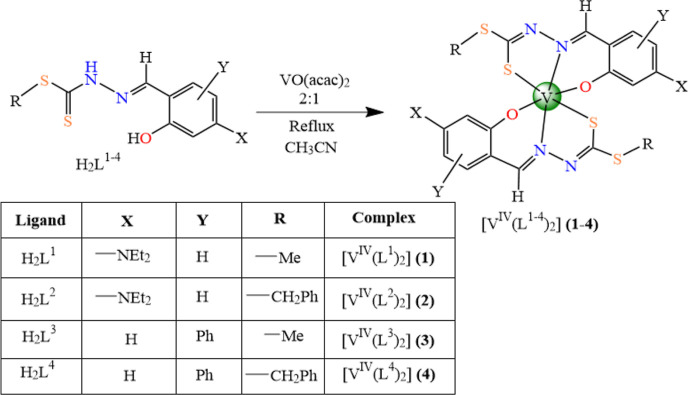
Abstract

A series of mononuclear non-oxido vanadium(IV) complexes, [VIV(L1–4)2] (1–4), featuring tridentate bi-negative ONS chelating S-alkyl/aryl-substituted dithiocarbazate ligands H2L1–4, are reported. All the synthesized non-oxido VIV compounds are characterized by elemental analysis, spectroscopy (IR, UV–vis, and EPR), ESI-MS, as well as electrochemical techniques (cyclic voltammetry). Single-crystal X-ray diffraction studies of 1–3 reveal that the mononuclear non-oxido VIV complexes show distorted octahedral (1 and 2) or trigonal prismatic (3) arrangement around the non-oxido VIV center. EPR and DFT data indicate the coexistence of mer and fac isomers in solution, and ESI-MS results suggest a partial oxidation of [VIV(L1–4)2] to [VV(L1–4)2]+ and [VVO2(L1–4)]−; therefore, all these three complexes are plausible active species. Complexes 1–4 interact with bovine serum albumin (BSA) with a moderate binding affinity, and docking calculations reveal non-covalent interactions with different regions of BSA, particularly with Tyr, Lys, Arg, and Thr residues. In vitro cytotoxic activity of all complexes is assayed against the HT-29 (colon cancer) and HeLa (cervical cancer) cells and compared with the NIH-3T3 (mouse embryonic fibroblast) normal cell line by MTT assay and DAPI staining. The results suggest that complexes 1–4 are cytotoxic in nature and induce cell death in the cancer cell lines by apoptosis and that a mixture of VIV, VV, and VVO2 species could be responsible for the biological activity.
Short abstract
The synthesis, stability, and chemical transformations in solution, protein interaction, and cytotoxicity of a series of non-oxido [VIV(L1−4)2] complexes of tridentate ONS dithiocarbazate ligands is reported. The results indicate that a mixture of [VIV(L1−4)2], [VV(L1−4)2]+, and [VVO2(L1−4)2]− species is formed, which may be responsible for the observed activity.
Introduction
Vanadium is a biologically relevant trace element required by different organisms.1 It exists in enzymes such as vanadium-dependent haloperoxidases and nitrogenases2−5 and is probably involved in the regulation of phosphate metabolism, thereby playing crucial roles in the human organism.6−9 Over the past few years, vanadium compounds have also been demonstrated to have various therapeutic effects,9−15 and the correlation between the structure and activity,16 reactivity and activity,17 as well as the biotransformation of these complexes in blood have been investigated.18−27
The interaction of metal complexes with proteins plays crucial roles in pharmaceutical and biomedical applications4,28−33 and may affect the action of enzymes and proteins in the organism.34 Serum albumins are the major soluble proteins in the blood and have an utmost important role in biological processes; for example, studies have revealed that the in vivo circulatory half-life of some metallodrugs was extended as a result of binding with serum albumin.35,36
Some oxidovanadium(IV) complexes, particularly [VIVO(4,7-Me2phen)2(SO4)] (Metvan, where 4,7-Me2phen = 4,7-dimethyl-1,10-phenanthroline), were introduced as promising anticancer drugs for testicular and ovarian cancer,37 but they never proceeded to the clinic. There is interest in the study of the pharmaceutical roles of several vanadium complexes,5,38−46 such as [bis(cyclopentadienyl)vanadium(IV)] dichloride, [VIVO(phen)(H2O)2]2+,47 and [VIVO(phen)2(SO4)]48 (phen = 1,10-phenanthroline), which exhibited potent antitumor activity against the human nasopharyngeal carcinoma-KB cell line and the clonogenic NALM-6 cell line, respectively. However, more recently, the relevance of addressing the consequences of the probable hydrolysis, ligand exchange, and redox reactions of complexes of labile metal ions in in vitro and in vivo conditions have been highlighted,25,49−52 emphasizing the need to properly disclose which are the relevant biological active species. Namely, incubation media used in studies with mammalian cells also contain appreciable amounts of bovine serum albumin (BSA), which competes for the binding to the metal ions present, often changing the nature of metal-containing species that enter inside cells.51,52
Vanadium in its most stable higher oxidation states (+IV and +V) is oxophilic and forms several types of oxido species both in the solid state and in solution, containing strong terminal V=O bonds. It is worth noting that in contrast to those VIV/VV-oxido species, only very few non-oxido VIV and VV complexes formed by ONS/SS/OS donor ligands have been isolated and structurally characterized.53−60 The tendency to form the strong “terminal” V=O bond under most experimental conditions, particularly in water-containing media, makes the formation of non-oxido vanadium(IV) complexes much less probable, particularly under biologically relevant conditions. Even after the discovery of amavadin,61 a non-oxido octa-coordinated VIV complex isolated from the mushroom Amanita muscaria,62,63 studies of their biological activities are still scarce,43,64−67 and the determination of the active complex species has been rarely attempted.16
Over the last years, the interest in the study of ONS donor ligands has increased significantly due to their ligation properties and potential applications of their transition metal complexes in pharmacology and medicine.46,68−80 For example, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, well known as triapine, is one of the most extensively studied drugs81 and has been tested in nearly 30 clinical phases I/II trials as an antiproliferative agent.82 Likewise, di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC-TSC) has also entered clinical phase I studies as a chemotherapeutic agent.83,84 Out of this class of compounds, similar to thiosemicarbazones, S-substituted dithiocarbazates (DTCs) are also well known for their antitumor, antimicrobial, antifungal, and antibacterial activities.68,71−76,79,80,85 Moreover, N’-(phenyl-pyridin-2-yl-methylene)-hydrazine carbodithioic acid methyl ester (PHCM) was also found to enhance radiation-induced cell death of an NSCLC cell line, H460, both in vivo and in vitro. Interestingly, the possible use in bio-imaging and in the treatment of pancreatic cancer of a dithiocarbazate-copper complex has been recently published.86
Considering the potential therapeutic use of this type of compounds and our expertise in the field of biological and pharmacological applications of vanadium compounds,64,65,87−98 herein we report a new series of non-oxido vanadium(IV) complexes, [VIV(L1–4)2] (1–4), formed by S-alkyl/aryl substituted dithiocarbazate ligands (H2L1–4). All these compounds were characterized through elemental analysis, mass spectrometry, spectroscopic techniques, and cyclic voltammetry. Structural evidence for the formation of non-oxido VIV complexes was obtained by the SC-XRD characterization of [VIV(L1–3)2] (1–3). To the best of our knowledge, this is the second study involving the structural characterization by single-crystal X-ray diffraction analysis (SC-XRD) of non-oxido vanadium(IV) complexes with ONS donor ligands.53
Under biological conditions, vanadium complexes often undergo chemical transformations like hydrolysis, redox reactions, ligand-exchange, and so forth, which have limited the experimentation of V-based drugs in clinical practice.3,5,14,41,42,65 For example, recently, focus has been given to the products formed under biological media by the hydrolysis/oxidation of non-oxido VIV-complexes, which have demonstrated to be responsible for the biological properties.16,43,65,66 Thus, the possibility of hydrolysis and redox processes of new non-oxido and oxidovanadium(IV) complexes is worth of being explored. Therefore, in this work, we address the chemical transformations, disclosing the solution phase stability of complexes [VIV(L1–4)2] through various physico-chemical techniques. The synthesized complexes 1–4 were also tested for their interaction with BSA and in vitro antiproliferative activity on HT-29 (colon cancer), HeLa (cervical cancer), and NIH-3T3 (mouse embryonic fibroblast) cell lines.
Experimental Section
General Methods and Materials
Most chemicals were obtained from commercial sources. Carbon disulfide (HiMedia), hydrazine hydrate (Loba Chemie), methyl iodide (Sigma-Aldrich), benzyl chloride, and 4-(diethylamino)salicylaldehyde (Sigma-Aldrich) and 2-hydroxy-1-naphthaldehyde (Sigma-Aldrich) were used as received. [VIVO(acac)2], where acac stands for acetylacetonate, was prepared as described in the literature.99 Reagent grade solvents were dried and distilled under nitrogen prior to use according to standard procedures.100 HPLC grade DMSO and CH3CN were used for spectroscopic and electrochemical studies, whereas EtOH and CH3CN were employed for synthesis of ligand precursors101 and metal complexes, respectively. BSA for the study of interactions with the compounds was purchased from Sigma-Aldrich. Cell lines (HT-29, HeLa, and NIH-3T3) were obtained from National Centre of Cell Science (NCCS), Pune, India. Dulbecco’s phosphate buffered saline (DPBS), Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), antibiotic-antimitotic solution, and trypsin EDTA solution were procured from Himedia, Mumbai, India, while 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium (MTT) were purchased from Sigma-Aldrich, India. Elemental analysis measurements were performed on A Vario EL cube CHNS elemental analyzer. A PerkinElmer Spectrum RXI spectrophotometer was used for recording the IR spectra, and 1H and 13C NMR spectra were recorded on a Bruker Ultrashield 400 MHz spectrometer in the presence of internal standard SiMe4. Electronic spectra were obtained on a Lambda25 PerkinElmer spectrophotometer. EPR spectra were recorded at 120 K with an X-band Bruker EMX spectrometer equipped with a HP 53150A microwave frequency counter and a variable temperature unit, the instrumental setting being: microwave frequency 9.40–9.41 GHz; microwave power 20 mW; time constant 81.92 ms; modulation frequency 100 kHz; modulation amplitude 4 Gauss. Positive- (+) and negative-ion (−) mode ESI-MS spectra were recorded with a high-resolution Q Exactive Plus Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific) with a flow rate of infusion into the ESI chamber of 5.00 μL/min and a recording range of 50–1000 m/z. The instrumental conditions for positive-ion mode spectra were: spray voltage 2300 V; capillary temperature 250 °C; sheath gas 5–10 (arbitrary units); auxiliary gas 3 (arbitrary units); sweep gas 0 (arbitrary units); probe heater temperature 50 °C. For negative-ion mode spectra, they were: spray voltage −1900 V; capillary temperature 250 °C; sheath gas 20 (arbitrary units); auxiliary gas 5 (arbitrary units); sweep gas 0 (arbitrary units); probe heater temperature 14 °C. The redox properties were studied under nitrogen by cyclic voltammetry using a three-compartment cell equipped with Pt wire (working and auxiliary) and Ag wire (reference) electrodes, interfaced with VoltaLab PST050 equipment. The cyclic voltammograms of complexes 1–4 and free ligands (H2L3–4) were obtained from electrolyte solutions of NBu4BF4 in CH2Cl2 (0.10 M). The potentials were measured in Volt (±10 mV) vs saturated calomel electrode (SCE) at 200 mV/s scan rate using [Fe(η5-C5H5)2]0/+ as an internal reference.
Synthesis of Ligand Precursors (H2L1–4)
The dithiocarbazate compounds H2L1–2 were prepared in a fair yield by the condensation of 4-(diethylamino)salicylaldehyde with the corresponding S-alkyl/aryl substituted dithiocarbazates [S-methyldithiocarbazate (H2L1) and S-benzyldithiocarbazate (H2L2)], while H2L3–4 were synthesized by the reaction of 2-hydroxy-1-naphthaldehyde with the respective dithiocarbazates [S-methyldithiocarbazate (H2L3) and S-benzyldithiocarbazate (H2L4)] in a 1:1 ratio in dry ethanol by adopting a reported procedure.102 The resulting yellowish compounds were filtered and then washed with ethanol and finally dried over fused CaCl2 in a desiccator.
H2L1
Yield: 74%. Anal. Calcd for C13H19N3OS2: C, 52.49; H, 6.44; N, 14.13; S, 21.56. Found: C, 52.28; H, 6.72; N, 14.48; S, 21.23. IR (KBr pellet, cm–1): 3642 ν(O–H); 3189 ν(N–H); 1630 ν(C=N); 1494 ν(C–O/phenolate); 1317 ν(C=S). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 13.13 (s, 1H, NH), 10.13 (s, 1H, OH), 8.31 (s, 1H, HC=N−), 7.31–6.12 (m, 3H, Aromatic), 3.31 (m, 4H, −CH2–N), 2.51 (s, 3H, CH3–S), 1.11 (m, 6H, CH3). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 194.48, 159.79, 151.31, 147.81, 131.13, 106.51, 104.86, 97.66, 44.35, 17.12, 12.98.
H2L2
Yield: 82%. Anal. Calcd C19H23N3OS2: C, 61.09; H, 6.21; N, 11.25; S, 17.17. Found: C, 61.28; H, 6.28; N, 11.41; S, 17.49. IR (KBr pellet, cm–1): 3683 ν(O–H); 3109 ν(N–H); 1624 ν(C=N); 1516 ν(C–O/phenolate); 1340 ν(C=S). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 13.18 (s, 1H, NH), 10.09 (s, 1H, OH), 8.31 (s, 1H, HC=N−), 7.42–6.07 (m, 8H, Aromatic), 4.49 (s, 2H, CH2–S), 3.36 (m, 4H, CH2–N), 1.08 (m, 6H, CH3). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 192.70, 159.76, 151.36, 147.74, 137.45, 130.89, 129.64, 129.53, 129.45, 106.46, 104.87, 97.54, 44.35, 37.85, 12.95.
H2L3
Yield: 76%. Anal. Calcd C13H12N2OS2: C, 56.49; H, 4.38; N, 10.14; S, 23.20. Found: C, 56.53; H, 4.40; N, 10.20 S, 23.18. IR (KBr pellet, cm–1): 3445 ν(O–H); 3156 ν(N–H); 1620 ν(C=N); 1577 ν(C–O/phenolate); 1325 ν(C=S). 1H NMR (400 MHz, 157 DMSO-d6): δ (ppm) 13.42 (s, 1H, NH), 11.08 (s, 1H, OH), 9.19 (s, 1H, HC=N−), 8.80–7.23 (m, 6H, Aromatic), 2.50 (s, 3H, CH3–S). 13C NMR (100 MHz, DMSO- d6): δ (ppm) 196.72, 158.69, 146.23, 134.21, 131.72, 129.36, 128.71, 128.61, 124.18, 123.73, 118.70, 109.83, 17.38.
H2L4
Yield: 80%. Anal. Calcd C19H16N2OS2: C, 64.74; H, 4.58; N, 7.95; S, 18.19. Found: C, 64.80; H, 4.55; N, 7.96; S, 18.23. IR (KBr pellet, cm–1): 3458 ν(O–H); 3157 ν(N–H); 1622 ν(C=N); 1572 ν(C–O/phenolate); 1327 ν(C=S). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 13.46 (s, 1H, NH), 11.09 (s, 1H, OH), 9.20 (s, 1H, HC=N−), 8.71–7.21 (m, 11H, Aromatic), 4.59 (s, 2H, CH2–S). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 194.73, 157.25, 145.20, 136.09, 132.66, 131.72, 130.55, 127.34, 127.20, 126.80, 125.74, 124.60, 123.69, 120.19, 120.71, 117.25, 116.11, 112.89, 45.15.
Synthesis of Complexes [VIV(L1–4)2] (1–4)
The non-oxido vanadium(IV) compounds, formulated as [VIV(L1–4)2], were synthesized by mixing the ONS donor dithiocarbazates H2L1–4 with [VIVO(acac)2] in stoichiometric amounts (2:1 molar ratio) in CH3CN and allowing it to reflux for 3 h. Greenish black-colored crystalline compounds (1–4) were directly obtained from the reaction mixtures. They were then collected by filtration, washed properly with hexane, and dried over CaCl2. Suitable crystals of monomeric 1–3 compounds for single-crystal X-ray measurements were collected from their respective reaction pots containing CH3CN solvent.
[VIV(L1)2] (1)
Yield: 70%. Anal. Calcd for C26H34N6O2S4V: C, 48.66; H, 5.34; N, 13.09; S, 19.98. Found: C, 48.61; H, 5.33; N, 13.15; S, 19.99. IR (KBr pellet, cm–1): 1612 ν(C=N). UV–vis (DMSO) [λmax, nm (ε, M–1 cm–1)]: 629 (472), 529 (659), 390 (2157). ESI-MS (CH3CN): m/z 664.0960 [M + Na]+.
[VIV(L2)2] (2)
Yield: 76%. Anal. Calcd for C38H42N6O2S4V: C, 57.48; H, 5.33; N, 10.58; S, 16.15. Found: C, 57.51; H, 5.34; N, 10.63; S, 16.13. IR (KBr pellet, cm–1): 1613 ν(C=N). UV–vis (DMSO) [λmax, nm (ε, M–1 cm–1)]: 628 (450), 526 (730), 401 (2046). ESI-MS (CH3CN): m/z 816.1585 [M + Na]+.
[VIV(L3)2] (3)
Yield: 72%. Anal. Calcd for C26H20N4O2S4V: C, 52.08; H, 3.36; N, 9.34; S, 21.39. Found: C, 52.09; H, 3.33; N, 9.29; S, 21.40. IR (KBr pellet, cm–1): 1592 ν(C=N). UV–vis (DMSO) [λmax, nm (ε, M–1 cm–1)]: 653 (397), 522 (472), 382 (1869), 333 (2487). ESI-MS (CH3CN): m/z 621.9814 [M + Na]+.
[VIV(L4)2] (4)
Yield: 74%. Anal. Calcd for C38H28N4O2S4V: C, 60.70; H, 3.75; N, 7.45; S, 17.06. Found: C, 60.73; H, 3.71; N, 7.49; S, 17.09. IR (KBr pellet, cm–1): 1602 ν(C=N). UV–vis (DMSO) [λmax, nm (ε, M–1 cm –1)]: 644 (401), 528 (611), 385 (1932), 334 (2531). ESI-MS (CH3CN): m/z 774.0427 [M + Na]+.
X-ray Crystallography
Crystal and intensity data on complexes 1–3 were collected on a standard Bruker Kappa APEX II CCD-based 4-circle X-ray diffractometer using graphite-monochromated Mo-Kα radiation. Data were corrected for Lorentz and polarization effects; no extinction corrections were applied, but absorption correction was taken into account on a semi-empirical basis using equivalent scans with Rint = Σ|Fo2 – Fo2(mean)|/Σ[Fo2] and Rσ = Σ[σ(Fo2)]/Σ[Fo2].
The structures were solved by direct methods (SHELXS103) and refined by full-matrix least squares techniques against Fo2 (SHELXL-97 and SHELXL-2014). Atomic scattering factors were taken from International Tables for Crystallography.104 The final agreement indices are R1 = Σ||Fo| – |Fc||/Σ|Fo| and wR2 = {Σ[w(Fo2 – Fc2)2]/Σ[(wFo2)2]}1/2, and weighting function used is w = 1/[σ2(Fo2) + (aP)2 + bP] with P = (maxFo2 + 2Fc2)/3. Goof = S = {Σ[w(Fo2 – Fc2)2]/(n–p)}1/2, where n is the number of reflections and p is the total number of parameters refined. Although the hydrogen atoms could be localized in difference Fourier syntheses, those of the organic moieties were refined in geometrically optimized positions riding on the corresponding carbon atoms, with C–H distances at T = 293 K/100 K of 0.96/0.98 Å (−CH3), 0.97/0.99 Å (−CH2−), 0.93/0.95 Å (−CHsp2), and common isotropic thermal displacement parameters for chemical equivalent H-atoms. Figures were drawn using DIAMOND105 and Mercury.106 In the ball-and-stick models, all atoms are drawn as thermal displacement ellipsoids of the 50% level with exception of the hydrogen atoms, which are shown as spheres of arbitrary radii. Hydrogen bonds are drawn in red as dashed sticks. Crystallographic data as well as structure solution and refinement details are summarized in Table S1. XP (SIEMENS Analytical X-ray Instruments, Inc.1994) was used for structure representations.
Fluorescence Competition Titrations
The interaction between complexes 1–4 and BSA was studied using fluorescence quenching spectroscopy on a PerkinElmer LS 55 instrument. The instrumental response was corrected by means of a correction function provided by the manufacturer. The fluorescence experiments were carried out at room temperature and are steady-state measurements. Electronic absorption spectra were measured on a PerkinElmer Lambda35.
Fatty acid free BSA stock solution was prepared in PBS buffer (10 mM, pH 7.4), and the concentration was determined spectrophotometrically considering the extinction coefficient 44,309 M–1 cm–1 at 280 nm.107 Stock solutions of the complexes (ca. 8 × 10–4 M) were prepared in DMSO and further diluted (1:10) immediately before running the experiments. Aliquots of each complex (ca. 80 μM in DMSO) were added directly to a cuvette containing the BSA solution (ca. 1–2 μM) in PBS buffer to obtain complex/BSA molar ratios ranging from 0.1 to 3.5. After addition of the complex, the solutions were allowed to stand for 5 min before measurements. Fluorescence emission spectra were measured at room temperature with λex = 295 nm between 310 and 500 nm. Fluorescence spectra containing the same amount of complex but no BSA were recorded and subtracted from each corresponding emission spectrum of the mixtures. The DMSO amount in the titrations was always lower than 5% (v/v). UV–vis spectra were measured for each solution, and the absorbance at excitation and emission wavelengths were used to make corrections for inner filter effects and reabsorption.108,109
The Stern–Volmer equation was used to fit the fluorescence quenching data109
| 1 |
where F0 and F are the
fluorescence intensities of BSA in the absence
and presence of quencher, respectively, [Q] is the
quencher concentration (the vanadium complex in our systems), and KSV is the Stern–Volmer quenching constant,
obtained from the slope of the 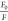 vs [Q] plot.
vs [Q] plot.
The binding constants (KBC) were estimated through linearization of the quenching data, considering the equilibrium between free and bound quencher molecules when they bind independently to n equivalent sites of BSA
| 2 |
Computational Studies
DFT Calculations
The geometry of non-oxido vanadium(IV) [VIV(L1–4)2], non-oxido vanadium(V) [VV(L1–4)2]+, and dioxidovanadium(V) [VVO2(L1–4)]− complexes was optimized with Gaussian 16, rev. B01,110 at the DFT theory level using the hybrid B3P86 functional combined with the split-valence 6-311g basis set. This method has been successfully applied and discussed in the literature for the geometry prediction of vanadium species.111,112 For all the structures, minima were verified through frequency calculations. The 51V hyperfine coupling tensor A of all [VIV(L1–4)2] complexes was predicted with ORCA software version 4.0115 using the B2PLYP functional and 6-311g(d,p) basis set, as suggested previously.114 The percent deviation (PD) of the absolute calculated value (|Az|calcd) from the absolute experimental value (|Az|exptl) was obtained as 100 × [(|Az|calcd – |Az|exptl)/|Az|exptl]. UV–vis vertical excitations were simulated on the time-dependent density functional theory (TD-DFT) framework. The simulations were carried out on the previously optimized geometries using the range separated CAM-B3LYP functional and the 6-31+g(d) basis set, according to the method proposed in the literature.53 The predicted electronic spectra were generated using Gabedit software,115 and the molecular orbitals (MOs) involved in the transitions were simulated performing a Mulliken population analysis (MPA) with Gaussian 16 at the same level of theory used for the TD-DFT calculations and identified with the AOMix package (vers. 6.52).116
Molecular Docking on BSA
Docking assays toward BSA were carried out with GOLD 5.8 software,117 according to the procedures recently reported.118−122 The calculations were based on the XRD structure of BSA (PDB code: 4F5S),123 removing all the crystallographic waters and adding hydrogen atoms with the UCSF Chimera program.124 Non-covalent classical dockings, in which the complexes [VIV(L1–4)2]/[VV(L1–4)2]+ and [VVO2(L1–4)]− can interact only through second-coordination sphere interactions, were carried out. The blind exploration was performed on the rigid protein, building four evaluation spheres of 20 Å containing globally the whole protein. Genetic algorithm (GA) parameters have been set in 50 GA runs and a minimum of 100,000 operations. The other parameters of GA were set to default. The scoring (Fitness of GoldScore) was determined through the recent validated versions of GoldScore accounting for vanadium-complexes surface interactions.121 The best solutions (binding poses), were evaluated taking into account the mean (Fmean) and the highest value (Fmax) of the scoring and population of the cluster containing the best pose.
Cytotoxicity Experiments
MTT Assay
HT-29 (colon cancer), HeLa (cervical cancer), and NIH-3T3 (mouse embryonic fibroblasts) cells were maintained in DMEM supplemented with 10% FBS, penicillin–streptomycin solution in a humidified (95% humidity) CO2 incubator (5% CO2). Thereafter, cells were harvested from the logarithmic phase and seeded into a 96-well plate at a concentration of 8 × 104 cells per well. After overnight seeding, cells were treated with different concentrations (2.5, 5, 10, 20, and 50 μM) of the tested ligands and vanadium complexes (H2L1–4 and 1–4) for 48 h. The compounds’ stock solutions were prepared in DMSO. DMEM was used to prepare the final working concentration of the compounds, so that the final amount of DMSO is less than 2%. The effect of the complexes on the viability of the cells was evaluated through MTT dye reduction assay by measuring the optical density at 595 nm.93 DMSO alone, used to dissolve the complexes, was also employed for the control group treatment.
Nuclear Staining Assay
Morphological changes of the nucleus and its structural integrity during cell death were analyzed by the DAPI staining method.91,93 Briefly, the cells (HeLa and HT-29) were treated with the tested compounds 1–4 at a concentration of 10 μM and were kept for 24 h. After this, fixation of the cells was done, followed by washing with PBS. Subsequently, the cells were stained with DAPI and kept in dark for 10 min. Finally, the cells were washed twice with PBS and taken for imaging in a Leica TCS SP8 confocal laser scanning microscope with 25× magnification.
Apoptosis Assay
The apoptotic population induced by complexes 1 and 2 in HT-29 cells was evaluated using Annexin V-FITC Apoptosis Detection Kit (Abgenex) by a flow cytometer (BD Accuri C6 flow cytometer; BectoneDickinson).125 HT-29 cells (3 × 105 cells/well) were cultured in a 6-well plate for 24 h. After the cells were adhered, different concentrations (5 and 10 μM) of the tested complexes were treated and incubated for further 48 h. Subsequently, the cells were collected by trypsinization and then suspended in 1X binding buffer. Finally, the suspension was stained with 5 μL of Annexin V and PI at room temperature and incubated for 15 min in the dark. Then, the samples were immediately analyzed under a BD Accuri C6 flow cytometer.
Results and Discussion
Synthesis
The non-oxido vanadium(IV) complexes (1–4) were synthesized by the reaction of tridentate dibasic ONS donor dithiocarbazates H2L1–4 with [VIVO(acac)2] in a 2:1 molar ratio in CH3CN medium (Scheme 1). All compounds are stable in the solid state and do not oxidize under air. They are soluble in polar aprotic solvents like DMSO, DMF, DCM, THF, and CH3CN and are partially soluble in aqueous media. The solid-state characterization of all compounds was done through elemental analysis, FTIR, and also with single-crystal X-ray diffraction studies of 1–3, while the solution phase characterization was carried out through UV–vis, ESI-MS spectrometry, and EPR spectroscopy. Details of all the characterizations are described in the following sections.
Scheme 1. Schematic Representation of the Synthesis of Complexes [VIV(L1–4)2] (1–4).
IR Spectroscopy
The spectral data of compounds 1–4 are summarized in the Experimental Section. Some of the IR bands of the complexes show a shift in wavenumbers, compared to those of their respective dithiocarbazate precursors (H2L1–4). A band in the region 3683–3445 cm–1 can be assigned to the stretching vibration of the aromatic O–H of the free ligands, which is absent in the corresponding metal complexes due to the deprotonation and coordination. The spectra exhibit the characteristic bands of the dithiocarbazate ligands, which include a strong signature band at ∼1630–1620 cm–1 of the ν(C=N) vibration,126 together with a strong band in the region 1577–1494 cm–1 due to the C–O phenolic group.53 These bands shift toward lower frequency upon coordination to the metal center. The absence of the strong characteristic ν(V=O) stretching band in the region 900–1000 cm–1 indicates the formation of a non-oxido vanadium center in 1–4.53,64,65,127
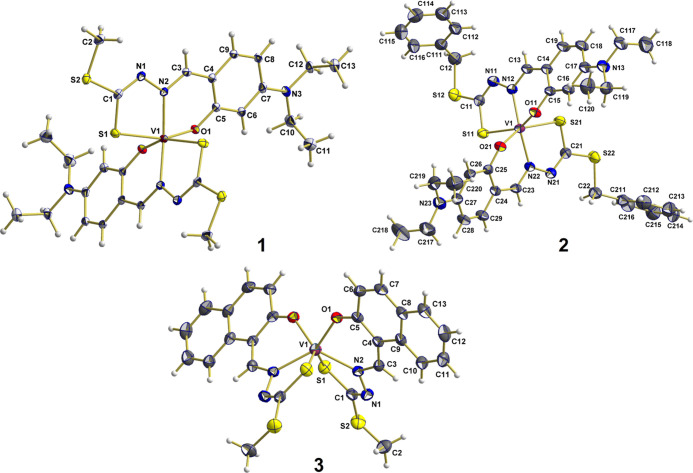
Single-Crystal X-ray Diffraction Analysis of Complexes 1–3
The solid-state structures of 1 (monoclinic, C2/c), 2 (triclinic, P1̅), and 3 (monoclinic, P2/n) were characterized by SC-XRD techniques. An overview of the solid-state conformation of all three complexes is shown in Figure 1, and selected bond distances and angles are given in Table 1. The three complexes belong to three different point groups (1 = Ci, 2 = C1, and 3 = C2), thus exhibiting different symmetry elements (1 = inversion center at the position of the central vanadium atom, 2 = asymmetric, and 3 = a twofold rotation axes through the vanadium atom and in between both ligands).
Figure 1.
Ball-and-stick models of the discrete, monomeric complexes found in the SC-XRD structures of 1, 2, and 3, showing the atom labeling scheme of the asymmetric unit. All non-hydrogen atoms are drawn as thermal displacement ellipsoids of the 50% level, while hydrogen atoms have been drawn as spheres of arbitrary radii.
Table 1. Selected Bond Distances and Angles for Complexes 1, 2, and 3.
| complex | 1 | 2 |
3 | |
|---|---|---|---|---|
| prefix m of ligand | not applied | m = 1 | m = 2 | not applied |
| Bond Distances (Å) | ||||
| V(1)–O(m1) | 1.923(2) | 1.926(1) | 1.937(1) | 1.894(2) |
| V(m1)–N(m2) | 2.047(2) | 2.071(2) | 2.064(2) | 2.104(2) |
| V(m1)–S(m1) | 2.3524(6) | 2.3434(7) | 2.3500(7) | 2.3519(8) |
| Bond Angles (°) | ||||
| O(m1)–V(1)–O(m1)a | 78.27(9)° | 78.36(6)° | [−O(2)] | 83.3(1)° |
| S(m1)–V(1)–S(m1)a | 108.37(4)° | 112.68(3)° | [−S(12)] | 138.77(5)° |
| N(m2)–V(1)–N(m2)a | 155.1(1)° | 148.04(7)° | [−N(22)] | 125.2(1)° |
| O(m1)–V(1)–S(m1) | 155.6(1)° | 148.9(1)° | 149.5(1)° | 125.3(1)° |
Symmetry operators used to generate equivalent atoms: −x+1, y, −z+1/2 for 1, −x+1/2, y, −z+1/2 for 3. For 2, bond angles are given between chemical equivalents atoms O(1)–O(2), S(11)–S(12), and N(12)–N(22).
The molecular structures of all three compounds contain discrete monomeric vanadium(IV) species coordinated by two tridentate, twofold negative ONS-donor Schiff base ligands. The Schiff bases coordinate to vanadium via the O-atom of their deprotonated hydroxyl-OH, the imine N-atom, and the S-atom of the sulfide moieties. In each case, the coordination spheres around the V atoms are strongly distorted. The geometries may be best described as distorted octahedral for 1 and 2 with the O- and S-atoms in cis- and the N-atoms in trans-positions, while 3 exhibits a distorted trigonal prismatic geometry with the O-atoms in cis-positions at one edge of the prism, while the S- and N-atoms are cis to each other on the other edges of the prism, as shown in Figure S1.
As a result of these different coordination geometries, bond lengths and angles in the coordination spheres of the vanadium atoms differ significantly (Table 1). Namely, considerable differences are observed in the case of bond angles as a result of different coordination geometries, mostly related to the bond angles between the central vanadium atom and two terminal donors (O, S) atoms of the ligands. In this context, the corresponding values [155.6(1)° for 1 and 148.9(1)°/149.5(1)° for 2] are typical for the octahedral vanadium coordination with all three donor atoms in a meridional arrangement [ideal value: 180°], while the value of 125.3(1)° for 3 corresponds quite well with a trigonal-prismatic vanadium coordination [ideal value of a regular trigonal prism: 125°]. Additionally, bond lengths are also affected. While differences are small (Δ = 0.009 Å) for the vanadium–sulfur distances [d(V–S) range from 2.3434(7) Å for 2 to 2.3524(6) Å for 1], those for the vanadium–oxygen and vanadium–nitrogen distances are much greater [d(V–O) range from 1.894(2) Å for 3 to 1.937(1) Å for 2 with Δ = 0.043 Å, while d(V–N) range from 2.047(2) for 1 to 2.104(2) for 3 with Δ = 0.057 Å]. To a certain extent, this may be ascribed to the different temperatures at which the crystal structures were determined, but this is not fully consistent with the behavior of 1 (T = 100 K) and 2 and 3 (T = 296 K). Therefore, other effects must be taken into account. Notably, EPR analysis and DFT simulations indicate that at least two isomers with comparable stability, close to the meridional and facial limit, coexist in the solutions, from where the solid complexes are isolated (vide infra); thus, the isomeric selection experimentally observed could be ascribed to stabilizing crystal packing effects. Here, it is important to highlight that the characterized isomers are defined referring to the limit cases represented by meridional and facial arrangement, even though complexes 1–3 correspond to intermediate structures.
In this context, we highlight two features of the crystal structures of 1–3. The first one concerns the different conformations of the two crystallographic independent ligands found in the crystal structure of 2, indicated with m in Table 1, as they show high conformation flexibility within the ligands (Figure S2), not only in the domain of the sulfide moiety with their low rotation barriers around the sulfur–carbon single bonds, but to a lesser extent also associated to the conjugated double bond system, which obviously may adopt different orientations for coordination.
A second feature is found in the crystal structure of 1 where the formation of dimeric centrosymmetric supramolecules results from the π-interaction of their phenyl moieties (Figure S3), which are almost coplanar to each other with six intermolecular carbon–carbon distances ranging from 3.358 to 3.411 Å. Moreover, similar intermolecular distances (3.257 Å) are found for the nitrogen and carbon atoms attached to the phenyl groups.
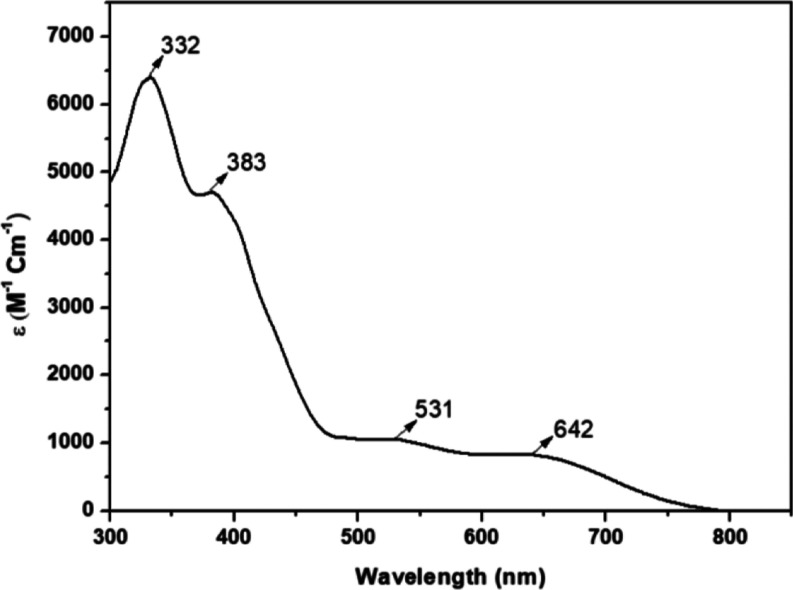
UV–vis Spectroscopy
Electronic absorption spectra of 1–4 were recorded in DMSO and show similarities. As a representative example, the UV–vis spectrum of 3 is shown in Figure 2, and those of 1, 2, and 4 are reported in Figure S4. In the wavelength range 330–650 nm, three absorptions were observed for complexes 1 and 2, whereas four bands are detected for 3 and 4. As pointed out in the literature, for non-oxido vanadium(IV) complexes, the UV–vis spectrum is dominated by ligand-to-metal charge transfer (LMCT) transitions, with large values of the molar absorption coefficient (generally, ε > 1000 M–1 cm–1 in the visible and ε > 5000 M–1 cm–1 in the UV region), from MOs centered on the ligands to V-d based orbitals.53,128 TD-DFT calculations carried out on 1–4 confirmed these findings and indicated that all the absorption bands are strongly mixed and correspond to a combination of various electronic transitions.
Figure 2.
UV–vis spectrum of [VIV(L3)2] (3) in DMSO recorded with a concentration of 1.5 × 10–4 M and path length = 1 cm.
In the visible region, the absorptions in the range 624–642 nm (ε = 159–844 M–1 cm–1) and 530–532 nm (ε = 265–1080 M–1 cm–1) correspond to excitations from ligand-centered MOs, as from highly delocalized π orbitals (for example, L1-π and L1’-π) to V-dz2 or V-dxz.65 The bands at 398 nm for 1 and 2 (with molar absorption coefficients of 7966 and 6557 M–1 cm–1, respectively) and between 332 and 384 nm for 3 and 4 (with molar absorption coefficients of range of 6402–4707 M–1 cm–1) are countersigned by values of ε significantly higher than 4000 M–1 cm–1.65 They are LMCT absorptions mixed with ligand-to-ligand charge transfers. The principal transitions involve MOs with characters V-dz2, V-dxz, and V-dyz for vanadium and MOs with π character for the ligands.
ESI-Mass Spectroscopy
ESI-MS spectra of the compounds 1–4 were recorded in several solvents or mixtures of solvents: CH3CN, DMSO, MeOH/H2O 90/10 (v/v), and DMSO/H2O 90/10 (v/v) in the positive- and negative-ion mode. The ESI-MS(+) and ESI-MS(−) spectra of 1 in CH3CN, recorded with a vanadium concentration of 50 μM, are reported in Figure 3. In the positive-ion mode spectrum, the major peaks are at m/z = 641.11, 642.11, 664.10, and 680.07. No peaks ascribable to the free ligand were detected, and this indicates the stability of the complex to hydrolysis even at these low metal concentrations. The peak at m/z 641.11 is assigned to the oxidized species [VV(L1)2]+ (simulation in panel a of Figure S5), while that at m/z 642.11 can be explained with [VIV(L1)2] + H+ and/or to the isotopic pattern of [VV(L1)2]+ (panels b and c of Figure S5). The high-resolution instrument used in this work allowed us to distinguish between the two options; even though the simulation seems to be identical up to two decimal figures, it is significantly different when four decimal figures are considered. In particular, a peak at m/z 642.1094 is expected for [VV(L1)2]+ and at m/z 642.1138 for [VIV(L1)2] + H+, which therefore contribute to the signals in the range m/z 641–645 in a negligible mode (Figure S6). To the best of our knowledge, this is one of the first cases in which ESI-MS allows to discriminate unambiguously two complexes in two different metal oxidation states. Instead, the signals at m/z 664.11 and at m/z 680.07 can be assigned without any ambiguity to the adducts [VIV(L1)2] + Na+ and [VIV(L1)2] + K+ (simulations in Figures S7 and S8). The values of m/z for all the species detected by ESI-MS are listed in Table 2. Summarizing the results, [VIV(L1)2] (1) can undergo partial oxidation to [VV(L1)2]+.
Figure 3.

ESI-MS spectra in the positive-ion mode (a) and negative-ion mode (b) recorded on complex 1 dissolved in CH3CN (50 μM).
Table 2. Identified Species in the Positive- and Negative-Ion Mode ESI-MS Spectra of Complexes 1–4a.
| ion | composition | experimental m/zb | calculated m/zb | error (ppm)c |
|---|---|---|---|---|
| [VV(L1)2]+ | C26H34N6O2S4V | 641.1066 | 641.1060 | 0.9 |
| [VIV(L1)2] + Na+ | C26H34N6NaO2S4V | 664.0960 | 664.0958 | 0.3 |
| [VIV(L1)2] + K+ | C26H34KN6O2S4V | 680.0699 | 680.0697 | 0.3 |
| [VVO2(L1)]− | C13H17N3O3S2V | 378.0161 | 378.0156 | 1.3 |
| [VV(L2)2]+ | C38H42N6O2S4V | 793.1688 | 793.1686 | 0.3 |
| [VIV(L2)2] + Na+ | C38H42N6NaO2S4V | 816.1585 | 816.1584 | 0.1 |
| [VIV(L2)2] + K+ | C38H42KN6O2S4V | 832.1323 | 832.1323 | 0.0 |
| [VVO2(L2)]− | C19H21N3O3S2V | 454.0480 | 454.0469 | 2.4 |
| [VV(L3)2]+ | C26H20N4O2S4V | 598.9911 | 598.9903 | 1.3 |
| [VIV(L3)2] + Na+ | C26H20N4NaO2S4V | 621.9814 | 621.9801 | 2.1 |
| [VIV(L3)2] + K+ | C26H20KN4O2S4V | 637.9561 | 637.9540 | 3.3 |
| [VVO2(L3)]− | C13H10N2O3S2V | 356.9586 | 356.9578 | 2.2 |
| [VV(L4)2]+ | C38H28N4O2S4V | 751.0533 | 751.0529 | 0.5 |
| [VIV(L4)2] + Na+ | C38H28N4NaO2S4V | 774.0427 | 774.0413 | 1.8 |
| [VVO2(L4)]− | C19H14N2O3S2V | 432.9899 | 432.9891 | 1.8 |
The data are referred to the mass spectra recorded in CH3CN.
Experimental and calculated m/z values refer to the monoisotopic peak with the highest intensity.
Error in ppm with respect to the experimental value, calculated as 106 × [Experimental (m/z) – calculated (m/z)]/calculated (m/z).
The negative-ion spectrum shows few signals and a major peak at m/z = 378.02 (Figure 3b). The simulations allowed us to determine the stoichiometry of this species which is assigned to [VVO2(L1)]−, formed upon the oxidation of [VIV(L1)2]. The simulation of the isotopic pattern is represented in Figure S9.
The behavior of complexes 2–4 is comparable, and the observed species are listed in Table 2. The results in the mixture MeOH/H2O 90/10 (v/v) are globally similar, but it should be mentioned that the presence of water decreases the solubility of the complexes and favors the oxidation to VV, as shown in Figure S10.
A few aspects deserve to be emphasized: (i) the possibility that the observed oxidation process partially occurs in-source, during the recording of the spectra, cannot be excluded;120,129 (ii) the tridentate coordination of the ONS ligand to V is quite strong, and the hydrolysis occurs at low extension, both in CH3CN and in MeOH/H2O 90/10 (v/v), even at the low metal concentrations used for recording the spectra; (iii) a non-oxido vanadium(V) species is formed in solution; (iv) the formation of non-oxido vanadium(V), a hard metal ion, is rather unusual and few examples have been reported in the literature up to now;130,131 and (v) under our experimental conditions, [VIV(L1)2] forms adducts only with Na+ and K+ ions but not with H+.
NMR Spectroscopy
Proton NMR spectra of H2L1–4 were recorded in DMSO-d6. These spectra exhibit a resonance in the range δ = 13.46–13.13 ppm attributable to −NH groups,132 one singlet peak of the phenolic −OH groups in the region δ = 11.09–10.08 ppm due to intermolecular hydrogen bonding, and another sharp singlet at δ = 9.20–8.31 ppm due to the azomethine −CH protons.53,64,89,127,132−138 The aromatic proton signals of the free ligands are clearly observed in the expected range between δ 8.80 and 6.07 ppm. For the aliphatic protons, two multiplets are observed in the range δ = 3.36–3.31 ppm for −N–CH2 and δ = 1.08–1.11 ppm for the −CH3 protons of the diethylamino fragment in the case of H2L1–2. Singlets for the CH2S– protons of H2L2 and H2L4 are observed around ∼4.45 ppm, which are absent in the spectra of H2L1 and H2L3.132 Compounds H2L1 and H2L3 contain one extra singlet in the aliphatic region near 2.51 ppm due to the −SCH3 protons.53,126
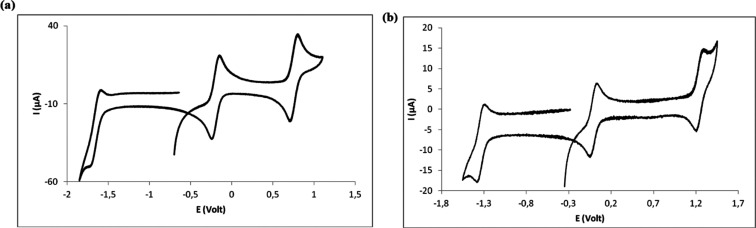
Electrochemical Studies
The cyclic voltammograms of 1–4 were obtained from Bu4NBF4/CH2Cl2 (0.10 M) electrolyte solutions under dinitrogen at a scan rate of 200 mV s–1. The potentials were measured in Volt (±10 mV) vs SCE using [Fe(η5-C5H5)2]0/+ (E1/2ox = 0.475 V) as an internal reference. The redox potential data are summarized and displayed in Table 3. All complexes present reversible anodic waves in the range 0.74–1.18 V, attributable to the VIV → VV oxidation processes and cathodic reversible waves from −0.08 to −0.20 V, assignable to the VIV → VIII reduction64 (Table 3). One additional quasi-reversible lower potential cathodic wave is observed from −1.39 to −1.73 V, which is attributed to a ligand-centered reduction process. Such an attribution is based on the study of the redox properties of H2L3 and H2L4 as representative examples (Table 3 and Figure S11). The cyclic voltammograms obtained for the free ligands display irreversible cathodic processes with potentials that differ from those of the related complexes by ca. 5 mV or less (Table 3). In the complexes, such cathodic waves gain reversibility, a behavior earlier reported for the similar types of non-oxido vanadium(IV) complexes.53,65 Such a behavior suggests that the metal ion partially accommodates the increase in electron density without further changes in the ligand. The reversibility of the anodic and cathodic processes shows that no major structural modifications occur upon electron transfer from or to the hexa-coordinated VIV complexes as observed for several other non-oxido VIV species.139,140 Representative cyclic voltammograms of complexes 2 and 4 are displayed in Figure 4.
Table 3. Cyclic Voltammetry Data for 1–4 and H2L3–4a,b.
| complex/ligand | IIE1/2red | IE1/2red | E1/2ox |
|---|---|---|---|
| 1 | –1.68 | –0.20 | 0.75 |
| 2 | –1.73 | –0.20 | 0.74 |
| 3 | –1.49 | –0.12 | 1.10 |
| 4 | –1.39 | –0.08 | 1.18 |
| H2L3 | –1.47c | 1.27 | |
| H2L4 | –1.34c | 1.33 |
Values in Volt (±10 mV) vs SCE measured at a scan rate of 200 mV/s.
In Bu4NBF4/CH2Cl2 (0.10 M).
Epred.
Figure 4.
Cyclic voltammograms of (a) [VIV(L2)2] (2) and (b) [VIV(L4)2] (4) obtained from Bu4NBF4/CH2Cl2 (0.10 M).
The electrochemical behavior of 1–4 is similar to those of other non-oxido VIV complexes containing of ONS and ONO donor ligand systems reported earlier,53,65 where rather similar reversible oxidation and reduction processes (VIV → VV and VIV → VIII) were observed. Additionally, 1 and 2, which have diethylamine substituents at the phenyl ring (X = NEt2; Scheme 1), display considerably lower VIV → VV oxidation potentials (Table 3) than the unsubstituted VIV complexes (3 and 4). Such a trend is attributed to the high electron releasing character of the amine group in agreement with the pattern previously reported for VIV species formed by the phenyl amine (X = NH2)-substituted ONO ligands that also display lower potentials than their analogues.65 For 3 and 4, the anodic processes occur at potentials in the same range (1.1 V vs SCE) of the formerly reported dithiocarbazate species.53 For 1–4, the higher potential cathodic process occurs within a short range of potentials (from −0.2 to 0.0 V) either for dithiocarbazate or for aroylhydrazone complexes, in agreement with the VIV → VIII reduction being less sensitive to the characteristics of the substituents at the ligand than the VIV → VV oxidation.
Optimization of the Stable Structures in Solution
On the basis of the results obtained with single-crystal X-ray diffraction and ESI-MS data, the species stable in media containing moderate amounts of water are [VIV(L1–4)2], i.e. the same species isolated in the solid state, and [VV(L1–4)2]+ and [VVO2(L1–4)]−, derived from the partial oxidation of 1–4.
The structures of the complexes formed in solution were optimized by DFT methods following the procedure reported in the literature.111,112 As an example, those of [VIV(L1)2], [VV(L1)2]+, and [VVO2(L1)]− are depicted in Figure 5. Selected bond lengths and angles of all the optimized structures are collected in Tables S2–S4. Notably, the prediction of the geometric parameters is very good, as demonstrated by the superposition of the experimental and calculated structures of complex 1 (Figure 5, top left).
Figure 5.
DFT-optimized structures of mer-[VIV(L1)2], fac’-[VIV(L1)2], fac-[VIV(L1)2], mer-[VV(L1)2]+, and [VVO2(L1)]−. For mer-[VIV(L1)2] isomer (1), the superposition with the SC-XRD structure (in orange) is also shown.
It is interesting to observe that for [VIV(L1–4)2] (1–4), three different energy minima were found, one corresponding to the meridional arrangement of the ligands and two to the facial one. In Table 4, the angles between the external donors of the same ligand molecule are indicated; they should be 180° and 90° for mer and fac situations, respectively. Notably, for 1 and 2 complexes, the mer isomer is the most stable, while for 3 and 4, it is the fac isomer. This agrees well with the SC-XRD structures that show the same trend: in fact, the geometry of 1 and 2 is close to the meridional limit and that of 3 to the facial limit (see section “Single-Crystal X-ray Diffraction Analysis of Complexes 1–3”). The flexible structure of the ligands accounts for the coexistence of the two isomers. The free energy difference between the three isomers is very low, between 0.1 and 2.3 kcal mol–1 (Table 4); so, it is possible that more than one species exists in solution and subtle factors such as the solubility, the interaction between the solid complexes, and the crystal packing could favor the formation of one of the three isomers in the solid state.
Table 4. Bond Angles and Relative ΔG of Formation in Solution (ΔΔGaq) for the Isomers of Non-Oxido Complexes [VIV(L1–4)2].
|
trans anglesb,c |
||||
|---|---|---|---|---|
| isomera | N1–V–N2 | O1–V–S1 | O2–V–S2 | ΔΔGaqd |
| mer-[VIV(L1)2]a | 153.9 (155.1) | 156.3 (155.6) | 156.3 (155.6) | 0.0 |
| fac-[VIV(L1)2] | 121.4 | 123.1 | 120.6 | 0.7 |
| fac’-[VIV(L1)2] | 118.8 | 124.6 | 124.6 | 1.7 |
| mer-[VIV(L2)2]a | 154.0 (148.0) | 156.4 (148.9) | 156.6 (149.5) | 0.0 |
| fac-[VIV(L2)2] | 122.0 | 123.4 | 120.9 | 0.1 |
| fac’-[VIV(L2)2] | 118.8 | 124.6 | 124.6 | 1.0 |
| mer-[VIV(L3)2] | 161.7 | 164.0 | 164.0 | 0.9 |
| fac-[VIV(L3)2]a | 121.1 (125.2) | 122.0 (125.2) | 119.7 (125.2) | 0.0 |
| fac’-[VIV(L3)2] | 122.9 | 124.8 | 124.8 | 1.8 |
| mer-[VIV(L4)2] | 161.2 | 163.4 | 163.4 | 1.1 |
| fac-[VIV(L4)2]a | 121.2 | 122.0 | 119.8 | 0.0 |
| fac’-[VIV(L4)2] | 161.7 | 124.7 | 124.7 | 2.3 |
In bold, the most stable isomers in aqueous solution.
Values in degrees.
In parentheses, the experimental values extracted from SC-XRD structures.
Values in kcal mol–1.
Concerning [VV(L1–4)2]+, the structures are very similar to [VIV(L1–4)2], in agreement with the electrochemical results that indicate a reversible anodic wave for 1–4, while [VVO2(L1–4)]− are typical dioxidovanadium(V) complexes with a geometry intermediate between the square pyramid and the trigonal bipyramid.
EPR Spectroscopy
EPR spectra of complexes 1–4 were recorded at 120 K after dissolution in dichloromethane. Spin Hamiltonian parameters (factor g and 51V hyperfine coupling constant A) were extracted simulating the spectra with WinEPR software141 and were compared with the experimental ones. They are reported in Table 5, while the experimental spectra of 1–4 are represented in Figure 6.
Table 5. Experimental (Exptl) and DFT Calculated (Calcd) Spin Hamiltonian EPR Parameters for the Non-Oxido VIV Complexes [VIV(L1–4)2].
| isomer | gxexptl | gyexptl | gzexptl | Axexptla | Ayexptla | Azexptla | Azcalcda,b | PD (Az)c |
|---|---|---|---|---|---|---|---|---|
| mer-[VIV(L1)2] | 1.987 | 1.980 | 1.960 | –10.0 | –36.0 | –110.2 | –104.6 | –5.1 |
| fac-[VIV(L1)2] | 1.987 | 1.982 | 1.961 | –12.0 | –38.4 | –120.6 | –120.2 | –0.3 |
| fac’-[VIV(L1)2] | –115.6 | –4.2 | ||||||
| mer-[VIV(L2)2] | 1.986 | 1.980 | 1.960 | –9.8 | –35.7 | –110.0 | –104.4 | –5.1 |
| fac-[VIV(L2)2] | 1.986 | 1.982 | 1.961 | –11.8 | –38.1 | –120.7 | –120.2 | –0.4 |
| fac’-[VIV(L2)2] | –115.6 | –4.2 | ||||||
| mer-[VIV(L3)2] | d | d | d | d | d | d | –98.2 | d |
| fac-[VIV(L3)2] | 1.986 | 1.981 | 1.962 | –11.7 | –38.4 | –120.9 | –120.0 | –0.7 |
| fac’-[VIV(L3)2] | –116.7 | –3.5 | ||||||
| mer-[VIV(L4)2] | d | d | d | d | d | d | –97.2 | d |
| fac-[VIV(L4)2] | 1.986 | 1.982 | 1.962 | –12.0 | –38.2 | –120.6 | –120.0 | –0.5 |
| fac’-[VIV(L4)2] | –116.7 | –3.2 |
Values in 10–4·cm–1.
Calculated at the B2PLYP/6-311g(d,p) DFT theory level.
Percent deviation (PD) calculated as 100 × [(|Az|calcd – |Az|exptl)/|Az|exptl].
Isomer not present in solution.
Figure 6.

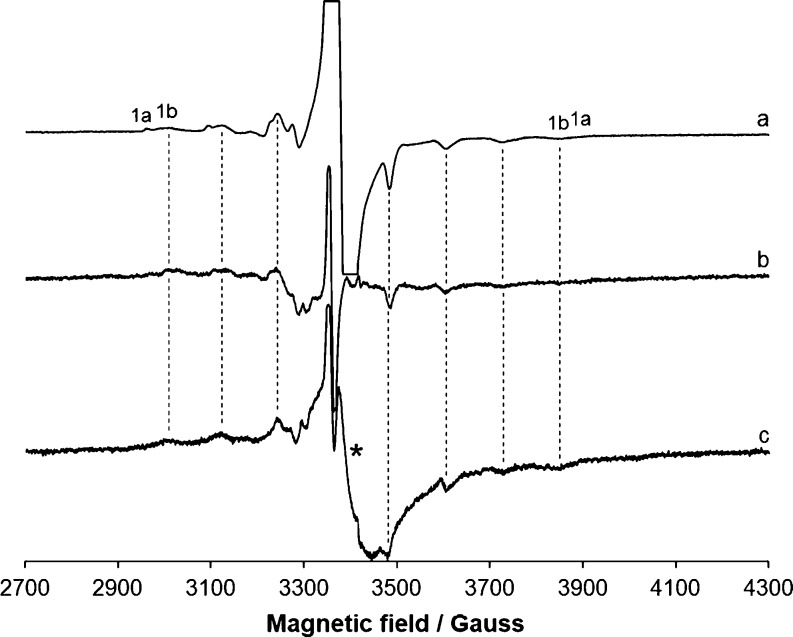
First derivative X-band anisotropic EPR spectra of (a) [VIV(L1)2] (1); (b) [VIV(L2)2] (2); (c) [VIV(L3)2] (3); and (d) [VIV(L4)2] (4). All the spectra were recorded in CH2Cl2 at 120 K with an approximate vanadium concentration of 8 × 10–4 M. With the dotted lines, the MI = −7/2, 7/2 resonances of the species 1a–4a and 1b–2b are indicated, while with the asterisk, the isotropic absorption due to the undissolved solid complex 3 is shown. The low- and high-field regions of the spectrum of 1 are amplified by 20 and 25 times, respectively, at the top of the figure.
It is noteworthy that, when 3 and 4 are dissolved in CH2Cl2, only one set of resonances is detected (species 3a and 4a in the traces c and d) with a value of A between 120 and 121 × 10–4 cm–1. In contrast, for 1 and 2, two complexes are observed, the first denoted by 1a and 2a, which coincide with those revealed in the systems containing 3 and 4, and the second ones denoted by 1b and 2b, with a smaller A constant, around 110 × 10–4 cm–1. The values of A, much lower than (140–145) × 10–4 cm–1, are typical of non-oxido vanadium(IV) complexes.114 Considering the results from X-ray diffraction analysis that indicate that two types of crystal structures are obtained, one close to the limit of the meridional isomerism (1 and 2) and another close to the facial arrangement (3, see section “Single-Crystal X-ray Diffraction Analysis of Complexes 1–3”), we propose that the resonances belong to the two isomeric forms of 1–4, those corresponding to the fac and mer structures.
In Figure 7, the experimental spectra of 1 and 4 (traces b and c) are compared with the simulated ones. Notably, the spectrum of the pure species 1b can be obtained subtracting the signals of 1a from the experimental spectrum of 1 and can be satisfactory simulated with WinEPR software (Table 5).
Figure 7.

First derivative X-band anisotropic EPR spectra of (a) simulated spectrum of 4a obtained with WinEPR software; (b) experimental spectrum of [VIV(L4)2] (4); (c) experimental spectrum of [VIV(L1)2] (1); (d) spectrum of the species 1b obtained from the experimental spectrum of 1 subtracting the signals of 1a; (e) simulated spectrum of 1b. With 1a and 4a, the MI = −7/2, 7/2 resonances of the fac isomers are indicated, while 1b denotes the MI = −7/2, 7/2 resonances of mer isomer. The experimental spectra were recorded in CH2Cl2 at 120 K with an approximate vanadium concentration of 8 × 10–4 M.
The A tensor of the 51V nucleus for non-oxido VIV complexes was computed for each structure stable in solution (isomers mer, fac, and fac’; see the previous section) through the method implemented into the ORCA package,113,142,143 using the functional B2PLYP coupled with the triple-ζ basis 6-311g(d,p) (Table 5). The B2PLYP/6-311g(d,p) combination gives high quality predictions of the largest component of the A tensor.114 The theory background was described elsewhere.144Ax, Ay, and Az are negative, as expected for VIV species, but in the text, the absolute values are reported for convenience of discussion.
By examining the data in Table 5, it emerges that the order of the constant |Az| is fac ∼ fac’ > mer, in agreement with what was experimentally observed. The prediction, expressed as percent deviation, is in the range 0–5%, in line with the data previously published; in particular, in all the cases, a slight underestimation of |Az| is expected.114 It must be observed that for 3 and 4, only the fac isomer should exist in solution having the large value of the hyperfine coupling constant (species 3a and 4a in Figure 6). This is in line with the X-ray diffraction determinations and DFT calculations, which suggest that, for these two complexes, the facial arrangement is more stable than the meridional one (Table 4). Instead, with 1 and 2, the two sets of resonances could be assigned to fac (species 1a and 2a) and mer isomers (1b and 2b), in agreement with SC-XRD and DFT data. Notably, the existence of the two isomers in solution, meridional and facial, has been proposed by Chaudhuri and co-workers for non-oxido VIV species formed by some ONO ligands.145
Solution Stability of Complexes 1–4
The stability of all complexes was established in water containing solvents through UV–vis, ESI mass spectrometry, and NMR and EPR spectroscopy.
UV–vis Studies
Solutions of complexes 1–4 were prepared in a mixture DMSO/H2O 10/90 (v/v) with a vanadium concentration of 1 mM. Their UV–vis spectra are depicted in Figure S12. The shape of the spectrum of 1 in this solvent mixture is very similar to the one measured in DMSO; therefore, we can conclude that the same type of coordination is retained.
When complex 1 was dissolved in a solution containing minimum essential medium (MEM, which does not contain FBS) instead of water, precipitation was evident from the beginning (Figure S13).
ESI-MS Studies
The aged solutions in MeOH/H2O 90/10 (v/v) were diluted in CH3CN and analyzed by ESI-MS (concentration 50 μM). For all complexes, the most important species remained those detected at t = 0 h, i.e., the non-oxido VIV complexes [VIV(L1–4)2]; the VV species [VV(L1–4)2]+, derived from the oxidation of the previous one, and [VVO2(L1–4)]− were also detected. At these experimental conditions, the non-oxido VIV complexes seem to be stable after 24 h with a slight increase in the oxidation process, as indicated by the increment of the relative amount of [VVO2(L1)2]− (Figure S14). This demonstrates that, under the biological experiments, the active species may be one or more of those present in solution: [VIV(L1–4)2], [VV(L1–4)2]+, and/or [VVO2(L1–4)]−. This was considered during the docking studies.
EPR Studies
EPR spectra were recorded 24 h after the dissolution of the most soluble compound, [VIV(L1)2] (1), in DMSO and in an aqueous solution in phosphate buffer at pH 7.4 (Figure 8). It must be observed that complex 1 is not completely soluble in phosphate buffer, but its solubility is enough to record a well-resolved EPR signal. The spectrum in DMSO is very similar to that in CH2Cl2 (cfr. traces in Figures 6 and 8), with the coexistence of fac (1a) and mer (1b) isomers. When the spectrum is recorded after 24 h, the pattern remains substantially unaltered, even though a significant decrease in the spectral intensity is detected (trace b of Figure 8). In aqueous solution, the behavior is more or less the same, and after 24 h, the resonances of [VIV(L1)2] are clearly visible (trace c of Figure 8).
Figure 8.
First derivative X-band anisotropic EPR spectra of (a) complex 1 dissolved in DMSO; (b) complex 1 dissolved in DMSO after 24 h; and (c) complex 1 dissolved in the phosphate buffer (pH 7.4) after 24 h. Fac and mer isomers are denoted with 1a and 1b, while with the dotted lines the MI = −7/2, −5/2, −3/2, 1/2, 3/2, 5/2, 7/2 resonances of the species 1b are shown. The presence of a minor amount of the undissolved solid causes the isotropic resonance centered around 3400 Gauss and indicated by the asterisk. The approximate vanadium concentration to record the spectra was 8 × 10–4 M.
The decrease of the signal intensity over time can be related to the oxidation of VIV to VV and the formation of the species [VV(L1)2]+ and [VVO2(L1)2]−, as revealed in the ESI-MS experiments described above.
BSA Binding Study
Fluorescence Competition Titrations of 1–4 with BSA
Fluorescence titration data for all systems are depicted in Figure 9. In all cases, significant quenching of BSA fluorescence is observed, due to its Trp residues, but no major spectral shifts were observed. As concluded in the next section on docking studies (vide infra), the binding of complexes may take place close to the Trp213 residue, this explaining the strong quenching of fluorescence experimentally observed.
Figure 9.
Fluorescence emission titration spectra of BSA, measured at room temperature: (a) 1 with [BSA] = 1.39 μM and [1]:[BSA] = 0.0–2.1; (b) 2 with [BSA] = 1.15 μM and [2]:[BSA] = 0.0–3.4; (c) 3 with [BSA] = 1.35 μM, and [3]:[BSA] = 0.0–3.4; and (d) 4 with [BSA] = 1.33 μM and [4]:[BSA] = 0.0–2.9. In all systems, progressive quenching (emission intensity decrease) was observed with the addition of the solutions containing 1–4.
Stern–Volmer quenching constants as well as the fitting parameter (r2) were determined by assuming that the species present in solution are the complexes [VIV(L1–4)2]; the results are included in Table 6. Figure 10 shows the Stern–Volmer plots for all systems. Complexes 1 and 3 show KSV constants of (1.1–1.2) × 105 M–1, while for 2 and 4,KSV are ca. 2.1 × 105 M–1, confirming the high affinity of complexes [VIV(L1–4)2] for BSA. Therefore, the group that seems to influence the most the strength of the interaction with BSA is the one connected to the sulfur atom: the replacement of CH3 by CH2–Ph increases twice the quenching constant. This interpretation is also supported by docking simulations.
Table 6. Binding and Fitting Parameters Obtained from the Fluorescence Titration Experiments, Measured at Room Temperature.
| complex | KSV (M–1) | r2 | KBC | n | r2 |
|---|---|---|---|---|---|
| 1 | 1.1 × 105 | 0.995 | 2.6 × 104 | 0.89 | 0.985 |
| 2 | 2.1 × 105 | 0.997 | 4.0 × 104 | 0.88 | 0.973 |
| 3 | 1.2 × 105 | 0.996 | 1.8 × 104 | 0.85 | 0.999 |
| 4 | 2.1 × 105 | 0.997 | 3.6 × 104 | 0.87 | 0.991 |
Figure 10.
Stern–Volmer
plots ( vs [Q]) for complexes 1–4.
vs [Q]) for complexes 1–4.
The double logarithm plot of log[(I0–I)/I] vs log[Q], where Q is the concentration of complexes 1–4 (see eq 2), is depicted in Figure S15 and allows us to obtain the binding constants KBC (Table 6). If it is assumed that the values of KBC, in the range (1.8–4.0) × 104, are correct, this would indicate a reversible binding of the metal complexes to BSA. It must be observed that, considering the existence in solution of [VV(L1–4)2]+ and [VVO2(L1–4)]− besides [VIV(L1–4)2], as suggested by ESI-MS and DFT techniques, a mixture of these species may contribute to the affinity of 1–4 to BSA.
Study of BSA Interaction through Docking Analysis
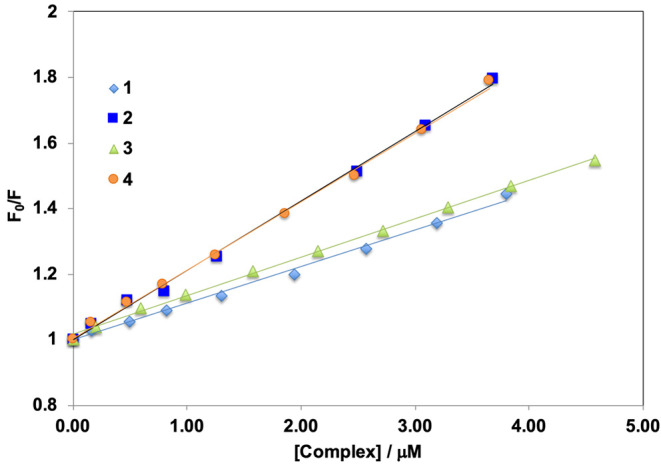
Dockings of the most stable isomers of [VIV(L1–4)2], derived from the SC-XRD structures and DFT calculations, toward BSA highlight several binding modes with scoring values (Fmax) ranging from 15.5 to 24.5 GoldScore units. Although the scoring values are similar indicating the absence of binding specificity, the affinity order mer-[VIV(L2)2] ∼ fac-[VIV(L3)2] > mer-[VIV(L1)2] ∼ fac-[VIV(L4)2] is predicted for this series of complexes. The best solutions are located at the interfaces of subdomains IIA/IIIA for [VIV(L1,3,4)2] (Figure 11a,c,d) and IIB/IIIA for mer-[VIV(L2)2] (Figure 11b). Additional solutions with slightly lower affinity are found at interface IA/IIA. All these interfacial regions are internal pockets reported to be common binding sites for ligands, including metal species.146,147 Notably, the adducts formed at these sites are close to Trp213, in line with the fluorescence quenching observed experimentally. Each adduct is stabilized inside the binding site by at least one hydrogen bond between the aza functionality of L1–4 with hydroxyl groups of Tyr or NH groups of Arg, Asn or Lys side chains (Table 7 and Tables S5–S8).
Figure 11.
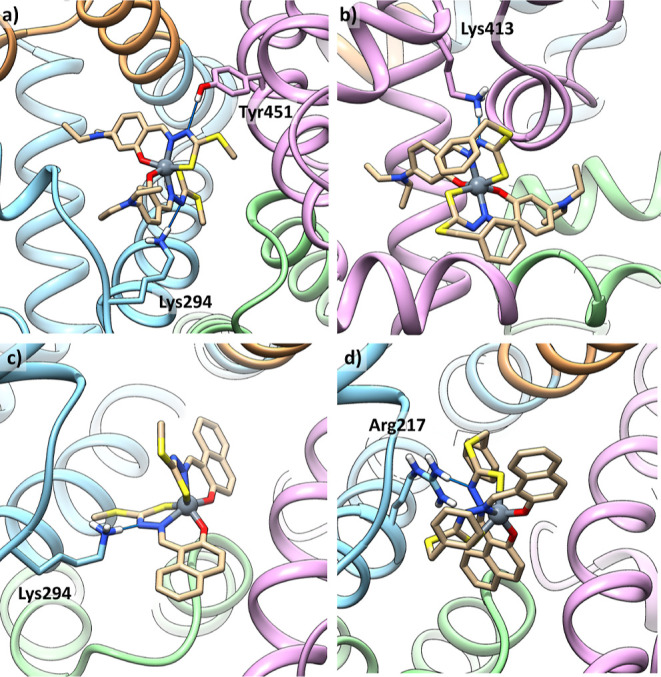
Best representative solutions of the most stable clusters for the interaction of [VIV(L1–4)2] with BSA: (a) mer-[VIV(L1)2] at the interface IIA/IIIA; (b) mer-[VIV(L2)2] at the interface IIB/IIIA; (c) fac-[VIV(L3)2] at the interface IIA/IIIA; and (d) fac-[VIV(L4)2] at the interface IIA/IIIA. Subdomains IIA, IIIA, IB, and IIB are depicted in cyan, purple, brown, and green, respectively. Interacting residues are also shown.
Table 7. Blind Docking Results for the Interaction of [VIV(L1–4)2] with Bovine Serum Albumin.
| complex | region | Fmaxa | Fmeanb | interactions | popc |
|---|---|---|---|---|---|
| [VIV(L1)2] | IIA/IIIA | 20.8 | 17.9 | NH3+–Lys294···NN; OH–Tyr451···NN’ | 43 |
| [VIV(L2)2] | IIB/IIIA | 24.5 | 20.3 | NH3+–Lys413···NN | 67 |
| [VIV(L3)2] | IIA/IIIA | 23.2 | 20.6 | NH3+–Lys294···NN | 63 |
| [VIV(L4)2] | IIA/IIIA | 19.4 | 14.7 | NH2–Arg217···NN | 17 |
Fitness value for the most stable pose of each cluster (Fmax).
Mean Fitness value of the GoldScore scoring function for each cluster (Fmean).
Number of solutions in the identified cluster.
Docking results for [VVO2(L1–4)]− show a similar trend, and the affinity order is [VVO2(L2)]− ∼ [VVO2(L1)]− > [VVO2(L3)]− ∼ [VVO2(L4)]−, with the best solutions located at the interfaces of subdomains IIA/IB for [VVO2(L1)]− (Figure 12a), IIA/IIB for [VVO2(L2–4)]− (Figure 12b,d), and IB/IIIB for [VVO2(L3)]− (Figure 12c). Also, for this series of complexes, additional solutions with slight lower affinity are found at interfaces IIIA/IB, IA/IIA, and IIA/IB. Similar to the non-oxido VIV species, with [VVO2(L1–4)]− hydrogen bonds are formed between the aza functionality of L1–4 and VV=Ooxido with hydroxyl groups of Tyr and Thr or NH groups of Arg, Asn, or Lys side chains (Table 8 and Tables S9–S12). For [VVO2(L1–4)]− too, the simulated adducts are in quite proximity of residue Trp213.
Figure 12.
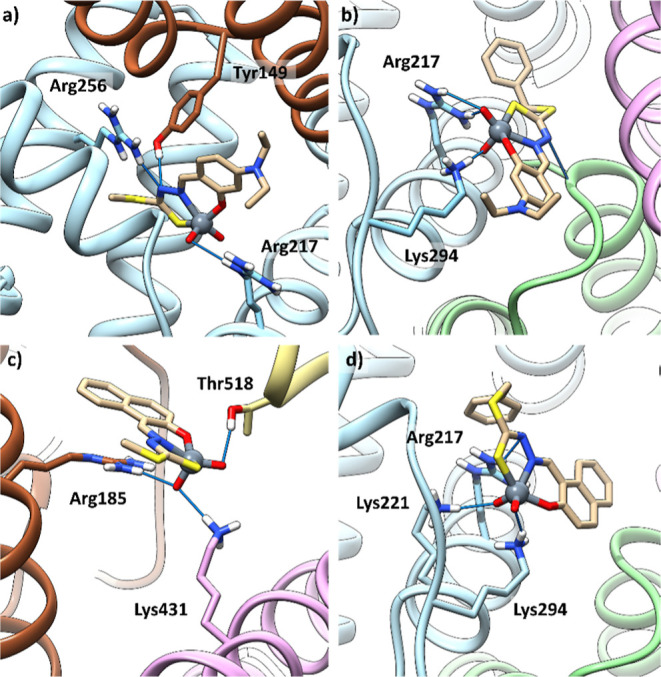
Best representative solutions of the most stable clusters for the interaction of [VVO2(L1–4)]− with BSA: (a) [VVO2(L1)]− at the interface IIA/IB; (b) [VVO2(L2)]− at the interface IIA/IIB; (c) [VVO2(L3)]− at the interface IB/IIIB; and (d) [VVO2(L4)]− at the interface IIA/IIB. Subdomains IIA, IIIA, IB, IIB, and IIIB are depicted in cyan, purple, brown, green and tan, respectively. Interacting residues are also shown.
Table 8. Blind Docking Results for the Interaction of [VVO2(L1–4)]− with Bovine Serum Albumin.
| complex | region | Fmaxa | Fmeanb | interactions | popc |
|---|---|---|---|---|---|
| [VVO2(L1)]− | IIA/IB | 22.8 | 21.4 | NH2–Arg217···VOeq/NN; HO–Tyr149···NN | 56 |
| [VVO2(L2)]− | IIA/IIB | 23.3 | 21.8 | NH3+–Lys294···VOax; NH2–Arg217···VOax | 4 |
| [VVO2(L3)]− | IB/IIIB | 21.3 | 17.6 | NH2–Arg185/NH3+–Lys431···VOax; OH–Thr518···VOeq | 46 |
| [VVO2(L4)]− | IIA/IIB | 21.9 | 20.8 | NH3+–Lys294···VOeq; NH3+–Lys221···VOax; NH2–Arg217···NN | 66 |
Fitness value for the most stable pose of each cluster (Fmax).
Mean Fitness value of the GoldScore scoring function for each cluster (Fmean).
Number of solutions in the identified cluster.
Concerning the oxidized species [VV(L1–4)2]+, solution analogues to those found for the [VIV(L1–4)2] are obtained. This is not surprising considering the high geometry similarity between the two series of complexes.
In Vitro Cytotoxicity Studies
MTT Assay
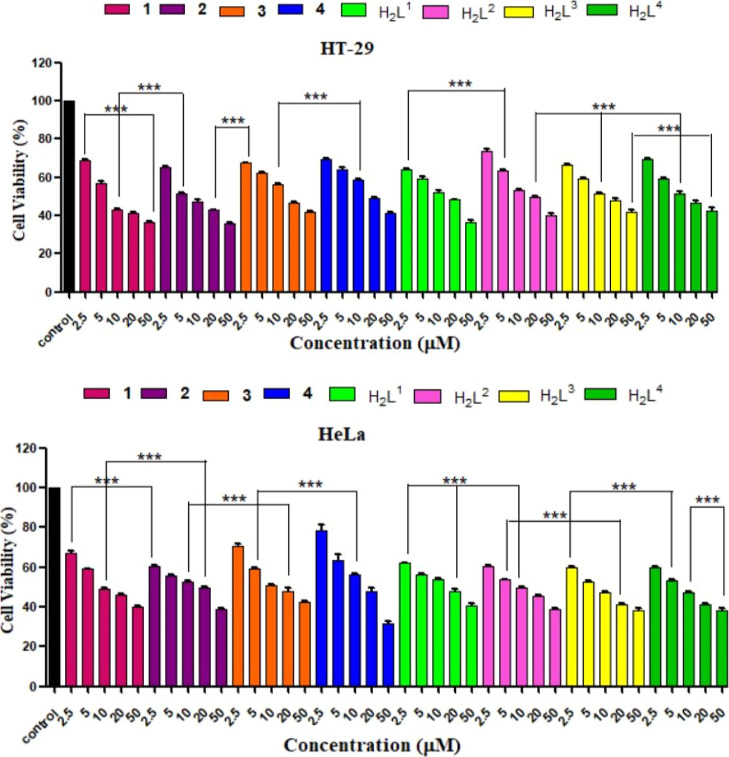
The cytotoxicity potentials of dithiocarbazate ligand precursors (H2L1–4) and non-oxido vanadium(IV) complexes 1–4 were examined through MTT assay against two cancer (HT-29 and HeLa) and one normal (NIH-3T3) cell lines to check the specificity of the metal species toward cancer cells. From Table 9 and Figures 13 and S16, it can be observed that compounds having the −NEt2 group attached in the ligand backbone (1 and 2) are more active than the ligands having the −Ph group (3 and 4). Therefore, in both cell lines, the cytotoxicity potential of 1–4 follows the order 2 > 1 > 3 > 4. The IC50 values of 1–4 are close in the two cancer cell lines, suggesting that these compounds do not show cell type-dependent nature. From the data depicted in Table 9, we can make other important observations, namely, that in cancer cells compounds 1 and 2 (derived from the ligands containing the −NEt2 group) are more active than their corresponding free ligands (H2L1–2), while the free ligands containing the −Ph group (H2L3–4) are more cytotoxic than their respective vanadium species (3 and 4). In other words, in the case of 3 and 4, complexation does not improve the cytotoxic activity like for 1 and 2.
Table 9. IC50 Values (μM) Calculated for the Ligand Precursors and Corresponding Vanadium Complexes Against the HT-29, HeLa, and NIH-3T3 Cells Upon 48 h of Exposurea.
| complex | HT-29 | selectivity index (SI) | HeLa | selectivity index (SI) | NIH-3T3 |
|---|---|---|---|---|---|
| H2L1 | 14.7 ± 0.6 | 1.6 | 15.7 ± 1.9 | 1.5 | 23.5 ± 0.6 |
| 1 | 7.2 ± 0.6 | 1.2 | 9.3 ± 0.2 | 0.9 | 8.7 ± 1.1 |
| H2L2 | 17.9 ± 0.8 | 1.3 | 16.5 ± 1.4 | 1.4 | 23.1 ± 2.3 |
| 2 | 6.1 ± 2.0 | 2.2 | 8.2 ± 0.2 | 1.6 | 13.3 ± 1.3 |
| H2L3 | 12.6 ± 2.1 | >4 | 6.3 ± 0.1 | >8 | >50 |
| 3 | 16.2 ± 0.4 | 0.6 | 11.1 ± 0.9 | 0.9 | 10.3 ± 2.2 |
| H2L4 | 13.1 ± 3.2 | 3.7 | 7.5 ± 0.3 | 6.6 | 49.2 ± 1.6 |
| 4 | 18.7 ± 1.6 | 0.6 | 17.1 ± 0.1 | 0.7 | 11.4 ± 3.1 |
| cisplatinb | 32.7 ± 0.6 | 25.5 ± 0.8 |
Figure 13.
Cytotoxicity profiles of complexes 1–4 for HT-29 and HeLa cell lines after 48 h of incubation. The cells were treated with varying concentrations (2.5, 5, 10, 20, and 50 μM) of the tested complexes, and the cell viability was measured using the MTT assay. Data are reported as the mean ± SD for n = 4; the symbol *** represents a statistical significance of p < 0.001 as compared to the control.
Additionally, it is found that compounds 1–4 are more cytotoxic toward the normal cells than the ligand precursors. This accounts for the selectivity index (SI value). Among the complexes, 2 is more selective toward both cancer cells as compared to its ligand precursor, H2L2 (Table 9). These results provide the clear idea that compound 2, having high cytotoxicity and better SI, is the most promising complex of the series. Also, BSA binding studies, obtained through experimental and DFT methods, are in line with the cytotoxicity results, indicating that 2 with higher protein binding affinity also shows higher cytotoxicity potential.
Another interesting thing observed is the direct relationship which apparently exists between the IC50 values and the gap (mV) between the oxidation and reduction potentials for the complexes 1–4, i.e., the lower the gap, the lower the IC50 values (Figure S17). The same trend is observed for the SI index.
It is also significant to discuss here that 1–4 show comparable or even better cytotoxic results than worldwide used chemotherapeutic drugs; for example, cisplatin under identical experimental conditions against HT-29 and HeLa cancer cell lines shows IC50 values of 32.7 ± 0.6 and 25.5 ± 0.8 μM, respectively.79,148 The present series of compounds also exhibits higher cytotoxicity than our previously reported non-oxido vanadium(IV) complexes with ONO donor ligands against cancer cell lines such as HeLa, A2780, and PC3.64,65 Additionally, the comparison between the cytotoxic activity of this new series of non-oxido vanadium(IV) complexes formed by ONS donors and other previously reported non-oxido VIV species shows that 1–4 have analogous or even better cytotoxicity.43,67
In the light of ESI-MS and DFT results, which suggested that, besides [VIV(L1–4)2], the oxidation products [VV(L1–4)2]+ and [VVO2(L1–4)]− exist in solution, this mixture of species could be responsible for the cytotoxic activity of 1–4. As the formation of a small amount of [VVO2(L1–4)]− implies the presence of an equivalent amount of free ligands in solution, cytotoxicity may also have the contribution of H2L1–4. However, because all IC50 values are in a rather narrow range (ca. 6–19 μM) and the amounts of free ligands formed are quite small, we expect the contribution of H2L1–4 to the cytotoxicity to be small.
Nuclear Staining Assay
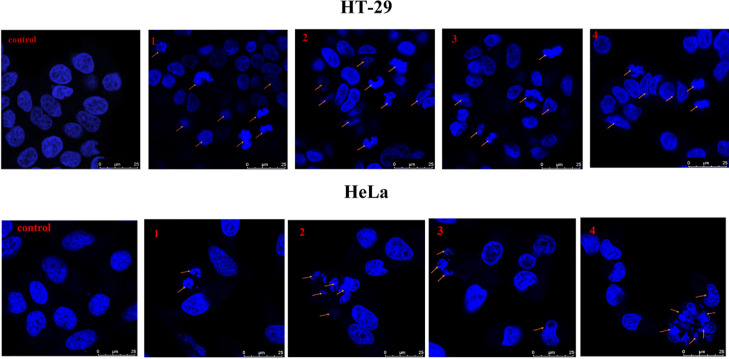
Apoptosis is normally considered as the “cleanest” pathway of cell death since the cellular contents do not leak; moreover, no inflammation takes place and the dead cells may be cleanly eliminated by the immune system. DAPI, a nuclear staining agent, is helpful to localize any nuclear alterations in cells, which further helps in the study of the mechanism of cell death in response to the treatment with the vanadium complexes. The DAPI staining images of 1–4 against HeLa and HT-29 cell lines are depicted in Figure 14. From the figure, the change in the nuclei morphology is clearly observed in the presence of the complexes, as compared to the control cell image. In the treated HT-29 cells, shrinking of the cells, fragmented, and condensed chromatin bodies are observed, while in HeLa cells—in addition to these nuclear changes—nuclear blebbing is also revealed after the treatment with 1–4. The observed nuclear changes are the indication of apoptotic cell death induced by the complexes. Also, in the case of HeLa, a decrease in the number of cells is noticed after the treatment with the metal drugs as compared to the control. Therefore, from all these observations, we can state that programmed cell death (apoptosis)149,150 occurs in cancer cells in response to the treatment with the synthesized vanadium drugs.
Figure 14.
Morphologies of HT-29 (upper panel) and HeLa (lower panel) cells treated with complexes 1–4 at a concentration of 10 μM for 24 h. The cells were visualized under a confocal microscope after staining with DAPI (blue color, nucleus). The scale bar corresponds to 25 μm.
Apoptosis Assay
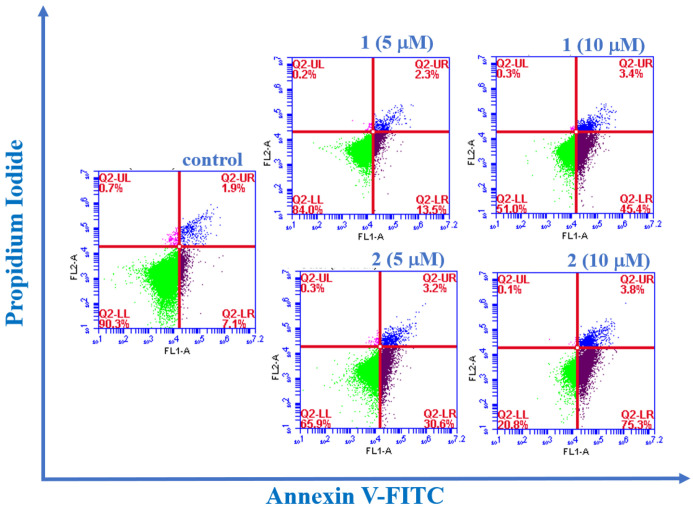
From the DAPI staining assay, we got the indication that the apoptosis mode of cell death has occurred in cancer cells (HT-29 and HeLa) after the treatment with 1–4. Therefore, to further confirm this and to determine the amount of apoptosis, annexin V FITC and PI double staining assay were performed in the HT-29 cell line on complexes 1 and 2 as representative examples. From Figure 15, it emerges that the sum of Q2(LR) and Q2(UR) regions is increased in both complexes as compared to the control; these two regions stand for early and late apoptosis cell percentages, respectively. The results suggest that the apoptotic amount is increased after the treatment with 1–4 and increases with the complex concentration from 5 to 10 μM. In particular, with 5 μM concentration, after the treatment with 1 and 2, the apoptotic cells are 15.8 and 33.8%, and with 10 μM, they are 48.8 and 79.1%, while only 9% was noticed in the control. Hence, the overall result indicates that concentration-dependent apoptotic cell death occurs in HT-29 cells upon being treated with 1 and 2.
Figure 15.
Quadrant graphs of cell apoptosis analysis in HT-29 cells treated with 5 and 10 μM concentrations of 1 and 2 for 48 h incubation time. Control indicates the untreated group.
Conclusions
In its most stable higher oxidation states, +IV and +V, vanadium forms many types of compounds containing V=O bonds, e.g., VIVO2+-, VVO3+-, and VVO2+-based complexes. In contrast, only very few non-oxido VIV or VV complexes have been isolated and structurally characterized. So, for these non-oxido complexes to be stable in the solid state or in water-containing solvents, preventing hydrolysis to form V-oxido species, the ligands must bind the V center quite strongly.
In this work, we report the preparation and characterization in the solid state and in solution of a group of new mononuclear non-oxido VIV complexes, [VIV(L1–4)2] (1–4), the ligands being tridentate ONS chelating S-alkyl/aryl-substituted dithiocarbazate compounds. Three of these complexes, [VIV(L1–3)2] (1–3), were analyzed by single-crystal X-ray diffraction studies, revealing that the coordination geometry around the V center is distorted octahedral (1 and 2) or trigonal prismatic (3). Additionally, considering the formed O2N2S2 coordination environment around the VIV center, 1 and 2 correspond to the mer isomers, while in 3, a fac arrangement is formed. Interestingly, EPR data and DFT calculations indicate the coexistence of mer and fac isomers in solution, with comparable stability; probably, subtle factors like the solubility and/or the crystal packing may determine the isolation of one of the two isomeric forms in the solid state. To the best of our knowledge, this is one of the first studies in which the mer and fac isomers of non-oxido VIV species are in equilibrium in solution, and this possibility should be considered in the future for the characterization of similar compounds.
It is worth noting that EPR, UV–vis, and ESI-MS data allowed to confirm that the obtained [VIV(L1–4)2] complexes are quite stable in solution for several hours, but suggested their partial oxidation to [VV(L1–4)2]+ and [VVO2(L1–4)]−, these changes proceeding faster as the relative amount of water and time increase.
Since we focus on the use vanadium compounds as pharmaceuticals, namely, for cancer treatment, the understanding of the interaction of complexes [VIV(L1–4)2] with proteins is relevant; this was studied with BSA by fluorescence quenching and computational methods. Docking calculations revealed non-covalent interactions with different regions of BSA, particularly with Tyr, Lys, Arg, Asn, and Thr residues. On the one hand, they allowed to confirm the fluorescence quenching results, since the adducts formed are found close to Trp213 residue; on the other hand, they highlighted that [VIV(L1–4)2], [VV(L1–4)2]+, and [VVO2(L1–4)]− interact with different strengths and different subdomains of the protein, depending on the vanadium species and on the ligand coordinated.
Concerning their possible use as anti-cancer agents, in vitro cytotoxicity of all the complexes (and free ligands for comparison) was assayed against HT-29 and HeLa cancer cell lines and compared with NIH-3T3 normal cells. The results suggested that all four complexes are cytotoxic and induce cell death by apoptosis. However, although the compounds were normally more active against the cancer cells than in the normal cells, the selectivity is not high. Concerning the identity of the active species triggering apoptosis, we highlight that, despite the considerable stability of the complexes [VIV(L1–4)2], oxidation/hydrolysis certainly takes place to some extent during the experiments in water. Therefore, a mixture of VIV, VV, and VVO2 species, being present in these systems, could be responsible for the activity observed. The small amounts of free ligands formed may also contribute. These results reinforce our recent conclusions that, regardless of the oxidation state of one of these administered vanadium drugs, a mixture of oxido and non-oxido VIV and VV species could be formed at the physiological conditions, their relative amount depending on the nature of the ligand and thermodynamic stability of the initial compound; therefore, the biological action should be interpreted and explained assuming the existence of more than one V species in solution, probably in the +IV and +V oxidation states.
Acknowledgments
R.D. thanks CSIR, Govt. of India [grant no. 01(3073)/21/EMR-II], for funding this research. J.C.P., I.C., and F.C. thank Centro de Química Estrutural which is financed by Portuguese funds from Fundação para a Ciência e a Tecnologia (FCT), projects UIDB/00100/2020, UIDP/00100/2020, LA/P/2020, and Programa Operacional Regional de Lisboa 2020. E.G., G.S., and F.P. thank Fondazione di Sardegna (project FdS2017Garribba).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.3c00753.
Tables with crystal data and refinement details for the X-ray structure determinations, with calculated structural details for [VIV(L1–4)2], [VVO2(L1–4)]−, and [VV(L1–4)2]+ complexes, docking results for the interaction of [VIV(L1–4)2] and [VVO2(L1–4)]− with BSA, figures with crystallographic details, UV–vis spectra of complexes 1–4, experimental and calculated ESI-MS spectra of 1, cyclic voltammogram of H2L4, double logarithm plot of log[(I0–I)/I] vs log[Q] for 1–4, cytotoxicity profiles of 1–4 for NIH-3T3 cell lines, and correlations between IC50 and gap between the oxidation and reduction potentials for 1–4 (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Rehder D.Bioinorganic Vanadium Chemistry; John Wiley & Sons, 2008. [Google Scholar]
- Eady R. R. Current status of structure function relationships of vanadium nitrogenase. Coord. Chem. Rev. 2003, 237, 23–30. 10.1016/s0010-8545(02)00248-5. [DOI] [Google Scholar]
- Mjos K. D.; Orvig C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. 10.1021/cr400460s. [DOI] [PubMed] [Google Scholar]
- Pessoa J. C.; Santos M. F. A.; Correia I.; Sanna D.; Sciortino G.; Garribba E. Binding of vanadium ions and complexes to proteins and enzymes in aqueous solution. Coord. Chem. Rev. 2021, 449, 214192. 10.1016/j.ccr.2021.214192. [DOI] [Google Scholar]
- Crans D. C.; Smee J. J.; Gaidamauskas E.; Yang L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 2004, 35, 849–902. 10.1002/chin.200420288. [DOI] [PubMed] [Google Scholar]
- Crans D. C. Antidiabetic, Chemical, and Physical Properties of Organic Vanadates as Presumed Transition-State Inhibitors for Phosphatases. J. Org. Chem. 2015, 80, 11899–11915. 10.1021/acs.joc.5b02229. [DOI] [PubMed] [Google Scholar]
- McLauchlan C. C.; Peters B. J.; Willsky G. R.; Crans D. C. Vanadium–phosphatase complexes: Phosphatase inhibitors favor the trigonal bipyramidal transition state geometries. Coord. Chem. Rev. 2015, 301–302, 163–199. 10.1016/j.ccr.2014.12.012. [DOI] [Google Scholar]
- Sánchez-Lombardo I.; Alvarez S.; McLauchlan C. C.; Crans D. C. Evaluating transition state structures of vanadium–phosphatase protein complexes using shape analysis. J. Inorg. Biochem. 2015, 147, 153–164. 10.1016/j.jinorgbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Pessoa J. C.; Etcheverry S.; Gambino D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. 10.1016/j.ccr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehder D. The potentiality of vanadium in medicinal applications. Future Med. Chem. 2012, 4, 1823–1837. 10.4155/fmc.12.103. [DOI] [PubMed] [Google Scholar]
- Crans D. C.; Yang L.; Haase A.; Yang X. Health benefits of vanadium and its potential as an anticancer agent. Met. Ions Life Sci. 2018, 18, 251–279. [DOI] [PubMed] [Google Scholar]
- Treviño S.; Díaz A.; Sánchez-Lara E.; Sanchez-Gaytan B. L.; Perez-Aguilar J. M.; González-Vergara E. Vanadium in biological action: chemical, pharmacological aspects, and metabolic implications in diabetes mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. 10.1007/s12011-018-1540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crans D. C.; Henry L.; Cardiff G.; Posner B. I. Developing vanadium as an antidiabetic or anticancer drug: A clinical and historical perspective. Met. Ions Life Sci. 2019, 19, 203–230. [DOI] [PubMed] [Google Scholar]
- Aureliano M.; Gumerova N. I.; Sciortino G.; Garribba E.; Rompel A.; Crans D. C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. 10.1016/j.ccr.2021.214143. [DOI] [Google Scholar]
- Amante C.; de Sousa-Coelho A. L.; Aureliano M. Vanadium and melanoma: A systematic review. Metals 2021, 11, 828. 10.3390/met11050828. [DOI] [Google Scholar]
- Yoshikawa Y.; Sakurai H.; Crans D. C.; Micera G.; Garribba E. Structural and redox requirements for the action of anti-diabetic vanadium compounds. Dalton Trans. 2014, 43, 6965–6972. 10.1039/c3dt52895b. [DOI] [PubMed] [Google Scholar]
- Levina A.; McLeod A. I.; Kremer L. E.; Aitken J. B.; Glover C. J.; Johannessen B.; Lay P. A. Reactivity–activity relationships of oral anti-diabetic vanadium complexes in gastrointestinal media: An X-ray absorption spectroscopic study. Metallomics 2014, 6, 1880–1888. 10.1039/c4mt00146j. [DOI] [PubMed] [Google Scholar]
- Sanna D.; Bíró L.; Buglyó P.; Micera G.; Garribba E. Transport of the anti-diabetic VO2+ complexes formed by pyrone derivatives in the blood serum. J. Inorg. Biochem. 2012, 115, 87–99. 10.1016/j.jinorgbio.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Sanna D.; Fabbri D.; Serra M.; Buglyó P.; Bíró L.; Ugone V.; Micera G.; Garribba E. Characterization and biotransformation in the plasma and red blood cells of VIVO2+ complexes formed by ceftriaxone. J. Inorg. Biochem. 2015, 147, 71–84. 10.1016/j.jinorgbio.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Koleša-Dobravc T.; Lodyga-Chruscinska E.; Symonowicz M.; Sanna D.; Meden A.; Perdih F.; Garribba E. Synthesis and characterization of VIVO complexes of picolinate and pyrazine derivatives. Behavior in the solid state and aqueous solution and biotransformation in the presence of blood plasma proteins. Inorg. Chem. 2014, 53, 7960–7976. 10.1021/ic500766t. [DOI] [PubMed] [Google Scholar]
- Willsky G. R.; Goldfine A. B.; Kostyniak P. J.; McNeill J. H.; Yang L. Q.; Khan H. R.; Crans D. C. Effect of vanadium(IV) compounds in the treatment of diabetes: in vivo and in vitro studies with vanadyl sulfate and bis(maltolato)oxovandium(IV). J. Inorg. Biochem. 2001, 85, 33–42. 10.1016/s0162-0134(00)00226-9. [DOI] [PubMed] [Google Scholar]
- Liboiron B. D.; Thompson K. H.; Hanson G. R.; Lam E.; Aebischer N.; Orvig C. New insights into the interactions of serum proteins with bis(maltolato)oxovanadium(IV): transport and biotransformation of insulin-enhancing vanadium pharmaceuticals. J. Am. Chem. Soc. 2005, 127, 5104–5115. 10.1021/ja043944n. [DOI] [PubMed] [Google Scholar]
- Kiss T.; Jakusch T.; Hollender D.; Dörnyei Á.; Enyedy É. A.; Pessoa J. C.; Sakurai H.; Sanz-Medel A. Biospeciation of antidiabetic VO(IV) complexes. Coord. Chem. Rev. 2008, 252, 1153–1162. 10.1016/j.ccr.2007.09.011. [DOI] [Google Scholar]
- Mehtab S.; Gonçalves G.; Roy S.; Tomaz A. I.; Santos-Silva T.; Santos M. F. A.; Romão M. J.; Jakusch T.; Kiss T.; Pessoa J. C. Interaction of vanadium(IV) with human serum apo-transferrin. J. Inorg. Biochem. 2013, 121, 187–195. 10.1016/j.jinorgbio.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Levina A.; Crans D. C.; Lay P. A. Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities. Coord. Chem. Rev. 2017, 352, 473–498. 10.1016/j.ccr.2017.01.002. [DOI] [Google Scholar]
- Sanna D.; Ugone V.; Micera G.; Buglyó P.; Bíró L.; Garribba E. Speciation in human blood of Metvan, a vanadium based potential anti-tumor drug. Dalton Trans. 2017, 46, 8950–8967. 10.1039/c7dt00943g. [DOI] [PubMed] [Google Scholar]
- Pessoa J. C.; Correia I. Misinterpretations in evaluating interactions of vanadium complexes with proteins and other biological targets. Inorganics 2021, 9, 17. 10.3390/inorganics9020017. [DOI] [Google Scholar]
- Scior T.; Abdallah H. H.; Mustafa S. F. Z.; Guevara-García J. A.; Rehder D. Are vanadium complexes druggable against the main protease Mpro of SARS-CoV-2?–A computational approach. Inorg. Chim. Acta 2021, 519, 120287. 10.1016/j.ica.2021.120287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggers E. Targeting proteins with metal complexes. Chem. Commun. 2009, 1001–1010. 10.1039/b813568a. [DOI] [PubMed] [Google Scholar]
- Sciortino G.; Maréchal J.-D.; Garribba E. Integrated experimental/computational approaches to characterize the systems formed by vanadium with proteins and enzymes. Inorg. Chem. Front. 2021, 8, 1951–1974. 10.1039/d0qi01507e. [DOI] [Google Scholar]
- Aureliano M.; Gumerova N. I.; Sciortino G.; Garribba E.; McLauchlan C. C.; Rompel A.; Crans D. C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2022, 454, 214344. 10.1016/j.ccr.2021.214344. [DOI] [Google Scholar]
- Santos M. F. A.; Sciortino G.; Correia I.; Fernandes A. C. P.; Santos-Silva T.; Pisanu F.; Garribba E.; Costa Pessoa J. Binding of VIVO2+, VIVOL, VIVOL2 and VVO2L Moieties to Proteins: X-ray/Theoretical Characterization and Biological Implications. Chem. - Eur. J. 2022, 28, e202200105 10.1002/chem.202200105. [DOI] [PubMed] [Google Scholar]
- Thompson K. H.; Orvig C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006, 100, 1925–1935. 10.1016/j.jinorgbio.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Costa Pessoa J.; Garribba E.; Santos M. F. A.; Santos-Silva T. Vanadium and proteins: uptake, transport, structure, activity and function. Coord. Chem. Rev. 2015, 301–302, 49–86. 10.1016/j.ccr.2015.03.016. [DOI] [Google Scholar]
- Hoogenboezem E. N.; Duvall C. L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. 10.1016/j.addr.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot A. M.; Kalinowski D. S.; Des Richardson R. Unraveling the mysteries of serum albumin–more than just a serum protein. Front. Physiol. 2014, 5, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla R. K.; Chen C.-L.; Dong Y.; Uckun F. M. In vivo antitumor activity of bis(4,7-dimethyl-1,10-phenanthroline)sulfatooxovanadium(IV) {METVAN [VO(SO4)(Me2-Phen)2]. Clin. Cancer Res. 2001, 7, 2124–2133. [PubMed] [Google Scholar]
- Gomathi Sankareswari V.; Vinod D.; Mahalakshmi A.; Alamelu M.; Kumaresan G.; Ramaraj R.; Rajagopal S. Interaction of oxovanadium(IV)–salphen complexes with bovine serum albumin and their cytotoxicity against cancer. Dalton Trans. 2014, 43, 3260–3272. 10.1039/c3dt52505h. [DOI] [PubMed] [Google Scholar]
- Sanna D.; Ugone V.; Lubinu G.; Micera G.; Garribba E. Behavior of the potential antitumor VIVO complexes formed by flavonoid ligands. 1. Coordination modes and geometry in solution and at the physiological pH. J. Inorg. Biochem. 2014, 140, 173–184. 10.1016/j.jinorgbio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Ni L.; Zhao H.; Tao L.; Li X.; Zhou Z.; Sun Y.; Chen C.; Wei D.; Liu Y.; Diao G. Synthesis, in vitro cytotoxicity, and structure–activity relationships (SAR) of multidentate oxidovanadium(IV) complexes as anticancer agents. Dalton Trans. 2018, 47, 10035–10045. 10.1039/c8dt01778f. [DOI] [PubMed] [Google Scholar]
- Ribeiro N.; Bulut I.; Cevatemre B.; Teixeira C.; Yildizhan Y.; André V.; Adão P.; Pessoa J. C.; Acilan C.; Correia I. Cu(II) and V(IV)O complexes with tri- or tetradentate ligands based on (2-hydroxybenzyl)-l-alanines reveal promising anticancer therapeutic potential. Dalton Trans. 2021, 50, 157–169. 10.1039/d0dt03331f. [DOI] [PubMed] [Google Scholar]
- Ugone V.; Sanna D.; Sciortino G.; Crans D. C.; Garribba E. ESI-MS Study of the Interaction of Potential Oxidovanadium(IV) Drugs and Amavadin with Model Proteins. Inorg. Chem. 2020, 59, 9739–9755. 10.1021/acs.inorgchem.0c00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankhoff K.; Ahmad A.; Weber B.; Biersack B.; Schobert R. Anticancer properties of a new non-oxido vanadium(IV) complex with a catechol-modified 3,3′-diindolylmethane ligand. J. Inorg. Biochem. 2019, 194, 1–6. 10.1016/j.jinorgbio.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Gambino D. Potentiality of vanadium compounds as anti-parasitic agents. Coord. Chem. Rev. 2011, 255, 2193–2203. 10.1016/j.ccr.2010.12.028. [DOI] [Google Scholar]
- Kioseoglou E.; Petanidis S.; Gabriel C.; Salifoglou A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301–302, 87–105. 10.1016/j.ccr.2015.03.010. [DOI] [Google Scholar]
- Papaioannou A.; Manos M.; Karkabounas S.; Liasko R.; Evangelou A. M.; Correia I.; Kalfakakou V.; Pessoa J. C.; Kabanos T. Solid state and solution studies of a vanadium(III)-L-cysteine compound and demonstration of its antimetastatic, antioxidant and inhibition of neutral endopeptidase activities. J. Inorg. Biochem. 2004, 98, 959–968. 10.1016/j.jinorgbio.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Sakurai H.; Tamura H.; Okatani K. Mechanism for a new antitumor vanadium complex: hydroxyl radical-dependent DNA cleavage by 1,10-phenanthroline vanadyl complex in the presence of hydrogen peroxide. Biochem. Biophys. Res. Commun. 1995, 206, 133–137. 10.1006/bbrc.1995.1019. [DOI] [PubMed] [Google Scholar]
- Dong Y.; Narla R. K.; Sudbeck E.; Uckun F. M. Synthesis, X-ray structure, and anti-leukemic activity of oxovanadium(IV) complexes. J. Inorg. Biochem. 2000, 78, 321–330. 10.1016/s0162-0134(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Le M.; Rathje O.; Levina A.; Lay P. A. High cytotoxicity of vanadium(IV) complexes with 1,10-phenanthroline and related ligands is due to decomposition in cell culture medium. J. Inorg. Biochem. 2017, 22, 663–672. 10.1007/s00775-017-1453-4. [DOI] [PubMed] [Google Scholar]
- Matos C. P.; Addis Y.; Nunes P.; Barroso S.; Alho I.; Martins M.; Matos A. P. A.; Marques F.; Cavaco I.; Costa Pessoa J.; et al. Exploring the cytotoxic activity of new phenanthroline salicylaldimine Zn(II) complexes. J. Inorg. Biochem. 2019, 198, 110727. 10.1016/j.jinorgbio.2019.110727. [DOI] [PubMed] [Google Scholar]
- Nunes P.; Correia I.; Cavaco I.; Marques F.; Pinheiro T.; Avecilla F.; Pessoa J. C. Therapeutic potential of vanadium complexes with 1,10-phenanthroline ligands, quo vadis? Fate of complexes in cell media and cancer cells. J. Inorg. Biochem. 2021, 217, 111350. 10.1016/j.jinorgbio.2020.111350. [DOI] [PubMed] [Google Scholar]
- Nunes P.; Correia I.; Marques F.; Matos A. P.; Dos Santos M. M. C.; Azevedo C. G.; Capelo J.-L.; Santos H. M.; Gama S.; Pinheiro T.; et al. Copper complexes with 1, 10-phenanthroline derivatives: underlying factors affecting their cytotoxicity. Inorg. Chem. 2020, 59, 9116–9134. 10.1021/acs.inorgchem.0c00925. [DOI] [PubMed] [Google Scholar]
- Kundu S.; Mondal D.; Bhattacharya K.; Endo A.; Sanna D.; Garribba E.; Chaudhury M. Nonoxido Vanadium(IV) Compounds Involving Dithiocarbazate-Based Tridentate ONS Ligands: Synthesis, Electronic and Molecular Structure, Spectroscopic and Redox Properties. Inorg. Chem. 2015, 54, 6203–6215. 10.1021/acs.inorgchem.5b00359. [DOI] [PubMed] [Google Scholar]
- Kang B.; Weng L.; Liu H.; Wu D.; Huang L.; Lu C.; Cai J.; Chen X.; Lu J. Syntheses, structures, and properties of vanadium, cobalt, and nickel compounds with 2-mercaptophenol. Inorg. Chem. 1990, 29, 4873–4877. 10.1021/ic00349a012. [DOI] [Google Scholar]
- Paine T. K.; Weyhermüller T.; Slep L. D.; Neese F.; Bill E.; Bothe E.; Wieghardt K.; Chaudhuri P. Nonoxovanadium(IV) and Oxovanadium(V) Complexes with Mixed O, X, O-Donor Ligands (X = S, Se, P, or PO). Inorg. Chem. 2004, 43, 7324–7338. 10.1021/ic040052f. [DOI] [PubMed] [Google Scholar]
- Stiefel E. I.; Dori Z.; Gray H. B. Octahedral vs. trigonal-prismatic coordination. Structure of (Me4N)2[V(mnt)3]. J. Am. Chem. Soc. 1967, 89, 3353–3354. 10.1021/ja00989a048. [DOI] [Google Scholar]
- Kondo M.; Minakoshi S.; Iwata K.; Shimizu T.; Matsuzaka H.; Kamigata N.; Kitagawa S. Crystal Structure of a Tris(dithiolene) Vanadium(IV) Complex Having Unprecedented D3h Symmetry. Chem. Lett. 1996, 25, 489–490. 10.1246/cl.1996.489. [DOI] [Google Scholar]
- Yan J.-A.; Chen Y.-S.; Chang Y.-H.; Tsai C.-Y.; Lyu C.-L.; Luo C.-G.; Lee G.-H.; Hsu H.-F. Redox Interconversion of Non-Oxido Vanadium Complexes Accompanied by Acid–Base Chemistry of Thiol and Thiolate. Inorg. Chem. 2017, 56, 9055–9063. 10.1021/acs.inorgchem.7b01040. [DOI] [PubMed] [Google Scholar]
- Zadrozny J. M.; Niklas J.; Poluektov O. G.; Freedman D. E. Multiple Quantum Coherences from Hyperfine Transitions in a Vanadium(IV) Complex. J. Am. Chem. Soc. 2014, 136, 15841–15844. 10.1021/ja507846k. [DOI] [PubMed] [Google Scholar]
- Zadrozny J. M.; Niklas J.; Poluektov O. G.; Freedman D. E. Millisecond Coherence Time in a Tunable Molecular Electronic Spin Qubit. ACS Cent. Sci. 2015, 1, 488–492. 10.1021/acscentsci.5b00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneifel H.; Bayer E. Stereochemistry and total synthesis of amavadin, the naturally occurring vanadium compound of Amanita muscaria. J. Am. Chem. Soc. 1986, 108, 3075–3077. 10.1021/ja00271a043. [DOI] [Google Scholar]
- Berry R. E.; Armstrong E. M.; Beddoes R. L.; Collison D.; Ertok S. N.; Helliwell M.; Garner C. D. The structural characterization of amavadin. Angew. Chem., Int. Ed. 1999, 38, 795–797. . [DOI] [PubMed] [Google Scholar]
- de C T Carrondo M. A. A. F.; Duarte M. T. L. S.; Pessoa J. C.; Silva J. A. L.; da Silva J. J. R. F.; Vaz M. C. T.; Vilas-Boas L. F. Bis-(N-hydroxy-iminodiacetate)vanadate(IV), a synthetic model of 'amavadin'. J. Chem. Soc. Chem. Commun. 1988, 1158–1159. 10.1039/c39880001158. [DOI] [Google Scholar]
- Dash S. P.; Pasayat S.; Bhakat S.; Roy S.; Dinda R.; Tiekink E. R. T.; Mukhopadhyay S.; Bhutia S. K.; Hardikar M. R.; Joshi B. N.; et al. Highly stable hexacoordinated nonoxidovanadium(IV) complexes of sterically constrained ligands: syntheses, structure, and study of antiproliferative and insulin mimetic activity. Inorg. Chem. 2013, 52, 14096–14107. 10.1021/ic401866x. [DOI] [PubMed] [Google Scholar]
- Banerjee A.; Dash S. P.; Mohanty M.; Sahu G.; Sciortino G.; Garribba E.; Carvalho M. F. N. N.; Marques F.; Costa Pessoa J.; Kaminsky W.; Brzezinski K.; Dinda R. New VIV, VIVO, VVO, and VVO2 Systems: Exploring their Interconversion in Solution, Protein Interactions, and Cytotoxicity. Inorg. Chem. 2020, 59, 14042–14057. 10.1021/acs.inorgchem.0c01837. [DOI] [PubMed] [Google Scholar]
- Griffin E.; Levina A.; Lay P. A. Vanadium(V) tris-3,5-di-tert-butylcatecholato complex: Links between speciation and anti-proliferative activity in human pancreatic cancer cells. J. Inorg. Biochem. 2019, 201, 110815. 10.1016/j.jinorgbio.2019.110815. [DOI] [PubMed] [Google Scholar]
- Biswas N.; Bera S.; Sepay N.; Pal A.; Halder T.; Ray S.; Acharyya S.; Biswas A. K.; Drew M. G. B.; Ghosh T. Simultaneous formation of non-oxidovanadium(IV) and oxidovanadium(V) complexes incorporating phenol-based hydrazone ligands in aerobic conditions. New J. Chem. 2020, 44, 3700–3716. 10.1039/c9nj06114b. [DOI] [Google Scholar]
- Li M. X.; Zhang L. Z.; Chen C. L.; Niu J. Y.; Ji B. S. Synthesis, crystal structures, and biological evaluation of Cu(II) and Zn(II) complexes of 2-benzoylpyridine Schiff bases derived from S-methyl-and S-phenyldithiocarbazates. J. Inorg. Biochem. 2012, 106, 117–125. 10.1016/j.jinorgbio.2011.09.034. [DOI] [PubMed] [Google Scholar]
- Rettondin A. R.; Carneiro Z. A.; Gonçalves A. C. R.; Ferreira V. F.; Oliveira C. G.; Lima A. N.; Oliveira R. J.; de Albuquerque S.; Deflon V. M.; Maia P. I. S. Gold(III) complexes with ONS-Tridentate thiosemicarbazones: Toward selective trypanocidal drugs. Eur. J. Med. Chem. 2016, 120, 217–226. 10.1016/j.ejmech.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Kalaivani P.; Prabhakaran R.; Dallemer F.; Poornima P.; Vaishnavi E.; Ramachandran E.; Padma V. V.; Renganathan R.; Natarajan K. DNA, protein binding, cytotoxicity, cellular uptake and antibacterial activities of new palladium(II) complexes of thiosemicarbazone ligands: effects of substitution on biological activity. Metallomics 2012, 4, 101–113. 10.1039/c1mt00144b. [DOI] [PubMed] [Google Scholar]
- Low M. L.; Maigre L.; Tahir M. I. M.; Tiekink E. R. T.; Dorlet P.; Guillot R.; Ravoof T. B.; Rosli R.; Pagès J.-M.; Policar C.; et al. New insight into the structural, electrochemical and biological aspects of macroacyclic Cu(II) complexes derived from S-substituted dithiocarbazate Schiff bases. Eur. J. Med. Chem. 2016, 120, 1–12. 10.1016/j.ejmech.2016.04.027. [DOI] [PubMed] [Google Scholar]
- Ramilo-Gomes F.; Addis Y.; Tekamo I.; Cavaco I.; Campos D. L.; Pavan F. R.; Gomes C. S. B.; Brito V.; Santos A. O.; Domingues F.; et al. Antimicrobial and antitumor activity of S-methyl dithiocarbazate Schiff base zinc(II) complexes. J. Inorg. Biochem. 2021, 216, 111331. 10.1016/j.jinorgbio.2020.111331. [DOI] [PubMed] [Google Scholar]
- Malik M. A.; Lone S. A.; Wani M. Y.; Talukdar M. I. A.; Dar O. A.; Ahmad A.; Hashmi A. A. S-benzyldithiocarbazate imine coordinated metal complexes kill Candida albicans by causing cellular apoptosis and necrosis. Bioorg. Chem. 2020, 98, 103771. 10.1016/j.bioorg.2020.103771. [DOI] [PubMed] [Google Scholar]
- Low M. L.; Maigre L.; Dorlet P.; Guillot R.; Pagès J.-M.; Crouse K. A.; Policar C.; Delsuc N. Conjugation of a new series of dithiocarbazate Schiff base copper(II) complexes with vectors selected to enhance antibacterial activity. Bioconjug. Chem. 2014, 25, 2269–2284. 10.1021/bc5004907. [DOI] [PubMed] [Google Scholar]
- Low M. L.; Paulus G.; Dorlet P.; Guillot R.; Rosli R.; Delsuc N.; Crouse K. A.; Policar C. Synthesis, characterization and biological activity of Cu(II), Zn(II) and Re(I) complexes derived from S-benzyldithiocarbazate and 3-acetylcoumarin. BioMetals 2015, 28, 553–566. 10.1007/s10534-015-9831-2. [DOI] [PubMed] [Google Scholar]
- Basha M. T.; Chartres J. D.; Pantarat N.; Akbar Ali M.; Mirza A. H.; Kalinowski D. S.; Richardson D. R.; Bernhardt P. V. Heterocyclic dithiocarbazate iron chelators: Fe coordination chemistry and biological activity. Dalton Trans. 2012, 41, 6536–6548. 10.1039/c2dt12387h. [DOI] [PubMed] [Google Scholar]
- Easmon J.; Pürstinger G.; Heinisch G.; Roth T.; Fiebig H. H.; Holzer W.; Jäger W.; Jenny M.; Hofmann J. Synthesis, cytotoxicity, and antitumor activity of copper(II) and iron(II) complexes of 4N-azabicyclo[3.2.2]nonane thiosemicarbazones derived from acyl diazines. J. Med. Chem. 2001, 44, 2164–2171. 10.1021/jm000979z. [DOI] [PubMed] [Google Scholar]
- Greenbaum D. C.; Mackey Z.; Hansell E.; Doyle P.; Gut J.; Caffrey C. R.; Lehrman J.; Rosenthal P. J.; McKerrow J. H.; Chibale K. Synthesis and structure-activity relationships of parasiticidal thiosemicarbazone cysteine protease inhibitors against Plasmodium falciparum, Trypanosoma brucei, and Trypanosoma cruzi. J. Med. Chem. 2004, 47, 3212–3219. 10.1021/jm030549j. [DOI] [PubMed] [Google Scholar]
- Sahu G.; Patra S. A.; Lima S.; Das S.; Görls H.; Plass W.; Dinda R. Ruthenium(II)-dithiocarbazates as Anticancer Agents: Synthesis, Solution Behavior, and Mitochondria-targeted Apoptotic Cell Death. Chem. - Eur. J. 2023, 29, e202202694 10.1002/chem.202202694. [DOI] [PubMed] [Google Scholar]
- Gou Y.; Jia X.; Hou L. X.; Deng J. G.; Huang G. J.; Jiang H. W.; Yang F. Dithiocarbazate–FeIII, −CoIII, −NiII, and −ZnII Complexes: Design, Synthesis, Structure, and Anticancer Evaluation. J. Med. Chem. 2022, 65, 6677–6689. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Wong J.; Lovejoy D. B.; Kalinowski D. S.; Richardson D. R. Chelators at the cancer coalface: desferrioxamine to Triapine and beyond. Clin. Cancer Res. 2006, 12, 6876–6883. 10.1158/1078-0432.ccr-06-1954. [DOI] [PubMed] [Google Scholar]
- Traynor A. M.; Lee J.-W.; Bayer G. K.; Tate J. M.; Thomas S. P.; Mazurczak M.; Graham D. L.; Kolesar J. M.; Schiller J. H. A phase II trial of Triapine(NSC# 663249) and gemcitabine as second line treatment of advanced non-small cell lung cancer: Eastern Cooperative Oncology Group Study 1503. Invest. New Drugs 2010, 28, 91–97. 10.1007/s10637-009-9230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson P. J.; Kalinowski D. S.; Lane D. J. R.; Kovacevic Z.; Seebacher N. A.; Fouani L.; Sahni S.; Merlot A. M.; Richardson D. R. The renaissance of polypharmacology in the development of anti-cancer therapeutics: Inhibition of the “Triad of Death” in cancer by di-2-pyridylketone thiosemicarbazones. Pharmacol. Res. 2015, 100, 255–260. 10.1016/j.phrs.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Stacy A. E.; Palanimuthu D.; Bernhardt P. V.; Kalinowski D. S.; Jansson P. J.; Richardson D. R. Zinc(II)–thiosemicarbazone complexes are localized to the lysosomal compartment where they transmetallate with copper ions to induce cytotoxicity. J. Med. Chem. 2016, 59, 4965–4984. 10.1021/acs.jmedchem.6b00238. [DOI] [PubMed] [Google Scholar]
- Maryam M.; Tan S. L.; Crouse K. A.; Mohamed Tahir M. I.; Chee H. Y. Synthesis, characterization and evaluation of antidengue activity of enantiomeric Schiff bases derived from S-substituted dithiocarbazate. Turk. J. Chem. 2020, 44, 1395–1409. 10.3906/kim-2006-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Y.; Chen M.; Li S.; Deng J.; Li J.; Fang G.; Yang F.; Huang G. Dithiocarbazate-Copper Complexes for Bioimaging and Treatment of Pancreatic Cancer. J. Med. Chem. 2021, 64, 5485–5499. 10.1021/acs.jmedchem.0c01936. [DOI] [PubMed] [Google Scholar]
- Banerjee A.; Mohanty M.; Lima S.; Samanta R.; Garribba E.; Sasamori T.; Dinda R. Synthesis, structure and characterization of new dithiocarbazate-based mixed ligand oxidovanadium(IV) complexes: DNA/HSA interaction, cytotoxic activity and DFT studies. New J. Chem. 2020, 44, 10946–10963. 10.1039/d0nj01246g. [DOI] [Google Scholar]
- Dash S. P.; Panda A. K.; Dhaka S.; Pasayat S.; Biswas A.; Maurya M. R.; Majhi P. K.; Crochet A.; Dinda R. A study of DNA/BSA interaction and catalytic potential of oxidovanadium(V) complexes with ONO donor ligands. Dalton Trans. 2016, 45, 18292–18307. 10.1039/c6dt03228a. [DOI] [PubMed] [Google Scholar]
- Dash S. P.; Panda A. K.; Pasayat S.; Majumder S.; Biswas A.; Kaminsky W.; Mukhopadhyay S.; Bhutia S. K.; Dinda R. Evaluation of the cell cytotoxicity and DNA/BSA binding and cleavage activity of some dioxidovanadium(V) complexes containing aroylhydrazones. J. Inorg. Biochem. 2015, 144, 1–12. 10.1016/j.jinorgbio.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Lima S.; Banerjee A.; Mohanty M.; Sahu G.; Kausar C.; Patra S. K.; Garribba E.; Kaminsky W.; Dinda R. Synthesis, structure and biological evaluation of mixed ligand oxidovanadium(IV) complexes incorporating 2-(arylazo) phenolates. New J. Chem. 2019, 43, 17711–17725. 10.1039/c9nj01910c. [DOI] [Google Scholar]
- Mohanty M.; Maurya S. K.; Banerjee A.; Patra S. A.; Maurya M. R.; Crochet A.; Brzezinski K.; Dinda R. In vitro cytotoxicity and catalytic evaluation of dioxidovanadium(V) complexes in an azohydrazone ligand environment. New J. Chem. 2019, 43, 17680–17695. 10.1039/c9nj01815h. [DOI] [Google Scholar]
- Patra S. A.; Mohanty M.; Banerjee A.; Kesarwani S.; Henkel F.; Reuter H.; Dinda R. Protein binding and cytotoxic activities of monomeric and dimeric oxido-vanadium(V) salan complexes: Exploring the solution behavior of monoalkoxido-bound oxido-vanadium(V) complex. J. Inorg. Biochem. 2021, 224, 111582. 10.1016/j.jinorgbio.2021.111582. [DOI] [PubMed] [Google Scholar]
- Roy S.; Böhme M.; Dash S. P.; Mohanty M.; Buchholz A.; Plass W.; Majumder S.; Kulanthaivel S.; Banerjee I.; Reuter H.; et al. Anionic Dinuclear Oxidovanadium(IV) Complexes with Azo Functionalized Tridentate Ligands and μ-Ethoxido Bridge Leading to an Unsymmetric Twisted Arrangement: Synthesis, X-ray Structure, Magnetic Properties, and Cytotoxicity. Inorg. Chem. 2018, 57, 5767–5781. 10.1021/acs.inorgchem.8b00035. [DOI] [PubMed] [Google Scholar]
- Sahu G.; Banerjee A.; Samanta R.; Mohanty M.; Lima S.; Tiekink E. R. T.; Dinda R. Water-Soluble Dioxidovanadium(V) Complexes of Aroylhydrazones: DNA/BSA Interactions, Hydrophobicity, and Cell-Selective Anticancer Potential. Inorg. Chem. 2021, 60, 15291–15309. 10.1021/acs.inorgchem.1c01899. [DOI] [PubMed] [Google Scholar]
- Sahu G.; Tiekink E. R. T.; Dinda R. Study of DNA Interaction and Cytotoxicity Activity of Oxidovanadium(V) Complexes with ONO Donor Schiff Base Ligands. Inorganics 2021, 9, 66. 10.3390/inorganics9090066. [DOI] [Google Scholar]
- Lima S.; Banerjee A.; Sahu G.; Patra S. A.; Sahu K.; Sasamori T.; Sciortino G.; Garribba E.; Dinda R. New mixed ligand oxidovanadium(IV) complexes: Solution behavior, protein interaction and cytotoxicity. J. Inorg. Biochem. 2022, 233, 111853. 10.1016/j.jinorgbio.2022.111853. [DOI] [PubMed] [Google Scholar]
- Patra S. A.; Banerjee A.; Sahu G.; Mohanty M.; Lima S.; Mohapatra D.; Görls H.; Plass W.; Dinda R. Evaluation of DNA/BSA interaction and in vitro cell cytotoxicity of μ2-oxido bridged divanadium(V) complexes containing ONO donor ligands. J. Inorg. Biochem. 2022, 233, 111852. 10.1016/j.jinorgbio.2022.111852. [DOI] [PubMed] [Google Scholar]
- Sahu G.; Patra S. A.; Mohanty M.; Lima S.; Pattanayak P. D.; Kaminsky W.; Dinda R. Dithiocarbazate based oxidomethoxidovanadium(V) and mixed-ligand oxidovanadium(IV) complexes: Study of solution behavior, DNA binding, and anticancer activity. J. Inorg. Biochem. 2022, 233, 111844. 10.1016/j.jinorgbio.2022.111844. [DOI] [PubMed] [Google Scholar]
- Bryant B. E.; Fernelius W. C.; Busch D. H.; Stoufer R. C.; Stratton W.. Vanadium(IV) Oxy(acetylacetonate). In Inorganic Syntheses; Moeller T., Ed.; Inorganic Syntheses; John Wiley & Sons, Inc, 1957; pp 113–116. [Google Scholar]
- Armarego W. L. F.; Chai C. L. L.. Purification of Laboratory Chemicals, 6th ed.; Elsevier; Butterworth Heinemann: Amsterdam, Boston, and Paris, 2009. [Google Scholar]
- Smith T. S.; LoBrutto R.; Pecoraro V. Paramagnetic spectroscopy of vanadyl complexes and its applications to biological systems. Coord. Chem. Rev. 2002, 228, 1–18. 10.1016/s0010-8545(01)00437-4. [DOI] [Google Scholar]
- Ali M. A.; Livingstone S. E.; Phillips D. J. Metal chelates of dithiocarbazic acid and its derivatives. VI. Antiferromagnetic and ferromagnetic interactions in some copper(II) complexes of salicylaldehyde and acetylacetone Schiff bases derived from s-methyldithiocarbazate. Inorg. Chim. Acta 1973, 7, 179–186. 10.1016/s0020-1693(00)94808-4. [DOI] [Google Scholar]
- Altomare A.; Burla M. C.; Camalli M.; Cascarano G. L.; Giacovazzo C.; Guagliardi A.; Moliterni A. G.; Polidori G.; Spagna R. SIR97: a new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. 10.1107/s0021889898007717. [DOI] [Google Scholar]
- Wilson A. J. C.; Geist V.. International Tables for Crystallography. Volume C: Mathematical, Physical and Chemical Tables. Kluwer Academic Publishers, Dordrecht/Boston/London,1992. [Google Scholar]
- Putz H.; Brandenburg K. DIAMOND-Crystal and molecular structure visualization. Crystal Impact-GbR, Kreuzherrenstr 2006, 102, 53227. [Google Scholar]
- Macrae C. F.; Bruno I. J.; Chisholm J. A.; Edgington P. R.; McCabe P.; Pidcock E.; Rodriguez-Monge L.; Taylor R.; van de Streek J.; Wood P. A. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. 10.1107/s0021889807067908. [DOI] [Google Scholar]
- Putnam F. W.The Plasma Proteins, 2nd ed.; Academic Press, New York; Vol. I, 1975. [Google Scholar]
- Coutinho A.; Prieto M. Ribonuclease T1 and alcohol dehydrogenase fluorescence quenching by acrylamide: A laboratory experiment for undergraduate students. J. Chem. Edu. 1993, 70, 425. 10.1021/ed070p425. [DOI] [Google Scholar]
- Valeur B.; Berberan-Santos M. N.. Molecular Fluorescence: Principles and Applications; John Wiley & Sons, 2012. [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Keith T.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, 2010;.
- Micera G.; Garribba E. The effect of the functional, basis set, and solvent in the simulation of the geometry and spectroscopic properties of VIVO2+ complexes. chemical and biological applications. Int. J. Quantum Chem. 2012, 112, 2486–2498. 10.1002/qua.23237. [DOI] [Google Scholar]
- Micera G.; Garribba E. Application of DFT Methods in the Study of VIVO2+–Peptide Interactions. Eur. J. Inorg. Chem. 2010, 2010, 4697–4710. 10.1002/ejic.201000382. [DOI] [Google Scholar]
- Neese F.ORCA – an Ab Initio, DFT and Semiempirical Program Package, version 5.0; Max-Planck-Institute for Chemical Energy Conversion, Mülheim a. d. Ruhr, 2021;.
- Sanna D.; Sciortino G.; Ugone V.; Micera G.; Garribba E. Nonoxido VIV complexes: prediction of the EPR spectrum and electronic structure of simple coordination compounds and Amavadin. Inorg. Chem. 2016, 55, 7373–7387. 10.1021/acs.inorgchem.6b00409. [DOI] [PubMed] [Google Scholar]
- Allouche A.-R. Gabedit–A graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. 10.1002/jcc.21600. [DOI] [PubMed] [Google Scholar]
- Gorelsky S. I.AOMix: Program for Molecular Orbital Analysis, version 6.52, 2011http://www.sg-chem.net/.
- Jones G.; Willett P.; Glen R. C.; Leach A. R.; Taylor R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- Sciortino G.; Sanna D.; Ugone V.; Micera G.; Lledós A.; Marechal J.-D.; Garribba E. Elucidation of binding site and chiral specificity of oxidovanadium drugs with lysozyme through theoretical calculations. Inorg. Chem. 2017, 56, 12938–12951. 10.1021/acs.inorgchem.7b01732. [DOI] [PubMed] [Google Scholar]
- Sciortino G.; Rodríguez-Guerra Pedregal J.; Lledós A.; Garribba E.; Maréchal J.-D. Prediction of the interaction of metallic moieties with proteins: An update for protein-ligand docking techniques. J. Comput. Chem. 2018, 39, 42–51. 10.1002/jcc.25080. [DOI] [PubMed] [Google Scholar]
- Sanna D.; Ugone V.; Sciortino G.; Buglyó P.; Bihari Z.; Parajdi-Losonczi P. L.; Garribba E. VIVO complexes with antibacterial quinolone ligands and their interaction with serum proteins. Dalton Trans. 2018, 47, 2164–2182. 10.1039/c7dt04216g. [DOI] [PubMed] [Google Scholar]
- Sciortino G.; Sanna D.; Ugone V.; Lledós A.; Maréchal J.-D.; Garribba E. Decoding surface interaction of VIVO metallodrug candidates with lysozyme. Inorg. Chem. 2018, 57, 4456–4469. 10.1021/acs.inorgchem.8b00134. [DOI] [PubMed] [Google Scholar]
- Sciortino G.; Garribba E.; Marechal J.-D. Validation and applications of protein–ligand docking approaches improved for metalloligands with multiple vacant sites. Inorg. Chem. 2018, 58, 294–306. 10.1021/acs.inorgchem.8b02374. [DOI] [PubMed] [Google Scholar]
- Bujacz A. Structures of bovine, equine and leporine serum albumin. Acta Cryst. Sect. D 2012, 68, 1278–1289. 10.1107/s0907444912027047. [DOI] [PubMed] [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Couch G. S.; Greenblatt D. M.; Meng E. C.; Ferrin T. E. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Chen J.; Peng F.; Zhang Y.; Li B.; She J.; Jie X.; Zou Z.; Chen M.; Chen L. Synthesis, characterization, cellular uptake and apoptosis-inducing properties of two highly cytotoxic cyclometalated ruthenium(II) β-carboline complexes. Eur. J. Med. Chem. 2017, 140, 104–117. 10.1016/j.ejmech.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Majumder S.; Pasayat S.; Panda A. K.; Dash S. P.; Roy S.; Biswas A.; Varma M. E.; Joshi B. N.; Garribba E.; Kausar C.; et al. Monomeric and Dimeric Oxidomolybdenum(V and VI) Complexes, Cytotoxicity, and DNA Interaction Studies: Molybdenum Assisted C=N Bond Cleavage of Salophen Ligands. Inorg. Chem. 2017, 56, 11190–11210. 10.1021/acs.inorgchem.7b01578. [DOI] [PubMed] [Google Scholar]
- Dash S. P.; Majumder S.; Banerjee A.; Carvalho M. F. N.; Adão P.; Pessoa J. C.; Brzezinski K.; Garribba E.; Reuter H.; Dinda R. Chemistry of monomeric and dinuclear non-oxido vanadium(IV) and oxidovanadium(V) aroylazine complexes: exploring solution behavior. Inorg. Chem. 2016, 55, 1165–1182. 10.1021/acs.inorgchem.5b02346. [DOI] [PubMed] [Google Scholar]
- Karpishin T. B.; Stack T. D.; Raymond K. N. Octahedral versus trigonal prismatic geometry in a series of catechol macrobicyclic ligand-metal complexes. J. Am. Chem. Soc. 1993, 115, 182–192. 10.1021/ja00054a025. [DOI] [Google Scholar]
- Sojo L. E.; Chahal N.; Keller B. O. Oxidation of catechols during positive ion electrospray mass spectrometric analysis: Evidence for in-source oxidative dimerization. Rapid Commun. Mass Spectrom. 2014, 28, 2181–2190. 10.1002/rcm.7011. [DOI] [PubMed] [Google Scholar]
- Smith P. D.; Berry R. E.; Harben S. M.; Beddoes R. L.; Helliwell M.; Collison D.; Garner C. D. New vanadium-(IV) and -(V) analogues of Amavadin. J. Chem. Soc., Dalton Trans. 1997, 4509–4516. 10.1039/a703683c. [DOI] [Google Scholar]
- Leggett C. J.; Parker B. F.; Teat S. J.; Zhang Z.; Dau P. D.; Lukens W. W.; Peterson S. M.; Cardenas A. J. P.; Warner M. G.; Gibson J. K.; et al. Structural and spectroscopic studies of a rare non-oxido V(V) complex crystallized from aqueous solution. Chem. Sci. 2016, 7, 2775–2786. 10.1039/c5sc03958d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik N. R.; Ghosh S.; Raychaudhuri T. K.; Chaudhuri S.; Drew M. G. B.; Mandal S. S. Chemical, electrochemical and structural studies of some cis-dioxomolybdenum(VI) complexes of two tridentate ONS chelating ligands. J. Coord. Chem. 2007, 60, 2177–2190. 10.1080/00958970701258192. [DOI] [Google Scholar]
- Dash S. P.; Roy S.; Mohanty M.; Carvalho M. F. N.; Kuznetsov M. L.; Pessoa J. C.; Kumar A.; Patil Y. P.; Crochet A.; Dinda R. Versatile reactivity and theoretical evaluation of mono- and dinuclear oxidovanadium(V) compounds of aroylazines: electrogeneration of mixed-valence divanadium(IV,V) complexes. Inorg. Chem. 2016, 55, 8407–8421. 10.1021/acs.inorgchem.6b01001. [DOI] [PubMed] [Google Scholar]
- Saswati S.; Adão P.; Majumder S.; Dash S. P.; Roy S.; Kuznetsov M. L.; Costa Pessoa J.; Gomes C. S. B.; Hardikar M. R.; Tiekink E. R. T.; et al. Synthesis, structure, solution behavior, reactivity and biological evaluation of oxidovanadium(IV/V) thiosemicarbazone complexes. Dalton Trans. 2018, 47, 11358–11374. 10.1039/c8dt01668b. [DOI] [PubMed] [Google Scholar]
- Dash S. P.; Panda A. K.; Pasayat S.; Dinda R.; Biswas A.; Tiekink E. R. T.; Mukhopadhyay S.; Bhutia S. K.; Kaminsky W.; Sinn E. Oxidovanadium(V) complexes of aroylhydrazones incorporating heterocycles: synthesis, characterization and study of DNA binding, photo-induced DNA cleavage and cytotoxic activities. RSC Adv. 2015, 5, 51852–51867. 10.1039/c4ra14369h. [DOI] [Google Scholar]
- Dash S. P.; Pasayat S.; Dash H. R.; Dash H. R.; Das S.; Butcher R. J.; Dinda R. Oxovanadium(V) complexes incorporating tridentate aroylhydrazoneoximes: synthesis, characterizations and antibacterial activity. Polyhedron 2012, 31, 524–529. 10.1016/j.poly.2011.10.017. [DOI] [Google Scholar]
- Dinda R.; Majhi P. K.; Sengupta P.; Pasayat S.; Ghosh S.; Falvello L. R.; Mak T. C. W. Alkali metal (Na+ and K+)-mediated supramolecular assembly of oxovanadium(V) complexes: Synthesis and structural characterization. Polyhedron 2010, 29, 248–253. 10.1016/j.poly.2009.08.008. [DOI] [Google Scholar]
- Dinda R.; Sengupta P.; Sutradhar M.; Mak T. C. W.; Ghosh S. Solution study of a structurally characterized monoalkoxo-bound monooxo-vanadium(V) complex: Spontaneous generation of the corresponding oxobridged divanadium(V,V) complex and its electroreduction to a mixed-valence species in solution. Inorg. Chem. 2008, 47, 5634–5640. 10.1021/ic702172p. [DOI] [PubMed] [Google Scholar]
- Friedrich A.; Hefele H.; Mickler W.; Mönner A.; Uhlemann E.; Scholz F. Voltammetric and potentiometric studies on the stability of vanadium(IV) complexes. A comparison of solution phase voltammetry with the voltammetry of microcrystalline solid compounds. Electroanalysis 1998, 10, 244–248. . [DOI] [Google Scholar]
- Galloni P.; Conte V.; Floris B. A journey into the electrochemistry of vanadium compounds. Coord. Chem. Rev. 2015, 301–302, 240–299. 10.1016/j.ccr.2015.02.022. [DOI] [Google Scholar]
- WinEPR SimFonia, version 1.25; Bruker Analytische Messtechnik GmbH, Karlsruhe, 1996.
- Neese F.; Wennmohs F.; Becker U.; Riplinger C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. 10.1063/5.0004608. [DOI] [PubMed] [Google Scholar]
- Neese F. Software update: The ORCA program system–Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606 10.1002/wcms.1606. [DOI] [Google Scholar]
- Micera G.; Garribba E. Is the spin-orbit coupling important in the prediction of the 51V hyperfine coupling constants of VIVO2+ species? ORCA versus Gaussian performance and biological applications. J. Comput. Chem. 2011, 32, 2822–2835. 10.1002/jcc.21862. [DOI] [PubMed] [Google Scholar]
- Paine T. K.; Weyhermüller T.; Bill E.; Bothe E.; Chaudhuri P. Non-Oxo Vanadium(IV) Complexes of Aminebis(phenolate) [O,N,O] Donor Ligands and Solution Studies of Isostructural VIV and MnIV Complexes. Eur. J. Inorg. Chem. 2003, 2003, 4299–4307. 10.1002/ejic.200300400. [DOI] [Google Scholar]
- Wang J.; Gou Y.; Zhang Z.; Yu P.; Qi J.; Qin Q.; Sun H.; Wu X.; Liang H.; Yang F. Developing an Anticancer Copper(II) Multitarget Pro-Drug Based on the His146 Residue in the IB Subdomain of Modified Human Serum Albumin. Mol. Pharm. 2018, 15, 2180–2193. [DOI] [PubMed] [Google Scholar]
- Gou Y.; Qi J.; Ajayi J.-P.; Zhang Y.; Zhou Z.; Wu X.; Yang F.; Liang H. Developing anticancer copper(II) pro-drugs based on the nature of cancer cells and the human serum albumin carrier IIA subdomain. Mol. Pharm. 2015, 12, 3597–3609. 10.1021/acs.molpharmaceut.5b00314. [DOI] [PubMed] [Google Scholar]
- El-Afify M. E.; Elsayed S. A.; Shalaby T. I.; Toson E. A.; El-Hendawy A. M. Synthesis, characterization, DNA binding/cleavage, cytotoxic, apoptotic, and antibacterial activities of V(IV), Mo(VI), and Ru(II) complexes containing a bioactive ONS-donor chelating agent. Appl. Organometal. Chem. 2021, 35, e6082 10.1002/aoc.6082. [DOI] [Google Scholar]
- Acilan C.; Cevatemre B.; Adiguzel Z.; Karakas D.; Ulukaya E.; Ribeiro N.; Correia I.; Pessoa J. C. Synthesis, biological characterization and evaluation of molecular mechanisms of novel copper complexes as anticancer agents. Biochem. Biophys. Acta - Gen Subj. 2017, 1861, 218–234. 10.1016/j.bbagen.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Matos C. P.; Yildizhan Y.; Adiguzel Z.; Pavan F. R.; Campos D. L.; Pessoa J. C.; Ferreira L. P.; Tomaz A. I.; Correia I.; Acilan C. New ternary iron(III) aminobisphenolate hydroxyquinoline complexes as potential therapeutic agents. Dalton Trans. 2019, 48, 8702–8716. 10.1039/c9dt01193e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.