Abstract
Background:
Hyperbilirubinemia is a common problem in neonates that causes hospitalization. The aim of this study was to investigate the effects of concentrated pomegranate juice (CPJ) consumption by breastfeeding mothers on neonatal hyperbilirubinemia.
Materials and Methods:
In this open-labeled, add-on, randomized clinical trial, 86 breastfeeding mothers and their neonates were allocated into two groups. In the control group, neonates received phototherapy. Besides neonates’ phototherapy in the intervention group, their mothers received CPJ (1 tablespoon [15 g] three times a day) up to discharge. The bilirubin level was the primary outcome. The duration of phototherapy, the duration of hospital stay, and the need for exchange transfusions were secondary outcomes.
Results:
CPJ reduced the bilirubin level of hospitalized neonates within 48 h after consumption (P = 0.048, standard mean difference = 0.648). It also resulted in reduced duration of hospital stay and faster discharge of the neonates. Furthermore, in 48 h after discharge, bilirubin was significantly lower in the CPJ group (P = 0.003, partial eta squared = 0.123).
Conclusion:
Compared to the control group, consumption of CPJ by lactating mothers whose infants underwent phototherapy resulted in lower bilirubin levels, shorter hospital stay, and faster discharge.
Keywords: Complementary therapies, hyperbilirubinemia, infant, jaundice, Persian medicine, Punica granatum
INTRODUCTION
Neonatal jaundice that is seen in 60% of term and 80% of premature neonates is often benign.[1] Bilirubin exists in both direct and indirect forms. Indirect bilirubin is difficult to excrete in the urine because it does not dissolve in water and passes the blood–brain barrier. Therefore, in high concentrations, if left untreated, it can be neurotoxic.[2] Compared to adults, neonates are more sensitive to oxidative reactions. In addition, neonates have a shorter lifespan of red blood cells, which increases unconjugated bilirubin levels.[2]
The goal of treatment in neonatal hyperbilirubinemia is to prevent serum bilirubin levels from reaching neurotoxic levels.[1] Common treatments for neonatal jaundice include phototherapy,[1] exchange transfusions,[3] and medications (phenobarbital, metalloporphyrin, and clofibrate).[4,5] Phototherapy can be associated with complications such as erythematous skin rash, hyperthermia, eye injury, increased insensible fluid loss, diarrhea, weight loss, and hypocalcemia.[6] Exchange transfusions are performed at the time of failure of phototherapy to reduce neonatal bilirubin levels[3] but are associated with possible complications such as metabolic acidosis, hypoglycemia, hypocalcemia, thrombocytopenia, arrhythmia, necrotizing enterocolitis, infection, and even death.[7]
Complimentary or alternative medicines include a wide range of services that are used to improve health services in many countries, which are used alongside conventional medicine to prevent, treat, and restore the health of patients.[8]
Numerous treatments for different types of jaundice are mentioned in traditional medicine references, including Yin et al.,[9] chicory, cucumber seeds, violets, tamarind, mangosteen, milk thistle, peppermint, Portulaca oleracea juice, malt, barberry, jujube, barley flour,[10] Fumaria officinalis, and pomegranate.[11]
Pomegranate with the scientific name of Punica granatum contains components such as anthocyanins, amino acids, potassium, ellagitannins, gallic acid, punicalagin, and ascorbic acid[12] and has high antioxidant properties.[13,14,15]
According to traditional Persian medicine (PM), jaundice is caused by the hotness of the liver.[16,17] Since the pomegranate reduces the heat of the liver, it can help reduce jaundice.[18,19] Neonatal jaundice in PM should also be treated with the mother's treatment in order to prevent complications.[20]
A study has been conducted on the effects of pomegranate on reducing the incidence of neonatal hyperbilirubinemia by consuming its extract by pregnant mothers. In this study, consumption of pomegranate extract by mothers from 34 weeks of gestational age reduced the need for phototherapy and also decreased the mean bilirubin in the neonates compared with the control group.[18] However, the use of this extract by infants or lactating mothers has not been studied to evaluate its effectiveness in reducing bilirubin.
In this study, we investigated the effects of the consumption of concentrated pomegranate juice (CPJ) by breastfeeding mothers on neonatal hyperbilirubinemia.
MATERIALS AND METHODS
Trial design
This prospective, randomized, open-labeled, add-on, parallel-arm clinical trial was performed from December 2020 to February 2021 in Shafizadeh Children's Hospital, Babol University of Medical Sciences (BUMS), North of Iran.
It was approved by the ethics committee of BUMS with the code: IR.MUBABOL.HRI.REC.1398.367 and was also registered in the IRCT with the code: IRCT20200105046009N1. All mothers and also the fathers were given written consent forms.
Participants
Inclusion criteria
Mothers with term infants (gestational age over 37 weeks and birth weight over 2500 g), infants with total serum bilirubin level above 15 mg/dl, and infants over 72 h of age with breastfeeding under phototherapy for the treatment of hyperbilirubinemia in the neonatal ward in Shafizadeh Children's Hospital, BUMS, North of Iran, were included in the study.
Exclusion criteria
Mothers with infants with hemolytic disease (ABO and Rh incompatibility and G6PD deficiency), direct hyperbilirubinemia, jaundice in the first 24 h of life, sepsis, respiratory distress, systemic diseases such as liver, heart, and hypothyroidism, history of blood transfusion, history of using phenobarbital, herbal medicines during the first 72 h of birth, and sensitivity to eating pomegranate juice in mothers were excluded from the study.
Dropout criteria
Dropout criteria of this study were as follows: lack of irregular use of pomegranate juice by mothers (<3 times a day), cessation of breastfeeding for any reason, and consumption of any other herbal medicines during the study by the mother.
Entering the study
The demographic data of neonates and their mothers were recorded before entering the study. Blood samples were taken from mothers to assess blood groups and from infants to determine the probable cause of jaundice. Examinations in neonates included serum levels of total and direct bilirubin, blood groups and Coombs test, G6PD level, TSH, T4, peripheral blood smear, and reticulocyte count.
Intervention
Phototherapy was performed in the standard way and according to the local guideline by phototherapy devices with the same power in two groups of intervention and control neonates until the end of the hospital stay. Five-lamp phototherapy devices with a lifespan of 2000 h from the Philips Company of Germany were used for this study. In the intervention group, in addition to phototherapy (such as the control group), the mothers of neonates received CPJ.
Intervention group
Mothers of neonates with hyperbilirubinemia who were under phototherapy used CPJ, 1 tablespoon (15 g) three times a day (before each meal) until discharge of the neonates. The dose was based on the traditional practices by traditional medicine practitioners.
Preparation and dosage
Sweet fresh pomegranates were bought in autumn (November 2020) from the Behshahr region, North of Iran. The fruit species with the scientific name of P. granatum L. and with the code HNB 11,484 was registered in the herbarium of Nowshahr National Botanical Garden. In a hygienic condition, after peeling, pomegranate arils were separated, and then, pomegranate juice was extracted in a state where the seeds were not crushed. Then, it was concentrated at a gentle temperature in the Bain Marie apparatus to a point where its Brix reached 60%. The microbial limit tests were performed in the Professional Center of Analysis, Institute of Medicinal Plants, Karaj, Iran, and in accordance with the United States Pharmacopeia (USP40), the concentrate was within the allowable range. Finally, the produced concentrated juice was poured into 500 g containers and packed and kept in the refrigerator until consumption.
Determination of phenolic and flavonoid contents
An in-house spectrophotometric analysis method (as presented in supplement) was performed for the total phenolic compound and total flavonoid determination in the dried concentrate in Professional Center of Analysis, Institute of Medicinal Plants, Karaj, Iran. Total phenol content was 13.56 ± 0.33 mg/g (equivalent as gallic acid) and total flavonoid was 1.39 ± 0.32 mg/g (equivalent as catechin).
High-performance liquid chromatography quantification of gallic acid
Gallic acid content was analyzed in the prepared dry concentrate by high-performance liquid chromatography analysis via an in-house method in Professional Center of Analysis, Institute of Medicinal Plants, Karaj, Iran (appendix), based on a previous method.[21] The obtained extract contained gallic acid in the amount of 0.42 ± 0.02%.
Control group
In the control group, only phototherapy was done for neonates.
Mothers in both the groups received the same recommendations in written sheets. It was suggested that they drink adequate fluids, eat fiber foods, and avoid constipation.
Primary outcome
Neonate's total bilirubin levels in blood were the primary outcome.
Secondary outcomes
The duration of phototherapy, the duration of hospital stay, and the need for exchange transfusions were the secondary outcomes.
Follow-up
Bilirubin level of neonates in the two study groups was checked every 12 h until discharge and 48 h after discharge with the Diaz manner (photometer) with the Faraz Kav treatment kit made in Iran.
Evaluation of serum levels of total bilirubin was done according to the local guideline,[22] so neonates were discharged after reaching below 10 mg/dl. Infants who, despite treatment with phototherapy, reach an increase in bilirubin to the point of exchange transfusion (20 mg/dl in infants with risk factors, and 25 mg/dl in infants without risk factors) as the failure of phototherapy were referred to exchange transfusion. Other routine cares for both the groups were the same.
Sample size
The sample size, taking into account the expected average changes of 3.5 and standard deviation of 5, based on the formula for comparing the means, was at least 34, and considering the drop rate of 25%, the sample size was considered 43 in each group and a total of 86 infants.[23]
Randomization
Performed block randomization was done for two groups in blocks of 4. Patients were randomly divided into two groups of CPJ and control. Random permuted blocks (blocks containing 2 of A [CPJ] and B [control], ratio 1:1) were prepared and eligible samples were assigned to two groups. The cards with different quadruple combinations of letters A and B were produced. The random sequence was generated by online software sealed envelope (https://www.sealedenvelope.com/simple-randomiser/v1/lists).
Blinding
Since the drug was administered to only one group, blinding was not possible in this study.
Statistical methods
Data were analyzed using SPSS V.22 software (SPSS Inc., Chicago, Ill., USA). We present the descriptive analysis of quantitative data with mean and standard deviation and for qualitative variables with frequency (percentage). To evaluate and compare the variables in the two groups, at the beginning of the study, independent t-test (in case of abnormality: U Mann–Whitney) and Chi-square (if necessary, Fisher's exact test) were used. To evaluate the effect of the intervention on neonatal jaundice (bilirubin), generalized estimating equations were used. We used Kaplan-Meir analysis of covariance to evaluate the effect of CPJ on bilirubin changes at 48 hours after discharge between groups. Survival analysis (log-rank test) and Cox regression were used to compare the length of hospital stay in the two treatment groups. Controlling the effect of birth weight as a potential confounder, we used repeated measures analysis and entered this parameter as a covariate to the model. Furthermore, we entered the maternal weight as a covariate to the Cox regression to control its effect on the duration of hospital stay.
In addition, for sensitivity analysis, all steps of the above analysis were performed once with the per-protocol (PP) method and once with the intention to treat (ITT) method. In PP, all individuals who were present at the end of treatment and completed the study protocol correctly were analyzed. In the ITT method, all subjects who entered the study and the randomization process were included in the analysis. Since there was no missing data in this study and all infants completed phototherapy treatment, the results of PP and ITT analysis were the same. In all cases, the significance level was considered less than 0.05.
To calculate the effect size of relative risk (RR), absolute risk reduction (ARD), and number needed to treat (NNT) effects, considering that the criterion for neonatal discharge is reaching a level of bilirubin below 10, in this study, a recovery level of 10 was considered. The mentioned cases were calculated and evaluated two times of 48 h and 72 h after hospitalization.
RESULTS
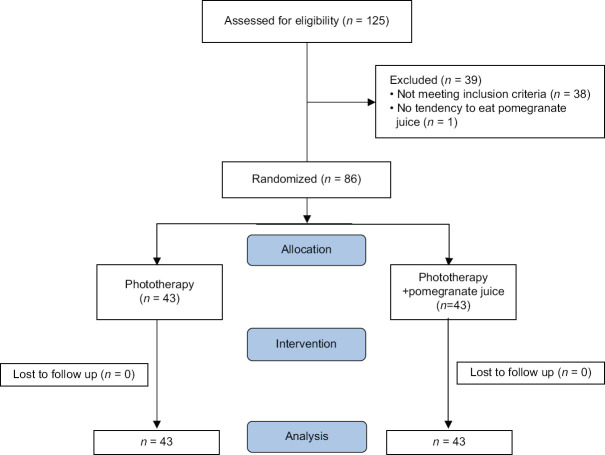
Out of 125 neonates referred to Amirkola Children's Hospital, 86 patients (43 in the intervention group and 43 in the control group) randomly entered the study. The flowchart of the study is shown in Figure 1. Thirty-nine neonates were boys (45.34%) and 47 were girls (54.66%). There was no statistical difference between the two groups in demographic data. The details are shown in Table 1.
Figure 1.
The flowchart of the study
Table 1.
The baseline data in two groups of phototherapy and phototherapy + concentrated pomegranate juice
| Demographic data | Phototherapy (n=43) | Phototherapy + CPJ (n=43) | P |
|---|---|---|---|
| Neonate | |||
| Sex, male, n (%) | 19 (44.2) | 20 (46.5) | 0.829 |
| Sex, female, n (%) | 24 (55.8) | 23 (53.5) | |
| Age at hospitalization, days, mean±SD | 5.31±2.09 | 5.64±1.94 | 0.456 |
| Weight (g), mean±SD | 3099.1±348.68 | 2982±238.27 | 0.120 |
| Total bilirubin at admission (mg/dL), mean±SD | 17.61±2.54 | 18.01±2.08 | 0.487 |
| Mother | |||
| Delivery, n (%) | |||
| Cesarian | 29 (67.45) | 28 (65.12) | 0.820 |
| NVD | 14 (32.55) | 15 (34.88) | |
| Weight (kg), mean±SD | 80.39±12.25 | 78.21±15.41 | 0.470 |
CPJ=Concentrated pomegranate juice; SD=Standard deviation; NVD=Normal vaginal delivery
Primary outcome
In both the groups, bilirubin levels decreased during the hospital stay. However, a significant difference between the two groups began 48 h after the start of phototherapy in the CPJ group (P = 0.048, standard mean difference (SMD) =0.648). This difference has increased over time (P = 0.003, SMD = 1.191) [Figure 2].
Figure 2.
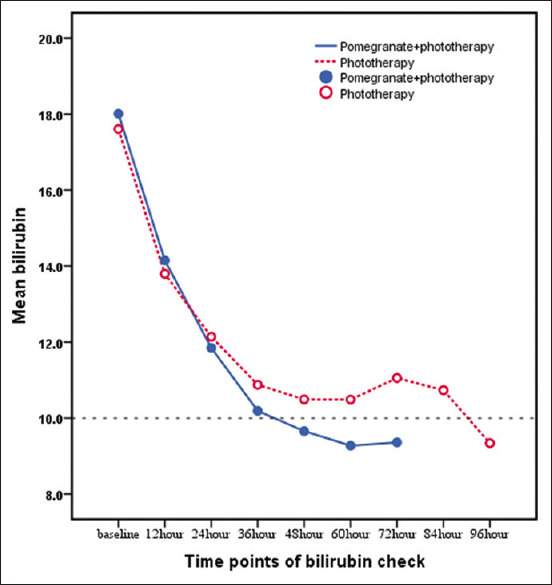
Trend of bilirubin changes during hospitalization in the two groups of phototherapy and phototherapy + CPJ. CPJ = Concentrated pomegranate juice
The bilirubin level in the two groups at the time of discharge was not significantly different (P = 0.758) which was due to the similar protocol in both the groups for discharge (serum bilirubin <10 mg/dl). However, in 48 h after discharge (compared with the bilirubin at discharge time), bilirubin was significantly lower in the CPJ group (P = 0.003, partial eta squared = 0.123). The details are shown in Table 2.
Table 2.
The bilirubin levels in two groups of phototherapy and phototherapy + concentrated pomegranate juice
| Bilirubin level assessment (mg/dL), (n), mean±SD | Phototherapy | Phototherapy + CPJ | P a | P b |
|---|---|---|---|---|
| At the time of hospitalization | 17.61±2.54 (n=43) | 18.01±2.08 (n=43) | 0.487 | 0.005 |
| After hospitalization (h) | ||||
| 12 | 13.79±1.78 (n=43) | 14.15±1.71 (n=43) | 0.338 | |
| 24 | 11.99±2.06 (n=43) | 11.85±1.38 (n=43) | 0.486 | |
| 36 | 10.88±1.71 (n=41) | 10.19±1.38 (n=41) | 0.106 | |
| 48 | 10.49±1.49 (n=30) | 9.66±1.03 (n=23) | 0.048 | |
| 60 | 11.06±1.56 (n=22) | 9.36±1.28 (n=17) | 0.003 | |
| At discharge time | 9.33±1.00 (n=43) | 9.27±0.88 (n=43) | 0.758 | 0.003 |
| 48 h after discharge | 10.87±2.38 (n=43) | 9.41±1.68 (n=43) | 0.003 |
aUsing EM means of the GEE post hoc tests; bBetween-group comparison with GEE analysis. Significant P values are shown as bold text. CPJ=Concentrated pomegranate juice; SD=Standard deviation; GEE=Generalized estimating equations; EM=Expectation–Maximization
Secondary outcomes
The mean duration of phototherapy in all study neonates (both the groups) was 58.4 ± 2.13 (median: 58) h. The mean duration of phototherapy in the CPJ group was 52.02 ± 2.46 h (median = 50, confidence interval [CI] 95% =47.19–56.85) that was significantly shorter than the phototherapy group that was 64.87 ± 3.21 h (median = 60, CI 95% =58.58–71.16) (P = 0.003). The details of survival analysis of duration of phototherapy are shown in Figure 3.
Figure 3.
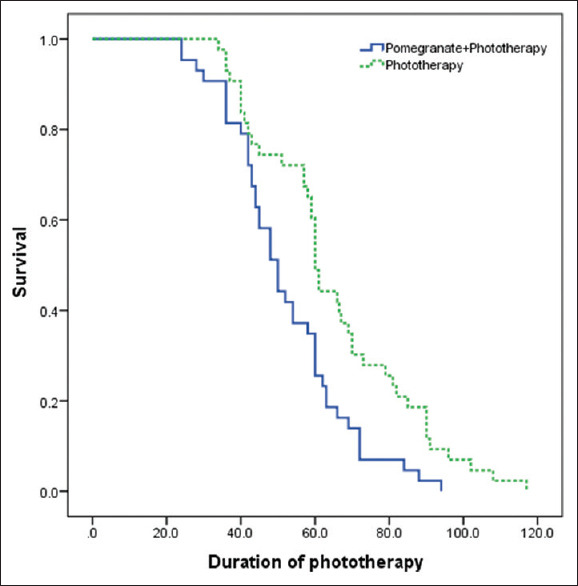
Survival chart of phototherapy duration (in terms of hours) in the two groups
In the CPJ group, all neonates were discharged up to 96 h after the start of treatment, while in the control group, this period was a maximum of 114 h of hospitalization.
The effect of consuming CPJ on bilirubin was different based on different birth weights. For each gram of neonate's weight gain, the amount of bilirubin decreased by a factor of 0.001 over time (P = 0.036). However, the maternal weight does not affect the duration of hospitalization of infants (P = 0.967) and the rate of bilirubin reduction (P = 0.737).
Furthermore, the sex of the infant did not affect bilirubin reduction in both the intervention and control groups (P = 0.233).
In the first 48 h, ARD was 16.28% (CI 95% = −3.41–35.96% and RR = 1.28). This means that in the CPJ group, a higher probability of recovery was at the rate of 16.28%. In the first 72 h, ARD was 13.95% (CI 95% =0.73%–27.18% and RR = 1.17) which means that, in the CPJ group, there was a 13.95% higher probability of recovery.
Exacerbation of hyperbilirubinemia requiring exchange transfusions was not observed in either group. There were no complications attributable to pomegranate in the group receiving CPJ.
DISCUSSION
In this randomized, parallel-group, add-on clinical trial, oral consumption of CPJ by breastfeeding mothers significantly reduced the bilirubin level of hospitalized neonates due to hyperbilirubinemia within 48 h after consumption. It also reduced the duration of hospital stay and faster discharge of the neonates.
Based on our best knowledge, this study is the first trial using CPJ in breastfeeding mothers. However, our results are confirmed with the results of the Seeram et al. and Henning et al.'s studies that showed the early detection of polyphenolic compounds in breast milk after consuming pomegranate juice by the mothers. In the study of Seeram et al., it was shown that after 6 h of consuming pomegranate juice, ellagitannin rises in the blood and after 24 h excreted through urine.[24] Henning et al. also measured the polyphenolic compounds of pomegranate in breast milk on days 7 and 14 after consuming pomegranate juice. They showed that pomegranate juice increases biomarkers such as urolithin-A and ellagitannin, which, due to their antioxidant properties, can play an effective role in strengthening the baby's immune system and reducing oxidative reactions. In addition, this study showed that in some patients, ellagitannin is secreted in breast milk on the 1st day after consumption.[25] Furthermore, according to an animal study, pomegranate increases the total antioxidant content of milk in ewes.[26]
NNT was 7 in the first 48 h of the study, indicating that in the first 48 h of hospitalization, from every 7 neonates enrolled, 1 recovered. NNT was 8 in the first 72 h of treatment, indicating that in the first 72 h, of every 8 neonates who entered the study, 1 recovered.
Only neonate's weight affects on bilirubin levels in the CPJ group (decrease by 0.001/gram of weight), but other factors such as maternal weight and infant sex did not affect the treatment process.
The results of our study match with the results of Manouchehrian et al. on the effectiveness of pomegranate juice consumption by pregnant women on neonatal jaundice. In their study, 80 healthy pregnant women between the ages of 18 and 35 were divided into intervention and control groups. Pomegranate extract was given from the 34th week of pregnancy up to the delivery. On the 5th day after birth, bilirubin levels were measured. Compared with the control group, the intervention group needed less phototherapy and had a significantly lower mean bilirubin level.[18]
Pomegranate is a plant native to Iran. Based on PM theories, the etiology of jaundice is the increased warmness of the liver. Therefore, as the Mizaj (temperament) of pomegranate is cold and has an affinity to the liver, it can reduce jaundice. Furthermore, the results of our study approve the theory of PM that proposes the best way of managing infants via modifying their breastfeeding mothers.[16,20]
Pomegranate contains many active ingredients including gallic acid, tannins, antioxidants, phenols, and ascorbic acid (Vitamin C). Since the antioxidant system of neonates is weak, neonates are more prone to oxidative damage that can cause hemoglobin damage and hyperbilirubinemia. It seems that pomegranate can be effective in modulating oxidative stress reactions and the regeneration of antioxidants in the body.
In the study of Khadem Al-Hosseini et al., 500 mg of Vitamin C was given to pregnant mothers once a day from the 34th week of pregnancy. Vitamin C with probably antioxidant effects reduced neonatal bilirubin levels on the 5th day of the study, compared with the control group.[27] In our study, bilirubin levels were measured in neonates every 12 h until discharge. Although changes in bilirubin levels were observed in the first 36 h of hospitalization, the first significant effect of CPJ on reducing bilirubin levels was observed after 48 h of its consumption. It can be due to the delayed secretory phase of CPJ in breast milk.
Perhaps if in our study, regarding ethical considerations, neonates could have consumed CPJ (as in the Monsef et al.'s[28] study), the effect of lowering bilirubin levels would have appeared sooner.
In a clinical trial study conducted by Monsef et al., concomitant use of Biliniser drops both by mothers and neonates was more effective in reducing neonatal hyperbilirubinemia compared with the groups receiving the drops just in mothers or neonates.[28]
No side effects were seen in the mothers and neonates in our study. No exchange transfusion was performed in any infants in the two groups.
Given that in our study, the reducing effects of CPJ on bilirubin levels remained stable for up to 48 h after discharge, it may be possible to discharge neonates with higher levels of bilirubin that can cause in reducing the hospital stay, the cost of hospitalization, and reducing the stress of separation of mothers and neonates. Of course, it needs further studies.
Since the average length of hospital stay of neonates in the intervention group was reduced by an average of 12 h (from 2.7 days to 2.1 days), it can be important in general policymaking in this field to reduce the need for new hospital wards and beds.
One of the limitations of our study was that the antioxidant content of breast milk was not measured during the consumption of CPJ. Measuring serum levels of gallic acid, tannins, and other active ingredients of pomegranate in the milk of breastfeeding mothers could help make better conclusions.
Evaluating the bilirubin level 48 h after discharge was one of the most important strengths of our study. Evaluating more times after discharge is suggested for future studies.
CONCLUSION
The results of this study show that consumption of CPJ in breastfeeding mothers whose neonates underwent phototherapy led to a decrease in bilirubin levels, reduced length of hospital stay, and faster discharge than the control group.
Financial support and sponsorship
It was funded by BUMS.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank stuffs of the neonatal ward of Shafizadeh Children's Hospital, Babol, Iran.
REFERENCES
- 1.Robert K. Nelson Textbook of Pediatrics, 2-Volume Set. 21st ed. Philadelphia, USA: Joseph St. Geme; 2019. [Google Scholar]
- 2.Ansong-Assoku B, Shah SD, Adnan M, Ankola PA. Neonatal Jaundice. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [Last updated on 2022 Feb 19]. Available from: https://www.ncbi.nlm.nih. gov/books/NBK532930 . [PubMed] [Google Scholar]
- 3.Zahed Pasha Y, Alizadeh-Tabari S, Zahed Pasha E, Zamani M. Etiology and therapeutic management of neonatal jaundice in Iran: A systematic review and meta-analysis. World J Pediatr. 2020;16:480–93. doi: 10.1007/s12519-020-00339-3. [DOI] [PubMed] [Google Scholar]
- 4.Dennery PA. Pharmacological interventions for the treatment of neonatal jaundice. Semin Neonatol. 2002;7:111–9. doi: 10.1053/siny.2002.0098. [DOI] [PubMed] [Google Scholar]
- 5.Aygün E, Semerci SY. Prolonged Jaundice in Newborn. Topics on Critical Issues in Neonatal Care. 2022:49. [Google Scholar]
- 6.Abohussein HA, Fahmy BS, Khalil AM, Fahmy YA. Effect of phototherapy on electrolytes, liver and kidney functions during treatment of neonatal hyperbilirubinemia. A prospective-analytical study. Ann Neonatol J. 2022;9:1–9. [Google Scholar]
- 7.Boskabadi H, Khodashenas E, Bagheri F, Behgam N, Zakerihamidi M. Evaluation of hematologic factors and bilirubin following exchange transfusion in neonatal hyperbilirubinemia. Transfus Apher Sci. 2022;61:103451. doi: 10.1016/j.transci.2022.103451. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019. Gneva: World Health Organization; 2019. [Google Scholar]
- 9.Yin J, Wennberg RP, Miller M. Induction of hepatic bilirubin and drug metabolizing enzymes by individual herbs present in the traditional Chinese medicine, Yin Zhi Huang. Dev Pharmacol Ther. 1993;20:186–94. doi: 10.1159/000457561. [DOI] [PubMed] [Google Scholar]
- 10.Mohsenzadeh A, Javidi KJ, Yari F. Effect Of barely flour on jaundice in full-term neonates. Yafte. 2005;25:17–22. [Google Scholar]
- 11.Nabavizadeh SH, Nabavi M. The effect of herbal drugs on neonatal jaundice. Iranian Journal of Pharmaceutical Research. 2010;Supplement 2:39–40. [Google Scholar]
- 12.Kostka T, Ostberg-Potthoff JJ, Briviba K, Matsugo S, Winterhalter P, Esatbeyoglu T. Pomegranate (Punica granatum L.) extract and its anthocyanin and copigment fractions-free radical scavenging activity and influence on cellular oxidative stress. Foods. 2020;9:1617. doi: 10.3390/foods9111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposto S, Veneziani G, Taticchi A, Urbani S, Selvaggini R, Sordini B, et al. Chemical composition, antioxidant activity, and sensory characterization of commercial pomegranate juices. Antioxidants (Basel) 2021;10:1381. doi: 10.3390/antiox10091381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argov-Argaman N, Cohen-Zinder M, Leibovich H, Yishay M, Eitam H, Agmon R, et al. Dietary pomegranate peel improves milk quality of lactating ewes: Emphasis on milk fat globule membrane properties and antioxidative traits. Food Chem. 2020;313:125822. doi: 10.1016/j.foodchem.2019.125822. [DOI] [PubMed] [Google Scholar]
- 15.Sihag S, Pal A, Saharan V. Antioxidant properties and free radicals scavenging activities of Pomegranate (Punica granatum L.) peels: An in-vitro study. Biocatal Agric Biotechnol. 2022;42:102368. [Google Scholar]
- 16.Tabari A. In: Ferdows al-Hekmah Fi Al-Tibb (Paradise of Wisdom on Medicine) 1st ed. Siddiqi MZ, editor. Berlin: Aftab Press; 1928. pp. 167–8. [Google Scholar]
- 17.Rezaeizadeh H, Alizadeh M, Naseri M, Ardakani MR Shams. “The traditional Iranian medicine point of view on health and disease.”. 2009:169–72. [Google Scholar]
- 18.Manouchehrian M, Shakiba M, Shariat M, Lotfi MH, Kamalinejad M, Babaeian M. The effect of pomegranate paste on neonatal jaundice incidence: A clinical trial in women during pregnancy. Int J Clin Med. 2017;8:144. [Google Scholar]
- 19.Aghili Mohammad Hosein. Qarabadin-e-Kabir. Tehran: Research Institute for Islamic and Complementary Medicine Tehran; 2007. [Google Scholar]
- 20.Ibn-e-Sina A. Al-Qanun Fit-Tib [The Canon of Medicine] Beirut, Lebanon: Alaalami Beirut lib Press; 2055. [Google Scholar]
- 21.Monajjemi M, Ilkhani A, Aminin AN, Eghdami A, Mollaamin F, Rezaei S. High-performance liquid chromatography (HPLC) nano analysis of antioxidant compounds of Iranian medicinal plants. J Med Plant Res. 2012;6:3459–63. [Google Scholar]
- 22.Ahmadpour KM, Zahedpasha Y, Peydayesh S, Mazloomi A. Assessment of bilirubin to albumin ratio as a criterion for exchange transfusion in severe neonatal hyperbilirubinemia. Int J Pediatr. 2017;5:5053–70. [Google Scholar]
- 23.Slusher TM, Vreman HJ, Brearley AM, Vaucher YE, Wong RJ, Stevenson DK, et al. Filtered sunlight versus intensive electric powered phototherapy in moderate-to-severe neonatal hyperbilirubinaemia: A randomised controlled non-inferiority trial. Lancet Glob Health. 2018;6:e1122–31. doi: 10.1016/S2214-109X(18)30373-5. [DOI] [PubMed] [Google Scholar]
- 24.Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136:2481–5. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 25.Henning S, Wallenstein M, Weigel N, Johnson C, Yang J, Lee R, et al. Appearance of ellagic acid metabolites from pomegranate juice in breast milk: A case report. Ann Clin Case Rep. 2019;4:1738. [Google Scholar]
- 26.Valenti B, Luciano G, Morbidini L, Rossetti U, Codini M, Avondo M, et al. Dietary pomegranate pulp: Effect on ewe milk quality during late lactation. Animals (Basel) 2019;9:283. doi: 10.3390/ani9050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khadem Al-Hosseini M, Rahideh ST, Saadati A, Rahmati N, Azadeh F, Janani L, et al. The effect of vitamin C supplementation in the last month of pregnancy on neonatal bilirubin levels; a double-blind randomized clinical trial. Complement Ther Med. 2020;50:102359. doi: 10.1016/j.ctim.2020.102359. [DOI] [PubMed] [Google Scholar]
- 28.Monsef A, Eghbalian F, Rahimi N. Comparison of purgative manna drop and phototherapy with phototherapy treatment of neonatal jaundice: A randomized double-blind clinical trial. Osong Public Health Res Perspect. 2019;10:152–7. doi: 10.24171/j.phrp.2019.10.3.06. [DOI] [PMC free article] [PubMed] [Google Scholar]