Abstract
Background:
Across the world, people are exposed to pesticide residues in agricultural products. Various materials are used to deal with effects of these residues. Considering the wide use of dichlorvos and acetamiprid in crops, pesticide residues in cucumber and its effects on the biochemical parameters of mice were calculated, and the protective role of donkey colostrum (DC) to deal with the pesticide effects was investigated.
Materials and Methods:
Dichlorvos (4 ml/l) and acetamiprid (0.5 g/l) residues, after spraying cucumber plants, were 0.5 and 1.5 mg/kg, respectively. For 60 days, the mentioned doses were used in the drinking water of 4 groups of mice. No substances were added to mice drinking water in the control group while dichlorvos and acetamiprid groups received 0.5 and 1.5 mg/kg of pesticide, respectively, and the mixed group received a combination of two pesticides. In order to investigate the protective role of DC, 0.2 ml of colostrum was given to each of the groups in a similar division and timing.
Results:
In the biochemical sector, albumin (control 2.96, dichlorvos 1.86, acetamiprid 2.00, and mix 1.6 g/dl) and total protein levels reduced. Alanine aminotransferase (control 41.8, dichlorvos 56.2, acetamiprid 58.4, and mix 68 iu/l) and aspartate aminotransferase levels increased. In the protective role of colostrum, albumin (control 2.96, dichlorvos 2.74, acetamiprid 2.80, and mix 2.50 g/dl) and alanine aminotransferase changes (control 41.8, dichlorvos 43.4, acetamiprid 46.0, and mix 52.2 iu/l) were recorded (P = 0.0001).
Conclusion:
Adding pesticides to mice drinking water causes liver disorders and DC can be effective in protecting these damages.
Keywords: Biochemical parameters, colostrum, food, pesticides, protection
INTRODUCTION
Pesticides are widely used throughout the world, especially underdeveloped and developing countries and pesticide demand are increasing due to the current system of crop production, which prioritizes high agricultural yields. The widespread use of pesticides increases their residues in agricultural products. The risks of this issue are greater in products that are consumed fresh and raw.[1] These risks affect various organs of the body. The liver is among these organs, the function of which has a significant impact on the evaluation of health status.[2]
Organophosphate pesticides and neonicotinoids are used to control a wide range of plant pests in both greenhouses and fields.[3] Researches show that the residue of diazinon pesticide on vegetables and fruits such as cucumber and tomato is higher than limit maximum residue.[4] By measuring the blood proteins of mice exposed to diazinon pesticide, the results illustrate liver damage and disorder.[5] Furthermore, the investigation of neonicotinoid pesticide residues in fresh fruits and vegetables showed that they are limit maximum residue.[6] Mice exposed to thiamethoxam showed changes in their liver enzymes, which indicated liver damage.[7]
To reduce the harmful effects of pesticides from the therapeutic properties of various substances such as coffee, plant extracts, honey, and its products have been used.[8] In recent years, the use of mammalian milk and colostrum has become very popular due to its therapeutic properties. Donkey milk has a composition similar to human milk and its biological activities, such as antimicrobial, antiviral, anti-inflammatory, antiproliferative, and antioxidant activity, have a special place. The chemical properties of donkey colostrum (DC) create a suitable platform for healing inflammations. Lactose is the most representative element of donkey's milk. In addition, DC contains essential amino acids, and lysozyme and lactoferrin present in colostrum increase the body's immunity. The low amount of saturated fatty acids and the high concentration of unsaturated fatty acids in DC compared to other milks increases anti-atherogenic and anti-thrombogenic activities which play a significant role in autoimmune processes and reducing chronic inflammations.[9]
Ming et al.[10] investigate the preventive and therapeutic effects of camel milk on liver damage. The analysis of serum and liver biochemical indicators showed that the consumption of camel milk before and after liver damage could play a role in healing through regulating the inflammatory and immune factors in the livers of rats. In comparative, the results indicated that donkey milk is second to human milk in the treatment of inflammatory conditions, oxidative stress, antioxidant enzyme activity, and detoxification.[11]
According to the investigations, dichlorvos (from the organophosphate group) and Acetamiprid (from the neonicotinoid group) are among the most widely used pesticides in the study area (Isfahan) for food production, which are used in excess of the recommended dose and without observing the preharvest interval (PHI). For this purpose, first, the residue of these pesticides was obtained on greenhouse cucumbers at twofold the recommended dose and without observing PHI. Then, the effect of pesticide residues on the biochemical parameters of Balb/C male mice was calculated. In the end, the protective role of DC to deal with the effects caused by pesticides was investigated in different treatment groups.
MATERIALS AND METHODS
Cucumber plant cultivation
The plant used in this study to assess the pesticide residue was Sana cultivar of cucumber, which is widely consumed. The cucumber was cultivated in two stages of seedling production and its transfer to the main location. Both stages were done inside a greenhouse with 20 × 2 × 2.5 dimensions, respective day and night temperatures of 25°C ± 5°C and 18°C ± 2°C, relative humidity of 50%–60%, 16/8 light, and dark conditions. The arable land of the region was used for the generation of seedling and pots. After 45 days, the seedlings were ready for transfer to the main pots, which were made of polyvinyl chloride (PVC) with a height of 40 cm and lid diameter of 30 cm. The plants were irrigated every 2 days.
Determining the dose
To measure the residues of acetamiprid and dichlorvos in greenhouse cucumber, a total of 27 pots of cucumber plants were used as experimental units, including three treatments and three replications (each including three pots). Cucumber plants were sprayed with pesticides during the fruiting stage. Treatments for each agent included concentrations two-fold the recommended as well as a control treatment (water). For spraying, SeeSa sprayer with a volume of 2.5 liters was used. The control group in which the experimental units were sprayed with water. The dichlorvos group was the pots that were sprayed with a concentration equal to twofold the recommended dose (4 ml/l) of dichlorvos pesticide. The acetamiprid group was the pots that were sprayed with a concentration equal two-fold to the recommended dose (0.5 g/l) of acetamiprid pesticide.
Twenty-four hours after spraying the pots, one cucumber (three in each replication) was taken from each pot and placed in separate plastic bags, and after labeling, the samples were transferred to the laboratory. In the laboratory, all three cucumbers were mixed in a replicator with a mixer and the residues of pesticide in the sample were measured by the method of 17,026 QuEChERS and AGILENT GC MS. In this study, dichlorvos was utilized with emulsifiable concentrate formulation, purity of 50%, PHI of 3 days, and acetamiprid as water-soluble powder formulation, purity of 20% and PHI of 14 days from golsang company that is widely used in the region.
Biochemical experiments
Chromatographic data were used for biochemical measurement. The analysis results of pesticides in cucumber showed that the amount of pesticides was higher in doses twofold maximum residue limit (MRL) of Codex standard (0.5 ppm for Dichlorvos and 1.5 ppm for Acetamiprid), which is equivalent to the same amount of pesticide was used in drinking water of mice. For this purpose, 20 male Balb/C mice with an average weight of 30–35 g aged 10–12 weeks were purchased from Royan Research Institute in Isfahan. To adapt the animals to the environment, they were placed in standard cages at 22°C ± 3°C, 12 h of light/dark, and 40 ± 10 humidity for 1 month. Food and water were provided to mice on a daily basis.
For blood tests, mice were randomly divided into four groups of five samples as follows:
Control group: No agent was added to the drinking water of this group
Dichlorvos group: 0.5 ppm dichlorvos was added to the drinking water daily
Acetamiprid group: 1.5 ppm of acetamiprid was added to the drinking water daily
Mix group: Every other day, the equivalent dose of group 1 and 2 agents was added to the drinking water daily and alternately (1 day 0.5 ppm dichlorvos and the next day 1.5 ppm of Acetamiprid).
Donkey colostrum
The colostrum that was used in this research was obtained from a donkey breeding farm in Karaj city and was transported to the laboratory in sterile flasks. Then it was immediately frozen in special containers in the required units.
Protective effect of colostrum
For this purpose, 25 mice were divided into 5 groups of five members and were subjected to different treatments for 2 months. Water and DC groups received 0.2 ml of drinking water and colostrum by gavage, respectively. Dichlorvos + DC group received 0.5 ppm of dichlorvos in drinking water and 0.2 ml of colostrum per day by gavage simultaneously. Acetamiprid + DC group received 1.5 ppm of acetamiprid pesticide in drinking water and 0.2 ml of colostrum per day simultaneously. Mix + DC group alternately received 0.5 ppm of dichlorvos and 1.5 ppm of acetamiprid in drinking water the next day as well as 0.2 ml of colostrum per day.
Measurement of some biological parameters
All mice in the treatment groups were measured in terms of weight and this was repeated at the end of the period (60 days). In addition, during the experiment, the amount of food consumption by these treatment groups was measured and their nutritional efficiency was calculated at the end of the period.
Blood sampling
Blood samples were taken from different groups of mice for 60 days in vitro. For this purpose, the mice were anesthetized and then the place of the heart was determined by the tip of the finger. After that, blood samples were taken directly from the animal's heart with a 5 cc syringe. All stages of maintenance and blood sampling of mice were performed in accordance with the rules approved by the Ethics Committee of the Islamic Azad University, Isfahan (Khorasgan) Branch, Iran, with the code of IR.IAU.KHUISF.REC.1400.305.
The samples were analyzed with the BT3000 AutoAnalyzer and Pars Azmun test kit. For this purpose, first of all, samples were prepared by centrifuging them at 3000 rpm (revolutions per minute) for 10 min. Then, International Federation of Clinical Chemistry and Laboratory Medicine method was used to evaluate medical enzymes without adding Pyridoxal-5-phosphate by examining a wavelength of 340–405 nm. Bromocresol green method was used for albumin and Biuret method was used for total protein based on the wavelength of 546–700 nm. It is noteworthy that the Sebia device and capillary electrophoresis method were used for Globulin group variants.
Data analysis
Statistical analysis was done using SPSS software for Windows (version 22). The difference between the groups was assessed by one-way ANOVA and least significant difference test at significance level of P < 0.05 and P < 0.01 was employed to group the treatments.
RESULTS
The residual acetamiprid and dichlorvos according to the analysis of the chromatography device, MRL and the dose of agents added to mice drinking water in the biochemical section has been shown in Table 1. In twofold doses of Acetamiprid and Dichlorvos, the residual agents are higher than the allowed level in greenhouse cucumber. The residual pesticide at twofold recommended dose in Dichlorvos and Acetamiprid is 0.51 ± 0.005 and 1.51 ± 0.005, respectively. Therefore, this concentration of agent was used in the drinking water of mice.
Table 1.
The amount of pesticides residual in greenhouse cucumber after 24 h according to chromatographic analysis
| Groups | Pesticides residues (ppm)±SD | MRL (ppm) | Dose of pesticides added to drinking water (ppm) |
|---|---|---|---|
| Control | 0.01±0.00 | - | - |
| Dichlorvos (4 mL/L) | 0.51±0.005* | 0.05 | 0.5 |
| Acetamiprid (0.5 g/L) | 1.51±0.005* | 0.3 | 1.5 |
| P | 0.0001 | - | - |
*Significant difference with the control group (P<0.01). Statistical test: One-way ANOVA and LSD. Mean±SD. MRL=Maximum residue limit; LSD=Least significant difference; SD=Standard deviation
The effect of different pesticide and colostrum treatments on weight changes of mice at the end of the experimental period is illustrated in Figure 1. A comparison of the average weight change of mice whose drinking water was pesticide shows weight loss. This reduction was more severe in acetamiprid and Mix groups. Also, the treatments that received DC experienced weight compensation, which was more in Acetamiprid + DC group (P < 0.01).
Figure 1.

Comparison of weight changes in groups treated with pesticide and those treated with pesticide and colostrum during 60 days. Different letters (a-c) indicate the significance of treatment groups (P < 0.01). Statistical testing: One way ANOVA and LSD P = 0.0001. The Dichlorvos and Acetamiprid groups received 0.5 and 1.5 ppm, respectively, and the mix group received both doses (0.5 and 1.5) alternately. The dichlorvos + DC, acetamiprid + DC, and mix + DC groups received 0.2 ml of donkey colostrum per day in addition to pesticides. DC: Donkey colostrum, LSD: Least significant difference
Figure 2 shows the amount of food consumed by the groups treated with dichlorvos and acetamiprid pesticides which were significantly different with those treated with pesticide and colostrum at 99% confidence level. The results showed that the decrease in the amount of consumed food in the groups receiving pesticides was more severe in the mix group and its compensation was greater in the groups receiving DC in the Dichlorvos + DC group.
Figure 2.
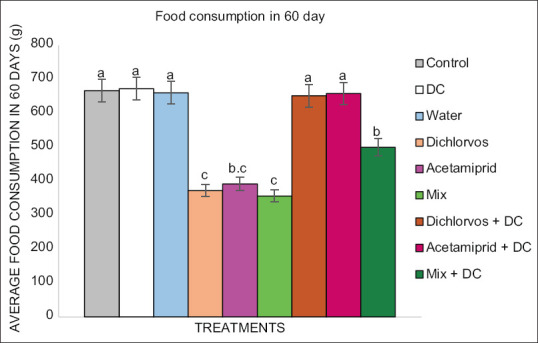
Comparison of food consumption in treated groups with pesticide and treated groups with pesticide and colostrum during 60 days. Different letters (a-c) indicate the significance of treatment groups (P < 0.01). Statistical testing: One way ANOVA and LSD P = 0.0001. The Dichlorvos and Acetamiprid groups received 0.5 and 1.5 ppm, respectively, and the mix group received both doses (0.5 and 1.5) alternately. The Dichlorvos + DC, Acetamiprid + DC, and Mix + DC groups received 0.2 ml of donkey colostrum per day in addition to pesticides. DC: Donkey colostrum, LSD: Least significant difference
The relative body weight (RBW) and feeding efficiency or efficiency of conversion of ingested food in treated mice with dichlorvos and acetamiprid pesticides and the treated groups with pesticide and colostrum are shown in Table 2. The relative weight loss in the Mix group is more intense compared to other groups receiving the pesticide. Furthermore, DC has been able to compensate for the relative weight loss more in acetamiprid + DC group than in other groups. Compared to other groups, the efficiency of food consumption in the acetamiprid group has the most severe decrease and the most compensation in the acetamiprid + DC group. (P < 0.01).
Table 2.
The computing relative body weight and efficiency of conversion of ingested food in treatment groups after 60 days
| Groups | IBW (g) | FBW (g) | RBW | ECI |
|---|---|---|---|---|
| Control | 32.4±1.94 | 41.2±2.58 | 127.66±13.14a | 1.28±0.39a |
| DC | 35.6±1.94 | 44.6±3.04 | 125.28±5.57a | 137±0.46a |
| Water | 35.2±0.83 | 43.0±1.41 | 122.17±3.80a | 1.17±0.17a |
| Dichlorvos | 37.8±5.21 | 37.8±4.96 | 100.13±3.72c | –0.04±0.35b |
| Acetamiprid | 36.2±3.63 | 35.4±0.54 | 98.46±8.48c | –0.35±1.00b |
| Mix | 34.8±3.89 | 34.0±2.64 | 98.15±7.43c | –0.34±0.74b |
| Dichlorvos + DC | 33.6±1.34 | 39.2±1.30 | 116.70±1.92a,b | 0.85±0.06a |
| Acetamiprid + DC | 32.4±1.67 | 38.4±1.67 | 118.57±2.54a,b | 0.90±0.09a |
| Mix + DC | 34.0±1.41 | 36.6±1.81 | 107.62±1.45b,c | 0.51±0.10a,b |
| P | 0.07 | 0.0001 | 0.0001 | 0.0001 |
Different letters (a-c) indicate the significance of treatment groups (P<0.01). ECI: Added weight amount/eaten food amount×100. RBW: Final body weight/initial body weight×100. Statistical test: One-way ANOVA and LSD. The dichlorvos and acetamiprid groups received 0.5 and 1.5 ppm, respectively, and the mix group received both doses (0.5 and 1.5) alternately. The dichlorvos + DC, acetamiprid + DC, and mix + DC groups received 0.2 ml of donkey colostrum per day in addition to pesticides. Mean±SD. IBW=Initial body weight; FBW=Final body weight; DC=Donkey colostrum; ECI=Efficiency of conversion of ingested food; RBW=Relative body weight; LSD=Least significant difference; SD=Standard deviation
Table 3 shows the changes in biochemical parameters of mice treated with ichlorvos and Acetamiprid pesticides as well as the contribution of colostrum to these changes. The mix group showed more changes in all biochemical parameters compared to other treatments that received pesticide. In the treatments that received DC, albumin and the ratio of albumin to globulin in acetamiprid + DC group had the most changes. In addition, the Dichlorvos + DC group indicated the most changes in total protein and liver enzymes (P < 0.05).
Table 3.
Changes in biochemical parameters in treatment groups after 60 days
| Groups | Albumin (g/dL) | Total (g/dL) | Ratio A/G (g/dL) | AST (IU/L) | ALT (IU/L) |
|---|---|---|---|---|---|
| Control | 2.96±0.25a,b | 6.06±0.19a,b | 0.96±0.13a,b | 155.6±31.04d,e | 41.8±5.06d |
| DC | 3.08±0.08a | 6.22±0.08a | 0.97±0.03a | 138.6±22.44e | 38.8±2.38d |
| Water | 2.92±0.13a,b | 5.96±0.05a,b | 0.95±0.10a,b | 157.8±33.22d,e | 42.6±3.50d |
| Dichlorvos | 1.86±0.11d | 4.98±0.38d | 0.60±0.11d | 229.0±37.47b,c | 56.2±4.26b |
| Acetamiprid | 2.00±0.22d | 4.92±0.13d | 0.68±0.11c,d | 263.8±73.57b | 58.4±7.43b |
| Mix | 1.60±0.14e | 4.48±0.31e | 0.55±0.06d | 337.8±40.73a | 68.0±8.06a |
| Dichlorvos + DC | 2.74±0.16b | 5.82±0.23b,c | 0.90±0.14a,b | 171.4±29.27d,e | 43.4±2.79d |
| Acetamiprid + DC | 2.80±0.25b | 5.80±0.17b,c | 0.94±0.16a,b | 196.6±18.43c,d | 46.0±4.63c,d |
| Mix + DC | 2.50±0.21c | 5.62±0.16c | 0.80±0.09b,c | 245.0±30.47b,c | 52.2±6.76b,c |
| P | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
Different letters (a-e) indicate the significance of treatment groups (P<0.05). Statistical test: One-way ANOVA and LSD. Mean±SD. The dichlorvos and acetamiprid groups received 0.5 and 1.5 ppm, respectively, and the mix group received both doses (0.5 and 1.5) alternately. The dichlorvos + DC, acetamiprid + DC, and mix + DC groups received 0.2 mL of donkey colostrum per day in addition to pesticides. Total=Total protein; A/G=Ratio of albumin to globulin; AST=Aspartate transaminase; ALT=Alanine transaminase; LSD=Least significant difference; SD=Standard deviation; DC=Donkey colostrum
DISCUSSION
The purpose of this research was to measure the residue of dichlorvos and acetamiprid pesticides in greenhouse cucumber and to investigate the effect of residual pesticides on the biochemical parameters of mice and also the protective role of colostrum in these parameters. Farmers consider using a higher dose of pesticides, increasing spraying, or mixing different compounds as a general solution when faced with pest problems or to achieve a high-quality crop.[12] Therefore, in most cases, the residual pesticides on agricultural products, especially fruits and vegetables that are consumed raw, reach the consumer with excess pesticide residues. To better understand residual pesticide risks, the biochemical analysis such as measuring blood proteins and liver enzymes related to liver function can provide a clearer picture of health because the liver is the largest vital organ in the body that plays an important role in maintaining the metabolic homeostasis and balancing the internal environment. Therefore, hepatic dysfunction is considered a serious health problem.[13]
According to the results the residues of acetamiprid and dichlorvos in greenhouse cucumber after 24 h at two-fold the recommended dose were higher than the Maximum Residue Limit by Codex and the National Organization for Standardization. In this regard, 21% of the fruits and vegetables that were evaluated for residual Imidacloprid (6.18–6.21 mg/kg) and acetamiprid (2.98–3.21 mg/kg), the residual agents on them–were higher than MRL.[6] In another study on pesticide residues in farm vegetables, residues of chlorpyrifos (3–3.5 mg/kg) and cypermethrin (0.1–0.2 mg/kg) were detected above the allowable set limit.[14] Pesticide residues higher than the allowable limit in agricultural products of underdeveloped and developing countries are more likely due to increasing population and food needs, lack of training of producers and financial incentives, absence of regulatory bodies, and noncompliance with PHI.[1] In Iran, in a study, the residues of Diazinon and Carbaryl were higher than the allowable level, which was 0.37 and 0.72 μg/g for cucumber and tomato, respectively.[4] The situation is the same in most underdeveloped and developing countries. Galani et al.[15] in the western region of Cameroon evaluated the residues of 99 toxins in 72 samples from 12 crops. The residues of 62 agents were found in the products, 12 of which were prohibited. Twenty-one pesticides exceeded the European Union limit and 22 agents were found in all 6 sampling sites. Malathion and a type of DDT were found in all samples and sites. Peppers, beans, celery, and soybeans were examples of crops containing residual toxins.
Based on the results, the effect of residual Dichlorvos and Acetamiprid at two-fold recommended dose on mice caused weight loss and decreases food consumption, which results in RBW loss and lower food efficiency. Besides, the treatment groups that received DC were able to regain their lost weight by increasing feeding, which resulted in the increase in RBW and nutrition efficiency.
Relative weight loss and food consumption have also been observed by exposing mice to Malathion, 500 and 200 mg/kg,[16] and various pesticides.[17] Nutritional indicators can be used as a useful tool to improve understanding of the physiology of nutrition and the effects of various (pesticides) on the body. Therefore, the possible reasons for weight loss and reduced food intake can be expressed in the form of degradation of lipids and proteins due to exposure to pesticides and the resulting oxidative stress.[18] On the other hand, colostrum plays its role in improving nutrition and increasing the weight by reducing the effect of oxidative stress.[19]
In this study, the addition of dichlorvos and acetamiprid to drinking water in twice recommended doses to mice reduced serum albumin in all treatment groups, and the Mix treatment showed a significant difference with the other two groups receiving the pesticide. Albumin is the most abundant single protein of normal plasma that usually accounts for up to two-third of total plasma protein. Impaired synthesis in malnutrition, malabsorption, and hepatic impairment or reduced level of albumin due to its loss in protein-losing nephropathy leads to the severe imbalance of intravascular oncotic pressure.[2] Reduced albumin level has also been observed with exposure of mice to pyrethroid and diazinon (70–100 mg/kg) in another study.[20] Liver damage and hepatic disorders are possible causes of albumin depletion.[5] Reduced albumin level is a major prognostic marker of cirrhosis and low serum albumin levels are usually associated with primary liver tissue injury. A decrease of albumin level is usually associated with the reduction of total serum proteins.[2]
Our results on total protein levels of all treatment groups indicate a significant reduction in all groups compared to the control group. In this parameter, the Mix treatment showed a significant difference with the other two groups receiving the agent. These findings are consistent with studies in rats exposed to sumithion (85 mg/kg) and paracetamol (50 mg/kg).[21] Decreases in total serum albumin and protein have also been observed in mice exposed to Diazinon (12.50 mg/kg) and Sodium fluoride (500 ppm).[18] A decrease in albumin synthesis, which reflects the reduction in total protein, may be due to a decrease in the number of cells responsible for albumin synthesis as a result of necrosis. Albumin as an antioxidant may be used in the process of combating oxidation due to the entry of toxins into the body. Exposure to toxins and the development of hypoproteinemia, which is consistent with previous studies, have been attributed in part to lymphocytic leukopenia. By stating these cases, the added toxin can be considered as a factor compromising the body's immunity.[22]
Furthermore, the ratio of albumin to globulin in all treatment groups shows a significant decrease compared to the control group. This is consistent with previous studies on the effect of Chlorpyrifos (6.75 and 21.3 mg/kg) on rats and mice.[22,23] Measuring the two major protein components of serum (albumin and globulin) and albumin to globulin ratio are highly valuable. The importance of this parameter is reflected in the fact that disorders related to serum protein compounds in various diseases are usually manifested by a decrease in albumin and an increase in one or more globulin components.[2] Therefore, the researchers attributed the decrease in the ratio of albumin to globulin to liver and kidney disorders as well as liver damage or loss of protein in the urine due to kidney damage.[22,23]
The results of this study indicate that adding dichlorvos and acetamiprid in two-fold the recommended dose increases alanine transaminase (ALT) and aspartate transaminase (AST) levels. Furthermore, the third treatment (Mix) showed a significant difference with the other two groups receiving the agent. Increasing ALT and AST levels have also been observed in mice exposed to hepatotoxicity in other studies.[24] In the study by Kartheek and David[25] where mice were exposed to fipronil (6.46–32.33 mg/kg) and the study of Jaiswal et al.[26] in which mice were exposed to carbofuran (1.5 mg/kg), the results were associated with increased aspartate aminotransferase and alanine aminotransferase levels. The level of these enzymes is usually low in the blood; therefore, if the liver cells are damaged, the level of enzymes is expected to increase in the bloodstream due to their leakage from the damaged cells. Therefore, the increase in the level of these enzymes in the blood may be due to impaired liver function and disrupted biosynthesis of the mentioned enzymes through altered permeability of the liver membrane.[27]
Mice fed with DC showed increasing concentration of albumin as well as rising albumin to globulin and total protein ratios but decreased ALT and AST levels compared to mice that received only the insecticide. These results are consistent with the research by Alharbi et al.[28] and Karabacak et al.[29] These findings indicate that colostrum has a beneficial effect on acute liver damage caused by pesticides. Adding acetamiprid and dichlorvos residues in cucumber to the drinking water of rats causes oxidative stress, leads to the increase in oxygen free radicals, and disrupts its balance with antioxidants. Moreover, the increase in oxygen free radicals causes lipid peroxidation, which in turn increases oxidative stress. The outcome of these processes is liver damage, changing the concentration of blood proteins and liver enzymes. Antioxidant enzymes such as superoxide dismutase and glutathione in the liver are the main defense mechanisms against reactive oxygen species. Colostrum significantly increases the activity of these enzymes in a liver damaged by pesticides and balances oxygen free radicals and antioxidants; colostrum also prevents peroxidation damage and inhibits the strong inflammatory response by reducing fat.[19] As a result, colostrum suppresses cell apoptosis by eliminating free radicals, inhibiting lipid peroxidation, and increasing antioxidant activity, eliciting a defensive response against liver inflammation that results in concentration regulation of blood proteins and liver enzymes. Also, the role of colostrum in boosting the body's immune system and stimulating the release of immunoglobulins can be indicative of its protective role against the damage caused by toxins.[30]
CONCLUSION
Improper and inadvertent use of pesticides especially in underdeveloped and developing countries is contrary to ecological principles and can be the source of several problems. Although the use of pesticides has increased agricultural production, residues of these agents enter the human body together with food. Therefore, monitoring programs must be constantly implemented for the presence of pesticide residues in food to ensure the MRL and the intake of these substances through the diet. According to the results of this study, adding the residues of Dichlorvos and Acetamiprid obtained in cucumber to the drinking water of mice not only affected biological changes (weight loss and food consumption) but also caused changes in blood proteins and liver enzymes. Therefore, the addition of these pesticides to the drinking water of mice can be considered a factor of liver damage and disorders as well as reduced immunity. On the other hand, it can be stated that DC has the potential to be developed as a prospective practical food to reduce liver damage. Therefore, it is recommended that producers of agricultural products, especially products that are consumed raw and fresh, use the recommended doses of pesticides and pay special attention to the management of pesticide consumption.
Most of the research on the health consequences of pesticide toxicity has been based on their lethal dose. However, in this research, these results were obtained based on the residue of pesticides in a widely consumed food item (cucumber) in accordance with scientific guidelines, which can illustrate a more appropriate picture of the harms of using them. In addition, the use of DC, due to its special characteristics, can promise the introduction of a “medicinal food.”
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sarkara S, Dias J, Keeley J, Mohring N, Jansen K. The Use of Pesticides in Developing Countries and Their Impact on Health and the Right to Food. Policy Department for External Relations Directorate General for External Policies of the Union PE 653.622. 2021 January [Google Scholar]
- 2.McPherson R, Pincus M. Henry's Clinical Diagnosis and Management by Laboratory Methods. 24th ed. Elsevier; 2021. [Google Scholar]
- 3.Nguyen TT, Rosello C, Bélanger R, Ratti C. Fate of residual pesticides in fruit and vegetable waste (FVW) processing. Foods. 2020;9:1468. doi: 10.3390/foods9101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amrollahi H, Pazoki R, Imani S. Pesticide multiresidue analysis in tomato and cucumber samples collected from fruit and vegetable markets in Tehran, Iran. Middle East J Rehabil Health Stud. 2018;6:e64271. doi: 10.5812/mejrh.64271. [In Press] [Google Scholar]
- 5.Assaraj SH, Alattar HA, Farid AS, Fararah KM. Influence of lactoferrin on immune response in rats intoxicated by diazinon. Benha Vet Med J. 2018;34:169–81. [Google Scholar]
- 6.Jallow MF, Awadh DG, Albaho MS, Devi VY, Ahmad N. Monitoring of pesticide residues in commonly used fruits and vegetables in Kuwait. Int J Environ Res Public Health. 2017;14:833. doi: 10.3390/ijerph14080833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elhamalawy OH, Al-Anany FS, El Makawy AI. Thiamethoxam-induced hematological, biochemical, and genetic alterations and the ameliorated effect of Moringa oleifera in male mice. Toxicol Rep. 2022;9:94–101. doi: 10.1016/j.toxrep.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaya E, Yılmaz S, Ceribasi S. Protective role of propolis on low and high dose furan-induced hepatotoxicity and oxidative stress in rats. J Vet Res. 2019;63:423–31. doi: 10.2478/jvetres-2019-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martini M, Altomonte I, Licitra R, Salari F. Nutritional and nutraceutical quality of donkey milk. J Equine Vet Sci. 2018;65:33–7. [Google Scholar]
- 10.Ming L, Qi B, Hao S, Ji R. Camel milk ameliorates inflammatory mechanisms in an alcohol-induced liver injury mouse model. Sci Rep. 2021;11:22811. doi: 10.1038/s41598-021-02357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinchese G, Cavaliere G, Canani RB, Matamoros S, Bergamo P, De Filippo C, et al. Human, donkey and cow milk differently affects energy efficiency and inflammatory state by modulating mitochondrial function and gut microbiota. J Nutr Biochem. 2015;26:1136–46. doi: 10.1016/j.jnutbio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Thorburn C. The rise and demise of integrated pest management in rice in Indonesia. Insects J. 2015;6:381–408. [Google Scholar]
- 13.Mukherji A, Bailey SM, Staels B, Baumert TF. The circadian clock and liver function in health and disease. J Hepatol. 2019;71:200–11. doi: 10.1016/j.jhep.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leupraserta LB, Monmorab S, Puydechab M, Chapmana RS, Wattasit Siriwong A, Taneepanichskula S. Innovative Pesticide Kit Model for Vegetable Farm Safety Surveillance Program. Singapore: ICESD; 2014. Feb 19-21, [Google Scholar]
- 15.Galani JH, Houbraken M, Wumbei A, Djeugap JF, Fotio D, Spanoghe P. Evaluation of 99 pesticide residues in major agricultural products from the western highlands zone of Cameroon using QuEChERS method extraction and LC-MS/MS and GC-ECD analyses. Foods. 2018;7:184. doi: 10.3390/foods7110184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim Ali1 R, Abdelbasset Ibrahim M. Malathion induced testicular toxicity and oxidative damage in male mice: The protective effect of curcumin. Egypt J Forensic Sci. 2018 doi: 10.1186/s41935-018-0099-x. [Google Scholar]
- 17.Wang M, Guckland A, Murfitt R, Ebeling M, Sprenger D, Foudoulakis M, et al. Relationship between magnitude of body weight effects and exposure duration in mammalian toxicology studies and implications for ecotoxicological risk assessment. Environ Sci Eur. 2019:31. Doi: 10.1186/s12302-019-0221-1. [Google Scholar]
- 18.Abdel-Tawab HM, Heikal TM, Omara EA. Physiological and histopathological changes in the liver of male rats exposed to paracetamol and diazinon? Asian Pac J Trop Biomed. 2012;2:S1683–90. Doi: 10.1016/S2221-1691(12)60478-X. [Google Scholar]
- 19.Wu T, Li J, Li Y, Song H. Antioxidant and hepatoprotective effect of swertiamarin on carbon tetrachloride-induced hepatotoxicity via the Nrf2/HO-1 pathway. Cell Physiol Biochem. 2017;41:2242–54. doi: 10.1159/000475639. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Hussain SA, Ali D, Omar SY, Shaik U, Alghamdi HA, et al. Induced alteration of rat erythrocyte membrane with effect of pyrethroid based compounds. Saudi J Biol Sci. 2020;27:3669–75. doi: 10.1016/j.sjbs.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassar AM, Salim YM, Eid KS, Shaheen HM, Saati AA, Hetta HF, et al. Ameliorative effects of honey, propolis, pollen, and royal jelly mixture against chronic toxicity of sumithion insecticide in white albino rats. Molecules. 2020;25:2633. doi: 10.3390/molecules25112633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambali SF. Ameliorative Effect of Vitamins C and E on Neurotoxicological, Hematological and Biochemical Changes Induced by Chronic Chlorpyrifos in Wistar Rats, PhD Dissertation, Ahmadu Bello University, Zaria. 2009 [Google Scholar]
- 23.Mansour SA, Mossa AH. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pest Biochem Physiol. 2010;96:14–23. [Google Scholar]
- 24.Elgaml AS, Hassan WH, Abdelaziz S, Hashish E. Parkinsonia aculeata L. aqueous extract alleviated the hepatotoxicity induced by acetaminophen in albino rats. Comp Clin Pathol. 2020;29:1–7. [Google Scholar]
- 25.Kartheek RM, David M. Assessment of fipronil toxicity on wistar rats: A hepatotoxic perspective. Toxicol Rep. 2018;5:448–56. doi: 10.1016/j.toxrep.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaiswal K, Gupta V, Siddiqi N, Pandey R, Sharma B. Hepatoprotective effect of citrus limon fruit extract against carbofuran induced toxicity in wistar rats. Chin J Biol. 2015;2015:686071. [Google Scholar]
- 27.Ilavenil S, Al-Dhabi NA, Srigopalram S, Ock Kim Y, Agastian P, Baru R, et al. Acetaminophen induced hepatotoxicity in Wistar rats – A proteomic approach. Molecules. 2016;21:161. doi: 10.3390/molecules21020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alharbi YM, El-Zahar KM, Mousa HM. Beneficial effects of fermented camel and cow's milk in lipid profile, liver, and renal function in hypercholesterolemic rats. Fermentation. 2022;8:171. [Google Scholar]
- 29.Karabacak M, Kanbur M, Eraslan G, Siliğ Y, Soyer Sarıca Z, Tekeli MY, et al. The effects of colostrum on some biochemical parameters in the experimental intoxication of rats with paracetamol. Environ Sci Pollut Res Int. 2018;25:23897–908. doi: 10.1007/s11356-018-2382-7. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Ma Q, Liu G, Wang C. Effects of donkey milk on oxidative stress and inflammatory response. J Food Biochem. 2022;46:e13935. doi: 10.1111/jfbc.13935. [DOI] [PubMed] [Google Scholar]


