Abstract
Nasopharyngeal carcinoma (NPC) is an epithelial tumor located in the nasopharynx and is highly associated with Epstein-Barr virus (EBV) infection. Although radiotherapy alone can cure ~90% of patients with early-stage disease, >70% of patients with NPC have locoregionally advanced or metastatic disease at the first diagnosis due to the insidious and aggressive nature of NPC. After comprehensive radiochemotherapy, 20-30% of patients with advanced NPC still fail treatment, mainly due to recurrence and/or metastasis (R/M). Conventional salvage treatments, such as radiotherapy, chemotherapy and surgery, are suboptimal and frequently accompanied by severe adverse effects and limited efficacy. In recent years, immunotherapy has emerged as a promising treatment modality for R/M NPC. An increasing number of clinical studies have investigated the safety and efficacy of immunotherapy for advanced NPC and have shown considerable progress. In the present review, the rationale for the use of immunotherapy to treat NPC was summarized and the current status, progress and challenges of NPC clinical research on different immunotherapeutic approaches were highlighted, including immune checkpoint inhibitors, vaccines, immunomodulators, adoptive cell transfer and EBV-specific monoclonal antibodies. The comprehensive overview of immunotherapy in NPC may provide insight for clinical practice and future investigation.
Keywords: nasopharyngeal carcinoma, Epstein-Barr virus, immunotherapy, clinical trial, treatment
1. Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial tumor of the head and neck with a well-defined geographical distribution (1). According to global cancer statistics from 2020, >75% of NPC cases are diagnosed in Southeast Asia and Southern China (2). NPC is thought to be mediated by the interaction of Epstein-Barr virus (EBV) infection, environmental factors (diet and smoking) and genetic susceptibility [high-risk human leukocyte antigen (HLA) allotypes] (Fig. 1) (3). The non-keratinizing subtype accounts for >95% of NPCs in endemic areas, while it accounts for 75% in the United States (4). This unique geographic distribution is etiologically attributable to genetic and environmental factors (5-8). In addition, EBV infection is ubiquitous in non-keratinizing NPC and has an important pathogenic role (9). Furthermore, the interaction of unique genetic factors and EBV variants may lead to an increased risk of persistent infection and clonal expansion of EBV-infected epithelial cells, ultimately culminating in an invasive phenotype promoting NPC progression (10,11). Of note, improvements in diagnostic imaging technology, the widespread use of intensity-modulated radiotherapy (IMRT) and the optimization of chemotherapy regimens have significantly improved the survival of patients with NPC (12-14). Approximately 90% of patients with early-stage disease can be cured by IMRT alone (15). However, due to the hidden anatomical location of the nasopharynx and atypical early symptoms, >70% of patients with NPC are at stage III or IV at the time of initial diagnosis (16). Approximately 20-30% of patients with advanced NPC still fail treatment, mainly because of recurrence and/or metastasis (R/M) (17).
Figure 1.
Pathogenesis of nasopharyngeal carcinoma. Genetically susceptible nasopharyngeal epithelial cells undergo malignant transformation upon acquisition of persistent latent Epstein-Barr virus infection and exposure to environmental carcinogens, which enable cellular transformation and clonal expansion. The figure was created with BioRender. LMP1/2, latent membrane protein 1/2; BARF1, BamH1-A right frame 1; EBNA, Epstein-Barr nuclear antigen 1; MHC, major histocompatibility complex.
Conventional salvage treatments for R/M NPC include radiotherapy, chemotherapy and surgery (Fig. 2), but none are satisfactory. Current NCCN Head and Neck Cancer Clinical Practice Guidelines® (https://www.nccn.org/) recommend gemcitabine plus cisplatin (GP) (Category 1 recommendation) as the preferred first-line systemic treatment for R/M NPC. However, the prognosis of these patients remains suboptimal, with a median overall survival (OS) of ~20-30 months (18,19). Furthermore, the outcome of patients with advanced recurrent disease who undergo salvage IMRT is poor, with a 5-year survival rate of ~30%, and radiation-induced injuries are also serious, with 46-70% of all deaths being due to severe radiotherapy sequelae (20,21). Therefore, R/M has become a challenge and research priority in the clinical management of NPC. Novel treatment strategies are desperately needed to improve the prognosis of patients with advanced NPC.
Figure 2.
Treatment for NPC. Conventional treatment for nasopharyngeal carcinoma includes radiotherapy, chemotherapy, surgery and targeted therapy. Immunotherapy has emerged as a new strategy for the treatment of advanced NPC in recent years. The figure was created with BioRender. NPC, nasopharyngeal carcinoma.
In recent years, immunotherapy has sparked a revolution in the clinical management of cancer. Immunotherapy activates the immune response in the tumor microenvironment by altering the biological properties of immune effector cells, thereby inhibiting or killing cancer cells (22). At present, immunotherapy has emerged as a promising treatment modality for R/M NPC. An increasing number of clinical studies have investigated the safety and efficacy of immunotherapy for advanced NPC and have shown considerable progress. In general, immunotherapy for NPC may be divided into two major approaches: i) Active immunotherapy, which relies on the ability of the patient's immune system to mount a specific immune response to tumor-associated antigens and includes immune checkpoint inhibitors (ICIs), vaccines and immunomodulators; and ii) passive immunotherapy, which uses exogenous lymphocytes or antibodies to mediate immune responses and immune stimulation, such as adoptive cell transfer (ACT) and EBV-specific monoclonal antibodies (Fig. 3). In the present article, the available clinical evidence on different immunotherapeutic approaches for NPC was reviewed and summarized to provide insight for future investigation.
Figure 3.
Classification of immunotherapy for NPC. NPC, nasopharyngeal carcinoma; EBV, Epstein-Barr virus; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; LAG-3, Lymphocyte activation gene-3; TIM-3, T-cell immunoglobulin- and mucin-domain-containing molecule-3; CTL, cytotoxic T lymphocytes; TIL, tumor-infiltrating lymphocytes; CIK, cytokine-induced killer; NK, natural killer; CAR-T, chimeric antigen receptor-modified T; TCR-T, T-cell receptor-engineered T.
2. Rationale of immunotherapy for NPC
Studies have indicated that NPC is primarily suitable for immunotherapy due to the following reasons: The expression of EBV antigens and CD4+/CD8+ T-cell target proteins (23,24), massive lymphocytic infiltration (25), programmed death ligand-1 (PD-L1) expression of up to 89-95% (26) and the presence of several key immune molecules (CD40, CD70, CD80 and CD86) that regulate T-cell activation (27). In China, >95% of NPCs are undifferentiated nonkeratinizing carcinomas and are widely considered to be associated with EBV infection (12). EBV exists in a type II latency state and has an important role in inducing NPC development (Fig. 1) (1). EBV-infected nasopharyngeal epithelial cells usually express EBV antigens, including Epstein-Barr nuclear antigen 1 (EBNA1), latent membrane protein 1/2 (LMP1/2) and BamH1-A right frame 1 (BARF1) (28). EBNA1 induces the transformation of nasopharyngeal epithelial cells into NPC cells and is associated with invasion and metastasis of NPC (29). EBNA1 is a major target of CD4+ T cells (30). LMP1 promotes cancer cell growth during NPC development and promotes NPC cells to interact with surrounding stromal cells and induce invasion, angiogenesis and immune regulation (31). LMP1 is detected in 100% of preinvasive NPC and ~50% of advanced diseases (32). LMP2 contains substantial CD8+ T-cell epitopes and is therefore considered to be a major target for CD8+ T cells (33).
NPC is characterized by numerous immune infiltrates in the primary tumor, including T cells, B cells, dendritic cells (DCs), monocytes and eosinophils (34). However, due to the immunosuppressive tumor microenvironment, NPC cells can continue to proliferate (35). Data from whole-exome sequencing and single-cell sequencing studies have gradually revealed the immune profile of NPC (36,37). The immunosuppressive tumor microenvironment in NPC is infiltrated by dysfunctional and depleted CD8+ T cells and effector T cells that overexpress suppressive immune checkpoint signaling molecules, such as programmed cell death protein-1 (PD-1) or its ligand PD-L1, as well as various chemokines and cytokines. The depletion and weakness of immune cells allow NPC cells to evade immune clearance by the host.
3. Active immunotherapy
ICIs
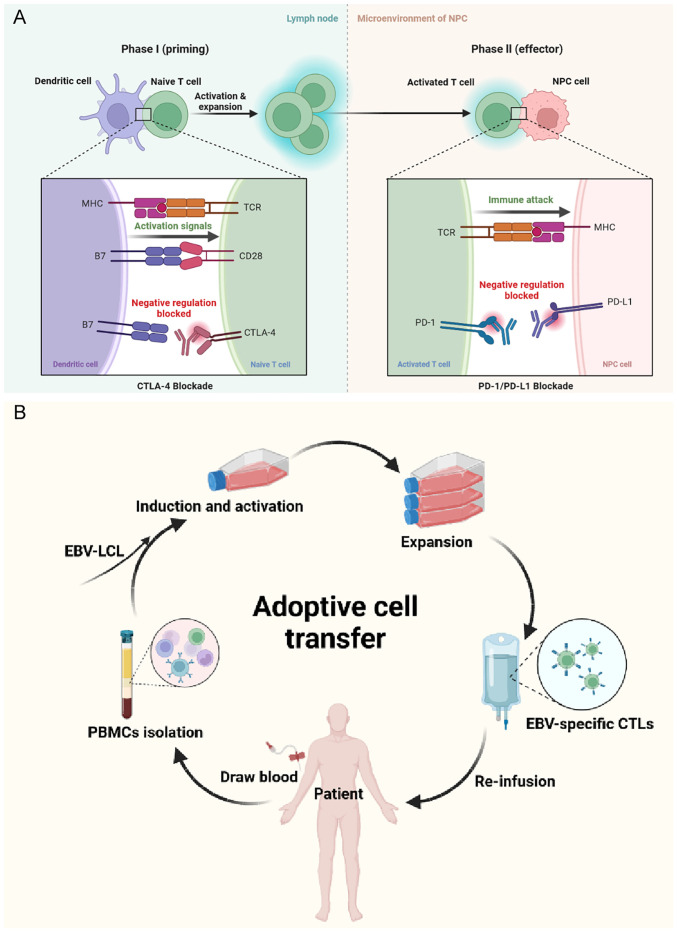
Based on current clinical research evidence, the most promising approach to activate therapeutic antitumor immunity in NPC is ICIs. Immune checkpoints refer to a plethora of inhibitory pathways in the immune system. They are essential for maintaining self-tolerance and regulating the duration and magnitude of physiological immune responses in peripheral tissues (38). However, cancer cells frequently evade immunity by suppressing immune responses through immune checkpoints. In NPC, immune checkpoints that have been extensively studied include PD-1/PD-L1 and cytotoxic T-lymphocyte (CTL)-associated antigen-4 (CTLA-4). ICIs acting on these targets can effectively inhibit the binding of immunosuppressive signals to the corresponding ligands, thereby blocking the transduction of immunosuppressive signals, weakening the negative immune regulation in the tumor microenvironment, relieving the suppressive state of T cells and preventing immune escape (Fig. 4A).
Figure 4.
Mechanism of (A) immune checkpoint inhibitors (CTLA-4, PD-1 and PD-L1 inhibitors) and (B) adoptive cell transfer of cytotoxic T lymphocytes. The figure was created with BioRender. MHC, major histocompatibility complex; TCR, T-cell receptor; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; EBV, Epstein-Barr virus; LCL, lymphoblastoid B-cell line; CTLs, cytotoxic T-lymphocytes; PBMCs, peripheral blood mononuclear cells.
Anti-PD-1/PD-L1 antibody
PD-1, a member of the CD28 superfamily, is an inhibitory receptor expressed on the surface of antigen-activated T cells. PD-1 has a vital role in limiting normal host adaptive immunity and preventing autoimmune and autoinflammatory responses (39,40). PD-L1 is generally expressed on the surface of antigen-presenting cells and cancer cells (41). The PD-1/PD-L1 interactions have a leading role in the inhibition of T-cell responses in vivo, particularly in the tumor microenvironment (38,41-43). The results of published clinical trials of PD-1/PD-L1 inhibitors for R/M NPC are summarized in Table I.
Table I.
Published clinical trials of PD-1/PD-L1 inhibitors in recurrence and/or metastatic nasopharyngeal carcinoma.
| Setting/trial identifier | Phase | Treatment | Sample size | ORR, % | Median PFS, months | Median OS, months | Grade ≥3 TRAEs, % |
|---|---|---|---|---|---|---|---|
| Third- or later-line | |||||||
| NCT02054806 (KEYNOTE-028) | Ib | Pembrolizumab | 27 | 25.9 | 6.5 | 16.5 | 29.6 |
| NCT02339558 (NCI-9742) | II | Nivolumab | 44 | 20.5 | 2.8 | 17.1 | 22.0 |
| NCT03558191 (CAPTAIN) | I | Camrelizumab | 156 | 28.2 | 3.7 | 17.4 | 15.4 |
| Second-line | |||||||
| NCT02721589 | I | Camrelizumab | 93 | 34.0 | 5.6 | NA | 16.0 |
| NCT02593786 (CheckMate 077) | I/II | Nivolumab | 32 | 12.5 | 3.5 | NR | 3.1 |
| CTR20160872 | I/II | Tislelizumab | 21 | 42.9 | 10.4 | NR | NA |
| NCT02915432 (POLARIS-02) | II | Toripalimab | 190 | 20.5 | 1.9 | 17.4 | 14.2 |
| NCT02605967 | II | Spartalizumab | 122 | 17.1 | 1.9 | 25.2 | 16.8 |
| NCT04586088 | II | Camrelizumab+apatinib | 58 | 65.5 | 10.4 | NR | 58.6 |
| First-line | |||||||
| NCT03121716 | I | Camrelizumab+GP | 24 | 91.0 | NR | NA | 87.0 |
| NCT03707509 (CAPTAIN-1st) | III | Camrelizumab+GP | 263 | 87.3 | 9.7 | Immature | 94.0 |
| NCT03581786 (JUPITER-02) | III | Toripalimab+GP | 289 | 77.4 | 11.7 | Immature | 89.0 |
PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; TRAE, treatment-related adverse effect; NA, not available; NR, not reached; GP, gemcitabine and cisplatin.
Third- or later-line treatment for R/M NPC: Anti-PD-1/PD-L1 antibody monotherapy
The landmark KEYNOTE-028 study (44) was a nonrandomized, multi-cohort, phase Ib trial investigating the efficacy and safety of pembrolizumab in 27 patients with NPC. Key eligibility criteria included unresectable or metastatic disease, failure of prior standard therapy and PD-L1 expression in 1% or more of cancer cells or tumor-infiltrating lymphocytes (TILs). During a median follow-up of 20 months, the objective response rate (ORR) was 25.9% and the median progression-free survival (PFS) and median OS were 6.5 and 16.5 months, respectively. PD-L1 expression was associated with treatment outcome. Treatment-related adverse events (TRAEs) occurred in 20 patients (74.1%) and 8 patients (29.6%) experienced grade ≥3 TRAEs. Pembrolizumab is the first anti-PD-1 antibody approved by the US Food and Drug Administration (FDA). A randomized phase III study (NCT02611960) of pembrolizumab monotherapy vs. standard chemotherapy in patients with platinum-pretreated R/M NPC (Keynote-122) is ongoing.
The NCI-9742 study (45) was an international, multicenter, phase II study exploring the antitumor activity of nivolumab monotherapy in patients with multiple pretreated R/M NPC. In all 44 patients, the overall ORR was 20.5%, with median PFS and median OS of 2.8 and 17.1 months, respectively. The survival rate was not associated with PD-L1 expression or plasma EBV-DNA clearance. Grade ≥3 TRAEs occurred in 22% of patients.
A phase II clinical study (CAPTAIN study) was conducted to investigate the antitumor activity and predictive biomarkers of camrelizumab (SHR-1210) in NPC (46). A total of 156 patients with R/M NPC who had failed at least two lines of chemotherapy were enrolled. The ORR was 28.2%. The median duration of response (DoR) was not reached. The median PFS and OS were 3.7 and 17.4 months, respectively. Grade ≥3 TRAEs were reported in 24 patients (15.4%). The combination of both major histocompatibility complex (MHC)-II-positive cell density and PD-L1 expression may allow for better patient selection.
The above studies suggest that anti-PD-1/PD-L1 antibody monotherapy has clinically meaningful antitumor activity as a third- or later-line treatment in patients with R/M NPC. Similar studies are being conducted using bintrafusp alfa [a bifunctional fusion protein targeting transforming growth factor-β (TGF-β) and PD-L1] (NCT04396886) and penpulimab (AK105, anti-PD-1 antibody) (NCT03866967).
Exploration of second-line treatment for R/M NPC
Fang et al (47) reported the safety and preliminary antitumor activity of camrelizumab alone as a second-line treatment for patients with R/M NPC. In this trial, 15 (16%) of 93 patients had grade 3 or 4 TRAEs. With a median follow-up of 9.9 months, 31 (34%) of 91 evaluable patients had overall remission and the median PFS was 5.6 months.
In addition, Ma et al (48) conducted a phase I/II study of nivolumab in Chinese patients with previously treated R/M NPC. Grade ≥3 TRAEs were reported in 1 (3.1%) of all 32 patients. The ORR was 12.5% and the disease control rate (DCR) was 65.6%. The median follow-up duration was 7.5 months. The median PFS was 3.5 months and the median OS was not reached.
An open-label, non-comparative, phase I/II study was designed to examine the safety, tolerability and antitumor activity of tislelizumab in adult Chinese patients with advanced solid tumors (49). The ORR of the 21 patients with NPC was 42.9%, with a median DoR of 8.3 months and a median follow-up of 4.8 months. The median PFS was 10.4 months and the median OS had not been reached. Response to tislelizumab was seen in multiple tumor types, including NPC, regardless of PD-L1 expression.
The POLARIS-02 study (50) was a single-arm, multicenter, phase II study evaluating the antitumor activity, safety and biomarkers of toripalimab in standard chemotherapy-refractory R/M NPC. Grade 3-5 TRAEs occurred in 27 (14.2%) of all 190 patients. The ORR was 20.5%, the median DoR was 12.8 months, the median PFS was 1.9 months and the median OS was 17.4 months. The ORR was higher in the PD-L1-positive group (27.1%). However, the ORR in the PD-L1-negative group was also close to 20% (19.4%), suggesting that PD-L1-negative individuals could also benefit from toripalimab. In addition, patients with a baseline EBV-DNA titer <10,000 IU/ml, baseline lactate dehydrogenase level ≤2 upper limits of normal and PD-L1 positivity were more likely to benefit from toripalimab. Early plasma EBV-DNA copy number reduction was associated with a favorable response.
A phase II randomized study was conducted to assess the PFS of spartalizumab (PDR001, an anti-PD-1 antibody) vs. chemotherapy in 122 patients with nonkeratinizing R/M NPC who progressed on/after platinum-based chemotherapy (51). Eligible patients were randomly allocated to the spartalizumab or chemotherapy arm at a 2:1 ratio. Fewer grade 3/4 TRAEs occurred in the spartalizumab group than in the chemotherapy arm (16.8 vs. 41.0%). However, spartalizumab did not improve PFS or OS compared to chemotherapy (median PFS, 1.9 vs. 6.6 months, P=0.915; median OS, 25.2 vs. 15.5 months, P=0.138). The ORRs were 17.1 and 35.0% in the spartalizumab and chemotherapy groups, respectively. The median DoR was longer in the spartalizumab group than in the chemotherapy group (10.2 vs. 5. months), suggesting an initial durable antitumor effect of spartalizumab in NPC. However, these results were not conclusive, as the study was not sufficiently powered to assess the significance of these endpoints.
Recently, Ding et al (52) reported the results of an open-label, single-arm, phase II study investigating the activity and safety of camrelizumab in combination with apatinib in 58 patients with R/M NPC who had failed first-line therapy. The DCR was 86.2% with an ORR of 65.5% (80.0% in patients with locoregional recurrence vs. 54.5% in patients with metastatic lesions). The median PFS was 10.4 months. Nasopharyngeal necrosis was the most common and important cause of apatinib discontinuation and was significantly associated with nasopharyngeal recurrence and reirradiation. These findings suggest that camrelizumab combined with apatinib showed promising antitumor activity and manageable toxicity. Immunotherapy combined with antiangiogenesis therapy may be a promising immunotherapy combination strategy for R/M NPC.
First-line treatment for R/M NPC: GP plus anti-PD-1/PD-L1 antibody
Fang et al (47) conducted a phase I trial to investigate the safety and preliminary antitumor activity of camrelizumab in combination with gemcitabine and cisplatin as first-line therapy for R/M patients with NPC. In this trial, 20 of 23 patients (87%) had grade 3 or 4 TRAEs. With a median follow-up time of 10.2 months, 20 of 22 evaluable patients (91%) had overall remission and 22 patients (100%) achieved disease control. The median time to respond was 1.6 months. The median PFS was not reached at the time of data cutoff. Based on the success of this previous trial, they conducted a multicenter, randomized, double-blind, phase III trial (CAPTAIN-1st) comparing camrelizumab with placebo in combination with GP as first-line treatment in 263 patients with R/M NPC (53). The results indicated that the median PFS was significantly longer in the camrelizumab group than in the placebo group (9.7 vs. 6.9 months, P=0.0002). Serious TRAEs were reported in 59 (44%) of 134 patients in the camrelizumab group compared with 48 (37%) of 129 patients in the placebo group.
JUPITER-02 (54) is a multicenter randomized phase III trial designed to investigate the antitumor activity and safety of toripalimab or placebo in combination with GP as first-line treatment for patients with NPC. In the study, 289 patients with R/M NPC and no previous chemotherapy for R/M disease were enrolled. The median PFS was significantly improved in the toripalimab group compared to the placebo group (11.7 vs. 8.0 months, P=0.0003), particularly in the PD-L1-positive subgroup. The risk of death was reduced by 40% in the toripalimab group compared to the placebo group. The ORR was significantly higher in the toripalimab group than in the placebo group (77.4 vs. 66.4%, P=0.0335). The incidence of grade ≥3 TRAEs was similar between the toripalimab and placebo groups (89.0 vs. 89.5%).
The above-mentioned studies suggest that GP plus anti-PD-1 antibody may be a promising standard of care for patients with R/M NPC in the first-line setting. Similar studies are being conducted using nivolumab (NCT04458909) and tislelizumab (anti-PD-1 antibody) (NCT03924986).
Treatment for unresectable locally recurrent NPC
Hua et al (55) conducted a single-arm phase II trial to investigate the efficacy and safety of toripalimab in combination with IMRT for unresectable locally recurrent NPC. A total of 25 patients were included. With a median follow-up duration of 14.6 months, 19 patients (79.2%) achieved overall remission and 23 patients (95.8%) achieved disease control at 3 months post-IMRT. The 12-month PFS was as high as 91.8%. Grade ≥3 late TRAEs were nasopharyngeal wall necrosis (28.0%), nasal bleeding (12.0%) and trismus (4.0%). Due to the small sample size and lack of a control group, larger randomized controlled trials are needed to confirm the results.
Zhou et al (56) enrolled 28 patients with unresectable locally recurrent NPC who had progressed after second-line chemotherapy. They found that ICIs alone or combined with chemotherapy showed promising antitumor activity with low toxicity. However, chemotherapy plus ICIs did not improve complete response or partial response compared to ICIs alone.
Anti-PD-1/PD-L1 antibodies in locoregionally advanced NPC (LANPC)
Clinical trials on the addition of anti-PD-1/PD-L1 antibodies to chemoradiotherapy are being conducted in different settings of LANPC at initial diagnosis. Four phase III trials are evaluating camrelizumab, sintilimab or toripalimab in combination with induction chemotherapy, concurrent chemoradiotherapy (CCRT), or as maintenance after radical treatment (NCT03427827, NCT04453826, NCT03700476 and NCT04557020, respectively). The results of these different clinical trials may identify an optimal multidisciplinary management approach in the era of immunotherapy for NPC. Breakthroughs in the field of LANPC by ICIs are to be expected.
Anti-CTLA-4 antibody
CTLA-4 is a secondary receptor of B7 (CD80 and CD86) and is homologous to the CD28 receptor. However, CTLA-4 transmits different costimulatory signals from CD28. During T-cell activation, the most important function of CTLA-4 is to compete with CD28 for binding to CD80/CD86 molecules on the surface of antigen-presenting cells, thereby negatively regulating T-cell activation signals (57,58). Cancer cells can activate CTLA-4 to inactivate activated T cells, allowing immune escape (59).
To date, two CTLA-4 inhibitors, ipilimumab and tremelimumab, have been approved by the FDA for the treatment of advanced melanoma and hepatocellular carcinoma (60-62). However, reports on the application of anti-CTLA-4 anti-bodies in NPC are scarce. A case-control study showed that CTLA-4 polymorphism was associated with susceptibility to NPC (63). Ahmed et al (64) found that high CTLA-4 expression was significantly associated with disease progression and worsening OS. A phase II trial (NCT03097939) to test the hypothesis that ipilimumab in combination with nivolumab is effective in advanced NPC is ongoing. Furthermore, four phase I/II clinical trials are exploring the safety, tolerability and anti-tumor activity of AK104 (PD-1/CTLA-4 bispecific antibody), cadonilimab (PD-1/CTLA-4 bispecific antibody), IBI310 (CTLA-4 antibody) and XmAb20717 (PD-1/CTLA-4 bispecific antibody) in R/M NPC (NCT04220307, NCT05587374, NCT04945421 and NCT03517488).
Other ICIs in NPC
Lymphocyte activation gene-3 (LAG-3) and PD-1 are distinct suppressive immune checkpoints that are often coexpressed on TILs, thereby promoting tumor-mediated T-cell exhaustion and immune escape (65-67). Opdualag is the first FDA-approved antibody against LAG-3 (68), making LAG-3 the third clinical immune checkpoint after PD-1 and CTLA-4. Only one registered clinical trial of anti-LAG-3 antibody is evaluating the safety and tolerability of XmAb22841 (LAG-3/CTLA-4 bispecific antibody) plus pembrolizumab in patients with advanced solid tumors, including NPC (NCT03849469).
Another major regulatory checkpoint is T-cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM-3) (69,70). Tim-3 is a coinhibitory receptor whose expression is increased when patients develop adaptive resistance to anti-PD-1 antibodies (71). Inhibition of its ligand, galectin-9, selectively rejuvenates TILs by interfering with the interaction between PD-1 and TIM-3 (72). As galectin-9 is specifically expressed in NPC cells (73), TIM-3/galectin-9 interaction is a promising approach to overcome resistance to PD-1/PD-L1 inhibitors. Currently, only one registered clinical trial is investigating the role of anti-TIM-3 antibodies in NPC, a phase II trial evaluating the efficacy and safety of TQB2618 (anti-TIM-3 antibody) in combination with penpulimab in patients with R/M NPC (NCT05563480).
In general, the ongoing exploration of ICIs has focused on investigating the long-term effects of these agents on the toxicity of chemotherapy and/or radiotherapy, the optimal sequence of chemotherapy and/or radiotherapy, the optimal duration of treatment, the role of predictive biomarkers in selecting subgroups of patients more likely to benefit from treatment, and the cost-effectiveness of these ICIs in routine practice (1). It is reasonable to assume that combining different ICIs may be a more effective treatment option for patients with R/M NPC (74).
Vaccines
Cancer vaccines are active immunotherapies that use cancer cells or tumor antigenic substances to activate the immune system and induce a specific immune response. Currently, NPC vaccines are mainly divided into prophylactic vaccines and therapeutic vaccines.
Prophylactic vaccines
Five membrane proteins (gp350, gH, gL, gB and gp42) are needed to infect B cells with EBV, whereas four membrane proteins (BMFR2, gH, gL and gB) are needed to infect epithelial cells with EBV (Fig. 1). These proteins are expressed in EBV and may be good targets for EBV-preventive vaccines (75-77). Cui et al (78-80) indicated that serum EBV-neutralizing titers were higher in rabbits inoculated with trimeric and monomeric EBV gH/gL, trimeric gB and tetrameric gp350(1-470) than in those inoculated with monomeric gp350 (1-470). EBV gH/gL and gB may be better targets for EBV-preventive vaccines than gp350 alone and the combination of gp350 with gH/gL and gB may yield a more potent EBV-preventive vaccine. These data strongly suggest that the combination of the EBV core fusion mechanism envelope protein gH/gL and trimeric gB is a promising EBV prophylactic vaccine.
Therapeutic vaccines
Therapeutic vaccines for NPC mainly target EBNA1, LMP1 or LMP2 (81). One approach is to inject DCs containing EBV antigens. Lin et al (33) and Li et al (82) demonstrated that DC vaccines induced or promoted specific T-cell responses and were well tolerated in patients with NPC. Chia et al (83) conducted a phase II study to evaluate the safety and efficacy of an adenovirus-ΔLMP1-LMP2 transduced DC vaccine in patients with metastatic NPC. They found clinical responses in only 3 of 16 patients. The other strategy is an injection of viral vectors containing EBV antigens. Two phase I trials (84,85) confirmed the safety and immunogenicity in NPC of the recombinant vaccinia virus MVA-EL, which encodes an EBNA1/LMP2 fusion protein. A phase I trial (NCT01800071) and a phase II trial (NCT01094405) are ongoing to further evaluate the efficacy and duration of the immune response to the MVA-EBNA1/LMP2 vaccine in NPC. An ongoing phase I study (NCT03282617) is recruiting patients with advanced NPC who will receive a DC vaccine called CD137L-DC-EBV-VAX.
In general, despite the progress with EBV vaccines, DC-based vaccines have few targets and are costly to prepare, while recombinant viral vectors, although having a broad range of epitopes, may yield reduced immune function after repeated immunization. Of note, different routes of administration should be systematically examined to facilitate EBV vaccination. Future EBV vaccines containing gp350, gH/gL, gB EBNA1 and LMP1/2 are expected to be the direction of EBV vaccine research.
Immunomodulators
Immunomodulators are nonspecific and include mainly cytokines and oncolytic viruses. Cytokine therapy may alter the concentration of cytokines in the immune microenvironment and enhance the antitumor immune response. A study evaluated the multifunctional antitumor effects of the cytokine recombinant interferon-α1b (IFN-α1b) (86). Results from in vitro and in vivo experiments showed that IFN-α1b inhibited cell growth, promoted apoptosis and necrosis, suppressed tumor growth and metastasis, reduced intratumor microvascular density and prolonged survival. Oncolytic viruses are a class of viruses that replicate in cancer cells through different regulatory mechanisms and lyse cancer cells without affecting normal cell proliferation. T-VEC, the first oncolytic viral treatment approved by the US FDA, was shown to be effective in advanced melanoma (87). Liu et al (88) found that an oncolytic adenovirus enhanced antiangiogenic and antitumor effects by rescuing the selective replication of replication-deficient adenovirus encoding endothelial repressor in NPC cells. A third-generation oncolytic virus, G47Δ (HSV-1), demonstrated a reliable antitumor effect in NPC in vitro and in vivo (89). With the development of genetic engineering technology and virology, oncolytic viruses are expected to become an important tool for comprehensive tumor therapy.
4. Passive immunotherapy
ACT
As viral proteins are almost exclusively expressed in cancer cells, ACT that targets EBV antigens holds therapeutic promise in the treatment of NPC. ACT is a passive immunotherapy that mainly adopts CTLs, TILs, cytokine-induced killer (CIK) cells and natural killer (NK) cells. The corresponding active immune cells are isolated from patients, screened and expanded in vitro to induce highly specific antitumor immune cells, which are then injected back into the patient to kill cancer cells (Fig. 4B). Genetically modified cellular immunotherapy is also a form of ACT and includes chimeric antigen receptor-modified T (CAR-T) cell therapy, T-cell receptor-engineered T (TCR-T) cell therapy and other cellular immunotherapies.
CTLs
Numerous clinical studies (90-96) have confirmed the feasibility, efficacy and safety of EBV-CTL immunotherapy for refractory NPC (Table II). In addition, Louis et al (97) and Secondino et al (98) found that the clinical benefit of EBV-CTLs was not improved by prior administration of lymphodepleting chemotherapy in patients with R/M NPC. The adenovirus-based vector AdE-LMPpoly, which encodes CTL epitopes fused from LMP1 and LMP2 to truncated EBNA1, has been used to generate CTLs for ACT. Smith et al (99,100) showed that ACT in combination with AdE1-LMPpoly was safe and well tolerated and may provide a clinical benefit in patients with R/M NPC. A phase I/II study (101) found that 2 patients with R/M NPC unexpectedly showed a strong response to their previously failed chemotherapy regimens after failing EBV-CTL immunotherapy. This finding suggests that EBV-CTL immunotherapy may contribute to chemosensitizing the tumor and prime the patient's immune system. Of note, Chia et al (102) showed that a combination chemotherapy and EBV-CTL immunotherapy regimen for patients with R/M NPC improved the response rates by up to 71.4%. A multi-center phase III randomized controlled trial is ongoing to evaluate gemcitabine and carboplatin followed by EBV-CTL immunotherapy (NCT02578641). Several novel strategies to increase CTL activity in NPC are being explored in clinical trials, such as LMP-, BARF1- and EBNA1-specific CTLs (NCT02287311), most closely HLA-matched allogeneic CTLs (NCT01447056) and TGF-β-resistant CTLs (NCT02065362).
Table II.
Published clinical trials on adoptive EBV-CTL immunotherapy for NPC.
| Author, year | Design | Treatment | Sample size | Condition | Efficacy | Safety | (Refs.) |
|---|---|---|---|---|---|---|---|
| Chua, 2001 | Pilot | EBV-specific CTLs | 4 | R/M EBV+ NPC | 3 EBV burden decrease; 3 died of PD (9-21 months after EBV-CTLs) | No ≥G3 toxicity | (90) |
| Comoli, 2004 | Preliminary | EBV-specific CTLs | 1 | Relapsed EBV+ NPC | SD | Temporary worsening of local tumor symptoms | (91) |
| Straathof, 2005 | Phase I | EBV-specific CTLs | 10 | Stage III/IV EBV+ NPC in remission or with R/R history | 4 pts with previous remission remain disease-free, DFS: 19-27 months; 6 pts with previous R/R NPC: 2 CR; 1 PR; 1 SD; 2 no response | 1 pts: Tumor swelling requiring tracheostomy | (92) |
| Comoli, 2005 | Phase I | EBV-specific CTLs | 10 | Stage IV EBV+ NPC | 2 PR; 4 SD; 4 PD | 2 pts: Inflammatory reaction at tumor sites | (93) |
| Louis, 2010 | Phase I/II | EBV-specific CTLs | 23 | R/R EBV+ NPC | 5 remain in remission; 3 recurrent disease; 3 CR; 2 CRu; 2 PR; 3 SD; 5 PD | 1 pts: Tumor swelling requiring tracheostomyp | (94) |
| Lutzky, 2014 | Preliminary | EBV-specific CTLs | 1 | R/M EBV+ NPC | PR | No ≥G3 toxicity | (95) |
| Eom, 2016 | Phase I | EBV/LMP2A-specific CD8+ T cells (EBViNT) | 4 | R/R EBV+ NPC | 3 PD at week 4; 1 SD at week 4, but PD at week 8 | No ≥G3 toxicity | (96) |
| Louis, 2009 | Phase I | Anti-CD45 mAb followed by EBV-specific CTLs | 8 | R/M EBV+ NPC | 1 CR; 2 SD; 5 PD | 1 pts: Transient lymph node swelling | (97) |
| Secondino, 2012 | Phase II | Fludarabine-cyclophosphamide followed by EBV-specific CTLs | 11 | Stage IV, EBV+ NPC | 2 PR; 4 SD; 5 PD | 4 pts: G3 neutropenia; 2 pts: Tumor swelling | (98) |
| Smith, 2012 | Phase I | EBV-specific CTLs | 14 | R/M EBV+ NPC | 10 SD; mPFS: 4.5 months; median OS: 17.4 months | No ≥G3 toxicity | (99) |
| Smith, 2017 | Phase II | AdE1-LMPpoly vector-based CTLs | 29 | 9 pts: no or minimal residual NPC (N/MRD) 20 pts: active R/M NPC (ARMD) | N/MRD: 6 maintain response; 3 PD; ARMD: 12 SD; 8 PD; All: mPFS: 5.5 months; median OS: 38.1 months | 2 pts: G3 lung abscess | (100) |
| Huang, 2017 | Phase I/II | EBV-specific CTLs | 21 | R/M EBV+ NPC | 1 CR; 2 SD; 18 PD; (mPFS: 2.2 months; median OS: 16.7 months) | No ≥G3 toxicity | (101) |
| Chia, 2014 | Phase II | Gemcitabine-carboplatin followed by EBV-specific CTLs | 38 | R/M EBV+ NPC | 3 CR; 22 PR; 11 SD; 1 PD; 1 NA; (3-year OS 37.1%) | No ≥G3 toxicity | (102) |
NPC, nasopharyngeal carcinoma; EBV, Epstein-Barr virus; CTL, cytotoxic T-lymphocytes; LMP, latent membrane protein; mAb, monoclonal antibody; R/M, recurrent/metastatic; R/R, relapsed/refractory; pts, patients; PD, progressive disease; SD, static disease; CR, complete response; PR, partial response; CRu, unconfirmed complete response; NA, not assessed; DFS, disease-free survival; OS, overall survival; mPFS, median progression-free survival; G3, grade 3.
TILs
A retrospective study (103) found that low CD3+ TIL infiltration was highly associated with shorter disease-free survival (DFS) and OS in patients with R/M NPC. Li et al (104) evaluated the safety and antitumor activity of TIL immunotherapy after CCRT in 20 patients with LANPC. They found that 19 patients exhibited objective antitumor responses and 18 patients had a DFS of >12 months. This study suggests that TIL immunotherapy after CCRT produces sustained antitumor activity and anti-EBV immune responses. A phase II trial of TILs following CCRT in patients with LANPC (NCT02421640) is underway.
CIK cells
CIK cells are in vitro expanded and activated T-lymphocytes obtained by the sequential incubation of peripheral blood mononuclear cells with various cytokines (105). Li et al (106,107) demonstrated that sequential CIK immunotherapy was effective in improving the therapeutic efficacy of GP in patients with metastatic NPC. A phase II trial is underway to investigate the efficacy of DC-CIK immunotherapy in the treatment of solid tumors, including NPC (NCT04476641).
NK cells
Lim et al (108) conducted a phase I study of expanded NK cells in combination with cetuximab for R/M NPC. The results indicated that cetuximab combined with autologous NK cells was well tolerated in NPC. Promising results were observed in 3 of the 7 subjects who demonstrated durable stable disease. Another forthcoming clinical trial also used highly active NK cells in combination with cetuximab in refractory NPC (NCT03007836).
CAR-T cells
One of the most promising approaches in antitumor therapy is CAR-T-cell therapy, where T cells are redirected against tumors after the engineered expression of CARs (109). CAR-T cells are not restricted by MHC and can directly recognize tumor cell surface antigens. To date, six CAR-T-cell products have been approved by the FDA for use in patients with relapsed and/or refractory B-cell malignancies (110-115). However, their effects on solid tumors have not been satisfactory. The low expression rates of immunosuppressive factors in the tumor microenvironment and tumor-associated antigens on the surface of solid tumor cells and the intracellular nature of most cellular proteins have limited the clinical application of CAR-T-cell therapy in solid tumors. Studies have confirmed that the main barriers to CAR-T-cell product development are related to antigen escape and intrinsic T-cell dysfunction (116). In NPC, Guo et al (117) designed a CAR construct that targets the oncofetal antigen 5T4 (5T4-28Z) and generated CAR-transduced CIK cells. The results indicated that 5T4-28Z-CIK cells were able to efficiently attack NPC cells in vitro, suggesting that they may be an attractive tool for developing an efficient therapy for NPC. Chen et al (118) successfully produced LMP2A CAR-T cells that exhibited marked targeted cytotoxicity against LMP2A-positive NPC cells. Tang et al (119,120) demonstrated that LMP1-specific CAR-T cells mediated antitumor effects against LMP1-positive NPC cells in vitro and in vivo. These results are encouraging. At least four registered clinical studies of CAR-T cells for the treatment of NPC are currently in progress (Table III).
Table III.
Clinical trials of CAR-T/TCR-T cell therapy in NPC.
| Trial identifier | Phase | Treatment | Condition | Estimated enrollment | Completion date | Status | Location |
|---|---|---|---|---|---|---|---|
| NCT02980315 | Phase I/II | LMP1-CAR-T cells | EBV+ NPC | 20 | December 2017 | Unknown | China |
| NCT05239143 | Phase I | P-MUC1C-ALLO1 CAR-T cells |
Advanced or metastatic solid tumors including NPC | 100 | April 2039 | Recruiting | United States |
| NCT04107142 | Phase I | Haplo/allogeneic NKG2DL-targeting chimeric antigen receptor-grafted γδ T-cells | R/R solid tumor including NPC | 10 | March 2021 | Unknown | Malaysia |
| NCT05587543 | Early phase I | CAR-T/TCR-T cells | R/R EBV+ NPC | 24 | October 2030 | Not yet recruiting | China |
| NCT03648697 | Phase II | EBV-TCR-T (YT-E001) cells | R/M EBV+ NPC | 20 | October 2021 | Unknown | China |
| NCT04509726 | Phase I/II | EBV-specific TCR-T cell with cytokine auto-secreting element | R/M EBV+ NPC | 20 | August 2023 | Recruiting | China |
| NCT03925896 | Phase I | LMP2 antigen-specific TCR-T cells | R/M EBV+ NPC | 27 | August 2022 | Unknown | China |
CAR-T, chimeric antigen receptor-modified T; TCR-T, T cell receptor-engineered T; NPC, nasopharyngeal carcinoma; EBV, Epstein-Barr virus; LMP, latent membrane protein; MUC1C, mucin1 cell surface-associated C-terminal; NKG2DL, natural killer group 2D ligand; R/R, relapsed/refractory; R/M, recurrent/metastatic.
TCR-T cells
Unlike CAR T-cell therapy, TCR T-cell therapy has shown encouraging potential in the treatment of solid tumors (121,122). TCR T cells have distinct advantages over CAR T cells in the treatment of solid tumors (123): i) TCR T cells can recognize antigens expressed on the cell surface and in intracellular compartments; ii) TCR T cells are more capable of invading cancer cells; iii) the intracellular proteins targeted by TCR T cells are tumor-specific; and iv) the neurotoxicity of TCR T-cell therapy is less severe than that of CAR T-cell therapy. Based on excellent efficacy data from a randomized phase III trial (124), the FDA approved tebentafusp for patients with HLA-A*02:01-positive metastatic uveal melanoma. Four ongoing clinical trials are evaluating TCR T-cell therapy for the treatment of NPC (Table III). An early phase I trial was designed to investigate the safety and efficacy of EBV CAR T/TCR T cells in the treatment of recurrent/refractory EBV-positive NPC (NCT05587543). A phase I/II study (NCT03648697) was conducted to evaluate the safety and tolerability of EBV-TCR-T (YT-E001) in patients with EBV-positive R/M NPC. Two phase I/II clinical trials are investigating the role of LMP2-specific TCR T-cells in EBV-positive R/M NPC (NCT04509726, NCT03925896).
Other emerging cellular immunotherapy strategies, such as CAR-NK and CAR-macrophage immunotherapy, should be evaluated in NPC for their promising clinical efficacy in other cancers (125,126). Overall, treatment specificity and targeting are the focus of current ACT studies and the direction of future research. Although ACT has demonstrated good effcacy with limited side effects, it is associated with technical and financial limits.
EBV-specific monoclonal antibody
Tumor therapeutic anti-bodies can target specific antigens on the surface of cancer cells and kill them through antibody-dependent cell-mediated cytotoxicity and complement-dependent cell-mediated cytotoxicity. Turrini et al (127) identified a BARF1-specific monoclonal antibody as a novel immunotherapeutic tool for EBV-associated tumors through preclinical models. Ahmed et al (128) constructed a dimeric T-cell-engaging bispecific antibody (DiBsAb) targeting LMP2A. Their results suggested that DiBsAb may be a leading candidate for the treatment of EBV-associated malignancies.
5. Conclusion
R/M remains the biggest challenge for NPC. Fortunately, NPC is an 'immune-hot' tumor and the unique immune environment of NPC provides rational targets for immunotherapy. ICIs can non-specifically activate the antitumor immune response by removing or attenuating the negative regulators of immunoreactive cells. Thus, ICIs, as pan-cancerous antitumor agents, were found to have equally promising efficacy in NPC. More research is needed to find suitable biomarkers for predicting the efficacy of ICIs as monotherapy or combination therapy to maximize personalized immunotherapy in NPC. Furthermore, based on current findings, ACT and therapeutic vaccines have shown early success in treating a small subset of patients with refractory NPC; immunomodulators and EBV-specific monoclonal antibodies against NPC remain in the exploratory phase and their clinical translation still has some way to go. Of note, EBV-specific vaccines, ACTs and monoclonal antibodies induce specific antitumor responses by directly or indirectly targeting tumor antigens. These EBV-specific agents have few targets and are costly and technically immature to produce. In addition, factors such as mutations in oncogenes, limited antigen expression, weak immunogenicity, effector cell self-dysfunction and immunosuppressive microenvironment in different populations of NPC and route of drug administration contribute to the efficacy and safety of EBV-specific immunotherapy. Finally, immunotherapy undoubtedly offers hope for patients with R/M NPC for whom no standard treatment is available. In clinical practice, certain patients with effective immunotherapy resumed immunotherapy after relapse on discontinuation and still achieved good results. Future studies may consider combination immunotherapy to enhance the clinical response, such as ICIs in combination with ACT (129) or CAR T-cell therapy in combination with an oncolytic virus (130). Therefore, the exploration of the molecular and cellular drivers of immune escape in NPC may lead to innovative therapeutic options to improve the outcomes for patients with NPC.
Acknowledgments
Not applicable.
Funding Statement
This study was supported by the National Natural Science Foundation of China-Science and Technology Development Fund, Macau SAR (grant no. 81661168011) and the Regional Innovation and Cooperation Project of Sichuan Province (grant no. 2021YFQ0037).
Availability of data and materials
Not applicable.
Authors' contributions
HH, YY and XD performed the literature review and wrote the manuscript. HH and ZH revised the figures and tables. YCC, ZW and HH revised the manuscript. HH and TL were involved in the conception of the study. All authors contributed to the article and have read and approved the submitted version. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wong KCW, Hui EP, Lo KW, Lam WKJ, Johnson D, Li L, Tao Q, Chan KCA, To KF, King AD, et al. Nasopharyngeal carcinoma: An evolving paradigm. Nat Rev Clin Oncol. 2021;18:679–695. doi: 10.1038/s41571-021-00524-x. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Renaud S, Lefebvre A, Mordon S, Moralès O, Delhem N. Novel therapies boosting T cell immunity in epstein barr virus-associated nasopharyngeal carcinoma. Int J Mol Sci. 2020;21:4292. doi: 10.3390/ijms21124292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks JE, Phillips JL, Menck HR. The National Cancer Data Base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer. 1998;83:582–588. doi: 10.1002/(SICI)1097-0142(19980801)83:3<582::AID-CNCR29>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Wang HY, Chang YL, To KF, Hwang JS, Mai HQ, Feng YF, Chang ET, Wang CP, Kam MK, Cheah SL, et al. A new prognostic histopathologic classification of nasopharyngeal carcinoma. Chin J Cancer. 2016;35:41. doi: 10.1186/s40880-016-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsao SW, Yip YL, Tsang CM, Pang PS, Lau VM, Zhang G, Lo KW. Etiological factors of nasopharyngeal carcinoma. Oral Oncol. 2014;50:330–338. doi: 10.1016/j.oraloncology.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Chang ET, Liu Q, Cai Y, Zhang Z, Chen G, Xie SH, Cao SM, Shao JY, Jia WH, et al. Oral hygiene and risk of nasopharyngeal carcinoma-A Population-based case-control study in China. Cancer Epidemiol Biomarkers Prev. 2016;25:1201–1207. doi: 10.1158/1055-9965.EPI-16-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang ET, Liu Z, Hildesheim A, Liu Q, Cai Y, Zhang Z, Chen G, Xie SH, Cao SM, Shao JY, et al. Active and passive smoking and risk of nasopharyngeal carcinoma: A population-based case-control study in Southern China. Am J Epidemiol. 2017;185:1272–1280. doi: 10.1093/aje/kwx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu M, Yao Y, Chen H, Zhang S, Cao SM, Zhang Z, Luo B, Liu Z, Li Z, Xiang T, et al. Genome sequencing analysis identifies Epstein-Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat Genet. 2019;51:1131–1136. doi: 10.1038/s41588-019-0436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo KW, Chung GT, To KF. Deciphering the molecular genetic basis of NPC through molecular, cytogenetic, and epigenetic approaches. Semin Cancer Biol. 2012;22:79–86. doi: 10.1016/j.semcancer.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Tsang CM, Lui VWY, Bruce JP, Pugh TJ, Lo KW. Translational genomics of nasopharyngeal cancer. Semin Cancer Biol. 2020;61:84–100. doi: 10.1016/j.semcancer.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Miao J, Xiao X, Hu J, Zhang G, Peng Y, Lu S, Liang Y, Huang S, Han F, et al. Impact on xerostomia for nasopharyngeal carcinoma patients treated with superficial parotid lobe-sparing intensity-modulated radiation therapy (SPLS-IMRT): A prospective phase II randomized controlled study. Radiother Oncol. 2022;175:1–9. doi: 10.1016/j.radonc.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Miao J, Zhao C, Wang L. Response to Gargi S Sarode, Sachin C Sarode, and Rahul Anand's Letter to the Editor of Radiotherapy and Oncology regarding the paper titled 'Impact on xerostomia for nasopharyngeal carcinoma patients treated with superficial parotid lobe-sparing intensity-modulated radiation therapy (SPLS-IMRT): A prospective phase II randomized controlled study' by Huang et al. Radiother Oncol. 2022;177:253. doi: 10.1016/j.radonc.2022.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Miao J, Huang H, Chen B, Xiao X, Zhu M, Liang Y, Xiao W, Huang S, Peng Y, et al. Long-term survivals, toxicities and the role of chemotherapy in Early-stage nasopharyngeal carcinoma patients treated with Intensity-modulated radiation therapy: A retrospective study with 15-year Follow-up. Cancer Res Treat. 2022;54:118–129. doi: 10.4143/crt.2021.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan JJ, Ng WT, Zong JF, Chan LL, O'Sullivan B, Lin SJ, Sze HC, Chen YB, Choi HC, Guo QJ, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122:546–558. doi: 10.1002/cncr.29795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Zhang Y, Lai SZ, Li WF, Hu WH, Sun R, Liu LZ, Zhang F, Peng H, Du XJ, et al. 10-year results of therapeutic ratio by Intensity-modulated radiotherapy versus two-dimensional radiotherapy in patients with nasopharyngeal carcinoma. Oncologist. 2019;24:e38–e45. doi: 10.1634/theoncologist.2017-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, Peng P, Wu X, Lin Q, Xi X, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: A multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388:1883–1892. doi: 10.1016/S0140-6736(16)31388-5. [DOI] [PubMed] [Google Scholar]
- 19.Hong S, Zhang Y, Yu G, Peng P, Peng J, Jia J, Wu X, Huang Y, Yang Y, Lin Q, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as First-line therapy for recurrent or metastatic nasopharyngeal carcinoma: Final overall survival analysis of GEM20110714 phase III study. J Clin Oncol. 2021;39:3273–3282. doi: 10.1200/JCO.21.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua YJ, Han F, Lu LX, Mai HQ, Guo X, Hong MH, Lu TX, Zhao C. Long-term treatment outcome of recurrent nasopharyngeal carcinoma treated with salvage intensity modulated radiotherapy. Eur J Cancer. 2012;48:3422–3428. doi: 10.1016/j.ejca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Xiao W, Liu S, Tian Y, Guan Y, Huang S, Lin C, Zhao C, Lu T, Han F. Prognostic significance of tumor volume in locally recurrent nasopharyngeal carcinoma treated with salvage intensity-modulated radiotherapy. PLoS One. 2015;10:e0125351. doi: 10.1371/journal.pone.0125351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164:1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith C, Wakisaka N, Crough T, Peet J, Yoshizaki T, Beagley L, Khanna R. Discerning regulation of cis- and trans-presentation of CD8+ T-cell epitopes by EBV-encoded oncogene LMP-1 through self-aggregation. Blood. 2009;113:6148–6152. doi: 10.1182/blood-2009-02-203687. [DOI] [PubMed] [Google Scholar]
- 24.Münz C, Bickham KL, Subklewe M, Tsang ML, Chahroudi A, Kurilla MG, Zhang D, O'Donnell M, Steinman RM. Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191:1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AZE, Tan LSY, Lim CM. Cellular-based immunotherapy in Epstein-Barr virus induced nasopharyngeal cancer. Oral Oncol. 2018;84:61–70. doi: 10.1016/j.oraloncology.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Outh-Gauer S, Alt M, Le Tourneau C, Augustin J, Broudin C, Gasne C, Denize T, Mirghani H, Fabre E, Ménard M, et al. Immunotherapy in head and neck cancers: A new challenge for immunologists, pathologists and clinicians. Cancer Treat Rev. 2018;65:54–64. doi: 10.1016/j.ctrv.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Agathanggelou A, Niedobitek G, Chen R, Nicholls J, Yin W, Young LS. Expression of immune regulatory molecules in Epstein-Barr virus-associated nasopharyngeal carcinomas with prominent lymphoid stroma. Evidence for a functional interaction between epithelial tumor cells and infiltrating lymphoid cells. Am J Pathol. 1995;147:1152–1160. [PMC free article] [PubMed] [Google Scholar]
- 28.Cui X, Snapper CM. Epstein Barr Virus: Development of vaccines and immune cell therapy for EBV-associated diseases. Front Immunol. 2021;12:734471. doi: 10.3389/fimmu.2021.734471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Tian WD, Xu X, Nie B, Lu J, Liu X, Zhang B, Dong Q, Sunwoo JB, Li G, et al. Epstein-Barr virus nuclear antigen 1 (EBNA1) protein induction of epithelial-mesenchymal transition in nasopharyngeal carcinoma cells. Cancer. 2014;120:363–372. doi: 10.1002/cncr.28418. [DOI] [PubMed] [Google Scholar]
- 30.Fu T, Voo KS, Wang RF. Critical role of EBNA1-specific CD4+ T cells in the control of mouse Burkitt lymphoma in vivo. J Clin Invest. 2004;114:542–550. doi: 10.1172/JCI22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young LS, Yap LF, Murray PG. Epstein-Barr virus: More than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16:789–802. doi: 10.1038/nrc.2016.92. [DOI] [PubMed] [Google Scholar]
- 32.Yoshizaki T, Kondo S, Endo K, Nakanishi Y, Aga M, Kobayashi E, Hirai N, Sugimoto H, Hatano M, Ueno T, et al. Modulation of the tumor microenvironment by Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma. Cancer Sci. 2018;109:272–278. doi: 10.1111/cas.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin CL, Lo WF, Lee TH, Ren Y, Hwang SL, Cheng YF, Chen CL, Chang YS, Lee SP, Rickinson AB, et al. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 2002;62:6952–6958. [PubMed] [Google Scholar]
- 34.Le QT, Colevas AD, O'Sullivan B, Lee AWM, Lee N, Ma B, Siu LL, Waldron J, Lim CM, Riaz N, et al. Current treatment landscape of nasopharyngeal carcinoma and potential trials evaluating the value of immunotherapy. J Natl Cancer Inst. 2019;111:655–663. doi: 10.1093/jnci/djz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gourzones C, Barjon C, Busson P. Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer Biol. 2012;22:127–136. doi: 10.1016/j.semcancer.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Li YY, Chung GT, Lui VW, To KF, Ma BB, Chow C, Woo JK, Yip KY, Seo J, Hui EP, et al. Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nat Commun. 2017;8:14121. doi: 10.1038/ncomms14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen YP, Yin JH, Li WF, Li HJ, Chen DP, Zhang CJ, Lv JW, Wang YQ, Li XM, Li JY, et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res. 2020;30:1024–1042. doi: 10.1038/s41422-020-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 41.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. J Clin Investigation. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, Le Tourneau C, Mehnert JM, Algazi A, van Brummelen EMJ, Saraf S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: Results of the KEYNOTE-028 Study. J Clin Oncol. 2017;35:4050–4056. doi: 10.1200/JCO.2017.73.3675. [DOI] [PubMed] [Google Scholar]
- 45.Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, Foster NR, Riess JW, Agulnik M, Chang AYC, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: An international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742) J Clin Oncol. 2018;36:1412–1418. doi: 10.1200/JCO.2017.77.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Zhou T, Chen X, Li J, Pan J, He X, Lin L, Shi YR, Feng W, Xiong J, et al. Efficacy, safety, and biomarker analysis of camrelizumab in previously treated recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN study) J Immunother Cancer. 2021;9:e003790. doi: 10.1136/jitc-2021-003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, Xiong J, Li P, Zhao H, Huang Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: Results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19:1338–1350. doi: 10.1016/S1470-2045(18)30495-9. [DOI] [PubMed] [Google Scholar]
- 48.Ma Y, Fang W, Zhang Y, Yang Y, Hong S, Zhao Y, Tendolkar A, Chen L, Xu D, Sheng J, et al. A Phase I/II Open-label study of nivolumab in previously treated advanced or recurrent nasopharyngeal carcinoma and other solid tumors. Oncologist. 2019;24:891–e431. doi: 10.1634/theoncologist.2019-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen L, Guo J, Zhang Q, Pan H, Yuan Y, Bai Y, Liu T, Zhou Q, Zhao J, Shu Y, et al. Tislelizumab in Chinese patients with advanced solid tumors: An open-label, non-comparative, phase 1/2 study. J Immunother Cancer. 2020;8:e000437. doi: 10.1136/jitc-2019-000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC, Liao W, Jiang Y, Lin XY, Zhang QY, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: A phase II clinical trial (POLARIS-02) J Clin Oncol. 2021;39:704–712. doi: 10.1200/JCO.20.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Even C, Wang HM, Li SH, Ngan RK, Dechaphunkul A, Zhang L, Yen CJ, Chan PC, Chakrabandhu S, Ma BBY, et al. Phase II, randomized study of spartalizumab (PDR001), an Anti-PD-1 antibody, versus chemotherapy in patients with recurrent/metastatic nasopharyngeal cancer. Clin Cancer Res. 2021;27:6413–6423. doi: 10.1158/1078-0432.CCR-21-0822. [DOI] [PubMed] [Google Scholar]
- 52.Ding X, Zhang WJ, You R, Zou X, Wang ZQ, Ouyang YF, Peng L, Liu YP, Duan CY, Yang Q, et al. Camrelizumab plus apatinib in patients with recurrent or metastatic nasopharyngeal carcinoma: An Open-label, Single-arm, phase II study. J Clin Oncol. 2023;41:2571–2582. doi: 10.1200/JCO.22.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Qu S, Li J, Hu C, Xu M, Li W, Zhou T, Shen L, Wu H, Lang J, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): A multi-centre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021;22:1162–1174. doi: 10.1016/S1470-2045(21)00302-8. [DOI] [PubMed] [Google Scholar]
- 54.Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, Li J, Shi YR, Jin F, Xu R, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: A multicenter randomized phase 3 trial. Nat Med. 2021;27:1536–1543. doi: 10.1038/s41591-021-01444-0. [DOI] [PubMed] [Google Scholar]
- 55.Hua Y, You R, Wang Z, Huang P, Lin M, Ouyang Y, Xie Y, Zou X, Liu Y, Duan C, et al. Toripalimab plus intensity-modulated radiotherapy for recurrent nasopharyngeal carcinoma: An open-label single-arm, phase II trial. J Immunother Cancer. 2021;9:e003290. doi: 10.1136/jitc-2021-003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou X, Niu X, Liu P, Ou D, Zhu Y, Wang X. Is immune therapy plus chemotherapy more effective than immune therapy alone for unresectable recurrent nasopharyngeal carcinoma? Front Immunol. 2021;12:762663. doi: 10.3389/fimmu.2021.762663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Investigation. 2015;125:3377–3383. doi: 10.1172/JCI80012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Li X, Ma Z, Wang C, Yang Q, Byrne-Steele M, Hong R, Min Q, Zhou G, Cheng Y, et al. CTLA-4 expression by B-1a B cells is essential for immune tolerance. Nat Commun. 2021;12:525. doi: 10.1038/s41467-020-20874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 61.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DW, Lim HY, Yau T, et al. Safety, Efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J Clin Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao M, Qi F, Chen X, Luo Z, Zhang L, Zheng C, Hu S, Jiang X, Zhou M, Tang J. Functional polymorphism of cytotoxic T-lymphocyte antigen 4 and nasopharyngeal carcinoma susceptibility in a Chinese population. Int J Immunogenet. 2010;37:27–32. doi: 10.1111/j.1744-313X.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed MM, Gebriel MG, Morad EA, Saber IM, Elwan A, Salah M, Fakhr AE, Shalaby AM, Alabiad MA. Expression of immune checkpoint regulators, cytotoxic T-lymphocyte Antigen-4, and programmed Death-Ligand 1 in Epstein-Barr Virus-associated Nasopharyngeal Carcinoma. Appl Immunohistochem Mol Morphol. 2021;29:401–408. doi: 10.1097/PAI.0000000000000903. [DOI] [PubMed] [Google Scholar]
- 65.Yu X, Huang X, Chen X, Liu J, Wu C, Pu Q, Wang Y, Kang X, Zhou L. Characterization of a novel anti-human lymphocyte activation gene 3 (LAG-3) antibody for cancer immunotherapy. MAbs. 2019;11:1139–1148. doi: 10.1080/19420862.2019.1629239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, Rutkowski P, Gogas HJ, Lao CD, De Menezes JJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solinas C, De Silva P, Bron D, Willard-Gallo K, Sangiolo D. Significance of TIM3 expression in cancer: From biology to the clinic. Semin Oncol. 2019;46:372–379. doi: 10.1053/j.seminoncol.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Chen TC, Chen CH, Wang CP, Lin PH, Yang TL, Lou PJ, Ko JY, Wu CT, Chang YL. The immunologic advantage of recurrent nasopharyngeal carcinoma from the viewpoint of Galectin-9/Tim-3-related changes in the tumour microenvironment. Sci Rep. 2017;7:10349. doi: 10.1038/s41598-017-10386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276:97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang R, Sun L, Li CF, Wang YH, Yao J, Li H, Yan M, Chang WC, Hsu JM, Cha JH, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12:832. doi: 10.1038/s41467-021-21099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang CX, Huang DJ, Baloche V, Zhang L, Xu JX, Li BW, Zhao XR, He J, Mai HQ, Chen QY, et al. Galectin-9 promotes a suppressive microenvironment in human cancer by enhancing STING degradation. Oncogenesis. 2020;9:65. doi: 10.1038/s41389-020-00248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018;29:71–83. doi: 10.1093/annonc/mdx686. [DOI] [PubMed] [Google Scholar]
- 75.Shannon-Lowe C, Rowe M. Epstein Barr virus entry; kissing and conjugation. Curr Opin Virol. 2014;4:78–84. doi: 10.1016/j.coviro.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Cohen JI. Vaccine development for epstein-barr virus. Adv Exp Med Biol. 2018;1045:477–493. doi: 10.1007/978-981-10-7230-7_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jean-Pierre V, Lupo J, Buisson M, Morand P, Germi R. Main targets of interest for the development of a prophylactic or therapeutic epstein-barr virus vaccine. Front Microbiol. 2021;12:701611. doi: 10.3389/fmicb.2021.701611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui X, Cao Z, Sen G, Chattopadhyay G, Fuller DH, Fuller JT, Snapper DM, Snow AL, Mond JJ, Snapper CM. A novel tetrameric gp350 1-470 as a potential Epstein-Barr virus vaccine. Vaccine. 2013;31:3039–3045. doi: 10.1016/j.vaccine.2013.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cui X, Cao Z, Chen Q, Arjunaraja S, Snow AL, Snapper CM. Rabbits immunized with Epstein-Barr virus gH/gL or gB recombinant proteins elicit higher serum virus neutralizing activity than gp350. Vaccine. 2016;34:4050–4055. doi: 10.1016/j.vaccine.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 80.Cui X, Cao Z, Ishikawa Y, Cui S, Imadome KI, Snapper CM. Immunization with Epstein-barr virus core fusion machinery envelope proteins elicit high titers of neutralizing activities and protect humanized mice from lethal dose EBV challenge. Vaccines (Basel) 2021;9:285. doi: 10.3390/vaccines9030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dasari V, Sinha D, Neller MA, Smith C, Khanna R. Prophylactic and therapeutic strategies for Epstein-Barr virus-associated diseases: Emerging strategies for clinical development. Expert Rev Vaccines. 2019;18:457–474. doi: 10.1080/14760584.2019.1605906. [DOI] [PubMed] [Google Scholar]
- 82.Li F, Song D, Lu Y, Zhu H, Chen Z, He X. Delayed-type hypersensitivity (DTH) immune response related with EBV-DNA in nasopharyngeal carcinoma treated with autologous dendritic cell vaccination after radiotherapy. J Immunother. 2013;36:208–214. doi: 10.1097/CJI.0b013e31828bd87b. [DOI] [PubMed] [Google Scholar]
- 83.Chia WK, Wang WW, Teo M, Tai WM, Lim WT, Tan EH, Leong SS, Sun L, Chen JJ, Gottschalk S, et al. A phase II study evaluating the safety and efficacy of an adenovirus-ΔLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann Oncol. 2012;23:997–1005. doi: 10.1093/annonc/mdr341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hui EP, Taylor GS, Jia H, Ma BB, Chan SL, Ho R, Wong WL, Wilson S, Johnson BF, Edwards C, et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 2013;73:1676–1688. doi: 10.1158/0008-5472.CAN-12-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor GS, Jia H, Harrington K, Lee LW, Turner J, Ladell K, Price DA, Tanday M, Matthews J, Roberts C, et al. A recombinant modified vaccinia ankara vaccine encoding Epstein-Barr Virus (EBV) target antigens: A phase I trial in UK patients with EBV-positive cancer. Clin Cancer Res. 2014;20:5009–5022. doi: 10.1158/1078-0432.CCR-14-1122-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X, Lu J, He ML, Li Z, Zhang B, Zhou LH, Li Q, Li G, Wang L, Tian WD, et al. Antitumor effects of interferon-alpha on cell growth and metastasis in human nasopharyngeal carcinoma. Curr Cancer Drug Targets. 2012;12:561–570. doi: 10.2174/156800912800673293. [DOI] [PubMed] [Google Scholar]
- 87.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 88.Liu RY, Zhou L, Zhang YL, Huang BJ, Ke ML, Chen JM, Li LX, Fu X, Wu JX, Huang W. An oncolytic adenovirus enhances antiangiogenic and antitumoral effects of a replication-deficient adenovirus encoding endostatin by rescuing its selective replication in nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2013;442:171–176. doi: 10.1016/j.bbrc.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 89.Wang JN, Hu P, Zeng MS, Liu RB. Anti-tumor effect of oncolytic herpes simplex virus G47delta on human nasopharyngeal carcinoma. Chin J Cancer. 2011;30:831–841. doi: 10.5732/cjc.011.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chua D, Huang J, Zheng B, Lau SY, Luk W, Kwong DL, Sham JS, Moss D, Yuen KY, Im SW, et al. Adoptive transfer of autologous Epstein-Barr virus-specific cytotoxic T cells for nasopharyngeal carcinoma. Int J Cancer. 2001;94:73–80. doi: 10.1002/ijc.1430. [DOI] [PubMed] [Google Scholar]
- 91.Comoli P, De Palma R, Siena S, Nocera A, Basso S, Del Galdo F, Schiavo R, Carminati O, Tagliamacco A, Abbate GF, et al. Adoptive transfer of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T cells with in vitro antitumor activity boosts LMP2-specific immune response in a patient with EBV-related nasopharyngeal carcinoma. Ann Oncol. 2004;15:113–117. doi: 10.1093/annonc/mdh027. [DOI] [PubMed] [Google Scholar]
- 92.Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, Gresik MV, Gee AP, Russell HV, Brenner MK, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus-specific T lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 93.Comoli P, Pedrazzoli P, Maccario R, Basso S, Carminati O, Labirio M, Schiavo R, Secondino S, Frasson C, Perotti C, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942–8949. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- 94.Louis CU, Straathof K, Bollard CM, Ennamuri S, Gerken C, Lopez TT, Huls MH, Sheehan A, Wu MF, Liu H, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother. 2010;33:983–990. doi: 10.1097/CJI.0b013e3181f3cbf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lutzky VP, Crooks P, Morrison L, Stevens N, Davis JE, Corban M, Hall D, Panizza B, Coman WB, Coman S, et al. Cytotoxic T cell adoptive immunotherapy as a treatment for nasopharyngeal carcinoma. Clin Vaccine Immunol. 2014;21:256–259. doi: 10.1128/CVI.00121-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eom HS, Choi BK, Lee Y, Lee H, Yun T, Kim YH, Lee JJ, Kwon BS. Phase I clinical trial of 4-1BB-based adoptive T-cell therapy for epstein-barr virus (EBV)-positive tumors. J Immunother. 2016;39:140–148. doi: 10.1097/CJI.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Louis CU, Straathof K, Bollard CM, Gerken C, Huls MH, Gresik MV, Wu MF, Weiss HL, Gee AP, Brenner MK, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009;113:2442–2450. doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Secondino S, Zecca M, Licitra L, Gurrado A, Schiavetto I, Bossi P, Locati L, Schiavo R, Basso S, Baldanti F, et al. T-cell therapy for EBV-associated nasopharyngeal carcinoma: Preparative lymphodepleting chemotherapy does not improve clinical results. Ann Oncol. 2012;23:435–441. doi: 10.1093/annonc/mdr134. [DOI] [PubMed] [Google Scholar]
- 99.Smith C, Tsang J, Beagley L, Chua D, Lee V, Li V, Moss DJ, Coman W, Chan KH, Nicholls J, et al. Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res. 2012;72:1116–1125. doi: 10.1158/0008-5472.CAN-11-3399. [DOI] [PubMed] [Google Scholar]
- 100.Smith C, Lee V, Schuessler A, Beagley L, Rehan S, Tsang J, Li V, Tiu R, Smith D, Neller MA, et al. Pre-emptive and therapeutic adoptive immunotherapy for nasopharyngeal carcinoma: Phenotype and effector function of T cells impact on clinical response. Oncoimmunol. 2017;6:e1273311. doi: 10.1080/2162402X.2016.1273311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang J, Fogg M, Wirth LJ, Daley H, Ritz J, Posner MR, Wang FC, Lorch JH. Epstein-Barr virus-specific adoptive immunotherapy for recurrent, metastatic nasopharyngeal carcinoma. Cancer. 2017;123:2642–2650. doi: 10.1002/cncr.30541. [DOI] [PubMed] [Google Scholar]
- 102.Chia WK, Teo M, Wang WW, Lee B, Ang SF, Tai WM, Chee CL, Ng J, Kan R, Lim WT, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther. 2014;22:132–139. doi: 10.1038/mt.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Al-Rajhi N, Soudy H, Ahmed SA, Elhassan T, Mohammed SF, Khoja HA, Ghebeh H. CD3+T-lymphocyte infiltration is an independent prognostic factor for advanced nasopharyngeal carcinoma. BMC Cancer. 2020;20:240. doi: 10.1186/s12885-020-06757-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J, Chen QY, He J, Li ZL, Tang XF, Chen SP, Xie CM, Li YQ, Huang LX, Ye SB, et al. Phase I trial of adoptively transferred tumor-infiltrating lymphocyte immunotherapy following concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Oncoimmunology. 2015;4:e976507. doi: 10.4161/23723556.2014.976507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Introna M. CIK as therapeutic agents against tumors. J Autoimmun. 2017;85:32–44. doi: 10.1016/j.jaut.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 106.Li JJ, Gu MF, Pan K, Liu LZ, Zhang H, Shen WX, Xia JC. Autologous cytokine-induced killer cell transfusion in combination with gemcitabine plus cisplatin regimen chemotherapy for metastatic nasopharyngeal carcinoma. J Immunother. 2012;35:189–195. doi: 10.1097/CJI.0b013e318241d9de. [DOI] [PubMed] [Google Scholar]
- 107.Li Y, Pan K, Liu LZ, Li YQ, Gu MF, Zhang H, Shen WX, Xia JC, Li JJ. Sequential Cytokine-induced killer cell immunotherapy enhances the efficacy of the gemcitabine plus cisplatin chemotherapy regimen for metastatic nasopharyngeal carcinoma. PLoS One. 2015;10:e0130620. doi: 10.1371/journal.pone.0130620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lim CM, Liou A, Poon M, Koh LP, Tan LK, Loh KS, Petersson BF, Ting E, Campana D, Goh BC, et al. Phase I study of expanded natural killer cells in combination with cetuximab for recurrent/metastatic nasopharyngeal carcinoma. Cancer Immunol Immunother. 2022;71:2277–2286. doi: 10.1007/s00262-022-03158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. 'Off-the-shelf' allogeneic CAR T cells: Development and challenges. Nat Rev Drug Discov. 2020;19:185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 110.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. The New England journal of medicine. 2017;377:2531–44. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in Children and Young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, Ibrahimi S, Mielke S, Mutsaers P, Hernan dez-Ilizaliturri F, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–2308. doi: 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 114.Munshi NC, Anderson LD, Jr, Shah N, Madduri D, Berdeja J, Lonial S, Raje N, Lin Y, Siegel D, Oriol A, et al. Idecabtagene Vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 115.Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, Stewart AK, Hari P, Htut M, Lesokhin A, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet. 2021;398:314–324. doi: 10.1016/S0140-6736(21)00933-8. [DOI] [PubMed] [Google Scholar]
- 116.Brown CE, Mackall CL. CAR T cell therapy: Inroads to response and resistance. Nat Rev Immunol. 2019;19:73–74. doi: 10.1038/s41577-018-0119-y. [DOI] [PubMed] [Google Scholar]
- 117.Guo X, Zheng H, Luo W, Zhang Q, Liu J, Yao K. 5T4-specific chimeric antigen receptor modification promotes the immune efficacy of cytokine-induced killer cells against nasopharyngeal carcinoma stem cell-like cells. Sci Rep. 2017;7:4859. doi: 10.1038/s41598-017-04756-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen Y, Chen RJ, Huang XC, Tang GX, Kuai XW, Zhang MJ, Zhang DW, Tang Q, Zhu J, Feng ZQ. Construction of latent membrane protein 2A chimeric antigen receptor-T cells and their lethal effects on nasopharyngeal carcinoma cells. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;53:925–930. doi: 10.3760/cma.j.issn.1673-0860.2018.12.010. In Chinese. [DOI] [PubMed] [Google Scholar]
- 119.Tang X, Zhou Y, Li W, Tang Q, Chen R, Zhu J, Feng Z. T cells expressing a LMP1-specific chimeric antigen receptor mediate antitumor effects against LMP1-positive nasopharyngeal carcinoma cells in vitro and in vivo. J Biomed Res. 2014;28:468–475. doi: 10.7555/JBR.28.20140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang X, Tang Q, Mao Y, Huang X, Jia L, Zhu J, Feng Z. CD137 Co-stimulation improves the antitumor effect of LMP1-specific chimeric antigen receptor T cells in vitro and in vivo. Onco Targets Ther. 2019;12:9341–9350. doi: 10.2147/OTT.S221040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu H, Pan C, Song W, Liu D, Li Z, Zheng L. Novel strategies for immuno-oncology breakthroughs with cell therapy. Biomarker Res. 2021;9:62. doi: 10.1186/s40364-021-00316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsimberidou AM, Van Morris K, Vo HH, Eck S, Lin YF, Rivas JM, Andersson BS. T-cell receptor-based therapy: An innovative therapeutic approach for solid tumors. J Hematol Oncol. 2021;14:102. doi: 10.1186/s13045-021-01115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y, Liu Z, Wei W, Li Y. TCR engineered T cells for solid tumor immunotherapy. Exp Hematol Oncol. 2022;11:38. doi: 10.1186/s40164-022-00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, Sullivan RJ, Ochsenreither S, Dummer R, Kirkwood JM, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385:1196–1206. doi: 10.1056/NEJMoa2103485. [DOI] [PubMed] [Google Scholar]
- 125.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Turrini R, Merlo A, Martorelli D, Faè DA, Sommaggio R, Montagner IM, Barbieri V, Marin O, Zanovello P, Dolcetti R, et al. A BARF1-specific mAb as a new immunotherapeutic tool for the management of EBV-related tumors. Oncoimmunology. 2017;6:e1304338. doi: 10.1080/2162402X.2017.1304338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahmed M, Lopez-Albaitero A, Pankov D, Santich BH, Liu H, Yan S, Xiang J, Wang P, Hasan AN, Selvakumar A, et al. TCR-mimic bispecific antibodies targeting LMP2A show potent activity against EBV malignancies. JCI Insight. 2018;3:e97805. doi: 10.1172/jci.insight.97805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smith C, McGrath M, Neller MA, Matthews KK, Crooks P, Le Texier L, Panizza B, Porceddu S, Khanna R. Complete response to PD-1 blockade following EBV-specific T-cell therapy in metastatic nasopharyngeal carcinoma. NPJ Precision Oncol. 2021;5:24. doi: 10.1038/s41698-021-00162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rezaei R, Esmaeili Gouvarchin Ghaleh H, Farzanehpour M, Dorostkar R, Ranjbar R, Bolandian M, Mirzaei Nodooshan M, Ghorbani Alvanegh A. Combination therapy with CAR T cells and oncolytic viruses: A new era in cancer immunotherapy. Cancer Gene Ther. 2022;29:647–660. doi: 10.1038/s41417-021-00359-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.