 patients undergoing thrombectomy could not be matched due to insufficient control patients with basilar artery occlusion.
Of 52 included patients, 31 (60%) were male, and the mean (SD) age was 10.3 (4.4) years. Matching was achieved for 26 children undergoing thrombectomy with 26 controls. Of the 26 patients in each group, 24 had LVO involving the anterior circulation. Two matched patients underwent a second thrombectomy procedure, due to recurrent occlusion in the same angiographic location within 96 hours of the original occlusion; each achieved successful recanalization at both of their procedures. Because the primary outcome was measured at 3 months poststroke, these 2 patients were each counted once only for pediatric mRS outcome data.
There was no significant difference between the case and control groups for age, sex, site or side of occlusion, intravenous or intra-arterial thrombolysis status, stroke etiology, baseline ASPECTS, or time since last seen well until initial clinical presentation. More patients in the thrombectomy group received intravenous thrombolysis compared with the control group, but this difference was not statistically significant (4 vs 1; P = .16). Baseline demographic and imaging data for each group are summarized in Table 1.
Table 1. Baseline Demographic, Imaging, and Clinical Characteristics.
| Characteristic | No. (%) | P valuea | |
|---|---|---|---|
| Thrombectomy group | Control group | ||
| Patients, No. | 26 | 26 | NA |
| Thrombectomy procedures, No. | 28b | 0 | NA |
| Sex | |||
| Female | 10 (38.5) | 11 (42.3) | .78 |
| Male | 16 (61.5) | 15 (57.7) | |
| Age, y | |||
| Mean (SD) | 10.9 (4.4) | 9.8 (4.4) | .37 |
| Median (range) | 12.5 (3.1-16.0) | 10.5 (1.9-17.0) | |
| Age group, y | |||
| 0-2 | 0 | 1 (3.8) | .62 |
| 3-6 | 6 (23.1) | 5 (19.2) | |
| 7-11 | 6 (23.1) | 9 (34.6) | |
| 12-17 | 14 (53.8) | 11 (42.3) | |
| Thrombolysis status | |||
| Intravenous tPA given | 4 (15.4) | 1 (3.8) | .16 |
| Intra-arterial tPA given | 0 | 2 (7.7) | .15 |
| Intravenous or intra-arterial tPA given | 4 (15.4) | 3 (11.5) | .69 |
| Site of occlusion | |||
| Intracranial ICA | 10 (38.4) | 10 (38.4) | >.99 |
| MCA, proximal to mid M1 | 9 (34.6) | 9 (34.6) | |
| MCA, M1-2 junction to M2 | 5 (19.2) | 5 (19.2) | |
| Basilar/vertebrobasilar | 2 (7.7) | 2 (7.7) | |
| Side of occlusion | |||
| Left | 14 (53.8) | 13 (50.0) | .96 |
| Right | 10 (38.4) | 11 (42.3) | |
| Midline | 2 (7.7) | 2 (7.7) | |
| Baseline ASPECTS, No./total No. (%) | |||
| CT available | 22/28 (78.6) | 17/26 (65.4) | .13 |
| DWI available | 12/28 (42.9) | 21/26 (80.8) | |
| Both CT and DWI available | 6/28 (21.4) | 12/26 (46.2) | |
| ASPECTS, mean (SD)c | 6.8 (2.25) | 6.7 (2.74) | .94 |
| ASPECTS ≤5 | 6/28 (21.4) | 7/26 (26.9) | .64 |
| Stroke etiology | |||
| Cardio-embolic | 10 (38.5) | 8 (30.8) | .66 |
| Idiopathic | 5 (19.2) | 3 (11.5) | |
| Dissection | 4 (15.4) | 7 (26.9) | |
| Other | 7 (26.9)d | 8 (30.8)e | |
| Time since LSW, mean (SD), min | |||
| Initial clinical presentation | 157 (159) | 226 (346) | .43 |
| Arrival at pediatric hospital | 344 (400) | 554 (655) | .24 |
| Initial angiographic imaging | 301 (318) | 661 (909) | .07 |
| Clinical presentation timing, No./total No. (%) | |||
| <6 h | 24/28 (85.7) | 16/26 (61.5) | NA |
| 6-24 h | 4/28 (14.3) | 8/26 (30.8) | |
| >24 h | 0 | 2/26 (7.7) | |
| Total <24 h | 28/28 (100) | 24/26 (92.3) | |
| Selection criteria fulfilled | |||
| MR-CLEAN1 | 15 (53.6) | 8 (30.8) | NA |
| DAWN19 | 5 (17.9) | 6 (23.1) | NA |
| AHA class I-IIb criteria, No./total No. (%)20 | 22/28 (78.6) | 16/26 (61.5) | .17 |
| Excluded for ASPECTS ≤5 | 6 (21.4) | 7 (26.9) | NA |
| Excluded for presentation >24 h | 0 | 2 (7.7) | NA |
Abbreviations: AHA, American Heart Association; ASPECTS, Alberta Stroke Program Early CT Score; CT, computed tomography; DAWN, DWI or CTP Assessment With Clinical Mismatch in the Triage of Wake-Up and Late Presenting Stroke Undergoing Neurointervention With Trevo Study19; DWI, diffusion-weighted imaging; ICA, internal carotid artery; LSW, last seen well; MCA, middle cerebral artery; MR-CLEAN, Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischaemic Stroke in the Netherlands Study1; NA, not applicable; tPA, tissue plasminogen activator.
P values calculated using χ2 or t tests, as appropriate.
Two patients underwent a second thrombectomy procedure within 96 hours of the initial procedure, in both cases due to reocclusion in the same angiographic location (right intracranial ICA and left proximal M1, respectively). For the purposes of measuring clinical outcome at 3 months and final follow-up, both patients were counted once only.
For pooled ASPECTS analyses in patients with both baseline CT and DWI available, the final imaging modality used for acute clinical decision-making was included. Both patients with posterior circulation occlusion in each group had ASPECTS of 10.
A total of 7 patients undergoing thrombectomy with stroke etiology classified as other included 4 with paradoxical embolus, 1 with acute thrombosis following radiotherapy for adjacent suprasellar pilocytic astrocytoma, 1 with hypercoagulable state, and 1 with extracorporeal membrane oxygenation system–related embolus.
A total of 8 control patients with stroke etiology classified as other included 3 with focal cerebral arteriopathy with superimposed acute thrombotic occlusion, 2 with large-vessel vasculitis, 1 with hypercoagulable state, 1 with drug-induced vasculopathy, and 1 with Bowhunter syndrome with secondary thrombotic occlusion.
Primary Outcome
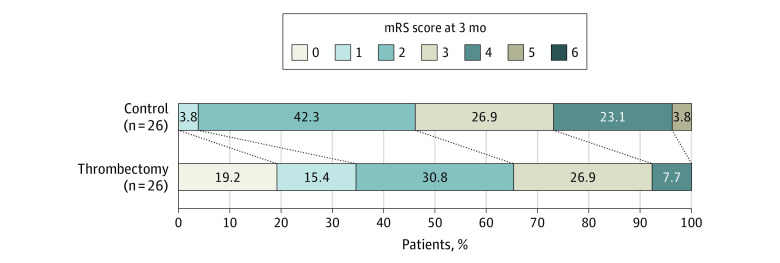
Pediatric patients with LVO who underwent thrombectomy were significantly more likely to have a better functional outcome on the pediatric mRS at 3 months after stroke than control patients with LVO managed with medical therapy alone (OR, 3.76; 95% CI, 1.32-10.67; P = .01). The absolute risk reduction for a poor outcome (pediatric mRS of 3 to 6) with thrombectomy at 3 months was 19.2% (number needed to treat, 6). There was no significant difference in the rate of sICH (1 in each group). Better functional outcomes in the thrombectomy group at 3 months were also confirmed when comparing PSOM scores (OR, 4.72; 95% CI, 1.69-13.15; P = .003). Primary outcome results are detailed in Table 2 and the Figure.
Table 2. Pediatric Modified Rankin Scale (mRS) Scores at 3 Months Following Stroke.
| Outcome | No. (%) | OR (95% CI) | P valuea | |
|---|---|---|---|---|
| Thrombectomy group (n = 26) | Control group (n = 26) | |||
| Pediatric mRS score 3 mo poststroke | ||||
| 0 | 5 (19.2) | 0 | 3.76 (1.32-10.67) | .01 |
| 1 | 4 (15.4) | 1 (3.8) | ||
| 2 | 8 (30.8) | 11 (42.3) | ||
| 3 | 7 (26.9) | 7 (26.9) | ||
| 4 | 2 (7.7) | 6 (23.1) | ||
| 5 | 0 | 1 (3.8) | ||
| 6 | 0 | 0 | ||
| 0-2 | 17 (65.4) | 12 (46.2) | NA | NA |
| 3-6 | 9 (34.6) | 14 (53.8) | NA | NA |
| PSOM score 3 mo poststroke | ||||
| 0 | 5 (19.2) | 0 | 4.72 (1.69-13.15) | .003 |
| 0.5 | 4 (15.4) | 1 (3.8) | ||
| 1.0-2.5 | 8 (30.8) | 11 (42.3) | ||
| 3.0-5.0 | 7 (26.9) | 7 (26.9) | ||
| 6.0-7.0 | 2 (7.7) | 6 (23.1) | ||
| 8.0-9.0 | 0 | 1 (3.8) | ||
| 10.0 | 0 | 0 | ||
| Primary safety outcome | ||||
| Symptomatic ICHb | 1 (3.8) | 1 (3.8) | NA | >.99 |
Abbreviations: ICH, intracranial hemorrhage; NA, not applicable; OR, odds ratio; PSOM, Pediatric Stroke Outcome Measure.
P values calculated using ordinal linear regression or χ2 tests, as appropriate.
The definition used for symptomatic ICH was from the European-Australian Co-operative Acute Stroke Study III trial.16 Symptomatic ICH was defined as any apparently extravascular blood in the brain or within the cranium that was associated with clinical deterioration, as defined by an increase of 4 points or more in the score on the National Institute of Health Stroke Scale, or that led to death and that was identified as the predominant cause of the neurological deterioration.16
Figure. Pediatric Modified Rankin Scale (mRS) Scores Measured 3 Months After Stroke Onset in the Thrombectomy and Control Groups.
Secondary Outcomes
Patients undergoing mechanical thrombectomy were significantly more likely to have a better functional outcome on the pediatric mRS at final available follow-up than control patients (mean [SD] follow-up, 33.0 [26.7] months; OR, 3.65; 95% CI, 1.25-10.68; P = .02). The absolute risk reduction for a poor outcome with thrombectomy at final follow-up was 26.9% (number needed to treat, 4). These better outcomes in the thrombectomy group were also confirmed when comparing final PSOM scores (OR, 4.33; 95% CI, 1.56-12.01; P = .005). Control patients had significantly longer final follow-up than patients undergoing thrombectomy (mean [SD] follow-up of 46.4 [29.0] vs 17.3 [15.7] months; P < .001). Secondary outcome results are detailed in Table 3.
Table 3. Pediatric Modified Rankin Scale (mRS) Scores at Final Available Follow-up.
| Outcome | No. (%) | OR (95% CI) | P valuea | |
|---|---|---|---|---|
| Thrombectomy group (n = 26) | Control group (n = 26) | |||
| Pediatric mRS score at final follow-up | ||||
| 0 | 7 (26.9) | 1 (3.8) | 3.65 (1.25-10.68) | .02 |
| 1 | 2 (7.7) | 3 (11.5) | ||
| 2 | 13 (50.0) | 11 (42.3) | ||
| 3 | 3 (11.5) | 9 (34.6) | ||
| 4 | 0 | 1 (3.8) | ||
| 5 | 0 | 0 | ||
| 6 | 1 (3.8) | 1 (3.8) | ||
| 0-2 | 22 (84.6) | 15 (57.7) | NA | NA |
| 3-6 | 4 (15.4) | 11 (42.3) | NA | NA |
| PSOM score at final follow-up | ||||
| 0 | 7 (26.9) | 1 (3.8) | 4.33 (1.56-12.01) | .005 |
| 0.5 | 2 (7.7) | 3 (11.5) | ||
| 1.0-2.5 | 13 (50.0) | 11 (42.3) | ||
| 3.0-5.0 | 3 (11.5) | 9 (34.6) | ||
| 6.0-7.0 | 0 | 1 (3.8) | ||
| 8.0-9.0 | 0 | 0 | ||
| 10.0 | 1 (3.8) | 1 (3.8) | ||
| Timing of final follow-up poststroke, mean (SD), mo | 19.7 (15.7) | 46.4 (29.0) | NA | <.001 |
| Secondary safety outcomes | ||||
| Craniectomy | 5 (19.2) | 6 (23.1) | NA | .73 |
| Major access site complicationsb | 2 (7.7) | 0 | NA | .15 |
Abbreviations: NA, not applicable; OR, odds ratio; PSOM, Pediatric Stroke Outcome Measure.
P values calculated using ordinal linear regression, χ2, or t tests, as appropriate.
Major access site complications were defined as a complication arising from the arterial access site requiring blood transfusion, surgery, or endovascular treatment. In the thrombectomy arm, 1 patient had a retroperitoneal hemorrhage requiring blood transfusion and placement of a covered stent. The second patient developed a delayed common femoral artery pseudoaneurysm while taking anticoagulation, successfully treated with percutaneous thrombin injection.
No significant difference was present between groups for decompressive craniectomy status (thrombectomy group, 5 [19.2%]; control group, 6 [23.1%]; P = .73). Two patients undergoing thrombectomy had major access site complications (Table 3); neither patient had long-term sequelae from these complications.
In the thrombectomy group, the rate of successful recanalization (modified Treatment in Cerebral Ischaemic score of 2b/3) was 89.3% (25 of 28 procedures). The mean (range) time from last seen well to final recanalization was 695 (130-3840) minutes. The mean reduction in pediatric NIHSS scores at 24 hours postprocedure (data available for 18 of 28 procedures) was 6.1 (5.3) points. While there was heterogeneity of thrombectomy techniques used, most procedures (23 of 28) used a stent-retriever. Secondary outcomes specific to the thrombectomy group only are detailed in Table 4.
Table 4. Technical Outcomes in the Thrombectomy Group.
| Outcome | Thrombectomy group, No. (%) |
|---|---|
| Patients, No. | 26 |
| Thrombectomy procedures, No. | 28 |
| Symptom onset in hospital | 7 (26.9)a |
| Time to clinical presentation, mean (range), min | |
| LSW to arrival at peripheral hospital ED (8 patients) | 247.6 (27-549) |
| LSW to arrival at pediatric/interventional hospital (21 patients)a | 345.9 (51-1170) |
| Peripheral hospital arrival to interventional hospital arrival (9 patients)a | 457.9 (95-960) |
| Time to initial angiographic imaging, mean (range), min | |
| LSW to initial angiographic imaging (CTA, MRA, or DSA) (28 episodes) | 300.9 (34-1264) |
| Initial ED arrival to initial angiographic imaging (20 episodes) | 175.4 (25-1048) |
| Inpatient LSW to initial angiographic imaging (8 episodes) | 221.9 (34-725) |
| Time to recanalization, mean (range), min (28 procedures) | |
| LSW to final recanalization | 695.0 (130-3840) |
| Initial angiographic imaging to DSA suite arrival | 318.4 (10-3105) |
| DSA suite arrival to groin puncture | 24.2 (4-62) |
| Groin puncture to final recanalization | 53.3 (5-135) |
| Time from LSW to final recanalization, time groups (n = 28 procedures) | |
| <6 h | 14 (50.0) |
| 6-12 h | 7 (25.0) |
| 12-24 h | 5 (17.9) |
| >24 h | 2 (7.1) |
| Preprocedure pediatric NIHSS score | |
| Mean (range) | 11.8 (0-22)b |
| Missing data, No. | 7 |
| Postprocedure pediatric NIHSS score at 24 hc | |
| Mean (range) | 4.6 (0-19) |
| Missing data, No. | 9 |
| Reduction in pediatric NIHSS score from prestroke to poststroke | |
| Mean (range) | 6.1 (−3 to 19) |
| Missing data, No. | 10 |
| Recanalization status (mTICI score) | |
| 0 | 0 |
| 1 | 0 |
| 2a | 3 (10.7) |
| 2b | 11 (39.3) |
| 3 | 14 (50.0) |
| Successful recanalization (mTICI score of 2b/3), No./total No. (%) | 25/28 (89.3) |
| No. of passesd | |
| Mean (SD) | 1.9 (1.57) |
| Median (range) | 1.0 (0-7) |
| Most successful recanalization technique | |
| Aspiration only | 3 (10.7) |
| Stent-retriever only | 6 (21.4) |
| Combined stent-retriever and aspiration | 17 (60.7) |
| Balloon angioplasty | 1 (3.6) |
| Intravenous tPA onlyd | 1 (3.6) |
| Additional flow-diverter stent placement | 2 (7.1) |
| Postprocedure intracranial hemorrhage on CT or MRI | |
| Asymptomatic, petechial | 5 (19.2) |
| Asymptomatic, subarachnoid | 2 (7.7) |
| Asymptomatic, lobar | 1 (3.8) |
| Symptomatic, lobar | 1 (3.8) |
| Decompressive craniectomy | |
| For malignant edema | 4 (15.4) |
| For symptomatic lobar hemorrhage | 1 (3.8) |
| Major access site complications | |
| Retroperitoneal hemorrhage | 1 (3.8) |
| Femoral artery pseudoaneurysm | 1 (3.8) |
Abbreviations: CT, computed tomography; CTA, computed tomography angiography; DSA, digital subtraction angiography; ED, emergency department; LSW, last seen well; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; mTICI, modified Treatment in Cerebral Ischaemic; NIHSS, National Institute of Health Stroke Scale; tPA, tissue plasminogen activator.
Seven patients had symptomatic onset of stroke as a hospital inpatient. One patient underwent 2 thrombectomy procedures due to 2 inpatient episodes of occlusion within 96 hours in the same vessel. A second patient had initial symptom onset at home and was treated with thrombectomy but then had recurrent occlusion within 96 hours in the same vessel while an inpatient, leading to a second procedure. A third patient had symptom onset while an inpatient at a peripheral hospital, where they underwent initial angiographic imaging prior to transfer to a pediatric hospital for thrombectomy.
One patient who received thrombectomy had a pediatric NIHSS score of 0 at the time of final treatment decision-making. This patient had a flow-limiting dissection with adjacent thrombus in the intracranial internal carotid artery and presented with mild contralateral hemiparesis that resolved within several hours and was initially managed with heparin infusion. However, 2 additional MRI studies and a CT perfusion study performed in the next 48 hours demonstrated progressive large areas of watershed infarct developing in the ipsilateral deep white matter, and the patient described subjective episodes of receptive dysphasia at night. Based on this, it was decided to proceed to thrombectomy and flow-diverter placement, with successful recanalization at 3840 minutes since last seen well.
Pediatric NIHSS score at 24 hours included scores measured between 20 and 36 hours postprocedure.
One patient who received bridging intravenous tPA en route to the angiography suite after consenting to mechanical thrombectomy had an mTICI score of 3 and successful recanalization on the initial angiographic run and received zero passes.
Discussion
In this case-control study of pediatric acute LVO stroke, mechanical thrombectomy resulted in significantly better functional outcomes than medical therapy alone at 3 months (OR, 3.76; 95% CI, 1.32-10.67; P = .01). These improved outcomes were sustained into the long-term at final available follow-up (OR, 3.65; 95% CI, 1.25-10.68; P = .02). Through appropriately matching patients undergoing thrombectomy and controls by site and side of occlusion, age, and sex, the impact of selection bias was markedly reduced. In addition, the groups were well balanced for thrombolysis status, baseline ASPECTS, and timing of initial clinical presentation.
Mechanical Thrombectomy in Children
The Save ChildS Study, a large single-arm retrospective cohort study of pediatric mechanical thrombectomy, published results from 73 children across 27 centers.10 They reported favorable clinical outcomes (mRS score of 0 to 2) in 80% of patients at discharge and 86% at 6 months poststroke.10 To our knowledge, the largest cohort published (n = 190) was a national inpatient database analysis from the US (2010-2019) in which 55.3% of patients had favorable functional outcomes.8 These pediatric results are superior to those published in adult randomized trials (favorable outcomes in 46.0%).13
Variable selection criteria, differences in stroke etiology, enhanced neuroplasticity, and collaterals in children may account in part for this variance.6,8,10,12,21 However, selection bias is likely present, partly reflected in the disproportionate representation of adolescents in pediatric cohorts (42.5% to 76.3%).8,9,10,12 Children younger than 5 years represented less than 6% of thrombectomy cases in the US inpatient analysis discussed above, despite forming 37.5% of the overall stroke cohort.8
Reduced angiographic diameters22 and limited equipment choices may dampen enthusiasm for intervention in young children. However, newer low-profile distal aspiration catheters and smaller stent-retrievers are likely compatible with known angiographic diameters in toddlers and preschool children.22,23 In our study, children younger than 6 years represented 23.1% of both patients undergoing thrombectomy and controls. No neonates or infants were included in our study.
In the thrombectomy group, 6 of 26 patients had a baseline low ASPECTS of 1 to 5 (3 of 6 on computed tomography and 3 of 6 on DWI), of which 5 patients had a favorable final pediatric mRS score of 2 and 1 patient had a pediatric mRS score of 3. Thus, large infarct extent on baseline imaging did not necessarily portend a poor long-term clinical outcome for pediatric patients who received thrombectomy. Exclusion of pediatric patients based on ASPECTS or infarct volume appears difficult to justify but warrants further research.
A total of 6 patients undergoing thrombectomy were excluded during the matching process because there were insufficient control patients with basilar artery occlusion. This may reflect a more aggressive treatment approach toward basilar occlusion. However, their exclusion limits the applicability of our results in the posterior circulation.
Natural History of Pediatric LVO Stroke
To address the shortfall in natural history data, we previously published a population-based study of pediatric patients with acute arterial ischemic stroke.9 In the conservatively managed cohort (26 of 39 patients), unfavorable clinical outcomes (pediatric mRS score of 3 to 6) occurred in 73.1% of patients at 3 months.9 This updated case-control study further confirms the poor outcomes in pediatric patients with LVO managed conservatively (mRS score of 3 to 6 in 14 [53.8%] at 3 months and 11 [42.3%] at final follow-up), even when control patients are extensively matched to patients undergoing thrombectomy. There was an overlap between the natural history and case-control studies for 12 of 26 patients undergoing thrombectomy and 14 of 26 control patients.
Timing of Clinical Presentation
In this case-control study, all 26 patients undergoing thrombectomy and 24 control patients (92.3%) presented within 24 hours since last seen well. Therefore, most patients with LVO presented early enough to be eligible for thrombectomy using adult selection criteria. In addition, 22 of 28 patients undergoing thrombectomy (78.6%) and 16 of 26 control patients (61.5%) fulfilled selection criteria (other than age) for American Heart Association class I-IIb recommendations supporting thrombectomy in adult stroke,20 with most exclusions resulting from low ASPECTS. Given the strength of collaterals in children, it may not be appropriate to restrict thrombectomy access using adult timelines, and perfusion imaging may be useful in late-presenting cases.24
In this study, 26 of 28 thrombectomy procedures (92.9%) were completed less than 24 hours since last seen well (Table 4). The remaining 2 patients both had flow-limiting dissections of the intracranial ICA with adjacent thrombus and were initially managed with anticoagulation prior to clinical decline. Both patients had excellent outcomes (pediatric mRS score of 0).
There was no significant difference between the thrombectomy and control groups for time since last seen well to initial clinical presentation (mean [SD] time, 157 [159] vs 226 [346] minutes; P = .43). However, the groups diverged for time to arrival at a dedicated pediatric hospital (mean [SD] time, 344 [400] vs 554 [655] minutes; P = .24) and to initial angiographic/vascular imaging (mean [SD] time, 301 [318] vs 661 [909] minutes; P = .07). There were nonsignificantly greater transfer and imaging delays in the control group. These results suggest that pediatric patients with LVO are presenting in appropriate time for thrombectomy (50 of 52 within 24 hours), but our triage, transfer, imaging, and decision-making pathways require streamlining. Such improvements would be aided by strengthened treatment guidelines and referral networks.
Safety Outcomes
In this study, there was no significant difference between the case and control groups for sICH or decompressive craniectomy status. There were 2 major access site complications in the thrombectomy group (1 retroperitoneal hemorrhage and 1 femoral pseudoaneurysm) that both required endovascular/percutaneous treatment. In both instances, the patients were anticoagulated for cardiac disease. Overall, the risk-benefit profile still favored thrombectomy.
Limitations
This study is impacted by several limitations. First, despite extensive matching of patients with controls, the absence of randomization leaves an ongoing degree of selection bias, such as preferential allocation of thrombectomy to patients with lower triage and transfer times. Second, conversion of PSOM to pediatric mRS scores and retrospective derivation of pediatric mRS scores from clinical records are more prone to bias. In particular, the PSOM is a summated score across 5 functional domains, with potential loss of subtle differences when converted to a single pediatric mRS score.25 Third, dichotomous classification of pediatric mRS outcomes into favorable (score of 0 to 2) and unfavorable (score of 3 to 6) is not validated in children as it is in adults, and therefore ordinal regression was used for the primary analysis. There are also differing views in the literature regarding what constitutes good vs bad outcomes in children with stroke.26 Fourthly, pediatric NIHSS data were not available for most control patients and as such could not be used in the matching process.
Fifthly, the dedicated modified pediatric ASPECTS (a magnetic resonance imaging–based system27) was not used, as the modified system incorporates anterior cerebral artery and posterior cerebral artery territories, but none of our matched patients had anterior cerebral artery or posterior cerebral artery occlusion (all anterior circulation patients had occlusion of the internal carotid artery or middle cerebral artery). Sixthly, control patients had significantly longer final follow-up than patients undergoing thrombectomy (46.4 [29.0] vs 17.3 [15.7]; P < .001). Additionally, only 2 patients in each group had posterior circulation occlusions, limiting applicability of the results in this subgroup.
Most patients in this study were assessed by pediatric neurologists and treated by interventionists with pediatric experience. The superior outcomes demonstrated with thrombectomy, therefore, should not be generalized to settings without pediatric neurology expertise.28 Specific subgroups that may benefit (or not benefit) from thrombectomy need to be further defined. These results may not be applicable to children younger than 2 years or with focal cerebral arteriopathy, as they were not represented in the thrombectomy cohort.
Conclusions
In the absence of a randomized trial, this case-control study demonstrates better clinical outcomes with thrombectomy than medical management for pediatric patients aged 2 to 18 years with anterior circulation LVO stroke.
eTable. Conversion Table From PSOM to Pediatric mRS
eAppendix. Study Protocol
Data Sharing Statement
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 6.Ferriero DM, Fullerton HJ, Bernard TJ, et al. ; American Heart Association Stroke Council and Council on Cardiovascular and Stroke Nursing . Management of stroke in neonates and children: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2019;50(3):e51-e96. doi: 10.1161/STR.0000000000000183 [DOI] [PubMed] [Google Scholar]
- 7.Medley TL, Miteff C, Andrews I, et al. Australian Clinical Consensus Guideline: the diagnosis and acute management of childhood stroke. Int J Stroke. 2019;14(1):94-106. doi: 10.1177/1747493018799958 [DOI] [PubMed] [Google Scholar]
- 8.Dicpinigaitis AJ, Gandhi CD, Pisapia J, et al. Endovascular thrombectomy for pediatric acute ischemic stroke. Stroke. 2022;53(5):1530-1539. doi: 10.1161/STROKEAHA.121.036361 [DOI] [PubMed] [Google Scholar]
- 9.Bhatia KD, Briest R, Goetti R, et al. Incidence and natural history of pediatric large vessel occlusion stroke: a population study. JAMA Neurol. 2022;79(5):488-497. doi: 10.1001/jamaneurol.2022.0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sporns PB, Sträter R, Minnerup J, et al. Feasibility, safety, and outcome of endovascular recanalization in childhood stroke: the Save ChildS study. JAMA Neurol. 2020;77(1):25-34. doi: 10.1001/jamaneurol.2019.3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoirah H, Shallwani H, Siddiqui AH, et al. Endovascular thrombectomy in pediatric patients with large vessel occlusion. J Neurointerv Surg. 2019;11(7):729-732. doi: 10.1136/neurintsurg-2018-014320 [DOI] [PubMed] [Google Scholar]
- 12.Kossorotoff M, Kerleroux B, Boulouis G, et al. ; KidClot Group . Recanalization treatments for pediatric acute ischemic stroke in France. JAMA Netw Open. 2022;5(9):e2231343. doi: 10.1001/jamanetworkopen.2022.31343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 14.Rivkin MJ, deVeber G, Ichord RN, et al. Thrombolysis in pediatric stroke study. Stroke. 2015;46(3):880-885. doi: 10.1161/STROKEAHA.114.008210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bigi S, Fischer U, Wehrli E, et al. Acute ischemic stroke in children versus young adults. Ann Neurol. 2011;70(2):245-254. doi: 10.1002/ana.22427 [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 17.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355(9216):1670-1674. doi: 10.1016/S0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 18.Barber PA, Hill MD, Eliasziw M, et al. ; ASPECTS Study Group . Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76(11):1528-1533. doi: 10.1136/jnnp.2004.059261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 20.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 21.Bhatia K, Kortman H, Blair C, et al. Mechanical thrombectomy in pediatric stroke: systematic review, individual patient data meta-analysis, and case series. J Neurosurg Pediatr. Published online August 9, 2019. doi: 10.3171/2019.5.PEDS19126 [DOI] [PubMed] [Google Scholar]
- 22.He L, Ladner TR, Pruthi S, et al. Rule of 5: angiographic diameters of cervicocerebral arteries in children and compatibility with adult neurointerventional devices. J Neurointerv Surg. 2016;8(10):1067-1071. doi: 10.1136/neurintsurg-2015-012034 [DOI] [PubMed] [Google Scholar]
- 23.Haussen DC, Eby B, Al-Bayati AR, et al. A comparative analysis of 3MAX aspiration versus 3 mm Trevo Retriever for distal occlusion thrombectomy in acute stroke. J Neurointerv Surg. 2020;12(3):279-282. doi: 10.1136/neurintsurg-2019-014990 [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Mlynash M, Christensen S, et al. Hyperacute perfusion imaging before pediatric thrombectomy: analysis of the Save ChildS study. Neurology. 2023;100(11):e1148-e1158. doi: 10.1212/WNL.0000000000201687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitchen L, Westmacott R, Friefeld S, et al. The Pediatric Stroke Outcome Measure: a validation and reliability study. Stroke. 2012;43(6):1602-1608. doi: 10.1161/STROKEAHA.111.639583 [DOI] [PubMed] [Google Scholar]
- 26.Slim M, Fox CK, Friefeld S, et al. ; SIPS Investigators . Validation of the Pediatric Stroke Outcome Measure for classifying overall neurological deficit. Pediatr Res. 2020;88(2):234-242. doi: 10.1038/s41390-020-0842-5 [DOI] [PubMed] [Google Scholar]
- 27.Beslow LA, Vossough A, Ichord RN, et al. Association of pediatric ASPECTS and NIH Stroke Scale, hemorrhagic transformation, and 12-month outcome in children with acute ischemic stroke. Neurology. Published online August 13, 2021. doi: 10.1212/WNL.0000000000012558 [DOI] [PubMed] [Google Scholar]
- 28.Roach ES, Bernard T, deVeber G. Defining a pediatric stroke center. Pediatr Neurol. 2020;112:11-13. doi: 10.1016/j.pediatrneurol.2020.08.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Conversion Table From PSOM to Pediatric mRS
eAppendix. Study Protocol
Data Sharing Statement