Abstract
Background
Measures for improving medication safety in outpatient care are often complex and involve medication reviews. Over the period 2016–2022 (with a preceeding one-year pilot phase), an interprofessional medication management program—the Medicines Initiative Saxony–Thuringia (Arzneimittelinitiative Sachsen-Thüringen, ARMIN)—was implemented in two German federal states. More than 5000 patients received a medication review by the end of 2019 by a team composed of physicians and pharmacists and were provided with joint, continuous care thereafter.
Methods
In the framework of a retrospectively registered cohort study, the mortality and hospitalizations of this population (5033 patients) were studied using routine data from a statutory health insurer (observation period 2015–2019) and compared with those of a control group (10 039 patients) determined from the routine data by propensity score matching. Mortality was compared by survival analysis (Cox regression), and hospitalization rates were compared in terms of event probabilities within two years of enrollment in the medication management program. Robustness was tested in multiple sensitivity analyses.
Results
Over the observation period, 9.3% of the ARMIN participants and 12.9% of persons in the control group died (hazard ratio of the adjusted Cox regression, 0.84; 95% confidence interval [0.76; 0.94], P = 0.001). In the first two years after inclusion, the ARMIN participants were hospitalized just as often as the persons in the control group (52.4% versus 53.4%; odds ratio from the adjusted model, 1.04 [0.96; 1.11], P = 0.347). The effects were consistent in sensitivity analyses.
Conclusion
In this retrospective cohort study, participation in the ARMIN program was associated with a lower risk of death. Exploratory analyses provide clues to the potential origin of this association.
Medication review interventions, as a core element for improving medication safety in outpatient care (1, 2), are designed to identify and resolve potential drug related problems (DRPs) (2). There is currently a lack of uniform standards of how and by whom medication reviews are to be provided in the patient care setting (3), so it is difficult to compare study results and to derive recommendations (4).
In the past, medication reviews have been particularly successful where there has been close interprofessional collaboration (5– 7). Yet, at the same time, some DRPs appear to be resolvable only over the course of time, thus favoring the concept of medication reviews with continuous follow-ups (8). Medication review studies have produced positive outcomes for clinical biomarkers (e.g. blood pressure) and patient-related endpoints (e.g. medication adherence), in addition to medication-related endpoints (9). Clinical endpoints, such as hospitalization and mortality were investigated comparatively rarely, with no clear effects arising from them either (4). A meta-analysis of community pharmacist-based medication reviews revealed that only three of 40 randomized controlled trials (RCT) reported on mortality as an endpoint, with one trial showing no effect and two being underpowered (4). Another meta-analysis found that medication reviews (as part of setting-independent deprescribing interventions with the purpose of systematically reducing potentially inappropriate medications) may have lowered all-cause mortality by an absolute 1.4% (odds ratio: 0.74; 95% confidence interval [0.58; 0.95]) but had at most a minor impact on hospitalizations – both statements were rated with a low certainty of evidence (10).
In Germany, general practitioners and community pharmacists have offered interprofessional medication management as part of the Medicines Initiative Saxony-Thuringia (Arzneimittelinitiative Sachsen-Thüringen, ARMIN) since 2015. Mortality and hospitalizations of the enrolled patients were now compared with the corresponding results of a propensity-score matched (PSM) control group.
Methods
The ARMIN program is a model project on the legal basis of Section 63 of the German Social Security Code Part V, agreed in 2014 between regional physician and pharmacist associations in cooperation with the AOK PLUS statutory health insurance fund and consisting of three modules. The present assessment focuses on patients who enrolled in Module 3, the continuous medication management program, to be supervised by a distinct primary physician–pharmacist pair (box). The ARMIN project has already been reported in detail elsewhere (11); the eMethods section contains a detailed description of data, endpoint operationalization, and statistical analysis. The STROBE/RECORD reporting guidelines are to be found in the eResultssection Table S1. The analysis reported in the present article is part of the comprehensive evaluation of the ARMIN project (12).
BOX. Study intervention.
ARMIN medication management program includes the following components:
-
The pharmacist responsible for the patient first records all their medication (including self-medication) as part of a “brown bag” review and conducts a pharmaceutical medication safety assessment according to the medication review guideline of the German Federal Chamber of Pharmacists. This involves checking for medication-related problems such as
potential drug interactions
double medication
assessment of dosage information for plausibility.
The responsible pharmacist then draws up a provisional medication plan which is transmitted in digital format to the physician via the so-called medication plan server. Both health care professionals can communicate digitally with each other by adding comments in the medication plan.
The physician then conducts a medical medication safety assessment in compliance with the practice guideline “Multimedication”. This involves checking for overuse, underuse and misuse, with due regard to current diagnoses and risk factors. Where necessary, other attending physicians are contacted, and any knowledge gaps with regard to the patient’s diseases and therapy are addressed.
The medication review is concluded by finalizing the medication plan and handing it to the patient.
This is followed by an ongoing medication review by both healthcare professionals and the recording of an up-to-date medication plan.
The data analysis used routine secondary health care data provided by AOK PLUS. This type of data has been used in previous routine data analyses independent of ARMIN (13, 14). In addition to health insurance data, ARMIN-related data regarding the involved healthcare professionals was also made available.
The data of all patients in this retrospective cohort study who had enrolled for the medication management up to the end of 2018 (N = 5180) fulfilled the inclusion criteria and were eligible for analysis. A control group was assigned by PSM to the enrolled ARMIN participants with a planned ratio of 1 : 2.5 (figure 1). The observation period started on the index date (enrollment date for ARMIN patients, randomly assigned starting date for controls). The primary endpoints were mortality and hospitalizations (the latter being within two years of enrollment). Robustness of the results was tested in sensitivity analyses, which included alternative operationalization of the endpoints, comparison of adjusted with unadjusted analyses, and aggregated analyses together with analyses at patient level (eMethods).
Figure 1.
Flowchart for defining the analysis population using propensity score matching (PSM). Potential control subjects, taken from the distribution of the enrollment data of the Medicines Initiative Saxony-Thuringia (ARMIN) from 2015 to the end of 2018, were randomly assigned a start date for follow-up when they were tested for comparability by PSM. These start dates are the index dates of the analysis population which were followed-up in routine data from AOK PLUS until the end of 2019 (information taken from death registries covered an additional quarter until 31.03.2020).
The following secondary endpoints were analyzed:
How hospitalizations differed in their incidence (their number over the entire follow-up period), dynamics (time until first hospitalization), and cause (potentially related to an adverse drug event [15])
Whether there were differences in utilization of healthcare services, and
How selected indicators for medication safety changed in each group (adherence based on the prescriptions, occurrence of drug interactions, prescription for potentially inappropriate medication in accordance with the PRISCUS list 1.0 [16]), overuse and misuse (START/STOPP [Screening Tool to Alert to Right Treatment / Screening Tool of Older Persons’ Prescriptions]), operationalized according to [15]).
The primary endpoints were compared with regard to their time-to-event outcome (mortality in Cox regression analysis) and probability of an event (hospitalization in logistic regression analysis). A weighted regression analysis using inverse probability of censorship weights (IPCW, 17) accounted for the possibility that patients were not followed up for the full two years. Generally, regression analyses were further adjusted for relevant prognostic variables (for example, patient age, for details see eMethods). Statistical tests were two-tailed, and 95-percent confidence intervals were calculated with an alpha-error of 0.05; p-values below 0.05 were considered significant. All statistical analyses were performed using the R-software environment Version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
PSM yielded an analysis population of 5033 ARMIN participants and 10 039 controls. The groups were successfully balanced; the standardized differences were outside the targeted range only for age, and with 72.6 ± 11.6 years the ARMIN patients were around one year younger than the control persons (73.7 ± 11.4; eResults Figure S1, eResults Figure S2). The analysis population comprised 15 072 patients with an average observation period of 30.0 ± 9.7 months (table 1).
Table 1. Patient characteristics of the analysis population formed by PSM.
| ARMIN (N = 5033) | Controls(N = 10 039) | Total(N = 15 072) | |
| Demographics | |||
| – male sex, N (%) | 2231 (44.3) | 4450 (44.3) | 6681 (44.3) |
| – age in years, mean ± SD | 72.6 ± 11.6 | 73.7 ± 11.4 | 73.4 ± 11.4 |
| Selected comorbidities and clinical characteristics taken from inpatient and outpatient diagnoses in the year before the index date | |||
| Elixhauser comorbidity score (17), mean ± SD | 6.3 ± 2.8 | 6.4 ± 3.0 | 6.4 ± 3.0 |
| Utilization of the health care system (in the year before the index date) | |||
| Number of previous all-cause hospitalizations per patient | |||
| – Median (IQR) | 0 (0, 1) | 1 (0, 1) | 1 (0, 1) |
| – Mean ± SD | 0.7 ± 1.1 | 1.0 ± 1.5 | 0.9 ± 1.4 |
| Number of previous specific* hospitalizations per patient | |||
| – Median (IQR) | 0 (0, 0) | 0 (0, 1) | 0 (0, 0) |
| – Mean ± SD | 0.3 ± 0.7 | 0.4 ± 0.9 | 0.4 ± 0.8 |
| High utilization of outpatient healthcare services, N (%) | 2151 (42.7) | 4283 (42.7) | 6434 (42.7) |
| Medication (in the year before the index date) | |||
| Number of different ATC codes per patient | |||
| – Median (IQR) | 9 (7, 13) | 9 (7, 13) | 9 (7, 13) |
| – Mean ± SD | 10.2 ± 4.4 | 10.0 ± 4.8 | 10.1 ± 4.7 |
* Specific hospitalizations were defined as admissions potentially related to adverse drug reactions to currently prescribed drugs, according to Meid et al. 2018 (15) ARMIN,Medicines Initiative Saxony-Thuringia; ATC, Anatomic Therapeutic Chemical classification system; IQR, interquartile range; N, number;
PSM, propensity score matching; SD, standard deviation
Mortality and hospitalizations
During the observation period, 9.3% of patients in the ARMIN group (N = 469/5033) died, and 12.9% in the control group (N = 1300/10 039). The relative risk in the adjusted survival time analysis was reduced by 16% in the ARMIN group (hazard ratio 0.84; [0.76; 0.94], p = 0.001) (Figure 2a, eResults Table S3A). This corresponds to a covariate-adjusted absolute risk reduction of 1.52% and a number needed to treat (NNT) of 66.
In the two-year follow-up observation period after enrollment, 52.4% of the ARMIN participants were hospitalized at least once (N = 2639/5033) compared with 53.4% of the control persons (N = 5359/10 039); with regard to the entire observation period, this affected 56.8% and 59.3% of the patients, respectively. After taking other prognostic variables into account, there was no group difference in the likelihood of being hospitalized within two years (odds ratio 1.04 [0.96; 1.11], p = 0.347) (Figure 2b, eResults Table S4A).
Sensitivity analysis
In line with the main analysis, the probability of dying within two years was also reduced in the ARMIN group (eResults Table S3B). Also, the non-parametric Kaplan-Meier analysis showed a reduction in mortality (figure 3). An association remained even when, as a per-protocol analysis, the 320 patients in the ARMIN group who left the program prematurely were excluded (hazard ratio 0.88 [0.7; 0.98]), as was the case after excluding deaths within 30 days of enrollment (hazard ratio 0.84 [0.75; 0.94]). An exploratory analysis showed an increasing effect over time (eResults Figure S2). When examining the aggregated hospitalization rate, no difference over time was observed between the ARMIN and the control group (eResults Figure S3).
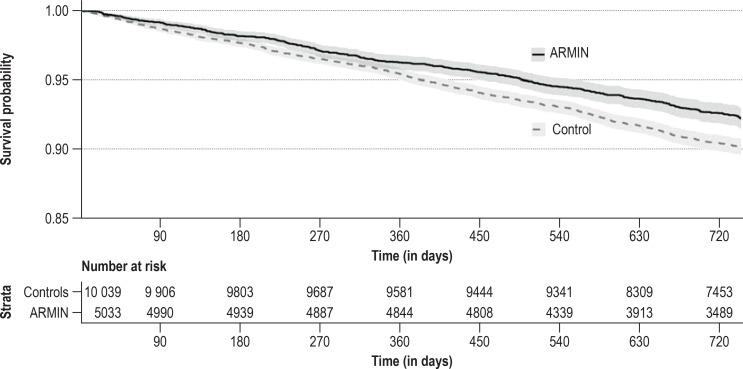
Figure 3.
Kaplan-Meier representation of survival times. Survival probabilities are shown stratified in the propensity score-matched analysis population for the ARMIN group (black continuous line) and the control group (dashed gray line) (log rank test: p <0.001; the difference between the groups for the restricted mean survival times [38] is 80 days, p <0.001 for individual values amongst ARMIN patients of 1720 days and amongst controls of 1640 days). ARMIN, Medicines Initiative Saxony-Thuringia
Secondary endpoints
Hospitalizations differed between the groups with regard to both numbers and dynamics: Hospitalized persons in the ARMIN group were admitted to hospital earlier (hazard ratio from time-to-first-event analysis 1.05 [1.01; 1.10], eResults Table S4B) and more often (incidence rate ratio from an analysis of count data for the absolute number of hospitalizations 1.06 [1.03; 1.09], eResults Table S4C). When extended lifespan is considered in a time-to-event analysis in the presence of competing risks, then the probability of hospitalization was increased by 8% in line with the intensified care provided (hazard ratio based on a cause-specific hazard model 1.08 [1.02; 1.13]). The associations with prognostic variables were comparable for all-cause hospitalizations and hospitalizations potentially related to adverse drug events (eResults Tables S4A/C and S5A/C, eResults Figure S4, eResults Figure S5). Regarding utilization of outpatient health care services, the annual contacts with general practitioners and specialists for the ARMIN group were more frequent (16.6 and 6.6, respectively) than for the control group (15.0 and 4.8, respectively), and the incidence rates of general practitioner visits increased in comparison with the previous year (+ 1.3 in the ARMIN group versus -0.4 in the control group) (table 2). Whereas the average number of different pharmacies visited was marginally lower in the ARMIN group (1.1 versus 1.2), the average total number of contacts with pharmacies was higher among patients in the ARMIN group (and also increased more strongly after ARMIN enrollment [+ 2.0 versus + 0.6, Table 2]).
Table 2. Secondary endpoints in the ARMIN and control groups.
| Total | ARMIN(N = 5033) | Controls(N = 10 039) | p-value | |
| Number of specific* hospitalizations of patients during the entire follow-up | ||||
| – Median (IQR) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | |
| – Mean ± SD | 0.4 ± 0.5 | 0.4 ± 0.5 | 0.4 ± 0.5 | pWilcoxon = 0.860 |
| Utilization of health care in the follow-up observation period (and comparison with the previous year) | ||||
|
Total pharmacy visits Incidence rate/patient year (absolute change from previous year) |
14.6 (+1.1) | 15.5 (+2.0) | 14.1 (+0.6) | pPoisson <0.001 |
|
Visits to different pharmacies Incidence rate/patient year (absolute change from previous year) |
1.2 (−0.8) | 1.1 (−0.8) | 1.2 (−0.8) | pPoisson <0.001 |
|
Total contacts with general practitioners Incidence rate/patient year (absolute change from previous year) |
15.5 (+0.2) | 16.6 (+1.3) | 15.0 (−0.4) | pPoisson <0.001 |
|
Contacts with different general practitioners Incidence rate/patient year (absolute change from previous year) |
1.3 (−0.6) | 1.6 (−0.2) | 1.1 (−0.8) | pPoisson <0.001 |
|
Total contacts with specialists Incidence rate/patient year (absolute change from previous year) |
5.4 (−1.8) | 6.6 (−0.6) | 4.8 (−2.5) | pPoisson <0.001 |
* Specific hospitalizations were defined as admissions potentially related to adverse drug reactions to currently prescribed drugs, according to Meid et al. 2018 (15); pPoisson denotes a group comparison using a Poisson model for the incidence of visits in the follow-up observation period, controlling for the number of events in the previous year.
ARMIN, Medicines Initiative Saxony-Thuringia; IQR, interquartile range; SD, standard deviation
Various indicators of medication safety changed during the follow-up observation period (eResults Figure S5): the situation improved more often for the ARMIN participants in terms of exposure days with potential misuse and overuse (62.4 % versus 58.4 %; descriptive p-value = 0.003) and with regard to medication adherence (43.3% versus 41.6%; descriptive p-value = 0.001), and they participated more frequently in specific disease management programs (DMPs) (5.3% versus 2.8%; descriptive p-value <0.001). No group differences were evident in the number of patients with potentially inappropriate medications administered (PRISCUS; 59.5% versus 58.6%; descriptive p-value = 0.320), drug interactions (23.8% versus 24.1%; descriptive p-value = 0.927), or exposure days with potential underuse (START criteria) (2.5% versus 2.8%; descriptive p-value = 0.252).
Discussion
The impact of medication reviews or medication management programs on mortality or hospitalizations has so far hardly been investigated. In this regard, RCTs with small sample sizes (for example, intervention groups with 56–315 participants) (18– 24) or (too) short observation periods of typically six months were possibly underpowered to demonstrate any statistical significance (4), and a rather low certainty of evidence was reported in meta-analyses (10).
In the present retrospective cohort study, 5033 patients were followed-up over an average of 30 months; compared with the control group generated by PSM technique, the risk of dying in the ARMIN group during the observation period was only 0.84 times as high. This outcome was also adjusted for age, thus taking into account that the control group was on average about one year older. Risk of hospitalization was comparable in the two groups, yet patients hospitalized in the ARMIN cohort were admitted to hospital earlier and in absolute numbers more often than control persons. These results were also consistent in magnitude and significance in sensitivity analyses.
Some possible explanations emerging from the secondary endpoints appear to be of such relevance that they should be assessed further in future RCTs. For instance, our secondary data analysis suggests that patients in the ARMIN group were supervized more closely since they utilized more healthcare services during the observation period and their participation in the DMP increased more strongly (5.3 % versus 2.8 %). Although the rate of participation in DMPs was already high initially (>70% in both groups), this may nevertheless have affected the outcome, given that DMPs as a single measure have previously been associated with a survival benefit in several studies (25– 28). We too saw relevant risk reductions in mortality by DMPs (type 2 diabetes mellitus hazard ratio 0.85 [0.77; 0.94]; coronary heart disease 0.81 [0.73; 0.90]). This data therefore indicates that ARMIN participants were in contact with healthcare professionals more often and more closely, suggesting that critical situations may have been recognized earlier, and in the event could have resulted in (necessary) hospitalizations more rapidly—as is also shown in the data.
Furthermore, improvement in some of the assessed medication safety indicators was also detected: For example, exposure to STOPP criteria decreased more strongly in the ARMIN population than in the control group, and health insurance data demonstrated that prescriptions were filled more regularly regarding potentially important long-term treatments. Both markers have been associated in earlier studies with a risk of death (29– 32) and hospitalization (30, 32, 33). This association has not been demonstrated for other medication safety indicators, such as the prescription of potentially inappropriate medication according to PRISCUS (34), for which no improvement was observed in the ARMIN group either. Furthermore, with regard to assessing drug interactions, it is uncertain whether a lower prevalence would indeed have indicated a positive effect, given that many serious interactions can also be managed by appropriate monitoring, dose reduction, or temporary discontinuation.
Irrespective of all these considerations, it is not possible to assess the extent to which changes in drug therapy may have had a direct impact on endpoints—especially since these did not include any specifications, such as reason for hospitalization or cause of death.
It is also not possible to quantify the effect of the continuous interprofessional supervision which followed the initial medication review. Our results seem to indicate a growing effect over time. However, the question of whether and, if so, to what extent the continuous interprofessional supervision contributed to this observation cannot be answered causally. It would be difficult to measure the scope of, or any change in, the interprofessional supervision. It is estimated that one primary physician–pharmacist pair supervised a median of 21 insured persons during the medication management program, though the range was very wide (1–136 patients).
Given these surprisingly clear results, it would therefore seem all the more important to conduct prospective studies with a similar interprofessional intervention in the future and collect specific detailed information on medication and endpoints—such as cause of death or reason for hospitalization—as well as on subsequent changes in medication. Variables typically not available in secondary data should also be identified and quantified. These include, for example, sociodemographic data—such as educational or marital status—and attitudes of the participants which might influence their motivation to take part or their willingness to implement interventions. Relevant estimators for potential transferability of the approach should also be recorded, such as recruitment rates or time spent per patient.
Strengths and weaknesses
The strengths and weaknesses of the present study result largely from the data set and the study design. The latter was retrospective and observational, so a prospective confirmation of the results by RCTs, which would take multiplicity issues into account, is yet to follow. However, the higher level of evidence of RCTs is offset by their stricter inclusion and exclusion criteria and close monitoring of study-related interventions, making potential effects difficult to replicate under everyday settings (efficacy-effectiveness gap). Moreover, given that obtaining sufficiently large case numbers for RCTs can be challenging, there is increasing debate about whether this type of study is really optimal for evaluating the effectiveness of complex health care interventions (35, 36).
It was not possible to directly measure some of the medication safety endpoints with the available data. They had to be roughly estimated using drug dispensation data as no information was on hand regarding actual drug administration patterns. This was the case, for example, when it came to assessing medication adherence and analyzing drug interactions. Furthermore, participant selection is crucial for the transferability of the findings to routine care. This study involved patients insured by the AOK PLUS statutory health insurance fund which covers about 50% of patients in the participating federal states. They were recruited by their primary care physicians and pharmacists and had consented to participate in the interprofessional medication management program. This presupposes a certain degree of selection which could result in the observed effects being overestimated (because in general a more motivated, more approachable patient population was addressed) or even underestimated (because those patients who would benefit in particular from optimization of their medication and a closer interprofessional supervision were possibly not addressed). A similar selection may also be assumed at the level of the health care professionals since, for example, only about 8% of all potential physicians actually participated in the medication management project.
As part of PSM, potentially confounding variables were considered as comprehensively as possible and relevant potential confounders were also once again used for adjustment in the subsequent regression analyses (doubly robust). Nevertheless, it seems likely that other important prognostic and potentially confounding variables were not included—sociodemographic data, such as educational or marital status, readiness for change, motivation, and implementation quality of the intervention—because they were either not captured at all (omitted-variable bias) or not precisely enough (misclassification bias). However, the results of this analysis were also robust in all sensitivity analyses and so distinct that any single cause of bias would have to be very high to have any impact (E-value [37] of 1.67 with a lower confidence interval limit of 1.32). On the other hand, there may also be a large number of unknown confounders (which cannot be measured using routine data).
And last but no least, this study is limited by the fact that no causal conclusions can be drawn from the available data, particularly with regard to treatment-related changes: So it is uncertain which changes in medication were initiated by whom and how, whether they were maintained, and whether they had an effect on the morbidity of the patients.
Overview
This retrospective cohort study demonstrates a lower risk of death for patients enrolled in the ARMIN medication management program in comparison with control patients identified by PSM. The generated signals should now be assessed in prospective, controlled studies to also address potential structural healthcare and mechanistic associations. The aim here should be to identify the potential determining factors for effectiveness in this complex intervention and identify any subgroups of patients with particular benefit from the program.
Collaborators
Members of the ARMIN study team: Christiane Eickhoff, Uta Müller, Martin Schulz (ABDA – Federal Union of German Associations of Pharmacists); Andreas Fuchs, Dorit Braun, Ulf Maywald (AOK PLUS);
Catharina Döhler, Mike Mätzler (Association of Statutory Health Insurance Physicians of Saxony); Anja Auerbach, Urs Dieter Kuhn, Anke Möckel (Association of Statutory Health Insurance Physicians of Thuringia); Christine Honscha, Susanne Donner (Pharmacist Association of Saxony); Stefan Fink, Kathrin Wagner (Pharmacist Association of Thuringia); Walter E. Haefeli, Andreas D. Meid, Robert Moecker, Carmen Ruff, Hanna M. Seidling, Felicitas Stoll, Marina Weissenborn, Lucas Wirbka (University Hospital of Heidelberg); Petra Kaufmann-Kolle, Anja Klingenberg, Jona Frasch (aQua Institute for Applied Quality Improvement and Research in Health Care).
Funding
The Department of Clinical Pharmacology and Pharmacoepidemiology of the University Hospital of Heidelberg and the aQua Institute for Applied Quality Improvement and Research in Health Care received funding from the AOK Plus, ABDA, the Association of Statutory Health Insurance Physicians of Saxony and the Association of Statutory Health Insurance Physicians of Thuringia to conduct the evaluation as external evaluators.
Figure 2.
Forest plot of the regression models for risk of mortality (a) and hospitalizations (b), adjusted for the presented variables. The point estimates for hazard ratios (see eResults Table S3) and odds ratios (see eResults Table S4) are shown, together with associated 95% confidence intervals. ARMIN, Medicines Initiative Saxony-Thuringia
Acknowledgments
Translated from the original German by Dr Grahame Larkin MD
Acknowledgments
The authors would like to thank all participating members of the health care professions and their patients.
Footnotes
Conflict of interest statement
RM was reimbursed for presentations by the Regional Chamber of Pharmacists for Baden-Wurttemberg. He was reimbursed travel expenses and conference delegate fees by the German Society for Clinical Pharmacy.
HMS was reimbursed for presentations or further education courses and travel expenses by Chambers of Pharmacists or professional associations (e.g., ADKA [Federal Association of German Hospital Pharmacists] or GSASA [Swiss Association of Public Health Administration and Hospital Pharmacists]. She also performs honorary committee activities for the German Coalition for Patient Safety, the German Society for Hospital Pharmacists, the Drug Commission of German Pharmacists, and in the Coordination Group of the Action Plan on Drug Therapy Safety (AMTS) of the German Federal Ministry of Health (BMG).
The other authors declare that no conflict of interest exists.
References
- 1.Dautzenberg L, Bretagne L, Koek HL, et al. Medication review interventions to reduce hospital readmissions in older people. J Am Geriatr Soc. 2021;69:1646–1658. doi: 10.1111/jgs.17041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griese-Mammen N, Hersberger KE, Messerli M, et al. PCNE definition of medication review: reaching agreement. Int J Clin Pharm. 2018;40:1199–1208. doi: 10.1007/s11096-018-0696-7. [DOI] [PubMed] [Google Scholar]
- 3.Imfeld-Isenegger TL, Soares IB, Makovec UN, et al. Community pharmacist-led medication review procedures across Europe: characterization, implementation and remuneration. Res Social Adm Pharm. 2020;16:1057–1066. doi: 10.1016/j.sapharm.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Al-Babtain B, Cheema E, Hadi MA. Impact of community-pharmacist-led medication review programmes on patient outcomes: a systematic review and meta-analysis of randomised controlled trials. Res Social Adm Pharm. 2022;18:2559–2568. doi: 10.1016/j.sapharm.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Delgado-Silveira E, Vélez-Díaz-Pallarés M, Muñoz-García M, Correa-Pérez A, Álvarez-Díaz AM, Cruz-Jentoft AJ. Effects of hospital pharmacist interventions on health outcomes in older polymedicated inpatients: a scoping review. Eur Geriatr Med. 2021;12:509–544. doi: 10.1007/s41999-021-00487-3. [DOI] [PubMed] [Google Scholar]
- 6.Kwint HF, Bermingham L, Faber A, Gussekloo J, Bouvy ML. The relationship between the extent of collaboration of general practitioners and pharmacists and the implementation of recommendations arising from medication review: a systematic review. Drugs Aging. 2013;30:91–102. doi: 10.1007/s40266-012-0048-6. [DOI] [PubMed] [Google Scholar]
- 7.Sloeserwij VM, Hazen ACM, Zwart DLM, et al. Effects of non-dispensing pharmacists integrated in general practice on medication-related hospitalisations. Br J Clin Pharmacol. 2019;85:2321–2331. doi: 10.1111/bcp.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidling HM, Wien K, Fabricius J, et al. Short and mid-term impact of community pharmacy-based medication reviews on medication- and patient-related outcomes in Germany. Int J Clin Pharmacol Ther. 2021;59:188–197. doi: 10.5414/CP203841. [DOI] [PubMed] [Google Scholar]
- 9.Jokanovic N, Tan EC, Sudhakaran S, et al. Pharmacist-led medication review in community settings: an overview of systematic reviews. Res Social Adm Pharm. 2017;13:661–685. doi: 10.1016/j.sapharm.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Bloomfield HE, Greer N, Linsky AM, et al. Deprescribing for community-dwelling older adults: a systematic review and meta-analysis. J Gen Intern Med. 2020;35:3323–3332. doi: 10.1007/s11606-020-06089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller U, Schulz M, Mätzler M. [Electronically supported co-operation of physicians and pharmacists to improve medication safety in the ambulatory setting: The „Arzneimittelinitiative Sachsen-Thüringen“ (ARMIN)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61:1119–1128. doi: 10.1007/s00103-018-2780-5. [DOI] [PubMed] [Google Scholar]
- 12.Öffentlichkeitsarbeit/kbb. Evaluation für das Modellprojekt ARMIN. www.arzneimittelinitiative.de/ueber-armin/aktuelle-nachrichten/2079-evaluation-fuer-das-modellprojekt-armin/ (last accessed on 15 August 2022) [Google Scholar]
- 13.Hardtstock F, Myers D, Li T, et al. Real-world treatment and survival of patients with advanced non-small cell lung cancer: a German retrospective data analysis. BMC Cancer. 2020;20 doi: 10.1186/s12885-020-06738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilke T, Groth A, Fuchs A, Pfannkuche M, Maywald U. Persistence with VKA treatment in newly treated atrial fibrillation patients: an analysis based on a large sample of 38,076 German patients. Eur J Clin Pharmacol. 2017;73:1437–1447. doi: 10.1007/s00228-017-2307-2. [DOI] [PubMed] [Google Scholar]
- 15.Meid AD, Groll A, Heider D, et al. Prediction of drug-related risks Using Clinical Context Information in Longitudinal Claims Data. Value Health. 2018;21:1390–1398. doi: 10.1016/j.jval.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010;107:543–551. doi: 10.3238/arztebl.2010.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vock DM, Wolfson J, Bandyopadhyay S, et al. Adapting machine learning techniques to censored time-to-event health record data: a general-purpose approach using inverse probability of censoring weighting. J Biomed Inform. 2016;61:119–131. doi: 10.1016/j.jbi.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz M, Griese-Mammen N, Anker SD, et al. Pharmacy-based interdisciplinary intervention for patients with chronic heart failure: results of the PHARM-CHF randomized controlled trial. Eur J Heart Fail. 2019;21:1012–1021. doi: 10.1002/ejhf.1503. [DOI] [PubMed] [Google Scholar]
- 19.van der Meer HG, Wouters H, Pont LG, Taxis K. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: a randomised controlled trial. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019042. e019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland R, Brooksby I, Lenaghan E, et al. Effectiveness of visits from community pharmacists for patients with heart failure: HeartMed randomised controlled trial. BMJ. 2007;334 doi: 10.1136/bmj.39164.568183.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care—-the POLYMED randomised controlled trial. Age Ageing. 2007;36:292–297. doi: 10.1093/ageing/afm036. [DOI] [PubMed] [Google Scholar]
- 22.Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoes AW, Leufkens HG. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J Card Fail. 2003;9:404–411. doi: 10.1054/s1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 23.Olesen C, Harbig P, Buus KM, Barat I, Damsgaard EM. Impact of pharmaceutical care on adherence, hospitalisations and mortality in elderly patients. Int J Clin Pharm. 2014;36:163–171. doi: 10.1007/s11096-013-9898-1. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijden A, de Bruijne MC, Nijpels G, Hugtenburg JG. Cost-effectiveness of a clinical medication review in vulnerable older patients at hospital discharge, a randomized controlled trial. Int J Clin Pharm. 2019;41:963–971. doi: 10.1007/s11096-019-00825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George PP, Heng BH, Lim TK, et al. Evaluation of a disease management program for COPD using propensity matched control group. J Thorac Dis. 2016;8:1661–1671. doi: 10.21037/jtd.2016.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirsch F, Becker C, Schramm A, Maier W, Leidl R. Patients with coronary artery disease after acute myocardial infarction: effects of continuous enrollment in a structured Disease Management Program on adherence to guideline-recommended medication, health care expenditures, and survival. Eur J Health Econ. 2020;21:607–619. doi: 10.1007/s10198-020-01158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miksch A, Laux G, Ose D, et al. Is there a survival benefit within a German primary care-based disease management program? Am J Manag Care. 2010;16:49–54. [PubMed] [Google Scholar]
- 28.Drabik A, Büscher G, Thomas K, Graf C, Müller D, Stock S. Patients with type 2 diabetes benefit from primary care-based disease management: a propensity score matched survival time analysis. Popul Health Manag. 2012;15:241–247. doi: 10.1089/pop.2011.0063. [DOI] [PubMed] [Google Scholar]
- 29.De Vera MA, Bhole V, Burns LC, Lacaille D. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014;78:684–698. doi: 10.1111/bcp.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002606. e002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreira ATA, Pinto CR, Lemos ACM, Assunção-Costa L, Souza GS, Martins Netto E. Evidence of the association between adherence to treatment and mortality among patients with COPD monitored at a public disease management program in Brazil. J Bras Pneumol. 2021;48 doi: 10.36416/1806-3756/e20210120. e20210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang CH, Umegaki H, Watanabe Y, et al. Potentially inappropriate medications according to STOPP-J criteria and risks of hospitalization and mortality in elderly patients receiving home-based medical services. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211947. e0211947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly WN, Ho MJ, Bullers K, Klocksieben F, Kumar A. Association of pharmacist counseling with adherence, 30-day readmission, and mortality: a systematic review and meta-analysis of randomized trials. J Am Pharm Assoc (2003) 2021;61 doi: 10.1016/j.japh.2021.01.028. 340-50.e5. [DOI] [PubMed] [Google Scholar]
- 34.Schmiedl S, Rottenkolber M, Szymanski J, et al. Preventable ADRs leading to hospitalization—results of a long-term prospective safety study with 6,427 ADR cases focusing on elderly patients. Expert Opin Drug Saf. 2018;17:125–137. doi: 10.1080/14740338.2018.1415322. [DOI] [PubMed] [Google Scholar]
- 35.Franklin JM, Schneeweiss S. When and how can real world data analyses substitute for randomized controlled trials? Clin Pharmacol Ther. 2017;102:924–933. doi: 10.1002/cpt.857. [DOI] [PubMed] [Google Scholar]
- 36.Serhal S, Mitchell B, Krass I, et al. Rethinking the gold standard—the feasibility of randomized controlled trials within health services effectiveness research. Res Social Adm Pharm. 2022;18:3656–3668. doi: 10.1016/j.sapharm.2022.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R Package for computing e-values. Epidemiology. 2018;29 e45-e7. doi: 10.1097/EDE.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32:2380–2385. doi: 10.1200/JCO.2014.55.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]