Abstract
Background:
The role of pulse pressure (PP) ‘widening’ at older and younger age as a cardiovascular risk factor is still controversial. Mean PP, as determined from repeated blood pressure (BP) readings, can be expressed as a sum of two components: ‘elastic PP’ ((elPP) and ‘stiffening PP’ (stPP) associated, respectively, with stiffness at the diastole and its relative change during the systole. We investigated the association of 24-h ambulatory PP, elPP, and stPP (‘PP variables’) with mortality and composite cardiovascular events in different age classes.
Method:
Longitudinal population-based cohort study of adults with baseline observations that included 24-h ambulatory BP. Age classes were age 40 or less, 40–50, 50–60, 60–70, and over 70 years. Co-primary endpoints were total mortality and composite cardiovascular events. The relative risk expressed by hazard ratio per 1SD increase for each of the PP variables was calculated from multivariable-adjusted Cox regression models.
Results:
The 11 848 participants from 13 cohorts (age 53 ± 16 years, 50% men) were followed for up for 13.7 ± 6.7 years. A total of 2946 participants died (18.1 per 1000 person-years) and 2093 experienced a fatal or nonfatal cardiovascular event (12.9 per 1000 person-years). Mean PP, elPP, and stPP were, respectively, 49.7, 43.5, and 6.2 mmHg, and elPP and stPP were uncorrelated (r = −0.07). At age 50–60 years, all PP variables displayed association with risk for almost all outcomes. From age over 60 years to age over 70 years, hazard ratios of of PP and elPP were similar and decreased gradually but differently for pulse rate lower than or higher than 70 bpm, whereas stPP lacked predictive power in most cases. For age 40 years or less, elPP showed protective power for coronary events, whereas stPP and PP predicted stroke events. Adjusted and unadjusted hazard ratio variations were similar over the entire age range.
Conclusion:
This study provides a new basis for associating PP components with outcome and arterial properties in different age groups and at different pulse rates for both old and young age. The similarity between adjusted and unadjusted hazard ratios supports the clinical usefulness of PP components but further studies are needed to assess the prognostic significance of the PP components, especially at the young age.
Keywords: aging, arterial wall mechanics, blood pressure, cardiovascular disease, hypertension, mortality, pulse pressure, risk factors, stiffness
INTRODUCTION
Pulse pressure (PP), the difference between SBP and DBP, becomes wider after 50 years of age [1], but it remains an elusive cardiovascular risk factor with findings inconsistent across studies [2]. PP predicts cardiovascular outcomes, mainly in elderly individuals, as determined using office [3-6], ambulatory [7-9], and home BP measurements [10]. However, in randomly recruited populations, ambulatory PP did not add to risk stratification below age 60, whereas in the elderly, PP was a weak risk factor, with levels below 64 mmHg probably being innocuous [2]. Very recently, we have shown that the relative risks of death, a cardiovascular endpoint, and stroke, associated with ambulatory PP, decreased from 50 to 75 years of age by a factor of 3 to 5, whereas both absolute risk and PP increased [11].
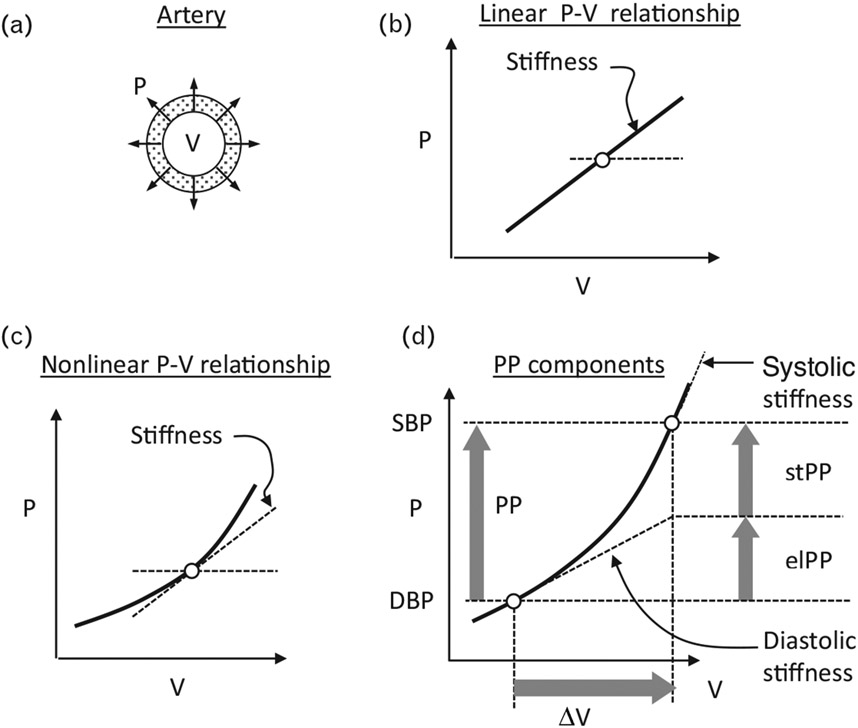
It is widely accepted that at older age, arterial stiffness is an important determinant of PP [12,13]. For the purposes of the present article, arterial stiffness is the resistance offered by the arterial wall to its circumferential stretch by the distending pressure developed during the early systole, in response to the left ventricular ejection. Conceptually, given the relationship between the distending pressure and arterial volume (standing also for cross-sectional lumen area, or lumen diameter), arterial stiffness is defined, at a given pressure level, by the ratio between incremental changes in the said pressure and volume (Fig. 1). During early systole, and for normal or higher pressure, arterial stiffness increases dynamically from its value at the DBP (‘diastolic stiffness’) to its SBP value (‘systolic stiffness’) [14], reflecting the well known nonlinear pressure–volume relationship (Fig. 1) observed in arteries both in vivo and ex vivo [14-20]. This behavior is explained by the parallel arrangement of elastin and collagen in the arterial wall that resist differently to stretch: Elastin fibers display pressure-independent stiffness (called here ‘elastic’ behavior), whereas the recruitment of collagen fibers (via smooth muscles) in response to the increased stretch, generates stiffness that increases dynamically with the wall stretch (called here ‘stiffening’ behavior) [21]. More details are found in the Discussion section. In other terms, Fig. 1 shows that the observed nonlinear arterial pressure–volume relationship can be viewed, in general, as a superposition of ‘elastic relationship’ (straight line) and ‘stiffening relationship’ (curved line). According to this description, PP can be viewed as the sum of two components representing these behaviors: ‘elastic PP’ (elPP) and ‘stiffening PP’ (stPP). The quantitative estimation of elPP and stPP from repeated BP readings was previously described [22], and is summarized here (Supplemental Methods, http://links.lww.com/HJH/C43). The predictive power of the PP components was evaluated in two previous studies [22,23]. It should be mentioned that the pressure dependence of arterial stiffness was also observed using pulse wave velocity (PWV) measurements performed at fiduciary points on the pressure waveform [14,24]. Therefore, the widespread determination of arterial stiffness from PWV measured at the foot of the pulse wave [25], that is, at the DBP, reflects diastolic stiffness, which is smaller than PWV measured at the SBP that determines the systolic stiffness [14,19].
FIGURE 1.
The concept of pulse pressure components [22]. (a) Illustration of the distending arterial pressure (P) and volume (V) that stands also for cross-sectional lumen area, or lumen diameter. (b) and (c) show, respectively, a linear P–V and nonlinear P–V relationship. At each selected pressure (empty circle), stiffness is defined as the ratio between small incremental changes in P and V, so-called ‘tangent slope’, which is the tangent of the angle between the tangent to the curve, at that point, and the x-axis. Thus, in (b), stiffness is simply the slope of the line, characterizing a simple elastic tube having constant elasticity (‘elastic behavior’), whereas in (c), stiffness increases for higher pressure (or volume), characterizing the ‘stiffening behavior’ displayed by real arteries (see Introduction). (d) The variation of the distending arterial pressure from its diastolic level to systolic level (DBP to SBP, respectively) during the early systole, which is the pulse pressure (PP); the corresponding volume change (‘pulse volume’ ΔV), and the stiffness change from its ‘diastolic’ to ‘systolic’ level. This diagram enables to view, in general, the P–V relationship during the early systole, as a superposition of a linear relationship (‘elastic’) having the slope of the diastolic stiffness and a nonlinear relationship (the original P–V relationship minus the elastic relationship). This procedure splits a given PP into the illustrated ‘elastic component’ (elPP, equals to ΔV · [diastolic stiffness]), and a ‘stiffening component’ (stPP equals to PP-elPP) expressing the extra pressure required to overcome the increased resistance of the arterial wall because of the increased stiffness using the given formulas for quantitative determination of elPP and stPP (Supplemental Methods, http://links.lww.com/HJH/C43). PP, pulse pressure.
Using the International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) [26], the main objective of the present study was to evaluate the association between PP components determined from ambulatory BP and cardiovascular morbidity and mortality for different age classes and endpoints. We hypothesize that PP components have different associations with different outcomes at different age classes that may associate risk with arterial stiffness and its pressure dependence.
METHODS
Study participants
All 13 cohort studies included in IDACO (12 countries from Europe, Asia, and South America) [26] (Supplemental Table 1, http://links.lww.com/HJH/C43) received ethical approval from the responsible institutional review boards in their country of origin and adhered to the principles of the Declaration of Helsinki [27]. Participants provided written informed consent. In accordance with current national regulations, review boards either waived or provided ethical clearance for secondary use of data to be included in the IDACO resource. The construction of the IDACO database was described previously [26]. IDACO-eligible studies that received ethical approval and qualified for inclusion were those performed in random population samples or those conducted in professional groups representative of a community. At least one baseline ambulatory blood pressure recording and information on cardiovascular risk factors and subsequent fatal and nonfatal outcomes should be available [26]. Information on the population-sampling methods, timelines, country of recruitment, and the ambulatory BP measures were detailed in the Expanded Methods section in Supplement 1, http://links.lww.com/HJH/C43 of a previous publication [28].
Eligibility for the present analysis required a baseline ambulatory BP recording with at least six daytime and three night-time readings [29], availability of the 24-h mean and standard deviation (SD) of SBP, DBP, and pulse rate, and age not younger than 18 years.
Blood pressure and other measurements
Methods used for conventional and ambulatory blood pressure, and details of the ambulatory BP monitoring by cohort were described, respectively, in the Expanded Methods Section, and eTable 2 of Supplement 1, http://links.lww.com/HJH/C43 in a previous publication [29]. Determination of the status of smoking and drinking habits, diabetes mellitus, taking antihypertensive drugs, level of serum cholesterol, and history of cardiovascular diseases was described previously [2]. SBP, DBP, and PP were weighed mean 24-h data using the time interval between successive measurements as weighting factors, and mean arterial pressure (MAP), defined as DBP + PP/3, will be called collectively hereafter ‘BP variables’. The same method of determination was applied for pulse rate. Unless stated differently, BP variables and pulse rate refer hereafter to 24-h averages of ambulatory measurements in individuals or groups.
Ascertainment of endpoints
Vital status and the incidence of fatal and nonfatal events were ascertained from the appropriate sources in each country, as described previously [26].
The co-primary endpoints in the present analysis were all-cause mortality and a composite cardiovascular out-come consisting of cardiovascular mortality, including sudden death, nonfatal coronary events, coronary revascularization, heart failure, and stroke. Secondary end-points included cardiovascular mortality, fatal and nonfatal coronary endpoints, and fatal and nonfatal stroke, excluding transient ischemic attack. The relationship between the said endpoints is illustrated in Supplemental Figure 1, http://links.lww.com/HJH/C43. All events were validated against hospital files or medical records held by primary care physicians or specialists. In all outcome analyses, only the first event within each category was considered.
Statistical analysis
The PP component concept is explained and illustrated by Fig. 1, and the formulas required for the determination of PP components from repeated BP measurements, based on exponential pressure–volume relationship, are provided with a worked example (Supplemental Methods, http://links.lww.com/HJH/C43) [22,30,31]. We applied the Kolmogorov–Smirnov test to assess the normality of distributions. For comparison of means and proportions, we applied the large-sample z test and Fisher exact test, respectively. The baseline characteristics used as covariables for adjustment in multivariate analyses included sex, age (continuous), BMI (continuous), current status of smoking (yes/no) and drinking alcohol (yes/no), serum total cholesterol (continuous), antihypertensive drug intake (yes/no), history of cardiovascular disease (yes/no), diabetes mellitus (yes/no), and mean 24-h DBP and pulse rate (both continuous) derived from the same recordings as PP and its components. Missing values of variables from the above covariables (not including DBP and pulse rate) were previously interpolated using the following procedure [2]: after stratification for cohort and sex, missing values of BMI and serum total cholesterol were interpolated from the regression slopes on age. In participants with unknown status of smoking, drinking, diabetes mellitus, or history of cardiovascular disease, an indicator (dummy) variable was set to the cohort-specific and sex-specific mean of the codes (0,1). Missing values that were interpolated included BMI (n = 33), serum cholesterol (n = 822), smoking (n = 79) and drinking (n = 826) status, diabetes mellitus (n = 5), and history of cardiovascular disease (n = 1). To adjust for the cohorts (Supplemental Table 1, http://links.lww.com/HJH/C43), we set indicator (dummy) variables for the individual cohort and included for each age class and endpoint the contributing cohorts. The event rate R was defined as the number of events per 1000 person-years, and its 95% confidence interval given by R ± 1.96 · (R/T)1/2, where T equals (Sum of the follow-up durations in years)/1000. The following five age classes were analyzed: age 40 years, 40 < age ≤50 (‘40–50’), 50 < age ≤60 (‘50–60’), 60 < age ≤70 (‘60–70’), and age over 70 years. Using multivariate Cox regression models, we determined the covariable-adjusted hazard ratio of each PP variable for age class and endpoint per 1SD increase, which was calculated for each PP variable for all participants, that is, the tested PP variable had the standardized form (PP variable)/SD. Hazard ratios of the two PP components were included in the same Cox regression model after assessing that they are weakly correlated. The validity of the proportional hazard assumption was assessed by the linearity of the log–log plot of the cumulative hazard versus the follow-up time. All analyses were performed using SYSTAT 12 (SYSTAT Software Inc., San Jose, California, USA). Statistical significance was a two-sided P value of less than 0.05.
RESULTS
Baseline characteristics of participants
Of the 13 728 people included in the database, we excluded 1786 for having an ambulatory BP recording with fewer than six daytime or three night-time readings, and additionally, 94 adolescents (age <18 years) who were without event. This left 11 848 individuals for statistical analysis with baseline characteristics presented in Table 1. The study population included roughly 50% women and consisted of 60% Europeans, 20% Asians, and 20% South Americans. The number of participants per cohort is provided in Supplemental Table 1 (http://links.lww.com/HJH/C43). The median number of ambulatory readings [5th–95th percentile intervals] was 55 [34–81] for 24-h, 28 [15–41] for daytime, and 11 [5–13] for night-time readings. Mean 24-h ambulatory SBP and DBP and MAP were, respectively, 123.6 ± 14.4, 73.9 ± 8.6 and 90.4 ± 9.8 mmHg, and pulse rate 75.5 ± 9.2 bpm.
TABLE 1.
Baseline characteristics of participants by age class and overall
| Characteristics | Age class (years) | All Ages | ||||
|---|---|---|---|---|---|---|
| ≤40 | 40–50 | 50–60 | 60–70 | >70 | ||
| Number in age class | 2635 | 2028 | 2470 | 2263 | 2452 | 11 848 |
| Number with characteristic (%) | ||||||
| Women | 1423 (54.0) | 1108 (54.6) | 1383 (56.0) | 1192 (52.7) | 777 (31.7) | 5883 (49.7) |
| Europeans | 1837 (69.7) | 1260 (62.1) | 1386 (56.1) | 1060 (46.8) | 1571 (64.1) | 7114 (60.0) |
| Asians | 273 (10.3) | 427 (21.1) | 637 (25.8) | 626 (27.7) | 452 (18.4) | 2415 (20.4) |
| South Americans | 525 (20.0) | 341 (16.8) | 447 (18.1) | 577 (25.5) | 429 (17.5) | 2319 (19.6) |
| Current smoking | 764 (29.1) | 687 (34.1) | 693 (28.0) | 570 (25.2) | 488 (19.9) | 3202 (27.0) |
| Drinking alcohol | 1123 (42.6) | 988 (48.7) | 1179 (47.7) | 1101 (48.7) | 1124 (45.8) | 5515 (46.5) |
| On antihypertensive treatment | 54 (2.1) | 172 (8.5) | 535 (21.7) | 644 (28.5) | 949 (38.7) | 2354 (19.9) |
| Diabetes mellitus | 41 (1.6) | 75 (3.7) | 181 (7.3) | 263 (11.6) | 306 (12.5) | 866 (7.3) |
| History of cardiovascular disease | 76 (2.8) | 69 (3.4) | 251 (10.2) | 409 (18.1) | 509 (20.8) | 1314 (11.1) |
| Mean (SD) of characteristic | ||||||
| Age (years) | 29.9 ± 5.0 | 44.3 ± 2.9 | 54.5 ± 3.0 | 63.8 ± 3.0 | 73.2 ± 3.8 | 52.9 ± 15.9 |
| BMI (kg/m2) | 23.8 ± 4.0 | 25.4 ± 4.3 | 26.2 ± 4.5 | 26.0 ± 4.5 | 25.6 ± 4.0 | 25.4 ± 4.4 |
| Serum cholesterol (mg/dl) | 193.5 ± 38.0 | 212.5 ± 40.7 | 221.0 ± 42.4 | 224.8 ± 47.1 | 222.0 ± 43.8 | 214.4 ± 44.0 |
| 24-h SBP (mmHg) | 115.3 ± 9.9 | 119.4 ± 12.3 | 123.7 ± 13.6 | 127.6 ± 14.0 | 132.2 ± 15.1 | 123.6 ± 14.4 |
| 24-h DBP (mmHg) | 70.3 ± 7.1 | 74.0 ± 8.8 | 75.8 ± 9.1 | 75.2 ± 8.6 | 74.4 ± 8.4 | 73.9 ± 8.6 |
| 24-h PP (mmHg) | 45.0 ± 6.7 | 45.4 ± 7.1 | 47.9 ± 8.1 | 52.4 ± 9.6 | 57.8 ± 11.2 | 49.7 ± 10.0 |
| 24-h MAP (mmHg) | 85.3 ± 7.5 | 89.1 ± 9.5 | 91.8 ± 10.1 | 92.6 ± 9.7 | 93.6 ± 9.7 | 90.4 ± 9.8 |
| 24-h PR (bpm) | 75.1 ± 9.1 | 73.9 ± 8.8 | 72.5 ± 8.5 | 71.3 ± 8.8 | 69.5 ± 9.6 | 72.5 ± 9.2 |
| 24-h elastic PP (mmHg) | 43.5 ± 7.2 | 40.7 ± 6.2 | 41.3 ± 6.9 | 44.0 ± 8.3 | 47.5 ± 10.4 | 43.5 ± 8.3 |
| 24-h stiffening PP (mmHg) | 1.5 ± 3.9 | 4.7 ± 9.9 | 6.6 ± 5.2 | 8.4 ± 5.7 | 10.3 ± 6.8 | 6.23 ± 6.22 |
BP, blood pressure; PP, pulse pressure; PR, pulse rate; MAP, mean arterial pressure derived as DBP with PP/3. Current smoking (the daily use of smoking materials), drinking alcohol-containing beverages occasionally or daily, the use of antihypertensive medications and a history of cardiovascular disease were assessed at baseline by questionnaire. Diabetes was a self-reported diagnosis, use of antidiabetic drugs, fasting blood glucose of at least 126 mg/dl (7.0 mmol/l), random blood glucose of at least 200 mg/dl (11.1 mmol/l), or diabetes documented in practice or hospital records. BMI was body weight in kilograms divided by body height in square meters. The 24-h BP was recorded with validated oscillometric devices (see more details in a previous publication) [29]. Serum cholesterol was measured by automated methods in certified laboratories. The elastic PP and stiffening PP were determined from the mean 24-h PP and the SD of the 24-h SBP and DBP readings (Supplemental Methods, http://links.lww.com/HJH/C43).
Basic statistics of pulse pressure variables
The mean ± SD of PP, elPP, and stPP for all participants were, respectively, 49.7 ± 10.0, 43.5 ± 8.3, and 6.23 ± 6.22 mmHg. The mean stPP was 12.5% of mean PP. Table 2 shows that for the whole study population, elPP and stPP correlated with PP and SBP, respectively, strongly (r = 0.79 and 0.65, respectively) and moderately (r = 0.56 and 0.43, respectively), and very weakly with DBP (r = 0.07 to 0.19), and with each other (r = −0.075). The last result justifies the inclusion of elPP and stPP as independent predictors in the same Cox model. Furthermore, the correlation between PP variables and pulse rate was weak to very weak (r = −0.22 to −0.084).
TABLE 2.
Correlations between blood pressure variables, pulse pressure variables and pulse rate
| PP | elPP | stPP | DBP | SBP | MAP | |
|---|---|---|---|---|---|---|
| elPP | 0.785 | |||||
| stPP | 0.560 | −0.075 | ||||
| DBP | 0.189 | 0.174 | 0.071 | |||
| SBP | 0.808 | 0.650 | 0.431 | 0.731 | ||
| MAP | 0.505 | 0.419 | 0.253 | 0.943 | 0.912 | |
| PR | −0.207 | −0.084 | −0.222 | 0.154 | −0.051 | 0.065 |
Data were determined using 24-h ambulatory measurements for the whole population (N = 11 848). The relationship between two variables, as evaluated by Pearson correlation coefficient r, is considered very weak for r = 0.00–0.19; weak for r = 0.20–0.39; moderate for 0.40–0.59; strong for r = 0.60–0.79; and very strong for r = 0.80–1.0 (Evans JD, 1996. Straightforward Statistics for the Behavioral Sciences. Brooks/Cole Publishing, Pacific Grove). PP, pulse pressure; elPP, elastic PP; stPP, stiffening PP; MAP, mean arterial pressure (equals DBP + PP/3); PR; pulse rate.
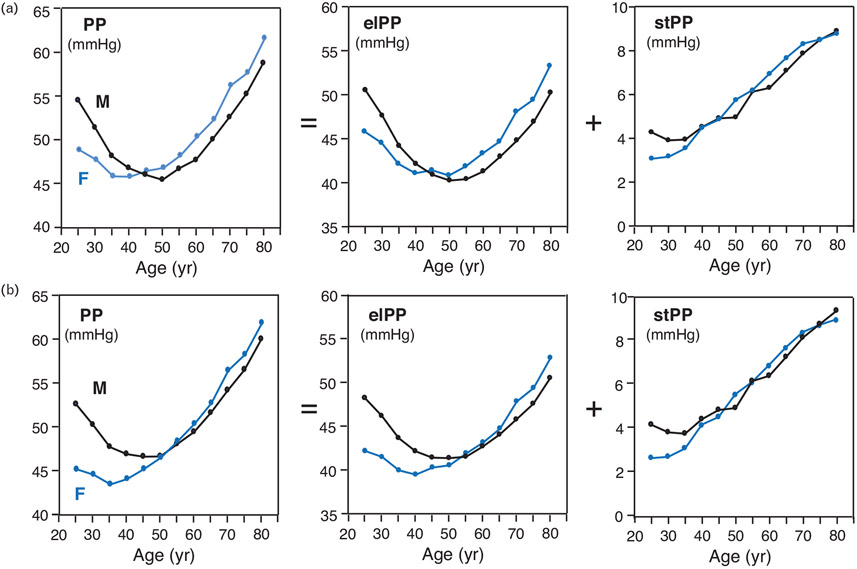
Variation of pulse pressure variables with age stratified by sex
Figure 2 depicts these variations either fully adjusted (a) or adjusted to cohorts only (b). PP and elPP showed curvilinear relationships with age with nadirs around 50 and 40 years of age for male and female individuals, respectively, where the male and female individual curves crossed each other at 45 years in Fig. 2a, and at 50–60 years in Fig. 2b. For age younger than these crossing values, both PP and elPP were higher in male individuals than in female individuals but lower in male individuals than in female individuals for age over the crossing values. For both sexes, stPP was rather constant below age 30 years (higher value for males) but from age 30 years onwards, it increased linearly with age with similar values for both sexes. The exclusion of the DBP of individuals, as an adjustor, caused very small shift (smaller or about 1 mmHg) in the relative position of the male and female curves and had no effect on the stPP curve.
FIGURE 2.
Variation of pulse pressure variables with age stratified by sex. Data points represent means of each pulse pressure (PP) variable for age grouped in 5-year intervals, fully adjusted in (a) and adjusted to cohorts only in (b), determined using two-way ANOVA taking grouped age and sex as the independent variables. The covariables in (a) were cohorts, BMI, smoking and drinking, serum cholesterol, antihypertensive drug intake, history of cardiovascular disease and diabetes mellitus, and mean 24-h DBP and heart rate. The covariables for (b) were merely cohorts. Values of grouped age were ‘25’, ‘30’, …, and ‘80’, standing for, respectively, age 27.5 or less, 27.5 less than age, 32.5 or less, …, and age more than 77.5 years. The black and blue lines correspond, respectively, to males (‘M’) and females (‘F’). The ‘ = ’ and ‘ + ’ symbols reminds that PP is the sum of elPP and stPP. elPP, elastic pulse pressure; stPP, stiffening PP.
Applying the full adjustment separately to age older and younger than 50 years resulted in patterns that resemble those depicted in Fig. 2a. The fully-adjusted ratio stPP/elPP increased monotonically from ~0.1 at age = 25 years to ~0.20 at age = 80 years, similarly for both sexes. The exclusion of 24-h average DBP of individuals as an adjustor caused very small shift (smaller or about 1 mmHg) in the relative position of the male and female curves and had no effect on the stPP.
Incidence of events
Table 3 shows that the number of person-years of follow-up totaled 162 367. Over a median follow-up of 13.7 years (5th–95th percentile interval, 3.7–26.0), 2946 deaths occurred, of which 1102 (37.0%) were cardiovascular, including 156 heart failure deaths. Of the 2093 fatal and nonfatal cardiovascular events, 932 were coronary events (44.5%), including 392 deaths from ischemic heart disease and 87 sudden deaths, and 846 (40.4%) were stroke events, including 296 deaths (Supplemental Figure 1, http://links.lww.com/HJH/C43). The event rates are expressed as number of events per 1000 person-years given as mean [95% confidence interval (CI)] in Table 3, and plotted graphically in Supplemental Figure 2, http://links.lww.com/HJH/C43, with the best fitted exponential curves displaying very high correlation (R2 > 0.994 for all endpoints). These curves show an increase in the event rate by 2.3–3.7-fold per 10 years of age, where the three highest values corresponded to the fatal events.
TABLE 3.
Incidence of events and rate by baseline age class
| Events | Age class (years) | All participants | ||||
|---|---|---|---|---|---|---|
| ≤40 | 40–50 | 50–60 | 60–70 | >70 | ||
| Number at risk | 2635 | 2028 | 2470 | 2263 | 2452 | 11848 |
| Total person-years/1000 | 29.7 | 43.7 | 36.3 | 30.0 | 29.3 | 162.4 |
| Total mortality | ||||||
| Number of deaths | 42 | 102 | 322 | 736 | 1744 | 2946 |
| Rate (per 1000 person-years) | 1.18 [0.82–1.54] | 3.29 [2.65–3.93] | 8.87 [7.90–9.84] | 24.5 [22.8–26.3] | 59.5 [56.7–62.3] | 18.1 [17.5–18.8] |
| Cardiovascular events | ||||||
| Number of events | 26 | 89 | 283 | 502 | 1193 | 2093 |
| Rate (per 1000 person-years) | 0.73 [0.45–1.01] | 2.87 [2.27–3.47] | 7.80 [6.89–8.70] | 16.7 [15.3–18.2] | 40.7 [38.4–43.0] | 12.9 [12.3–13.4] |
| CV mortality | ||||||
| Number of deaths | 7 | 17 | 104 | 235 | 739 | 1102 |
| Rate (per 1000 person-years) | 0.20 [0.05–0.35] | 0.55 [0.29–0.81] | 2.87 [2.31–3.42] | 7.83 [6.83–8.83] | 25.2 [23.4–27.0] | 6.79 [6.39–7.19] |
| Coronary events | ||||||
| Number of deaths | 12 | 51 | 153 | 211 | 505 | 932 |
| Rate (per 1000 person-years) | 0.34 [0.15–0.53] | 1.65 [1.20–2.10] | 4.21 [3.54–4.88] | 7.03 [6.67–7.39] | 17.2 [15.7–18.7] | 5.74 [5.34–6.11] |
| Stroke events | ||||||
| Number of events | 10 | 30 | 99 | 232 | 475 | 846 |
| Rate (per 1000 person-years) | 0.28 [0.11–0.45] | 0.97 [0.62–1.32] | 2.73 [2.19–3.26] | 7.73 [6.73–8.73] | 16.2 [14.8–17.7] | 5.21 [4.86–5.56] |
| Stroke mortality | ||||||
| Number of deaths | 3 | 3 | 31 | 69 | 190 | 296 |
| Rate (per 1000 person-years) | 0.08 [−0.01 to 0.17] | 0.10 [−0.01 to 0.21] | 0.85 [0.55–1.15] | 2.30 [1.76–2.84] | 6.48 [5.56–7.41] | 1.82 [1.52–2.03] |
Data are rates given with 95% confidence interval calculated using the formulas shown in the Statistical Analysis section.
Hazard ratios associated with pulse pressure variables
Table 4 shows the multivariable-adjusted hazard ratio expressing, for a given age class and endpoint, the relative risk for reaching an outcome associated with a 1SD increase in a PP variable.
TABLE 4.
Hazard ratios for primary and secondary endpoints by age class
| Endpoint | PP variable |
Age class (years) | Ratio of HR at >70 years to HR at 50–60 years |
P value of HR trend for age greater than 50 years |
||||
|---|---|---|---|---|---|---|---|---|
| ≤40 | 40–50 | 50–60 | 60–70 | >70 | ||||
| Number at risk | 2635 | 2028 | 2470 | 2263 | 2452 | |||
| Total mortality | N | 42 | 102 | 322 | 736 | 1744 | ||
| PP | 0.83 [0.45–1.51] (0.54) | 0.97 [0.68–1.38] (0.87) | 1.35 [1.16–1.57] (0.0001) | 1.18 [1.09–1.29] (0.0001) | 1.09 [1.05–1.14] (0.00008) | 0.81 | 0.003 | |
| N = 2946 | elPP | 0.80 [0.47–1.35] (0.40) | 0.98 [0.71–1.35] (0.90) | 1.20 [1.03–1.40] (0.018) | 1.20 [1.10–1.30] (0.00003) | 1.09 [1.05–1.14] (0.00001) | 0.91 | 0.014 |
| stPP | 1.07 [0.60–1.90] (0.81) | 0.97[0.70–1.35] (0.87) | 1.32 [1.13–1.53] (0.0004) | 1.04[0.95–1.14] (0.36) | 1.02 [0.97–1.07] (0.38) | 0.77 | 0.061 | |
| CV events | N | 26 | 89 | 283 | 502 | 1193 | ||
| PP | 0.63 [0.30–1.31] (0.22) | 1.00 [0.69–1.44] (1.00) | 1.50 [1.29–1.75] (<0.000001) | 1.28 [1.16–1.41] (0.000001) | 1.14 [1.09–1.20] (<0.000001) | 0.76 | 0.0006 | |
| N = 2093 | elPP | 0.56 [0.29–1.08] (0.086) | 0.95 [0.68–1.35] (0.78) | 1.41 [1.22–1.63] (0.000004) | 1.27[1.16–1.40] (0.000001) | 1.13 [1.08–1.19] (<0.000001) | 0.80 | 0.0001 |
| stPP | 1.12 [0.57–2.23] (0.74) | 1.08 [0.77–1.51] (0.66) | 1.28 [1.09–1.50] (0.002) | 1.11[1.00–1.23] (0.061) | 1.06 [1.00–1.12] (0.045) | 0.83 | 0.44 | |
| CV mortality | N | 7 | 17 | 104 | 235 | 739 | ||
| PP | 0.51 [0.12–2.22] (0.37) | 0.51 [0.21–1.25] (0.14) | 1.58 [1.21–2.03] (0.0006) | 1.44 [1.25–1.65] (<0.000001) | 1.16 [1.09–1.24] (0.000005) | 0.73 | 0.018 | |
| N = 1102 | elPP | 0.58 [0.17–2.00] (0.39) | 0.42 [0.17–1.01] (0.054) | 1.50 [1.16–1.94] (0.002) | 1.46[1.29–1.67] (<0.000001) | 1.15 [1.08–1.22] (0.000004) | 0.77 | 0.0015 |
| stPP | 0.56 [0.11–2.78] (0.48) | 0.99 [0.46–2.14] (0.98) | 1.26 [0.97–1.54] (0.082) | 1.09 [0.94–1.26] (0.27) | 1.06 [0.99–1.14] (0.080) | 0.84 | 0.88 | |
| Coronary events | N | 12 | 51 | 153 | 211 | 505 | ||
| PP | 0.36 [0.12–1.1] (0.074) | 0.95 [0.58–1.56] (0.83) | 1.50 [1.22–1.84] (0.00009) | 1.23 [1.07–1.43] (0.0045) | 1.20 [1.11–1.29] (0.000001) | 0.80 | 0.13 | |
| N = 932 | elPP | 0.38 [0.14–0.99] (0.047) | 0.88 [0.55–1.40] (0.59) | 1.43 [1.19–1.73] (0.0002) | 1.25 [1.08–1.43] (0.002) | 1.17 [1.09–1.25] (0.00001) | 0.82 | 0.031 |
| stPP | 0.84 [0.27–2.64] (0.76) | 1.11 [0.71–1.74] (0.64) | 1.23 [1.00–1.52] (0.049) | 1.03 [0.89–1.20] (0.68) | 1.11[1.02–1.20] (0.011) | 0.90 | 0.63 | |
| Stroke events | N | 10 | 30 | 99 | 232 | 475 | ||
| PP | 3.06 [1.03–9.09] (0.044) | 1.40 [0.76–2.58[ (0.28) | 1.51 [1.14–1.99[ (0.004) | 1.44 [1.24–1.67] (0.000002) | 1.18 [1.09–1.28] (0.00008) | 0.78 | 0.007 | |
| N = 846 | elPP | 1.86 [0.67–5.21] (0.24) | 1.20 [0.66–2.18] (0.55) | 1.35 [1.03–1.78] (0.031) | 1.35 [1.17–1.56] (0.00006) | 1.19 [1.11–1.28] (0.000001) | 0.88 | 0.066 |
| stPP | 3.99 [1.20–13.3] (0.024) | 1.29 [0.75–2.22] (0.36) | 1.37 [1.02–1.83] (0.033) | 1.26 [1.07–1.48) (0.0044) | 1.00 [0.92–1.10] (0.92) | 0.73 | 0.005 | |
| Stroke mortality | N | 3 | 3 | 31 | 69 | 190 | ||
| PP | 0.93 [0.08–11.5] (0.96) | 0.61 [0.10–3.54] (0.58) | 1.85 [1.18–2.92] (0.008) | 1.48 [1.12–1.95] (0.005) | 1.19 [1.03–1.36] (0.015) | 0.64 | 0.12 | |
| N = 296 | elPP | 0.94 [0.10–9.14] (0.96) | 0.63 [0.10–3.99] (0.63) | 1.69 [1.04–2.72] (0.033) | 1.54 [1.20–1.97] (0.0007) | 1.23 [1.10–1.38] (0.0004) | 0.73 | 0.12 |
| stPP | 0.97 [0.05–19.0] (0.98) | 0.77 [0.13–4.64] (0.78) | 1.45 [0.91–2.30] (0.12) | 0.97 [0.70–1.33] (0.84) | 0.93 [0.80–1.08] (0.36) | 0.64 | 0.23 | |
Data are the mean adjusted hazard ratio (HR) with [95% confidence interval] and P value (in parentheses) for given PP variable, endpoint, and age class. HR expresses the relative risk associated with a 1SD increment in a PP variable calculated for all participants. SD was 10.0, 8.3, and 6.22mmHg for PP, elPP, and stPP, respectively (see Table 1). Data in bold font mark hazard ratios for which P is less than 0.05. The PP components, elPP and stPP, were included in the same Cox model. Adjustments included cohort, sex, age, BMI, smoking and drinking, serum total cholesterol, antihypertensive drug intake, history of cardiovascular disease and diabetes mellitus, and mean 24-h DBP and heart rate. N is the number of events. CV, cardiovascular; y, years; PP, 24-h mean pulse pressure; elPP, and stPP are, respectively, the ‘elastic’ and ‘stiffening’ PP components described in Fig. 1 and the formulas for its calculations are provided (Supplemental Methods, http://links.lww.com/HJH/C43). P value of hazard ratio trend for age over 50 years was derived from an interaction term between the PP variable and age class added to the Cox models.
For age older than 50 years, all hazard ratios were associated with risk (hazard ratio > 1) and displayed for all endpoints a decrease from the first to the last age class, that is, from age 50–60 to age older than 70 years (mean hazard ratio 1.20–1.85 to 1.09–1.23, respectively, for both PP and elPP). This trend reached statistical significance for both PP and elPP for the primary endpoints and cardiovascular mortality but not for stPP, with the exception of stroke events. Predictive power was demonstrated for hazard ratios of PP and elPP for all endpoints, and for stPP in most endpoints at age class 50–60 years but only twice for age older than 60 years (cardiovascular mortality and stroke events).
For age class 40–50 years, none of the PP variables was associated with risk for any of the endpoints. In contrast, for age 40 years or less, elPP showed a protective effect (hazard ratio <1) for coronary events [hazard ratio, mean [95% CI] 0.38 [0.14–0.99]), and stPP and PP were associated with a risk for stroke events (3.99 [1.2–13.3] and 3.06 [1.03–9.09], respectively).
Supplemental Table 2, http://links.lww.com/HJH/C43 shows the unadjusted hazard ratios for primary and secondary endpoints by age class, that is, including only PP variables only as predictors in the Cox models. It appears that the variation of the unadjusted hazard ratios by age and endpoint is similar to that of the adjusted ones in Table 4. It is noteworthy that the unadjusted hazard ratios for stPP and PP displayed strong association with stroke events at age class 40–50 years, with no parallel finding for adjusted hazard ratio.
We further investigated the effect of mean 24-h ambulatory pulse rate on the hazard ratios of the PP variables by recalculating hazard ratio for the primary endpoints in the subgroups of ‘lower PR’ (pulse rate ≤70 bpm) and ‘higher pulse rate’ (pulse rate >70 bpm) by age class (pulse rate of 70 bpm is close to the mean value shown in Table 1). Results are reported in Supplemental Tables 3a and 3b, http://links.lww.com/HJH/C43 together with hazard ratios for all pulse rate values (‘All PR’) that are the same as in Table 4.
Supplemental Table 3a, http://links.lww.com/HJH/C43 shows that for total mortality in age class 50–60 years, all PP variables were associated with the risk merely for the higher pulse rate subgroup (no predictive power for lower pulse rate); in age class 60–70 years, PP and elPP displayed association with risk for both higher and lower pulse rate that was stronger for the higher PR, and had predictive power only for lower PR at age older than 70 years. The trend of hazard ratio reduction between age classes 50–60 and older than 70 years was significant only for higher pulse rate. For age 50 years or less, hazard ratio had predictive power only for elPP and PP in age class 40–50 years and lower pulse rate (1.91 [1.12–3.27], and 2.06 [1.13–3.73], respectively).
A similar pattern of pulse rate effect of the hazard ratio was found for cardiovascular events at age older than 50 years (Supplemental Table 3b, http://links.lww.com/HJH/C43), where the trend of hazard ratio reduction between age classes 50–60 and older than 70 years was significant only for elPP and PP at higher pulse rate. However, a different pattern was observed for age 50 years or less, where hazard ratio had predictive power (protective effect) only for elPP and PP at age 40 years or less and lower pulse rate (0.27 [0.11–0.70], and 0.21 [0.07–0.65], respectively).
Supplemental Table 4, http://links.lww.com/HJH/C43 shows sensitivity analyses of the primary endpoints, including only untreated participants without history of cardiovascular disease at baseline or only 24-h ambulatory recordings with at least 10 daytime and 5 night-time readings that produced confirmatory results.
DISCUSSION
Previous findings on the dynamic association between the 24-h ambulatory PP and outcomes in participants older than 50 years [11] were expanded here by applying the analysis also to ‘elastic PP component’ and ‘stiffening PP component’ associated, respectively, with two different aspects of arterial stiffness: arterial stiffness at the diastole, and the dynamic stiffness change during the systole, where PP is the sum of elPP and stPP (Fig. 1).
The variation of the PP variables with age stratified by sex showed a strong coupling that largely differed between patients younger or older than age 45–55 years for PP and elPP, and over age 35–40 years for stPP (depending on the sex) with or without adjustment to clinical and demographic variables (Fig. 2).
PP components demonstrated different associations with cardiovascular morbidity and mortality for different age classes and outcomes, where in adults younger than 50 years, PP components showed either predictive power for risk or protective effect or for different endpoints (Table 4). Between 50 to over 70 years of age, all PP variables decreased in a pulse-rate-dependent way (Supplementary Tables 3a and b, http://links.lww.com/HJH/C43). For age 50–60 years, both PP components displayed association with risk, but for age over 60 years, the elastic component, which reflects the diastolic stiffness, but not stPP, dominated the association of PP with risk for all endpoints.
The prognostic significance of the PP components was previously investigated by two studies: study 1 – with Israeli hypertensive patients referred for 24-h ambulatory monitoring from an urban population consisting of European, Mediterranean, and North African ethnicities [22], and study 2 – a study conducted in Japan, as a part of the Ohasama study, with a rural Japanese general population without history of cardiovascular disease [23]. In the sub-group of participants older than about 70 years, and PR lower than about 70 bpm, predictive power for total mortality was found in study (1) for stPP, but not for elPP, and the opposite result in study (2), that is, predictive power for elPP, but not for stPP, as in the present study. The discrepancy between the first and the present study calls for analysis of multiple factors that differentiate the studies from one another, which is beyond the scope of the present article.
Physiological basis of the pulse pressure components
The PP component concept stems from the nonlinear form of the pressure–volume relationship in arteries, which can be explained in a simplified way by the arterial wall stress borne by elastin and collagen fibers in response to left-ventricular ejection: elastin is stretched as a purely elastic material, that is, displaying a linear pressure–volume relationship during the systole (Fig. 1). In parallel, upon increasing the pressure (or volume), greater numbers of collagen fibers, initially slack when unloaded, are ‘recruited’ when loaded, reach their maximum length, and carry a higher portion of the arterial wall stress. As stretched collagen fibers are about 1000-fold stiffer than elastin fibers, this recruitment process superimposed on the elastin-stretching gives the pressure–volume relationship its typical nonlinear form [14-17,21,32-34]. Functionally, these mechanical characteristics not only enable the arteries to smoothen the pressure and flow pulsation generated by the intermittent nature of the ventricular ejection at normal and low pressures (cushioning function) but also prevent over-distension of, and damage to arteries caused by increased pressure (or strain) [34,35]. Furthermore, the recruitment of collagen is enabled and controlled by smooth muscles that connect in series the collagen fibers having tone controlled by neural sympathetic activity [17,33,36], which may vary the mechanical properties of the arteries acutely [37]. In fact, the nonlinearity of the arterial pressure–volume relationship is reduced upon reducing the smooth muscle tone [21]. According to the present view, elPP corresponds to the stress generated in parallel by the elastin and collagen at their DBP state in response to the pulse volume (ΔV in Fig. 1). Thus, the ratio elPP/ΔV is the arterial stiffness at the DBP, whereas stPP is the excess contribution to the PP of the stress developed in the collagen fibers recruited during the early systole, that is, between the DBP and SBP. Finally, it is noteworthy to clarify the potential effect of the so-called ‘reflected waves’ on the PP variables: reflected waves are widely believed to affect occasionally the pulse waveform at the early systole, and thus PP and its components, elPP and stPP. However, the ratio stPP/elPP derived from the relationship between arterial pressure and volume can be shown to be a pressure-independent arterial property (Supplemental Methods, http://links.lww.com/HJH/C43), and thus unlikely to be affected by reflected waves.
Interpreting the effect of aging on the relationship between arterial properties, pulse pressure variables, and outcomes
This attempt is should be read with caution as it seems that the PP variables and outcomes vary with age, sex, and pulse rate in parallel with variations in the arterial wall structure that were reported in literature but were not measured in the present study.
Normal aging involves a number of structural and functional modifications such as: elastin fragmentation, possibly reflecting mechanical fatigue/failure caused by the pulsatile wall strain experienced by the aorta over about three billion cardiac cycles during a lifetime [38]; an increase in the content of the collagen, and cross-bridging between the collagen fibers that increase stiffness with aging [39], which may be reflected by the increase of PWV with aging [40,41]; an increase in the muscle sympathetic neural activity that makes the pressure–volume relationship more ‘curved’ in older age [33], and also increases arterial stiffness [37,42]; an increase in the arterial stiffness is associated with the wall thickening that reduces the storage of blood by the arteries during the early systole that buffers the pressure pulsatility (the so-called ‘cushioning function’). This process is compensated, in part, by an increase in the lumen area [43].
Some of these ‘normal aging modifications’ are coupled and controlled by multiple processes that are superimposed on the background of genetic predisposition and lifestyle influences to generate association between arterial properties and the risk of cardiovascular morbidity and mortality [36,38,43-46], such as calcification, glycation, and lipid peroxidation. These structural modifications impair the cushioning function and have important consequences for cardiovascular health, including isolated systolic hypertension, excessive penetration of pulsatile energy into the microvasculature of target organs that operate at low vascular resistance, and abnormal ventricular–arterial interactions that promote left ventricular remodeling, dysfunction, and failure [44-46]. The observed increase in the event rate versus age (Table 3), to which an exponential function fits remarkably well for all endpoints (Fig. 2), is known for humans as well as for other species, and was predicted, in the general case, by a model describing the risk of mortality by a process of accumulated damage and repair at a limited rate [47].
The U-shaped age dependency of the PP variables similar to the present finding (Fig. 2) was reported previously in unmedicated hypertensive patients with a similar age range [48]. The PP widening for age over 50 years was associated with greater stroke volume resulting in larger stretch of compliant arteries, whereas for age over 50 years, the PP widening in spite of the lower stroke volume was attributed to higher arterial stiffness [48]. Age and sex dependence of PP similar to our findings are found in literature [40,49,50]. Its similarity to the corresponding variations of elPP (Fig. 2) is explained by the relatively small size of stPP that increases almost linearly with age but lacks sex dependence for age over 35 years. The increase of stPP with age may be rationalized by the age-dependent increased number of collagen fibers participating the recruitment process, as well as smooth muscle cell hypertrophy. The age dependence of the PP components was previously reported [22], but its sex dependence is reported here for the first time.
The protective effect demonstrated by elPP for cardiovascular events at lower pulse rate for age class 40 years or less (Supplementary Table 3b, http://links.lww.com/HJH/ C43) may reflect the elevation of stiffness that prevents excessive arterial wall stretch for greater stroke volume. Such protective effect has been reported previously [45]. On the other hand, the large relative risk associated with stPP and PP for stroke events in this age class (Table 4) may point to a specific disorder. However, the paucity of events in these two cases justifies dedicated studies for young participants with a larger sample size.
We speculate that the peaked hazard ratios of all PP variables in age class 50–60 years reflect structural and functional modifications of the arterial wall that take place, probably because of the transfer of loading from elastin and collagen, and the start of large age-related decrease in stroke volume [48], leading to reduced functionality of the arterial system and increased risk. In this age range, SBP increases steeply whereas DBP displays moderate reduction, with parallel increase of PP and elPP. The demonstrated dominance of the association of elPP with the relative risk for age over 50 years supports the view that the prognostic power of PP is associated with pressure-independent stiffness. It is likely that the gradually increased stability of the arterial wall structure with aging (greater collagen content, cross-bridging between collagen fibers, reduction in recruitment of collagen fibers, and the thicker arterial wall) reduces the association with risk of elPP and PP and especially stPP, as observed, suggesting that most of the increase in the PP variables for age over 50 years is associated with normal aging. Our previous publication reached the same conclusion [11].
The main limitation of our study is the small number of participants below 50 years of age with a relatively low incidence of events that limits the ability to study the prognostic significance of the PP variables at this age range. Additional limitations are that the IDACO database available for the analysis did not include treatment-specific data other than antihypertensive drug intake (yes/no), and the height of participants, which prevented analysis of the combined effect of sex and height on the prognostic power of the PP variables for different age groups,. Further studies are required for better understanding of the interplay between PP components, the vascular wall structure, its modification by clinical and demographical factors, and outcomes.
In conclusion, the PP components, which stand for two different aspects of arterial stiffness, are associated differently with the relative risk of cardiovascular morbidity and mortality for different age classes and outcomes, which is in accord with our study hypothesis. In adults younger than 50 years, PP components show predictive power for risk or protective effect for different endpoints. Between 50 to over 70 years, all PP variables are reduced in a pulse rate-dependent way. For age 50–60 years, both PP components are associated with risk, but for age over 60 years, the elastic component, which reflects the diastolic stiffness, dominates the prognostic significance of PP for all outcomes.
This study provides a new basis for associating PP components with both outcomes and arterial properties at different age groups and pulse rates for both old and young age. Results support the need for further studies to assess the prognostic significance of the PP components, especially at the young age, at which there were too few cases to allow solid conclusions. The fact that the pattern of variation of the fully adjusted hazard ratios of the PP components over age class and endpoints (Table 4) was similar to that of the unadjusted hazard ratios that increases the confidence in the clinical usefulness of these measures.
Supplementary Material
ACKNOWLEDGEMENTS
Source of funding: the funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Belgium: European Union (HEALTH-F7–305507 HOMAGE), European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET), European Research Area Net for Cardiovascular Diseases (JTC2017-046-PROACT) and Research Foundation Flanders, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13); China: The National Natural Science Foundation of China (grants 81770455, 82070432, and 82070435), the Ministry of Science and Technology (2018YFC1704902), Beijing, China, and by the Shanghai Commissions of Science and Technology (grant 19DZ2340200) and the Shanghai Bureau of Health (a Grant for Leading Academics); The Czech Republic: European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007–201550) and Charles University Research Fund (project P36); Denmark: Danish Heart Foundation (grant 01-2-9-9A-22914) and Lundbeck Fonden (grant R32-A2740); Ireland: the Irish Allied Bank; Italy: European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007–201550); Japan: Ministry of Culture, Sports, Science and Technology (17H04126, 17H06533, 17K15853, 17K19930, 18K09674, 18K09904, 18K17396, 19K19466, 19H03908, 19K10662, and 20K08612), Japan Atherosclerosis Prevention Fund (Comprehensive Research on Cardiovascular and Lifestyle Related Diseases), Ministry of Health, Labor and Welfare (H29–Junkankitou–Ippan–003 and 20FA1002), Ministry of Agriculture, Forestry and Fisheries (NouEi 2-02), Academic Contributions from Pfizer Japan Inc., ACRO Incubation Grants of Teikyo University; and scholarship donations from Chugai Pharmaceutical Company and Daiichi Sankyo Company; Poland (Gdańsk): European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007–201550); Poland (Kraków): European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007–201550) and Foundation for Polish Science; The Russian Federation: European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007–201550); Uruguay: Asociación Española Primera en Salud; Venezuela: The National Institute of Aging and the Fogarty International Center (grant 1 R01AG036469 A1), the National Institutes of Health and National Institute of Aging (grant 1 R03 AG054186-01), FONACIT, Caracas (grant G-97000726), and FundaConCiencia, Maracaibo (grant LOCTI/008-2008). The Research Institute Alliance for the Promotion of Preventive Medicine (APPREMED), Mechelen, Belgium received a nonbinding grant from OMRON Healthcare, Co., Ltd., Kyoto, Japan.
Abbreviations:
- BP
blood pressure
- elPP
elastic pulse pressure
- HR
hazard ratio
- PP
pulse pressure
- PWV
pulse wave velocity
- stPP
stiffening pulse pressure
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Franklin SS, Gustin W 4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96:308–315. [DOI] [PubMed] [Google Scholar]
- 2.Gu YM, Thijs L, Li Y, Asayama K, Boggia J, Hansen TW, et al. International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators. Outcome-driven thresholds for ambulatory pulse pressure in 9938 participants recruited from 11 populations. Hypertension 2014; 63:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benetos A, Rudnichi A, Safar M, Guize L. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension 1998; 32:560–564. [DOI] [PubMed] [Google Scholar]
- 4.Assmann G, Cullen P, Evers T, Petzinna D, Schulte H. Importance of arterial pulse pressure as a predictor of coronary heart disease risk in PROCAM. Eur Heart J 2005; 26:2120–2126. [DOI] [PubMed] [Google Scholar]
- 5.Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000; 160:1085–1089. [DOI] [PubMed] [Google Scholar]
- 6.Domanski M, Norman J, Wolz M, Mitchell G, Pfeffer M. Cardiovascular risk assessment using pulse pressure in the first national health and nutrition examination survey (NHANES I). Hypertension 2001; 38:793–797. [DOI] [PubMed] [Google Scholar]
- 7.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Pede S, Porcellati C. Ambulatory pulse pressure: a potent predictor of total cardiovascular risk in hypertension. Hypertension 1998; 32:938–983. [DOI] [PubMed] [Google Scholar]
- 8.Khattar RS, Swales JD, Dore C, Senior R, Lahiri A. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation 2001; 104:783–789. [DOI] [PubMed] [Google Scholar]
- 9.Staessen JA, Thijs L, O’Brien ET, Bulpitt CJ, de Leeuw PW, Fagard RH, et al. , Syst-Eur Trial Investigators. Ambulatory pulse pressure as predictor of outcome in older patients with systolic hypertension. Am J Hypertens 2002; 15:835–843. [DOI] [PubMed] [Google Scholar]
- 10.Hozawa A, Ohkubo T, Nagai K, Kikuya M, Matsubara M, Tsuji I, et al. Prognosis of isolated systolic and isolated diastolic hypertension as assessed by self-measurement of blood pressure at home: the Ohasama study. Arch Intern Med 2000; 160:3301–3306. [DOI] [PubMed] [Google Scholar]
- 11.Melgarejo JD, Thijs L, Wei DM, Bursztyn M, Yang WY, Li Y, et al. Relative and absolute risk to guide the management of pulse pressure, an age-related cardiovascular risk factor. Am J Hypertens 2021; 34:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dart AM, Kingwell BA. Pulse pressure–a review of mechanisms and clinical relevance. J Am Coll Cardiol 2001; 37:975–984. [DOI] [PubMed] [Google Scholar]
- 13.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 2003; 107:2864–2869. [DOI] [PubMed] [Google Scholar]
- 14.Hermeling E, Vermeersch SJ, Rietzschel ER, de Buyzere ML, Gillebert TC, van de Laar RJ, et al. The change in arterial stiffness over the cardiac cycle rather than diastolic stiffness is independently associated with left ventricular mass index in healthy middle-aged individuals. J Hypertens 2012; 30:396–402. [DOI] [PubMed] [Google Scholar]
- 15.Hallock P, Benson IC. Studies on the elastic properties of human isolated aorta. J Clin Invest 1937; 16:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolinsky H, Glagov S. Structural basis for the static mechanical properties of the aortic media. Circ Res 1964; 14:400–413. [DOI] [PubMed] [Google Scholar]
- 17.Bank AJ, Wang H, Holte JE, Mullen K, Shammas R, Kubo SH. Contribution of collagen, elastin, and smooth muscle to in vivo human brachial artery wall stress and elastic modulus. Circulation 1996; 94:3263–3270. [DOI] [PubMed] [Google Scholar]
- 18.Gavish B, Izzo JL Jr. Arterial stiffness: going a step beyond. Am J Hypertens 2016; 29:1223–1233. [DOI] [PubMed] [Google Scholar]
- 19.Hermeling E, Hoeks AP, Winkens MH, Waltenberger JL, Reneman RS, Kroon AA, Reesink KD. Noninvasive assessment of arterial stiffness should discriminate between systolic and diastolic pressure ranges. Hypertension 2010; 55:124–130. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech 1980; 13:175–184. [DOI] [PubMed] [Google Scholar]
- 21.VanBavel E, Siersma P, Spaan JA. Elasticity of passive blood vessels: a new concept. Am J Physiol Heart Circ Physiol 2003; 285:H1986–H2000. [DOI] [PubMed] [Google Scholar]
- 22.Gavish B, Bursztyn M. Ambulatory pulse pressure components: concept, determination and clinical relevance. J Hypertens 2019; 37:765–774. [DOI] [PubMed] [Google Scholar]
- 23.Bursztyn M, Kikuya M, Asayama K, Satoh M, Gavish B, Ohkubo T. Do estimated 24-h pulse pressure components affect outcome? The Oha-sama study. J Hypertens 2020; 38:1286–1292. [DOI] [PubMed] [Google Scholar]
- 24.Anliker M, Histand MB, Ogden E. Dispersion and attenuation of small artificial pressure waves in the canine aorta. Circ Res 1968; 23:539–551. [DOI] [PubMed] [Google Scholar]
- 25.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. , American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 2015; 66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thijs L, Hansen TW, Kikuya M, et al. The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit 2007; 12:255–262. [DOI] [PubMed] [Google Scholar]
- 27.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 28.Yang WY, Melgarejo JD, Thijs L, Zhang ZY, Boggia J, Wei FF, et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA 2019; 322:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang WY, Thijs L, Zhang ZY, Asayama K, Boggia J, Hansen TW, et al. , International Database; on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators. Evidence-based proposal for the number of ambulatory readings required for assessing blood pressure level in research settings: an analysis of the IDACO database. Blood Press 2018; 27:341–350. [DOI] [PubMed] [Google Scholar]
- 30.Gavish B Effect of arterial mechanics on the pulse pressure: a model view. J Clin Hypertens 2007; 9:190A. [Google Scholar]
- 31.Gavish B, Ben-Dov IZ, Kark JD, Mekler J, Bursztyn M. The association of a simple blood pressure-independent parameter derived from ambulatory blood pressure variability with short-term mortality. Hypertens Res 2009; 32:488–495. [DOI] [PubMed] [Google Scholar]
- 32.Roach MR, Burton AC. The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol 1957; 35:681–690. [PubMed] [Google Scholar]
- 33.Bank AJ, Kaiser DR, Rajala S, Cheng A. In vivo human brachial artery elastic mechanics: effects of smooth muscle relaxation. Circulation 1999; 100:41–47. [DOI] [PubMed] [Google Scholar]
- 34.Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res 2012; 5:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holzapfel GA. Collagen in arterial walls: biomechanical aspects. In: Fratzl P, editor. Collagen. Boston, MA: Springer; 2008. pp. 285–324. [Google Scholar]
- 36.Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev 2017; 97:1555–1617. [DOI] [PubMed] [Google Scholar]
- 37.Holwerda SW, Luehrs RE, DuBose L, Collins MT, Wooldridge NA, Stroud AK, et al. Elevated muscle sympathetic nerve activity contributes to central artery stiffness in young and middle-age/older adults. Hypertension 2019; 73:1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface 2013; 10:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aronson D Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 2003; 21:3–12. [DOI] [PubMed] [Google Scholar]
- 40.Avolio AP, Kuznetsova T, Heyndrickx GR, Kerkhof PLM, Li JK. Arterial flow, pulse pressure and pulse wave velocity in men and women at various ages. Adv Exp Med Biol 2018; 1065:153–168. [DOI] [PubMed] [Google Scholar]
- 41.Kim EJ, Park CG, Park JS, Suh SY, Choi CU, Kim JW, et al. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: invasive study. J Hum Hypertens 2007; 21:141–148. [DOI] [PubMed] [Google Scholar]
- 42.Tsioufis C, Dimitriadis K. Sympathetic system–related artery stiffness: understanding the Gordian knot of vascular ageing. Hypertension 2019; 73:975–976. [DOI] [PubMed] [Google Scholar]
- 43.Thijssen DH, Carter SE, Green DJ. Arterial structure and function in vascular ageing: are you as old as your arteries? J Physiol 2016; 594:2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chirinos JA. Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res 2012; 5:243–255. [DOI] [PubMed] [Google Scholar]
- 45.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation 2003; 107:139–146. [DOI] [PubMed] [Google Scholar]
- 46.Duca L, Blaise S, Romier B, Laffargue M, Gayral S, El Btaouri H, et al. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res 2016; 110:298–308. [DOI] [PubMed] [Google Scholar]
- 47.Ledberg A Exponential increase in mortality with age is a generic property of a simple model system of damage accumulation and death. PLoS One 2020; 15:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alfie J, Waisman GD, Galarza CR, Cámera MI. Contribution of stroke volume to the change in pulse pressure pattern with age. Hypertension 1999; 34:808–812. [DOI] [PubMed] [Google Scholar]
- 49.Saladini F, Fania C, Mos L, Mazzer A, Casiglia E, Palatini P. Office pulse pressure is a predictor of favorable outcome in young- to middle-aged subjects with stage 1 hypertension. Hypertension 2017; 70:537–542. [DOI] [PubMed] [Google Scholar]
- 50.Skurnick JH, Aladjem M, Aviv A. Sex differences in pulse pressure trends with age are cross-cultural. Hypertension 2010; 55:40–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.