Abstract

We demonstrated that the loading amounts and concentrations of reactant 1,3-cyclohexanedione affect reaction rates and outcomes. In certain cases, reactions with higher concentrations of 1,3-cyclohexanedione were slower than those with lower concentrations. By minimizing the use of the cyclic 1,3-dione derivatives and by tuning the reaction concentration, the acid catalyst was reduced to 0.1 mol % to afford the desired products in high yields, and the reaction scope was expanded.
Understanding the factors that affect catalyst loading is necessary for the development of catalysts and catalyzed chemical transformations and for minimization of the amount of catalyst used. Cyclic 1,3-diketone derivatives, such as 1,3-cyclohexanedione, are versatile reactants, and various transformations of these molecules have been developed for the synthesis of a range of molecules.1−4 We recently observed that cyclic 1,3-diketone derivatives that have a reactive α-methylene group on their β-diketone moiety serve as more than just reactants.5 Those cyclic 1,3-diketone derivatives function as buffering molecules in non-aqueous solutions.5 Here we report that the loading and concentration of reactant 1,3-cyclohexanedione affect the reaction rate and the amount of catalyst required (Scheme 1). By minimizing the use of the cyclic 1,3-dione derivatives and by tuning the concentrations of the reactants and the catalyst, the catalyst amount was cut by 450-fold compared to that used under previously reported conditions, and the desired products were obtained in higher yields with higher diastereoselectivities in shorter reaction times as described below. In addition, with the use of less catalyst, the scope of the reaction was expanded.
Scheme 1. Previously Reported Reactions That Required an Acid Catalyst Loading of 0.45 equiv and This Work That Results in the Required Catalyst Loading of 0.001 equiv.

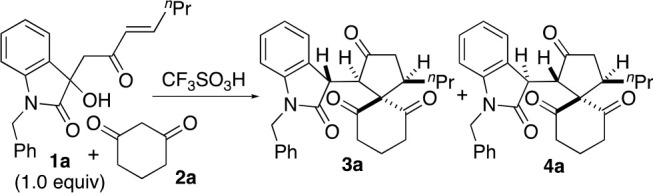
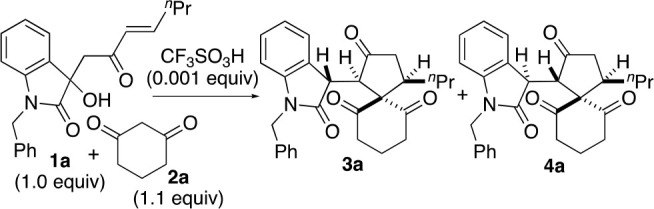
We recently reported acid-catalyzed formal (4+1) cycloaddition reactions that led to the formation of spiro[4,5]decanes (Scheme 1a).6 Enone derivatives 1 were used as the C4 reactants, and 1,3-cyclohexanedione or related cyclic 1,3-diketone derivatives were used as the C1 reactants. The conditions required 0.45 equiv of acid catalyst CF3SO3H when the reactions of 1 (1.0 equiv) with cyclic 1,3-dione derivative 2 (3.0 equiv) were performed to afford products 3.6 Depending on the conditions and substituents on 1, products 4 (the diastereomers of 3) were also formed; isomerization of 3 to 4 often occurred during the reaction.6 Whereas both 3 and 4 are useful compounds, which have been used for the synthesis of functionalized polycyclic derivatives by further transformations,6,7 selective formation of 3 is required for the synthesis of molecules that are derived from 3.
We sought to understand why relatively high loading of the acid catalyst (0.45 equiv) was required. We hypothesized that the acid was neutralized by 2 on the basis of the buffering functions of 2 in non-aqueous solutions5 (Scheme 1, proposed mechanisms) and that minimizing the loading of 2 would reduce the amounts of the acid catalyst necessary for the formation of 3. The rapid dynamics of the tautomerization equilibrium of 2 would allow 2 to interact with and neutralize the acid or base that is added as the catalyst. A high loading of 2 and/or a high concentration of 2 should shift the equilibrium of the binding of 2 with the acid or base toward the bound form, inhibiting the interactions between the acid or base and the reactants and/or the intermediates that are necessary for the catalysis. Thus, we investigated how the loading and concentration of 2 influence the reactions of 2 catalyzed by an acid or a base.
To test the hypothesis, the reactions of 1a and 2a in CDCl3 were performed with various loadings of 2a and the acid catalyst CF3SO3H, and the formation of 3a and 4a was monitored (Table 1). When the loading of 2a was 3.0 equiv relative to 1a, the use of lower loadings of the acid catalyst decreased the yield of 3a at 60 min (Table 1, entries 1 and 4–7). The formation of 3a in the reaction using 1.0 equiv of 2a relative to 1a in the presence of 0.01 equiv (1.0 mol %) of the acid catalyst was faster than that in the reaction using 3.0 equiv of 2a in the presence of the same amount of the acid (Table 1, entry 8 vs entry 7). The addition of 2b (2.0 equiv) in the reaction using 2a (1.0 equiv) in the presence of the acid (0.01 equiv) resulted in a decrease in the rate of formation of 3a and 4a (Table 1, entry 9 vs entry 8). In addition, when the amount of the acid catalyst was 0.001 equiv (0.1 mol %) relative to 1a, the reactions with lower concentrations of 2a gave product 3a in higher yields at the same reaction times (at 30 or 100 min) (Table 1, entries 10–13). With the use of only 1.1 equiv of 2a relative to 1a, the amount of the acid catalyst was able to be reduced to only 0.1 mol % without decreasing the rate of formation of 3a compared to the reactions using 3.0 equiv of 2a (Table 1, entry 13 vs entry 1). The amount of the acid catalyst was able to be reduced 450-fold when 1.1 equiv of 2a was used relative to 1a. The reaction with 1.1 equiv of 2a in the presence of the acid catalyst (0.001 equiv) (Table 1, entry 13) was cleaner than the reactions with exactly 1.0 equiv of 2a relative to 1a in the presence of the acid (0.01 equiv) (Table 1, entry 8); the use of a small excess of 2a suppressed side reactions.
Table 1. Evaluations of Conditions for the Reaction of 1a and 2a to Afford 3aa.
| entry | 2a (equiv) | CF3SO3H (equiv) | time (min) | yield of 3a (%)b | yield of 4a (%)b |
|---|---|---|---|---|---|
| 1 | 3.0 | 0.45 | 60 | 70 | 0 |
| 150 | 94 | 6 | |||
| 2c | 3.0 | 0.45 | 60 | 60 | 0 |
| 150 | 94 | 6 | |||
| 3c | 3.0 | 0.45 | 82 | 40 | 1 |
| 147 | 69 | 3 | |||
| 4 | 3.0 | 0.20 | 60 | 46 | 0 |
| 5 | 3.0 | 0.10 | 60 | 39 | 0 |
| 6 | 3.0 | 0.05 | 60 | 32 | 0 |
| 7 | 3.0 | 0.01 | 60 | 31 | 0 |
| 120 | 60 | 0 | |||
| 240 | 94 | 6 | |||
| 8 | 1.0 | 0.01 | 48 | 45 | 2 |
| 75 | 78 | 5 | |||
| 9d | 1.0 | 0.01 | 48 | 14 | 0 |
| 75 | 24 | 1 | |||
| 10e | 3.0 | 0.001 | 30 | 18 | 0 |
| 100 | 38 | 0 | |||
| 11e | 2.0 | 0.001 | 30 | 23 | 0 |
| 100 | 58 | 1 | |||
| 12e | 1.5 | 0.001 | 30 | 29 | 2 |
| 100 | 88 | 2 | |||
| 13e | 1.1 | 0.001 | 30 | 30 | 0 |
| 100 | 90 | 2 |
For the reactions, to a solution of 2a (indicated equivalents relative to 1a) and CF3SO3H (indicated equivalents relative to 1a) in CDCl3 (1.0 mL), 1a (0.029 mmol, 1.0 equiv) was added, and the mixture was heated at 60 °C. See the Supporting Information for details.
Determined by 1H NMR analysis.
To a solution of 1a (0.029 mmol, 1.0 equiv) and 2a (indicated equivalents) in CDCl3 (1.0 mL) (entry 2) or in C6D5CD3 (1.0 mL) (entry 3), CF3SO3H (indicated equivalents) was added, and the mixture was heated at 60 °C.
2b (2.0 equiv) was added.
A 0.11 mmol scale reaction.

Next, the effects of the concentrations of the reaction on the formation of 3a were evaluated (Table 2). The experiment shown in entry 13 of Table 1 had the same concentrations as the experiment shown in entry 2 of Table 2. The yields of 3a at 30 min were determined to compare the rates of formation of 3a. The reaction performed under 2-fold more concentrated conditions was approximately 1.6-fold faster on the basis of the formation of 3a at 30 min (Table 2, entry 4 vs entry 2). However, the reaction with 4-fold more concentrated conditions resulted in a reaction rate similar to that of the initial conditions (Table 2, entry 2 vs entry 5). These results suggest that the concentration of 2a affects the acidic environment of the reaction on the basis of the buffering function of 2a. The reaction in the presence of 0.1 mol % of the acid catalyst under the conditions shown in entry 4 of Table 2 was identified to be the fastest reaction among those evaluated. The use of optimized reaction conditions (Table 2, entry 4) resulted in the formation of 3a in a high yield before the formation of 4a from 3a by isomerization became significant.
Table 2. Effects of Concentrations in the Reaction on the Formation of 3aa.
| entry | CDCl3 (mL) | 2a (mM) | CF3SO3H (mM) | time (min) | yield of 3a (%)b | yield of 4a (%)b |
|---|---|---|---|---|---|---|
| 1 | 8.0 | 16 | 0.014 | 30 | 29 | 0 |
| 2 | 4.0 | 31 | 0.028 | 30 | 31 | 0 |
| 3 | 3.0 | 42 | 0.038 | 30 | 42 | 0 |
| 4 | 2.0 | 63 | 0.057 | 30 | 51 | 0 |
| 60 | 97 | 1 | ||||
| 5 | 1.0 | 126 | 0.11 | 30 | 33 | 1 |
For the reactions, to a solution of 2a (0.13 mmol, 1.1 equiv) and CF3SO3H (0.00011 mmol, 0.001 equiv) in CDCl3 (indicated volume), 1a (0.11 mmol, 1.0 equiv) was added, and the mixture was heated at 60 °C. See the Supporting Information for details.
Determined by 1H NMR analysis.
Next, using the optimized conditions, various oxindole-derived spiro[4,5]decanes 3 were synthesized by the formal (4+1) cycloaddition reactions of 1 and 2a in the presence of acid catalyst CF3SO3H (0.1 mol %) (Scheme 2). The reaction time to afford 3 was shorter under these conditions than under previously reported conditions6 with 3.0 equiv of 2a and 0.45 equiv of CF3SO3H. Products 3, including those bearing functional groups, such as olefin, chloro, furyl, thienyl, and imido (3d, 3e, 3h, 3i, and 3l, respectively), were obtained in high yields as single diastereomers. In addition, no additional acid other than CF3SO3H (0.1 mol %) was required to afford products bearing pyridyl or dimethylaminophenyl substitution (3j or 3k, respectively). With an acid catalyst loading of 0.1 mol %, the reactions of functionalized substrates to form 3 were enabled.
Scheme 2. Scope of the Formation of 3 from 1 and 2 in the Presence of a Catalyst Loading of 0.001 equiv.

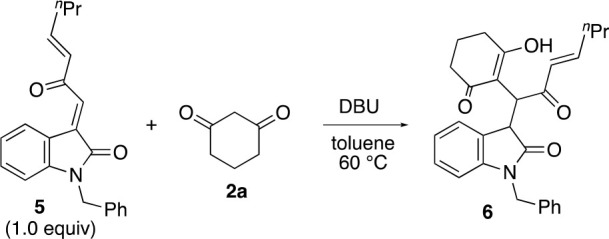
In addition, the loading amounts and the concentration of 2a also affected reactions of 2a catalyzed by a base. We previously reported DBU-catalyzed addition of 2a to enone derivative 5 to afford 6(1j) (Table 3). During the optimization of the conditions, lower concentrations and less loading of 2a resulted in a faster reaction rate and a higher yield of the desired product within the same reaction time or a shorter reaction time (Table 3, entry 3 vs entries 1 and 2). These results indicate that 2a also interacts with the base catalyst and that the loading amounts and concentrations of reactant 2a affect the outcomes of the base-catalyzed reactions.
Table 3. Effects of Concentrations for the Reaction of 5 and 2a to Afford 6a.
| entry | toluene (mL) | 2a (equiv; mM) | DBU (equiv; mM) | time (h) | yield of 6 (%)b |
|---|---|---|---|---|---|
| 1c | 0.5 | 1.5; 135 | 0.1; 9.0 | 2 | 76 |
| 2c | 1.0 | 1.5; 67 | 0.1; 4.5 | 3 | 94 |
| 3c | 1.0 | 1.05; 47 | 0.1; 4.5 | 2 | 96 |
For the reactions, to a solution of 5 (0.045 mmol, 1.0 equiv) and 2a (0.067 mmol, 1.5 equiv or 0.047 mmol, 1.05 equiv) in toluene (indicated volume), DBU (0.0045 mmol, 0.1 equiv) was added, and the mixture was heated at 60 °C.
Determined by 1H NMR analysis.
Results reported in ref (1j) are interpreted (concentrations are calculated for this study).
In summary, we have demonstrated that the loading amount and the concentration of reactant 1,3-cyclohexanedione affect reaction rates and outcomes and that, for certain cases, the reactions with higher concentrations of 1,3-cyclohexanedione are slower than those with lower concentrations. Our results indicate that when cyclic 1,3-diketone derivatives are used as reactants in non-aqueous solutions, they have buffering functions. Limiting the loading of 1,3-cyclohexanedione allowed reduction of the acid catalyst loading to 0.1 mol % and, in combination with tuning of the concentration of the reaction, resulted in the formation of the desired products in high yields as single diastereomers in short reaction times. In addition, the limited loading of 1,3-cyclohexanedione and the use of a less loading of the acid catalyst enabled the expansion of the scope of the reaction. In addition, limiting the loading of 1,3-cyclohexanedione also allowed the use of less loading of the base catalyst. Thus, for either acid- or base-catalyzed reactions that use 1,3-cyclohexanedione as the reactant, the loading amounts and concentrations of 1,3-cyclohexanedione influence the catalyst loading required, the reaction rates, and the reaction outcomes. For the development of the reactions of 1,3-cyclohexanedione derivatives that have a reactive α-methylene group on their β-diketone moiety, the buffering functions of the 1,3-cyclohexanedione derivatives must be considered.
Acknowledgments
The authors thank Dr. Michael Chandro Roy (Research Support Division, Okinawa Institute of Science and Technology Graduate University) for mass analyses. This study was supported by the Okinawa Institute of Science and Technology Graduate University.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c00838.
Experimental procedures, characterization data of compounds, and NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Wang L.; Zhong J.; Lin X. Atroposelective Phosphoric Acid Catalyzed Three-Component Cascade Reaction: Enantioselective Synthesis of Axially Chiral N-Arylindoles. Angew. Chem., Int. Ed. 2019, 58, 15824–15828. 10.1002/anie.201909855. [DOI] [PubMed] [Google Scholar]; b Raut V. S.; Jean M.; Vanthuyne N. N.; Roussel C.; Constantieux T.; Bressy C.; Bugaut X.; Bonne D.; Rodriguez J. Enantioselective Syntheses of Furan Atropisomers by an Oxidative Central-to-Axial Chirality Conversion Strategy. J. Am. Chem. Soc. 2017, 139, 2140–2143. 10.1021/jacs.6b11079. [DOI] [PubMed] [Google Scholar]; c Mo Y.; Liu S.; Liu Y.; Ye L.; Shi Z.; Zhao Z.; Li X. Highly Stereoselective Synthesis of 2,3-Dihydrofurans via a Cascade Michael Addition-Alkylation Process: A Nitro Group as the Leaving Group. Chem. Commun. 2019, 55, 6285–6288. 10.1039/C9CC01509D. [DOI] [PubMed] [Google Scholar]; d Hsiao C.-C.; Liao H.-H.; Rueping M. Enantio- and Diastereoselective Access to Distant Stereocenters Embedded within Tetrahydroxanthenes: Utilizing ortho-Quinone Methides as Reactive Intermediates in Asymmetric Brønsted Acid Catalysis. Angew. Chem., Int. Ed. 2014, 53, 13258–13263. 10.1002/anie.201406587. [DOI] [PubMed] [Google Scholar]; e Cañellas S.; Ayats C.; Henseler A. H.; Pericàs M. A. A. Highly Active Polymer-Supported Catalyst for Asymmetric Robinson Annulations in Continuous Flow. ACS Catal. 2017, 7, 1383–1391. 10.1021/acscatal.6b03286. [DOI] [Google Scholar]; f El-Sepelgy O.; Haseloff S.; Alamsetti S. K.; Schneider C. Brønsted Acid Catalyzed, Conjugate Addition of β-Dicarbonyls to In Situ Generated ortho-Quinone Methides-Enantioselective Synthesis of 4-Aryl-4H-Chromenes. Angew. Chem., Int. Ed. 2014, 53, 7923–7927. 10.1002/anie.201403573. [DOI] [PubMed] [Google Scholar]; g Bradshaw B.; Etxebarria-Jardi G.; Bonjoch J.; Viozquez S. F.; Guillena G.; Najera C. Efficient Solvent-Free Robinson Annulation Protocols for the Highly Enantioselective Synthesis of the Wieland–Miescher Ketone and Analogues. Adv. Synth. Catal. 2009, 351, 2482–2490. 10.1002/adsc.200900321. [DOI] [Google Scholar]; h Chouthaiwale P. V.; Aher R. D.; Tanaka F. Catalytic Enantioselective Formal (4 + 2) Cycloaddition by Aldol-Aldol Annulation of Pyruvate Derivatives with Cyclohexane-1,3-Diones to Afford Functionalized Decalins. Angew. Chem., Int. Ed. 2018, 57, 13298–13301. 10.1002/anie.201808219. [DOI] [PubMed] [Google Scholar]; i Aher R. D.; Ishikawa A.; Yamanaka M.; Tanaka F. Catalytic Enantioselective Construction of Decalin Derivatives by Dynamic Kinetic Desymmetrization of C2-Symmetric Derivatives through Aldol-Aldol Annulation. J. Org. Chem. 2022, 87, 8151–8157. 10.1021/acs.joc.2c00889. [DOI] [PubMed] [Google Scholar]; j Sohail M.; Tanaka F. Dynamic Kinetic Asymmetric Transformation of Racemic Diastereomers: Diastereo- and Enantioconvergent Michael-Henry Reactions to Afford Spirooxindoles Bearing Furan-Fused Rings. Angew. Chem., Int. Ed. 2021, 60, 21256–21260. 10.1002/anie.202108734. [DOI] [PubMed] [Google Scholar]
- a Mandal S.; Saha S.; Jana C. K. Diastereoselective and Reversed Regioselective Annulations of N-Alkyl Anilines to Julolidines and Lilolidines. Org. Lett. 2020, 22, 4883–4887. 10.1021/acs.orglett.0c01731. [DOI] [PubMed] [Google Scholar]; b Sha Q.; Arman H.; Doyle M. P. Three-Component Cascade Reactions with 2,3-Diketoesters: A Novel Metal-Free Synthesis of 5-Vinyl-pyrrole and 4-Hydroxy-indole Derivatives. Org. Lett. 2015, 17, 3876–3879. 10.1021/acs.orglett.5b01855. [DOI] [PubMed] [Google Scholar]; c Smith A. B.; Dorsey B. D.; Ohba M.; Lupo A. T. Jr; Malamas M. S. Preparation, Reactivity, and Spectral Properties of 1,3-Dioxin Vinylogous Esters: Versatile β-Ketovinyl Cation Equivalents. J. Org. Chem. 1988, 53, 4314–4325. 10.1021/jo00253a024. [DOI] [Google Scholar]; d Lo C.-L.; Akula P. S.; Hong B.-C.; Lee G.-H.; Chien S.-Y. Total Synthesis of Ulodione A via a Double-Alkylation and DABCO Promoted Ring-Expansion Rearrangement Sequence. Org. Lett. 2022, 24, 3353–3357. 10.1021/acs.orglett.2c01038. [DOI] [PubMed] [Google Scholar]; e Capomolla S. S.; Lim N.-K.; Zhang H. Single-Step Synthesis of 5,6,7,8-Tetrahydroindolizines via Annulation of 2-Formylpiperidine and 1,3-Dicarbonyl Compounds. Org. Lett. 2015, 17, 3564–3567. 10.1021/acs.orglett.5b01671. [DOI] [PubMed] [Google Scholar]; f Kasare S.; Bankar S. K.; Ramasastry S. S. V. Expeditious Metal-Free Access to Functionalized Polycyclic Acetals under Mild Aqueous Conditions. Org. Lett. 2014, 16, 4284–4287. 10.1021/ol501986f. [DOI] [PubMed] [Google Scholar]; g Chebanov V. A.; Saraev V. E.; Desenko S. M.; Chernenko V. N.; Knyazeva I. V.; Groth U.; Glasnov T. N.; Kappe C. O. Tuning of Chemo- and Regioselectivities in Multicomponent Condensations of 5-Aminopyrazoles, Dimedone, and Aldehydes. J. Org. Chem. 2008, 73, 5110–5118. 10.1021/jo800825c. [DOI] [PubMed] [Google Scholar]; h Sun F.; Shao X.; Li Z. Access to Indenofurans and Indenopyridines via Annulation of Heterocyclec Ketene Aminals, o-Phthalaldehyde and Cyclic 1,3-Diketones. RSC Adv. 2016, 6, 15382–15389. 10.1039/C5RA23393C. [DOI] [Google Scholar]
- a Nishikawa K.; Kikuta K.; Tsuruta T.; Nakatsukasa H.; Sugahara S.; Kume S.; Morimoto Y. Asymmetric Total Synthesis of Toxicodenane A by Samarium Iodide-Induced Barbier-Type Cyclization and Its Cell-Protective Effect against Lipotoxicity. Org. Lett. 2022, 24, 531–535. 10.1021/acs.orglett.1c03924. [DOI] [PubMed] [Google Scholar]; b Wei L.; Xiao M.; Xie Z. Total Syntheses of (−)-Spirooliganones A and B. Org. Lett. 2014, 16, 2784–2764. 10.1021/ol501050s. [DOI] [PubMed] [Google Scholar]; c Boyko Y. D.; Huck C. J.; Ning S.; Shved A. S.; Yang C.; Chu T.; Tonogai E. J.; Hergenrother P. J.; Sarlah D. Synthetic Studies on Selective, Proapoptotic Isomalabaricane Triterpenoids Aided by Computational Techniques. J. Am. Chem. Soc. 2021, 143, 2138–2155. 10.1021/jacs.0c12569. [DOI] [PubMed] [Google Scholar]; d Guo L.-D.; Hu J.; Zhang Y.; Tu W.; Zhang Y.; Pu F.; Xu J. Enantioselective Total Synthesis of (−)-Caldaphnidine O via a Radical Cyclization Cascade. J. Am. Chem. Soc. 2019, 141, 13043–13048. 10.1021/jacs.9b07558. [DOI] [PubMed] [Google Scholar]; e Nie W.; Gong J.; Chen Z.; Liu J.; Tian D.; Song H.; Liu X.-Y.; Qin Y. Enantioselective Total Synthesis of (−)-Arcutinine. J. Am. Chem. Soc. 2019, 141, 9712–9718. 10.1021/jacs.9b04847. [DOI] [PubMed] [Google Scholar]; f Bradshaw B.; Etxebarria-Jardi G.; Bonjoch J. Total Synthesis of (−)-Anominine. J. Am. Chem. Soc. 2010, 132, 5966–5967. 10.1021/ja101994q. [DOI] [PubMed] [Google Scholar]; g Burns D. J.; Mommer S.; O’Brien P.; Taylor R. J. K.; Whitwood A. C.; Hachisu S. Stereocontrolled Synthesis of the AB Rings of Samaderine C. Org. Lett. 2013, 15, 394–397. 10.1021/ol303385a. [DOI] [PubMed] [Google Scholar]
- a Kong H.-H.; Zhu C.; Deng S.; Xu G.; Zhao R.; Yao C.; Xiang H.-M.; Zhao C.; Qi X.; Xu H. Remote Enantioselective [4 + 1] Annulation with Copper Vinylvinylidene Intermediates. J. Am. Chem. Soc. 2022, 144, 21347–21355. 10.1021/jacs.2c09572. [DOI] [PubMed] [Google Scholar]; b Thies N.; Haak E. Ruthenium-Catalyzed Synthesis of 2,3-Cyclo[3]dendralenes and Complex Polycycles from Propargyl Alcohols. Angew. Chem., Int. Ed. 2015, 54, 4097–4101. 10.1002/anie.201412207. [DOI] [PubMed] [Google Scholar]; c Zhang F.; Wei Y.; Wu X.; Jiang H.; Wang W.; Li H. Hollow Zeolitic Imidazolate Framework Nanospheres as Highly Efficient Cooperative Catalysts for [3 + 3] Cycloaddition Reactions. J. Am. Chem. Soc. 2014, 136, 13963–13966. 10.1021/ja506372z. [DOI] [PubMed] [Google Scholar]
- Sohail M.; Tanaka F. Control of Chemical Reactions by Using Molecules that Buffer Non-aqueous Solutions. Chem. Eur. J. 2020, 26, 222–229. 10.1002/chem.201903552. [DOI] [PubMed] [Google Scholar]
- Huang J.-R.; Sohail M.; Taniguchi T.; Monde T.; Tanaka F. Formal (4 + 1) Cycloaddition and Enantioselective Michael-Henry Cascade Reactions To Synthesize Spiro[4,5]decanes and Spirooxindole Polycycles. Angew. Chem., Int. Ed. 2017, 56, 5853–5857. 10.1002/anie.201701049. [DOI] [PubMed] [Google Scholar]
- Sohail M.; Tanaka F. Dynamic Stereoselective Annulation via Aldol-Oxa-Cyclization Cascade Reaction to Afford Spirooxindole Pyran Polycycles. Commun. Chem. 2019, 2, 73. 10.1038/s42004-019-0177-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.


