Abstract
Background
The authors’ remaining affiliations can be found at the end of the article.
In Germany, each year over 3000 patients with malignant and non-malignant hematologic and systemic diseases are treated by allogeneic hematopoietic cell transplantation (HCT). Genetic donor–recipient disparities, especially those concerning variable human leukocyte antigens (HLA), mediate both an immunotherapeutic effect and the risk of damage to healthy tissues (“graft-versus-host disease”). The adoption of evidence-based strategies for donor selection has been crucial for the continuous improvement of survival rates after allogeneic HCT, with over 50% of patients transplanted for standard indications—such as early-stage acute myeloid leukemia—alive at three years post-transplant.
Methods
The PubMed database was selectively searched for literature on immunogenetic and clinical factors relevant to allogeneic HCT, as part of the process of establishing a German consensus statement on HCT donor selection.
Results
The most important factor in donor selection is a match for the five major HLA loci (HLA-A, -B, -C, -DR, -DQ), either in genetically HLA-identical siblings or in unrelated but fully HLA-compatible donors from international registries. Additional selection criteria for the latter include compatibility for the HLA-DP locus, donor age and sex, cytomegalovirus serostatus, and blood group. Related donors identical for only 50% of the HLA genes (haploidentical donors) as well as unrelated donors with a single HLA mismatch are both valid alternatives although they are associated with an up to 10% higher risk of mortality.
Conclusion
The refinement of donor selection strategies has been instrumental for the continuous improvement of patient survival rates after allogeneic HCT witnessed over the past decades. An interdisciplinary approach to donor selection based on up-to-date scientific evidence is crucial for optimizing patient outcomes.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de.
The deadline for submissions is 13 April 2024.
Allogeneic hematopoietic cell transplantation plays a key role in the treatment of of malignant and benign hematological, immunological, and selected metabolic disorders (e.g., mucopolysaccharidosis type 1). Each year, this form of therapy is used to treat over 3000 patients in Germany and more than 17 000 patients in Europe as a whole (figure 1) (1). Allogeneic hematopoietic cell transplantation is a clinically and biologically complex procedure, by which the hematopoietic system of a genetically distinct healthy individual is engrafted in the patient to be treated (figure 2) (2). In contrast to solid organ transplantation, in which organ replacement is the primary therapeutic objective, allogeneic hematopoietic cell transplantation for hematologic malignancies is aimed at immune control of the underlying neoplastic disease. This process is referred to as graft versus tumor reaction or graft versus leukemia (GvL) effect. Acute myeloid leukemia (AML) is the most frequent (40.3%) indication for allogeneic hematopoietic cell transplantation in Germany (1). An important documentation of the GvL effect was provided by a matched-pair analysis (3), which showed significant improvement of the 7-year relapse-free survival of patients with AML in first complete remission from 33% when treated by conventional chemotherapy alone to 52% after allogeneic hematopoietic cell transplantation (3). In contrast, survival rates decrease to around 30% in patients with AML who undergo transplantation at an advanced stage of disease (i.e., after two or more previous complete remissions) (table 1) (4). This underlines the importance for the primary physician to inform patients who could benefit from allogeneic hematopoietic cell transplantation about this possible form of treatment and to establish contacts with a specialized center as appropriate. This is also wise in light of the fact that survival rates of AML patients after allogeneic hematopoietic cell transplantation have improved from around 20% to over 50% in the past two decades (table 1) (4, 5).
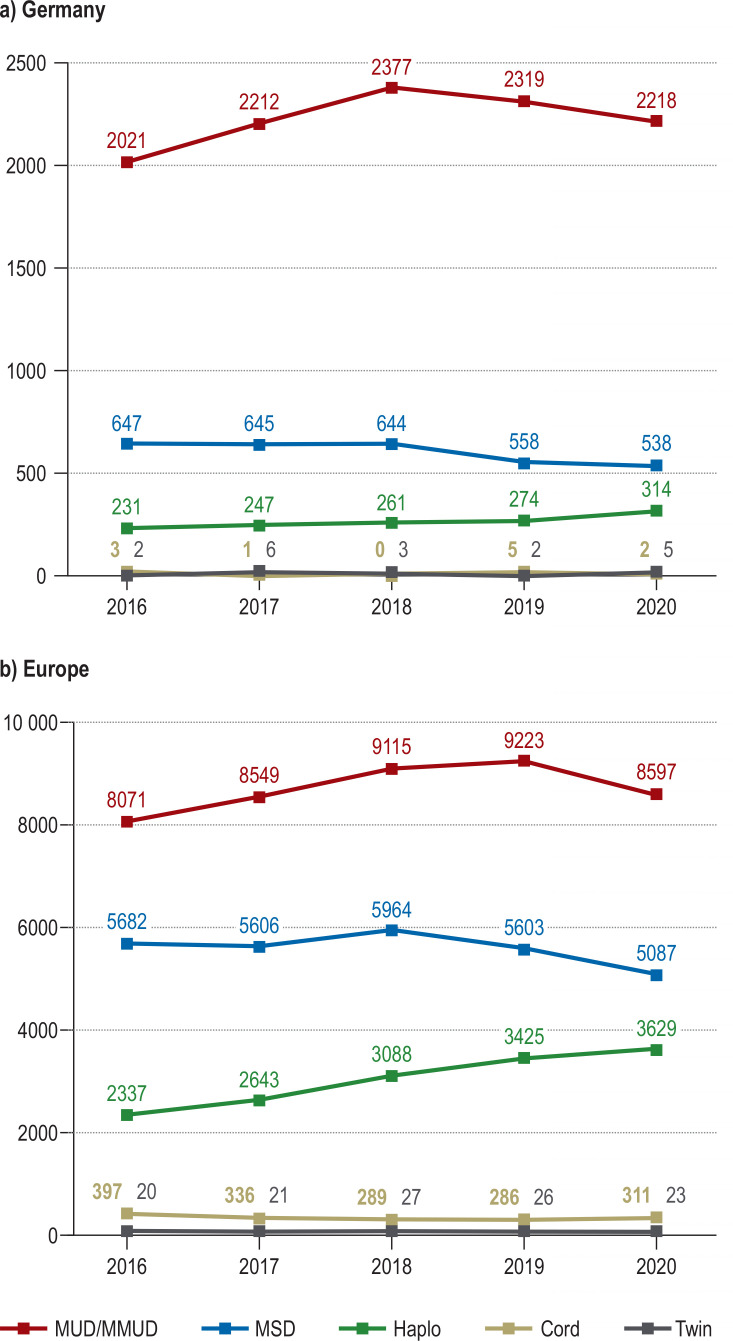
Figure 1.
Allogeneic hematopoietic cell transplant activity in Germany and in Europe by donor type. Shown are the numbers of reported first allogeneic hematopoietic cell transplants (not including second transplants) for adult and pediatric patients between 2016 and 2020.
a) Transplant activity in Germany, amounting to a total of 15 535 transplants in the past 5 years and 3 107 annual transplants on average
b) Transplant activity in Europe (including Germany), amounting to a total of 88 355 transplants in the past 5 years and 17 671 annual transplants on average
MUD/MMUD, Matched unrelated donor/mismatched unrelated donor, i.e., HLA-compatible or HLA-incompatible unrelated donor; MSD, matched sibling donor, i.e., HLA-identical sibling; Haplo, haploidentical related donor; Cord, umbilical cord blood donor; Twin, monozygotic twin donor
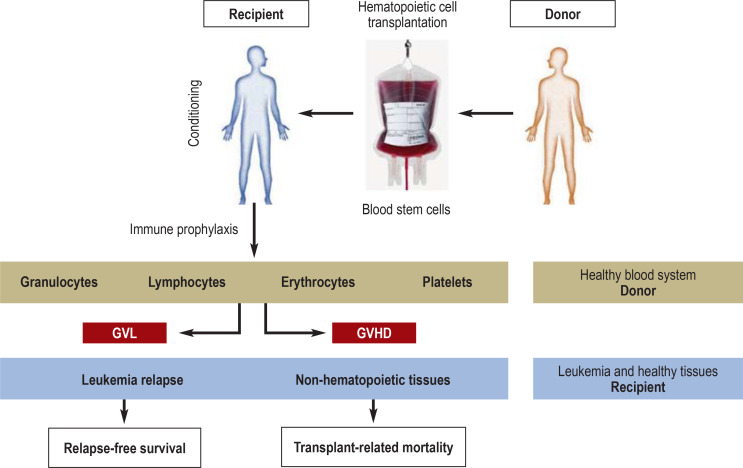
Figure 2.
The principle of allogeneic hematopoietic cell transplantation. The procedure aims at eradicating the underlying hematologic malignancy along with replacement of the patient‘s (blue) hematopoietic system by that of a healthy, genetically different donor (brown). The transplanted hematopoietic system therefore carries the genetic traits of the donor, while all other tissues (non-hematopoietic tissues and any residual leukemia cells) carry the genetic characteristics of the recipient. The recipient undergoes so-called pretransplant conditioning by chemotherapy with or without total-body irradiation, followed by pharmacological immune prophylaxis for days or weeks after transplantation. Donor immune cells, in particular T lymphocytes, can recognize patient-specific major and/or minor histocompatibility antigens, thereby mediating an anti-tumor effect (graft versus leukemia, GvL) and/or damage to healthy tissues, in particular skin, liver, or gut (graft versus host disease, GvHD). The balance between GvL and GvHD is crucial for relapse-free survival and transplant-related mortality.
Table 1. Survival rates of adult patients with acute myeloid leukemia following allogeneic hematopoietic cell transplantation by donor type.
| Disease stage at time of hematopoietic cell transplantation*1 | |||
| Donor type*2 | First remission | Second remission | No remission/recurrence |
| HLA-identical sibling | 58% (N = 5 317) | 54% (N = 1 226) | 31% (N = 1 721) |
| HLA-compatible unrelated donor | 56% (N = 7 441) | 54% (N = 1 940) | 31% (N = 2 463) |
| Partially HLA-compatible unrelated donor | 50% (N = 1 314) | 42% (N = 5 077) | 26% (N = 549) |
| Haploidentical related donor | 53% (N = 572) | 55% (N = 1 977) | 28% (N = 706) |
*1 Hematopoietic cell transplants performed between 2009 and 2019 at centers in the USA, predominantly under GvHD prophylaxis with calcineurin inhibitors (4). Data expressed as Kaplan–Meier probabilities of 3-year survival, followed in parentheses by the number of patients in the group. *2 Donor types according to Table 2. GvHD, Graft versus host disease; HLA, human leukocyte antigens
The principal targets of the GvL effect are genetically variable histocompatibility antigens, in particular human leukocyte antigen (HLA) molecules (6). However, the desired therapeutic GvL effect can be opposed by off-target activity against non-hematopoietic tissue structures, both mediated by donor T lymphocytes, clinically resulting in graft versus host disease (GvHD) (figure 2). GvHD is responsible for up to 15% of mortality after transplantation (4). Moreover, especially the chronic form of GvHD can severely limit the quality of life of the patients affected (7).
The strategy of donor selection plays a central role in minimizing the risk of GvHD and best exploiting GvL. Donation of hematopoietic cells from bone marrow or growth-hormone mobilized peripheral blood involves no organ loss and is generally tolerated well (8). This is a fundamental difference to the donation of solid organs. Since the pool of hematopoietic cell donors is much larger than that of organ donors—comprising the patient’s family members or unrelated donors from (inter)national registries that currently embrace over 40 million volunteers—a compatible donor can be found for the majority of patients (table 2). Since the legal eligibility criteria for stem cell donors are very similar to those for blood donors, primary care physicians have a potentially important role to play in conveying information regarding stem cell donation to relevant target groups.
Table 2. Donor types for allogeneic hematopoietic cell transplantation by HLA status.
| Related donors | Unrelated donors | |||||
| Monozygotic twins | HLA-identical siblings | Haploidentical family | HLA-compatible | Partially HLA-compatible | Umbilical cord blood | |
| Major antigens | 0 | 0 | ≤ 6 | 0 | 1–5 | 0–5 |
| Minor antigens | None | + | + | ++ | ++ | ++ |
| Probability | < 1% | 25% | > 90% | 75% | > 90% | > 90% |
Unrelated donors: Stem cell donors who are not family members; haploidentical family: difference for an entire HLA haplotype, i.e., for one of the two chromosomes carrying the six HLA loci (HLA-A, -B, -C, -DR, -DQ, -DP); HLA-compatible: shown is the possible number of differences for the five most relevant HLA loci (HLA-A, -B, -C, -DR, -DQ); in around 80% of cases there is also a difference for one or two HLA-DP alleles (21); major antigens: total number of HLA differences; minor antigens: polymporphic peptides encoded by variable genes different in donor and recipient (11);their number is lower in related (+) than in unrelated (++) donors owing to the greater genetic similarity between related than between unrelated individuals. Probability: the likelihood of identifying at least one of the respective types of donors for a patient in Germany; the percentages are based on the experience of German transplant centers and may vary according to ethnicity; HLA, human leukocyte antigens
Recent years have seen the development of modern forms of immunosuppression and ex-vivo manipulation of hematopoietic cell products (e.g., selective removal of potentially toxic immune cell populations). This has made it possible to include also haploidentical family members (e.g., parents or biological children) into the donor pool. Haploidentical donors share with the patient only one of the two chromosomes bearing the HLA characteristics (table 2). Since one or more haploidentical family members can be found for nearly all patients, they are increasingly being used as donors across the world (including Germany) (figure 1) (1, 4, 9).
The complex associations between donor characteristics and transplantation outcomes are partly controversial, but of important clinical relevance. However, randomized controlled trials (RCT) on donor selection are the exception. On the one hand, the statistical power required to appreciate the frequently small effect sizes is impractically large for RCT. On the other hand, donor characteristics are multifactorial, making it unrealistic to randomize for specific factors. For these reasons, our current knowledge is based predominantly on retrospective studies, the principal findings of which are presented in this review article. These data also form the basis for a large-scale consensus statement on stem cell donor selection (10– 12).
Immunogenetic criteria for stem cell donor selection
Definition and diagnosis of histocompatibility
The HLA system plays a key role for histocompatibility in allogeneic hematopoietic cell transplantation. To date, 35 820 different variants at the six loci HLA-A, -B, -C, -DR, -DQ, and -DP have been described (13). Most of the amino acid variation of HLA molecules is located in the so-called antigen-binding pocket, which binds peptide fragments of intra- and extracellular proteins and presents them to the immune system. Peptides encoded by interindividually variable genes represent the “minor histocompatibility antigens” (14). In contrast, “major histocompatibility antigens” are variable amino acids in the HLA molecules (6). Both minor and major antigens are targeted by donor immune cells after allogeneic hematopoietic cell transplantation, which can give rise to the above-mentioned GvL and GvHD effects. Variable HLA molecules can also be detected by antibodies that may develop as a result of immunization, e.g., in the context of pregnancy or blood transfusion. If these antibodies are directed against the HLA types of the donor, they are called donor-specific antibodies (DSA) (15). DSA play a role especially in solid organ transplantation, due to the usually incomplete HLA compatibility between donor and recipient. Now that hematopoietic cell donors with HLA differences are increasingly being used, DSA should also be avoided in allogeneic hematopoietic cell transplantation from donors with HLA differences.
Laboratory tests before transplantation are mainly focused on HLA typing of donor and recipient and on determination of the patient’s DSA. So-called high-resolution HLA typing, i.e., the unambiguous DNA sequencing of at least those HLA gene regions that code for the variable antigen-binding pocket, is nowadays based mainly on high-throughput sequencing methods (16). Likewise, the characterization of DSA can now be accomplished by semi-automated procedures with high sensitivity and specificity (15). HLA typing and DSA detection must be carried out in an HLA laboratory accredited by one of the international societies of immunogenetics. This laboratory also establishes the degree of compatibility between the patient and potential family donors (table 1). The likelihood that two siblings are HLA-identical (i.e., share both parental haplotypes), HLA-haploidentical (i.e., share only one of the two parental haplotypes), or HLA-diverse (i.e., share neither of the two parental haplotypes) is 25%, 50%, and 25%, respectively. Alternatively or in parallel, a worldwide search for an HLA-compatibile unrelated donor (i.e., a donor identical for at least the HLA gene regions encoding the variable antigen-binding pocket) will be facilitated through the German National Bone Marrow Donor Registry (Zentrales Knochenmarkspenderregister, ZKRD) (17).
The relative proportion of transplantations from unrelated donors compared with family donors has been considerably higher in Germany than in Europe as a whole over the past 5 years (71.8% versus 49.3%; Figure 1). Possible reasons for this include the high proportion of German donors in the global pool (17) and the comparably low number of children per family in Germany (18). Accordingly, the relative percentage of transplantations from HLA-identical siblings and from haploidentical family donors was lower in Germany (19.5% and 8.5%, respectively) than in Europe as a whole (31.6% and 17.1%). Nevertheless, the use of haploidentical related donors has witnessed a significant increase over recent years also in Germany, whereas the relevance of HLA-compatible umbilical cord blood as the source of hematopoietic cells is negligible in this country (figure 1) (1).
Relevance of HLA compatibility in allogeneic hematopoietic cell transplantation
In allogeneic hematopoietic cell transplants performed for hematological malignancies, the main objective is efficient immune control of the underlying disease by GvL, with concomitantly low collateral damage to healthy tissues by GvHD (figure 2). This is best achieved by limited HLA differences between donor and recipient. For instance, it has been shown that hematopoietic cell transplantation between monozygotic, genetically identical twins who differ neither in major nor in minor histocompatibility antigens (table 2) and do not develop GvHD is associated with an up to three times higher risk of leukemia relapse than transplantation from HLA-identical siblings (19). HLA disparity, however, increases also the risk of GvHD or graft rejection. Nevertheless, the disadvantages of HLA disparity can be compensated to a certain extent by means of appropriate strategies for immune suppression. When classical calcineurin-based platforms of immune prophylaxis are used, the best results are achieved with donors fully compatible for eight out of eight alleles at the four loci HLA-A, -B, -C, and -DR. The overall mortality risk, i.e., death from any cause including relapse, infection, multiorgan failure, GvHD, and rejection, increases by around 10% with each incompatible HLA allele (20– 22). In contrast, HLA-DQ mismatches play a less prominent role, although they were also associated with increased mortality in a meta-analysis (23). As for HLA-DP, so-called permissive, i.e., clinically tolerated mismatches that have been identified over recent years appear to be associated with a favorable balance between GvL and GvHD, and therefore have become established as a secondary criterion for donor selection (24, 25).
The past decade has seen the development of a new platform of immune prophylaxis based on post-transplant cyclophosphamide (PTCy), allowing for successful transplantation also across major HLA barriers (26). The mechanism of action relies on functional suppression and elimination of donor immune cells activated early after transplantation (26). A retrospective study showed only slightly inferior 3-year overall survival of AML patients after PTCy-based haploidentical transplantation than after calcineurin inhibitor-based HLA-identical sibling transplantation (48% versus 55%) (27). Likewise, another study comparing transplants from different donors under uniform PTCy-based immune prophylaxis showed 2-year overall survival rates of 54% for haploidentical donors, approximately in the range expected for allogeneic hematopoietic cell transplantation (table 1), which were even higher (67%) for HLA-compatible unrelated donors (28). These data suggest that the use of PTCy might also improve the outcome of HLA-compatible unrelated donor transplantation. This important question is being investigated in ongoing RCT, the results of which are to be awaited.
The encouraging results reported for PtCy immune prophylaxis in haploidentical hematopoietic cell transplantation explain the increasing clinical use of this transplant modality in Germany and other countries (figure 1) (9). On the same line, PtCy is increasingly being used as platform for partially HLA-compatible unrelated donor transplantation (4). In the case of HLA disparities, donors that can be targeted by patient DSA should be avoided owing to the elevated risk of delayed engraftment or graft failure (29). If this is impossible, appropriate desensitization procedures, e.g., plasmapheresis, should be considered prior to transplantation. Since the recipient’s immune system (including the antibody-forming cells) is replaced by that of the donor (figure 2), continued desensitization is usually unnecessary.
Clinical criteria for the selection of stem cell donors
Donor age
After HLA compatibility and DSA, the next most important factor impacting overall survival in unrelated donor transplantation is donor age (30, 31). Overall survival improves by 3% for each decade of decrease in donor age. In contrast, donor age is less considered for the selection of sibling donors, given that donor and patient age are mostly similar anyway in this setting. The results of two large registry studies suggest that in elderly patients it may even be advantageous to use young unrelated donors in preference to HLA-identical siblings (32, 33).
Haploidentical donors can be family members of different age groups, i.e., parents, siblings, or children of the patient (table 2). The current state of knowledge indicates that at least in patients > 40 years of age, higher donor age increases the risk of post-transplant mortality (34). In contrast, donor age plays a less prominent role in pediatric haploidentical hematopoietic cell transplantation, since here the relatively young parents are generally the first to be considered as donors. In pediatric transplantation from HLA-identical siblings, a donor beyond the age of majority is preferable both on legal and on ethical grounds. For similar reasons, an adolescent capable of agreement or consent should be preferred to under-age siblings. Moreover, donor weight can be an issue in pediatric allogeneic hematopoietic cell transplantation, and the possibility to harvest an adequate amount of cells from an under-age donor should be ascertained a priori in this setting.
Other clinical parameters
If there are only slight differences in age among the available HLA-identical or HLA-compatible donors without DSA, other clinical criteria can be considered, although they cannot be ranked due to the lack of conclusive evidence. For instance, male donors should be preferred for male patients in order to avoid GvHD reactivity of female donor lymphocytes against antigens encoded by the Y chromosome. The donor serostatus for cytomegalovirus (CMV) is relevant especially for seronegative patients, for whom a seronegative donor should be preferred to avoid CMV transmission and the associated risks (35). Conversely, a CMV-seropositive donor should be selected for a CMV-seropositive recipient (36). The extent to which these donor preferences are affected by modern methods of pharmacological CMV prophylaxis has not yet been established. Viral reactivations other than from CMV can also be clinically relevant, especially after HLA-mismatched transplantation. The viral serostatus therefore represents an additional clinically relevant selection criterion, especially for haploidentical transplantation (37). This also preserves the option of transferring virus-specific immune cells following transplantation (38). Finally, a number of studies have found blood group differences to be associated with slightly elevated risks of mortality and GvHD (30), although the effect is much less pronounced than the HLA-associated effects. Owing to these findings and the existence of other risks following hematopoietic cell transplantation with ABO-incompatible donors (particularly hemolysis and erythrocyte aplasia), it is recommended to select a tolerable ABO constellation between donor and recipient. If no ABO-major-compatible donor is available, bone marrow should be avoided as stem cell source for adult patients, because the erythrocyte depletion necessary in these circumstances can have a negative impact on the stem cell dose (39). ABO compatibility, however, is secondary to HLA compatibility and absence of DSA, donor age (the primary factor after HLA), HLA-DP, donor sex (for male patients), and CMV serostatus, with no specific ranking among the latter three.
Selection of hematopoietic stem cell donors
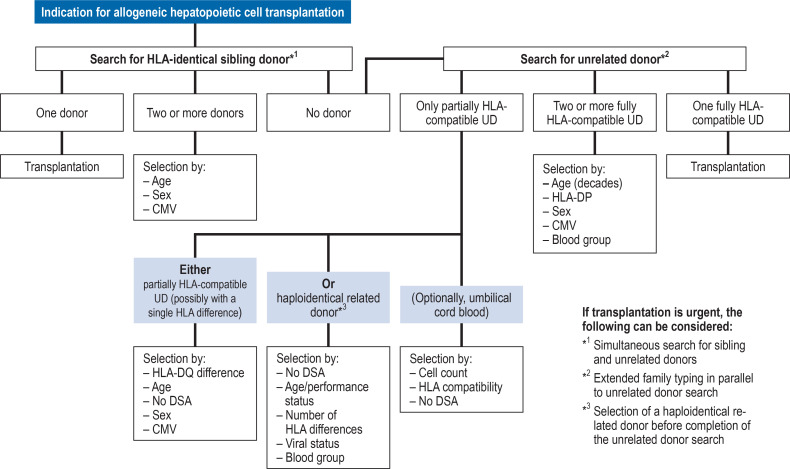
A flow chart was created to describe the guidelines for stem cell donor selection based on the data presented above (10– 12) (figure 3). HLA-identical siblings are the stem cell donors of choice. If none are available, a search for an unrelated donor or an alternative related donor should be initiated. The likelihood of identifying an HLA-compatible unrelated donor varies according to the genetic background of the recipient (40). If two ore more unrelated donors compatible for the five relevant HLA loci are available, donor age, HLA-DP, donor sex, CMV serostatus, and blood group should be considered. If no unrelated donor fully compatible for all five HLA loci can be found, partially HLA-compatible unrelated donors can be used. In this case, donors with a single HLA difference, ideally for HLA-DQ, should be preferred. At the same time, the availability of a haploidentical related donor should be investigated. The decision whether to use a partially HLA-compatible unrelated donor or a haploidentical related donor depends on the patient’s individual circumstances and the experience of the transplant center. In both cases the recipient must be tested for the presence of DSA to the donor’s incompatible HLA.
Figure 3.
Flow chart for stem cell donor selection. The chart shows the currently employed modus operandi, corresponding to the state of knowledge explained and referenced in the text, which formed the basis of a larger-scale consensus statement on stem cell donor selection (10– 12). Factors marked in red should be considered with priority; the remaining factors are listed in random order. CMV, Cytomegalovirus; DSA, donor-specific antibodies; HLA, human leukocyte antigen; UD, unrelated donor
Perspective
In view of the rapid pace of development in allogeneic hematopoietic cell transplantation, including both novel immunosuppressive strategies for GvHD prophylaxis and the dynamic progress in immunogenetics, this review article is only a snapshot of the current situation. Continuous adjustment will be necessary as new evidence emerges. Furthermore, other forms of adoptive cell therapies beyond allogeneic hematopoietic cell transplantation are increasingly being adopted clinically. These types of treatments will also have to be considered in future updates of our state of knowledge.
The authors’ remaining affiliations
Pediatric Working Group for Hematopoietic Cell Transplantation and Cell Therapy (PAS&ZT), Frankfurt am Main, and Pediatric Hematopoietic Cell Therapy, Department of Pediatric Oncology, Pediatric Hematology, and Clinical Immunology, University Hospital Düsseldorf: Prof. Dr. med. Roland Meisel
German National Bone Marrow Donor Registry, Ulm: PD Dr. med. Joannis Mytilineos
German Working Group for Hematopoietic Stem Cell Transplantation und Cellular Therapy e.V. (DAG-HSZT), Hamburg: Prof. Dr. med. Peter Dreger, Prof. Dr. med. Dr. h.c. Nicolaus Kröger
Division of Hematopoietic Stem Cell Transplantation, Department of Medicine V, University Hospital Heidelberg: Prof. Dr. med. Peter Dreger
Department of Hematopoietic Stem Cell Transplantation, University Hospital Hamburg-Eppendorf: Prof. Dr. med. Dr. h.c. Nicolaus Kröger
Funding
This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft; DFG FL 843/1–1), the German José Carreras Leukemia Foundation (DJCLS 20R/2019 and DJCLS 11 R/2021), the Dr. Werner Jackstädt Foundation, and the Joseph Senker Foundation to KF.
Questions on the article in issue 15/2023: Donor Selection for Allogeneic Hematopoietic Cell Transplantation.
The submission deadline is 13 April 2024. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Approximately how many hematopoietic cell transplants are performed each year in Germany?
1000
3000
5000
7000
9000
Question 2
What does the acronym GvL stand for in this article?
Graft versus lymphocytes
Graft versus lesion
Graft versus lymphoma
Graft versus leukemia
Graft versus lymphosarcoma
Question 3
Which of the following diseases is the most common indication for allogeneic hematopoietic cell transplantation in Germany?
Chronic myeloid leukemia
Hodgkin lymphoma
Non-Hodgkin lymphoma
Smoldering myeloma
Acute myeloid leukemia
Question 4
Approximately how high is the 3-year survival rate for patients with acute myeloid leukemia in early stage (first remission) after hematopoietic cell transplantation?
20%
30%
50%
70%
80%
Question 5
Which donor cells predominantly mediate graft versus host disease?
Erythrocytes
Granulocytes
B Lymphocytes
T Lymphocytes
Plasma cells
Question 6
After human leukocyte antigens (HLA) and donor-specific antibodies (DSA), which of the following clinical parameters of the donor is most important for overall survival following hematopoietic cell transplantation?
Cytomegalovirus serostatus
Viral status
Blood group
Sex
Age
Question 7
Which of the following HLA gene loci is least important for the choice of donor?
HLA-A
HLA-B
HLA-DR
HLA-DP
HLA-C
Question 8
How many HLA gene loci should be considered in the selection of a donor for allogeneic hematopoietic cell transplantation?
Two
Three
Six
Eight
Twelve
Question 9
What is the likelihood that two siblings are HLA-identical, i.e., that they have inherited the same haplotype from each of their two parents?
10%
25%
33%
50%
75%
Question 10
Which of the following terms is used to describe sharing of one HLA haplotype but not the other between a patient and a related donor?
HLA-identical
HLA-heteroidentical
HLA-homozygotic
HLA-haploidentical
HLA-heterozygotic
Acknowledgments
Translated from the original German by David Roseveare
Acknowledgments
We are grateful to all members of the three working groups who jointly collected and discussed the evidence summarized here, which resulted in a large-scale consensus paper on stem cell donor selection. For the German Immunogenetics Society (DGI): Dr. Esteban Arrieta-Bolaños (Essen), PD Dr. Francis Ayuk (Hamburg), PD Dr. Daniel Fürst (Ulm), Dr. Monika Füssel (Dresden), Prof. Peter A. Horn (Essen); for the German Working Group for Hematopoietic Stem Cell Transplantation und Cellular Therapy (DAG-HSZT): Prof. Wolfgang Bethge (Tübingen), Prof. Martin Bornhäuser (Dresden), Prof. Guido Kobbe (Düsseldorf), PD Dr. Hellmut Ottinger (Essen), Prof. Johannes Schetelig (Dresden), Prof. Matthias Stelljes (Münster), Dr. Eva Wagner-Drouet (Mainz), Prof. Robert Zeiser (Freiburg); for the Pediatric Working Group for Hematopoietic Cell Transplantation and Cell Therapy (PAS&ZT): Prof. Matthias Eyrich (Würzburg), Prof. Peter Lang (Tübingen).
Footnotes
Conflict of interest statement
KF has received payments for consultancy from the DKMS and for reviewing research grants submitted to the Wilhelm Sander Foundation. She is deputy chair of the German Registry for Hematopoietic Cell Tranplantation (DRST). She has received reimbursement of travel and accommodation costs as invited speaker at the respective conferences of the American Society for Hematology (ASH) and the Center for International Blood and Marrow Transplant Research (CIBMTR). She has received research support via third-party funding through the Joseph Senker Foundation.
THT is one of the three representatives of the German search teams for unrelated stem cell donors.
RM has received payments for consultancy from Bellicum Pharmaceuticals, Bluebird Bio, Celgene/Bristol-Myers Squibb, Medac, Novartis, and Vertex; for lectures from Vertex; and for the conduct of commissioned clinical trials (via third-party funding) by Crispr Therapeutics, Vertex, Novartis, Miltenyi Biotec, Celgene/Bristol-Myers Squibb, and Kite/Gilead. He is a committee member of the Pediatric Diseases Working Party (PDWP) of the European Bone Marrow Transplantion Group (EBMT).
JM has received funding from the DFG, the Else Kröner Foundation, the osé Carreras Leukemia Foundation, and the Sander Foundation.
PD is spokesperson of the German Working Group for Hematopoietic Stem Cell Transplantation and Cellular Therapy e.V. (DAG-HSZT), member of the advisory board of the German Society of Hematology and Medical Oncology (DGHO), board member of the DRST, and member of the Permanent Commission “Guidelines for Production and Use of Hematopoietic Cell Preparations” (period of office 2022–2025) of the Scientific Advisory Committee of the German Medical Association.
NK has received payments for consultancy from the DKMS (e.g., for supporting a study) and as grant reviewer for the DFG. He has received reimbursement of congress attendance fees from EBMT, ASH, ASTCT, and WMDA. and of travel costs from ZKRD and WMDA. He chairs the DRST and is a former president (up to March 2022) of the EBMT.
References
- 1.Deutsches Register für Stammzelltransplantationen e. V. Jahresbericht. www.drst.de/drst/download/jb2021.pdf (last accessed on 2 March 2023) 2021 [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 3.Stelljes M, Krug U, Beelen DW, et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol. 2014;32:288–296. doi: 10.1200/JCO.2013.50.5768. [DOI] [PubMed] [Google Scholar]
- 4.Auletta JJ, Kou J, Chen M, Shaw BE. Current use and outcome of hematopoietic stem cell transplantation. CIBMTR US summary slides. 2021 [Google Scholar]
- 5.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersdorf EW. Optimal HLA matching in hematopoietic cell transplantation. Curr Opin Immunol. 2008;20:588–593. doi: 10.1016/j.coi.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377:2565–2579. doi: 10.1056/NEJMra1703472. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt AH, Mengling T, Hernandez-Frederick CJ, et al. Retrospective analysis of 37,287 observation years after peripheral blood stem cell donation. Biol Blood Marrow Transplant. 2017;23:1011–1020. doi: 10.1016/j.bbmt.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Passweg JR, Baldomero H, Chabannon C, et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transplant. 2022;57:742–752. doi: 10.1038/s41409-022-01604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutsche Gesellschaft für Immungenetik (DGI) www.immungenetik.de/index.php/vorstand/docman2/oeffentlich/dgi-empfehlungen/1220-konsensus-stammzellspenderauswahl-2021/file (last accessed on 2 March 2023) [Google Scholar]
- 11.Deutsche Arbeitsgemeinschaft für Hämatopoetische Stammzelltransplantation und Zelluläre Therapien (DAG-HSZT) www.dag-kbt.de/files/downloads/Konsensus%20Stammzellspenderauswahl%20_2021_09_29_final%20exp.pdf (last accessed on 2 March 2023) [Google Scholar]
- 12.Deutsche Arbeitsgemeinschaft für Pädiatrische Stammzelltransplantation und Zelltherapie PAS&ZT) www.gpoh.de/sites/gpoh/content/e6/e13/e53/e264450/KonsensusStammzellspenderauswahl_2021_09_29_finalexp.pdf (last accessed on 2 March 2023) [Google Scholar]
- 13.Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh SG. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 2015;43:D423–D431. doi: 10.1093/nar/gku1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spierings E. Minor histocompatibility antigens: past, present, and future. Tissue Antigens. 2014;84:374–360. doi: 10.1111/tan.12445. [DOI] [PubMed] [Google Scholar]
- 15.Gladstone DE, Bettinotti MP. HLA donor-specific antibodies in allogeneic hematopoietic stem cell transplantation: challenges and opportunities. Hematology Am Soc Hematol Educ Program. 2017;2017:645–650. doi: 10.1182/asheducation-2017.1.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxter-Lowe LA. The changing landscape of HLA typing: understanding how and when HLA typing data can be used with confidence from bench to bedside. Hum Immunol. 2021;82:466–477. doi: 10.1016/j.humimm.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Association WMDA. www.wmda.info (last accessed on 2 March 2023) 2022 [Google Scholar]
- 18.Fowid. Geburtenraten in Europa. https://fowid.de/meldung/geburtenraten-europa-node3332 (last accessed on 2 March 2023) [Google Scholar]
- 19.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 20.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 21.Fürst D, Müller C, Vucinic V, et al. High-resolution HLA matching in hematopoietic stem cell transplantation: a retrospective collaborative analysis. Blood. 2013;122:3220–3229. doi: 10.1182/blood-2013-02-482547. [DOI] [PubMed] [Google Scholar]
- 22.Ayuk F, Beelen DW, Bornhäuser M, et al. Relative impact of HLA matching and Non-HLA donor characteristics on outcomes of allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2018;24:2558–2567. doi: 10.1016/j.bbmt.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Tie R, Zhang T, Yang B, et al. Clinical implications of HLA locus mismatching in unrelated donor hematopoietic cell transplantation: a meta-analysis. Oncotarget. 2017;8:27645–27660. doi: 10.18632/oncotarget.15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleischhauer K, Shaw BE. HLA-DP in unrelated hematopoietic cell transplantation revisited: challenges and opportunities. Blood. 2017;130:1089–1096. doi: 10.1182/blood-2017-03-742346. [DOI] [PubMed] [Google Scholar]
- 25.Dehn J, Spellman S, Hurley CK, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood. 2019;134:924–934. doi: 10.1182/blood.2019001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13:10–24. doi: 10.1038/nrclinonc.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashidi A, Hamadani M, Zhang MJ, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019;3:1826–1836. doi: 10.1182/bloodadvances.2019000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gooptu M, Romee R, St Martin A, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood. 2021;138:273–282. doi: 10.1182/blood.2021011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gladstone DE, Zachary AA, Fuchs EJ, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19:647–652. doi: 10.1016/j.bbmt.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127:260–267. doi: 10.1182/blood-2015-08-663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw BE, Logan BR, Spellman SR, et al. Development of an unrelated donor selection score predictive of survival after HCT: donor age matters most. Biol Blood Marrow Transplant. 2018;24:1049–1056. doi: 10.1016/j.bbmt.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kröger N, Zabelina T, de Wreede L, et al. Allogeneic stem cell transplantation for older advanced MDS patients: improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia. 2013;27:604–609. doi: 10.1038/leu.2012.210. [DOI] [PubMed] [Google Scholar]
- 33.Guru Murthy GS, Kim S, Hu ZH, et al. Relapse and disease-free survival in patients with myelodysplastic syndrome undergoing allogeneic hematopoietic cell transplantation using older matched sibling donors vs younger matched unrelated donors. JAMA Oncol. 2022;8:404–411. doi: 10.1001/jamaoncol.2021.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canaani J, Savani BN, Labopin M, et al. Donor age determines outcome in acute leukemia patients over 40 undergoing haploidentical hematopoietic cell transplantation. Am J Hematol. 2018;93:246–253. doi: 10.1002/ajh.24963. [DOI] [PubMed] [Google Scholar]
- 35.Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59:473–481. doi: 10.1093/cid/ciu364. [DOI] [PubMed] [Google Scholar]
- 36.Kalra A, Williamson T, Daly A, et al. Impact of donor and recipient cytomegalovirus serostatus on outcomes of antithymocyte globulin-conditioned hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:1654–1663. doi: 10.1016/j.bbmt.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 37.McCurdy SR, Fuchs EJ. Selecting the best haploidentical donor. Semin Hematol. 2016;53:246–251. doi: 10.1053/j.seminhematol.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Gössling KL, Fouz H, Kyrillopoulou O, et al. Clearance of treatment refractory adenoviremia via adenovirus-specific donor t-cell transfer during aplasia after alphabetaTCR-CD19-depleted stem cell transplantation. Clin Infect Dis. 2019;68:1406–1409. doi: 10.1093/cid/ciy820. [DOI] [PubMed] [Google Scholar]
- 39.Logan AC, Wang Z, Alimoghaddam K, et al. ABO mismatch is associated with increased nonrelapse mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:746–754. doi: 10.1016/j.bbmt.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the US. registry. N Engl J Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]