Abstract
Mol-scale oxyfunctionalization of cyclohexane to cyclohexanol/cyclohexanone (KA-oil) using an unspecific peroxygenase is reported. Using AaeUPO from Agrocybe aegerita and simple H2O2 as an oxidant, cyclohexanol concentrations of more than 300 mM (>60% yield) at attractive productivities (157 mM h–1, approx. 15 g L–1 h–1) were achieved. Current limitations of the proposed biooxidation system have been identified paving the way for future improvements and implementation.
Keywords: biocatalysis, peroxygenase, oxyfunctionalization, cyclohexane, upscaling, bulk chemical
Introduction
Biocatalysis is increasingly considered as an alternative to traditional chemical methodologies. In particular, the high selectivity of many enzymes is especially valued for the synthesis of chiral, value-added products.1−3 As a consequence, the overwhelming majority of biocatalytic processes in the chemical industry deals with the synthesis of fine or specialty chemicals or pharmaceutical intermediates. Bulk chemical applications such as the synthesis of acrylamide are scarce.4
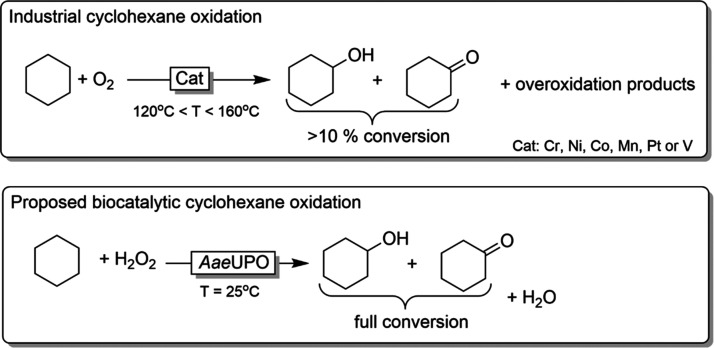
The biocatalytic oxyfunctionalization of cycloalkanes, for example, is occasionally addressed in the literature5−9 but so far has not been considered as an alternative to existing industrial practice. Especially in the case of this transformation, high chemoselectivity would be highly desirable.10 The main issue for the chemical oxidation of cyclohexane lies with the increasing reactivity of the oxidation products. In other words, the rate of overoxidation of products is faster than the rate of desired oxidation of starting materials, thereby making isolation of intermediate products such as cyclohexanol or cyclohexanone challenging. Today, the technically implemented solution to this problem is to limit conversions to less than 10% and thereby minimize reagent loss in undesired overoxidation products (Scheme 1).10 Obviously, the unreacted starting material is recycled, which however also adds complexity to the production system.
Scheme 1. Oxidation of Cyclohexane.
Outlined are the established aerobic oxidation procedures (upper) and the proposed biocatalytic alternative (lower).
Selective enzyme catalysts may represent a solution to this selectivity issue. In the past, especially cytochrome P450 monooxygenases (P450 MOs)11,12 have been considered as catalysts for the selective oxyfunctionalization of cycloalkanes.13−20 Though excellent results with full conversion and high selectivity have been achieved, the space time yields tend to be low, in the range of a few millimolar product formations per hour and low final product titers generally in the range of 5–10 mM.21 Next, the complex molecular architecture of many P450 MOs22 and also their dependency on molecular oxygen challenge their practical application at scale.23−25 So-called unspecific peroxygenases (UPOs, E.C. 1.11.2.)26,27 also catalyze the oxyfunctionalization of (cyclo)alkanes at high selectivity28 but at the same time only need hydrogen peroxide as the stoichiometric oxidant instead of the complex electron transfer chain to reductively activate molecular oxygen. Particularly, the UPO from Agrocybe aegerita (AaeUPO, PaDa-I variant)29,30 is an attractive biocatalyst for the oxyfunctionalization of, for example, cyclohexane.
Previously, we31 and the group of Hofrichter28 reported the selective, peroxygenase-catalyzed oxidation of cyclohexane yielding only cyclohexanol and cyclohexanone [i.e., ketone-alcohol (KA) oil] as products. However, in these studies, the substrate loading and consequently the product concentrations were in the lower millimolar (μmol) range and thus far too low for any preparative application.
Given the extraordinary stability and activity of AaeUPO,32 we set out to evaluate whether this enzyme may enable multi-mol-scale synthesis of KA-oil (Scheme 1).
Materials and Methods
Preparation of the Recombinant UPO from Agrocybe aegerita (AaeUPO, PaDa-I)
The biocatalyst (expression-engineered variant of the peroxygenase from Agrocybe aegerita, AaeUPO PaDa-I mutant) originated from a 2500 L pilot-scale cultivation of recombinant Pichia pastoris X-33.33 The concentrated supernatant was lyophilized at 0.1 mbar and −28 °C using a Christ Alpha 2–4 lyophilizer (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). For the 11 L reactions, 536 g of lyophilized enzyme with a total AaeUPO-amount of 456 μmol (0.85 μmolAaeUPO g–1lyophilisate) was used.
CO-Difference Spectra
AaeUPO concentrations were determined from carbon monoxide (CO)-difference spectra using the extinction coefficient at 445 nm of ε445 = 107 mM–1 cm–1.34 950 μL of protein sample, diluted in 100 mM KPi-buffer, was filled into plastic cuvettes, placed in a photometer, and blank recorded (base subtraction). After zeroing, the sample was exposed to CO for a few seconds. Next, 50 μL of 1 M sodium dithionite stock solution was added, and a difference spectrum between 400 and 500 nm was recorded. The measurements were continued until a constant absorption maximum was obtained.
H2O2 Quantification Assay
The concentration of H2O2 in the reactor was measured at different time points using a Pierce quantitative peroxide assay kit (catalog number 232802, Thermo Scientific Pierce, Rockford, IL, USA). The working reagent (WR) was prepared by mixing 100 μL of reagent A with 10 mL of reagent B as described in the kit. Samples were withdrawn every 15 min from the reactor for H2O2 analysis. Two dilutions were prepared for each sample, and the analysis of each dilution was performed in duplicate. 20 μL of sample was incubated with 200 μL of premixed WR in a 96-well plate and incubated at 25 °C for 15 mins.
The H2O2 concentration was determined by measuring the absorbance at 240 nm using a molar extinction coefficient of 43.6 M–1 cm–1.35 A standard curve was made with H2O2 concentrations ranging from 0 to 130 μM. The absorbance of standards and samples was measured at 595 nm using a microplate reader (SPECTRO star Nano; BMG LABTECH, Germany). The slope of the standard curve was used for quantification of the H2O2 concentration.
100 mL Scale Reactions
The AaeUPO-mediated hydroxylation of cyclohexane on a 100 mL scale was performed in a SYSTAG jacketed lab reactor (250 mL operational volume) at 25 °C and 300 rpm mixing speed. 100 mL of reaction solution contained 100 mM KPi buffer (pH 6), 50 vol % acetonitrile, 10–20 μM rAaeUPO (concentrated supernatant), and 500 mM cyclohexane. H2O2 solutions were freshly prepared prior to the experiment and continuously fed from a 4.5 M or 50 wt % stock solution with a H2O2-dosing rate of 50 and 200 mM h–1, respectively. The amount of added H2O2 per hour was kept constant at 1.2 g which corresponds roughly to 1.1 mL. The reaction was monitored for up to 48 h. At different time points, samples from the aqueous phase were withdrawn, extracted with 500 μL of ethyl acetate containing 5 mM of the internal standard n-dodecane (IS), and analyzed via achiral GC. Reaction mixtures were also qualitatively analyzed for H2O2 accumulation by color change using Quantofix peroxide 100 test strips (Macherey-Nagel, Düren, Germany).
AaeUPO-Mediated Oxidation at 11 L Scale under Preparative Scale Conditions
The UPO-mediated oxidation of cyclohexane was upscaled to a 11 L scale in a 35 L jacketed glass reactor (Figure 1). In total, two runs were performed.
Figure 1.
35 L-reactor used for the reaction on 10 L scale. (a) Process flow diagram (T1: temperature sensor, pH 01: pH sensor and display, P1: pump, HT in/out: hot water in/out, R-01: reactor). (b) Photograph of the reactor setup. (c) Photograph of the pump setup.
Fed-Batch 1
258 g of lyophilized AaeUPO (PaDa-I variant) was dissolved in a total volume of around 5 L of 100 mM KPi, pH 6 and added to a 35 L jacketed glass reactor. Afterward, 5.5 L of acetonitrile was pumped at 0.5 L per min using a Watson Marlow peristaltic pump 503 S (Watson-Marlow, Falmouth, UK). The agitation speed was set at 225 rpm to maintain the same power input of 2 W/L as the 100 mL batches. Then, 600 mL of cyclohexane was added using the same pump (total volume 10.7 L). The reaction was started by pumping H2O2 from a 12.75 M solution with a flow rate of 120 mL/h using a Watson-Marlow peristaltic pump 520 S (Watson-Marlow, Falmouth, UK). Every 15 min, a 5 mL sample was taken and analyzed for H2O2 concentration via a photometric assay. The product concentration was analyzed every hour for the first 2 h and subsequently every 30 min via GC-FID. The reaction was stopped after 3.75 h.
Fed-Batch 2
278 g of lyophilized AaeUPO (PaDa-I variant) was dissolved in a total volume of around 5 L of 100 mM KPi, pH 6 and placed in 35 L jacketed glass reactor. Afterward, 5.5 L of acetonitrile was pumped at 0.5 L per min using a Watson Marlow peristaltic pump 503 S (Watson-Marlow, Falmouth, UK). The agitation speed was set at 225 rpm to maintain the same power input of 2 W/L as the 100 mL batches. Then, 630 mL of cyclohexane was added using the same pump (total volume 10.7 L). The reaction was started by pumping H2O2 from a 12.75 M solution with a flow rate of 120 mL/h. Every 15 min, a 5 mL sample was taken and analyzed for H2O2 concentration via a photometric assay. The product concentration was analyzed every hour for the first 2 h and subsequently every 30 min via GC-FID. The reaction was stopped after 3.75 h.
Product Analysis
For GC analysis, a Shimadzu GC-2010 plus/FID equipped with an Agilent CP-Wax 52GB column (50 m × 0.53 mm × 2.0 μm) with N2 as the carrier gas was used. The temperature gradient was described in key points as follows: (Split 10): 90 °C hold 3 min, 10 °C/min to 180 °C hold 1 min, 30 °C/min to 230 °C hold 1 min, retention times: 10.4 min cyclohexanol, 9.1 min cyclohexanone, 6.7 min n-dodecane (IS).
Results
To test the feasibility of the proposed AaeUPO-catalyzed oxidation of cyclohexane, we used a previously reported batch of AaeUPO produced at pilot scale.33 We envisioned an initial cyclohexane concentration of 0.5 M. Since AaeUPO exhibits an exceptional stability toward acetonitrile,32 we decided using acetonitrile (50% vol/vol) as the cosolvent to improve the solubility of the reagents. H2O2 was added continuously (fed-batch mode) from a concentrated stock solution (Table 1). We deem potential safety issues to be low (at least in the small-scale experiments presented here) as the H2O2 is constantly consumed by the enzymatic reaction.
Table 1. Influence of H2O2 Feeding Rate and Enzyme Concentration on a 100 mL Scalea.
| experiment | [AaeUPO] [μM] | [cyclohexane] [mM] | H2O2-feeding rate [mM h–1] | time [h] | total product concentration [mM] | productivity [g L–1 h–1] | turnover number (TN = molProduct × molrAaeUPO–1) |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 500 | 50 | 24 | 70.7 | 0.3 | 7000 |
| 2 | 20 | 500 | 50 | 24 | 390 | 1.6 | 19,500 |
| 3 | 20 | 500 | 200 | 4 | 373 | 9.3 | 18,650 |
| 4 | 20 | 3 × 500 (0, 6, 28 h) | 20 | 24 | 269 | 1.1 | 13,450 |
Reaction conditions: [substrate] = 500 mM, [KPi, pH 6] = 100 mM, [ACN] = 50 vol %, [AaeUPO] = 10–20 μM, [H2O2-feeding rate] = 20–200 mM h–1 (4–17.6 M aqueous stock solution) starting volume 100 mL, 25 °C, 300 rpm, duplicate measurements. The detailed time courses are listed in the Supporting Information (Figures S1–S4).
In the first experiment, we used 10 μM (ca. 0.45 g L–1) AaeUPO and a H2O2 feed rate of 50 mM h–1 (Table 1, entry 1, Figure S1). The initial product formation rate was approx. 37 mM h–1 corresponding to 74% of the nominal H2O2 addition rate. However, the reaction rate dropped significantly over time and after approx. 5 h essentially ceased. This was accompanied by the accumulation of H2O2 in the reaction mixture. As a result, only 70 mM cyclohexanol: cyclohexanone (11.2:1) were found after 24 h. We reasoned that the H2O2 addition rate may have exceeded the catalytic activity of AaeUPO for cyclohexane hydroxylation resulting in a steady increase in the H2O2 concentration. The accumulation of H2O2 inactivates the enzyme, decreasing further the catalytic rate and thereby increasing the rate of accumulation of H2O2. This escalates the rate of enzyme inactivation leading to the cessation of the reaction. Therefore, in the next experiment, we doubled the enzyme concentration to 20 μM while maintaining all other conditions the same (Table 1, entry 2, Figure S2). Indeed, this resulted in a significantly more robust reaction with linear product accumulation (40.5 mM h–1 corresponding to >80% H2O2 yield) for at least 6 h.
This result encouraged us to increase the H2O2 feeding rate even more to 200 mM h–1 (Table 1, entry 3, Figure S3). Within 4 h, 373 mM product (332 mM cyclohexanol and 41 mM cyclohexanone) was formed corresponding to a productivity of 93 mM h–1 (9.3 g L–1 h–1). The reaction completely ceased after 4 h indicating complete inactivation of the biocatalyst. It is worth mentioning that in this experiment after completion, trace amounts (estimated less than 10 mM) of dual hydroxylation products (1,3- and 1,4-cyclohexanediol) were observed (Figure S8).
The reaction so far is limited by the still comparably low substrate concentration and the poor mass balance (mostly due to evaporation of the starting material), which in the current reactor setup is difficult to resolve. Initial attempts to address the loss of starting material in a fed-batch approach (Table 1, entry 4, Figure S4) largely failed as no improvement of the reaction robustness or overall product formation rate was achieved. Possibly, the occurrence of a liquid–liquid interface caused AaeUPO inactivation or reduced mass transfer. Further investigations aiming at understanding and resolving this issue are currently underway.
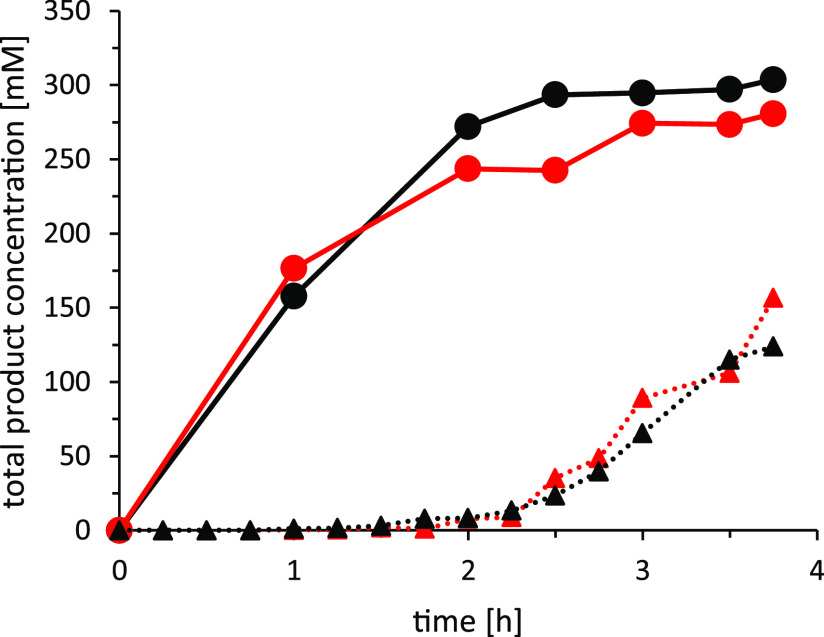
To demonstrate the scalability of the proposed AaeUPO-catalyzed oxyfunctionalization of cyclohexane, we performed two reactions with a working volume of 10.7 L in a 35 L reactor adopting the reaction conditions of Table 1, entry 3 (Figure 2). The average initial (1 h) product formation rate was at least 157 mM h–1 (approx. 15 g L–1 h–1). This corresponds well to the formal H2O2 addition rate (ca. 150 mM h–1) indicating that the H2O2 utilization was complete (close to 100% yield in H2O2). Hence, the H2O2 yield was significantly higher than in the smaller scale experiments (Table 1) with H2O2 yields ranging between 70 and 80%. In the latter case, the well-known catalase-activity of AaeUPO may account for the observation.36 Currently, we are lacking a plausible explanation for this upscaling effect. Interestingly within this initial period, practically only cyclohexanol was formed (cyclohexanol: cyclohexanone <25), whereas later cyclohexanone formation became more dominant (final ratio of cyclohexanol: cyclohexanone was approx. 10). However, the rate of product formation reduced in the next hour. As noted previously, this may be attributed to the depletion of the cyclohexane starting material (boiling point: 81 °C), which could not be quantified with our present analytical setup [due to similar boiling points of acetonitrile (boiling point: 82 °C) and the extraction solvent ethyl acetate (boiling point: 77 °C),37 separation of cyclohexane from the cosolvents on GC was not possible]. After 2 h, the product formation rate decreased considerably concomitant with the accumulation of H2O2.
Figure 2.
rAaeUPO-catalyzed oxidation of cyclohexane on a 10.9 L scale. Two individual batches are shown (black, red). (black circle, red circle): total product formed; (black triangle, red triangle): H2O2 concentration. Reaction conditions: 500 mM substrate (starting) concentration, 50 vol % ACN, 100 mM potassium phosphate buffer, pH 6.0, 20 μM AaeUPO (concentrated supernatant), H2O2 dosing rate: 120 mL h–1 from 12.75 M stock, (pump flow rate was 2 mL per min). The reaction was stirred at 225 rpm at 25 °C. The product concentration was calculated from calibration curves. GC analysis on achiral column (CP-Wax 52GB).
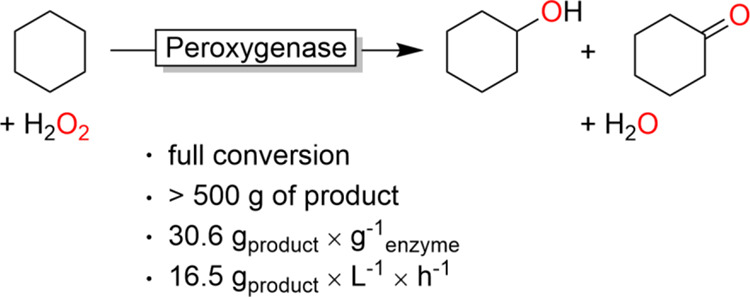
Overall, 290 mM (average of two experiments) cyclohexanol/cyclohexanone was obtained corresponding to approx. 520 g of product and a yield of 58% (based on 500 mM initial starting material concentration). Hence, a product to catalyst ratio of 30.6 gproduct g–1AaeUPO can be estimated (TTNAaeUPO = 13,000 mol mol–1).
Admittedly, the reaction presented here is not (yet) applicable for commercial-scale synthesis of the bulk chemical KA-oil. Significant improvements in process analytics (such as the quantification of the starting material and in situ H2O2 quantification to adjust the H2O2 dosing), substrate loading (e.g., by using two-liquid phase systems of ideally achieving solvent-free reaction conditions),38−40 and improving the catalyst usage (e.g., by further improving the H2O2-addition strategy) to minimize its cost contribution41 will be necessary to render the proposed biocatalytic oxyfunctionalization of cyclohexane industrially relevant. However, we are convinced that simple measures such as an adjusted ratio of H2O2 feed rate and AaeUPO concentration and in situ product removal42 will enhance the productivity and catalyst usage significantly. Furthermore, engineered variants of AaeUPO43 and suitable immobilization strategies44 will further improve the economic attractiveness.
It is, however, also worth mentioning that the oxyfunctionalization rates and product titers achieved in this study, to the best of our knowledge, surpass those reported for P450 MO-catalyzed pendants by orders of magnitude, thereby demonstrating the synthetic potential of the proposed peroxygenase technology.
Conclusions
Overall, in this contribution, we have demonstrated the mol-scale biocatalytic hydroxylation of cyclohexane using the peroxygenase from Agrocybe aegerita (AaeUPO, PaDa-I). To the best of our knowledge, this is the first time an unspecific peroxygenase has been used at this scale. The promising results obtained in this preliminary study underscore the potential of peroxygenases as industrial catalysts.
Acknowledgments
This project has received funding from the European Union (ERC, PeroxyZyme, No 101054658) and under the European Union’s Horizon 2020 research and innovation programme (Fluizyme, grant agreement no. 101021024). Views and opinions expressed are however those of the authors only and do not necessarily reflect those of the European Union or the European Research Council. Neither the European Union nor the granting authority can be held responsible for them. We thank Mr. Satish Kumar Kodiripaka for performing the H2O2 quantitation.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.oprd.3c00135.
AaeUPO-catalyzed oxidation of cyclohexane to cyclohexanol (blue) and cyclohexanone (gray) in 100 mL scale; qualitative tracking of H2O2 in the reaction mixture; calibration curve for cyclohexanol; calibration curve for cyclohexanone; and GCMS analysis of the AaeUPO-mediated conversion of cyclohexane after 6 h (PDF)
Author Contributions
F.H., M.A., H.B., and J.M.W. conceived the project and supervised the work. T.H. and R.V.O. planned and carried out most experiments. T.H. analyzed most of the data and drafted the manuscript. J.V. led the upscaling at DTU. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Hauer B. Embracing Nature’s Catalysts: A Viewpoint on the Future of Biocatalysis. ACS Catal. 2020, 10, 8418–8427. 10.1021/acscatal.0c01708. [DOI] [Google Scholar]
- Nestl B. M.; Hammer S. C.; Nebel B. A.; Hauer B. New Generation of Biocatalysts for Organic Synthesis. Angew. Chem., Int. Ed. 2014, 53, 3070–3095. 10.1002/anie.201302195. [DOI] [PubMed] [Google Scholar]
- Wu S.; Snajdrova R.; Moore J. C.; Baldenius K.; Bornscheuer U. T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem., Int. Ed. 2021, 60, 88–119. 10.1002/anie.202006648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanefeld U.; Hollmann F.; Paul C. E. Biocatalysis making waves in organic chemistry. Chem. Soc. Rev. 2022, 51, 594–627. 10.1039/D1CS00100K. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Fang L.; Wang F.; Deng Y.; Jiang Z.; Li A. Transforming Inert Cycloalkanes into α,ω-Diamines by Designed Enzymatic Cascade Catalysis. Angew. Chem., Int. Ed. 2023, 62, e202215935 10.1002/anie.202215935. [DOI] [PubMed] [Google Scholar]
- Brehm J.; Lewis R. J.; Richards T.; Qin T.; Morgan D. J.; Davies T. E.; Chen L.; Liu X.; Hutchings G. J. Enhancing the Chemo-Enzymatic One-Pot Oxidation of Cyclohexane via In Situ H2O2 Production over Supported Pd-Based Catalysts. ACS Catal. 2022, 12, 11776–11789. 10.1021/acscatal.2c03051. [DOI] [Google Scholar]
- Salamanca D.; Bühler K.; Engesser K.-H.; Schmid A.; Karande R. Whole-cell biocatalysis using the Acidovorax sp. CHX100 Δ6HX for the production of ω-hydroxycarboxylic acids from cycloalkanes. New Biotech. 2021, 60, 200–206. 10.1016/j.nbt.2020.10.009. [DOI] [PubMed] [Google Scholar]
- Staudt S.; Burda E.; Giese C.; Müller C. A.; Marienhagen J.; Schwaneberg U.; Hummel W.; Drauz K.; Gröger H. Direktoxidation von Cycloalkanen zu Cycloalkanonen mit Sauerstoff in Wasser. Angew. Chem., Int. Ed. 2013, 52, 2359–2363. 10.1002/anie.201204464. [DOI] [PubMed] [Google Scholar]
- Ilie A.; Agudo R.; Roiban G. D.; Reetz M. T. P450-catalyzed regio- and stereoselective oxidative hydroxylation of disubstituted cyclohexanes: creation of three centers of chirality in a single CH-activation event. Tetrahedron 2015, 71, 470–475. 10.1016/j.tet.2014.11.067. [DOI] [Google Scholar]
- Musser M. T.Cyclohexanol and Cyclohexanone. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH. [Google Scholar]
- Urlacher V. B.; Girhard M. Cytochrome P450 monooxygenases: an update on perspectives for synthetic application. Trends Biotechnol. 2012, 30, 26–36. 10.1016/j.tibtech.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Alwaseem H.; Fasan R.. Engineered Cytochromes P450 for Biocatalysis. In Protein Engineering: Tools and Applications; Wiley, 2021. [Google Scholar]
- Heuschkel I.; Hanisch S.; Volke D. C.; Löfgren E.; Hoschek A.; Nikel P. I.; Karande R.; Bühler K. Pseudomonas taiwanensis biofilms for continuous conversion of cyclohexanone in drip flow and rotating bed reactors. Eng. Life Sci. 2021, 21, 258–269. 10.1002/elsc.202000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretschneider L.; Heuschkel I.; Wegner M.; Lindmeyer M.; Bühler K.; Karande R.; Bühler B. Conversion of Cyclohexane to 6-Hydroxyhexanoic Acid Using Recombinant Pseudomonas taiwanensis in a Stirred-Tank Bioreactor. Front. Catal. 2021, 1, 683248 10.3389/fctls.2021.683248. [DOI] [Google Scholar]
- Zhang Z.; Li Q.; Wang F.; Li R.; Yu X.; Kang L.; Zhao J.; Li A. One-pot biosynthesis of 1,6-hexanediol from cyclohexane by de novo designed cascade biocatalysis. Green Chem. 2020, 22, 7476–7483. 10.1039/D0GC02600J. [DOI] [Google Scholar]
- Karande R.; Salamanca D.; Schmid A.; Buehler K. Biocatalytic conversion of cycloalkanes to lactones using an in-vivo cascade in Pseudomonas taiwanensis VLB120. Biotechnol. Bioeng. 2018, 115, 312–320. 10.1002/bit.26469. [DOI] [PubMed] [Google Scholar]
- Karande R.; Debor L.; Salamanca D.; Bogdahn F.; Engesser K.-H.; Buehler K.; Schmid A. Continuous cyclohexane oxidation to cyclohexanol using a novel cytochrome P450 monooxygenase from Acidovorax sp. CHX100 in recombinant P. taiwanensis VLB120 biofilms. Biotechnol. Bioeng. 2016, 113, 52–61. 10.1002/bit.25696. [DOI] [PubMed] [Google Scholar]
- Schröder G. C.; Smit M. S.; Opperman D. J. Harnessing heme chemistry: Recent advances in the biocatalytic applications of cytochrome P450 monooxgenases. Curr. Opin. Green Sustain. Chem. 2023, 39, 100734 10.1016/j.cogsc.2022.100734. [DOI] [Google Scholar]
- Maseme M. J.; Pennec A.; van Marwijk J.; Opperman D. J.; Smit M. S. CYP505E3: A Novel Self-Sufficient ω-7 In-Chain Hydroxylase. Angew. Chem., Int. Ed. 2020, 59, 10359–10362. 10.1002/anie.202001055. [DOI] [PubMed] [Google Scholar]
- Pennec A.; Hollmann F.; Smit M. S.; Opperman D. J. One-pot Conversion of Cycloalkanes to Lactones. ChemCatChem 2015, 7, 236–239. 10.1002/cctc.201402835. [DOI] [Google Scholar]
- Burek B. O.; Dawood A. W. H.; Hollmann F.; Liese A.; Holtmann D. Process Intensification as Game Changer in Enzyme Catalysis. Front. Catal. 2022, 2, 858706 10.3389/fctls.2022.858706. [DOI] [Google Scholar]
- Holtmann D.; Hollmann F. The Oxygen Dilemma: A Severe Challenge for the Application of Monooxygenases?. ChemBioChem 2016, 17, 1391–1398. 10.1002/cbic.201600176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeque R. M.; Woodley J. M. The Effect of Dissolved Oxygen on Kinetics during Continuous Biocatalytic Oxidations. Org. Proc. Res. Dev. 2020, 24, 2055–2063. 10.1021/acs.oprd.0c00140. [DOI] [Google Scholar]
- Meissner M. P.; Nordblad M.; Woodley J. M. Online Measurement of Oxygen-Dependent Enzyme Reaction Kinetics. ChemBioChem 2018, 19, 106–113. 10.1002/cbic.201700577. [DOI] [PubMed] [Google Scholar]
- Toftgaard Pedersen A.; de Carvalho T. M.; Sutherland E.; Rehn G.; Ashe R.; Woodley J. M. Characterization of a continuous agitated cell reactor for oxygen dependent biocatalysis. Biotechnol. Bioeng. 2017, 114, 1222–1230. 10.1002/bit.26267. [DOI] [PubMed] [Google Scholar]
- Monterrey D. T.; Menés-Rubio A.; Keser M.; Gonzalez-Perez D.; Alcalde M. Unspecific peroxygenases: The pot of gold at the end of the oxyfunctionalization rainbow?. Curr. Opin. Green Sustain. Chem. 2023, 41, 100786 10.1016/j.cogsc.2023.100786. [DOI] [Google Scholar]
- Beltrán-Nogal A.; Sánchez-Moreno I.; Méndez-Sánchez D.; Gómez de Santos P.; Hollmann F.; Alcalde M. Surfing the wave of oxyfunctionalization chemistry by engineering fungal unspecific peroxygenases. Curr. Opin. Struct. Biol. 2022, 73, 102342 10.1016/j.sbi.2022.102342. [DOI] [PubMed] [Google Scholar]
- Peter S.; Karich A.; Ullrich R.; Grobe G.; Scheibner K.; Hofrichter M. Enzymatic one-pot conversion of cyclohexane into cyclohexanone: Comparison of four fungal peroxygenases. J. Mol. Catal. B: Enzym. 2014, 103, 47–51. 10.1016/j.molcatb.2013.09.016. [DOI] [Google Scholar]
- Ullrich R.; Nüske J.; Scheibner K.; Spantzel J.; Hofrichter M. Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes. Appl. Environ. Microbiol. 2004, 70, 4575–4581. 10.1128/AEM.70.8.4575-4581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Espeja P.; Garcia-Ruiz E.; Gonzalez-Perez D.; Ullrich R.; Hofrichter M.; Alcalde M. Directed Evolution of Unspecific Peroxygenase from Agrocybe aegerita. Appl. Environ. Microbiol. 2014, 80, 3496–3507. 10.1128/AEM.00490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churakova E.; Kluge M.; Ullrich R.; Arends I.; Hofrichter M.; Hollmann F. Specific photobiocatalytic oxyfunctionalization reactions. Angew. Chem., Int. Ed. 2011, 50, 10716–10719. 10.1002/anie.201105308. [DOI] [PubMed] [Google Scholar]
- Hilberath T.; van Troost A.; Alcalde M.; Hollmann F. Assessing Peroxygenase-Mediated Oxidations in the Presence of High Concentrations of Water-Miscible Co-Solvents. Front. Catal. 2022, 2, 882992 10.3389/fctls.2022.882992. [DOI] [Google Scholar]
- Tonin F.; Tieves F.; Willot S.; van Troost A.; van Oosten R.; Breestraat S.; van Pelt S.; Alcalde M.; Hollmann F. Pilot-Scale Production of Peroxygenase from Agrocybe aegerita. Org. Proc. Res. Dev. 2021, 25, 1414–1418. 10.1021/acs.oprd.1c00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieves F.; Tonin F.; Fernández-Fueyo E.; Robbins J. M.; Bommarius B.; Bommarius A. S.; Alcalde M.; Hollmann F. Energising the E-factor: The E+-factor. Tetrahedron 2019, 75, 1311–1314. 10.1016/j.tet.2019.01.065. [DOI] [Google Scholar]
- Lorence R. M.; Gennis R. B. Spectroscopic and quantitative analysis of the oxygenated and peroxy states of the purified cytochrome d complex of Escherichia coli. J. Biol. Chem. 1989, 264, 7135–7140. 10.1016/S0021-9258(18)83212-4. [DOI] [PubMed] [Google Scholar]
- Hofrichter M.; Ullrich R. Heme-thiolate haloperoxidases: versatile biocatalysts with biotechnological and environmental significance. Appl. Microbiol. Biotechnol. 2006, 71, 276–288. 10.1007/s00253-006-0417-3. [DOI] [PubMed] [Google Scholar]
- Lide D. R.CRC Handbook of Chemistry and Physics, 84th edition; CRC Press, 2003. [Google Scholar]
- Hobisch M.; van Schie M. M. C. H.; Kim J.; Røjkjær Andersen K.; Alcalde M.; Kourist R.; Park C. B.; Hollmann F.; Kara S. Solvent-Free Photobiocatalytic Hydroxylation of Cyclohexane. ChemCatChem 2020, 12, 4009–4013. 10.1002/cctc.202000512. [DOI] [Google Scholar]
- Nintzel F. E. H.; Wu Y.; Planchestainer M.; Held M.; Alcalde M.; Hollmann F. An alginate-confined peroxygenase-CLEA for styrene epoxidation. Chem. Commun. 2021, 57, 5766–5769. 10.1039/D1CC01868J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch M. C. R.; Tieves F.; Paul C. E.; Arends I. W.; Alcalde M.; Hollmann F. Peroxygenase-catalysed epoxidation of styrene derivatives in neat reaction media. ChemCatChem 2019, 11, 4519–4523. 10.1002/cctc.201901142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufvesson P.; Lima-Ramos J.; Nordblad M.; Woodley J. M. Guidelines and cost analysis for catalyst production in biocatalytic processes. Org. Proc. Res. Dev. 2010, 15, 266–274. 10.1021/op1002165. [DOI] [Google Scholar]
- Frolkova A. K.; Maevskii M. A.; Oshanina I. V.; Frolkova A. V. Cyclohexanone: The Main Methods of Obtaining and Extracting Target Products from a Reaction Mixture. Theo. Found. Chem. Eng. 2018, 52, 653–660. 10.1134/S0040579518040097. [DOI] [Google Scholar]
- Gomez de Santos P.; Mateljak I.; Hoang M. D.; Fleishman S. J.; Hollmann F.; Alcalde M. Repertoire of Computationally Designed Peroxygenases for Enantiodivergent C–H Oxyfunctionalization Reactions. J. Am. Chem. Soc. 2023, 145, 3443–3453. 10.1021/jacs.2c11118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis P.; Petrovai N.; Meyer L.-E.; Hobisch M.; Kara S. A holistic carrier-bound immobilization approach for unspecific peroxygenase. Front. Chem. 2022, 10, 985997 10.3389/fchem.2022.985997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.