Abstract
Background:
Acute pain is one of the main complaints of patients after total knee arthroplasty (TKA), which causes delayed mobility, increased morphine consumption, and subsequently increased costs. Therefore, the present study was performed to evaluate the preventive effect of preoperative celecoxib and gabapentin on reducing patient pain as a primary outcome after TKA surgery.
Materials and Methods:
This randomized, double-blind controlled clinical trial was performed on 270 patients with osteoarthritis that were candidates for TKA surgery allocated into three groups. In the first group, 900 mg of gabapentin was administered orally on a daily basis for 3 days before surgery. In the second group, 200 mg of oral celecoxib was administered twice daily for 3 days before surgery. In the third group, oral placebo was administered twice daily for 3 days before the surgery. The patients’ pain score and knee and its functional score were recoded.
Results:
The mean of reduction pain in gabapentin and celecoxib groups was significantly lower than that of the control group at 12, 24, and 48 h after surgery (P < 0.001); however, two groups were not significantly different from each other (P > 0.05). Furthermore, the two medication groups were not significantly different in this regard (P > 0.05). In addition, the knee score in the gabapentin group with the means of 85.40 ± 5.47 and the celecoxib group with the means of 87.03 ± 3.97 were significantly higher than those of the control group with the means of 78.90 ± 4.39 in the 1st month after the surgery (P < 0.001).
Conclusion:
According to the results of the present study, the preventive administration of gabapentin and celecoxib showed a significant and similar effectiveness on reducing patient pain after TKA surgery and on improving the KSS and quality of life scores.
Keywords: Celecoxib, gabapentin, knee function, pain, quality of life, total knee arthroplasty
INTRODUCTION
Total knee arthroplasty (TKA) is one of the most common surgical procedures in orthopedic surgery which is used to improve knee function and reduce pain in patients with end-stage knee osteoarthritis (KOA) or patients that experience chronic pain from severe joint damage.[1] The incidence of TKA is expected to increase by about 70% by the year 2030 in the United States.[2] Although many studies have revealed that TKA improves the quality of life and knee function in patients with many positive clinical outcomes, complications also occur during and after this surgery.[3,4,5]
In this regard, acute pain following TKA has been reported as one of the most common complications of this surgery.[6] In fact, since the incidence of chronic postoperative pain following TKA is in approximately 20% of the patients,[7] controlling postoperative pain is one of the main challenges in this respect. At present, opioids are the mainstay of postoperative pain management; however, the increased need for opioids as well as their increased complications hinders the rehabilitation process and increases the length of hospital stay, which in turn increases the cost of treatment and affects patient satisfaction.[8,9]
In addition, many studies have indicated that the preventive use of analgesics can help patients cope with their postoperative pain. The inhibition of the pain stimuli as well as afferent pain signals from the surgery site to prevent central pain sensitization is the main goal of this treatment.[1,10]
The results of previous studies are also indicative of the benefits of preventive analgesia in various surgical procedures such that a lower use of postoperative analgesics which has been reported in 60% of cases.[11,12]
The use of oral analgesics before surgery has been associated with positive results.[13] Celecoxib as an oral NSAID and gabapentin from the gabapentinoid class can be used as preventive analgesics.[10]
Some researchers have shown that gabapentinoids have an anxiolytic effect as well[14,15] and reduce hyperstimulation of nerve cells.[16] Moreover, it has been suggested that the administration of gabapentinoids preoperatively and postoperatively can reduce the incidence of chronic postoperative pain. In this regard, Buvanendran and Kroin studied pregabalin, as compared with placebo, on the day of surgery and 14 days after TKA and observed a reduction in neuropathic pain within 6 months after TKA.[17] In addition, some studies support the peripheral effects on primary afferent neurons.[18,19]
With respect to celecoxib, it can be stated that it is an NSAID with selective activity on the COX-2 system. Zhao et al. revealed that spinal COX-2 is involved in the development of tactile allodynia following nerve injury in rats.[20] Celecoxib has been proven to be effective in reducing toothache and the signs and symptoms of osteoarthritis and rheumatoid arthritis and is also associated with a much lower rate of gastrointestinal damage than NSAIDs.[21] Nonselective NSAIDs are analgesics commonly used for minor surgeries, which are administered as useful adjunctive analgesics for patients undergoing major surgeries and result in the reduction of pain and a decrease in the need for morphine.[10,21]
Gabapentin has a similar analgesic effect as well as fewer complications.[22,23] Despite the promising effects of these drugs, research addressing these drugs and their dosage for preventing pain in TKA surgery is limited.
Given different perceptions of pain by individuals and the existence of various factors affecting pain intensity such as the nature, duration, and extent of surgery, psychological aspects (stress and anxiety) and the effective role of preoperative pain prevention as well as the potential effects of celecoxib and gabapentin, the present study was performed with the aim of evaluating the effect of these two drugs on reducing patient pain, improving their knee function, and increasing their quality of life after TKA surgery.
MATERIALS AND METHODS
Study design and patients
The present study was a randomized double-blind controlled clinical trial. The study population included all patients with osteoarthritis that were referred to the orthopedic specialty referral clinic of Al-Zahra Hospital affiliated to the Isfahan University of Medical Sciences during 2020–2021. In the current study, 270 patients who fulfilled the inclusion criteria were randomly recruited and randomly allocated to the intervention groups (90 patients in each group). The sample size was determined for detecting a standardized effect size of at least 0.5 (the least anticipated difference between active treatments and control) in pain score as the primary outcome with a type one error rate of 5% and statistical power of 80%.[24]
The inclusion criteria consisted of having primary osteoarthritis, being a candidate for TKA surgery. The patients were not included in the study in case with drug addiction (substance use disorder), psychiatric disorders (including anxiety disorders, major depressive disorder, and bipolar disorder), history of kidney diseases (end-stage renal disease and glomerular filtration rate <30 ml/min), history of chronic neuropathy, KOA due to rheumatoid arthritis and infection, diabetes and morbid obesity (body mass index [BMI] >35 kg/m2), blood clotting, and with embolism or vascular phlebitis.
Moreover, they were excluded from the study in case of allergy to gabapentin and celecoxib, the incidence of any complications due to surgery (including patella fracture, patella tendon rupture, quadriceps tendon rupture, femoral periprosthetic fracture, tibial periprosthetic fracture, popliteal artery injury, intimal injury, and postoperative ischemia), and nonattendance in follow-up appointments.
In the present study, 4 patients in the gabapentin group, 5 patients in the control group, and 3 patients in the celecoxib group were excluded from the study due to their nonattendance in follow-up appointments [Figure 1].
Figure 1.
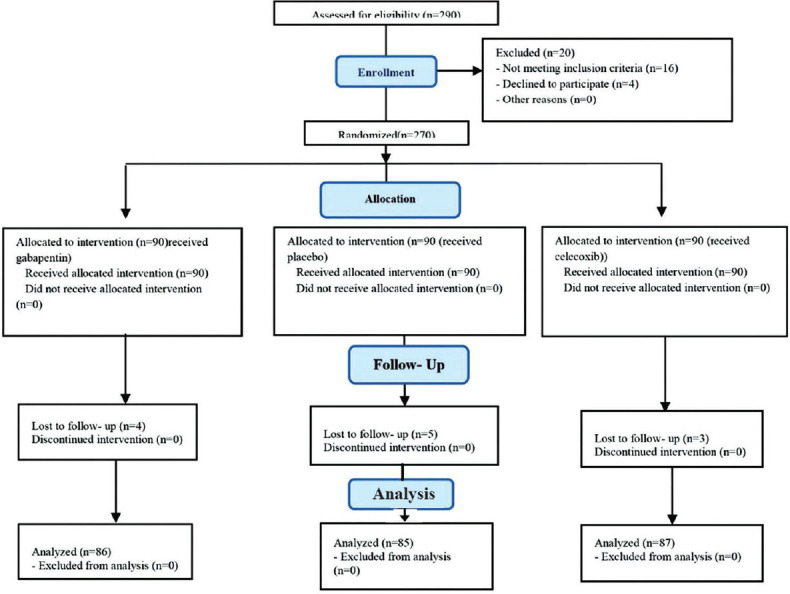
Consort flow diagram for recruitment of patients
After obtaining the code of ethics from the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.MED. REC.1399.948), the clinical trial code (IRCT20200217046523N10), and obtaining written informed consent from eligible patients, 270 patients in the study were randomly divided into three groups of 90 using the block randomization method with blocks of size 6. At the beginning of the study, patients’ demographic and clinical information including age, gender, and BMI was recorded. Moreover, before the intervention, patient anxiety and depression scores based on The Hospital Anxiety and Depression Scale (HADS), their knee scores and knee functional score in daily activities based on the Knee Society Score (KSS) criteria, and their quality of life score based on the 36-Item Short Form Health Survey (SF-36) were evaluated and recorded. In addition, the pain intensity in patients was recorded based on the Visual Analog Scale (VAS) with the score ranging from 0 (no pain) to 10 (severe pain).
Study interventions
Both patients and researchers were blinded to the type of the used medications. The interventions included oral gabapentin 300 mg, celecoxib 200 mg, and placebo which were all prepared in small capsules with the same appearance (Exir Pharmaceutical Co.). In the first group, 900 mg of oral gabapentin (Exir Pharmaceutical Co.) was administered daily for three days before surgery such that 600 mg and 300 mg were administered at 8 a.m. and 10 p.m., respectively. In the second group, 200 mg of oral celecoxib (Exir Pharmaceutical Co.) was administered twice daily for 3 days before surgery. In the third group, oral placebo was administered twice a day for 3 days before surgery.
Then, all patients underwent TKA surgery by a single professional surgery team. Anesthesia of all patients was uniformly performed epidurally with 15–20 mg of bupivacaine 5% before TKA.
It should be noted that about 5–7 patients in each group did not respond to spinal anesthesia and underwent general anesthesia.
Postoperative analgesia consisting of paracetamol 1000 mg + PCA morphine 2 mg/kg by BW with 5 min interval and the maximum dose of 6 mg/h was administered 2 h after the already-mentioned procedure. In case of nausea and vomiting, ondansetron 4 mg was administered intravenously.
In addition, physiotherapy sessions (From the 1st day after discharge) and all necessary medical recommendations were prescribed to patients equally. At discharge, the same medication regimen including acetaminophen 500 mg every 8 h and meloxicam 7.5 mg daily was prescribed for all patients.
Study instruments and outcome evaluation
Primary outcome
Pain intensity: The intensity of pain in patients was recorded according to the VAS criteria at 12, 24, and 48 h after surgery, at the time of discharge, and 1, 3, and 6 months after discharge.
Secondary outcomes
Quality of life
The quality of life of patients in our study was evaluated by using the 36 item SF-36 questionnaire. The SF-36 questionnaire assesses the quality of life across two main dimensions including physical health and mental health. Each domain in the SF-36 is scored based on a 0–100 scale and higher scores indicate better health conditions.[25] The validity and reliability of this questionnaire have been evaluated and confirmed in previous studies.[25,26] The quality of life score based on the SF-36 questionnaire for the study of participants was evaluated at before intervention and 1 month after hospital discharge.
Knee society score
KSS questionnaire includes items on functional ability, such as walking distance, stair climbing ability, and use of walking aids. The physical examination includes assessment of the range of motion, stability, alignment, and muscle power of the knee. These data are used to calculate a Knee Society Clinical Rating score, which consists of two scores, a knee score and a function score, ranging from 0 to 100 points (with 100 points being the best score). The function score allocates points for walking distance and stair-climbing ability and makes deductions for the use of a walking aid; a score of 100 represents unlimited walking distance and normal stair-climbing without the use of an aid. Fifty of the 100 points in the knee score reflect pain assessment (a score of 50 points represents no pain). Knee clinical scores and knee functional scores were recorded at before intervention and at 1, 3, and 6 months after hospital discharge. The validity and reliability of this questionnaire have been evaluated and confirmed in previous studies.[27,28]
Others study outcomes
Operative duration, the amount of intraoperative blood loss, postoperative blood loss, length of hospital stay, amount of morphine administered at 12, 24, and 48 h after surgery, and at the time of hospital discharge were recorded. The patients’ anxiety and depression score based on the HADS questionnaire was evaluated and recorded at before intervention and 1 month after hospital discharge. The total score of HADS ranges from 0 to 21 and higher scores are indicative of more severe anxiety and depressive disorders. The reliability and validity of the mentioned questionnaire have been confirmed by previous studies.[29,30] It should be mentioned that these questionnaires were administered and filled out by the patients in person at the relevant center or by a researcher on the telephone. Moreover, patients were interviewed about their emotions and problems in each visit, and a two-way interaction was formed between patients and specialists. In addition, complications such as nausea, vomiting, respiratory depression, and rates of blood transfusions were recorded in all three groups.
Statistical analysis
The collected data were entered into the Statistical analyses were carried out using SPSS v. 24 (IBM Corp., Armonk, USA). Data were presented as means ± standard deviation or frequency (percentage) for the continuous and categorical variables, respectively. The Kolmogorov − Smirnov test and Q-Q plot were used for evaluating normal distribution for the continuous variables. The one-way analysis of variance (ANOVA) test was used to compare the mean age, operative time, length of hospital stay, amount of intraoperative blood loss, and postoperative blood loss between the three groups and the Chi-squared test was used for comparing the categorical variables such as gender, depression, and anxiety. The repeated-measures ANOVA was employed to evaluate the variations in pain and KSS scores over the intervention period in each group and for between group comparisons. In the repeated-measures ANOVA, the sphericity preassumption was evaluated and when it was violated the Huynh-Feldt univariate modified degree of freedom was adopted. The paired samples t-test was used to compare the variations for those quantitative variables that have been evaluated only in two time points before the intervention. The ANOVA or analysis of covariance (ANCOVA) and Bonferroni pairwise post hoc tests were used for between group comparisons in terms of quality of life. A significance level of less than 0.05 was considered statistically significant in all analyses.
RESULTS
In the present study, the control group included 39 (45.9%) females, with a mean age of 61.38 ± 5.79 years, the gabapentin group consisted of 42 (48.8%) females, with a mean age of 60.73 ± 5.91 years, and the celecoxib group comprised 45 (51.7%) females and 42 (48.3%) males, with a mean age of 59.32 ± 5.89 years. There were no significant differences between the three groups in terms of age, sex, BMI, anxiety, and depression before surgery (P > 0.05) [Table 1].
Table 1.
Comparison of the basic demographic and clinical characteristics of patients in the three groups
| Characteristics | Control group (n=85) | Gabapentin group (n=86) | Celecoxib group (n=87) | P* |
|---|---|---|---|---|
| Age (year) | 61.38±5.79 | 60.73±5.91 | 59.32±5.89 | 0.065 |
| Sex, n (%) | ||||
| Female | 39 (45.9) | 42 (48.8) | 45 (51.7) | 0.746 |
| Male | 46 (54.1) | 44 (51.2) | 42 (48.3) | |
| BMI (kg/m2) | 28.05±2.08 | 27.95±2.66 | 27.45±2.27 | 0.194 |
| Anxiety, n (%) | ||||
| Preoperative | 10 (11.8) | 9 (10.5) | 7 (8.0) | 0.713 |
| Depression, n (%) | ||||
| Preoperative | 4 (4.7) | 1 (1.2) | 1 (1.1) | 0.190 |
*Resulted from one-way ANOVA and Chi-squared test for continuous and categorical data, respectively. Data are presented as mean±SD and frequency (%) for continuous and categorical, respectively. BMI=Body mass index; SD=Standard deviation; ANOVA=Analysis of variance
The mean pain score of patients in the gabapentin and celecoxib groups was significantly lower than that of the control group 12, 24, and 48 h after the surgery (P < 0.05). The mean of the two gabapentin and celecoxib groups was not significantly different 12, 24, and 48 h after the surgery (P > 0.05). In addition, the follow-up visits performed in the 1st month after hospital discharge indicated that the pain score of patients in the control group with the mean of 0.64 ± 0.74 was significantly higher than that of the gabapentin and celecoxib groups with the means of 0.08 ± 0.28 and 0.19 ± 0.39, respectively (P < 0.05). In the 3rd month after surgery, the pain was completely disappeared in the gabapentin group, and mild pain (0.02 ± 0.15) was reported in the celecoxib group; however, the pain score in the control group with the mean of 0.29 ± 0.56 was still higher than that of the other two groups (P < 0.05). With respect to 6 months after the surgery, the pain of patients in all three groups was very low and nonsignificant (P > 0.05). In addition, mean pain score of patients was significant over time in any of the groups (PTime < 0.05). Moreover, the interactive effect of time and intervention was not significant up to 48 h postoperative, while this effect was significant up to 6 months after hospital discharge (Ptime *group > 0.05) [Table 2 and Figure 2].
Table 2.
The comparison of mean pain score of patients in the three groups
| Pain score | Control group (n=85) | Gabapentin group (n=86) | Celecoxib group (n=87) | P 1 | P intervention | P time×intervention |
|---|---|---|---|---|---|---|
| Postoperative (h) | ||||||
| 12 | 3.73±1.05a | 2.46±0.79b | 2.71±0.93b | <0.001 | <0.001 | 0.07 |
| 24 | 2.89±0.95a | 1.64±0.66b | 1.78±0.65b | <0.001 | ||
| 48 | 2.37±0.94a | 0.99±0.68b | 1.03±0.72b | <0.001 | ||
| Hospital discharge | ||||||
| Immediately | 1.80±0.76a | 0.35±0.48b | 0.29±0.45b | <0.001 | <0.001 | |
| 1 month | 0.64±0.74a | 0.08±0.28b | 0.19±0.39b | 0.001 | <0.001 | |
| 3 months | 0.29±0.56a | 0b | 0.02±0.15b | 0.012 | ||
| 6 months | 0.14±0.35a | 0b | 0b | 0.110 | ||
| P time | <0.001 | <0.001 | <0.001 |
The different superscript letters indicating difference in mean pain score between two groups based on post hoc test. Pintervention, Ptime and Ptime×intervention obtained from repeated-measures ANOVA. Pintervention=Overall difference between three groups over the follow-up period; Ptime=The change in mean pain score in each intervention group over the follow-up period; Ptime×intervention=The interaction between time and intervention; P1=Resulted from one-way ANOVA for comparing mean pain score between three groups at each follow-up time point; ANOVA=Analysis of variance
Figure 2.
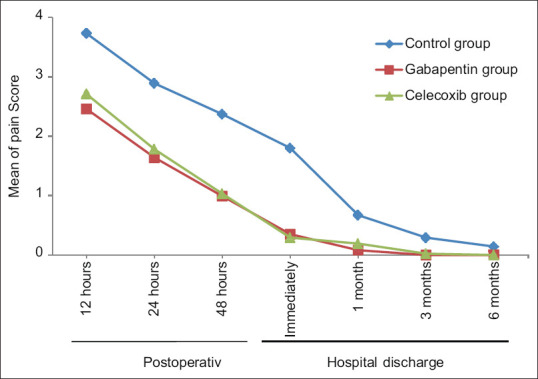
The mean pain score of patients in the three groups
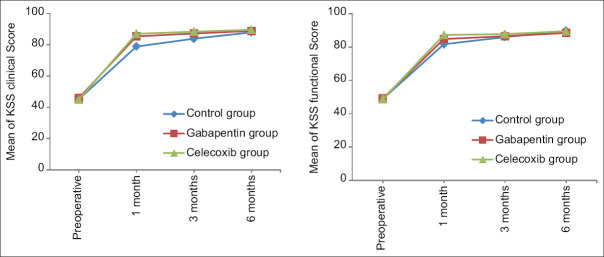
Evaluation of the knee clinical score and knee functional score based on the KSS criteria revealed that the three groups did not have significant differences in terms of clinical and functional evaluation of the knee before surgery (P > 0.05). However, in the 1st month after the intervention, the knee clinical score and knee functional score in the control group with the means of 78.90 ± 4.39 and 81.74 ± 4.49 were significantly lower than those of the gabapentin group with the means of 85.40 ± 5.47 and 84.83 ± 4.92 and the celecoxib group with the means of 87.03 ± 3.97 and 87.30 ± 3.73, respectively (P < 0.001). The mean score of these two criteria in the gabapentin group was much lower than that of the celecoxib group in the mentioned follow-up period (P < 0.05). Moreover, in the 3rd month after surgery, the knee clinical score of the control group with the mean of 83.89 ± 3.44 was significantly lower than that of the gabapentin and celecoxib groups with the means of 87.24 ± 4.18 and 88.37 ± 3.09, respectively (P < 0.05); however, there was no significant difference between the two groups (P > 0.05). In contrast, the knee clinical score in the 6th month and the knee functional score in the third and 6th months after surgery were not significantly different between the three groups (P > 0.05). In addition, changes in knee clinical and knee functional scores were significant over time in any of the groups (PTime < 0.05). Furthermore, their mean changes were not significantly different between the three groups (P1 < 0.05). Moreover, the interactive effect of time and intervention was significant (Ptime * group < 0.05) [Table 3 and Figure 3].
Table 3.
The comparison of the mean knee clinical and knee functional scores in three groups
| KSS score | Control group (n=85) | Gabapentin group (n=86) | Celecoxib group (n=87) | P 1 | P intervention | P time×intervention |
|---|---|---|---|---|---|---|
| KSS clinical score | ||||||
| Preoperative | 45.11±4.82 | 45.89±4.99 | 44.91±5.96 | 0.385 | <0.001 | <0.001 |
| 1 month | 78.90±4.39a | 85.40±5.47b | 87.03±3.97c | <0.001 | ||
| 3 months | 83.89±3.44a | 87.24±4.18b | 88.37±3.09b | <0.001 | ||
| 6 months | 87.96±3.54a | 88.85±2.93a | 89.64±2.67b | 0.009 | ||
| P time | <0.001 | <0.001 | <0.001 | |||
| KSS functional score | ||||||
| Preoperative | 48.94±4.49 | 49.24±5.33 | 48.69±5.51 | 0.763 | <0.001 | <0.001 |
| 1 month | 81.74±4.49a | 84.83±4.92b | 87.30±3.73b | <0.001 | ||
| 3 months | 86.02±4.23a | 86.53±4.53 | 87.77±5.19b | 0.043 | ||
| 6 months | 89.84±4.11 | 88.53±3.95 | 89.59±2.78 | 0.092 | ||
| P time | <0.001 | <0.001 | <0.001 |
The different superscript letters indicating difference in mean pain score between two groups based on post hoc test. Pintervention, Ptime, and Ptime×intervention obtained from repeated-measures ANOVA. P1=Resulted from one-way ANOVA for comparing mean pain score between three groups at each follow-up time point; Pintervention=Overall difference between three groups over the follow-up period; Ptime=The change in mean pain score in each intervention group over the follow-up period; Ptime×intervention=The interaction between time and intervention; ANOVA=Analysis of variance; KSS=Knee Society Score
Figure 3.
The mean knee clinical and knee functional scores of patients in the three groups
The results of evaluating the patients’ quality of life based on the SF-36 questionnaire in the two main dimensions of physical health and mental health among the three groups before and 1 month after surgery indicated that the patients’ quality of life in both domains was improved in all three groups (P < 0.05), except for mental health in the celecoxib group (P = 0.068) that was marginally reduced. However, there was not any significant difference between the groups before and 1 month after surgery for the mental health domain (P > 0.05). However, the patients’ quality of life in the physical health domain was significantly different between the three groups 1 month after intervention (P = 0.044) and a significant improvement was observed in the celecoxib group compared with the control and gabapentin groups (P < 0.05), based on the post hoc test) [Table 4].
Table 4.
The comparison of mean score of quality of life in the three groups
| SF36 two main domains | Control group (n=85) | Gabapentin group (n=86) | Celecoxib group (n=87) | P 2 |
|---|---|---|---|---|
| Physical health | ||||
| Preoperative | 38.97±4.52 | 39.41±3.94 | 39.55±4.32 | 0.651 |
| 1 month | 89.99±4.97a | 89.94±4.93a | 91.11±3.74b | 0.044 |
| P 1 | <0.001 | <0.001 | <0.001 | |
| Mental health | ||||
| Preoperative | 87.57±15.49 | 86.94±15.38 | 91.07±11.40 | 0.121 |
| 1 month | 89.29±12.82 | 88.38±13.53 | 92.09±9.87 | 0.702 |
| P 1 | <0.001 | <0.001 | 0.068 |
P1=Resulted from paired samples t-test for within group comparisons; P2=Resulted from ANCOVA for between group comparisons; adjustment was made for baseline quality of life; ANCOVA=Analysis of covariance; SF36=36 item short form health survey
Finally, the incidence of nausea and vomiting in the control group with 15.3% was higher than that of the gabapentin and celecoxib groups with 7% and 3.4%, respectively (P = 0.017). However, the incidence of respiratory depression and blood transfusion did not differ significantly between the three groups (P > 0.05). Furthermore, operation time, length of hospital stay, and intraoperative and postoperative blood loss were not significantly different between groups (P > 0.05). However, the dose of additional morphine received at 12, 24, and 48 h after surgery and at the time of hospital discharge in the control group was significantly higher than the other two groups (P < 0.05)[Table 5].
Table 5.
The comparison of complications and intra- and postoperative characteristics in the three groups
| Complication | Control group (n=85) | Gabapentin group (n=86) | Celecoxib group (n=87) | P* |
|---|---|---|---|---|
| Nausea and vomiting, n (%) | 13 (15.3) | 6 (7) | 3 (3.4) | 0.017 |
| Respiratory depression, n (%) | 7 (8.2) | 5 (5.8) | 2 (2.3) | 0.224 |
| Blood transfusion, n (%) | 5 (5.9) | 9 (10.5) | 9 (10.3) | 0.448 |
| Operative time (min) | 72.51±7.78 | 73.35±7.83 | 73.07±7.28 | 0.763 |
| Length of hospital stay | 3.73±0.75 | 3.37±0.77 | 3.76±0.85 | 0.112 |
| Morphine administration; mg | ||||
| 12 h | 4.35±0.47 | 3.48±0.81 | 3.17±0.53 | <0.001 |
| 24 h | 15.12±1.08 | 7.33±1.39 | 7.05±1.26 | <0.001 |
| 48 h | 20.91±1.29 | 10.59±1.66 | 9.82±1.48 | <0.001 |
| Discharge | 27.35±1.84 | 14.57±2.50 | 12.61±1.75 | <0.001 |
| Intraoperative blood loss (mL) | 217.88±65.68 | 208.97±59.25 | 195.76±66.05 | 0.074 |
| Postoperative blood loss (mL) | 251.98±58.06 | 237.50±58.03 | 250.93±60.24 | 0.200 |
*Resulted from Chi-squared and one-way ANOVA for categorical and continuous variables. ANCOVA=Analysis of covariance
DISCUSSION
The results of the present study revealed that the preventive administration of gabapentin and celecoxib before surgery had an important role in controlling patient pain. Moreover, there was no significant difference between the two intervention groups in terms of reducing patient pain scores. Consistent with the findings of the current study, previous studies indicated that celecoxib, when used for spinal fusion surgery and spinal disc surgery was associated with a significant reduction in pain and had a saving effect on opioid use.[31] Conversely, Huang et al. were unable to detect the degree of postoperative pain or morphine use in patients after radical prostatectomy when using oral rofecoxib (a COX-2 inhibitor) before surgery.[32]
The studies addressing the clinical pharmacokinetics of dexamethasone and celecoxib indicated that celecoxib could reach the highest plasma drug concentration after 2–4 h when a 200-mg dose was employed. An evident volume of distribution of 455 liters as well as the elimination half-life (t½) that ranged from 8 to 11 h could be reported in this respect.[33,34] The higher volume of distribution as well as t½ values for celecoxib could lead to a greater drug distribution through tissues as well as a slower elimination, which in turn cause an extended therapeutic effect in comparison with the dexamethasone regimen.[35]
Pilatti et al. also showed that the use of celecoxib before and after surgery was effective in the management of postoperative pain following open-flap debridement.[35]
In addition, Lubis et al. indicated that the pain score was lower in the groups receiving celecoxib and pregabalin as preventive analgesics as compared with the placebo group.[1] Similar results have been reported by Buvanendran and Kroin and Carmichael et al., who found that repeated the use of celecoxib and pregabalin successfully reduced acute and chronic postoperative pain.[17,36] Moreover, several meta-analysis studies have also highlighted the analgesic effect of preoperative gabapentin on pain over the first days after surgery.[37,38] In contrast, the results of another meta-analysis study demonstrated no preventive effect of pregabalin administration on pain reduction within 12 months after surgery.[39] Our study indicated that the short-term use of gabapentin before surgery had an effect on reducing pain and improving patients’ mental health within 1 month, although significant results in terms of improving anxiety or depression were not obtained in this study. Although the mechanism of action of gabapentinoids is not well-recognized, gabapentinoids may bind to the α2-δ subunit of voltage-sensitive calcium channels in dorsal horn presynaptic neurons,[40,41] inhibit neurotransmitter secretion, reduce pain transmission from dorsal neurons,[42] and thus reduce patient pain.[43]
It should be noted that due to the fact that various factors such as race, infection, and inflammatory response to TKA as well as psychological factors like anxiety and depression can be effective in the development and intensity of pain before and after surgery,[5,8,44] the current study opted to limit the effect of controllable factors. In fact, as it was indicated, anxiety and depression were very minor in patients of this study and were not significantly different between the three groups. Moreover, all patients in the study were of Iranian ethnicity and did not have any infection or inflammation caused by TKA. In addition, due to the administration of a higher dose of additional morphine in the control group as compared with the two intervention groups, this factor was adjusted along with the age and gender of patients. Thus, the distorting effect of these factors has been eliminated.
Furthermore, the results of the evaluation of the knee condition according to the KSS criteria also indicated that in general, this surgery could significantly improve the knee score and knee functional scores within 6 months. In addition, the preventive use of gabapentin and celecoxib along with this surgical procedure could significantly improve the knee and functional scores. Importantly, patients in the gabapentin group had a significantly higher knee and knee functional score than the celecoxib group in the 1st month after surgery. In fact, it can be stated that although there was no significant difference in the improvement of the knee functional score between the two types of interventions in the third and 6th month after surgery, the use of gabapentin before surgery had a better effect on the overall KSS in the 1st month after surgery (in a shorter period of time).
In this regard, Reuben et al. also stated that TKA surgery played a significant role in improving the overall KSS. In addition, they indicated that patients receiving celecoxib generally had a higher KSS than the placebo group at 12 months of the follow-up period.[45]
The results of another study revealed that in the 1st week after TKA, the KSS score in the celecoxib group was significantly higher than that of the hydrochloride group. The reported difference may be attributed to the significant pain reduction in patients receiving celecoxib during rest and joint activity (especially bending angle) in the 1st week. Therefore, the patients’ ROM in this group was more satisfactory.[46] Lubis et al. evaluated the knee function and its ROM and demonstrated that the combination of celecoxib and pregabalin, as compared to the control group, could play a significant role in increasing the scores of KSS and ROM.[1] Many other studies have also shown that gabapentin improved knee function after TKA surgery.[47,48] In fact, it can be stated that using a selective COX-2 inhibitor through inhibition of the inflammatory response and with the aid of gabapentinoids such as pregabalin or gabapentin can improve primary function by reducing over-excitability of the nerve cells and controlling pain. It is expected that patients will be able to be mobile early after the surgery with the use of preventive analgesics. It is well documented that early mobilization reduces complications such as DVT. This can enhance the healing process and in turn improve functional outcomes.[49]
The results of the present study revealed that by performing the TKA procedure, the patients’ quality of life with and without the use of preventive analgesics (gabapentin and celecoxib) were significantly improved. However, gabapentin was found to be more effective in improving their mental health than the control and celecoxib groups.
Significant improvements in patients’ quality of life are anticipated due to the improvement of the patients’ knee function and the reduction of their pain. However, the greater effect of gabapentin on patients’ mental health can be attributed to the effects of this drug on patients’ anxiety disorders.[50] However, it is crucial to mention that as few patients had depression or anxiety in the current study, the evaluation of the efficacy of drugs (especially gabapentin) on this group of patients was not associated with significant results; therefore, the researchers of this study failed to mention them. However, further studies are recommended in this respect.
Finally, the incidence of nausea and vomiting in the two intervention groups was significantly lower than that of the control group, which may be attributed to the use of lower doses of morphine after surgery. Moreover, the three groups were not significantly different in terms of the incidence of respiratory depression and need for blood transfusion.
Another study which was consistent with the present study indicated that the incidence of complications such as nausea, vomiting, and pruritus was lower in the celecoxib group as compared with the placebo group immediately after surgery.[45]
The results of another study also suggested that complications are dose-dependent and often occur in patients that use a combination of celecoxib and pregabalin for a long time.[1] Lee et al. compared the use of a combination of 400 mg celecoxib and 150 mg pregabalin with 400 mg celecoxib alone and found out that complications occurred in the combination group.[51]
It should be noted that although a large enough sample size of the present clinical trial along with the 6-month follow-up of patients can be expressed as the strengths of this study: the present study did not use different doses or combinations of the two drugs, which may be regarded as the limitations of the present study. In addition, the evaluation of the effect of gabapentin in patients with depression and anxiety undergoing the TKA procedure may be of great significance which needs to be studied further.
CONCLUSION
According to the results of the present study, the preventive use of gabapentin and celecoxib may have a significant role in reducing patient pain after TKA surgery, although the effect of these two drugs on pain reduction is not different. It is also imperative to note that the additional dose of morphine administered in these two groups was much lower than that of the control group, which resulted in a higher incidence of nausea and vomiting in the control group as compared with the intervention groups. In addition, the overall KSS score was significantly higher in the celecoxib group as compared with the gabapentin group in the 1st month of follow-up; however, no significant difference was found in the overall KSS score between the three groups in the third and 6th months after surgery. In addition, patient quality of life score also increased in all three groups 1 month after TKA surgery; however, the rate of mental health improvement was more significant in the gabapentin group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lubis AM, Rawung RB, Tantri AR. Preemptive analgesia in total knee arthroplasty: Comparing the effects of single dose combining celecoxib with pregabalin and repetition dose combining celecoxib with pregabalin: Double-blind controlled clinical trial. Pain Res Treat. 2018;2018:3807217. doi: 10.1155/2018/3807217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Lovald ST, Ong KL, Lau EC, Schmier JK, Bozic KJ, Kurtz SM. Mortality, cost, and health outcomes of total knee arthroplasty in Medicare patients. J Arthroplasty. 2013;28:449–54. doi: 10.1016/j.arth.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 4.Cram P, Lu X, Kaboli PJ, Vaughan-Sarrazin MS, Cai X, Wolf BR, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991-2008. JAMA. 2011;305:1560–7. doi: 10.1001/jama.2011.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjær Petersen K, Lunn TH, Husted H, Hansen LT, Simonsen O, Laursen MB, et al. The influence of pre- and perioperative administration of gabapentin on pain 3-4 years after total knee arthroplasty. Scand J Pain. 2018;18:237–45. doi: 10.1515/sjpain-2018-0027. [DOI] [PubMed] [Google Scholar]
- 6.Tsukada S, Wakui M, Hoshino A. Pain control after simultaneous bilateral total knee arthroplasty: A randomized controlled trial comparing periarticular injection and epidural analgesia. J Bone Joint Surg Am. 2015;97:367–73. doi: 10.2106/JBJS.N.00373. [DOI] [PubMed] [Google Scholar]
- 7.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2:e000435. doi: 10.1136/bmjopen-2011-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DP, O’Brien S, Doran E, Price AJ, Beard DJ, Murray DW, et al. Early postoperative predictors of satisfaction following total knee arthroplasty. Knee. 2013;20:442–6. doi: 10.1016/j.knee.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Korean Knee Society. Guidelines for the management of postoperative pain after total knee arthroplasty. Knee Surg Relat Res. 2012;24:201–7. doi: 10.5792/ksrr.2012.24.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White PF, Tang J, Wender RH, Zhao M, Time M, Zaentz A, et al. The effects of oral ibuprofen and celecoxib in preventing pain, improving recovery outcomes and patient satisfaction after ambulatory surgery. Anesth Analg. 2011;112:323–9. doi: 10.1213/ANE.0b013e3182025a8a. [DOI] [PubMed] [Google Scholar]
- 11.Katz J, Clarke H, Seltzer Z. Review article: Preventive analgesia: Quo vadimus? Anesth Analg. 2011;113:1242–53. doi: 10.1213/ANE.0b013e31822c9a59. [DOI] [PubMed] [Google Scholar]
- 12.Lee BH, Park JO, Suk KS, Kim TH, Lee HM, Park MS, et al. Pre-emptive and multi-modal perioperative pain management may improve quality of life in patients undergoing spinal surgery. Pain Physician. 2013;16:E217–26. [PubMed] [Google Scholar]
- 13.Fibel KH, Hillstrom HJ, Halpern BC. State-of-the-Art management of knee osteoarthritis. World J Clin Cases. 2015;3:89–101. doi: 10.12998/wjcc.v3.i2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nutt D, Mandel F, Baldinetti F. Early onset anxiolytic efficacy after a single dose of pregabalin: Double-blind, placebo- and active-comparator controlled evaluation using a dental anxiety model. J Psychopharmacol. 2009;23:867–73. doi: 10.1177/0269881108094722. [DOI] [PubMed] [Google Scholar]
- 15.Imani F, Rahimzadeh P. Gabapentinoids: Gabapentin and pregabalin for postoperative pain management. Anesth Pain Med. 2012;2:52–3. doi: 10.5812/aapm.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arendt-Nielsen L, Frøkjaer JB, Staahl C, Graven-Nielsen T, Huggins JP, Smart TS, et al. Effects of gabapentin on experimental somatic pain and temporal summation. Reg Anesth Pain Med. 2007;32:382–8. doi: 10.1016/j.rapm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22:588–93. doi: 10.1097/ACO.0b013e328330373a. [DOI] [PubMed] [Google Scholar]
- 18.Carlton SM, Zhou S. Attenuation of formalin-induced nociceptive behaviors following local peripheral injection of gabapentin. Pain. 1998;76:201–7. doi: 10.1016/s0304-3959(98)00043-8. [DOI] [PubMed] [Google Scholar]
- 19.Doleman B, Heinink TP, Read DJ, Faleiro RJ, Lund JN, Williams JP. A systematic review and meta-regression analysis of prophylactic gabapentin for postoperative pain. Anaesthesia. 2015;70:1186–204. doi: 10.1111/anae.13179. [DOI] [PubMed] [Google Scholar]
- 20.Coronel MF, Labombarda F, De Nicola AF, González SL. Progesterone reduces the expression of spinal cyclooxygenase-2 and inducible nitric oxide synthase and prevents allodynia in a rat model of central neuropathic pain. Eur J Pain. 2014;18:348–59. doi: 10.1002/j.1532-2149.2013.00376.x. [DOI] [PubMed] [Google Scholar]
- 21.Ferrer MD, Busquets-Cortés C, Capó X, Tejada S, Tur JA, Pons A, et al. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr Med Chem. 2019;26:3225–41. doi: 10.2174/0929867325666180514112124. [DOI] [PubMed] [Google Scholar]
- 22.Gordo AC, Walker C, Armada B, Zhou D. Efficacy of celecoxib versus ibuprofen for the treatment of patients with osteoarthritis of the knee: A randomized double-blind, non-inferiority trial. J Int Med Res. 2017;45:59–74. doi: 10.1177/0300060516673707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: A meta-analysis. Br J Anaesth. 2011;106:454–62. doi: 10.1093/bja/aer027. [DOI] [PubMed] [Google Scholar]
- 24.Karst M, Kegel T, Lukas A, Lüdemann W, Hussein S, Piepenbrock S. Effect of celecoxib and dexamethasone on postoperative pain after lumbar disc surgery. Neurosurgery. 2003;53:331–6. doi: 10.1227/01.neu.0000073530.81765.6b. [DOI] [PubMed] [Google Scholar]
- 25.Wagner AK, Wyss K, Gandek B, Kilima PM, Lorenz S, Whiting D. A Kiswahili version of the SF-36 health survey for use in Tanzania: Translation and tests of scaling assumptions. Qual Life Res. 1999;8:101–10. doi: 10.1023/a:1026441415079. [DOI] [PubMed] [Google Scholar]
- 26.Montazeri A, Vahdaninia M, Mousavi SJ, Omidvari S. The Iranian version of 12-item short form health survey (SF-12): Factor structure, internal consistency and construct validity. BMC Public Health. 2009;9:341. doi: 10.1186/1471-2458-9-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scuderi GR, Bourne RB, Noble PC, Benjamin JB, Lonner JH, Scott WN. The new knee society knee scoring system. Clin Orthop Relat Res. 2012;470:3–19. doi: 10.1007/s11999-011-2135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Culliton SE, Bryant DM, MacDonald SJ, Hibbert KM, Chesworth BM. Validity and internal consistency of the new knee society knee scoring system. Clin Orthop Relat Res. 2018;476:77–84. doi: 10.1007/s11999.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaviani H, Seyfourian H, Sharifi V, Ebrahimkhani N. Reliability and validity of anxiety and depression hospitalscales (HADS): Iranian patients with anxiety and depression disorders. Tehran Univ Med J. 2009;67:379–85. [Google Scholar]
- 30.Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The hospital anxiety and depression scale (HADS): Translation and validation study of the Iranian version. Health Qual Life Outcomes. 2003;1:14. doi: 10.1186/1477-7525-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuben SS, Connelly NR. Postoperative analgesic effects of celecoxib or rofecoxib after spinal fusion surgery. Anesth Analg. 2000;91:1221–5. doi: 10.1097/00000539-200011000-00032. [DOI] [PubMed] [Google Scholar]
- 32.Huang JJ, Taguchi A, Hsu H, Andriole GL, Jr, Kurz A. Preoperative oral rofecoxib does not decrease postoperative pain or morphine consumption in patients after radical prostatectomy: A prospective, randomized, double-blinded, placebo-controlled trial. J Clin Anesth. 2001;13:94–7. doi: 10.1016/s0952-8180(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 33.Gong L, Thorn CF, Bertagnolli MM, Grosser T, Altman RB, Klein TE. Celecoxib pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:310–8. doi: 10.1097/FPC.0b013e32834f94cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao H, Yu R, Tao Y, Nikolic D, van Breemen RB. Measurement of cyclooxygenase inhibition using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2011;54:230–5. doi: 10.1016/j.jpba.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilatti GL, André dos Santos F, Bianchi A, Cavassim R, Tozetto CW. The use of celecoxib and dexamethasone for the prevention and control of postoperative pain after periodontal surgery. J Periodontol. 2006;77:1809–14. doi: 10.1902/jop.2006.060128. [DOI] [PubMed] [Google Scholar]
- 36.Carmichael NM, Katz J, Clarke H, Kennedy D, Kreder HJ, Gollish J, et al. An intensive perioperative regimen of pregabalin and celecoxib reduces pain and improves physical function scores six weeks after total hip arthroplasty: A prospective randomized controlled trial. Pain Res Manag. 2013;18:127–32. doi: 10.1155/2013/258714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Straube S, Derry S, Moore RA, Wiffen PJ, McQuay HJ. Single dose oral gabapentin for established acute postoperative pain in adults. Cochrane Database Syst Rev. 2010;2010:CD008183. doi: 10.1002/14651858.CD008183.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannon CP, Fillingham YA, Browne JA, Schemitsch EH, Mullen K, Casambre F, et al. The efficacy and safety of gabapentinoids in total joint arthroplasty: Systematic review and direct meta-analysis. J Arthroplasty. 2020;35:2730–8.e6. doi: 10.1016/j.arth.2020.05.033. [DOI] [PubMed] [Google Scholar]
- 39.Martinez V, Pichard X, Fletcher D. Perioperative pregabalin administration does not prevent chronic postoperative pain: Systematic review with a meta-analysis of randomized trials. Pain. 2017;158:775–83. doi: 10.1097/j.pain.0000000000000838. [DOI] [PubMed] [Google Scholar]
- 40.Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010;49:661–9. doi: 10.2165/11536200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Weinbroum AA. Non-opioid IV adjuvants in the perioperative period: Pharmacological and clinical aspects of ketamine and gabapentinoids. Pharmacol Res. 2012;65:411–29. doi: 10.1016/j.phrs.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt PC, Ruchelli G, Mackey SC, Carroll IR. Perioperative gabapentinoids: Choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology. 2013;119:1215–21. doi: 10.1097/ALN.0b013e3182a9a896. [DOI] [PubMed] [Google Scholar]
- 43.Patel R, Dickenson AH. Mechanisms of the gabapentinoids and α 2 δ-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect. 2016;4:e00205. doi: 10.1002/prp2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baert IA, Lluch E, Mulder T, Nijs J, Noten S, Meeus M. Does pre-surgical central modulation of pain influence outcome after total knee replacement? A systematic review. Osteoarthritis Cartilage. 2016;24:213–23. doi: 10.1016/j.joca.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Reuben SS, Buvenandran A, Katz B, Kroin JS. A prospective randomized trial on the role of perioperative celecoxib administration for total knee arthroplasty: Improving clinical outcomes. Anesth Analg. 2008;106:1258–64. doi: 10.1213/ane.0b013e318165e208. [DOI] [PubMed] [Google Scholar]
- 46.Xu X, Sang W, Liu Y, Zhu L, Lu H, Ma J. Effect of celecoxib on surgical site inflammation after total knee arthroplasty: A randomized controlled study. Med Princ Pract. 2018;27:481–8. doi: 10.1159/000492922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke H, Pereira S, Kennedy D, Gilron I, Katz J, Gollish J, et al. Gabapentin decreases morphine consumption and improves functional recovery following total knee arthroplasty. Pain Res Manag. 2009;14:217–22. doi: 10.1155/2009/930609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ménigaux C, Adam F, Guignard B, Sessler DI, Chauvin M. Preoperative gabapentin decreases anxiety and improves early functional recovery from knee surgery. Anesth Analg. 2005;100:1394–9. doi: 10.1213/01.ANE.0000152010.74739.B8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mears SC, Edwards PK, Barnes CL. How to decrease length of hospital stay after total knee replacement. J Surg Orthop Adv. 2016;25:2–7. [PubMed] [Google Scholar]
- 50.Thiam WD, Teh JW, Bin Abd Razak HR, Tan HC. Correlations between functional knee outcomes and health-related quality of life after total knee arthroplasty in an Asian population. J Arthroplasty. 2016;31:989–93. doi: 10.1016/j.arth.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Lee JK, Chung KS, Choi CH. The effect of a single dose of preemptive pregabalin administered with COX-2 inhibitor: A trial in total knee arthroplasty. J Arthroplasty. 2015;30:38–42. doi: 10.1016/j.arth.2014.04.004. [DOI] [PubMed] [Google Scholar]