Abstract
Background:
This study aimed to investigate reference Doppler velocimetry indices (DVIs) of the fetal ductus venosus (DV) during 11–13 + 6 gestational weeks.
Materials and Methods:
In a prospective observation over referrals to a single tertiary care center in a 2-year interval, normal singleton pregnancies with fetal crown-rump lengths (CRLs) of 43–80 mm were examined by a single experienced sonographer for their DV pulsatility index (DVPI), DV resistance index (DVRI), and S-wave maximum velocity/A-wave minimum velocity (S/A ratio). Multinomial and quantile regression functions were used to analyze the effect of gestational age (estimated by CRL) on reference values (5th and 95th percentiles of the distribution in each gestational day/week). P < 0.05 was considered significant.
Results:
Over a sample of 415 participants with a mean/median gestational age of 12 + 1 weeks, no significant correlations were found between the CRL and DVIs using multinomial regression functions (linear model best fitted for all [DVPI: B coefficient = 0.001, P = 0.235] [DVRI: B coefficient = 0.001, P = 0.287] [DV S/A: B coefficient = 0.010, P = 283]). Quantile regression analyses of DVIs’ reference values were nonsignificant across the CRL range except for the DVRI ([5th regression line: coefficient = −0.004, P = 0.018] [95th regression line: coefficient = −0.001, P = 0.030]).
Conclusion:
Reference values for DVPI, DVRI, and DV S/A ratios were established as 0.80–1.39, 0.62–0.88, and 2.57–6.70, respectively. Future meta-analyses and multicenter studies are required to incorporate DV DVIs into an updated universal version of the practice.
Keywords: Aneuploidy, color Doppler ultrasonography, ductus venosus, first pregnancy trimester, prenatal diagnosis, pulsatility index, reference values
INTRODUCTION
Living creatures are primarily concerned with the health of their young, and humans have always shown a similar concern. Medical diagnostic tools continue to evolve as science and technology advance, providing novel tests to assess the health status of human beings before they are born. Since the introduction of ultrasonography (US), many questions have arisen regarding how US can help medical staff recognize the health status of fetuses and embryos.
Aneuploidy is a common chromosomal disorder, defined as extra, missing, or malconformed chromosomes, usually causing a nonviable embryo or fetus.[1,2,3] Then there are those born with aneuploidy, who suffer from syndromal disorders, imposing enormous physical, psychological, and financial burdens on them, their families, and society.[4,5,6]
Since aneuploidy might complicate every pregnancy, the American College of Obstetrics and Gynecology recommends routine screening for this category of anomalies during the 11–13 + 6 weeks of gestation.[7,8]
Nuchal translucency (NT) and visualization of the nasal bone (NB) are well-known, established US measures widely used to predict aneuploidy in combination with laboratory tests during the first pregnancy trimester.[9,10,11] However, it is not specific enough to indicate aneuploidy without the further need for aggressive tests such as chorion villus sampling or amniocentesis.[12,13] Although cell-free fetal DNA validation has been introduced recently as a noninvasive and highly accurate method for this purpose, it is con sidered high-cost and hard to afford within middle-to-low-income countries.[14,15] Besides, this method is not as informative to replace the current screening methods.[16]
A fetal circulatory system is formed through an intricate organogenesis process resulting from interactions between embryonic tissues.[17] Therefore, it is unsurprising to expect cardiovascular malfunctions among anomalous fetuses.[18]
During the routine recommended US screening for aneuploidy between 11 and 13 + 6 weeks of gestation, circulatory competence may be assessed using the Doppler pulsed-wave records of the blood flow through ductus venosus (DV).[19] The DV connects the umbilical vein to the inferior vena cava, which provides oxygenated blood for fetal circulation. Blood flow through DV shows a pulsatile feature throughout a beat-to-beat heart cycle; its characteristics could be reported qualitatively or quantitatively.[20]
The qualitative assessment focuses on the shape of a triphasic flow velocity waveform during a heartbeat cycle. The S and D waves represent maximum blood flow velocity during ventricular systole and early diastole, respectively. Fetal atrial contraction follows, producing a nadir in late diastole, which characterizes the A-wave. Reversal or absence of an anterograde blood flow at the A-wave during the first trimester of pregnancy is believed to associate with chromosomal abnormalities, congenital heart defects, and adverse pregnancy outcomes.[21]
DV pulsatility index (DVPI), DV resistance index (DVRI), and DV S/A ratio stand as the quantitative evaluators of blood flow through DV; the first two can be calculated using the following formulae:  Time-averaged maximum velocity (TAMV) is the area under a waveform diagram.[20]
Time-averaged maximum velocity (TAMV) is the area under a waveform diagram.[20]
Resembling the reversed A-wave, increased DVPI, DVRI, or DV S/A have been reported as predictors of an adverse pregnancy outcome.[22] These indices are continuous, unitless, and numerical measures of blood flow through the DV. As such, it is more precise and consistent than reporting waveform shapes.[23] Yet, reference values must be introduced to differentiate normal versus abnormal blood flow.
Incorporating into routine US assessments during the first trimester of pregnancy, DV Doppler velocity indices may enhance the predictive capabilities of regular screenings for aneuploidy, decreasing unnecessary invasive diagnostic procedures by reducing false-positive rates.[24,25] Some studies have suggested standard reference intervals for these indices between the 11 and 13 + 6 weeks of gestation; however, their results could not be applied universally. Thus, this study aims to establish reference DVPI, DVRI, and DV S/A ratio ranges between 11 and 13 + 6 weeks of gestation among ordinary Iranian fetuses.
METHODS
The national and local ethics committees for medical research approved the study's design as it was in line with the Declaration of Helsinki. Spoken and written informed consent was obtained from every eligible candidate to participate in this anterograde observational study.
From June 2018 to June 2020, all pregnant referrals to the US ward of Al-Zahra hospital, Isfahan (the biggest tertiary care center in Iran) were included in the study. Their referral was part of a routine screening program for chromosomal abnormalities during the first trimester (combined test). This program provides all pregnant women with NT thickness, maternal serum pregnancy-associated plasma protein-A, and free beta-human chorionic gonadotrophin measurements during 11–13 + 6 weeks of gestation.[12] To eliminate confounders, the following criteria were set as not-to-include after taking a careful history and examining the fetal ultrasonographic anatomy:
Multiple pregnancies
Inability to achieve satisfactory waveforms
Fetal crown-rump length (CRL) of less than 43 or more than 80 mm during US (concordant with the gestational age between 11 and 13 + 6 weeks based on the standards introduced by The International Fetal and Newborn Growth Consortium for the 21st Century)[26]
The presence of any discoverable fetal structural malformation or potential chromosomal abnormality during US: NT thickness of more than 95th percentile for gestational age, NB hypoplasia, or any other detectable major fetal abnormality (especially heart defects)
History of previous abnormal pregnancy outcomes, including stillbirth, miscarriages more than twice, or giving birth to chromosomally or functionally abnormal neonates
Any known maternal or paternal chromosomal abnormalities or translocations
History of receiving medications in the categories D or X for pregnancy since 4 months before the examination
History of maternal overt diabetes mellitus, documented hypertension, or confirmed cardiac defects (other than mild valvular stenosis or regurgitation)
Any high-risk combined test results for chromosomal abnormalities (adjusted risk >1:350)[27]
Absent or reversed A-wave during the DV Doppler US.
As the study was intended to introduce reference values for normal pregnancies with expected outcomes, all women included in the previous step were followed until the delivery. Meanwhile, the following exclusion criteria were applied to eliminate unhealthy cases:
Adverse pregnancy outcomes: Intrauterine growth restriction (IUGR), determined chromosomal or structural abnormality in the newborn by a pediatrician at the labor, miscarriages, or stillbirths
Women lost to follow-up.
Follow-up data were extracted from the hospital records. An interview was conducted over the phone with individuals whose profiles were unavailable in the hospital setting.
The transabdominal US combined with color and pulsed wave Doppler was performed for every patient. A GE Voluson Expert 730 system (GE Electric Medical System, Milwaukee, WI, USA) was used with an abdominal convex probe of 3.5 MHz. These specific scans were all carried out by a radiologist with over 10 years of experience in obstetric and venous Doppler US. CRL and NT thickness was measured for each patient using standard protocols.[28]
DVPI was measured as part of the first-trimester screening, fulfilling the below requirements:
Undertaking the procedure under fetal quiescence
Appropriate magnification so that the fetal thorax and abdomen occupy the entire screen
Achieving a right ventral mid-sagittal view of the fetal trunk
Locating the pulsed Doppler gate on the distal part of the umbilical sinus
Placing a small sample volume (0.5–1 mm) in the yellowish aliasing area immediately above the umbilical sinus. This measure was essential to avoid contamination from the adjacent veins.
At least a 30° insonation angle
Setting a low-frequency filter between 50 and 70 Hz
A sweep speed of 2–3 cm/s to spread out the waveforms widely
Adherence to the as low as a reasonably achievable principle, using the Food and Drug Administration (FDA)-approved thermal sensitive system.[29]
There should have been at least three typical high-quality DV waveforms obtained given the intrafetal variation so that manual tracing could be done and the machine could measure and record DVPI, DVRI, and S/A ratio [Figure 1].
Figure 1.
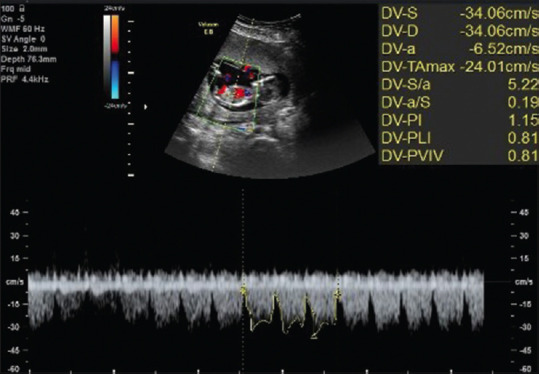
The color and pulsed Doppler ultrasonographic visualization of DV. A mid-sagittal view of the fetal trunk shows DV in the distal portion of the umbilical vein (upper part). Three typical DV waveforms were manually traced (lower part); DVPI, DVPLI (equivalent to DVRI), and DV S/A were extracted (upper right part). DV = Ductus venosus, DVPI = DV pulsatility index, DVPLI = DV preload index, DVRI = DV resistance index
The collected data, including maternal age, gestational age (estimated using CRL), fetal CRL, NT, DVPI, DVRI, and DV S/A ratio, were analyzed using IBM SPSS for Windows software package (v. 26, SPSS Inc., Chicago, IL, USA).
Quantitative and qualitative variables were reported as mean ± standard deviation and percentage/count, respectively.
The normality of each distribution was assessed by the Kolmogorov–Smirnov (n < 30) or Shapiro–Wilk test (n ≥ 30). For distributions other than Gaussian, median and interquartile ranges were reported.
Reference values were defined as between the 5th and 95th percentiles of the measured spectrum for the variables DVPI, DVRI, and S/A ratio, distributed over the CRL range. Multinomial regression analysis was used to identify the most accurate predicting model (the simplest model with the significantly highest R2) for each of the above indices based on the independent variable CRL. If a linear model was shown to best fit, the regression conditions, including homoscedasticity, Gaussian distribution of the dependent variable across each CRL in millimeters, and absence of outliers, were tried to be prepared.
Finally, median, 5th, and 95th percentile regression lines were designated to describe reference values across the CRL range by quantile regression function.
As it might be challenging to put reference values into practice for each millimeter of CRL, these values were further introduced for each gestational week-12, 13, and 14 in this study.
RESULTS
A total of 431 participants were eligible for inclusion, of whom three were later diagnosed with IUGR and two with abnormal morphology or structural defect after birth. Ten participants experienced miscarriage or stillbirth, and one was lost to follow-up. Table 1 provides the descriptive findings of this study at a glance.
Table 1.
Demographic and ultrasonographic indices within the study sample
| Mean±SD | Median (IQR) | Minimum–maximum | |
|---|---|---|---|
| Maternal age (year) | 28±5 | 27 (24–31) | 20–37 |
| NT (mm) | 1.30±0.28 | 1.30 (1.10–1.42) | 0.60–2.48 |
| CRL (mm) | 57.39±7.26 | 57 (51–63) | 43–80 |
| DVPI | 1.06±0.18 | 1.06 (0.95–1.14) | 0.1–1.89 |
| DVRI* | 0.77±0.76 | 0.78 (0.73–0.82) | 0.50–0.98 |
| DV S/A ratio* | 4.17±1.40 | 3.90 (3.22–4.79) | 1.80–10.18 |
*Missing data included 16 and 99 cases for DVRI and S/D ratio, respectively. None of the above variables showed normal distribution based on the Shapiro-Wilk test (P<0.05). CRL=Crown-rump length; DV=Ductus venosus; DVPI=DV pulsatility index; DVRI=DV resistance index; IQR=Interquartile range; SD=Standard deviation; NT=Nuchal translucency; S/A=S-wave maximum velocity/A-wave minimum velocity
Reference values for DV Doppler velocimetry indices (DVIs) are illustrated in Table 2, separately for each gestational week.
Table 2.
Estimated percentiles for ductus venosus Doppler velocity indices across gestational ages
| Gestational age* (week) | n | DVPI | DVRI† | DV S/A ratio‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| 5th | 50th | 95th | 5th | 50th | 95th | 5th | 50th | 95th | ||
| 12 | 160 | 0.76 | 1.03 | 1.36 | 0.66 | 0.78 | 0.88 | 2.54 | 3.87 | 6.68 |
| 13 | 207 | 0.81 | 1.06 | 1.39 | 0.61 | 0.78 | 0.88 | 2.57 | 3.95 | 6.70 |
| 14 | 48 | 0.76 | 1.10 | 1.45 | 0.61 | 0.78 | 0.92 | 2.41 | 3.88 | 6.94 |
| Total | 415 | 0.80 | 1.06 | 1.39 | 0.62 | 0.78 | 0.88 | 2.57 | 3.90 | 6.70 |
*Gestational age was estimated based on CRL; †DVRI had 3, 12 and 1 missing values within 12, 13 and 14 gestational weeks, respectively;. ‡S/A ratio had two, five, and three missing values within 12, 13 and 14 gestational weeks, respectively. DV=Ductus venosus; DVPI=DV pulsatility index; DVRI=DV resistance index; CRL=Crown-rump length; S/A=S-wave maximum velocity/A-wave minimum velocity
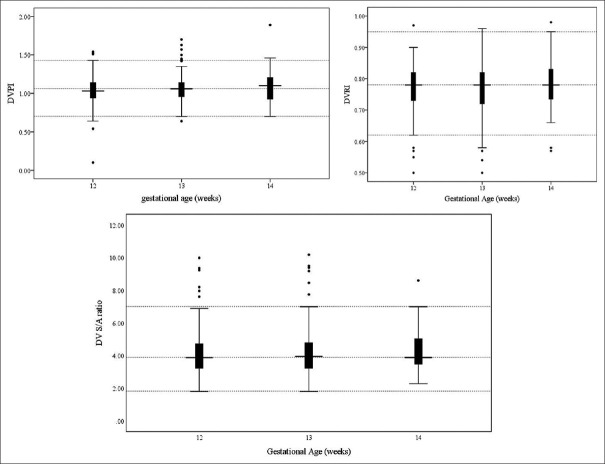
Linear regression model showed superior to multinomial models in predicting correlations between the CRL and the DV DVIs, proving no significant association (DVPI: B coefficient = 0.001, P = 0.235) (DVRI: B coefficient = 0.001, P = 0.287) (DV S/A: B coefficient = 0.010, P = 283). As none of the above variables was normally distributed, neither among the total sample nor among participants with the same gestational age (P < 0.05), box and whisker plots were generated to show the distribution of these variables over each gestational week [Figure 2].
Figure 2.
Box and whisker plots illustrating the distributions of ductus venosus Doppler velocity indices across each gestational week. CRL = Crown-rump length, DV = Ductus venosus, DVPI = Ductus venosus pulsatility index, DVRI = Ductus venosus resistance index
Positive otherwise non-significant correlations were found in all quantile regressions for DVPI and DV S/A ratio across the CRL. Negative correlations were reached in all quantile regressions for DVRI across the CRL; only the coefficients for the 5th and 95th percentile regressions were significant [Table 3].
Table 3.
The quantile regression analysis for fetal ductus venosus Doppler velocity indices
| Dependent variable | Percentile regression | CRL parameter estimates | |
|---|---|---|---|
|
| |||
| Coefficient (SE) [95% CI] | P | ||
| DVPI | 5th | 4.228E-18 (0.0035) [−0.007–0.007] | 1.000 |
| Median | 0.002 (0.0013) [−0.001–0.005] | 0.132 | |
| 95th | 0.003 (0.0039) [−0.005–0.011] | 0.422 | |
| DVRI | 5th | −0.004 (0.0018) [−0.008–−0.001] | 0.018* |
| Median | −3.128E-18 (0.0007) [−0.001–0.001] | 1.000 | |
| 95th | −0.001 (0.0006) [−0.002–0.000] | 0.030* | |
| DV S/A ratio | 5th | 0.002 (0.0098) [−0.017–0.022] | 0.805 |
| Median | 0.001 (0.0104) [−0.019–0.021] | 0.916 | |
| 95th | 0.012 (0.0552) [−0.096–0.120] | 0.827 | |
*Significant P-value for the estimated coefficient of the independent variable (CRL in mm). CI=Confidence interval; DV=Ductus venosus; DVPI=DV pulsatility index; DVRI=DV resistance index; SE=Standard error; CRL=Crown-rump length; S/A=S-wave maximum velocity/A-wave minimum velocity
DISCUSSION
About three decades have elapsed since the presentation of the DV Doppler flow velocity waveform during the first trimester of pregnancy as a predictor of fetal health,[30] yet supporting studies are much sparse in time and region; reference values for DV DVIs remain to be investigated during the first trimester of pregnancy and introduced in the context of a practice bulletin.
Herein, as part of a single-center longitudinal study over 415 participants, reference values for DV DVIs were estimated among healthy Iranian fetuses during 11–13 + 6 weeks of gestational age. The absolute velocity indices (S, D, or A-wave maximum velocity) have been extensively studied. However, these indices rely heavily on the insonation angle and the sonographer's experience. Therefore, fractional indices such as DVPI, DVRI, and DV S/A ratio can be reproduced with greater accuracy.[23]
Previous reports had encountered substantial challenges regarding estimating the reference values for Doppler indices, as these values did not demonstrate Gaussian distributions.[31] It was even more challenging when it came to specific gestational age groups because age-related changes in fetal hemodynamics necessitated the introduction of reference ranges for different gestational ages. Royston and Wright[32] described a standard method to construct normal ranges for fetal variables. However, they assumed the Gaussian distribution of the interest value across different gestational ages. They suggested logarithmic or fractional root transformation of data to achieve a Gaussian distribution and then converting it back to normal after the analysis. Quantile regression is an innovative feature that was added to SPSS in 2019. To the best of our knowledge, it is the first study that utilizes this function to introduce reference values for fetal variables. This function appeared to fit the data analysis because:
Could describe variables with distributions other than Gaussian or containing outliers, contrary to a linear regression model
Could reveal different levels of correlation in different percentiles of the dependent variable.
Obviously, the quantile regression function needs to be further investigated as a method to generate reference ranges for fetal variables. We believe that it will play a significant role in tackling the non-Gaussian distribution of the values of interest.
Several studies have investigated the DVPI reference values in various countries during the first trimester of pregnancy. Based on the evidence that this index differs significantly between normal and aneuploid fetuses, it could be inferred that DVPI is independent of gestational age during the 11th and 14th weeks of pregnancy. Similarly, our results showed no significant alteration of DVPI across the CRL range. We achieved reference values for this index that are pretty close to those reported by Axt-Fliedner et al.,[33] Peixoto et al.,[34] Prefumo et al.,[35] Sabria et al.,[36] and Teixeira et al.[37] However, our values were slightly higher than those of other studies.[22,23,38,39,40,41]
Such discordant results may be discussed physiologically or methodologically. Several studies have theoretically described the physiologic view. Because the developing organs require more blood flow, trophoblastic invasion of placental arteries should increase communication between the maternal and fetal circulations. Teixeira et al.[37] estimated the gestational age of 12 + 6 weeks (concordant with the CRL = 63 mm) as the time point for the beginning of this trophoblastic migration. Nevertheless, this point may differ among fetuses from different nationalities and the migration process is unlikely to mature until the second trimester.[34,39,42] Therefore, we may not be able to conclude a crude increasing or decreasing trend during this time of pregnancy.
The methodology-based discrepancies among the studies may be due to the variables’ operator- and instrument-dependent estimation. Hecher[43] stated that such inconsistencies made DV Doppler indices unreliable variables for estimating abnormal pregnancy outcomes during the first trimester. According to Maiz et al.,[44] a sonographer should perform at least 80 DV Doppler examinations to qualify as competent in reporting their findings. Furthermore, Sabria et al.[36] showed different estimated values in a single-center study at different yearly intervals. They attributed such a discrepancy to more accurate instruments and experienced sonographers in recent years. We believe that our results are noteworthy and reproducible, utilizing fractional indices, an expert operator, and an FDA-approved device.
The patient selection method is another source of discrepancy among the existing studies.[38] Although some studies have excluded merely fetuses with abnormal karyotypes or major structural abnormalities,[35] some others have strict exclusion criteria for participants, such as maternal conditions or minimal fetal abnormalities.[41] To avoid unwanted confounders, we applied relatively stringent standards for eligible participants. Nevertheless, such participants will likely be in better health than they should be to represent the average population.
Doppler indices other than DVPI are less discussed within the literature. It should be noted that although DVRI or the DV S/A ratio have not been directly compared between normal and abnormal fetuses, some studies have derived reference values for these indices and logically drew the conclusion that abnormal blood flow through DV-known to be a well-documented finding among anomalous fetuses-would affect DVRI and DV S/A ratio.
DVRI estimation formula is the same as the preload index discussed in earlier reports. Our results show a non-significant decrease in DVRI over the gestation period, in agreement with every other study.[23,33,39,42] As well, the reference values found in this study resembled those estimated by previous studies. Unexpected findings regarding this index were significant negative coefficients for the 5th and 95th percentile regression lines across the CRL range. Such discordance may be due to a concentration of data within the lower range of the CRL.
The DV S/A ratio showed a nonsignificant rise toward the higher gestational ages. Earlier studies have demonstrated nonsignificant changes within this index during the first trimester of pregnancy.[22,23,33,39] Our estimated reference ranges were much close to previous reports, a bit narrower than those of Turan et al.[22]
Taking all the evidence into account, Doppler findings of fetal vasculature are poorly explained by physiology.[22] In addition, a lack of universal multicenter studies and systematic reviews in this field during the past decade is surprising. For sure, future studies have the opportunity to enhance and globalize our current literature on this topic and put it into an updated standard protocol of practice.
To the best of our knowledge, this is the first study investigating reference values for DV DVIs among the Iranian population. Moreover, a novel method was introduced for evaluating reference intervals for the non-Gaussian distributions of fetal variables. As we had anticipated that using a novel approach to generate percentile regression lines as reference values could lead to discrepant results, multinomial regression functions were also applied, and box plots were developed to display our data conventionally. Another strength of the study was that it utilized a prospective design over an acceptable sample size.
Limitations of this study should be kept in mind when interpreting its results:
Strict inclusion criteria may have deviated our sample toward extraordinarily low-risk pregnancies
For those without hospital records for delivery, neonatal health was established by a telephone interview. Such a data acquisition was not precise enough.
CONCLUSION
In the 11–13 + 6 weeks of pregnancy, the DVIs DVPI, DVRI, and DV S/A ratio were determined independent of gestational age, and their reference values for Iranian fetuses have been established. Such results are not sufficient to translate directly into practice. However, they support the desire for large multicenter studies to develop universal guidelines to incorporate DV DVIs into an updated version of the practice.
Financial support and sponsorship
This work was supported by the Isfahan University of Medical Sciences [grant number 398250]. The university provided the required equipment for this study; no other financial or non-financial support was received from this or any other institution.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors appreciate the Isfahan University of Medical Sciences for providing the required equipment to conduct this study. Also, we must thank Professor Awat Feizi for his dedication to maximizing the preciseness of this study's methods and statistical analyses.
REFERENCES
- 1.MacLennan M, Crichton JH, Playfoot CJ, Adams IR. Oocyte development, meiosis and aneuploidy. Semin Cell Dev Biol. 2015;45:68–76. doi: 10.1016/j.semcdb.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott F, Bonifacio M, Sandow R, Ellis K, Smet ME, McLennan A. Rare autosomal trisomies: Important and not so rare. Prenat Diagn. 2018;38:765–71. doi: 10.1002/pd.5325. [DOI] [PubMed] [Google Scholar]
- 3.Vitez SF, Forman EJ, Williams Z. Preimplantation genetic diagnosis in early pregnancy loss(✰) Semin Perinatol. 2019;43:116–20. doi: 10.1053/j.semperi.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Akutsu SN, Miyamoto T, Oba D, Tomioka K, Ochiai H, Ohashi H, et al. iPSC reprogramming-mediated aneuploidy correction in autosomal trisomy syndromes. PLoS One. 2022;17:e0264965. doi: 10.1371/journal.pone.0264965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao G, Zhao Y, Huang W, Hu L, Wang G, Luo H. Health economic evaluation of noninvasive prenatal testing and serum screening for down syndrome. PLoS One. 2022;17:e0266718. doi: 10.1371/journal.pone.0266718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhytnik L, Peters M, Tilk K, Simm K, Tõnisson N, Reimand T, et al. From late fatherhood to prenatal screening of monogenic disorders: Evidence and ethical concerns. Hum Reprod Update. 2021;27:1056–85. doi: 10.1093/humupd/dmab023. [DOI] [PubMed] [Google Scholar]
- 7.American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics, Committee on Genetics, Society for Maternal-Fetal Medicine. Screening for fetal chromosomal abnormalities: ACOG practice bulletin, number 226. Obstet Gynecol. 2020;136:e48–69. doi: 10.1097/AOG.0000000000004084. [DOI] [PubMed] [Google Scholar]
- 8.Carlson LM, Vora NL. Prenatal diagnosis: Screening and diagnostic tools. Obstet Gynecol Clin North Am. 2017;44:245–56. doi: 10.1016/j.ogc.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekmekci E, Demirel E, Kelekci S. Nasal bone to nasal tip length ratio for describing nasal bone hypoplasia and predicting trisomy 21. Arch Med Sci. 2022;18:395–9. doi: 10.5114/aoms.2019.85655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Dong D, Sun Y, Hu C, Sun C, Wu Q, et al. Development and validation of a deep learning model to screen for trisomy 21 during thefirst trimester from nuchal ultrasonographic images. JAMA Netw Open. 2022;5:e2217854. doi: 10.1001/jamanetworkopen.2022.17854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Zhang L, Dong D, Li X, Wang J, Yin C, et al. Application of an individualized nomogram infirst-trimester screening for trisomy 21. Ultrasound Obstet Gynecol. 2021;58:56–66. doi: 10.1002/uog.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santorum M, Wright D, Syngelaki A, Karagioti N, Nicolaides KH. Accuracy offirst-trimester combined test in screening for trisomies 21, 18 and 13. Ultrasound Obstet Gynecol. 2017;49:714–20. doi: 10.1002/uog.17283. [DOI] [PubMed] [Google Scholar]
- 13.Practice bulletin no. 162: Prenatal diagnostic testing for genetic disorders. Obstet Gynecol. 2016;127:e108–22. doi: 10.1097/AOG.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 14.Kagan KO, Maier V, Sonek J, Abele H, Lüthgens K, Schmid M, et al. False-positive rate infirst-trimester screening based on ultrasound and cell-free DNA versusfirst-trimester combined screening with additional ultrasound markers. Fetal Diagn Ther. 2019;45:317–24. doi: 10.1159/000489121. [DOI] [PubMed] [Google Scholar]
- 15.Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J. Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell. 2016;164:57–68. doi: 10.1016/j.cell.2015.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suciu I, Galeva S, Abdel Azim S, Pop L, Toader O. First-trimester screening-biomarkers and cell-free DNA. J Matern Fetal Neonatal Med. 2021;34:3983–9. doi: 10.1080/14767058.2019.1698031. [DOI] [PubMed] [Google Scholar]
- 17.MacGrogan D, Münch J, de la Pompa JL. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat Rev Cardiol. 2018;15:685–704. doi: 10.1038/s41569-018-0100-2. [DOI] [PubMed] [Google Scholar]
- 18.Yates R. Fetal cardiac abnormalities and their association with aneuploidy. Prenat Diagn. 1999;19:563–6. doi: 10.1002/(sici)1097-0223(199906)19:6<563::aid-pd605>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Minnella GP, Crupano FM, Syngelaki A, Zidere V, Akolekar R, Nicolaides KH. Diagnosis of major heart defects by routinefirst-trimester ultrasound examination: Association with increased nuchal translucency, tricuspid regurgitation and abnormal flow in ductus venosus. Ultrasound Obstet Gynecol. 2020;55:637–44. doi: 10.1002/uog.21956. [DOI] [PubMed] [Google Scholar]
- 20.Seravalli V, Miller JL, Block-Abraham D, Baschat AA. Ductus venosus Doppler in the assessment of fetal cardiovascular health: An updated practical approach. Acta Obstet Gynecol Scand. 2016;95:635–44. doi: 10.1111/aogs.12893. [DOI] [PubMed] [Google Scholar]
- 21.Sidhu PS, Lui F. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022. Embryology, ductus venosus. [Google Scholar]
- 22.Turan OM, Turan S, Sanapo L, Willruth A, Berg C, Gembruch U, et al. Reference ranges for ductus venosus velocity ratios in pregnancies with normal outcomes. J Ultrasound Med. 2014;33:329–36. doi: 10.7863/ultra.33.2.329. [DOI] [PubMed] [Google Scholar]
- 23.Gürses C, Karadağ B, Erol O, İsenlik BS, Karadağ C. Reference ranges for flow velocities and the indices of the ductus venosus in low-risk pregnancies. J Turk Ger Gynecol Assoc. 2021;22:300–11. doi: 10.4274/jtgga.galenos.2021.2020.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timmerman E, Oude Rengerink K, Pajkrt E, Opmeer BC, van der Post JA, Bilardo CM. Ductus venosus pulsatility index measurement reduces the false-positive rate infirst-trimester screening. Ultrasound Obstet Gynecol. 2010;36:661–7. doi: 10.1002/uog.7706. [DOI] [PubMed] [Google Scholar]
- 25.Florjański J, Fuchs T, Zimmer M, Homola W, Pomorski M, Blok D. The role of ductus venosus Doppler flow in the diagnosis of chromosomal abnormalities during thefirst trimester of pregnancy. Adv Clin Exp Med. 2013;22:395–401. [PubMed] [Google Scholar]
- 26.Papageorghiou AT, Kennedy SH, Salomon LJ, Ohuma EO, Cheikh Ismail L, Barros FC, et al. International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown-rump length in thefirst trimester of pregnancy. Ultrasound Obstet Gynecol. 2014;44:641–8. doi: 10.1002/uog.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Biasio P, Siccardi M, Volpe G, Famularo L, Santi F, Canini S. First-trimester screening for down syndrome using nuchal translucency measurement with free beta-hCG and PAPP-A between 10 and 13 weeks of pregnancy – The combined test. Prenat Diagn. 1999;19:360–3. doi: 10.1002/(sici)1097-0223(199904)19:4<360::aid-pd556>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Matias A, Gomes C, Flack N, Montenegro N, Nicolaides KH. Screening for chromosomal abnormalities at 10-14 weeks: The role of ductus venosus blood flow. Ultrasound Obstet Gynecol. 1998;12:380–4. doi: 10.1046/j.1469-0705.1998.12060380.x. [DOI] [PubMed] [Google Scholar]
- 29.Bly S, Van den Hof MC, Diagnostic Imaging Committee, Society of Obstetricians and Gynaecologists of Canada. RETIRED: Obstetric ultrasound biological effects and safety. J Obstet Gynaecol Can. 2005;27:572–80. doi: 10.1016/s1701-2163(16)30716-2. [DOI] [PubMed] [Google Scholar]
- 30.Huisman TW, Stewart PA, Wladimiroff JW. Doppler assessment of the normal early fetal circulation. Ultrasound Obstet Gynecol. 1992;2:300–5. doi: 10.1046/j.1469-0705.1992.02040300.x. [DOI] [PubMed] [Google Scholar]
- 31.Maiz N, Wright D, Ferreira AF, Syngelaki A, Nicolaides KH. A mixture model of ductus venosus pulsatility index in screening for aneuploidies at 11-13 weeks’ gestation. Fetal Diagn Ther. 2012;31:221–9. doi: 10.1159/000337322. [DOI] [PubMed] [Google Scholar]
- 32.Royston P, Wright EM. How to construct ‘normal ranges’ for fetal variables. Ultrasound Obstet Gynecol. 1998;11:30–8. doi: 10.1046/j.1469-0705.1998.11010030.x. [DOI] [PubMed] [Google Scholar]
- 33.Axt-Fliedner R, Diler S, Georg T, Friedrich M, Diedrich K. Reference values of ductus venosus blood flow velocities and waveform indices from 10 to 20 weeks of gestation. Arch Gynecol Obstet. 2004;269:199–204. doi: 10.1007/s00404-003-0484-y. [DOI] [PubMed] [Google Scholar]
- 34.Peixoto AB, Caldas TM, Martins WP, Ferreira PC, Nardozza LM, Costa Fda S, et al. Reference range for the pulsatility index ductus venosus Doppler measurement between 11 and 13+6 weeks of gestation in a Brazilian population. J Matern Fetal Neonatal Med. 2016;29:2738–41. doi: 10.3109/14767058.2015.1103730. [DOI] [PubMed] [Google Scholar]
- 35.Prefumo F, Risso D, Venturini PL, De Biasio P. Reference values for ductus venosus Doppler flow measurements at 10-14 weeks of gestation. Ultrasound Obstet Gynecol. 2002;20:42–6. doi: 10.1046/j.1469-0705.2002.00710.x. [DOI] [PubMed] [Google Scholar]
- 36.Sabria J, Comas C, Barceló-Vidal C, Garcia-Posada R, Echevarria M, Gomez-Roig MD, et al. Updated reference ranges for the ductus venosus pulsatility index at 11-13 weeks. Fetal Diagn Ther. 2012;32:271–6. doi: 10.1159/000339413. [DOI] [PubMed] [Google Scholar]
- 37.Teixeira LS, Leite J, Viegas MJ, Faria MM, Chaves AS, Teixeira RC, et al. Ductus venosus Doppler velocimetry in thefirst trimester: A new finding. Ultrasound Obstet Gynecol. 2008;31:261–5. doi: 10.1002/uog.5245. [DOI] [PubMed] [Google Scholar]
- 38.Kalayci H, Yilmaz Baran Ş, Doğan Durdağ G, Yetkinel S, Alemdaroğlu S, Özdoğan S, et al. Reference values of the ductus venosus pulsatility index for pregnant women between 11 and 13(+6) weeks of gestation. J Matern Fetal Neonatal Med. 2020;33:1134–9. doi: 10.1080/14767058.2018.1517152. [DOI] [PubMed] [Google Scholar]
- 39.Pruksanusak N, Kor-anantakul O, Suntharasaj T, Suwanrath C, Hanprasertpong T, Pranpanus S, et al. A reference for ductus venosus blood flow at 11-13+6 weeks of gestation. Gynecol Obstet Invest. 2014;78:22–5. doi: 10.1159/000362273. [DOI] [PubMed] [Google Scholar]
- 40.Rozmus-Warcholinska W, Wloch A, Acharya G, Cnota W, Czuba B, Sodowski K, et al. Reference values for variables of fetal cardiocirculatory dynamics at 11-14 weeks of gestation. Ultrasound Obstet Gynecol. 2010;35:540–7. doi: 10.1002/uog.7595. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchiya M, Kikuchi A, Takakuwa K, Tanaka K. Increased pulsatility of the ductus venosus blood velocity in thefirst trimester is associated with the delivery of small for gestational age or low birth weight infants. J Obstet Gynaecol Res. 2010;36:1151–60. doi: 10.1111/j.1447-0756.2010.01299.x. [DOI] [PubMed] [Google Scholar]
- 42.Tseng CC, Wang HI, Wang PH, Yang MJ, Juang CM, Horng HC, et al. Ductus venosus Doppler velocimetry in normal pregnancies from 11 to 13 + 6 weeks’ gestation – A Taiwanese study. J Chin Med Assoc. 2012;75:171–5. doi: 10.1016/j.jcma.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Hecher K. Assessment of ductus venosus flow during thefirst and early second trimesters: What can we expect? Ultrasound Obstet Gynecol. 2001;17:285–7. doi: 10.1046/j.1469-0705.2001.00412.x. [DOI] [PubMed] [Google Scholar]
- 44.Maiz N, Kagan KO, Milovanovic Z, Celik E, Nicolaides KH. Learning curve for Doppler assessment of ductus venosus flow at 11 + 0 to 13 + 6 weeks’ gestation. Ultrasound Obstet Gynecol. 2008;31:503–6. doi: 10.1002/uog.5282. [DOI] [PubMed] [Google Scholar]