Abstract
Background
Congenital anomalies (CAs) increase the risk of death during infancy and childhood. This study aimed to evaluate the accuracy of using death certificates to estimate the burden of CAs on mortality for children under 10 years old.
Methods
Children born alive with a major CA between 1 January 1995 and 31 December 2014, from 13 population-based European CA registries were linked to mortality records up to their 10th birthday or 31 December 2015, whichever was earlier.
Results
In total 4199 neonatal, 2100 postneonatal and 1087 deaths in children aged 1–9 years were reported. The underlying cause of death was a CA in 71% (95% CI 64% to 78%) of neonatal and 68% (95% CI 61% to 74%) of postneonatal infant deaths. For neonatal deaths the proportions varied by registry from 45% to 89% and by anomaly from 53% for Down syndrome to 94% for tetralogy of Fallot. In children aged 1–9, 49% (95% CI 42% to 57%) were attributed to a CA. Comparing mortality in children with anomalies to population mortality predicts that over 90% of all deaths at all ages are attributable to the anomalies. The specific CA was often not reported on the death certificate, even for lethal anomalies such as trisomy 13 (only 80% included the code for trisomy 13).
Conclusions
Data on the underlying cause of death from death certificates alone are not sufficient to evaluate the burden of CAs on infant and childhood mortality across countries and over time. Linked data from CA registries and death certificates are necessary for obtaining accurate estimates.
Keywords: Epidemiology, Mortality
WHAT IS ALREADY KNOWN ON THIS TOPIC
Infant and child mortality is a public health concern, and congenital anomalies (CAs) contribute significantly to this mortality.
In Europe, CAs account for 26% of deaths in infants and over 10% of deaths in children aged under 10 years.
Data on cause of death on death certificates are frequently analysed to estimate the burden of disease in populations.
WHAT THIS STUDY ADDS
The recording of codes for CAs on European death certificates underestimates the proportion of deaths due to CAs by up to 30% in infants.
Only 70% of deaths in infants with CA had a CA recorded as the underlying cause of death.
Children aged 1–9 years with CA were less likely to have underlying cause of death recorded as a CA (49%).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This information should improve interpretation of mortality rates routinely reported for children with CAs in Europe.
Linkage of death certificates with CA registries should be used to evaluate the burden of CAs more accurately in future research.
Background
The contribution of congenital anomalies (CAs) to causes of early death is increasing as mortality from other causes declines globally.1 The Global Burden of Disease study estimated that, worldwide in 2010, CAs accounted for 6.4% of neonatal deaths, 2.2% of postneonatal and 2.5% of deaths in children under 5 years of age.2 In comparison, in Europe from 2000 to 2015, CAs were estimated to account for 26% of all deaths in infants, 16% in children aged 1–4 and 9% in children aged 5–9.3
The above estimates of the burden of disease in populations have all been derived from data on the underlying cause of death recorded on death certificates. Such data are often used to monitor any changes in primary and secondary prevention over time1 4 5 and for international comparisons.6 7 However, the accuracy of cause of death on death certificates has often been questioned, and a US study concluded that linking CA registries to death certificates was necessary to provide a comprehensive picture of the full burden of CAs on mortality in infants and children.8 This EUROlinkCAT study linked live births with a major CA reported to 13 (European Surveillance of Congenital Anomalies, EUROCAT) (European network for the epidemiological surveillance of CAs) registries9 to national/regional databases on vital statistics/mortality up to their 10th birthday. The aim was to determine what the children with CAs died from and whether their CA was mentioned anywhere on the death certificate. This information should improve interpretation of mortality rates routinely reported for children with CAs in Europe.
Method
Thirteen population-based EUROCAT CA registries from eight countries linked their data on live born children with a major CA, born between 1 January 1995 and 31 December 2014, to mortality records up to the child’s 10th birthday or to 31 December 2015, whichever was earlier (table 1). Ten registries linked to vital statistics containing civil registrations data (eg, births, deaths and emigrations) but three were able to link to death registrations only. Both deterministic and probabilistic linkage methods were used. Linkage rates were high, with eight registries linking over 95% of cases and only one registry linking less than 90% of the births. Additional details evaluating the linkage are provided elsewhere.10 11
Table 1.
Number of deaths and percentage by age at death in children with a major congenital anomaly reported by participating EUROCAT registries and databases
| Participating registries | Included birth years | No of livebirths | % of all live births linked | Only underlying cause of death provided | No of deaths | Percentage of deaths (%) | ||
| Infants <28 days |
Infants 28–364 days | Children 1–9 years | ||||||
| Denmark, Funen* | 1995–2014 | 2 425 | 100.0 | 150 | 58 | 30 | 11 | |
| Finland | 1995–2014 | 42 921 | 99.9 | 1 770 | 61 | 24 | 15 | |
| Italy, Emilia Romagna | 2008–2014 | 5 589 | 91.4 | 204 | 52 | 39 | 9 | |
| Italy, Tuscany | 2005–2014 | 4 312 | 87.2 | Yes | 148 | 48 | 36 | 16 |
| Malta | 1995–2014 | 2 718 | 91† | 241 | 69 | 20 | 11 | |
| Northern Netherlands* | 1995–2014 | 8 605 | 96.7 | Yes | 620 | 66 | 21 | 13 |
| Norway | 1999–2014 | 27 201 | 100.0 | 1 034 | 58 | 27 | 15 | |
| Spain, Basque Country | 1995–2014 | 5 904 | 94† | Yes | 411 | 52 | 37 | 11 |
| Spain, Valencian Region | 2007–2014 | 7 389 | 95† | Yes | 416 | 58 | 30 | 13 |
| UK, Thames Valley* | 2005–2013 | 3 988 | 96.5 | 295 | 56 | 30 | 14 | |
| UK, EMSY* | 2003–2012 | 11 587 | 97.3 | 910 | 52 | 32 | 16 | |
| UK, Wessex* | 2004–2014 | 4 729 | 91.7 | 330 | 51 | 33 | 15 | |
| UK, Wales* | 1998–2014 | 18 188 | 99.7 | 845 | 50 | 32 | 18 | |
| Total | 7 386 | 57 | 28 | 15 | ||||
*Numbers of deaths rounded to nearest multiple of 5 due to disclosure requirements.
†Estimated proportion as linkage was to mortality records only and completeness could not be directly estimated.
EMSY, East Midlands and South Yorkshire; EUROCAT, European Surveillance of Congenital Anomalies.
Classification of anomalies
The EUROCAT guide 1.4 specifies the coding of all major CAs into specific CA subgroups using ICD-10 (International Classification of Diseases, tenth revision) or ICD-9 (ninth revision) with the BPA (British Paediatric Association extension).12 A child is defined as having an isolated CA if (s)he has a CA in one organ system only or as part of a known sequence (eg, renal agenesis with pulmonary hypoplasia). A EUROCAT computer algorithm was used for classification of major CAs into isolated anomalies, multiple anomalies or genetic anomalies without a manual clinical review of the identified potential multiple CAs.13 The severe congenital heart defect (CHD) subgroup includes types of CHD’s selected due to their high mortality.14 Forty-six CA subgroups were analysed, including anomalies likely to be recorded as an underlying cause of death (such as trisomy 13 or 18) and anomalies less likely to be recorded on a death certificate (such as limb reduction defects or hypospadias).
Classification of cause of death
All causes of death were recorded using ICD-10 or ICD-9. Four registries were able to provide only the underlying cause of death. Other registries were able to report in addition to the underlying cause of death the primary/immediate cause of death, contributing cause of death and any other causes of death.
Deaths were categorised into neonatal (0–27 days), postneonatal (28–364 days) and child (365–3651 days). In England and Wales, the underlying cause for neonatal deaths was not specified and each cause was classified as related to infant, mother or either15; main causes were listed before other (secondary) causes. For this study, in Wales, the first cause of death related to the infant was taken to be the underlying cause of death. In England, the first mention of a CA (if present) was taken to be the underlying cause.
Seven categories of cause of neonatal and post-neonatal deaths, based on a modified version of the UK Office for National Statistics’ (ONS) classification of neonatal and postneonatal causes of death15 were used in this study (see online supplemental appendix A). The ‘All other conditions’ group was very heterogeneous. It included neoplasms, metabolic disorders, jaundice and endocrine disorders, and external causes.
bmjpo-2022-001617supp001.pdf (123.8KB, pdf)
Similarly, the classification of cause of death for children aged 1–9 in this study was based on the UK ONS classification of causes of death15 and was divided into 13 categories (see online supplemental appendix B).
bmjpo-2022-001617supp002.pdf (101.4KB, pdf)
To determine the accuracy of the CA recorded on death certificates, two paediatricians agreed a set of ICD-10 and ICD-9 codes corresponding to an ‘exact match’ for the child’s anomaly and a larger set of codes that were considered as an ‘acceptable match’ (see online supplemental appendix C). For example, for a child with spina bifida (ICD-10 code-Q05), if Q05 was on the death certificate this was considered an exact code; if there were codes for other neural tube defects Q00 (anencephaly) or Q01 (encephalocele) they were considered as acceptable codes. All the available causes of death were searched to determine if any exact or acceptable anomaly codes had been specified.
bmjpo-2022-001617supp003.pdf (110.3KB, pdf)
Statistical analysis
The analysis variables from each of the 13 registries were mapped and recoded to a common data model (full details are given in Morris et al 10) and analysed locally using common Stata syntax scripts. Aggregate data from each registry were submitted to a Central Results Repository based at Ulster University, UK using a secure portal where they were merged and uploaded to the study team for analysis.
The predicted proportions of deaths in children with a CA attributable to that anomaly was estimated from the population mortality rate (published by WHO) for children in the eight countries and the observed mortality rate for children with the specific CA (from an earlier EUROlinkCAT study16 as equal to (CA mortality—population mortality)/CA mortality. For example, if the mortality rate in children with anomalies is 10 times greater than that in the population, then for every 10 deaths in a group of children with anomalies you would expect only one death in a similar group of children without anomalies. Therefore, it could be said that the excess 9 deaths (10–1) were attributable to the anomaly, or 90% when expressed as a proportion of all deaths in children with anomalies (9/10). These were underestimates as the population mortality included children with CAs.
To estimate the overall proportion of each cause of death specified as the underlying cause of death, multilevel multinomial models were fitted with registry as a random effect. The models were fitted in Stata using the generalised structural equation model estimation command (gsem) for each CA separately. The observed information matrix was used to obtain the variance–covariance matrix of the estimates as it is the default method. The same models were used to estimate the overall proportion of death certificates with the exact and appropriate CA codes. For the group of all anomalies, the proportion of all neonatal deaths with CA as an underlying cause of death was compared across the different registries in a figure to illustrate the variation between registries, with the exact method used to estimate the 95% CIs.
Results
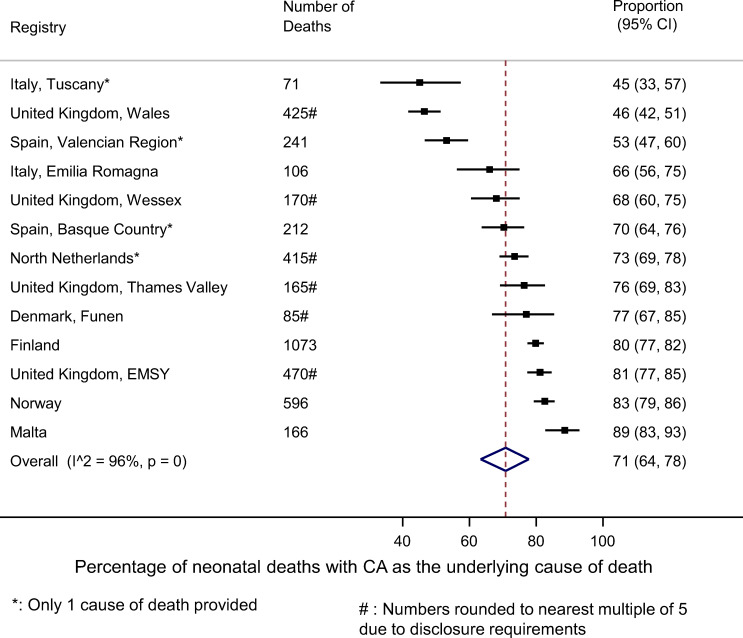
The 13 registries varied considerably in size due to the differences in populations covered and years of data available, from 1770 deaths in Finland to only 148 in Tuscany (table 1). The majority of deaths reported (57%) occurred in the neonatal period. Overall, 71% (95% CI 64% to 78%) of neonates who died had a CA coded as the underlying cause of death. However, this varied significantly according to registry from 89% in Malta to 45% in Tuscany (Italy) and 46% in Wales (UK) (figure 1). Registries providing only one cause of death had a lower proportion specifying a CA as the underlying cause of death. All registries, except for Wales, had under 7% or deaths attributed to immaturity. Wales had 15% of such deaths, which together with the low proportion of CAs, may be due to the Welsh coding of infant death certificates (see the Methods section).
Figure 1.
For neonates with a major congenital anomaly (CA): comparison of percentage of all neonatal deaths with ‘CA’ as the underlying cause of death on the death certificate by registry.
Figure 2 shows that the percentage of deaths with the underlying cause of death coded as a CA was slightly lower in the postneonatal period, 68% (95% CI 61% to 74%), and decreased to 49% (95% CI 42% to 57%) for children who died between the ages of 1 and 9. Variations by registry for the postneonatal period and for children aged 1–9 were similar to those in figure 1 in the neonatal period (see online supplemental appendix D)
Figure 2.
The distribution of causes of death in children with a major congenital anomaly (CA) according to the recorded underlying cause of death by age at death.
bmjpo-2022-001617supp004.pdf (343.3KB, pdf)
Table 2 provides the underlying cause of death for children with specific CAs. Deaths in neonates with severe CAs were likely to have the CA coded; for example, over 90% of deaths in neonates with trisomy 13, 18 or gastroschisis had an anomaly as the cause of death. Over 90% of births with cleft lip with or without cleft palate who died had CA as the cause of death, reflecting that these children were likely also to have had more severe anomalies, such as trisomy 13 or trisomy 18. For neonates with Down syndrome, 53% had an anomaly specified and 10% of deaths were due to immaturity. The pattern was similar for all infant deaths. For children aged 1–9, other causes of death were more prominent: for several anomalies, over 10% of deaths were due to infections. For children with Down syndrome, 17% of deaths were due to neoplasms. Table 2 also shows the predicted proportion of deaths attributable to the anomalies as estimated by comparing the mortality in these children to the population mortality. These proportions were above 90% for all anomalies apart from for deaths in neonates with a ventricular septal defect, cleft lip with or without cleft palate or Down syndrome, and deaths in infants with a cleft lip with or without cleft palate or multicystic renal dysplasia.
Table 2.
Percentages of children with congenital anomalies (CA) with 95% CI according to underlying cause of death by age at death (anomalies with <10 deaths occurring are not included)
| Anomaly | No deaths (100%) | Percentage cause of death (95% CI) | Predicted proportion attributable to the CA | ||||
| CA | Infections | Immaturity | Other | Missing cause of death | |||
| Neonatal deaths | |||||||
| All anomalies | 4199 | 71 (64 to 78) | 1 (1 to 2) | 7 (6 to 9) | 11 (8 to 14) | 9 (7 to 12) | 93 |
| Spina Bifida (isolated cases with or without hydrocephalus) | 19 | 79 (39 to 95) | 0 (0 to 0) | 11 (3 to 22) | 5 (1 to 19) | 5 (1 to 19) | 97 |
| Hydrocephalus | 138 | 64 (43 to 80) | 1 (0 to 4) | 7 (4 to 12) | 14 (8 to 20) | 14 (8 to 20) | 97 |
| Congenital heart defects (CHD) | 1937 | 79 (71 to 86) | 1 (1 to 2) | 3 (2 to 5) | 9 (7 to 13) | 7 (5 to 9) | 94 |
| Severe CHD | 1254 | 86 (78 to 91) | 0 (0 to 1) | 2 (1 to 3) | 6 (4 to 9) | 7 (4 to 10) | 98 |
| Transposition of great vessels (as only severe CHD) | 106 | 91 (66 to 98) | 1 (0 to 5) | 2 (0 to 7) | 4 (1 to 12) | 3 (1 to 10) | 97 |
| VSD (without severe CHD) | 291 | 75 (58 to 86) | 2 (1 to 5) | 5 (3 to 9) | 13 (8 to 19) | 5 (3 to 9) | 89 |
| ASD (without severe CHD) | 153 | 68 (45 to 84) | 4 (1 to 8) | 9 (4 to 15) | 14 (7 to 22) | 6 (3 to 12) | 90 |
| AVSD | 153 | 85 (65 to 94) | 0 (0 to 0) | 5 (2 to 11) | 6 (2 to 14) | 5 (2 to 11) | 97 |
| Tetralogy of Fallot | 63 | 94 (78 to 98) | 0 (0 to 0) | 0 (0 to 0) | 5 (2 to 13) | 2 (0 to 10) | 94 |
| Coarctation of aorta | 188 | 87 (75 to 93) | 0 (0 to 0) | 2 (1 to 5) | 7 (4 to 12) | 4 (2 to 8) | 96 |
| Cleft lip with or without cleft palate | 128 | 91 (71 to 98) | 0 (0 to 0) | 4 (1 to 13) | 2 (1 to 8) | 2 (0 to 7) | 89 |
| Cleft palate | 121 | 68 (46 to 83) | 1 (0 to 5) | 12 (6 to 18) | 11 (6 to 17) | 9 (5 to 15) | 92 |
| Oesophageal atresia | 110 | 82 (64 to 92) | 0 (0 to 0) | 5 (2 to 10) | 6 (3 to 12) | 7 (3 to 14) | 97 |
| Diaphragmatic hernia (isolated cases) | 186 | 89 (76 to 96) | 0 (0 to 0) | 2 (1 to 5) | 2 (1 to 6) | 6 (3 to 13) | 99 |
| Gastroschisis (isolated cases) | 25 | 92 (61 to 99) | 4 (1 to 20) | 0 (0 to 0) | 0 (0 to 0) | 4 (1 to 20) | 94 |
| Multicystic renal dysplasia | 92 | 67 (42 to 85) | 1 (0 to 6) | 9 (4 to 15) | 10 (5 to 17) | 13 (6 to 20) | 96 |
| Down syndrome | 102 | 53 (33 to 72) | 4 (2 to 7) | 10 (6 to 15) | 26 (17 to 34) | 7 (3 to 11) | 82 |
| Trisomy 13 | 156 | 90 (76 to 96) | 0 (0 to 0) | 1 (0 to 5) | 2 (1 to 6) | 6 (3 to 13) | 100 |
| Trisomy 18 | 330 | 93 (80 to 98) | 0 (0 to 0) | 1 (0 to 4) | 1 (0 to 4) | 5 (2 to 12) | 100 |
| Anomaly | No deaths (100%) | CA | Infections | Immaturity | Other | Missing cause of death | Predicted proportion attributable to the CA |
| Postneonatal deaths | |||||||
| All anomalies | 2100 | 68 (61 to 74) | 5 (4 to 6) | 4 (3 to 5) | 20 (16 to 22) | 4 (3 to 5) | 94 |
| Hydrocephalus | 85 | 55 (38 to 70) | 7 (4 to 11) | 6 (3 to 10) | 29 (22 to 34) | 2 (1 to 7) | 98 |
| Congenital heart defect | 1329 | 75 (68 to 82) | 4 (3 to 6) | 3 (2 to 4) | 15 (11 to 18) | 3 (2 to 4) | 97 |
| Severe CHD | 773 | 87 (79 to 93) | 3 (2 to 5) | 1 (0 to 2) | 6 (4 to 10) | 3 (2 to 6) | 99 |
| Transposition of great vessels (as only severe CHD) | 27 | 100 (100 to 100) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 97 |
| VSD (without severe CHD) | 289 | 67 (55 to 77) | 5 (3 to 8) | 5 (3 to 8) | 20 (15 to 25) | 2 (1 to 4) | 96 |
| ASD (without severe CHD) | 169 | 56 (41 to 69) | 2 (1 to 5) | 8 (5 to 11) | 30 (23 to 35) | 4 (2 to 7) | 96 |
| AVSD | 199 | 84 (67 to 93) | 4 (2 to 7) | 1 (0 to 3) | 9 (5 to 16) | 3 (1 to 6) | 99 |
| Tetralogy of Fallot | 85 | 98 (66 to 100) | 1 (0 to 9) | 0 (0 to 6) | 1 (0 to 12) | 0 (0 to 6) | 97 |
| Coarctation of aorta | 130 | 86 (67 to 95) | 5 (2 to 11) | 0 (0 to 0) | 6 (2 to 12) | 4 (1 to 10) | 98 |
| Cleft lip with or without cleft palate | 53 | 66 (45 to 80) | 2 (0 to 9) | 0 (0 to 0) | 26 (17 to 33) | 6 (2 to 12) | 88 |
| Cleft palate | 76 | 70 (49 to 84) | 3 (1 to 8) | 3 (1 to 8) | 16 (10 to 21) | 9 (5 to 15) | 95 |
| Oesophageal atresia | 45 | 66 (39 to 84) | 5 (1 to 11) | 2 (0 to 10) | 18 (10 to 24) | 9 (4 to 16) | 97 |
| Diaphragmatic hernia (isolated cases) | 12 | 83 (39 to 98) | 8 (1 to 30) | 0 (0 to 0) | 8 (1 to 30) | 0 (0 to 0) | 97 |
| Gastroschisis (isolated cases) | 20 | 82 (31 to 98) | 5 (1 to 17) | 5 (1 to 17) | 5 (1 to 17) | 5 (1 to 17) | 94 |
| Multicystic renal dysplasia | 11 | 41 (5 to 90) | 0 (0 to 0) | 24 (4 to 41) | 36 (6 to 54) | 0 (0 to 0) | 72 |
| Down syndrome | 165 | 71 (53 to 84) | 6 (3 to 10) | 5 (2 to 8) | 16 (9 to 23) | 2 (1 to 5) | 96 |
| Trisomy 13 | 35 | 94 (69 to 99) | 0 (0 to 0) | 0 (0 to 0) | 3 (0 to 15) | 3 (0 to 15) | 100 |
| Trisomy 18 | 136 | 93 (77 to 98) | 0 (0 to 0) | 1 (0 to 5) | 1 (0 to 6) | 5 (2 to 13) | 100 |
| Anomaly | No deaths 1–9 years (100%) | CA | Infections | Neoplasms | Nervous | Other | Missing cause of death | Predicted proportion attributable to the CA |
| Child deaths (1–9 years) | ||||||||
| All anomalies | 1087 | 49 (42 to 57) | 9 (8 to 10) | 5 (4 to 6) | 9 (8 to 11) | 23 (21 to 26) | 4 (3 to 5) | 99 |
| Hydrocephalus | 66 | 49 (25 to 73) | 13 (7 to 18) | 7 (3 to 11) | 10 (5 to 15) | 17 (10 to 21) | 5 (2 to 10) | 100 |
| Congenital heart defect | 474 | 63 (54 to 72) | 8 (6 to 10) | 3 (2 to 4) | 4 (3 to 5) | 19 (16 to 22) | 3 (2 to 5) | 99 |
| Severe CHD | 258 | 79 (67 to 87) | 5 (3 to 8) | 1 (0 to 3) | 2 (1 to 4) | 10 (7 to 13) | 3 (2 to 6) | 100 |
| VSD (without severe CHD) | 100 | 48 (31 to 66) | 13 (9 to 17) | 6 (3 to 10) | 6 (3 to 10) | 24 (18 to 27) | 2 (1 to 6) | 99 |
| ASD (without severe CHD) | 81 | 44 (28 to 61) | 10 (6 to 13) | 7 (4 to 11) | 2 (1 to 6) | 31 (25 to 32) | 5 (2 to 9) | 99 |
| AVSD | 70 | 79 (60 to 90) | 6 (2 to 12) | 0 (0 to 0) | 0 (0 to 0) | 11 (6 to 18) | 4 (2 to 10) | 99 |
| Tetralogy of Fallot | 51 | 71 (42 to 87) | 10 (5 to 15) | 2 (0 to 8) | 2 (0 to 8) | 12 (6 to 17) | 4 (1 to 10) | 100 |
| Coarctation of aorta | 44 | 70 (43 to 87) | 5 (1 to 12) | 0 (0 to 0) | 2 (0 to 10) | 14 (7 to 20) | 9 (4 to 16) | 99 |
| Cleft lip with or without cleft palate | 12 | 65 (7 to 98) | 17 (1 to 46) | 0 (0 to 0) | 0 (0 to 0) | 17 (1 to 46) | 0 (0 to 0) | 99 |
| Cleft palate | 41 | 67 (34 to 89) | 5 (1 to 12) | 0 (0 to 0) | 5 (1 to 12) | 16 (7 to 27) | 7 (2 to 15) | 91 |
| Oesophageal atresia | 23 | 47 (10 to 88) | 11 (2 to 19) | 0 (0 to 0) | 11 (2 to 19) | 26 (7 to 37) | 5 (1 to 15) | 99 |
| Down syndrome | 98 | 42 (28 to 56) | 14 (10 to 18) | 17 (13 to 21) | 0 (0 to 0) | 24 (20 to 28) | 2 (1 to 6) | 99 |
| Trisomy 18 | 17 | 94 (24 to 100) | 6 (0 to 76) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 99 |
ASD, atrial septal defect; AVSD, atrioventricular septal defect; VSD, ventricular septal defect.
Figure 3 shows the results for all causes (including underlying causes) of death to determine if any exact/acceptable CA codes were recorded for deaths up to age 10 years. As expected, no CA code indicating a limb reduction defect was provided for any of the 165 deaths among children with these anomalies. Five severe conditions (anencephaly, gastroschisis, diaphragmatic hernia, trisomy 13 and trisomy 18) had over 75% of exact codes recorded as cause of death. For other severe CAs (particularly CHDs), few deaths were attributed to the anomaly. For example, tetralogy of Fallot had an exact or acceptable code recorded for just 50% of deaths. There were also coding issues with several CAs, such as bilateral renal agenesis and atresia of bile ducts, resulting in over 30% of codes being ‘acceptable codes’ rather than exact codes.
Figure 3.
Anomaly codes on death certificates for children with a major congenital anomaly dying before their 10th birthday according to the acceptability of the ICD-9/ICD-10 code recorded (number of deaths). CHD, congenital heart defect; GA, gestational age; PDA, patent ductus arteriosus; ICD-9/ICD-10, version 9/10 of the International Classification of Diseases.
Discussion
This study found that only 70% of infants dying with a major CA had the underlying cause of death recorded as a CA, with higher percentages for those with anomalies known to be associated with high mortality, such as 86% for infants with severe CHD. Children aged 1–9 years with a major CA were less likely to have underlying cause of death recorded as a CA (49%) with other causes such as infections, trauma and cancer being more prominent.17 By comparing the mortality rate for children with anomalies from an earlier EUROlinkCAT study16 to published population rates it was possible to estimate that over 90% of deaths in children with anomalies are attributable to their anomaly. These results are consistent with the estimates for infant deaths in other studies, but estimates have not been reported for deaths in later childhood.8 18 19 Our results show that if only the underlying cause of death on death certificates is analysed, the impact of CAs on mortality is considerably underestimated for all ages and all anomalies.
Under-reporting is to be expected as it is not always clear if the CA is the underlying cause of death. For example, a child may die from an infection and it is difficult to distinguish if the CA is the underlying cause or not as the CA does increase the risk of severe infections, making infection either more likely or more severe.20 Similarly, many anomalies are associated with an increased risk of preterm birth. However, all registries apart from Wales, appeared to be consistent in assigning cause of death as the CA rather than the morbidity associated with being born preterm. Similar under-reporting of mortality and morbidity for children with chronic conditions has also been reported from healthcare databases.17 21–24
In addition to the under-reporting on death certificates, estimating the full burden of CAs will require including data on stillbirths, miscarriages and terminations of pregnancy for CAs.25
Another important finding was that if a CA was mentioned on the death certificate, it may not have been the exact code. For example, for CHDs a general code indicating a heart defect was often provided rather than the code for the specific cardiac defect. Care must be taken when analysing data from death certificates if there is no method of independently verifying the coding of the anomaly. A study in the USA, using information from death certificates, estimated that trisomy 18 and CHD were the two most common causes of infant death due to CAs in term born infants, accounting for 11% and 15% of CA deaths, respectively.6 In our study, the comparable figures using the diagnosed anomalies of the infants (not the anomalies recorded on the death certificates) are 8% for trisomy 18 and 32% for severe CHD. Inaccuracies on death certificates are expected with the accuracy depending on the person completing the form.26 27 The 60% of exact codes for children with a CHD in our study is similar to the 70% recorded by a recent study from the USA on deaths in infants with CHD.28 The occurrence of infections and neoplasms as underlying causes of death in infants with Down syndrome reflects their known increased risk of infections and leukaemia.29
Malta was the country most likely to record a CA as the underlying cause of death. This may be due to the fact that terminations of pregnancy for foetal anomaly is illegal in Malta, and therefore, more babies are born with severe life-threatening anomalies than in other countries where terminations are legal and the anomaly is likely to be detected by the prenatal screening.30 The Nordic countries (Denmark, Finland and Norway) were more likely to record CA as the underlying cause of death compared with the other European countries. In Denmark and Finland, the death certificates are completed online by using predefined ICD10 codes. Adopting this policy of standardised reporting in other countries may improve the accuracy and comparability of the data.
Strengths
The strength of this study is that it included data from 13 population-based CA registries in eight European countries. The analysis of linked cases enabled direct comparisons of the recorded anomaly codes on the death certificates with the CA diagnosis reported in the CA registries, assumed to be the gold standard. Earlier analyses of EUROlinkCAT data enabled attributable proportions to be estimated for the same CAs.
Limitations
A limitation of the study is that a child may have several anomalies and the one of interest may not be the underlying cause of death. Another limitation was the cause of death for infants was categorised into only seven categories resulting in the ‘all other conditions’ being heterogeneous including neoplasms, metabolic disorders, jaundice and endocrine disorders and external causes. This unfortunately limits the interpretation of the deaths in this category. As the study was conducted by standardising the data in all registries and then providing syntax scripts to analyse it, it was not possible to redefine more meaningful categories. In addition, four registries were only able to provide one cause of death, which may not have been the underlying cause of death.
Conclusion
Data on the underlying cause of death from death certificates alone are not sufficient to evaluate the burden of CAs on infant and childhood mortality across countries and over time. Linked data from CA registries and death certificates are necessary for obtaining accurate estimates.
Supplementary Material
Footnotes
Contributors: JT, ARissmann, SVG, JR, ML, JM and EG conceptualised and designed the study, coordinated and supervised data collection, carried out the initial analyses, drafted the initial manuscript and reviewed and revised the manuscript. AP, MS, AC, JG and AReid designed the data collection instruments, coordinated and supervised data collection, and reviewed and revised the manuscript. AA, DA, EB, IB, CC-C, HEKdW, MGatt, MGissler, AH, SJ, SKU, KK, RL, OM, AJN, DST, DGW, LY and OZ collected data, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. JM is the guarantor.
Funding: This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 733001.
Disclaimer: The views presented here are those of the authors only, and the European Commission is not responsible for any use that may be made of the information presented here.
Competing interests: None.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The aggregate data that support the findings of this study are available from the authors for scientifically valid requests and with permission of the participating registries of congenital anomalies. To apply for the data, please see https://www.eurolinkcat.eu/contactinformationanddatarequests.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All EUROCAT registries obtained ethical, governance and other permissions for the data linkage according to their national legislations and arrangements. University of Ulster obtained Ethics permission for the Central Results Repository on 15 September 2017 (Institute of Nursing and Health Research Ethics Filter Committee, number FCNUR-17-000).
References
- 1. Wang H, Bhutta ZA, Coates MM. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1725–74. 10.1016/S0140-6736(16)31575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pitt MJ, Morris JK. European trends in mortality in children with congenital anomalies: 2000–2015. Birth Defects Res 2021;113:958–67. 10.1002/bdr2.1892 [DOI] [PubMed] [Google Scholar]
- 4. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep 2019;68:1–77. [PubMed] [Google Scholar]
- 5. Khan SQ, Berrington de Gonzalez A, Best AF, et al. Infant and youth mortality trends by race/ethnicity and cause of death in the United States. JAMA Pediatr 2018;172:e183317. 10.1001/jamapediatrics.2018.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bairoliya N, Fink G. Causes of death and infant mortality rates among full-term births in the United States between 2010 and 2012: an observational study. PLOS Med 2018;15:e1002531. 10.1371/journal.pmed.1002531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kyu HH, Stein CE, Boschi Pinto C, et al. Causes of death among children aged 5-14 years in the WHO European region: a systematic analysis for the global burden of disease study 2016. Lancet Child Adolesc Health 2018;2:321–37. 10.1016/S2352-4642(18)30095-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Copeland GE, Kirby RS. Using birth defects registry data to evaluate infant and childhood mortality associated with birth defects: an alternative to traditional mortality assessment using underlying cause of death statistics. Birth Defects Res A Clin Mol Teratol 2007;79:792–7. 10.1002/bdra.20391 [DOI] [PubMed] [Google Scholar]
- 9. on behalf of the JRC Management Committee, Tucker FD, Morris JK, et al. EUROCAT: an update on its functions and activities. J Community Genet 2018;9:407–10. 10.1007/s12687-018-0367-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris JK, Garne E, Loane M, et al. Eurolinkcat protocol for a European population-based data linkage study investigating the survival, morbidity and education of children with congenital anomalies. BMJ Open 2021;11:e047859. 10.1136/bmjopen-2020-047859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loane M, Given JE, Tan J, et al. Linking a European cohort of children born with congenital anomalies to vital statistics and mortality records: a Eurolinkcat study. PLOS ONE 2021;16:e0256535. 10.1371/journal.pone.0256535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. EUROCAT . Guide 1.4. Available: https://eu-rd-platform.jrc.ec.europa.eu/eurocat/data-collection/guidelines-for-data-registration_en [Accessed 14 Feb 2018].
- 13. Garne E, Dolk H, Loane M, et al. Paper 5: surveillance of multiple congenital anomalies: implementation of a computer algorithm in European registers for classification of cases. Birth Defects Res A Clin Mol Teratol 2011;91 Suppl 1:S44–50. 10.1002/bdra.20777 [DOI] [PubMed] [Google Scholar]
- 14. Khoshnood B, Loane M, Garne E, et al. Recent decrease in the prevalence of congenital heart defects in Europe. J Pediatr 2013;162:108–13. 10.1016/j.jpeds.2012.06.035 [DOI] [PubMed] [Google Scholar]
- 15. Patel V. User guide to child mortality statistics. Office for National Statistics, 2018. [Google Scholar]
- 16. Glinianaia SV, Rankin J, Pierini A, et al. Ten-year survival of children with congenital anomalies: a European cohort study. Pediatrics 2022;149:e2021053793. 10.1542/peds.2021-053793 [DOI] [PubMed] [Google Scholar]
- 17. Hardelid P, Dattani N, Gilbert R, et al. Estimating the prevalence of chronic conditions in children who die in England, Scotland and Wales: a data linkage cohort study. BMJ Open 2014;4:e005331. 10.1136/bmjopen-2014-005331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Hu J, Druschel CM, et al. Twenty-five-year survival of children with birth defects in New York state: a population-based study. Birth Defects Res A Clin Mol Teratol 2011;91:995–1003. 10.1002/bdra.22858 [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Liu G, Canfield MA, et al. Racial/ethnic differences in survival of United States children with birth defects: a population-based study. J Pediatr 2015;166:819–26. 10.1016/j.jpeds.2014.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fraser LK, Fleming S, Parslow R. Changing place of death in children who died after discharge from Paediatric intensive care units: a national, data linkage study. Palliat Med 2018;32:337–46. 10.1177/0269216317709711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarvis S, Fraser LK. Comparing routine inpatient data and death records as a means of identifying children and young people with life-limiting conditions. Palliat Med 2018;32:543–53. 10.1177/0269216317728432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Cross PK, Druschel CM. Hospital discharge data: can it serve as the sole source of case ascertainment for population-based birth defects surveillance programs J Public Health Manag Pract 2010;16:245–51. 10.1097/PHH.0b013e3181b0b8a7 [DOI] [PubMed] [Google Scholar]
- 23. Salemi JL, Tanner JP, Sampat D, et al. The accuracy of hospital discharge diagnosis codes for major birth defects: evaluation of a statewide registry with passive case ascertainment. J Public Health Manag Pract 2016;22:E9–19. 10.1097/PHH.0000000000000291 [DOI] [PubMed] [Google Scholar]
- 24. Schneuer FJ, Lain SJ, Bell JC, et al. The accuracy of hospital discharge data in recording major congenital anomalies in Australia. Birth Defects Res 2021;113:1313–23. 10.1002/bdr2.1948 [DOI] [PubMed] [Google Scholar]
- 25. Boyle B, Addor M-C, Arriola L, et al. Estimating global burden of disease due to congenital anomaly: an analysis of European data. Arch Dis Child Fetal Neonatal Ed 2018;103:F22–8. 10.1136/archdischild-2016-311845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee H, Kim SH, Choi B, et al. Concordance between the underlying causes of death on death certificates written by three emergency physicians. Clin Exp Emerg Med 2019;6:218–25. 10.15441/ceem.18.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stevens JD, Landes SD. Assessing state level variation in signature authority and cause of death accuracy, 2005–2017. Prev Med Rep 2021;21:101309. 10.1016/j.pmedr.2020.101309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams JL, Torok RD, D’Ottavio A, et al. Causes of death in infants and children with congenital heart disease. Pediatr Cardiol 2021;42:1308–15. 10.1007/s00246-021-02612-2 [DOI] [PubMed] [Google Scholar]
- 29. Satgé D, Sommelet D, Geneix A, et al. A tumor profile in down syndrome. Am J Med Genet 1998;78:207–16. [PubMed] [Google Scholar]
- 30. Gatt M, England K, Grech V, et al. Contribution of congenital anomalies to neonatal mortality rates in Malta. Paediatr Perinat Epidemiol 2015;29:401–6. 10.1111/ppe.12206 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2022-001617supp001.pdf (123.8KB, pdf)
bmjpo-2022-001617supp002.pdf (101.4KB, pdf)
bmjpo-2022-001617supp003.pdf (110.3KB, pdf)
bmjpo-2022-001617supp004.pdf (343.3KB, pdf)
Data Availability Statement
Data are available on reasonable request. The aggregate data that support the findings of this study are available from the authors for scientifically valid requests and with permission of the participating registries of congenital anomalies. To apply for the data, please see https://www.eurolinkcat.eu/contactinformationanddatarequests.