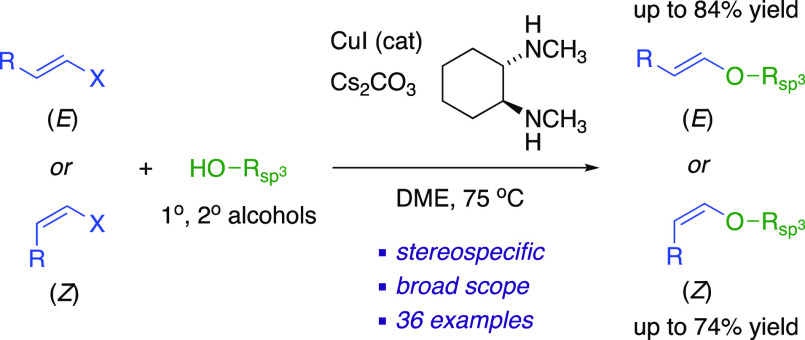
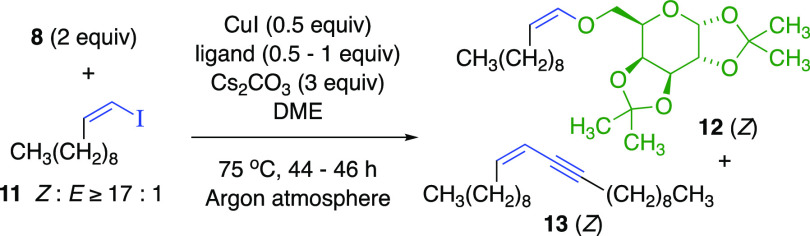
Abstract
CuI and trans-N,N′-dimethylcyclohexyldiamine catalyze the single-step C–O bond cross-coupling between 1,2-di- and trisubstituted vinylic halides with functionalized alcohols, producing acyclic vinylic ethers. This stereospecific transformation selectively gives each of the (E)- and (Z)-vinylic ether products from the corresponding vinyl halide precursors. This method is compatible with carbohydrate-derived primary and secondary alcohols and several other functional groups. The conditions are mild enough to reliably generate vinylic allylic ethers without promoting Claisen rearrangements.
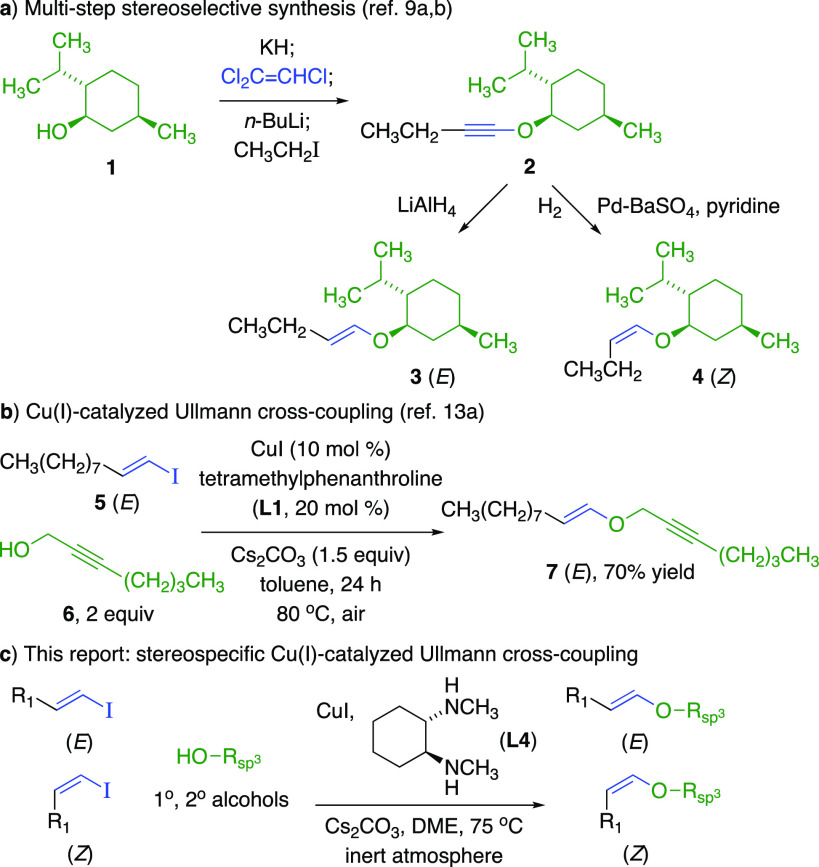
Vinylic ethers are electron-rich alkenes1 with applications including [3.3]-Claisen rearrangements,2 cyclization and cycloaddition processes,3,4 and bioorthogonal reactions.5 Plasmalogen phospholipid natural products feature acyclic (Z)-1,2-disubstituted vinylic ethers.6 A multistep synthetic route for acyclic disubstituted vinylic ethers via alkynyl ethers (2) provides each (E)- and (Z)-isomer (3, 4) from primary and secondary alcohols, but with limited functionality in the vinylic sector (Figure 1a).7 Other methods for acyclic disubstituted vinylic ethers have limited scope8 or poor stereoselectivity,9 restricting potential applications. Intermolecular single-step C–O bond cross-couplings provide aromatic ethers from phenols and/or aromatic synthons,10,11 but corresponding syntheses of substituted vinylic ethers from less acidic sp3-hybridized alcohols and substituted vinylic synthons are not well developed. Cu(II)-catalyzed Chan–Evans–Lam etherifications with vinylic boron synthons require one reactant in excess.12 Cu(I)-phenanthroline (L1)-catalyzed Ullmann cross-couplings provide (E)-disubstituted vinylic ethers, i.e., 7, although (Z)-disubstituted vinylic ethers cannot be prepared (Figure 1b).13 This report describes stereospecific Cu(I)-catalyzed C–O cross-couplings with functionalized primary and secondary alcohols, selectively producing (E)- and (Z)-1,2-disubstituted vinylic ethers (Figure 1c).
Figure 1.
Stereoselective syntheses of 1,2-disubstituted vinylic ethers from alcohols.
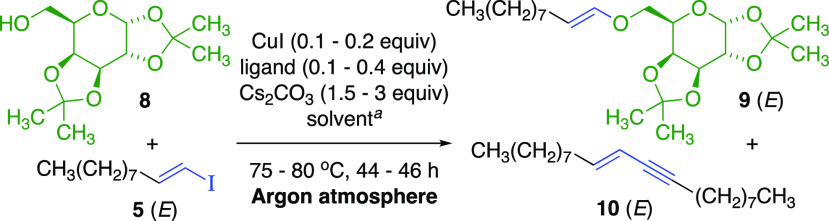
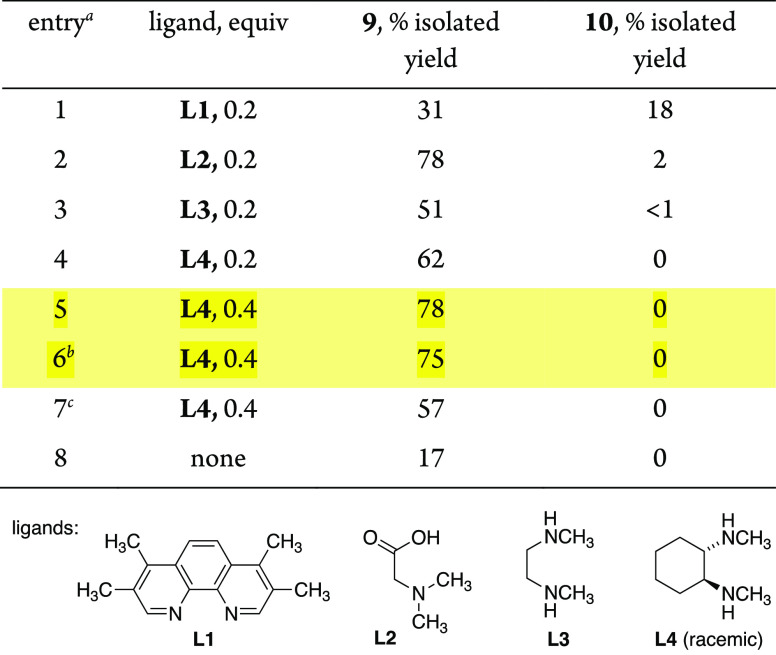
We initially applied CuI-phenanthroline (L1)-catalyzed literature conditions13 with d-galactose-derived primary alcohol 8 (Table 1). Cross-coupling with (E)-1-iodo-1-decene (5) required 120 °C temperature and strictly excluding air (see Supporting Information), giving (E)-vinylic ether 9 in modest yield (entry 1). Conjugated (E)-enyne 10 was a significant side product, consuming two equivalents of vinylic iodide,14 as a consequential limitation of the CuI-L1-catalyzed method. Hypothesizing that an anionic ligand may promote oxidative addition in the mechanism, N,N-dimethylglycine (L2)15 gave an excellent yield of (E)-vinylic ether 9 at lower temperature (entry 2). The neutral ligand 1,2-dimethylethylenediamine (DMEDA, L3) also produced vinylic ether 9 (entry 3). Enyne formation was completely suppressed with trans-N,N′-dimethylcyclohexyldiamine (L4, entry 4).16 Optimized conditions used 1,2-dimethoxyethane (DME, 0.7 M), 0.2 equiv of CuI, 0.4 equiv of L4 (CuI:L4 ratio = 1:2), and 3 equiv of Cs2CO3, under an argon atmosphere, providing (E)-vinylic ether 9 in excellent yield from equimolar vinylic iodide5and primary alcohol8 (entries 5 and 6). The corresponding (E)-vinylic bromide gave vinylic ether 9 in a slightly lower yield (entry 7). A control experiment with CuI and without ligand gave much lower yield (entry 8).
Table 1. Optimizing C–O Cross-Coupling of (E)-Vinylic Iodide 5 with Alcohol 8 to Give (E)-Vinylic Ether 9.
o-Xylene solvent at 120 °C in entry 1; tetraglyme solvent at 75–80 °C in entries 2–3; and DME solvent in entries 4–7.
Produced 1.05 g (2.6 mmol) of (E)-vinylic ether 9.
With (E)-1-bromo-1-decene (1 equiv).
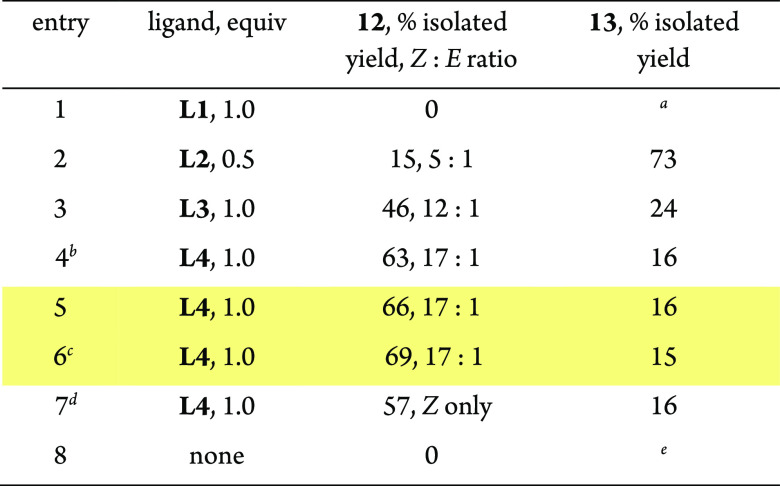
With (Z)-1-iodo-1-undecene (11), (Z)-vinylic ether 12 was not formed using ligand L1 (Table 2, entry 1), producing only a trace of enyne. With dimethylglycine (L2), (Z)-enyne 13 predominated over vinylic ether 12, from base-promoted anti-elimination of (Z)-vinylic iodide 11 (entry 2).17 However, neutral ligands L3 and L4 favored the (Z)-vinylic ether 12 over enyne 13 (entries 3 and 4). We used 2 equiv of alcohol 8, 0.5 equiv of CuI, and 1 equiv of L4 to outcompete the elimination side reaction (entries 5 and 6). Isomerically pure (Z)-1-iodo-1-decene gave exclusively (Z)-vinylic ether (entry 7), confirming the stereospecificity. A control experiment with CuI and without ligand only produced the terminal alkyne (entry 8).
Table 2. Optimizing C–O Cross-Coupling of (Z)-Vinylic Iodide 11 with Alcohol 8 to Give (Z)-Vinylic Ether 12.
Trace of enyne, E:Z = 5:1.
With 1.2 equiv of alcohol 8.
Produced 855 mg (2.1 mmol) of (Z)-vinylic ether 12.
With 100% (Z)-1-iodo-1-decene.
Only produced 1-undecyne.
Stereospecific CuI-L4-catalyzed conditions were tested for other primary alcohols with the E/Z pair of vinylic iodides 5 and 11 (Figure 2a). For comparison with the literature,13a CuI-L4-catalyzed cross-coupling of (E)-vinylic iodide 5 with 2-heptyn-1-ol (6) gave (E)-vinylic ether 7 in excellent yield. Moreover, CuI-L4 produced (Z)-isomer 14 from (Z)-vinylic iodide 11. Cross-couplings with cinnamyl alcohol at 70 °C generated vinylic allylic ethers 15 and 16with minimal thermal Claisen rearrangement. (E)-Vinylic ether 17 was prepared using only one equivalent of weakly nucleophilic trifluoroethanol.18 Vinylic ethers related to E/Z pairs 19–20 and 21–22 previously required multistep synthesis from alcohol precursors.9b,19 (E)-Vinylic ether syntheses were completely stereoselective. (Z)-Vinylic ether isomers were strongly favored from (Z)-11, although yields were generally lower due to enyne formation. Nonetheless, this (Z)-vinylic ether synthesis is a substantial advance, with only one previous example known for (Z)-vinylic ether from C–O cross-coupling.12b
Figure 2.
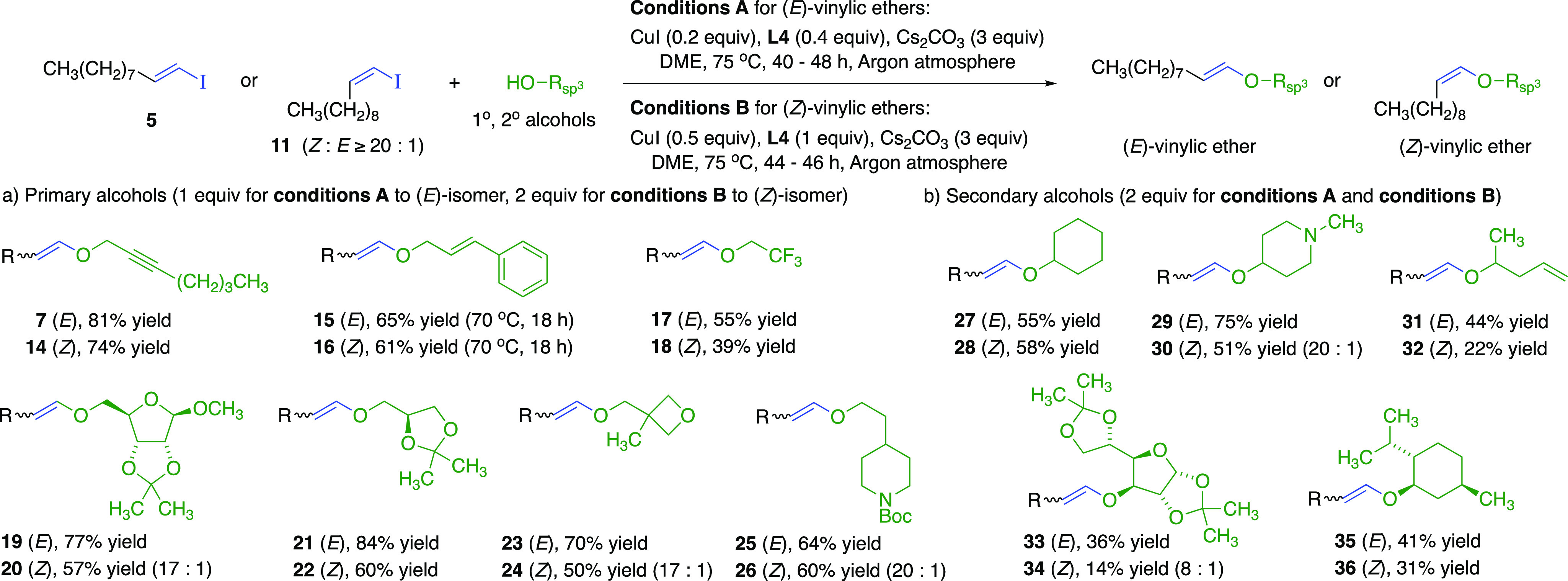
Scope of (E)- and (Z)-vinylic ethers synthesized from stereoselective cross-couplings with (a) primary alcohols and with (b) secondary alcohols, with isolated yields. (Z)/(E) ratios determined by 1H NMR analysis prior to chromatographic purification.
The scope of the CuI-L4-catalyzed process included several pairs of (E)- and (Z)-vinylic ethers 27–36 from secondary alcohols (Figure 2b), using two equivalents of secondary alcohols to maximize vinylic ether yields. Despite lower yields with sterically hindered secondary alcohols, this single-step process compared well with multistep routes to (E)- and (Z)-vinylic ethers related to 33–36.7b,9b This method is compatible with alcohols containing N-Boc-protected secondary amines and tertiary amines, giving E/Z pairs 25–26 and 29–30.
We also demonstrated CuI-L4 cross-couplings with E/Z pairs of other vinylic iodides, giving 37–38 and 39–40 (Figure 3a). (Z)-Cyclohexylvinyl iodide leading to (Z)-vinylic ether 38 was unexpectedly more reactive than the (E)-isomer. In contrast, glyceraldehyde-derived (E)-vinylic iodide gave a better yield of (E)-39 than for (Z)-40 from (Z)-vinylic iodide due to competing enyne formation. Trisubstituted (E)-1-iodo-2-methyldec-1-ene produced (E)-vinylic ethers 41–43 (Figure 3b). 1-Bromocyclohexene and 1-bromo-2-methyl-1-propene gave the trisubstituted vinylic ethers 44–46 (Figure 3c). Geraniol underwent cross-couplings to vinylic allylic ethers 43, 45, and 46 without triggering domino Claisen rearrangements.13a
Figure 3.
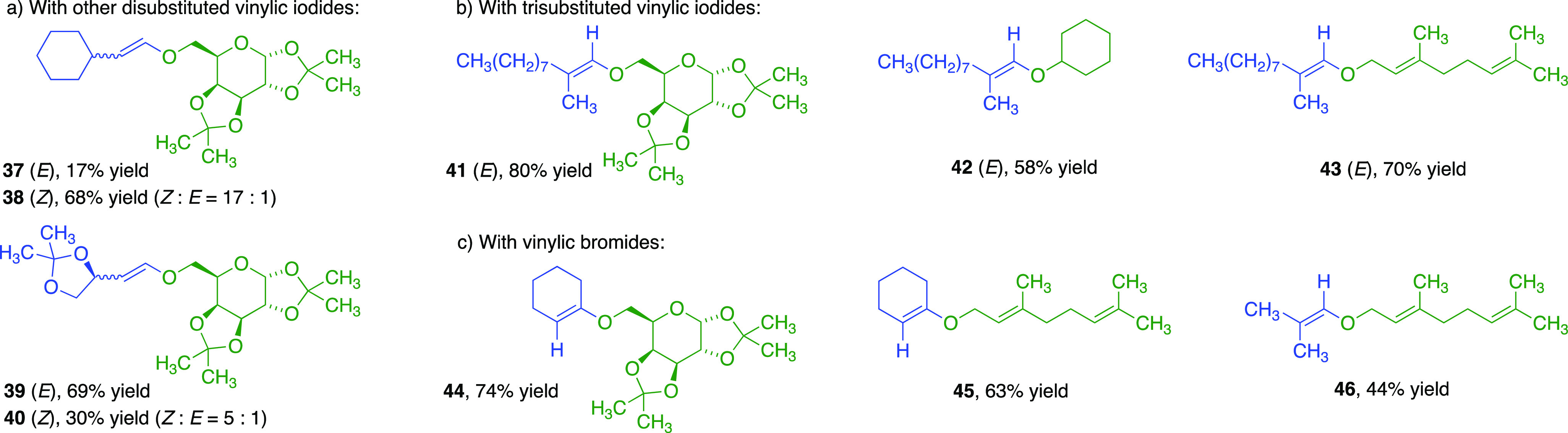
Scope of cross-coupling products with other vinylic halides.
In conclusion, CuI with trans-N,N′-dimethylcyclohexyldiamine (L4) promotes stereospecific C–O cross-couplings of vinylic halides with functionalized alcohols, giving vinylic ethers with high stereoselectivity for each (E)- and (Z)-isomer. The scope of this method is much broader than previous C–O cross-coupling syntheses of vinylic ethers.12,13 Notably, ligand L4 suppresses competing side reactions unique to 1,2-disubstituted vinylic halides. Future research will include mechanistic studies20 and synthetic applications of this method.
Acknowledgments
We dedicate this paper in memory of Prof. Hee-Yoon Lee (KAIST). The National Institute of General Medical Sciences of the National Institutes of Health under Award No. R21GM127971 has supported this research. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c01849.
Author Contributions
‡ These authors contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Winternheimer D. J.; Shade R. E.; Merlic C. A. Methods for Vinyl Ether Synthesis. Synthesis 2010, 2010 (15), 2497–2511. 10.1055/s-0030-1258166. [DOI] [Google Scholar]; b Lempenauer L.; Lemière G.; Duñach E. Cyclisation Reactions Involving Alkyl Enol Ethers. Adv. Synth. Catal. 2019, 361 (23), 5284–5304. 10.1002/adsc.201900478. [DOI] [Google Scholar]
- Geherty M. E.; Dura R. D.; Nelson S. G. Catalytic Asymmetric Claisen Rearrangement of Unactivated Allyl Vinyl Ethers. J. Am. Chem. Soc. 2010, 132 (34), 11875–11877. 10.1021/ja1039314. [DOI] [PubMed] [Google Scholar]
- a Redden A.; Perkins R. J.; Moeller K. D. Oxidative Cyclization Reactions: Controlling the Course of a Radical Cation-Derived Reaction with the Use of a Second Nucleophile. Angew. Chem., Int. Ed. 2013, 52 (49), 12865–12868. 10.1002/anie.201308739. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Maskeri M. A.; O’Connor M. J.; Jaworski A. A.; Bay A. V.; Scheidt K. A. A Cooperative Hydrogen Bond Donor–Brønsted Acid System for the Enantioselective Synthesis of Tetrahydropyrans. Angew. Chem., Int. Ed. 2018, 57 (52), 17225–17229. 10.1002/anie.201811383. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang Y.-Y.; Bode J. W. Olefin Amine (OLA) Reagents for the Synthesis of Bridged Bicyclic and Spirocyclic Saturated N-Heterocycles by Catalytic Hydrogen Atom Transfer (HAT) Reactions. J. Am. Chem. Soc. 2019, 141 (24), 9739–9745. 10.1021/jacs.9b05074. [DOI] [PubMed] [Google Scholar]
- a Harmata M.; Lee D. R.; Barnes C. L. Stereospecific Synthesis of Dienones via a Torquoselective Retro-Nazarov Reaction. Org. Lett. 2005, 7 (9), 1881–1883. 10.1021/ol050657v. [DOI] [PubMed] [Google Scholar]; b Zisopoulou S. A.; Andreou T.; Koftis T. V.; Gallos J. K.; Stathakis C. I. Hetero-Diels-Alder Addition of Ethyl 2-Nitrosoacylate to (Z)-Prop-1-enyl Ethers. Stereoselective Synthesis of a Precursor to Sacubitril. Synthesis 2023, 55 (10), 1507–1516. 10.1055/a-2029-0015. [DOI] [Google Scholar]
- a Wu H.; Alexander S. C.; Jin S.; Devaraj N. K. A Bioorthogonal Near-Infrared Fluorogenic Probe for mRNA Detection. J. Am. Chem. Soc. 2016, 138 (36), 11429–11432. 10.1021/jacs.6b01625. [DOI] [PubMed] [Google Scholar]; b Jiménez-Moreno E.; Guo Z.; Oliveira B. L.; Albuquerque I. S.; Kitowski A.; Guerreiro A.; Boutureira O.; Rodrigues T.; Jiménez-Osés G.; Bernardes G. J. L. Vinyl Ether/Tetrazine Pair for the Traceless Release of Alcohols in Cells. Angew. Chem., Int. Ed. 2017, 56 (1), 243–247. 10.1002/anie.201609607. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Neumann K.; Gambardella A.; Lilienkampf A.; Bradley M. Tetrazine-mediated bioorthogonal prodrug-prodrug activation. Chem. Sci. 2018, 9, 7198–7203. 10.1039/C8SC02610F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Van den Bossche J.; Shin J.; Thompson D. H. Improved Plasmalogen Synthesis Using Organobarium Intermediates. J. Org. Chem. 2007, 72 (13), 5005–5007. 10.1021/jo0705059. [DOI] [PubMed] [Google Scholar]; b Maeda S.; Mohri T.; Inoue T.; Asano Y.; Otoki Y.; Enomoto M.; Nakagawa K.; Kuwahara S.; Ogura Y. Synthesis of a plasmenylethanolamine. Biosci. Biotechnol. Biochem. 2021, 85 (6), 1383–1389. 10.1093/bbb/zbab037. [DOI] [PubMed] [Google Scholar]
- a Moyano A.; Charbonnier F.; Greene A. E. A Simple Preparation of Chiral Acetylenic Ethers. J. Org. Chem. 1987, 52 (13), 2919–2922. 10.1021/jo00389a048. [DOI] [Google Scholar]; b Kann N.; Bernardes V.; Greene A. E. Acetylenic Ethers from Alcohols and Their Reduction to Z- and E-Enol Ethers: Preparation of 1-Menthoxy-1-butyne from Menthol and Conversion to (Z)- and (E)-Menthoxy-1-butene. Org. Synth. 1997, 74, 13. 10.15227/orgsyn.074.0013. [DOI] [Google Scholar]; c Lipshutz B. H.; Tirado R. De Novo Approach to Dideoxyribosides. 5-Endo-trig-like Cyclizations of δ-Hydroxy Enol Ethers. J. Org. Chem. 1994, 59 (26), 8307–8311. 10.1021/jo00105a067. [DOI] [Google Scholar]
- a Okimoto Y.; Sakaguchi S.; Ishii Y. Development of a Highly Efficient Catalytic Method for Synthesis of Vinyl Ethers. J. Am. Chem. Soc. 2002, 124 (8), 1590–1591. 10.1021/ja0173932. [DOI] [PubMed] [Google Scholar]; b Khan R. K. M.; O’Brien R. V.; Torker S.; Li B.; Hoveyda A. H. Z- and Enantioselective Ring-Opening/Cross-Metathesis with Enol Ethers Catalyzed by Stereogenic-at-Ru Carbenes: Reactivity, Selectivity, and Curtin-Hammett Kinetics. J. Am. Chem. Soc. 2012, 134 (30), 12774–12779. 10.1021/ja304827a. [DOI] [PubMed] [Google Scholar]; c Ding W.; Chai J.; Wang C.; Wu J.; Yoshikai N. Stereoselective Access to Highly Substituted Vinyl Ethers via trans-Difunctionalization of Alkynes with Alcohols and Iodine(III) Electrophile. J. Am. Chem. Soc. 2020, 142 (19), 8619–8624. 10.1021/jacs.0c04140. [DOI] [PubMed] [Google Scholar]
- a Nicotra F.; Panza L.; Ronchetti F.; Russo G.; Toma L. Formation of Glycosides by Epoxidation-Ring Closure of Open-Chain Hydroxyenol Ethers obtained from Sugars. J. Chem. Soc., Perkin Trans. 1 1987, 1319–1324. 10.1039/p19870001319. [DOI] [Google Scholar]; b Pérez Sánchez I.; Turos E. Glycosylated vinyl ethers by the Julia-Lythgoe-Kocienski olefination: application to the synthesis of 2′,5′-dideoxydisaccharides and carbohydrated β-lactams. Tetrahedron: Asymmetry 2009, 20 (14), 1646–1660. 10.1016/j.tetasy.2009.05.039. [DOI] [Google Scholar]; c Bhosale V. A.; Waghmode S. B. Stereodivergent approach to Alzheimer’s therapeutic agent (R)-(−) and (S)-(+)-arundic acid employing chiral 4-pentenol derivatives as building blocks. Tetrahedron 2017, 73 (17), 2342–2348. 10.1016/j.tet.2017.03.002. [DOI] [Google Scholar]
- a Wolter M.; Nordmann G.; Job G. E.; Buchwald S. L. Copper-Catalyzed Coupling of Aryl Iodides with Aliphatic Alcohols. Org. Lett. 2002, 4 (6), 973–976. 10.1021/ol025548k. [DOI] [PubMed] [Google Scholar]; b Shafir A.; Lichtor P. A.; Buchwald S. L. N- versus O-Arylation of Aminoalcohols: Orthogonal Selectivity in Copper-Based Catalysts. J. Am. Chem. Soc. 2007, 129 (12), 3490–3491. 10.1021/ja068926f. [DOI] [PubMed] [Google Scholar]; c Chen Z.; Jiang Y.; Zhang L.; Guo Y.; Ma D. Oxalic Diamides and tert-Butoxide: Two Types of Ligands Enabling Practical Access to Alkyl Aryl Ethers via Cu-Catalyzed Coupling Reaction. J. Am. Chem. Soc. 2019, 141 (8), 3541–3549. 10.1021/jacs.8b12142. [DOI] [PubMed] [Google Scholar]; d Yoon H.; Galls A.; Rozema S. D.; Miller S. J. Atroposelective Desymmetrization of Resorcinol-Bearing Quinazolinones via Cu-Catalyzed C-O Bond Formation. Org. Lett. 2022, 24 (2), 762–766. 10.1021/acs.orglett.1c04266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a El Khatib M.; Molander G. A. Copper(II)-Mediated O-Arylation of Protected Serines and Threonines. Org. Lett. 2014, 16 (18), 4944–4947. 10.1021/ol5024689. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dimakos V.; Garrett G. E.; Taylor M. S. Site-Selective, Copper-Mediated O-Arylation of Carbohydrate Derivatives. J. Am. Chem. Soc. 2017, 139 (43), 15515–15521. 10.1021/jacs.7b09420. [DOI] [PubMed] [Google Scholar]
- a Quach T. D.; Batey R. A. Copper(II)-Catalyzed Ether Synthesis from Aliphatic Alcohols and Potassium Organotrifluoroborate Salts. Org. Lett. 2003, 5 (8), 1381–1384. 10.1021/ol034454n. [DOI] [PubMed] [Google Scholar]; b Shade R. E.; Hyde A. M.; Olsen J.-C.; Merlic C. A. Copper-Promoted Coupling of Vinyl Boronates and Alcohols: A Mild Synthesis of Allyl Vinyl Ethers. J. Am. Chem. Soc. 2010, 132 (4), 1202–1203. 10.1021/ja907982w. [DOI] [PubMed] [Google Scholar]; c Winternheimer D. J.; Merlic C. A. Alkoxydienes via Copper-Promoted Couplings: Utilizing an Alkyne Effect. Org. Lett. 2010, 12 (11), 2508–2510. 10.1021/ol100707s. [DOI] [PubMed] [Google Scholar]; d Bolshan Y.; Batey R. A. Copper-catalyzed cross-coupling of amides and potassium alkenyltrifluoroborate salts: a general approach to the synthesis of enamides. Tetrahedron 2010, 66 (27–28), 5283–5294. 10.1016/j.tet.2010.03.076. [DOI] [PubMed] [Google Scholar]
- a Nordmann G.; Buchwald S. L. A Domino Copper-Catalyzed C-O Coupling-Claisen Rearrangement Process. J. Am. Chem. Soc. 2003, 125 (17), 4978–4979. 10.1021/ja034809y. [DOI] [PubMed] [Google Scholar]; For applications, see:; b Wipf P.; Waller D. L.; Reeves J. T. Transition-Metal-Mediated Cascade Reactions: The Water-Accelerated Carboalumination-Claisen Rearrangement-Carbonyl Addition Reaction. J. Org. Chem. 2005, 70 (20), 8096–8102. 10.1021/jo051211v. [DOI] [PubMed] [Google Scholar]; c Lu C.; Su X.; Floreancig P. E. Stereocontrolled Cyanohydrin Ether Synthesis through Chiral Brønsted Acid-Mediated Vinyl Ether Hydrocyanation. J. Org. Chem. 2013, 78 (18), 9366–9376. 10.1021/jo4016002. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Amos S. G. E.; Nicolai S.; Waser J. Photocatalytic Umpolung of N- and O-substituted alkenes for the synthesis of 1,2-amino alcohols and diols. Chem. Sci. 2020, 11, 11274–11279. 10.1039/D0SC03655B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.; Li T.; Qu X.; Sun P.; Yang H.; Mao J. Copper(I)-catalyzed synthesis of 1,3-enynes via coupling between vinyl halides and alkynes or domino coupling of vinyl halides. Org. Biomol. Chem. 2011, 9, 7309–7312. 10.1039/c1ob06210g. [DOI] [PubMed] [Google Scholar]
- Ma D.; Cai Q. Copper/Amino Acid Catalyzed Cross-Couplings of Aryl and Vinyl Halides with Nucleophiles. Acc. Chem. Res. 2008, 41 (11), 1450–1460. 10.1021/ar8000298. [DOI] [PubMed] [Google Scholar]
- Diamine ligands L3 and L4 have been used for C–N cross-couplings with vinylic halides and intramolecular C–O cross-couplings.; a Jiang L.; Job G. E.; Klapars A.; Buchwald S. L. Copper-Catalyzed Coupling of Amides and Carbamates with Vinyl Halides. Org. Lett. 2003, 5 (20), 3667–3669. 10.1021/ol035355c. [DOI] [PubMed] [Google Scholar]; b Larsson P.-F.; Correa A.; Carril M.; Norrby P.-O.; Bolm C. Copper-Catalyzed Cross-Couplings with Part-per-Million Catalyst Loadings. Angew. Chem., Int. Ed. 2009, 48 (31), 5691–5693. 10.1002/anie.200902236. [DOI] [PubMed] [Google Scholar]; c Cai J.; Wang Z.-K.; Usman M.; Lu Z.-W.; Hu X.-D.; Liu W.-B. Enantioselective Synthesis of β-Quaternary Carbon-Containing Chromanes and 3,4-Dihydropyrans via Cu-Catalyzed Intramolecular C-O Bond Formation. Org. Lett. 2019, 21 (21), 8852–8856. 10.1021/acs.orglett.9b03549. [DOI] [PubMed] [Google Scholar]; d Wang L.; Lei X.; Wang Q.; Li Y. Copper-catalyzed cross-coupling of amino acid-derived amides with (Z)-vinyl iodides: Unexpected solvent effect and preparation of plocabulin. Tetrahedron 2021, 83, 131953. 10.1016/j.tet.2021.131953. [DOI] [Google Scholar]
- Shen R.; Lin C. T.; Bowman E. J.; Bowman B. J.; Porco J. A. Lobatamide C: Total Synthesis, Stereochemical Assignment, Preparation of Simplified Analogues, and V-ATPase Inhibition Studies. J. Am. Chem. Soc. 2003, 125 (26), 7889–7901. 10.1021/ja0352350. [DOI] [PubMed] [Google Scholar]
- Ding J.; You Y.; Weng Z. Copper-mediated trifluoroethoxylation of vinyl bromides. Tetrahedron Lett. 2016, 57 (15), 1724–1727. 10.1016/j.tetlet.2016.03.023. [DOI] [Google Scholar]
- Shin J.; Thompson D. H. Direct Synthesis of Plasmenylcholine from Allyl-Substituted Glycerols. J. Org. Chem. 2003, 68 (17), 6760–6766. 10.1021/jo026826w. [DOI] [PubMed] [Google Scholar]
- a Tye J. W.; Weng Z.; Giri R.; Hartwig J. F. Copper(I) Phenoxide Complexes in the Etherification of Aryl Halides. Angew. Chem., Int. Ed. 2010, 49 (12), 2185–2189. 10.1002/anie.200902245. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jones G. O.; Liu P.; Houk K. N.; Buchwald S. L. Computational Explorations of Mechanisms and Ligand-Directed Selectivities of Copper-Catalyzed Ullmann-Type Reactions. J. Am. Chem. Soc. 2010, 132 (17), 6205–6213. 10.1021/ja100739h. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yu H.-Z.; Jiang Y.-Y.; Fu Y.; Liu L. Alternative Mechanistic Explanation for Ligand-Dependent Selectivities in Copper-Catalyzed N- and O-Arylation Reactions. J. Am. Chem. Soc. 2010, 132 (51), 18078–18091. 10.1021/ja104264v. [DOI] [PubMed] [Google Scholar]; d Lefèvre G.; Franc G.; Tlili A.; Adamo C.; Taillefer M.; Ciofini I.; Jutand A. Contribution to the Mechanism of Copper-Catalyzed C–N and C–O Bond Formation. Organometallics 2012, 31 (22), 7694–7707. 10.1021/om300636f. [DOI] [Google Scholar]; e Giri R.; Brusoe A.; Troshin K.; Wang J. Y.; Font M.; Hartwig J. F. Mechanism of the Ullmann Biaryl Ether Synthesis Catalyzed by Complexes of Anionic Ligands: Evidence for the Reaction of Iodoarenes with Ligated Anionic CuI Intermediates. J. Am. Chem. Soc. 2018, 140 (2), 793–806. 10.1021/jacs.7b11853. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Ajitha M. J.; Pary F.; Nelson T. L.; Musaev D. G. Unveiling the Role of Base and Additive in the Ullmann-Type of Arene-Aryl C-C Coupling Reaction. ACS Catal. 2018, 8 (6), 4829–4837. 10.1021/acscatal.8b00837. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.