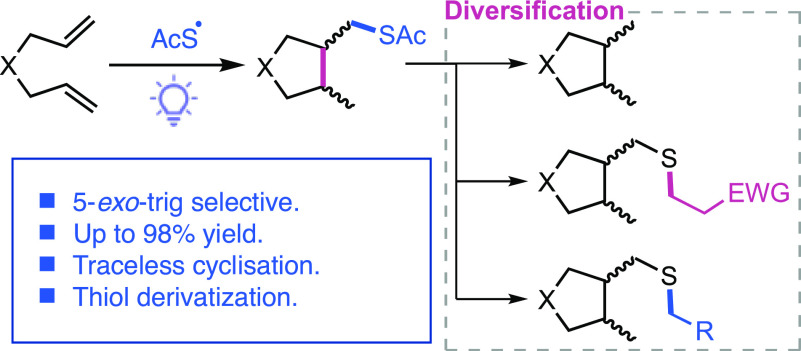
Abstract
Five-membered ring systems are ubiquitous throughout natural products and synthetic therapeutics, and thus, efficient methods to access this essential scaffold are required. Herein, we report the thioacid-mediated, 5-exo-trig cyclization of various 1,6-dienes, with high yields of up to 98%. The labile thioester functionality can be exploited to generate a free thiol residue which can be used as a functional handle or removed entirely to provide the traceless cyclized product.
Introduction
Cyclopentane, pyrrolidine, and tetrahydrofuran rings are ubiquitous scaffolds found across natural products and therapeutics and are therefore of considerable synthetic interest. In 2021, all of the “top-ten” small molecule pharmaceuticals, with cumulative sales of over $83.5 BN, contained at least one five-membered ring.1 Free-radical mediated cyclization reactions are widely utilized for the synthesis of functionalized ring systems with the cyclization of 1,6-di-unsaturated precursors attracting significant recent interest,2−6 in particular for applications in natural product synthesis.2,7−9 Thiyl radicals have received considerable attention as reactive intermediates with diverse applications in the fields of organic chemistry and chemical biology, as well as in polymer science.10−15 Cyclization of 1,6-dienes (Figure 1) through a cascade process initiated by thiyl radical addition to an alkene was first reported by Kuehne and Damon in 1977.16 Since then, a variety of conditions for this radical cascade reaction have been investigated. However, utilization of thioacids to furnish cyclic thioester derivatives suitable for further modification or concomitant desulfurization has not previously been reported. Moreover, detailed discussion on the scope and establishment of limitations remains elusive. Herein, we report a rapid and mild, 1,6-diene cyclization reaction propagated by sulfur-centered radicals, employing AcSH as the radical source. Furthermore, we investigate the impact of varying alkene substitution on the cyclization to provide insight into the limitations of such reaction-types, as well as computational insight into the origin of cis-selectivity. Finally, we exploit the thioacetate group present in the products of this reaction for further functionalization, including desulfurization, Michael addition followed by oxidation to sulfones, and SN2 chemistry.
Figure 1.
Radical-mediated 1,6-diene cyclization.
Results and Discussion
Our initial investigation focused on diallylmalonate 1 in the presence of thioacetic acid (AcSH) under UV-initiated conditions (Table 1). As expected, in the absence of UV irradiation and under an inert atmosphere, no cyclization was observed (entries 1 and 2). Irradiation in EtOAc in the presence of 2,2-dimethoxy-2-phenylacetophenone (DPAP) and 4-methoxyacetophenone (MAP) gave quantitative conversion to the cyclized product (entry 3). Use of either DPAP only or MAP only gave slightly reduced yields. Irradiation at 365 nm in the absence of DPAP and MAP furnished the cyclized product, albeit in reduced yield with a longer reaction time of 3 h (entry 6). Application of O2-initiated conditions previously utilized within the group17,18 gave reasonable conversion (entry 5). Blue LED photoactivation conditions were investigated with a range of initiators (entries 8–10) with Eosin Y and 9H-thioxanthen-9-one emerging as optimal initiators under these conditions (entry 8 and 10). Addition of TEMPO prevented any product formation, confirming the radical nature of the process. NMR timescale experiments over 2 h showed that this was sufficient time for consumption of the starting material while the control reaction in dark conditions showed no appreciable consumption of the alkene over 2 h.
Table 1. Optimization of Reaction Conditionsa.
| entry | additive | solvent | λ (nm) | conversion (%) |
|---|---|---|---|---|
| 1 | DPAP/MAP | CDCl3 | 0 | |
| 2b | CDCl3 | 0 | ||
| 3 | DPAP/MAP | EtOAc | 365 | 99 |
| 4 | DPAP | CDCl3 | 365 | 96 |
| 5 | MAP | CDCl3 | 365 | 94 |
| 6c | CDCl3 | 365 | 70 | |
| 7 | atm. O2 | EtOAc | 68 | |
| 8 | eosin Y | MeCN | 420 | 88 |
| 9 | acridine orange | CH2Cl2 | 420 | 41 |
| 10 | 9H-thioxanthen-9-one | CH2Cl2 | 420 | 86 |
Standard conditions: 0.1 M 1, DPAP (10 mol %), MAP (10 mol %), 2 h. Conversion measured by 1HNMR by integration of the product peak compared to the internal standard.
Inert atmosphere.
3 h reaction time.
Following optimization of the cyclization of the model substrate, we sought to evaluate the scope of the reaction (Scheme 1) in relation to heteroatomic substrates bearing N, O, or S atoms within the 1,6-diene backbone, in part due to their high frequency in therapeutics and natural products. Initial attempts to cyclize diallylamine were unsuccessful, although this is not surprising given the basic nature of the amine.19 Diallyl ether was successfully cyclized to give 2b with 60% yield; however, for diallyl sulfide, only traces of the desired product were detected, presumably due to the fragmentation of the beta-thioalkyl radical intermediate. Contrary to simple diallylamine, N-protected diallylamine-based substrates delivered the desired N-protected pyrrolidines in high yields of up to 98%. For instance, diallylacetamide cyclization yielded 2c in 89% yield. Likewise, trifluroacetylated and chloroacetylated derivatives gave excellent respective yields of 93% (2d) and 98% (2e). Boc and tosyl protection was also well tolerated, pyrrolidines 2f and 2g being obtained in 68 and 90% yields, respectively.
Scheme 1. Scope and Limitations for Thioacid-Initiated 1,6-Diene Cyclizations.

We then turned to investigation of varying the degree of substitution on the alkene groups of the 1,6-diene scaffold. A range of di- and tri-substituted alkene-bearing substrates was synthesized. These precursors bearing either methyl or phenyl substituents were subjected to optimized cyclization conditions. High levels of regioselectivity were observed when one of the two C=C bonds was nonterminal, with the addition of the acylthiyl radical taking place exclusively at the least sterically hindered alkenes (2h and 2i). Interestingly, precursor 1j presenting two internal, di-substituted alkenes furnished the desired cyclic compounds in good yield (57% for 2j). However, 1k having two trisubstituted alkenyl moieties gave an inseparable mixture of products containing cyclic and singly hydrothiolated species. Substitutions with phenyl groups at the alkene led to significant decreases in yields (2l and 2m), to the point of no reaction of the substrate bearing a phenyl substituent on both alkenes, most likely due to the formation of resonance-stabilized intermediates which inhibit the radical chain process, as well as due to the poorer reactivity of styrene-type alkenes to thiyl radicals.20
Brief investigation into variation of the thioacid component of the reaction demonstrated that this transformation is not unique to AcSH and can be used to generate a diverse range of thioesters. In the case of synthetically prepared thioacids, the corresponding S-trityl thioester was deprotected using 25% TFA in DCM in the presence of ethyldimethylsilane and dried in vacuo directly prior to use in the cyclization reaction without further purification. The aliphatic thioheptanoic acid gave reasonable yield of 3a at 64%, with a good d.r. of 8.5:1. The other aliphatic thioacid example 3b gave a very good yield of 80%, also with good d.r. of 8:1. Aromatic thioacids yielded 3c and 3d in moderate yields and good d.r. The glycine amino acid derivative yielded 3e in very good yield and good d.r. Importantly, these results demonstrate the tolerance of more sterically hindered thioacids than AcSH, often with improved d.r. This can facilitate the use of different thioacids to improve d.r. but at the potential expense of yield.
We also investigated the potential of this methodology for generation of larger ring systems. 1,7-Diene 4a was synthesized to potentially afford the larger, 6-membered ring. Subjecting this substrate to the cyclization conditions, however, gave no cyclized product, instead furnishing bis-hydrothiolated product 4b in 13% yield. This is likely due to must faster kinetics for attack of the thiyl radical on the alkene when compared to cyclization of the larger 6-membered system, noting that thiyl radical addition to alkenes is often a highly efficient process. Furthermore, the 6-membered transition state would require different structural conformation. The cyclization of substrates 5a and 5b containing a single α,β-unsaturated moiety was also investigated; however, neither yielded cyclic products. We then turned to investigation of cyclization of enyne 7. A small amount of alkene consumption was observed for this substrate when equimolar quantities of AcSH were used, but no cyclization product was obtained. Use of excess AcSH (3 equiv) gave complete consumption of both alkene and alkyne, but again, no cyclic products could be isolated. Despite the limitations of this methodology toward formation of larger ring sizes, these results show potentially beneficial selectivity for 1,6-diene systems over other unsaturated pi-systems.
The diastereoselectivities observed in cyclizations of hex-5-enyl radicals such as those investigated are often explained using the Beckwith–Houk model (Figure 2), which invokes a chairlike transition state in which substituents preferentially adopt pseudo-equatorial positions.21−23 DFT studies have since verified this model for protected N-substituted systems.24 Exo ring closure through this chairlike conformation then yields the cis product. Additionally, computational investigation into the observed exo selectivities was conducted for the thioacid-mediated system. The potential energy surface (PES) shows the lowest energy for the starting material conformation corresponding to the trans exo product, although the cis analogue lies only 0.4 kcal/mol higher. However, ΔG for the cis TS is marginally lower than that of the trans TS with respect to their starting conformations, at 10.7 and 10.8 kcal/mol, respectively. TS energies for the endo cyclization are significantly higher in both cis and trans cases. While the endo products are the more stable thermodynamic products, the exo products are the kinetic products in the case of this reaction. This can be attributed to the starting conformation for the exo more readily geometrically facilitating pre-TS assembly.
Figure 2.
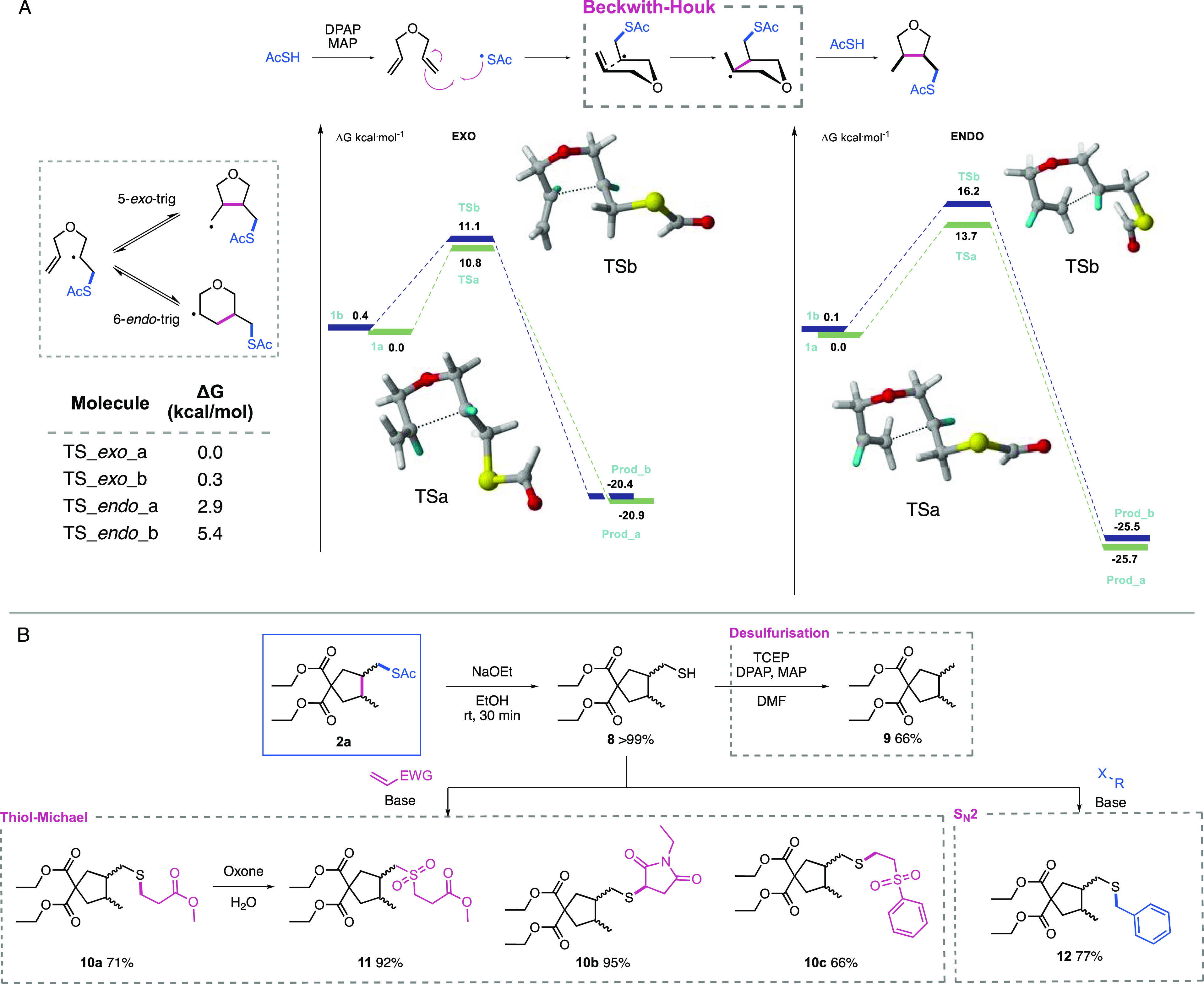
(A) Selectivity of 1,6-diene cyclizations. (B) Further diversification via the thiol handle.
Another key advantage of this methodology for generation of 5-membered rings is in the potential for further derivatization via the exocyclic sulfur atom (Figure 2). Facile deacetylation to cleave the thioester furnishes a thiol handle of great synthetic potential for either further modification of the 5-membereed ring or conjugation to other moieties. The thiol handle could then be exploited without any need for chromatographic purification. We investigated the desulfurization of these cyclized thiol products, furnishing the cyclized diene in a traceless manner. Photochemical desulfurization of thiol 8 with tris(2-carboxyethyl)phosphine hydrochloride (TCEP) gave traceless cyclic product 9 in 66% yield, amounting to a three-step 65% yield in traceless thioacid-mediated 1,6-diene cyclization. Such desulfurative conditions have been applied to generation of C-centered radicals for C–C bond formation,25 another application to which these cyclic thiol products are suited.
We then demonstrated these thiols as nucleophiles in two common classes of reaction; thiol-Michael and SN2. The simple Michael acceptor methyl acrylate proceeded with a good yield of 71% (10a). This was then readily converted to the corresponding sulfone 11 using oxone with a high yield of 92%, amounting to a 64% overall yield for the four steps from diene to sulfone. As a result, this cyclization-deacylation approach has facilitated the installation of further functionality, followed by conversion to a highly medicinally relevant sulfone. Maleimides represent another acceptor system commonly used in cysteine modification in peptides and proteins. Addition of thiol 8 to N-ethyl maleimide proceeded with excellent yield of 95% (10b). A further class of Michael acceptors includes vinyl sulfones, and reaction with phenyl vinyl sulfone proceeded with a yield of 66% (10c). SN2 reaction of thiol 8 with benzyl bromide demonstrated an additional route for further functionalization, proceeding with a good yield of 77% (12).
Conclusions
In conclusion, we have reported extensive study into the thioacid-mediated 5-exo-trig selective cyclization of 1,6-dienes. We have established the effects of varying alkene substitution on the yield of the cyclization reaction and investigated the origin of the observed exo selectivity via computational studies. We have demonstrated that through facile hydrolysis of the thioester, the corresponding thiol can be utilized for further derivatization or to access traceless cyclized products, offering facinating prospects for diversity-oriented synthesis and drug discovery.
Experimental Section
General Procedure
Commercial materials were obtained from Sigma-Aldrich, Fluorochem, Alfa-Aesar, or Fisher Scientific and used without further purification. Chromatographic separation was performed on Silica gel Florisil (200 mesh; Aldrich). Thin-layer chromatography (TLC) was performed on Merck 60 F254 silica gel plates and visualized by UV light, molybdenum, ninhydrin, or sulfuric acid staining. Dry solvents were obtained from a Pure Solv Micro Solvent Purification System. Deuterated solvents for use in NMR were purchased from Apollo Scientific. NMR data were obtained using a Bruker Advance 400 spectrometer and Bruker Ultrashield 600 and processed using Bruker TopSpin software. ESI mass spectra were acquired using a Bruker micrOTOF-Q III spectrometer interfaced to a Dionex UltiMate 3000 LC in positive and negative modes as required. The instrument was calibrated using a tune mix solution (Agilent Technologies ESI-l Low concentration tuning mix); this was also used as an internal lock mass. Masses were recorded over the range 100–2000 m/z. Operating conditions were as follows: end-plate offset 500 V capillary 4500 V, nebulizer 2.0Bar, dry gas 8.0 L/min, and dry temperature 180 °C. MicroTof control 3.2 and HyStar 3.2 software were used to carry out the analysis. UV reactions were performed in a Luzchem LZC-EDU (110 V/60 Hz) photoreactor housing 12 UV lamps centered at 365 nm. Reactions were performed in borosilicate glass and were centered in the reactor (approx. 10 cm from walls of the reactor).
General Procedure for Thioacid-Initiated 1,6-Diene Cyclization
To a 0.1 M solution of diene (1.0 equiv), DPAP (0.1 equiv), and MAP (0.1 equiv) in EtOAc, AcSH (1.2 equiv) was added. The mixture was then irradiated at 365 nm for 2 h and then concentrated in vacuo. The product was then purified by silica gel flash chromatography.
(2a) Diethyl-3-((acetylthio)methyl)-4-methylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from diethyl diallylmalonate (0.298 g, 1.24 mmol) and purified by silica gel flash chromatography in Hex/EtOAc (10%) to yield the product as a colorless oil (0.385 g, 98%). Rf = 0.23 (10% Hex/EtOAc). 1H NMR (400 MHz, CDCl3): δ 4.19–4.14 (m, 4H), 2.94 (dd, J = 13.2, 6.5 Hz, 1H), 2.78 (dd, J = 13.2, 6.5 Hz, 1H), 2.47–2.37 (m, 2H), 2.32 (s, 3H), 2.27–2.14 (m, 2H), 2.09–2.04 (m, 1H), 2.01–1.96 (m, 1H), 1.26–1.20 (t, J = 7.1 Hz, 6H), 1.04 (d, J = 5.9 Hz, 0.33H), 0.92 (d, J = 6.9 Hz, 2.67H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.8, 172.8, 172.7, 61.6, 58.9, 42.6, 41.3, 38.3, 36.2, 30.8, 29.9, 14.9, 14.2. HRMS: (ESI+) m/z calcd. For C15H25O5S ([M + H]+): 317.1417, found 317.1420.
(2b) S-((4-Methyltetrahydrofuran-3-yl)methyl)ethanethioate
Prepared following the general procedure from diallyl ether (0.200 g, 2.04 mmol) and purified by silica gel flash chromatography in Hex/EtOAc (2–5%) to yield the product as a colorless oil (0.212 g, 60%). Rf = 0.47 (20% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 4.04–3.95 (m, 2H), 3.53–3.47 (m, 1H), 3.38–3.33 (m, 1H), 3.14–3.09 (m, 1H), 2.88 (dd, J = 13.5, 7.8 Hz, 1H), 2.37 (s, 3H), 2.05–1.95 (m, 2H), 1.09 (d, J = 6.5 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3) δ 195.5, 75.2, 72.8, 46.8, 39.5, 31.2, 30.6, 16.9. HRMS: (APCI) m/z calcd. For C8H15O2S ([M + H]+): 175.0787, found 175.0796 υmax (ATR/cm–1): 2971 (C–H stretch), 2933 (C–H stretch), 1689 (C=O stretch).
(2c) S-((1-Acetyl-4-methylpyrrolidin-3-yl)methyl)ethanethioate
Prepared following the general procedure from 13a (0.0557 g, 0.40 mmol) and purified by silica gel flash chromatography in EtOAc to yield the product as a colorless oil (rotamers of diastereomers, 0.0761 g, 89%). Rf = 0.08 (EtOAc). 1H NMR (400 MHz, CDCl3): δ 3.85–3.80 (m, 0.32 H), 3.71–3.52 (m, 1.60 H), 3.35–2.98 (m, 3H), 2.90–2.80 (m, 1H), 2.49–2.36 (m, 4H), 2.11–1.85 (m, 4H), 1.14–1.11 (m, 1H), 1.06–1.01 (m, 2H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.3, 195.2, 169.5, 169.4, 169.1, 54.5, 54.2, 52.6, 52.3, 52.1, 50.6, 50.4, 48.9, 46.1, 44.4, 42.4, 40.6, 38.5, 37.1, 35.8, 34.3, 30.7, 30.7, 30.6, 30.1, 29.1, 27.9, 27.8, 22.3, 22.2, 22.1, 22.1, 16.2, 16.1, 13.3, 13.3. HRMS: (ESI+) m/z calcd. For C10H17NO2SNa ([M + Na]+): 238.0872, found 238.0874. υmax (ATR/cm–1): 2963 (C–H stretch), 1687 (C=O stretch), 1625 (C=O stretch).
(2d) S-((4-Methyl-1-(2,2,2-trifluoroacetyl)pyrrolidin-3-yl)methyl)ethanethioate
Prepared following the general procedure from 13d (0.0773 g, 0.40 mmol) and purified by silica gel flash chromatography in DCM to yield the product as a colorless oil (rotamers of diastereomers, 0.1000 g, 93%). Rf = 0.60 (DCM). 1H NMR (400 MHz, CDCl3): δ 3.97–3.65 (m, 2H), 3.51–2.98 (m, 3H), 2.90–2.81 (m, 1H), 2.54–2.38 (m, 4H), 2.14–1.94 (m, 1H), 1.16 (app-q, 1H), 1.06 (dd, J = 7.0, 2.1 Hz, 2H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.0, 194.9, 155.9, 155.6, 116.3 (q, J = 287.4 Hz), 54.1, 53.9, 53.3, 53.2, 53.2, 51.9, 50.4, 49.5, 49.4, 46.1, 43.4, 42.4, 39.7, 38.6, 35.9, 33.2, 30.6, 29.6, 29.4, 27.6, 27.3, 15.9, 15.7, 13.1, 12.9. HRMS: (ESI+) m/z calcd. For C10H14F3NO2SNa ([M + Na]+): 292.0590, found 292.0591. υmax (ATR/cm–1): 2966 (C–H stretch), 1684 (C=O stretch).
(2e) S-((1-(2-Chloroacetyl)-4-methylpyrrolidin-3-yl)methyl)ethanethioate
Prepared following the general procedure from 13e (0.0695 g, 0.40 mmol) and purified by silica gel flash chromatography in DCM/MeOH (0–5%) to yield the product as a colorless oil (rotamers of diastereomers, 0.0973 g, 98%). Rf = 0.60 (5% DCM/MeOH (v/v)). 1H NMR (400 MHz, CDCl3): δ 4.03–3.99 (m, 2H), 3.90–2.80 (m, 8H), 2.90–2.81 (m, 1H), 2.57–2.31 (m, 5H), 2.17–1.78 (m, 1H), 1.14 (t, J = 6.7 Hz, 1H), 1.05 (app-t, 2H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.2, 195.2, 165.1, 164.8, 53.7, 53.4, 53.3, 53.0, 51.2, 51.1, 49.9, 49.6, 46.2, 44.1, 42.6, 41.8, 41.7, 41.7, 40.6, 40.3, 38.6, 36.7, 36.0, 34.0, 30.7, 29.9, 29.7, 27.8, 27.6, 16.1, 10.4, 13.2, 13.2. HRMS: (ESI+) m/z calcd. For C10H16ClNO2SNa ([M + Na]+): 272.0482, found 272.0485. υmax (ATR/cm–1): 2963 (C–H stretch), 1686 (C=O stretch), 1646 (C=O stretch).
(2f) tert-Butyl-3-((acetylthio)methyl)-4-methylpyrrolidine-1-carboxylate
Prepared following the general procedure from 13b (0.0789 g, 0.40 mmol) and purified by silica gel flash chromatography in Hex/EtOAc (0–15%) to yield the product as a colorless oil (rotamers of diastereomers, 0.0737 g, 68%). Rf = 0.17 (10% EtOAc/Hexane (v/v)). 1H NMR (400 MHz, CDCl3): δ 3.64–3.59 (m, 0.75H), 3.52–4.43 (m, 1.25H), 3.19–3.11 (m, 1.55H), 3.02–2.79 (m, 2.55H), 2.40–2.29 (m, 4.17H), 1.98–1.92 (m, 0.79H), 1.48 (s, 5.70H), 1.47 (s, 3.30H), 1.09 (d, J = 6.20 Hz, 1.11H), 1.01 (d, J = 6.80, 1.81 Hz, 1.81H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.4, 195.3, 154.7, 154.4, 79.2, 53.0, 52.7, 50.7, 49.2, 30.6, 30.3, 28.5 28.0, 16.3, 13.3. HRMS: (ESI+) m/z calcd. For C13H23NO3SNa ([M + Na]+): 296.1291, found 296.1294. υmax (ATR/cm–1): 2972 (C–H stretch), 1687 (C=O stretch), 1131 (C–O stretch).
(2g) S-((4-Methy-1-tosylpyrrolidin-3-yl)methyl)ethanethioate
Prepared following the general procedure from 13c (0.2000 g, 0.79 mmol) and purified by silica gel flash chromatography in Hex/EtOAc (10%) to yield the product as a pale yellow oil (0.2330 g, 90%). Rf = 0.34 (10% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 7.69 (dd, J = 8.2, 2.6 Hz, 2H), 7.31 (dd, J = 8.4, 2.2 Hz 2H), 3.53–3.32 (m, 2H), 3.09–2.74 (m, 3H), 2.62 (m, 1H), 2.42 (d, J = 2.5 Hz, 3H), 2.30 (d, J = 4.2 Hz, 3H), 2.28–2.11 (m, 1H), 1.90–1.74 (m, 1H), 0.81 (d, J = 6.5 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.2, 143.6, 134.0, 133.8, 129.8, 127.6, 127.5, 54.8, 54.5, 41.9, 35.6, 30.7, 27.8, 21.6, 13.2. HRMS: (ESI+) m/z calcd. For C13H23NO3SNa ([M + Na]+): υmax (ATR/cm–1): 2884 (C–H stretch), 1688 (C=O stretch).
(2h) Diethyl-3-((acetylthio)methyl)-4-ethylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from 13g (0.2540 g, 1.00 mmol) and purified by silica gel flash chromatography in hex/EtOAc (2–5%) to yield the product as a colorless oil (0.2040 g, 62%). Rf = 0.28 (10% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 4.23–4.10 (m, 4H), 3.02 (dd, J = 13.2, 5.4 Hz, 1H), 2.62 (dd, J = 13.2, 10.1 Hz, 1H), 2.53–2.49 (m, 1H), 2.42–2.28 (m, 5H), 2.28–2.17 (m, 1H), 2.12 (dd, J = 13.6, 6.1 Hz, 1H), 2.07–1.89 (m, 2H), 1.52–1.37 (m, 1H), 1.36–1.15 (m, 7H), 0.97–0.83 (m, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.8, 172.8, 61.6, 58.8, 44.5, 41.8, 38.7, 38.7, 30.7, 29.6, 22.2, 14.1, 12.8. HRMS: (ESI+) m/z calcd. For C16H26O5SNa ([M + Na]+): 353.1399, found 353.1396. υmax (ATR/cm–1): 2972 (C–H stretch), 2874 (C–H stretch), 1725 (C=O stretch), 1689.
(2i) Diethyl-3-((acetylthio)methyl)-4-isopropylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from 13h (0.2680 g, 1.00 mmol) and purified by silica gel flash chromatography in hex/EtOAc (2–5%) to yield the product as a colorless oil (0.2280 g, 66%). Rf = 0.41 (10% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 4.26–4.08 (m, 4H), 3.17 (ddd, J = 13.0, 3.1, 1.5 Hz, 1H), 2.55–2.41 (m, 1H), 2.39–2.33 (m, 1H), 2.31 (s, 3H), 2.29–2.21 (m, 1H), 1.99–1.83 (m, 1H), 1.73–1.52 (m, 1H), 1.23 (td, J = 7.1, 3.9 Hz, 6H), 1.04 (d, J = 6.2 Hz, 3H), 0.94 (dd, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 196.1, 173.0, 61.7, 58.5, 52.0, 41.0, 38.6, 37.2, 30.7, 29.1, 28.8, 22.2, 21.8, 14.2. HRMS: (ESI+) m/z calcd. For C17H28O5SNa ([M + Na]+): 367.1555, found 367.1541. υmax (ATR/cm–1): 2963 (C–H stretch), 2160 (C–H stretch), 1722 (C=O stretch), 1683.
(2j) Diethyl-3-(1-(acetylthio)ethyl)-4-ethylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from 13i (0.2680 g, 1.00 mmol) and purified by silica gel flash chromatography in hex/EtOAc (2–5%) to yield the product as a colorless oil (0.1970 g, 57%). Rf = 0.33 (10% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 4.25–4.07 (m, 4H), 3.63–3.41 (m, 1H), 2.55–2.32 (m, 2H), 2.32–2.27 (m, 3H), 2.18 (dtd, J = 14.1, 7.2, 1.1 Hz, 1H), 2.13–2.01 (m, 1H), 1.98–1.88 (m, 2H), 1.36–1.30 (m, 3H), 1.30–1.18 (m, 6H), 0.96–0.80 (m, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.7, 172.7, 61.6, 58.4, 48.8, 43.1, 40.4, 37.6, 37.4, 36.9, 31.0, 30.9, 22.1, 21.6, 14.1, 12.3. HRMS: (ESI+) m/z calcd. For C17H28O5SNa ([M + Na]+): 367.1550, found 367.1544. υmax (ATR/cm–1): 2968 (C–H stretch), 2871 (C–H stretch), 1726 (C=O stretch), 1688.
(2k) Diethyl-3-(2-(acetylthio)propan-2-yl)-4-isopropylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from 13j (0.2960 g, 1.00 mmol) and purified by silica gel flash chromatography in hex/EtOAc (2–5%) to yield the product as a colorless oil (0.1730 g, 47%, inseparable mixture of products). Rf = 0.39 (10% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 4.21–4.05 (m, 4H), 2.74–2.60 (m, 1H), 2.42 (ddd, J = 13.5, 3.9, 2.5 Hz, 1H), 2.34 (s, 3H) 2.17–2.09 (m, 2H), 2.08–1.90 (m, 2H), 1.71–1.55 (m, 1H), 1.33–1.19 (m, 6H), 1.04–0.76 (m, 12H). 13C{1H} NMR (151 MHz, CDCl3): δ 194.2, 170.5, 61.7, 55.8, 51.4, 48.5, 34.9, 30.9, 30.6, 26.7, 26.6, 24.6, 18.4, 16.9, 14.2, 14.0. HRMS: (ESI+) m/z calcd. For C19H32O5SNa ([M + Na]+): 395.1863, found, 395.1878. υmax (ATR/cm–1): 2964 (C–H stretch), 1727 (C=O stretch), 1693.
(2l) Diethyl-4-((acetylthio)methyl)-3-methyl-3-phenylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from 13l (0.3160 g, 1.00 mmol) and purified by silica gel flash chromatography in hex/EtOAc (2–5%) to yield the product as a colorless oil (0.1374 g, 35%). Rf = 0.30 (10% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 7.37–7.27 (m, 3H), 7.24–7.17 (m, 2H), 4.29–4.09 (m, 4H), 3.42 (dd, J = 13.4, 1.3 Hz, 1H), 3.23 (dd, J = 16.3, 13.4 Hz, 1H), 3.03–2.86 (m, 1H), 2.73–2.59 (m, 1H), 2.58–2.48 (m, 1H), 2.47–2.24 (m, 2H), 2.25–2.19 (m, 3H), 1.35–1.13 (m, 6H), 1.01 (d, J = 6.8 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.2, 172.8, 172.3, 144.7, 128.4, 128.1, 127.9, 126.5, 61.8, 58.1, 52.4, 45.7, 45.0, 41.1, 34.0, 30.7, 30.6, 14.2. HRMS: (ESI+) m/z calcd. For C21H28O5SNa ([M + Na]+): 415.1555, found 415.1553. υmax (ATR/cm–1): 2979 (C–H stretch), 1724 (C=O stretch), 1690.
(2m) Diethyl-3-((acetylthio)methyl)-4-benzylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from 13k (0.1530 g, 0.48 mmol) and purified by silica gel flash chromatography in hex/EtOAc (2–5%) to yield the product as a colorless oil (0.0262 g, 14%). Rf = 0.16 (5% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 7.28 (dd, J = 8.3, 7.0 Hz, 2H), 7.21–7.18 (m, 3H), 4.22–4.10 (m, 4H), 3.11 (dd, J = 13.2, 6.3 Hz, 1H), 2.86 (dd, J = 13.2, 9.5 Hz, 1H), 2.80 (d, J = 8.4 Hz, 1H), 2.48–2.39 (m, 3H), 2.34 (d, J = 2.0 Hz, 3H), 2.33–2.27 (m, 1H), 2.27–2.15 (m, 2H), 2.14–2.03 (m, 1H), 1.29–1.20 (m, 9H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.7, 172.8, 172.6, 140.7, 122.9, 128.4, 126.0, 61.6, 61.5, 61.4 60.0, 58.5, 43.9, 42.4 39.7, 39.5, 38.5, 37.8, 35.0, 33.6, 33.5, 31.4, 30.6, 29.6, 14.2, 14.1. HRMS: (ESI+) m/z calcd. For C21H28O5SNa ([M + Na]+): 415.1555, found 415.1553. υmax (ATR/cm–1): 2979 (C–H stretch), 1723 (C=O stretch).
(3a) Diethyl-3-((heptanoylthio)methyl)-4-methylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from diethyl diallylmalonate (0.150 mL, 0.1489 g 0.62 mmol) and crude thioacid obtained via the general procedure for S-trityl deprotection from the trityl thioester 17a (0.2860 g, 0.74 mmol). The product was purified by silica gel flash chromatography in hex/EtOAc (2–5%) to yield the product as a colorless oil (0.1500 g, 65%). Rf = 0.31 (4% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 4.17 (m, 4H), 2.94 (dd, J = 13.2, 6.5 Hz, 2H), 2.78 (dd, J = 13.2, 8.6 Hz, 2H), 2.56–2.50 (m, 4H), 2.48–2.35 (m, 4H), 2.28–2.13 (m, 4H, CH2), 2.07 (dd, J = 13.2, 8.6 Hz, 2H), 1.99 (dd, J = 13.8, 5.7 Hz, 2H), 1.64 (dt, J = 15.0, 7.7 Hz, 4H), 1.36–1.20 (m, 12H), 1.04 (d, J = 5.9 Hz, 3H), 0.92 (d, J = 6.9 Hz, 6H), 0.88 (t, J = 6.9 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 199.6, 172.7, 61.6, 58.9, 44.3, 42.7, 41.3, 38.3, 36.2, 31.6, 29.5, 28.8, 25.8, 22.6, 14.9, 14.2. HRMS: (APCI) m/z calcd. For C20H35O5S ([M + H]+): 387.2205, found 387.2199. υmax (ATR/cm–1): 2958 (C–H stretch), 2931 (C–H stretch), 2873 (C–H stretch), 1729 (C=O), 1690 (C=O).
(3b) Diethyl-3-(((3-ethoxypropanoyl)thio)methyl)-4-methylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from diethyl diallylmalonate (80 μL, 0.0793 g, 0.33 mmol) and crude thioacid obtained via the general procedure for S-trityl deprotection from the trityl thioester 17b (0.1505 g, 0.4 mmol). The product was purified by silica gel flash chromatography in hex/EtOAc (2–10%) to yield the product as a yellow oil (0.0991 g, 80%). Rf = 0.31 (10% Hex/EtOAc (v/v). 1H NMR (400 MHz, CDCl3): δ 4.13–4.20 (m, 4H), 3.71 (t, J = 6.5 Hz, 2H), 3.48 (q, 7.0 Hz, 2H), 2.96 (dd, J = 13.6, 6.6 Hz, 1H), 2.78–2.84 (m, 3H), 2.37–2.47 (m, 2H), 2.14–2.27 (m, 2H), 2.04–2.10 (m, 1H), 1.98 (dd, J = 13.9, 5.7 Hz, 1H), 1.21–1.25 (dt, J = 7.1, 2.0 Hz, 6H), 1.18 (t, J = 7.1 Hz, 3H), 1.04 (d, J = 5.9 Hz, 3H), 0.91 (d, J = 6.9 Hz, 3H).13C{1H} NMR (151 MHz, CDCl3): δ 197.3, 172.6, 66.5, 65.9, 61.5, 58.9, 44.4, 42.4, 41.2, 38.2, 36.1, 29.5, 15.1, 14.8, 14.0. HRMS: (ESI) m/z calcd. For C18H30NaO6S ([M + Na]+): 397.1655, found 397.1666. υmax (ATR/cm–1): 2976 (C–H stretch), 1728 (C=O), 1689 (C=O), 1253 (C–O stretch).
(3c) Diethyl-3-((benzoylthio)methyl)-4-methylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from diethyl diallylmalonate (0.30 mL, 0.298 g, 1.24 mmol) and thiobenzoic acid (0.17 mL, 0.205 g, 1.49 mmol). The product was purified by silica gel flash chromatography in Hex/EtOAc (5% v/v) to yield the product as a pale yellow oil (0.190 g, 40%). Rf = 0.34 (10% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 7.98 (t, J = 8.0 Hz, 2H), 7.49 (tt, J = 7.4, 1.3 Hz, 1H), 7.47 (t, J = 7.7 Hz, 2H), 4.17–4.24 (m, 4H), 3.15–3.41 (m, 1H), 2.96–3.04 (m, 1H), 2.46–2.64 (m, 2H), 2.29–2.38 (m, 2H), 2.19 (dd, J = 13.5, 8.8 Hz, 1 H), 2.05 (dd, J = 13.7, 5.4 Hz, 1H), 1.24–1.28 (m, 6H), 1.01 (d, J = 6.8 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 191.8, 172.65, 172.63, 137.1, 133.3, 128.6, 127.2, 61.5, 58.9, 42.5, 41.2, 38.2, 36.2, 29.6, 17.8, 14.9, 14.0. HRMS: (APCI) m/z calcd. For C20H27O5S ([M + H]+): 379.1574, found 379.1576. υmax (ATR/cm–1): 2977 (C–H stretch), 1727 (C=O), 1662 (C=O), 1581 (Ar C–C stretch).
(3d) Diethyl-3-methyl-4-(((2-(p-tolyl)acetyl)thio)methyl)cyclopentane-1,1-dicarboxylate
Prepared following the general procedure from diethyl diallylmalonate (0.150 mL, 0.1489 g 0.62 mmol) and crude thioacid obtained via the general procedure for S-trityl deprotection from the trityl thioester 17c (0.3150 g, 0.74 mmol). The product was purified by silica gel flash chromatography in hex/EtOAc (10%) to yield the product as a colorless oil (0.1500 g, 65%). Rf = 0.27 (10% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 7.11–7.05 (m, 4H), 4.17 (qd, J = 7.2, 2.3 Hz, 4H), 2.96–2.91 (m, 3H,), 2.86–2.76 (m, 3H), 2.47–2.36 (m, 2H), 2.31 (s, 3H), 2.25–2.12 (m, 2H), 2.06 (dd, J = 13.3, 8.7 Hz, 1H), 1.99 (dd, J = 13.8, 5.7 Hz, 1H), 1.24 (td, J = 7.2, 1.2 Hz, 6H), 0.91 (d, J = 6.9 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 198.6, 172.7, 129.3, 128.3, 61.6, 58.9, 45.8, 42.6, 41.3, 38.3, 36.2, 31.2, 29.6, 21.1, 14.9, 14.2. HRMS: (APCI) m/z calcd. For C23H33O5S ([M + H]+): 421.2049, found 421.2049. υmax (ATR/cm–1): 2963 (C–H stretch), 1723 (C=O stretch).
(3e) Diethyl-3-((((((9H-fluoren-9-yl)methoxy)carbonyl)glycyl)thio)methyl)-4-methylcyclopentane-1,1-dicarboxylate
Prepared following the general procedure from diethyl diallylmalonate (0.150 mL, 0.1489 g 0.62 mmol) and crude thioacid obtained via the general procedure for S-trityl deprotection from the trityl thioester 17d (0.4140 g, 0.74 mmol). The product was purified by silica gel flash chromatography in hex/EtOAc (10%) to yield the product as a colorless oil (0.3000 g, 87%). Rf = 0.29 (10% Hex/EtOAc (v/v)). 1H NMR (400 MHz, CDCl3): δ 7.77 (d, J = 7.5 Hz, 2H), 7.61 (d, J = 7.4 Hz, 2H), 7.40 (t, J = 7.4 Hz, 2H), 7.32 (t, J = 7.2 Hz, 2H), 5.34 (br s, 1H), 4.44 (d, J = 7.0 Hz, 2H), 4.25 (t, J = 7.0 Hz, 1H), 4.20–4.11 (m, 6H), 3.02–2.97 (m, 1H, CH2), 2.87–2.82 (m, 1H), 2.48–2.37 (m, 2H), 2.28–2.15 (m, 2H), 2.11–2.05 (m, 1H), 2.01–1.96 (m, 1H), 1.27–1.20 (m, 6H), 0.92 (d, J = 6.9 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 197.4, 172.7, 172.7, 156.3, 143.9, 141.5, 127.9, 127.2, 125.2, 120.1, 67.5, 61.7, 58.9, 50.8, 47.3, 42.5, 41.3, 38.3, 36.3, 29.4, 14.9, 14.2. HRMS: (APCI) m/z calcd. For C30H36NO7S ([M + H]+): 554.2207, found 554.2209. υmax (ATR/cm–1): 3315 (N–H stretch), 2936 (C–H stretch), 1711 (C=O).
Acknowledgments
This work was supported by SSPC, The SFI Research Center for Pharmaceuticals, Grant No. 12/RC/2275_p2 (MN) and (EMS), and SFI 19/FFP/6667 (EMS).
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c00824.
General information; general synthetic procedures; diene substrate syntheses; trityl thioester syntheses; thioacid-mediated cyclization of 1,6-dienes; derivatization; computational work; references; and NMR spectra of novel compounds (PDF)
Author Present Address
§ Department of Chemistry, The University of Manchester, Oxford Road, Manchester, M13 9PL (LVD & CT), U. K
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. D.M.L. and M.D.N. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- McGrath N. A.; Brichacek M.; Njardarson J. T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ. 2010, 87, 1348–1349. 10.1021/ed1003806. [DOI] [Google Scholar]
- Lynch D. M.; Scanlan E. M. Thiyl Radicals: Versatile Reactive Intermediates for Cyclization of Unsaturated Substrates. Molecules 2020, 25, 3094. 10.3390/molecules25133094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet V.; Toullec P. Y.; Genêt J. P. Cycloisomerization of 1,n-Enynes: Challenging Metal-Catalyzed Rearrangements and Mechanistic Insights. Angew. Chem. Int. Ed. 2008, 47, 4268–4315. 10.1002/ANIE.200701589. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Meng Y. N.; Huang X. J.; Qin F. H.; Wu D.; Shao Q.; Guo Z.; Li Q.; Wei W. T. Radical Cyclization of 1,6-Dienes with Azobis(Alkylcarbonitriles) on Water under Additive-Free Conditions. Green Chem. 2020, 22, 4593–4596. 10.1039/D0GC00140F. [DOI] [Google Scholar]
- Wu S. P.; Wang D. K.; Kang Q. Q.; Ge G. P.; Zheng H.; Zhu M.; Li T.; Zhang J. Q.; Wei W. T. Sulfonyl Radical Triggered Selective Iodosulfonylation and Bicyclization of 1,6-Dienes. Chem. Commun. 2021, 57, 8288–8291. 10.1039/D1CC03252F. [DOI] [PubMed] [Google Scholar]
- Yu X. C.; Zheng Y. N.; Zhang J. H.; Zhang J.; Liu F. L.; Tang K.; Li T.; Wei W. T. Metal-Catalyst-Free Radical Cyclization of 1,6-Enynes for the Selective and Switchable Synthesis of Lactams in Water. ACS Sustainable Chem. Eng. 2022, 10, 6057. 10.1021/acssuschemeng.2c01021. [DOI] [Google Scholar]
- Harrowven D. C.; Lucas M. C.; Howes P. D. Total Syntheses of Aplysin and Debromoaplysin Using a Diastereoselective, Sulfur Mediated Radical Cyclisation Strategy. Tetrahedron Lett. 1999, 40, 4443–4444. 10.1016/S0040-4039(99)00768-6. [DOI] [Google Scholar]
- Harrowven D. C.; Hannam J. C.; Lucas M. C.; Newman N. A.; Howes P. D. A Thiyl Radical Mediated Cascade Sequence for the Co-Cyclisation of 1,6-Hexadienes with Sulfur Atom Transfer. Tetrahedron Lett. 2000, 41, 9345–9349. 10.1016/S0040-4039(00)01701-9. [DOI] [Google Scholar]
- Barrero A. F.; Arseniyadis S.; Herrador M. M.; Quílez Del Moral J. F.; Arteaga J. F.; Sánchez E. M. Sulfanyl Radical-Induced Cyclization of Linalyl Acetate to the Iridane Skeleton: A Short Synthesis of (±)-Dehydroiridomyrmecin. Synlett 2005, 2005, 591–594. 10.1055/s-2005-863729. [DOI] [Google Scholar]
- Dénès F.; Pichowicz M.; Povie G.; Renaud P. Thiyl Radicals in Organic Synthesis. Chem. Rev. 2014, 114, 2587–2693. 10.1021/cr400441m. [DOI] [PubMed] [Google Scholar]
- Hoyle C. E.; Bowman C. N. Thiol-Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- McLean J. T.; Benny A.; Nolan M. D.; Swinand G.; Scanlan E. M. Cysteinyl Radicals in Chemical Synthesis and in Nature. Chem. Soc. Rev. R. Soc. Chem. 2021, 50, 10857–10894. 10.1039/d1cs00254f. [DOI] [PubMed] [Google Scholar]
- Nolan M. D.; Scanlan E. M. Applications of Thiol-Ene Chemistry for Peptide Science. Front. Chem. 2020, 8, 583272 10.3389/fchem.2020.583272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D. A. Sulfur and Its Role In Modern Materials Science. Angew. Chem. Int. Ed. 2016, 55, 15486–15502. 10.1002/ANIE.201604615. [DOI] [PubMed] [Google Scholar]
- Glass R. S. Sulfur Radicals and Their Application. Top. Curr. Chem. 2018, 376, 22. 10.1007/S41061-018-0197-0. [DOI] [PubMed] [Google Scholar]
- Kuehne M. E.; Damon R. E. Thiyl Radical Induced Cyclizations of Dienes. Cyclization of α-Acoradiene, α-Bulnesene, and Geranyl Acetate to Cedrane, Patchulane, and Cyclogeranyl Acetate Products. J. Org. Chem. 1977, 42, 1825–1832. 10.1021/jo00431a001. [DOI] [Google Scholar]
- McCourt R. O.; Scanlan E. M. Atmospheric Oxygen Mediated Radical Hydrothiolation of Alkenes. Chem. −A Eur. J. 2020, 26, 15804–15810. 10.1002/CHEM.202002542. [DOI] [PubMed] [Google Scholar]
- Nolan M. D.; Mezzetta A.; Guazzelli L.; Scanlan E. M. Radical-Mediated Thiol-Ene ‘Click’ Reactions in Deep Eutectic Solvents for Bioconjugation. Green Chem. 2022, 24, 1456–1462. 10.1039/d1gc03714e. [DOI] [Google Scholar]
- Love D. M.; Kim K.; Goodrich J. T.; Fairbanks B. D.; Worrell B. T.; Stoykovich M. P.; Musgrave C. B.; Bowman C. N. Amine Induced Retardation of the Radical-Mediated Thiol-Ene Reaction via the Formation of Metastable Disulfide Radical Anions. J. Org. Chem. 2018, 83, 2912–2919. 10.1021/acs.joc.8b00143. [DOI] [PubMed] [Google Scholar]
- Northrop B. H.; Coffey R. N. Thiol-Ene Click Chemistry: Computational and Kinetic Analysis of the Influence of Alkene Functionality. J. Am. Chem. Soc. 2012, 134, 13804–13817. 10.1021/ja305441d. [DOI] [PubMed] [Google Scholar]
- Beckwith A. L. J.; Easton C. J.; Serelis A. K. Some Guidelines for Radical Reactions. J. Chem. Soc., Chem. Commun. 1980, 11, 482–483. 10.1039/C39800000482. [DOI] [Google Scholar]
- Beckwith A. L. J.; Lawrence T.; Serelis A. K. Stereoselectivity of Ring Closure of Substituted Hex-5-Enyl Radicals. J. Chem. Soc., Chem. Commun. 1980, 11, 484–485. 10.1039/C39800000484. [DOI] [Google Scholar]
- Spellmeyer D. C.; Houk K. N. A Force-Field Model for Intramolecular Radical Additions. J. Org. Chem. 1987, 52, 959–974. 10.1021/jo00382a001. [DOI] [Google Scholar]
- Shanks D.; Berlin S.; Beşev M.; Ottosson H.; Engman L. On the Origin of Cis Selectivity in the Cyclization of N-Protected 2-Substituted 3-Aza-5-Hexenyl Radicals: A Density Functional Study. J. Org. Chem. 2004, 69, 1487–1491. 10.1021/jo030294h. [DOI] [PubMed] [Google Scholar]
- Lopp J. M.; Schmidt V. A. Intermolecular Phosphite-Mediated Radical Desulfurative Alkene Alkylation Using Thiols. Org. Lett. 2019, 21, 8031–8036. 10.1021/acs.orglett.9b03018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.