Abstract
Background
Obesity is an increasing problem worldwide. The effective treatments for obesity mainly include diet, physical activity, behavioral intervention, pharmacotherapy, and bariatric surgery, which all have certain limitations. As a specific type of acupuncture therapy, acupoint catgut embedding (ACE) has gained substantial attention in the management of obesity in recent years. Previous studies suggested that ACE may be an effective obesity treatment. However, the evidence for the efficacy of ACE in abdominal obesity (AO) remains inadequate due to the paucity of high-quality studies.
Objective
This study aims to investigate the difference in the effectiveness of catgut embedding at acupoints and catgut embedding at nonacupoints in patients with AO and to further validate the efficacy and safety of ACE for AO.
Methods
This is a multicenter, double-blind, 16-week randomized controlled trial. A total of 92 eligible participants with AO will be randomly divided into 2 groups (1:1 allocation ratio). The ACE group will receive catgut embedding at acupoints and the control group will receive catgut embedding at nonacupoints. The intervention will be performed every 2 weeks for a total of 6 sessions. Follow-up will be performed every 2 weeks for a total of 2 visits. The primary outcome is waist circumference. Secondary outcomes include body weight, BMI, hip circumference, and the visual analog scale of appetite. Upon the completion of the trial, we will evaluate the effect of catgut embedding at acupoints or nonacupoints on obesity indicators in patients with AO. For treatment outcomes, an intention-to-treat analysis will be performed.
Results
The start of recruitment began in August 2019 and is expected to end in September 2023.
Conclusions
Although studies have been conducted to demonstrate the effectiveness of ACE in the treatment of obesity, the evidence for the efficacy of ACE in AO remains insufficient due to the quality of the studies. This rigorous normative randomized controlled trial will verify the effect of catgut embedding at acupoints or nonacupoints in patients with AO. The findings will provide credible evidence as to whether ACE is an effective and safe treatment for AO.
Trial Registration
Chinese Clinical Trial Registry ChiCTR1800016947; https://tinyurl.com/2p82257p
International Registered Report Identifier (IRRID)
DERR1-10.2196/46863
Keywords: acupoint catgut embedding, nonacupoints, abdominal obesity, randomized controlled trial, protocol, waist circumference
Introduction
Obesity has become a global health issue regardless of race, region, and age [1]. Globally, the prevalence of obesity in adults was approximately 13% (603.7 million). There were approximately 107.7 million children and adolescents who were obese [2,3]. The prevalence of obesity was about 20% or more in the United States, the Middle East and North Africa, the Caribbean, Polynesia, and Micronesia [4]. The increase of adults who are obese has leveled off in several high-income countries, but the incidence is dramatically on the rise in most low- and middle-income countries, particularly in urban regions [1,5]. As a major type of obesity, the prevalence of abdominal obesity (AO) in adults was 29.1% (28.6% in men and 29.6% in women) in China [6]. Obesity is a worldwide problem and a burden in many diseases [2,7,8]. Studies have demonstrated that obesity may lead to diabetes, subfertility, chronic spontaneous urticaria, nonalcoholic fatty liver disease, Alzheimer disease, cardiovascular diseases, and even cancer [2,9-18]. In addition to diseases, obesity can also affect disability-adjustment life-years and generate a large economic burden [4,19-21]. Obesity is also a persistent problem, where most children who are obese will remain obese in adolescence [22].
The effective treatments for obesity mainly include diet, physical activity, behavioral intervention, pharmacotherapy, and bariatric surgery [23-29]. Diet and physical activity require great self-discipline and enough time, which are difficult to adhere to. The effect of behavioral intervention for obesity is typically limited [30]. Side effects such as abdominal pain, vomiting, nervousness, insomnia, headache, dizziness, dysgeusia, and insomnia may accompany pharmacotherapy [31]. Pharmacotherapy could not be used for patients who are obese with complicated active cardiovascular disease, glaucoma, uncontrolled hypertension, anorexia, thyroid, and many other diseases [32,33]. For pregnant individuals and children, the safety of pharmacotherapy is still not guaranteed currently [34]. Bariatric surgery requires a high level of medical technology and may increase the risk of fractures [35-37]. Alternative therapies such as acupuncture, moxibustion, acupoint catgut embedding (ACE), and massage are increasingly used for the treatment of obesity [38-42].
ACE is a special acupuncture therapy that combined the absorbable surgical suture and acupoint, and the effective stimulation of every ACE treatment could persist for 2 weeks. Previous studies indicated that ACE was an efficient treatment for obesity, but the risk of bias was significantly high [43]. Therefore, this rigorous and normative randomized controlled trial (RCT) study is designed to verify the efficiency of catgut embedding at acupoints and nonacupoints in participants with AO. The findings will provide credible evidence as to whether ACE is an effective and safe treatment for AO.
Methods
Study Design and Setting
This multicenter, double-blind, 16-week RCT will be conducted from August 2019 to September 2023 in 4 clinical centers: The Second Affiliated Hospital of Yunnan University of Chinese Medicine, The Sports Trauma Specialist Hospital of Yunnan Province, Kunming Hospital of Traditional Chinese Medicine, and ShengAi Hospital of Traditional Chinese Medicine. A total of 92 eligible participants with AO will be assigned to 1 of 2 groups (catgut embedding at acupoints or nonacupoints) using central randomization (1:1 allocation ratio). Every participant will receive a 12-week intervention period and a 4-week follow-up. The flowchart of this study is shown in Figure 1.
Figure 1.

Flowchart of the trial. ACE: acupoint catgut embedding.
Ethics Approval
This protocol is in line with the Declaration of Helsinki and has been approved by the Hospital Ethics Committee of The Sports Trauma Specialist Hospital of Yunnan Province (2018CK-001) in June 2018. The study has been registered in the Chinese Clinical Trial Registry (ChiCTR1800016947). This protocol is compliant with the principles of the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials; see Multimedia Appendix 1) and CONSORT (Consolidated Standards for Reporting Trials) guidelines [44]. The SPIRIT checklist is presented in Multimedia Appendix 1 and the schedule is shown in Table 1.
Table 1.
Study schedule for data measurements.
|
|
Study period and time point (week) | ||||||||||||
|
|
|
Enrollment | Allocation | Treatment phase | Follow-up | ||||||||
|
|
|
–1 | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | ||
| Enrollment | |||||||||||||
|
|
Eligibility screen | ✓ |
|
|
|
|
|
|
|
|
|
||
|
|
Informed consent | ✓ |
|
|
|
|
|
|
|
|
|
||
|
|
Physical examination | ✓ |
|
|
|
|
|
|
|
|
|
||
|
|
Randomization |
|
✓ |
|
|
|
|
|
|
|
|
||
| Interventions | |||||||||||||
|
|
ACEa group (n=46) |
|
|
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
|
|
Control group (n=46) |
|
|
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Assessments | |||||||||||||
|
|
BWb | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
|
|
BMI | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
|
|
WCc | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
|
|
HCd |
|
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
|
|
VASe of appetite |
|
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Safety | |||||||||||||
|
|
AEf |
|
|
✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
|
|
||
aACE: acupoint catgut embedding.
bBW: body weight.
cWC: waist circumference.
dHC: hip circumference.
eVAS: visual analogue scale.
fAE: adverse event.
Participants
Participants who meet all the following criteria will be included: (1) aged between 18 and 60 years with simple obesity; (2) BMI ≥28 kg/m2 and waist circumference (WC) ≥90 cm for male participants and ≥85 cm for female participants; (3) agreed to participate in this study and signed written informed consent for this trial and catgut embedding therapy; and (4) did not participate in other trials within past 3 months. Participants will be excluded if they meet any one of the following criteria: (1) secondary obesity, such as obesity caused by endocrine disease (Cushing syndrome, thyroid disease, hypothalamic disease, pituitary disease, gonadal disease, etc) and medication (glucocorticoid or antipsychotics); (2) pregnancy, lactation, and childbirth within the past 6 months; (3) heart disease; hematopoietic system, liver, kidney, and other important organ diseases; and hypertension that is not effectively controlled; (4) severe mental and neurological diseases where the patient is unable or unwilling to cooperate; (5) allergy to alcohol and animal protein or immune diseases; and (6) received other weight loss treatment within the past 3 months.
Criteria for Elimination From the Study
Those who meet the following criteria should be eliminated from the study: (1) misdiagnosis and misrepresentation; (2) poor treatment tendency; (3) involvement with other treatments after being selected; and (4) participants’ withdrawal before the first test recording.
Recruitment and Randomization
All participants with eligible AO will be recruited from the 4 hospitals and the community. Recruitment methods mainly include posters, public notice boards, and WeChat public numbers in hospitals and communities. The randomization sequence will be generated by the Clinical Research Center of Yunnan University of Chinese Medicine using a computerized random number generator and placed in opaque envelopes. Stratified randomization will be performed for the 4 clinical centers. To ensure allocation concealment, randomization will be performed by an independent researcher. The research assistant will open the envelope when the participant begins treatment to determine the assigned group and will not disclose grouping information under any circumstances. The acupuncture therapist will be responsible for manipulation only.
Blinding and Informed Consent
Participants will be informed that they have a 1 in 2 chance of being assigned to 1 of 2 treatments: catgut embedding at acupoints or nonacupoints. The procedure and stimulation of the catgut embedding at acupoints are identical to catgut embedding at nonacupoints. Therefore, participants will be blinded to their treatment assignment. Both groups of participants will be assigned to different treatment rooms to prevent intercommunication. In addition, the acupuncturist, outcome assessor, and those involved in data collection and statistics will be blinded to treatment assignment throughout the trial. Research assistants with knowledge of grouping information will be asked not to communicate with participants or outcome assessors regarding treatment procedures and responses. All patients will be fully informed about the study (including the purpose of the trial and possible risks, etc) prior to participation in the trial, and they have the right to withdraw from the trial at any time without giving a reason. They will sign a written informed consent for voluntary participation prior to enrollment.
Sample Size Calculation
According to the existing literature [45], the mean BMI of simple obesity was 32.30 kg/m2, whereas after 12 weeks and 6 treatment of acupuncture combined with a low-energy diet, the mean BMI was 30.98 kg/m2, and the improvement value was 1.32 kg/m2. In contrast, the improvement value was 1.02 kg/m2 in a control group with a variation from 32.74 kg/m2 to 31.73 kg/m2 when treated by sham acupuncture combined with a low-energy diet, and the SD of the 2 groups was 0.31 kg/m2. In this project, the expected improvement value of the mean BMI of the Shu-Mu acupoints embedding group after treatment is 1.14 kg/m2 and that of the nonacupoints embedding group is 0.9 kg/m2, with the SD of each group being 0.31 kg/m2. The significant level is ɑ=.05, and the power of a test is 1 – β = 0.95. The sample size is 84 as calculated by German G*power software 3.1 [46]. According to the 10% dropout, the total sample is 92 cases, and 46 cases will be assigned to each group.
Interventions
All catgut embedding manipulations will be performed by an acupuncturist with national medical qualifications. The participants will receive treatment every 2 weeks for a total of 6 sessions.
ACE Group
The selection of acupoints for treatment in the ACE group will be based on the theory that obesity is closely related to the spleen, stomach, and large intestine in traditional Chinese medicine and the Shu-Mu acupoints allocation method commonly used in the current clinical treatment of obesity. The acupoint prescription includes BL20 (Pishu), BL21 (Weishu), BL25 (Dachangshu), RN12 (Zhongwan), ST25 (Tianshu), and LR13 (Zhangmen). All acupoints except Zhongwan are selected bilaterally, for a total of 11 acupoints. The locations of these acupoints are shown in Table 2 and Figure 2.
Table 2.
The locations of acupoints.
| Acupoints | Location |
| BL20 (Pishu) | In the upper back region, at the same level as the inferior border of the spinous process of the 11th thoracic vertebra (T11), 1.5 B-cun lateral to the posterior median line |
| BL21 (Weishu) | In the upper back region, at the same level as the inferior border of the spinous process of the 12th thoracic vertebra (T12), 1.5 B-cun lateral to the posterior median line |
| BL25 (Dachangshu) | In the lumbar region, at the same level as the inferior border of the spinous process of the fourth lumbar vertebra (L4), 1.5 B-cun lateral to the posterior median line |
| CV12 (Zhongwan) | On the upper abdomen, 4 B-cun superior to the center of the umbilicus, on the anterior median line |
| ST25 (Tianshu) | On the upper abdomen, 2 B-cun lateral to the center of the umbilicus |
| LR13 (Zhangmen) | On the lateral abdomen, inferior to the free extremity of the 11th rib |
Figure 2.
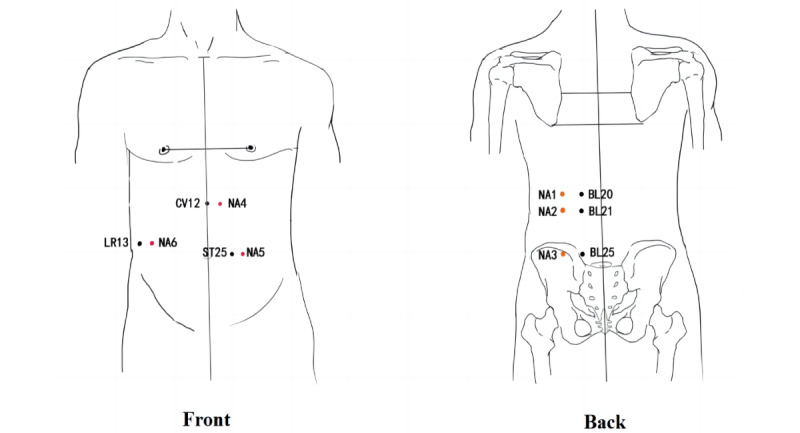
Locations of acupoints and nonacupoints (NAs). Acupoints: BL20: Pishu, BL21: Weishu, BL25: Dachangshu, CV12: Zhongwan, LR13: Zhangmen, and ST25: Tianshu.
Control Group
The acupoints in the control group will be selected based on the acupoints in the ACE group by moving each acupoint 2-3 cm outward horizontally in the median line (CV12 to the left side only) as a nonacupoints (not located on or overlapping with other meridians). They are labeled NA1, NA2, NA3, NA4, NA5, and NA6, respectively, for a total of 11 acupoints. The locations of these acupoints are shown in Table 3 and Figure 2, marked as red points.
Table 3.
The location of nonacupoints (NAs).
| Nonacupoint | Location |
| NA1 | The sitting position of the patient, in alignment with BL20 and the midpoint of the first and second lateral line of the bladder channel |
| NA2 | The sitting position of the patient, in alignment with BL21 and the midpoint of the first and second lateral line of the bladder channel |
| NA3 | The sitting position of the patient, in alignment with BL25 and the midpoint of the first and second lateral line of the bladder channel |
| NA4 | The supine position of the patient, in alignment with RN12 and the midpoint of the left kidney and stomach channel |
| NA5 | The supine position of the patient, in alignment with ST25 and the midpoint of the stomach and spleen channel |
| NA6 | The sitting position of the patient, in alignment with LR13; a vertical line is made from Zhangmen to the spleen channel and the midpoint of the vertical line |
Operation Instruments
The thread-embedding needle used in this study is an 8# disposable needle (Jiangxi Glance Medical Equipment Co Ltd Production), and the medical protein string is an absorbable collagen line with the specification of 2-0, 2 cm × 20 length (Jiangxi Longteng Biotechnology Co, Ltd).
Manipulation
The specific manipulation is as follows: participants are instructed to be placed in an appropriate position; the separate research assistant marks the acupoints or nonacupoints and routinely disinfects the skin around them; and a sterile sheet is spread, leaving only the buried area. Then, acupuncturists, who are unaware of the group allocation, take a sterile protein cord 1-2 cm long (the length depends on the location of the acupoints), place it on the front end of the trocar, connect the needle core, lift the partial skin with the thumb and forefinger of one hand, and pierce the needle with the other hand. When the piercing reaches the desired depth, apply appropriate push-and-twist techniques, push the needle core and remove the needle tube, and implant the sterile protein string in the subcutaneous tissue or muscle layer of the acupoints. After removing the needle, press the needle hole with a dry cotton ball for half a minute to stop the bleeding. Meanwhile, check that there is no thread residue exposure and no bleeding and then paste a bandage to protect the needle hole. It will be recommended that patients do not take a bath for 24 hours and keep the embedding place dry.
Outcomes and Assessment
Primary Outcome
The primary outcome is the change of WC every 2 weeks, from baseline to end points. To collect the measurements, participants will stand their body upright, feet at shoulder width. An inelastic, 1-mm minimal tapeline will be used, set on the midpoint between the upper border hipbone and lower border 12th rib of the right midaxillary line. At the end of normal exhalation, they will be measured around the abdomen in the horizontal direction and without pressing the skin. The WC will be measured in millimeters (mm) and accurate to 1 mm.
Secondary Outcomes
The following secondary outcomes will be assessed:
The change of body weight (BW) will be measured every 2 weeks, from baseline to end points. Participants will be fasting 2 hours before the assessment. Shoes will be taken off, and only light clothing should be worn in a warm room while standing upright in the middle of the calibrated scale. BW will be measured in kilograms (kg) and accurate to 10 g.
The change of BMI will be measured every 2 weeks, from baseline to end points: BMI = BW (kg) ÷ height2 (m2). Height will be measured after the weight measurement, with the participants standing upright and close to the measuring stick. Height will be measured from the top of the head to the bottom of the feet in meters (m) and accurate to 1 mm.
The change of hip circumference (HC) will be measured every 2 weeks, from baseline to end points. HC is the circumference of the most prominent point of the pelvis. Participants will stand upright, feet at shoulder width. The circumference at the end of the normal exhalation will be measured in millimeters (mm) and accurate to 1 mm.
The visual analog scale (VAS) of appetite will be scored every 2 weeks, from baseline to end points. Appetite will be assessed by VAS as reported in Doucet et al [47] (see Figure 3): no appetite and minimal intake (score of 0), slight appetite and a small amount of intake (score of 1-3), moderate appetite and moderate intake (score of 4-6), and strong appetite and huge intake (7-10 score of 7-10).
Figure 3.

The visual analogue scale (VAS) of appetite.
All of the above measurements and evaluations will be performed by a separate researcher in the morning, with the patient coming to the hospital without eating breakfast.
Safety Evaluation
Due to puncture injury and protein wire stimulation, participants may experience local sterile inflammatory reactions such as redness, swelling, fever, pain, or small amounts of exudate within 1-5 days, which are normal, generally do not require treatment, and are kept under close observation. If there is more exudate from the acupoints; localized redness; swelling; increased pain; increased temperature; or even protein line spillage, wound fat liquefaction, or nerve damage, treatment will be suspended when these adverse reactions occur. Participants will be treated aggressively until they have fully recovered and reported to the study center for evaluation by a medical professional to see if the trial can continue. Serious or unexpected adverse events will be verbally reported within 24 to 48 hours. All information will be classified as a safety event.
Data Management and Quality Control
The outcome evaluator will complete the initial data in the case report form and enter it into a Microsoft Excel spreadsheet. The protocol will be reviewed several times by an acupuncturist with the title of director and a statistical expert. To ensure the smooth conduct of the study, the study leader shall provide uniform training to all study personnel before the start of the study and provide detailed operational training on the project implementation protocol and observation indicators to familiarize them with the study process and specific implementation details to ensure the reliability of the study results. The project leader shall supervise and check the entire study process to confirm that all study data and case report forms are recorded and reported truthfully, accurately, completely, and consistently with the original data. Any deviation from the protocol will be reported to the ethics committee of The Sports Trauma Specialist Hospital of Yunnan Province, which will ultimately decide whether we must change the protocol or terminate the trial. To ensure participant compliance, all treatments and tests will be provided free of charge.
Statistical Analysis
All data will be analyzed by SPSS statistical software (version 19.0; IBM Corp). The clinical outcomes and baseline characteristics are based on the intention-to-treat population, which will include participants who have received at least one treatment. Continuous variables will be shown as the mean and SD, whereas the nonnormally distributed variables will be presented as the median and IQR. Categorical variables will be described as numbers and percentages. The normality of continuous variables will be determined using the Shapiro-Wilk test, Kolmogorov-Smirnov test, skewness, and kurtosis. The homogeneity of continuous variables will be determined by F-test, Brown-Forsythe, and Bartlett tests. Comparisons between groups will be made using independent samples 2-tailed t tests or nonparametric tests (Mann-Whitney U test). Repeated measures within groups (≥3) will be analyzed using one-way ANOVA or nonparametric (Friedman) tests. Paired 2-tailed t tests or nonparametric tests (Wilcoxon test) will be used for within-group pre-post comparisons. For categorical data, the chi-square test will be used to assess the significance of differences. Values of P<.05 will be considered significant using a 2-sided test.
Results
The start of recruitment began in August 2019 and is expected to end in September 2023.
Discussion
Expected Findings
In the past, obesity was considered a problem in high-income countries. However, recent studies reported that the prevalence of obesity is rapidly increasing in low- and middle-income countries [4,48-50]. As a type of obesity, AO is also related to cardiovascular diseases, cancer, and many other diseases [51-54].
ACE is a traditional Chinese medical practice that has been used in clinical practice since the mid-1960s. Compared with other treatments, ACE has the advantages of a short single-treatment time, long effective stimulation time, low impact on daily life, as well as being time-saving and low cost and having few side effects, which are well suited for the fast-paced modern society and become gradually favorable to patients who are obese. As a specific type of acupuncture therapy, ACE has gained substantial attention in the management of obesity in recent years. Research has demonstrated that ACE has a certain impact on obesity. First, it can reduce BW by regulating appetite and food intake. ACE stimulates specific acupoints, promoting the release of neurotransmitters and hormones to regulate feelings of hunger and satiety and ultimately reducing food intake [55]. Second, ACE can improve metabolic function. Studies have shown that acupoint stimulation can affect various physiological processes, including the endocrine, nervous, and immune systems, thus regulating energy and fat metabolism, improving insulin sensitivity, and enhancing blood lipid levels [41]. Additionally, ACE may also have positive effects on obesity by improving psychological well-being and sleep quality, among other factors [56]. However, current research has some limitations, including small sample sizes and inconsistent study designs [57]. Therefore, to better assess the effectiveness and safety of ACE treatment, further high-quality studies with larger sample sizes are needed.
The decrease of several indicators such as BW, WC, BMI, HC, and appetite is not only beneficial to personal health but also helps people who are obese to reshape their bodies [58]. Among them, BMI is the most commonly used indicator in obesity research, but it mainly reflects total body fat and cannot distinguish between fatty obesity and muscular obesity. HC is affected by both sex and age, and changes in abdominal fat cannot be accurately reflected promptly [59]. WC can directly reflect abdominal fat, so we use WC as the main outcome indicator and other related indicators as secondary outcome indicators. According to the classification adopted by the Chinese Medical Association of China in 2019, the inclusion criteria was set as WC ≥90 cm for male individuals and ≥85 cm for female individuals, rather than those of other Asian countries (WC ≥102 cm for male individuals and WC ≥88 cm for female individuals or WC ≥ 90cm for both male and female individuals) [6,60].
The combination of Shu and Mu acupoints is one of the classical methods of acupuncture and is mainly used in clinical practice to treat metabolic diseases of the viscera. According to the identification theory of traditional Chinese medicine, obesity is mainly caused by the dysfunction of the spleen, stomach, and large intestine, so we chose the Shu and Mu acupoints of these 3 internal organs as the treatment prescription. Our preliminary analysis of 175 obesity ACEs in the early stage also found that the existing high-frequency acupoints for obesity ACEs were mainly Shu and Mu acupoints of the spleen, stomach, and large intestine [61].
Strengths and Limitations
First, this study is a multicenter RCT designed to investigate the efficacy and safety of ACE in AO with a relatively long study period, and the results will provide powerful evidence for the clinical application of ACE in the treatment of obesity. Second, participants’ diet and exercise will not be intervened, which will allow participants to maintain their original lifestyle habits throughout and maximize the efficacy of ACE. Finally, to maintain the success of the double-blind method, contact between participants from different groups will be avoided. The acupuncturist, data evaluator, and statistician will also be hidden from the group assignment, which will minimize possible bias in the results.
Limitations of this study are as follows. First, the relatively short follow-up period of this study may not be comprehensive enough to assess the durability of ACE efficacy. Second, the selection of nonacupuncture acupoints as an intervention in the control group in this study may inevitably produce some efficacy as well [62,63]. Third, only patients with AO are selected as the study population in this study, and further studies on different types of obesity will be conducted at a later stage.
Conclusions
In summary, our trial aims to investigate the difference in the effectiveness of catgut embedding at acupoints and catgut embedding at nonacupoints in patients with AO. This finding will provide reliable evidence to support whether ACE is an effective and safe treatment for AO.
Acknowledgments
All authors are grateful for the support of all participants or participants who will join the trial, as well as the support of other institutions.
This study is funded by Youth Special of Yunnan Province Ten-thousand Plan (YNWR-QNBJ-2019-257) from the Human Resources & Social Security Department of Yunnan Province; “Liang Fanrong Expert Workstation” of Yunnan Province—Yunnan Provincial Science and Technology Plan Project (202305AF150072); Yunnan Provincial Science Plan Project—Joint of Traditional Chinese Medicine (2019FF002-021) from the Science & Technology Department of Yunnan Province; and “Liu Zili Famous Doctor” special talent program of the Yunnan Provincial Xing Dian Talent Support Program (Yunnan Party Talent Office [2022] No. 18). The funding agencies do not play any role in the design, collection, analysis, or writing manuscripts of the study.
TG and XZ (doczhangxh@163.com) are cocorresponding authors for this manuscript.
Abbreviations
- ACE
acupoint catgut embedding
- AO
abdominal obesity
- BW
body weight
- CONSORT
Consolidated Standards for Reporting Trials
- HC
hip circumference
- RCT
randomized controlled trial
- SPIRIT
Standard Protocol Items: Recommendations for Interventional Trials
- VAS
visual analogue scale
- WC
waist circumference
SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) checklist.
Data Availability
The data sets generated or analyzed in this study will not be publicly available. Consent and ethical approval for this study do not include a provision for the sharing of data from this study.
Footnotes
Authors' Contributions: TG and XZ are the principal investigators and were primarily responsible for the conception and design of this study; QL and GH wrote the original manuscript; RY, ZL, XP, XT, RZ, and YH participated in the implementation of the study and acquisition of data; XZ was primarily responsible for the analysis of the data; and CX provided ethical approval. All authors contributed to the revision and approved the final manuscript.
Conflicts of Interest: None declared.
References
- 1.Obesity. World Health Organization. 2014. Sep 5, [2020-03-10]. https://www.who.int/topics/obesity/en/
- 2.GBD 2015 Obesity Collaborators T, Afshin Ashkan, Forouzanfar Mohammad H, Reitsma Marissa B, Sur Patrick, Estep Kara, Lee Alex, Marczak Laurie, Mokdad Ali H, Moradi-Lakeh Maziar, Naghavi Mohsen, Salama Joseph S, Vos Theo, Abate Kalkidan H, Abbafati Cristiana, Ahmed Muktar B, Al-Aly Ziyad, Alkerwi Ala’a, Al-Raddadi Rajaa, Amare Azmeraw T, Amberbir Alemayehu, Amegah Adeladza K, Amini Erfan, Amrock Stephen M, Anjana Ranjit M, Ärnlöv Johan, Asayesh Hamid, Banerjee Amitava, Barac Aleksandra, Baye Estifanos, Bennett Derrick A, Beyene Addisu S, Biadgilign Sibhatu, Biryukov Stan, Bjertness Espen, Boneya Dube J, Campos-Nonato Ismael, Carrero Juan J, Cecilio Pedro, Cercy Kelly, Ciobanu Liliana G, Cornaby Leslie, Damtew Solomon A, Dandona Lalit, Dandona Rakhi, Dharmaratne Samath D, Duncan Bruce B, Eshrati Babak, Esteghamati Alireza, Feigin Valery L, Fernandes João C, Fürst Thomas, Gebrehiwot Tsegaye T, Gold Audra, Gona Philimon N, Goto Atsushi, Habtewold Tesfa D, Hadush Kokeb T, Hafezi-Nejad Nima, Hay Simon I, Horino Masako, Islami Farhad, Kamal Ritul, Kasaeian Amir, Katikireddi Srinivasa V, Kengne Andre P, Kesavachandran Chandrasekharan N, Khader Yousef S, Khang Young-Ho, Khubchandani Jagdish, Kim Daniel, Kim Yun J, Kinfu Yohannes, Kosen Soewarta, Ku Tiffany, Defo Barthelemy Kuate, Kumar G Anil, Larson Heidi J, Leinsalu Mall, Liang Xiaofeng, Lim Stephen S, Liu Patrick, Lopez Alan D, Lozano Rafael, Majeed Azeem, Malekzadeh Reza, Malta Deborah C, Mazidi Mohsen, McAlinden Colm, McGarvey Stephen T, Mengistu Desalegn T, Mensah George A, Mensink Gert B M, Mezgebe Haftay B, Mirrakhimov Erkin M, Mueller Ulrich O, Noubiap Jean J, Obermeyer Carla M, Ogbo Felix A, Owolabi Mayowa O, Patton George C, Pourmalek Farshad, Qorbani Mostafa, Rafay Anwar, Rai Rajesh K, Ranabhat Chhabi L, Reinig Nikolas, Safiri Saeid, Salomon Joshua A, Sanabria Juan R, Santos Itamar S, Sartorius Benn, Sawhney Monika, Schmidhuber Josef, Schutte Aletta E, Schmidt Maria I, Sepanlou Sadaf G, Shamsizadeh Moretza, Sheikhbahaei Sara, Shin Min-Jeong, Shiri Rahman, Shiue Ivy, Roba Hirbo S, Silva Diego A S, Silverberg Jonathan I, Singh Jasvinder A, Stranges Saverio, Swaminathan Soumya, Tabarés-Seisdedos Rafael, Tadese Fentaw, Tedla Bemnet A, Tegegne Balewgizie S, Terkawi Abdullah S, Thakur J S, Tonelli Marcello, Topor-Madry Roman, Tyrovolas Stefanos, Ukwaja Kingsley N, Uthman Olalekan A, Vaezghasemi Masoud, Vasankari Tommi, Vlassov Vasiliy V, Vollset Stein E, Weiderpass Elisabete, Werdecker Andrea, Wesana Joshua, Westerman Ronny, Yano Yuichiro, Yonemoto Naohiro, Yonga Gerald, Zaidi Zoubida, Zenebe Zerihun M, Zipkin Ben, Murray Christopher J L. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017 Jul 06;377(1):13–27. doi: 10.1056/NEJMoa1614362. https://europepmc.org/abstract/MED/28604169 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obesity and overweight. World Health Organization. [2020-03-10]. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight .
- 4.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017 Dec 16;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(17)32129-3 .S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015 Jun 2;66 Suppl 2(Suppl. 2):7–12. doi: 10.1159/000375143. doi: 10.1159/000375143.000375143 [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Wang Z, Wang X, Chen Z, Shao L, Tian Y, Dong Y, Zheng C, Li S, Zhu M, Gao R, China Hypertension Survey investigators Prevalence of abdominal obesity in China: results from a cross-sectional study of nearly half a million participants. Obesity (Silver Spring) 2019 Nov 24;27(11):1898–1905. doi: 10.1002/oby.22620. [DOI] [PubMed] [Google Scholar]
- 7.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann Markus, Anderson H Ross, Andrews Kathryn G, Aryee Martin, Atkinson Charles, Bacchus Loraine J, Bahalim Adil N, Balakrishnan Kalpana, Balmes John, Barker-Collo Suzanne, Baxter Amanda, Bell Michelle L, Blore Jed D, Blyth Fiona, Bonner Carissa, Borges Guilherme, Bourne Rupert, Boussinesq Michel, Brauer Michael, Brooks Peter, Bruce Nigel G, Brunekreef Bert, Bryan-Hancock Claire, Bucello Chiara, Buchbinder Rachelle, Bull Fiona, Burnett Richard T, Byers Tim E, Calabria Bianca, Carapetis Jonathan, Carnahan Emily, Chafe Zoe, Charlson Fiona, Chen Honglei, Chen Jian Shen, Cheng Andrew Tai-Ann, Child Jennifer Christine, Cohen Aaron, Colson K Ellicott, Cowie Benjamin C, Darby Sarah, Darling Susan, Davis Adrian, Degenhardt Louisa, Dentener Frank, Des Jarlais Don C, Devries Karen, Dherani Mukesh, Ding Eric L, Dorsey E Ray, Driscoll Tim, Edmond Karen, Ali Suad Eltahir, Engell Rebecca E, Erwin Patricia J, Fahimi Saman, Falder Gail, Farzadfar Farshad, Ferrari Alize, Finucane Mariel M, Flaxman Seth, Fowkes Francis Gerry R, Freedman Greg, Freeman Michael K, Gakidou Emmanuela, Ghosh Santu, Giovannucci Edward, Gmel Gerhard, Graham Kathryn, Grainger Rebecca, Grant Bridget, Gunnell David, Gutierrez Hialy R, Hall Wayne, Hoek Hans W, Hogan Anthony, Hosgood H Dean, Hoy Damian, Hu Howard, Hubbell Bryan J, Hutchings Sally J, Ibeanusi Sydney E, Jacklyn Gemma L, Jasrasaria Rashmi, Jonas Jost B, Kan Haidong, Kanis John A, Kassebaum Nicholas, Kawakami Norito, Khang Young-Ho, Khatibzadeh Shahab, Khoo Jon-Paul, Kok Cindy, Laden Francine, Lalloo Ratilal, Lan Qing, Lathlean Tim, Leasher Janet L, Leigh James, Li Yang, Lin John Kent, Lipshultz Steven E, London Stephanie, Lozano Rafael, Lu Yuan, Mak Joelle, Malekzadeh Reza, Mallinger Leslie, Marcenes Wagner, March Lyn, Marks Robin, Martin Randall, McGale Paul, McGrath John, Mehta Sumi, Mensah George A, Merriman Tony R, Micha Renata, Michaud Catherine, Mishra Vinod, Mohd Hanafiah Khayriyyah, Mokdad Ali A, Morawska Lidia, Mozaffarian Dariush, Murphy Tasha, Naghavi Mohsen, Neal Bruce, Nelson Paul K, Nolla Joan Miquel, Norman Rosana, Olives Casey, Omer Saad B, Orchard Jessica, Osborne Richard, Ostro Bart, Page Andrew, Pandey Kiran D, Parry Charles D H, Passmore Erin, Patra Jayadeep, Pearce Neil, Pelizzari Pamela M, Petzold Max, Phillips Michael R, Pope Dan, Pope C Arden, Powles John, Rao Mayuree, Razavi Homie, Rehfuess Eva A, Rehm Jürgen T, Ritz Beate, Rivara Frederick P, Roberts Thomas, Robinson Carolyn, Rodriguez-Portales Jose A, Romieu Isabelle, Room Robin, Rosenfeld Lisa C, Roy Ananya, Rushton Lesley, Salomon Joshua A, Sampson Uchechukwu, Sanchez-Riera Lidia, Sanman Ella, Sapkota Amir, Seedat Soraya, Shi Peilin, Shield Kevin, Shivakoti Rupak, Singh Gitanjali M, Sleet David A, Smith Emma, Smith Kirk R, Stapelberg Nicolas J C, Steenland Kyle, Stöckl Heidi, Stovner Lars Jacob, Straif Kurt, Straney Lahn, Thurston George D, Tran Jimmy H, Van Dingenen Rita, van Donkelaar Aaron, Veerman J Lennert, Vijayakumar Lakshmi, Weintraub Robert, Weissman Myrna M, White Richard A, Whiteford Harvey, Wiersma Steven T, Wilkinson James D, Williams Hywel C, Williams Warwick, Wilson Nicholas, Woolf Anthony D, Yip Paul, Zielinski Jan M, Lopez Alan D, Murray Christopher J L, Ezzati Majid, AlMazroa Mohammad A, Memish Ziad A. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8. https://europepmc.org/abstract/MED/23245609 .S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blüher Matthias. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019 May 27;15(5):288–298. doi: 10.1038/s41574-019-0176-8.10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 9.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S, Di Angelantonio E, Vander Hoorn Stephen, Lawes CMM, Ali MK, Mozaffarian D, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group; Asia-Pacific Cohort Studies Collaboration (APCSC) Diabetes Epidemiology: Collaborative analysis of Diagnostic criteria in Europe (DECODE) Emerging Risk Factor Collaboration (ERFC) Prospective Studies Collaboration (PSC) The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013 Jul 30;8(7):e65174. doi: 10.1371/journal.pone.0065174. https://dx.plos.org/10.1371/journal.pone.0065174 .PONE-D-12-31608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertil Steril. 2017 Apr;107(4):840–847. doi: 10.1016/j.fertnstert.2017.01.017. https://linkinghub.elsevier.com/retrieve/pii/S0015-0282(17)30060-2 .S0015-0282(17)30060-2 [DOI] [PubMed] [Google Scholar]
- 11.Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2018 Mar 09;16(1):22. doi: 10.1186/s12958-018-0336-z. https://rbej.biomedcentral.com/articles/10.1186/s12958-018-0336-z .10.1186/s12958-018-0336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang Y, Jung HS, Cho J, Zhang Y, Yun KE, Lazo M, Pastor-Barriuso Roberto, Ahn Jiin, Kim Chan-Won, Rampal Sanjay, Cainzos-Achirica Miguel, Zhao Di, Chung Eun Cheol, Shin Hocheol, Guallar Eliseo, Ryu Seungho. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am J Gastroenterol. 2016 Aug;111(8):1133–40. doi: 10.1038/ajg.2016.178.ajg2016178 [DOI] [PubMed] [Google Scholar]
- 13.Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018 Oct;69(4):896–904. doi: 10.1016/j.jhep.2018.05.036. https://linkinghub.elsevier.com/retrieve/pii/S0168-8278(18)32121-4 .S0168-8278(18)32121-4 [DOI] [PubMed] [Google Scholar]
- 14.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016 May 27;118(11):1752–70. doi: 10.1161/CIRCRESAHA.115.306883.CIRCRESAHA.115.306883 [DOI] [PubMed] [Google Scholar]
- 15.Bangalore S, Fayyad R, Laskey R, DeMicco DA, Messerli FH, Waters DD. Body-weight fluctuations and outcomes in coronary disease. N Engl J Med. 2017 Apr 06;376(14):1332–1340. doi: 10.1056/NEJMoa1606148. [DOI] [PubMed] [Google Scholar]
- 16.Alford S, Patel D, Perakakis N, Mantzoros CS. Obesity as a risk factor for Alzheimer's disease: weighing the evidence. Obes Rev. 2018 Feb 10;19(2):269–280. doi: 10.1111/obr.12629. [DOI] [PubMed] [Google Scholar]
- 17.Lapi F, Cassano N, Pegoraro V, Cataldo N, Heiman F, Cricelli I, Levi M, Colombo D, Zagni E, Cricelli C, Vena G. Epidemiology of chronic spontaneous urticaria: results from a nationwide, population-based study in Italy. Br J Dermatol. 2016 May 15;174(5):996–1004. doi: 10.1111/bjd.14470. [DOI] [PubMed] [Google Scholar]
- 18.Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, Ferlay J, Miranda JJ, Romieu I, Dikshit R, Forman D, Soerjomataram I. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015 Jan;16(1):36–46. doi: 10.1016/S1470-2045(14)71123-4. https://europepmc.org/abstract/MED/25467404 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colao A, Lucchese M, D'Adamo M, Savastano S, Facchiano E, Veronesi C, Blini V, Degli Esposti L, Sbraccia P. Healthcare usage and economic impact of non-treated obesity in Italy: findings from a retrospective administrative and clinical database analysis. BMJ Open. 2017 Feb 24;7(2):e013899. doi: 10.1136/bmjopen-2016-013899. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=28237961 .bmjopen-2016-013899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokdad Ali H, Ballestros Katherine, Echko Michelle, Glenn Scott, Olsen Helen E, Mullany Erin, Lee Alex, Khan Abdur Rahman, Ahmadi Alireza, Ferrari Alize J, Kasaeian Amir, Werdecker Andrea, Carter Austin, Zipkin Ben, Sartorius Benn, Serdar Berrin, Sykes Bryan L, Troeger Chris, Fitzmaurice Christina, Rehm Colin D, Santomauro Damian, Kim Daniel, Colombara Danny, Schwebel David C, Tsoi Derrick, Kolte Dhaval, Nsoesie Elaine, Nichols Emma, Oren Eyal, Charlson Fiona J, Patton George C, Roth Gregory A, Hosgood H Dean, Whiteford Harvey A, Kyu Hmwe, Erskine Holly E, Huang Hsiang, Martopullo Ira, Singh Jasvinder A, Nachega Jean B, Sanabria Juan R, Abbas Kaja, Ong Kanyin, Tabb Karen, Krohn Kristopher J, Cornaby Leslie, Degenhardt Louisa, Moses Mark, Farvid Maryam, Griswold Max, Criqui Michael, Bell Michelle, Nguyen Minh, Wallin Mitch, Mirarefin Mojde, Qorbani Mostafa, Younis Mustafa, Fullman Nancy, Liu Patrick, Briant Paul, Gona Philimon, Havmoller Rasmus, Leung Ricky, Kimokoti Ruth, Bazargan-Hejazi Shahrzad, Hay Simon I, Yadgir Simon, Biryukov Stan, Vollset Stein Emil, Alam Tahiya, Frank Tahvi, Farid Talha, Miller Ted, Vos Theo, Bärnighausen Till, Gebrehiwot Tsegaye Telwelde, Yano Yuichiro, Al-Aly Ziyad, Mehari Alem, Handal Alexis, Kandel Amit, Anderson Ben, Biroscak Brian, Mozaffarian Dariush, Dorsey E Ray, Ding Eric L, Park Eun-Kee, Wagner Gregory, Hu Guoqing, Chen Honglei, Sunshine Jacob E, Khubchandani Jagdish, Leasher Janet, Leung Janni, Salomon Joshua, Unutzer Jurgen, Cahill Leah, Cooper Leslie, Horino Masako, Brauer Michael, Breitborde Nicholas, Hotez Peter, Topor-Madry Roman, Soneji Samir, Stranges Saverio, James Spencer, Amrock Stephen, Jayaraman Sudha, Patel Tejas, Akinyemiju Tomi, Skirbekk Vegard, Kinfu Yohannes, Bhutta Zulfiqar, Jonas Jost B, Murray Christopher J L. The state of US health, 1990-2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018 Apr 10;319(14):1444–1472. doi: 10.1001/jama.2018.0158. https://europepmc.org/abstract/MED/29634829 .2678018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremmel M, Gerdtham U, Nilsson P, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017 Apr 19;14(4):435. doi: 10.3390/ijerph14040435. https://www.mdpi.com/resolver?pii=ijerph14040435 .ijerph14040435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, Pfäffle Roland, Kiess W, Körner Antje. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018 Oct 04;379(14):1303–1312. doi: 10.1056/NEJMoa1803527. [DOI] [PubMed] [Google Scholar]
- 23.Al-Khudairy L, Loveman E, Colquitt J, Mead E, Johnson R, Fraser H, Olajide Joan, Murphy Marie, Velho Rochelle Marian, O'Malley Claire, Azevedo Liane B, Ells Louisa J, Metzendorf Maria-Inti, Rees Karen. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst Rev. 2017 Jun 22;6(6):CD012691. doi: 10.1002/14651858.CD012691. https://europepmc.org/abstract/MED/28639320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, Singh S. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016 Jun 14;315(22):2424–34. doi: 10.1001/jama.2016.7602. https://europepmc.org/abstract/MED/27299618 .2528211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCrabb S, Lane C, Hall A, Milat A, Bauman A, Sutherland R, Yoong S, Wolfenden L. Scaling-up evidence-based obesity interventions: a systematic review assessing intervention adaptations and effectiveness and quantifying the scale-up penalty. Obes Rev. 2019 Jul 13;20(7):964–982. doi: 10.1111/obr.12845. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018 Jan 13;14(1):12–24. doi: 10.1038/nrendo.2017.122.nrendo.2017.122 [DOI] [PubMed] [Google Scholar]
- 27.Khorgami Z, Shoar S, Andalib A, Aminian A, Brethauer SA, Schauer PR. Trends in utilization of bariatric surgery, 2010-2014: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2017 May;13(5):774–778. doi: 10.1016/j.soard.2017.01.031.S1550-7289(17)30045-X [DOI] [PubMed] [Google Scholar]
- 28.Kyler KE, Bettenhausen JL, Hall M, Fraser JD, Sweeney B. Trends in volume and utilization outcomes in adolescent metabolic and bariatric surgery at children's hospitals. J Adolesc Health. 2019 Sep;65(3):331–336. doi: 10.1016/j.jadohealth.2019.02.021.S1054-139X(19)30131-4 [DOI] [PubMed] [Google Scholar]
- 29.Nguyen NT, Vu S, Kim E, Bodunova N, Phelan MJ. Trends in utilization of bariatric surgery, 2009-2012. Surg Endosc. 2016 Jul 10;30(7):2723–7. doi: 10.1007/s00464-015-4535-9.10.1007/s00464-015-4535-9 [DOI] [PubMed] [Google Scholar]
- 30.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, Levin BE, Perri MG, Rolls BJ, Rosenbaum M, Rothman AJ, Ryan D. NIH working group report: innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015 Jan 02;23(1):7–15. doi: 10.1002/oby.20967. https://europepmc.org/abstract/MED/25469998 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krentz AJ, Fujioka K, Hompesch M. Evolution of pharmacological obesity treatments: focus on adverse side-effect profiles. Diabetes Obes Metab. 2016 Jun 26;18(6):558–70. doi: 10.1111/dom.12657. [DOI] [PubMed] [Google Scholar]
- 32.Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018 Mar;6(3):237–248. doi: 10.1016/S2213-8587(17)30236-X.S2213-8587(17)30236-X [DOI] [PubMed] [Google Scholar]
- 33.van Gaal Luc, Dirinck E. Pharmacological Approaches in the Treatment and Maintenance of Weight Loss. Diabetes Care. 2016 Aug;39 Suppl 2:S260–7. doi: 10.2337/dcS15-3016.39/Supplement_2/S260 [DOI] [PubMed] [Google Scholar]
- 34.Mead E, Atkinson G, Richter B, Metzendorf M, Baur L, Finer N, Corpeleijn Eva, O'Malley Claire, Ells Louisa J. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016 Nov 29;11(11):CD012436. doi: 10.1002/14651858.CD012436. https://europepmc.org/abstract/MED/27899001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes D. Bone: fracture risk after bariatric surgery. Nat Rev Endocrinol. 2016 Oct 12;12(10):559–559. doi: 10.1038/nrendo.2016.134.nrendo.2016.134 [DOI] [PubMed] [Google Scholar]
- 36.Yu EW, Lee MP, Landon JE, Lindeman KG, Kim SC. Fracture risk after bariatric surgery: Roux-en-Y gastric bypass versus adjustable gastric banding. J Bone Miner Res. 2017 Jun 20;32(6):1229–1236. doi: 10.1002/jbmr.3101. https://europepmc.org/abstract/MED/28251687 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C, Wu D, Zhang J, Xu D, Xu W, Chen Y, Liu B, Li P, Li L. Changes in bone metabolism in morbidly obese patients after bariatric surgery: a meta-analysis. Obes Surg. 2016 Jan 16;26(1):91–7. doi: 10.1007/s11695-015-1724-5.10.1007/s11695-015-1724-5 [DOI] [PubMed] [Google Scholar]
- 38.Hsieh CH, Tseng CC, Shen JY, Chuang PY. Randomized controlled trial testing weight loss and abdominal obesity outcomes of moxibustion. Biomed Eng Online. 2018 Nov 06;17(Suppl 2):149. doi: 10.1186/s12938-018-0571-8. https://biomedical-engineering-online.biomedcentral.com/articles/10.1186/s12938-018-0571-8 .10.1186/s12938-018-0571-8 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Huang D, Liu Z, Xu B, Yuan J. Effect of acupuncture and moxibustion on severe obesity complicated with hyperlipidemia in different genders. Article in Chinese. Zhongguo Zhen Jiu. 2018 Jul 12;38(7):685–9. doi: 10.13703/j.0255-2930.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Huang W, Hu F, Jin Y, Hong Z, Zhou Z. Regularity of Acupoint Selection for Simple Obesity Treated by Acupoint Catgut Embedding Based on Complex Network Technology. Article in Chineses. Zhen Ci Yan Jiu. 2018 Sep 25;43(9):585–90. doi: 10.13702/j.1000-0607.170448. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Huang W, Wei D, Yang T, Zhou Z. Comparison of therapeutic effects of electroacupuncture and acupoint catgut embedding in redu-cing serum leptin and insulin levels in simple obesity patients. Article in Chinese. Zhen Ci Yan Jiu. 2019 Jan 25;44(1):57–61. doi: 10.13702/j.1000-0607.170768. [DOI] [PubMed] [Google Scholar]
- 42.Guo L, Fu X, Jiang Z, Xu A. Acupoint massage nursing conducive to improve curative effect of the obesity patients who are complicated with hypertension and are treated by oral drugs. Int J Clin Exp Med. 2015 Jul 15;8(7):11727–33. https://europepmc.org/abstract/MED/26380011 . [PMC free article] [PubMed] [Google Scholar]
- 43.Guo T, Ren Y, Kou J, Shi J, Tianxiao S, Liang F. Acupoint catgut embedding for obesity: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015 Aug 31;2015:401914–20. doi: 10.1155/2015/401914. doi: 10.1155/2015/401914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juszczak E, Altman DG, Hopewell S, Schulz K. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA. 2019 Apr 23;321(16):1610–1620. doi: 10.1001/jama.2019.3087.2731183 [DOI] [PubMed] [Google Scholar]
- 45.Abdi H, Zhao B, Darbandi M, Ghayour-Mobarhan M, Tavallaie S, Rahsepar A, Parizadeh Seyyed Mohammad Reza, Safariyan Mohammad, Nemati Mohsen, Mohammadi Maryam, Abbasi-Parizad Parisa, Darbandi Sara, Akhlaghi Saeed, Ferns Gordon A A. The effects of body acupuncture on obesity: anthropometric parameters, lipid profile, and inflammatory and immunologic markers. ScientificWorldJournal. 2012 Apr 29;2012:603539. doi: 10.1100/2012/603539. doi: 10.1100/2012/603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faul F, Erdfelder E, Buchner A, Lang A. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009 Nov;41(4):1149–60. doi: 10.3758/BRM.41.4.1149.41/4/1149 [DOI] [PubMed] [Google Scholar]
- 47.Doucet E, Imbeault P, St-Pierre S, Alméras N, Mauriège P, Richard D, Tremblay A. Appetite after weight loss by energy restriction and a low-fat diet-exercise follow-up. Int J Obes Relat Metab Disord. 2000 Jul 25;24(7):906–14. doi: 10.1038/sj.ijo.0801251. [DOI] [PubMed] [Google Scholar]
- 48.Tian Y, Jiang C, Wang M, Cai R, Zhang Y, He Z, Wang H, Wu D, Wang F, Liu X, He Z, An P, Wang M, Tang Q, Yang Y, Zhao J, Lv S, Zhou W, Yu B, Lan J, Yang X, Zhang L, Tian H, Gu Z, Song Y, Huang T, McNaughton LR. BMI, leisure-time physical activity, and physical fitness in adults in China: results from a series of national surveys, 2000-14. Lancet Diabetes Endocrinol. 2016 Jun;4(6):487–97. doi: 10.1016/S2213-8587(16)00081-4.S2213-8587(16)00081-4 [DOI] [PubMed] [Google Scholar]
- 49.GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016 Oct 08;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(16)31679-8 .S0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinsa GD, Goryakin Y, Fumagalli E, Suhrcke M. Obesity and socioeconomic status in developing countries: a systematic review. Obes Rev. 2012 Nov;13(11):1067–79. doi: 10.1111/j.1467-789X.2012.01017.x. https://europepmc.org/abstract/MED/22764734 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho JH, Shin CM, Han K, Yoon H, Park YS, Kim N, Lee DH. Abdominal obesity increases risk for esophageal cancer: a nationwide population-based cohort study of South Korea. J Gastroenterol. 2020 Mar 02;55(3):307–316. doi: 10.1007/s00535-019-01648-9.10.1007/s00535-019-01648-9 [DOI] [PubMed] [Google Scholar]
- 52.Hidayat K, Du X, Chen G, Shi M, Shi B. Abdominal obesity and lung cancer risk: systematic review and meta-analysis of prospective studies. Nutrients. 2016 Dec 15;8(12):810. doi: 10.3390/nu8120810. https://www.mdpi.com/resolver?pii=nu8120810 .nu8120810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohammadi H, Ohm J, Discacciati A, Sundstrom J, Hambraeus K, Jernberg T, Svensson Per. Abdominal obesity and the risk of recurrent atherosclerotic cardiovascular disease after myocardial infarction. Eur J Prev Cardiol. 2020 Dec;27(18):1944–1952. doi: 10.1177/2047487319898019. [DOI] [PubMed] [Google Scholar]
- 54.Randhawa AK, Ardern CI, Kuk JL. Changes in the prevalence of chronic conditions associated with abdominal obesity between 1999 and 2014. Clin Obes. 2020 Feb 09;10(1):e12349. doi: 10.1111/cob.12349. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Yu C, Li J, Tian Q, Du Y. Mechanism of action of acupuncture in obesity: a perspective from the hypothalamus. Front Endocrinol (Lausanne) 2021 Apr 2;12:632324. doi: 10.3389/fendo.2021.632324. https://europepmc.org/abstract/MED/33868169 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Yue H, Chen Y, Yin X, Xu S, Mi Y, Li Shan-Shan. Jianpi Peiyuan acupoint thread embedding therapy for perimenopausal obesity: a randomized controlled trial. Article in Chinese. Zhongguo Zhen Jiu. 2023 Mar 12;43(3):294–8. doi: 10.13703/j.0255-2930.20220526-0007. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Li X, Gou X, Chen C, Li Z, Zhao C, Huo W, Guo Y, Yang Y, Liu Z. Network meta-analysis of acupoint catgut embedding in treatment of simple obesity. Evid Based Complement Alternat Med. 2022 May 23;2022:6408073–16. doi: 10.1155/2022/6408073. doi: 10.1155/2022/6408073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caliendo M, Gehrsitz M. Obesity and the labor market: a fresh look at the weight penalty. Econ Hum Biol. 2016 Dec;23:209–225. doi: 10.1016/j.ehb.2016.09.004. https://strathprints.strath.ac.uk/59133/ S1570-677X(16)30134-4 [DOI] [PubMed] [Google Scholar]
- 59.Rerksuppaphol S, Rerksuppaphol Lakkana. Waist circumference, waist-to-height ratio and body mass index of Thai children: secular changes and updated reference standards. J Clin Diagn Res. 2014 Nov;8(11):PC05–9. doi: 10.7860/JCDR/2014/10017.5131. https://europepmc.org/abstract/MED/25584277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J, James WPT, Loria CM, Smith SC, International Diabetes Federation Task Force on EpidemiologyPrevention. Hational Heart‚ Lung‚Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009 Oct 20;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644.CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 61.Kou J, Guo T, Wen P, Wei Q, Zhu Y. Bibliometric analysis of the clinical literature of acupoint thread-embedding for simple obesity. Article in Chinese. Shanghai J Acu-Mox. 2016;35(9):1122–1125. doi: 10.13460/j.issn.1005-0957.2016.09.1122. [DOI] [Google Scholar]
- 62.Meissner K, Fässler Margrit, Rücker Gerta, Kleijnen J, Hróbjartsson Asbjorn, Schneider A, Antes G, Linde K. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. 2013 Nov 25;173(21):1941–51. doi: 10.1001/jamainternmed.2013.10391.1748829 [DOI] [PubMed] [Google Scholar]
- 63.Wu XK, Stener-Victorin E, Kuang HY, Ma HL, Gao JS, Xie LZ, Hou L, Hu Z, Shao X, Ge J, Zhang J, Xue H, Xu X, Liang R, Ma H, Yang H, Li W, Huang D, Sun Y, Hao C, Du S, Yang Z, Wang X, Yan Y, Chen X, Fu P, Ding C, Gao Y, Zhou Z, Wang CC, Wu T, Liu J, Ng EHY, Legro RS, Zhang H, PCOSAct Study Group Effect of acupuncture and clomiphene in Chinese women with polycystic ovary syndrome: a randomized clinical trial. JAMA. 2017 Jun 27;317(24):2502–2514. doi: 10.1001/jama.2017.7217. https://europepmc.org/abstract/MED/28655015 .2633915 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) checklist.
Data Availability Statement
The data sets generated or analyzed in this study will not be publicly available. Consent and ethical approval for this study do not include a provision for the sharing of data from this study.


