Abstract
Long waiting times due to ongoing organ shortage have led to increased utilization of locoregional therapies (LRTs) to bridge patients with hepatocellular carcinoma (HCC) to liver transplantation (LT). We performed this study to evaluate the impact of LRTs on post-LT outcomes. We conducted a retrospective study of patients who were transplanted for HCC at Stanford University Hospital between 2008 and 2018 (n = 302). We found that receipt of ≥5 LRTs was an independent and significant predictor of poor overall 5-year survival (58.3% vs. 83.3%; HR 2.26, p = .03), poor recurrence-free 5-year survival (51.9% vs. 80.4%; HR 2.12, p = .03), and was associated with higher rates of recurrence (25.0% vs. 7.4%, p = .001). Moreover, recurrent HCC was more likely to be the cause of death (58.3% vs. 41.7%, p = .04) in patients who received ≥5 LRTs. Also, patients who required ≥5 LRTs showed an overall lower rate of radiological complete response (46.9% vs. 97.8%, p = .001) and were more likely to have more advanced pathological stage tumors in the explant (65.6% vs. 29.6%, p < .001). In conclusion, receipt of ≥5 bridging LRTs prior to LT is associated with worse post-transplant clinical outcomes.
Keywords: Hepatocellular carcinoma, Liver cancer, Locoregional therapy, Transarterial chemoembolization, Transplant
1 |. INTRODUC TION
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality in the world.1
The incidence of HCC has been progressively increasing, especially in developed countries including the United States (US).2 Most of the patients who develop HCC have underlying liver cirrhosis from chronic viral hepatitis, alcoholic liver disease, and/or non-alcoholic fatty liver disease (NAFLD). Liver transplantation (LT) is the best treatment option for these patients who are suitable surgical candidates, as it provides a potential cure for both the cancer and cirrhosis. Hence, it is no surprise that HCC is the most common indication for LT and for wait-list placement in the US.3
The median waiting time from listing to LT for patients with HCC can vary significantly depending on the United Network for Organ Sharing (UNOS) region to which they belong. For instance, in regions 1 or 9 of the United States, which have long wait lists, more than 70% of the patients with HCC have been waiting for more than a year to undergo LT.4 In 2015, UNOS/OPTN policy was modified to mandate a 6-month delay before patients with HCC are given exception points, making wait times even longer.5
Locoregional therapies (LRTs) like transarterial chemoembolization (TACE) or ablation are used to prevent tumor progression while waiting on the list. Achieving lower viable tumor burden in the explant has been shown to be associated with lower risk for recurrence.6 Patients usually require multiple sessions of LRT to achieve and sustain remission of HCC. However, there is concern that treating tumors with multiple sessions of locoregional therapy over a prolonged period of time can paradoxically worsen clinical outcomes by selecting out more aggressive subclones.7,8 We still do not know which subset of patients undergoing LRT have worse outcomes, and this information has the potential to serve as a valuable pre-transplant predictor of outcomes.
We conducted this study to identify the potential predictors of long-term clinical outcomes in patients with HCC who underwent liver transplantation at our academic transplant center, with a special focus on the impact of locoregional therapy on post-transplant recurrence and survival.
2 |. METHODS
2.1 |. Patient selection
The study protocol was approved by the Institutional Review Board (IRB) at the School of Medicine, the Stanford University (Stanford, USA). This is a single-center retrospective study in adult patients with HCC who received a liver transplant at an academic transplant center (Stanford University Hospital) between 2008 and 2018 for a pre-transplant diagnosis of HCC. Patients were followed until death or until March 2020.
2.2 |. Data collection
Patient data abstracted from the electronic medical record for analysis included demographic data, etiology of liver disease, comorbidities, Child Pugh score, initial HCC staging, number and type of LRTs received, and pre- and post-transplant imaging data. Clinical outcomes assessed were post-LT HCC recurrence, recurrence-free survival (RFS), and overall survival (OS). Explant pathologic variables were extracted from the standardized pathology reports at our center and included total tumor number, maximum tumor diameter, grade, micro- and macro-vascular invasion, and American Joint Committee on Cancer (AJCC) tumor staging.
2.3 |. Clinical protocols
All images were reviewed by a group of liver radiologists at our transplant center, to determine radiographic tumor size and numbers. HCC diagnosis was made based on American Association for the Study of Liver Diseases (AASLD) guidelines.9 Patients who showed radiographic evidence of tumor thrombus or extrahepatic disease were removed from the transplant list. The mode of LRT the patients received was determined in a multidisciplinary tumor board which included members from transplant hepatology, medical oncology, transplant surgery, diagnostic, and interventional radiology. If lesions were located in a safely accessible location and had a diameter of 3 cm or less, radiofrequency ablation (RFA) was typically performed. Otherwise, transcatheter therapy was performed. Transarterial therapy with chemoembolization or radioembolization was performed for multifocal disease. The modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria were used to define response to LRT.10 The overall goal of treatment was to achieve complete radiologic response. Decisions regarding further treatment were made in the multidisciplinary tumor board, taking multiple factors into consideration, including residual tumor burden, hepatic reserve, response to previous treatment and anticipated time to transplantation.
2.4 |. Statistical analysis
Statistical Package for the Social Sciences (SPSS, IBM) was used to compare patient risk factors, demographics, and clinical outcomes. Categorical variables were described using frequencies and percentages, and statistical analyses of these variables were evaluated using Fisher’s exact test or a chi-squared test. Continuous variables were described by correlation distributions using medians with either minimum to maximum ranges or interquartile range (IQR) for non-normal distributions. Statistical differences between medians were calculated using SPSS non-parametric test for two independent medians. Kaplan Meier analysis was used for survival analyses, with the log-rank test being used to compare outcomes. Overall survival was defined as the duration between the date of LT and the date of death from any cause. Recurrence-free survival was defined as the duration between the date of LT and the date of recurrence or death from any cause. Univariate and multivariate Cox regression analyses were performed to investigate patient and tumor characteristics associated with the tumor recurrence or death. Through the time-to-event analysis, hazard ratios (HRs) and 95% confidence intervals (CIs) were generated. Statistically significant variables were determined to have p-Values <.05. However, a level of significance of 0.15 was used to determine the variables that would enter the multivariate analysis.
3 |. RESULTS
3.1 |. Baseline demographic features of patients transplanted for HCC
Between 2008 and 2018 a total of 302 patients were transplanted for HCC (Table 1). The median age at diagnosis of HCC was 60.0 years (range 17–73), and the median age at transplantation was 62.0 years (range 19–75). Study participants were mostly male (79.8% [n = 241]) and the majority of participants were either of Caucasian or Asian descent (68.9% [n = 208]) (Table 1). The most common etiology of HCC was viral hepatitis—hepatitis C (38.4% [n = 116]) and hepatitis B (18.5% [n = 56]). The majority of patients developed HCC in the background of cirrhosis (93.7% [n = 283]). Just over half of patients had a history of decompensated liver disease (52.6% [n = 159]), with ascites (42.7% [n = 129]) or hepatic encephalopathy (35.4% [n = 107]) being the top two causes. Most patients with HCC had good performance status—Eastern Cooperative Oncology Group (ECOG) 0–1 (92.3% [n = 279]). The majority of the patients had tumors within the Milan criteria at diagnosis (88.3% [n = 263]) and most patients were staged as Barcelona Clinic Liver Cancer (BCLC) 0-A at diagnosis (67.2% [n = 203]). The median time to transplantation was 18 months (IQR 11.0 months). In the overall cohort, the 5-year post-transplant survival was 81%, recurrence rate 9.3% (n = 28) and the 5-year recurrence-free survival was 77%. The median time to follow-up was 5.0 years (IQR 4.6).
TABLE 1.
Baseline characteristics of study participants
| Variable | Sub-category | n = 302 (%) | <5 LRT n = 270 (89.4%) | ≥5 LRT n = 32 (10.6%) | p-Value |
|---|---|---|---|---|---|
| Age | Median, At Diagnosis (Years) | 60.0 (17–73) | 60.0 (11) | 57.5 (11.0) | .212 |
| Median, At Transplantation | 62.0 (19–75) | 62.4 (10.5) | 59.3 (12.4) | .531 | |
| >65 years | 105 (34.8) | 93 (34.4) | 12 (37.5) | .731 | |
| >70 years | 33 (10.9) | 29 (10.7) | 4 (12.5) | .763 | |
| Gender | Male | 241 (79.8) | 213 (78.9) | 28 (87.5) | .251 |
| Female | 61 (20.2) | 57 (21.1) | 4 (12.5) | ||
| Race | Caucasian | 128 (42.4) | 114 (42.2) | 14 (43.8) | .809 |
| Asian | 80 (26.5) | 73 (27.0) | 7 (21.9) | ||
| African American | 94 (31.1) | 83 (30.7) | 11 (34.4) | ||
| American Indian | |||||
| Others | |||||
| Ethnicity | Hispanic | 85 (28.1) | 71 (26.3) | 14 (43.8) | .038 |
| Non-Hispanic | 217 (71.9) | 199 (73.7) | 18 (56.3) | ||
| Risk factors | Diabetes | 97 (32.1) | 86 (31.9) | 11 (34.4) | .773 |
| Hypertension | 130 (43.0) | 113 (41.9) | 17 (53.1) | .223 | |
| Hyperlipidemia | 48 (15.9) | 42 (15.6) | 6 (18.8) | .640 | |
| Obesity | 101 (33.4) | 89 (33.0) | 12 (37.5) | .607 | |
| Smoking | 123 (40.7) | 108 (40.0) | 15 (46.9) | .454 | |
| Metabolic Syndrome | 45 (14.9) | 38 (14.1) | 7 (21.9) | .241 | |
| Cirrhosis | Yes | 283 (93.7) | 254 (94.1) | 29 (90.6) | .437 |
| No | 19 (6.3) | 16 (5.9) | 3 (9.4) | ||
| Etiology of HCC | NAFLD | 32 (10.6) | 26 (9.6) | 3 (9.4) | > .99 |
| Hepatitis B | 56 (18.5) | 53 (19.6) | 7 (21.9) | .763 | |
| Hepatitis C | 116 (38.4) | 105 (38.9) | 11 (34.4) | .62 | |
| Alcoholic | 40 (13.2) | 85 (31.5) | 12 (37.5) | .491 | |
| Hepatitis C + Alcoholic | 49 (16.2) | 43 (15.9) | 6 (18.8) | .682 | |
| Cryptogenic | 3 (1.0) | 12 (4.4) | 1 (3.1) | > .99 | |
| Other | 6 (2.0) | 10 (3.7) | 0 (0.0) | .607 | |
| Decompensation from liver disease | None | 143 (47.4) | 126 (46.7) | 17 (53.1) | .489 |
| Ascites | 129 (42.7) | 120 (44.4) | 9 (28.1) | .078 | |
| Variceal Bleeding | 49 (16.2) | 44 (16.3) | 5 (15.6) | > .99 | |
| Hepatic Encephalopathy | 107 (35.4) | 100 (37.0) | 7 (21.9) | .09 | |
| Other | 7 (2.3) | 7 (2.6) | 0 (0.0) | >.99 | |
| Any Cause | 159 (52.6) | 144 (53.3) | 15 (46.9) | .489 | |
| Child Pugh class | A | 153 (50.7) | 135 (50.0) | 18 (56.3) | .327 |
| B | 103 (34.1) | 91 (33.7) | 12 (37.5) | ||
| C | 46 (15.2) | 44 (16.3) | 2(6.3) | ||
| BCLC staging | Very Early | 22 (7.3) | 22 (8.1) | 0 (0.0) | .146 |
| Early | 181 (59.9) | 161 (59.6) | 20 (62.5) | .754 | |
| Intermediate | 44 (14.6) | 34 (12.6) | 10 (31.5) | .005 | |
| Advanced | 9 (3.0) | 9 (3.3) | 0 (0.0) | .605 | |
| Terminal | 46 (15.2) | 44 (16.3) | 2 (6.3) | .192 | |
| AFP | At Diagnosis | 13.0 (34.0) | 12.5 (34.75) | 13.9 (27.25) | .532 |
| At Transplant | 6.1 (12.0) | 6.0 (12.0) | 7.0 (11.8) | .630 | |
| Milan (at diagnosis) | Within | 263 (88.3) | 242 (90.6) | 21 (67.7) | <.001 |
| Outside | 34 (11.4) | 24 (9.0) | 10 (32.3) | <.001 |
Note: Metabolic syndrome is defined as the presence of 2 or more of the following: diabetes, hypertension, hyperlipidemia, and obesity, with hyperlipidemia being a substitute for hypertriglyceridemia and obesity being a substitute for waist diameter.
median (minimum-maximum)
at transplantation, median (IQR).
Abbreviations: AFP, alfa-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HCC, Hepatocellular Carcinoma; LRT, Locoregional Therapy; MELD, Model for End-stage Liver Disease; NAFLD, Non-alcoholic fatty liver disease. The bold p values indicate statistically significance.
3.2 |. Profile of Locoregional therapies for HCC
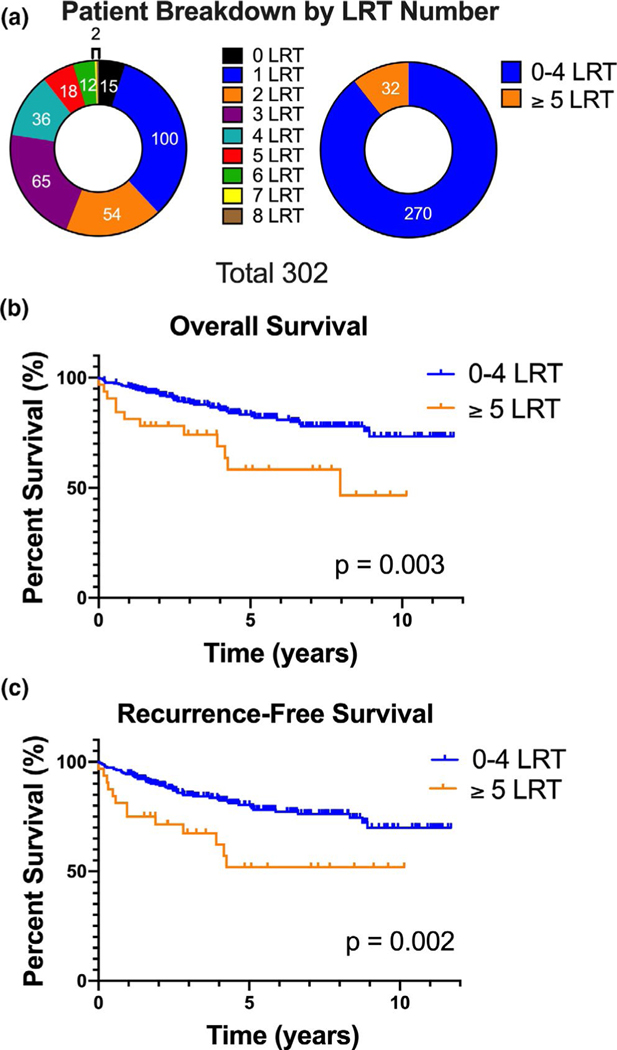
In our cohort, 95% (n = 287) of patients received bridging LRTs prior to transplantation. Fifteen patients (5%) did not receive any LRTs prior to transplantation due to decompensated liver disease. The median number of treatments per patient was 2 (IQR 2.0), 10.6% (n = 32) patients received ≥5 LRT (Figure 1A). Patients with tumors outside of the Milan criteria had a higher median number of LRTs (3 (IQR 3.0) vs. 2.0 (IQR 2.0), p < .001 and were more likely to receive ≥5 LRTs (29.4% vs. 8.0%, p < .001). The most common type of initial treatment was TACE (90.5% [n = 257]), followed by ablative therapies (8.1% [n = 23]) (Table S1). By the time of transplantation, 64.8% (n = 186) of patients treated with LRTs achieved a radiologic complete response (CR). Thirty-one patients (10.8% of treated patients) did not have mRECIST scores after their last LRT because they were transplanted shortly after treatment. Overall, LRTs reduced the number of viable lesions to 0 (33.4% [n = 101]) or 1 (39.4% [n = 119]) in most patients.
FIGURE 1.
Higher number of locoregional therapies (LRTs) prior to transplantation for hepatocellular carcinoma (HCC) predicts poor post-transplant survival. (A) Distribution of patients receiving 0 through 8 LRTs. Observed counts are: 0 LRT n = 15, 1 LRT n = 100, 2 LRT n = 54, 3 LRT n = 65, 4 LRT n = 36, 5 LRT n = 18, 6 LRT n = 12, 7 LRT n = 1, 8 LRT n = 1. (B) A comparison of the overall survival in patients receiving <5 (n = 270) or ≥5 LRTs (n = 32. (C) A comparison of the recurrence-free survival in patients receiving < 5 (n = 270) or ≥ 5 LRTs (n = 32)
3.3 |. Higher number of LRTs for HCC predicts poor post-transplant survival
We found that receipt of higher number of LRTs is associated with worse overall survival (OS) (p = .008; HR 1.24 [1.06–1.45]) and recurrence-free survival (RFS) (p = .007; HR 1.25 [1.06–1.42]). When patients were stratified by the number of LRTs, we found that receiving ≥5 LRTs was the threshold associated with worse OS and RFS (p = .002 and p = .003, respectively) (Figure 1B,C; Figure S1). We performed multivariate analysis for OS and RFS after adjusting for variables which could influence outcomes like age, gender, comorbidities, HCC etiology, BCLC tumor stage, Milan status, Child Pugh score, liver decompensation, and alfa-fetoprotein (AFP) (Tables 2 and 3). Receipt of ≥5 LRTs was still found to be an independent predictor of OS and RFS (58.3% vs. 83.3%; HR 2.26 (95% CI 1.08–4.70), p = .03) and RFS (51.9% vs. 80.4; HR 2.12 (95% CI 1.06–4.23), p = .03). Other factors predicting OS and RFS were age at transplantation ≥65 years (HR 2.52 (95% CI 1.39–4.59), p = .002; HR 2.32 (95% CI 1.34 −4.01), p = .003, respectively), hepatitis C (HR 2.35 (1.20–4.60), p = .01; HR 2.54 (1.38–4.67), p = .003), and vascular invasion (HR 2.67 (1.18–6.03), p = .02; HR 3.34 (1.62–6.70), p = .001, respectively), (Tables 2 and 3). Patients who received ≥5 LRTs were more likely to have tumor burden outside Milan criteria at diagnosis (32.3% vs. 9.0%, p < .001) and also have longer wait times on the list (24.0 months (IQR 22.8) vs. 17.0 months (IQR 11.0), p < .001). However, Milan status at diagnosis, tumor size, and number at diagnosis or time to transplantation were not determined to be predictors of OS or RFS (Tables 2 and 3). Thus, receipt of ≥5 LRTs was significantly and independently associated with poor overall and recurrence-free survival.
TABLE 2.
Univariate and multivariate analysis of pre-transplant factors influencing overall survival in patients transplanted for HCC
| Variables | Overall survival univariate analysis |
Overall survival multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| 5-year OS | HR | CI (95%) | p-Value | HR | CI (95%) | p-Value | ||
| Age | ≥65 | 71.4 vs. 85.0 | 2.21 | 1.31 –3.74 | .003 | 2.52 | 1.39–4.59 | .002 |
| Gender | 73.2 vs. 82.6 | .19 | ||||||
| Race | White | 77.5 vs. 83.1 | .58 | |||||
| Asian | 81.6 vs. 80.1 | .98 | ||||||
| African American | 66.7 vs. 86.3 | .84 | ||||||
| American Indian | ||||||||
| Other | ||||||||
| Ethnicity | 79.4 vs. 83.3 | .91 | ||||||
| Risk factors | Diabetes | 78.3 vs. 81.5 | .91 | |||||
| Hypertension | 85.3 vs. 77.6 | .91 | ||||||
| Hyperlipidemia | 82.0 vs. 80.3 | .94 | ||||||
| Obesity | 81.3 vs. 80.2 | .91 | ||||||
| Smoking | 77.0 vs. 83.2 | 0.66 | 0.39–1.11 | .12 | 0.83 | 0.45–1.54 | .56 | |
| HCC etiology | NAFLD | 75.0 vs. 81.0 | .94 | |||||
| Hepatitis C | 63.1 vs. 85.4 | 1.23–3.98 | .008 | 2.35 | 1.20–4.60 | .01 | ||
| Hepatitis B | 82.7 vs. 81.2 | .91 | ||||||
| Cirrhosis | 80.0 vs. 88.0 | .96 | ||||||
| Decompensation from liver disease | None | 80.7 vs. 80.5 | .91 | |||||
| Ascites | 78.5 vs. 82.2 | .91 | ||||||
| Variceal Bleeding | 80.9 vs. 79.8 | .94 | ||||||
| Hepatic | 79.7 vs. 81.0 | .91 | ||||||
| Encephalopathy | ||||||||
| Other | 41.7 vs. 81.2 | .97 | ||||||
| Child Pugh class | A | 79.2 | 1.00 | — | .23 | |||
| B | 84.3 | .33 | ||||||
| C | 76.3 | .30 | ||||||
| BCLC staging | Very Early | 81.1 | 1.00 | — | .64 | |||
| Early | 81.6 | .47 | ||||||
| Intermediate | 78.1 | .93 | ||||||
| Advanced | 100 | .35 | ||||||
| Terminal | 76.3 | .16 | ||||||
| Milan status at diagnosis | Outside | 72.3 vs. 82.2 | .35 | |||||
| AFP at transplantation | — | .10 | 1.00 | 1.00–1.00 | .53 | |||
| MELD at transplantation | — | .56 | ||||||
| LRT | 0 | 92.9 vs. 80.1 | .70 | |||||
| 1–4 | 83.3 vs. 58.3 | 0.38 | 0.20–0.72 | .003 | ||||
| ≥5 | 58.3 vs. 83.3 | 2.62 | 1.39–4.97 | .003 | 2.26 | 1.08–4.70 | .03 | |
| Downstaged | 78.2 vs. 88.5 | .69 | ||||||
| Response to therapy | PD | 78.4 vs. 81.5 | .54 | |||||
| Time from diagnosis to transplant | — | .24 | ||||||
| Grade (Explant) | Necrotic | 84.5 | 1.00 | — | .41 | 1.00 | — | .92 |
| Well-differentiated | 76.7 | .14 | 1.75 | 0.22–13.84 | .60 | |||
| Moderate differentiation | 79.8 | .14 | 1.58 | 0.20–12.44 | .66 | |||
| Poorly differentiated | 90 | .99 | 1.01 | 0.06–18.17 | >.99 | |||
| Vascular invasion (Explant) | 66.4 vs. 82.3 | 3.07 | 1.44–6.55 | .004 | 2.67 | 1.18–6.03 | .02 | |
| AJCC T stage (Explant) | T0 | 86.9 | 1.00 | — | .02 | 1.00 | — | .15 |
| T1 | 82.3 | 1.71 | 0.80–3.618 | .16 | 2.23 | 0.99–5.00 | .05 | |
| T2/T3/T4 | 72.8 | 2.749 | 1.35–5.58 | .005 | 1.64 | 0.72–3.72 | .24 | |
Note: Analysis for both models completed using Cox regression. Variables with p-Values <.15 were included in the multivariate model. Abbreviations: AFP, alfa-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CI, Confidence Interval; HCC, Hepatocellular Carcinoma; HR, Hazard Ratio; LRT, Locoregional Therapy; MELD, Model for End-stage Liver Disease; NAFLD, Non-alcoholic fatty liver disease; PD, Progressive Disease. The bold p values indicate statistically significance.
TABLE 3.
Univariate and multivariate analysis of pre-transplant factors influencing RFS in patients transplanted for HCC
| Recurrence-free survival univariate analysis |
Recurrence-free survival multivariate analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | 5-year OS | HR | CI (95%) | p-Value | HR | CI (95%) | p-Value | |
| Age | ≥65 | 69.3 vs. 81.3 | 1.93 | 1.18–3.16 | .009 | 2.32 | 1.34–4.01 | .003 |
| Gender | 70.0 vs. 79.3 | .18 | ||||||
| Race | White | 74.7 vs. 79.4 | .71 | |||||
| Asian | 76.8 vs. 77.5 | .94 | ||||||
| African American | 66.7 vs. 83.6 | .37 | ||||||
| American Indian | ||||||||
| Others | ||||||||
| Ethnicity | 76.1 vs. 77.7 | .94 | ||||||
| Risk factors | Diabetes | 77.5 vs. 77.5 | .94 | |||||
| Hypertension | 80.0 vs. 75.5 | .94 | ||||||
| Hyperlipidemia | 77.9 vs. 77.2 | .96 | ||||||
| Obesity | 79.0 vs. 76.5 | .94 | ||||||
| Smoking | 72.8 vs. 80.5 | .18 | ||||||
| HCC etiology | NAFLD | 72.3 vs. 77.8 | .81 | |||||
| Hepatitis C | 57.4 vs. 82.5 | 2.34 | 1.36–4.00 | .002 | 2.54 | 1.38–4.67 | .003 | |
| Hepatitis B | 77.7 vs. 78.2 | .71 | ||||||
| Cirrhosis | Yes | 77.2 vs. 77.2 | .96 | |||||
| Decompensation from liver disease | None | 78.8 vs. 76.0 | .94 | |||||
| Ascites | 74.6 vs. 79.5 | .94 | ||||||
| Variceal Bleeding | 73.9 vs. 78.1 | .94 | ||||||
| Hepatic Encephalopathy |
77.0 vs. 77.3 | .94 | ||||||
| Other | 47.6 vs. 78.1 | .97 | ||||||
| Child Pugh class | A | 76.2 | 1.00 | — | .42 | |||
| B | 80.9 | .28 | ||||||
| C | 73.1 | .67 | ||||||
| BCLC staging | Very Early | 77.7 | 1.00 | — | .90 | |||
| Early | 77.9 | .48 | ||||||
| Intermediate | 75.1 | .75 | ||||||
| Advanced | 100 | .96 | ||||||
| Terminal | 73.1 | .94 | ||||||
| Milan status at diagnosis | 68.6 vs. 79.2 | .21 | ||||||
| AFP at transplantation | — | .09 | 1.00 | 1.00–1.00 | .54 | |||
| MELD at transplantation | — | .85 | ||||||
| LRT | 0 | 74.7 vs. 77.2 | .49 | |||||
| 1–4 | 80.4 vs. 51.9 | 0.41 | 0.22–0.75 | .004 | ||||
| ≥ 5 | 51.9 vs. 80.4 | 2.64 | 1.43–4.84 | .002 | 2.12 | 1.06–4.23 | .03 | |
| Downstaged | 75.5 vs. 83.0 | .41 | ||||||
| Response to therapy | PD | 78.0 vs. 76.5 | .87 | |||||
| Time from diagnosis to transplant | — | .16 | ||||||
| Grade (Explant) | Necrotic | 82.3 | .35 | 1.00 | — | .31 | ||
| Well-differentiated | 73.4 | .15 | 1.88 | 0.90–3.92 | .09 | |||
| Moderate differentiation | 75.3 | .09 | 1.83 | 0.90–3.69 | .09 | |||
| Poorly differentiated | 80.0 | .48 | 1.82 | 0.40–8.24 | .44 | |||
| Vascular invasion (Explant) | 46.3 vs. 79.8 | 3.75 | 1.89–7.44 | < .001 | 3.34 | 1.62–6.70 | .001 | |
| AJCC T stage (Explant) | T0 | 85.9 | 1.00 | — | .002 | 1.00 | — | > .99 |
| T1 | 81.7 | 1.48 | 0.73–3.00 | .28 | 1.02 | 0.13–8.14 | .99 | |
| T2/T3/T4 | 64.1 | 2.99 | 1.56–5.72 | .001 | 1.03 | 0.12–8.69 | .98 | |
Note: Analysis for both models completed using Cox regression. Variables with p-Values <.15 were included in the multivariate model. Abbreviations: AFP, alfa-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CI, Confidence Interval; HCC, Hepatocellular Carcinoma; HR, Hazard Ratio; LRT, Locoregional Therapy; MELD, Model for End-stage Liver Disease; NAFLD, Non-alcoholic Fatty Liver Disease; PD, Progressive Disease; RFS, Recurrence-free Survival. The bold p values indicate statistically significance.
3.4 |. Patients receiving five or more LRTs have a higher incidence of HCC recurrence
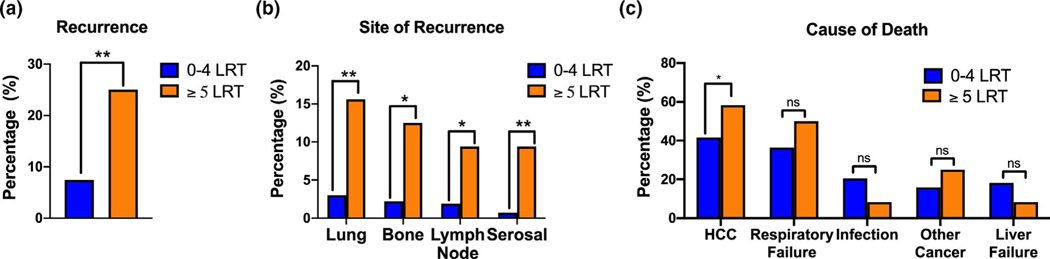
In the overall cohort, the cumulative recurrence rates at one and five years were 4.6% (n = 14) and 8.6% (n = 26), respectively. The post-transplant recurrence rate was higher among patients who received ≥5 LRTs (25.0% vs. 7.4%, p = .001) (Figure 2A). LRT ≥5 was determined to be independently predictive of HCC recurrence on multinomial logistic regression (HR 3.91 (95% CI 1.39–10.41), p = .009) (Table 4). AJCC stage and vascular invasion on explant were the other independent predictors of recurrence. Patients who received ≥5 LRT were more likely to have early recurrence, that is, recurrence before 24 months (21.9% vs. 5.2%, p < .001), and the mean time from transplantation to HCC recurrence was shorter (11.1 vs. 21.2 months, p = .05). Among those patients who recurred (9.3% [n = 28]), the most common sites of post-transplant HCC recurrence were the transplanted liver graft (53.6% [n = 15]), lungs (46.4% [n = 13]), and bones (35.7% [n = 10]). Patients who received ≥5 LRTs were more likely to develop extrahepatic HCC recurrence, with the lung (15.6% [n = 5] vs. 3.0% [n = 8], p = .001) and bone (12.5% [n = 4] vs. 2.2% [n = 6], p = .002) being the most common sites of recurrence (Figure 2B, Table S2). Lastly, recurrent HCC was the cause of death in a higher proportion of patients who had received ≥5 LRTs (58.3% [n = 7] vs. 41.7% [n = 5], p = .04) (Figure 2C).
FIGURE 2.
Receiving five or more locoregional therapies (LRTs) has a higher incidence of recurrence. (A) Overall hepatocellular carcinoma (HCC) recurrence rate stratified by <5 LRT and ≥5 LRTs. (B) A comparison of the sites of recurrence in patients with extrahepatic recurrence, stratified by <5 or ≥5 LRTs. Observed counts for each organ are: lung (n = 8 vs. n = 5), bone (n = 6 vs. n = 4), lymph (n = 5 vs. n = 3), and serosa (n = 2 vs. n = 3) for patient receiving <5 or ≥5 LRTs, respectively. Serosal includes pleura and peritoneum. (C) A comparison of the cause of death in patients receiving <5 or ≥5 LRTs. Includes HCC (n = 11 vs. n = 7), respiratory failure (n = 16 vs n = 6), infection (n = 9 vs. n = 1), other cancer (n = 7 vs. n = 3), and liver failure (n = 8 vs. n = 1), respectively. Death by respiratory failure includes causes attributed to cancer and infection. Other cancers causing death in this study include squamous cell carcinoma, lung adenocarcinoma, pancreatic cancer, lymphoma/leukemia, and prostate cancer. * p < .05, ** p < .01
TABLE 4.
Univariate and multivariate analysis of pre-transplant factors predicting HCC recurrence
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | Category | No Recurrence (n = 274) | Recurrence (n = 28) | p-Value | p-Value | HR (95% CI) |
| LRT | 0–4 | 250 (91.20%) | 20 (71.40%) | .005 | .009 | 3.91 (1.39–10.41) |
| ≥5 | 24 (8.80%) | 8 (28.60%) | ||||
| Age | >65 years | 93 (33.90%) | 12 (42.90%) | .405 | ||
| Etiology | Hepatitis C | 149 (54.40%) | 16 (57.10%) | .844 | ||
| Hepatitis B | 51 (18.60%) | 9 (32.10%) | .131 | |||
| NASH | 26 (9.50%) | 3 (10.70%) | .741 | |||
| ALD | 40 (14.8%) | 0 (0) | .832 | |||
| Child Pugh class | A | 135 (49.30%) | 18 (64.30%) | .318 | ||
| B | 96 (35.00%) | 7 (25.00%) | ||||
| C | 43 (15.70%) | 3 (10.70%) | ||||
| BCLC stage | 0- A | 182 (66.42%) | 21 (75.00%) | .749 | ||
| B | 40 (14.60%) | 4 (14.30%) | ||||
| C-D | 52 (18.90%) | 3 (10.70%) | ||||
| AFP at diagnosis | Mean (SEM) | 112.0 (35) | 228 (226) | .324 | ||
| AFP at transplant | Mean (SEM) | 30.6 (8.1) | 137 (71) | .149 | ||
| Cumulative tumor diameter at diagnosis | Mean (SEM) | 3.43 (0.13) | 3.38 (0.36) | .892 | ||
| Cumulative tumor diameter before LT | Mean (SEM) | 0.97 (0.096) | 1.68 (0.44) | .13 | ||
| Size of largest lesion at diagnosis | Mean (SEM) | 2.62 (0.06) | 2.54 (0.18) | .661 | ||
| Size of largest lesion before LT | Mean (SEM) | 0.89 (0.06) | 1.64 (0.36) | .05 | .166 | 0.81 (0.60–1.10) |
| Number of viable lesions at diagnosis | Mean (SEM) | 1.51 (0.04) | 1.48 (0.12) | .805 | ||
| Number of viable lesions before LT | Mean (SEM) | 0.62 (0.05) | 1.0 (0.23) | .124 | ||
| Progressive Disease after LRT | Yes | 137 (50%) | 14 (50%) | .147 | ||
| Vascular invasion | Yes | 15 (5.5%) | 8 (29.6%) | .001 | .010 | 4.17 (1.41–12.34) |
| AJCC stage | T 2–4 | 80 (29.6%) | 19 (70.4%) | .001 | .052 | 3.85 (1–15.00) |
Note: Analysis for both models completed using multinomial logistic regression. Variables with p-values <.15 were included in the multivariate model. Abbreviations: BCLC, Barcelona Class Liver Cancer Staging; HCC, hepatocellular carcinoma; LRTs, Locoregional Therapies. The bold p values indicate statistically significance.
3.5 |. Explanted liver demonstrates more advanced stage despite higher number of LRTs
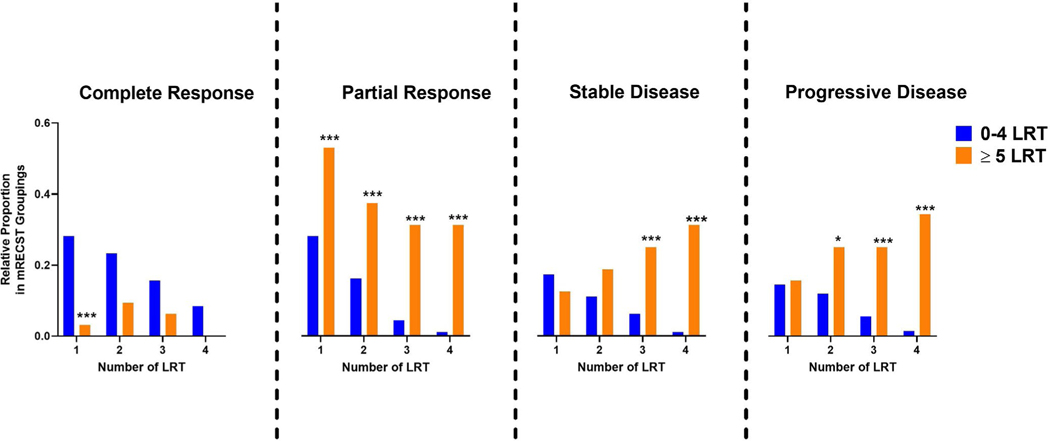
Patients who received ≥5 LRTs were generally poor responders to therapy. Table 5 shows a dynamic breakdown of mRECIST responses between patients who received ≥5 LRTs and <5 LRTs. The former group was less likely to achieve a radiological CR after either the first LRT (3.1% vs. 28.1%, p = .001) or after any of the first four LRTs (46.9% vs. 97.8%, p < .001) (Figure 3). By contrast, patients who received ≥5 LRTs were more likely to develop progressive disease (PD) during at least one of the LRTs they received (81.3% vs. 30.4%, p < .001). We did not find that PD after therapy was itself a predictor of overall survival (5 years-78.4% vs. 81.5%; p = .54) or recurrence-free survival (5 years-78.0% vs. 76.5%; p = .99) or recurrence (Table 4). Evaluation of explant histopathology showed no difference in tumor grade or vascular invasion (Table S3). But explants that had received ≥5 LRTs were more likely to have advanced AJCC tumor stage of the residual tumor (AJCC stage T2-T4 46.9% vs. 31.7%; p = .04). Advanced AJCC stage itself was not determined to be an independent predictor of OS (p = .24) or RFS (p = .98) Thus, patients who received ≥5 LRTs were less likely to respond to LRTs and had a more advanced AJCC stage in the explant.
TABLE 5.
Radiological Responses after LRTs in Patients Transplanted for HCC, stratified by < 5 or ≥ 5 LRTs
| Variable | LRT # | <5 LRT n = 270 (89.4%) | ≥5 LRT n = 32 (10.6%) | p-Value |
|---|---|---|---|---|
| Complete response | 1 | 76 (28.1) | 1 (3.1) | .001 |
| 2 | 63 (23.3) | 3 (9.4) | .07 | |
| 3 | 42 (15.6) | 2 (6.3) | .19 | |
| 4 | 23 (8.5) | 0 (0.0) | .15 | |
| Partial response | 1 | 76 (28.1) | 17 (53.1) | .004 |
| 2 | 44 (16.3) | 12 (37.5) | .004 | |
| 3 | 12 (4.4) | 10 (31.3) | <.001 | |
| 4 | 3 (1.1) | 10 (31.3) | <.001 | |
| Progressive disease | 1 | 47 (17.4) | 4 (12.5) | .62 |
| 2 | 30 (11.1) | 6 (18.8) | .21 | |
| 3 | 17 (6.3) | 8 (25.0) | <.001 | |
| 4 | 3 (1.1) | 10 (31.3) | <.001 | |
| Stable disease | 1 | 39 (14.4) | 5 (15.6) | .86 |
| 2 | 32 (11.9) | 8 (25.0) | .04 | |
| 3 | 15 (5.6) | 8 (25.0) | <.001 | |
| 4 | 4 (1.5) | 11 (34.4) | <.001 |
Abbreviations: CR, complete response; LRTs, Locoregional Therapies; PR, partial response; PD, progressive disease; SD, stable disease. The bold p values indicate statistically significance.
FIGURE 3.
Receiving five or more locoregional therapies (LRTs) is associated with worse radiological responses. Comparison of radiologic responses, as determined by mRECIST criteria, after LRT treatments, stratified by <5 or ≥5 LRTs. * p < .05, ** p < .01, *** p < .001
4 |. DISCUSSION
Locoregional therapies are increasingly used to bridge patients with HCC to transplant, given long wait times and ongoing organ shortage. We performed this study to evaluate the impact of receiving a higher number of LRTs on post-transplant clinical outcomes. In this study, we show that receiving five or more LRTs appears to be the threshold above which patients experience poor post-transplant survival and higher risk of HCC-related death. Evaluation of the radiological response patterns showed that patients who received ≥5 LRTs were consistently poor responders to LRT, and as a result had more advanced tumors in the explanted liver. Our data also shows that receiving ≥5 LRTs was associated not only with higher rates of recurrence and faster recurrence but also higher incidence of extrahepatic recurrence, implying these patients likely had aggressive tumor biology with possible systemic micrometastatic disease prior to transplantation. These results potentially have direct clinical implications in risk stratifying patients with HCC prior to LT and for improving patient selection for transplantation.
Hepatocellular carcinoma is unique, since a biopsy is not needed to make a diagnosis in most instances. But the disadvantage of this scenario is that we generally do not have access to information about the factors which strongly predict post-transplant outcomes, including the grade of tumor, presence of vascular invasion, or degree of pathologic response.6,11,12 Hence, it is crucial to determine which pre-transplant factors can serve as surrogate markers of aggressive tumor biology. Response to LRTs has been suggested to predict clinical outcomes,13,14 but identifying the highest risk subset of patients who receive LRTs remains challenging. We show here that the threshold of receiving more than five LRTs was an important predictor of poor overall survival and recurrence-free survival, even after adjusting for known predictors of survival like tumor stage, time to transplant, alfa-fetoprotein (AFP), vascular invasion and radiologic response. Although patients are treated with repeated LRTs to effectively bridge them to transplant and avoid wait-list drop-out from tumor progression, this strategy could paradoxically make the long-term clinical outcomes worse.
We demonstrate that the patients who received ≥5 LRTs repeatedly showed poor radiologic response, with only 3% showing complete response with initial treatment. This raises the possibility that the requirement for a high number of LRTs is likely a surrogate marker for tumors with primary chemoresistance. Tumors that require multiple LRTs have been shown to have poor vascularization, which likely limits the delivery of chemotherapeutic agents to the target.15 On the other hand, each subsequent LRT may also be selecting resistant clones within these tumors, since we found that most tumors which required ≥5 LRTs were likely to show progressive disease during subsequent therapies. Both of these scenarios lead to higher viable tumor burden, which implies higher potential for micrometastases from circulating tumor cells.16 Consistent with this, we observed earlier recurrence and higher rates of extrahepatic recurrence in patients receiving ≥5 LRTs, suggesting that recurrence arises from residual tumors rather than de-novo HCC. Larger studies will be needed to test these probable hypotheses.
We are reporting data from a center that has long wait times, since it is in region 5 of the United States. These data may still be applicable to centers which have shorter wait times and since higher median number of LRTs, in general, does predict outcomes. Also, with the UNOS policy constantly shifting toward more equitable distribution of organs and also with the rising incidence of HCC, it is conceivable that many centers may experience longer wait times for HCC in the future, thus making our results relevant. We also recognize that during the 10-year timespan of this study, the landscape of locoregional therapies has shifted, with increased use of Y-90 transarterial radioembolization (TARE). However, most of the patients in this study received TACE. These findings will need to be validated in groups that use other modes of LRT, like TARE, at higher rates. Another limitation is not having access to tumor biopsy prior to LRT, which does not allow us to determine whether these tumors were intrinsically resistant to therapy or if they acquired secondary resistance due to multiple LRTs. However, this is a general limitation of most HCC studies. This study does have biases inherent to a retrospective analysis, but using single-center data allow us to take a granular look at radiologic response after each therapy while ensuring that all the patients were treated under the same protocol and experienced similar wait times across the cohort. Lastly, our study has a relatively small sample size and our findings will need to be validated in larger external cohorts.
Our study thus identifies receipt of ≥5LRTs as a strong pre-transplant predictor of HCC recurrence and poor overall survival. We demonstrate the patients who receive >5 LRTs are less likely to respond to therapy, have more advanced histopathological features at the time of transplant despite receiving multiple therapies, and develop aggressive post-transplant extrahepatic recurrence. All these features suggest these tumors may have an intrinsically aggressive tumor biology, larger prospective studies will be needed to address this possibility. Furthermore, most policies focus on the number of tumors and size of tumors to determine transplant eligibility. But as shown in this study, even though tumors can be technically down staged to be within Milan criteria by employing multiple LRTs, tumor size itself might not be sufficient to predict clinical outcomes. Dynamic factors like response to therapy and the need for multiple therapies to attain remission need to be considered. In the setting of significant ongoing organ shortage, we urge caution while evaluating patients who need multiple therapies to attain HCC remission or consistently show lack of response to therapy. Further, if these patients are transplanted, they should be under close surveillance, especially for extrahepatic recurrence.
Supplementary Material
Funding information
RD-National Institutes of Health (NIH) grant CA222676 from the National Cancer Institute (NCI), American College of Gastroenterology Junior Faculty Career Development Grant.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
CONFLICT OF INTEREST
Authors declare no conflicts of interests.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Suo C, Mao X, et al. Global incidence trends in primary liver cancer by age at diagnosis, sex, region, and etiology, 1990–2017. Cancer. 2020;126(10):2267–2278. [DOI] [PubMed] [Google Scholar]
- 3.Yang JD, Larson JJ, Watt KD, et al. Hepatocellular carcinoma is the most common indication for liver transplantation and placement on the waitlist in the United States. Clin Gastroenterol Hepatol. 2017;15(5):767–775.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogen R, Lo M, DiNorcia J, et al. More than just wait time? Regional differences in liver transplant outcomes for hepatocellular carcinoma. Transplantation. 2019;103:747–754. [DOI] [PubMed] [Google Scholar]
- 5.Nagai S, Kitajima T, Yeddula S, et al. Effect of mandatory 6-month waiting period on waitlist and transplant outcomes in patients with hepatocellular carcinoma. Hepatology. 2020. 10.1002/hep.31223. [DOI] [PubMed] [Google Scholar]
- 6.DiNorcia J, Florman SS, Haydel B, et al. Pathologic response to pre-transplant locoregional therapy is predictive of patient outcome after liver transplantation for hepatocellular carcinoma: analysis from the US multicenter HCC transplant consortium. Ann Surg. 2020;271:616–624. [DOI] [PubMed] [Google Scholar]
- 7.Zen C, Zen Y, Mitry RR, et al. Mixed phenotype hepatocellular carcinoma after transarterial chemoembolization and liver transplantation. Liver Transpl. 2011;17:943–954. [DOI] [PubMed] [Google Scholar]
- 8.Agopian VG, Harlander-Locke MP, Ruiz RM, et al. Impact of pre-transplant bridging locoregional therapy for patients with hepatocellular carcinoma within milan criteria undergoing liver transplantation: analysis of 3601 patients from the US multicenter HCC transplant consortium. Ann Surg. 2017;266:525–535. [DOI] [PubMed] [Google Scholar]
- 9.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Clin Liv Dis. 2019;13:1. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Llovet J. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz C, Karaca CA, Iakobadze Z, et al. Factors affecting recurrence and survival after liver transplantation for hepatocellular carcinoma. Transpl Proc. 2018;50:3571–3576. [DOI] [PubMed] [Google Scholar]
- 12.Mehta N, Heimbach J, Harnois DM, et al. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol. 2017;3:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai Q, Avolio AW, Graziadei I, et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19:1108–1118. [DOI] [PubMed] [Google Scholar]
- 14.Yang K, Sung PS, You YK, et al. Pathologic complete response to chemoembolization improves survival outcomes after curative surgery for hepatocellular carcinoma: predictive factors of response. HPB. 2019;21:1718–1726. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, Kim JH, Choi S-Y, et al. Prediction of therapeutic response of hepatocellular carcinoma to transcatheter arterial chemoembolization based on pretherapeutic dynamic CT and textural findings. AJR Am J Roentgenol. 2017;209:W211–W220. [DOI] [PubMed] [Google Scholar]
- 16.Chiappini F Circulating tumor cells measurements in hepatocellular carcinoma. Int J Hepatol. 2012;2012:684802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.