Abstract
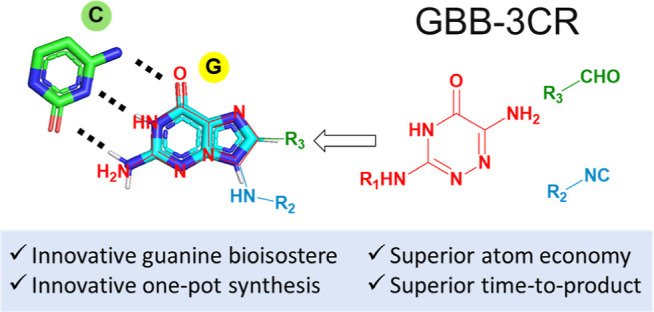
Guanine is one out of five endogenous nucleobases and of key interest in drug discovery and chemical biology. Hitherto, the synthesis of guanine derivatives involves lengthy multistep sequential synthesis of low overall diversity, resulting in the quest for innovation. Using a “single-atom skeletal editing” approach, we designed 2-aminoimidazo[2,1-f][1,2,4]triazin-4(3H)-one as a guanine isostere, conserving the biologically important HBA–HBD–HBD (HBA = hydrogen bond acceptor; HBD = hydrogen bond donor) substructure. We realized our design by a simple one-pot two-step method combining the Groebke-Blackburn-Bienaymé reaction (GBB-3CR) and a deprotection reaction to assemble the innovative guanine isosteres in moderate to good yields. Our innovative, diverse, short, and reliable multicomponent reaction synthesis will add to the toolbox of guanine isostere syntheses.
Introduction
Guanine (2-amino-1,9-dihydro-6H-purin-6-one) was first reported in 1844 by the German chemist Julius Bodo Unger and later structurally elucidated by Emil Fischer.1 Guanine is a purine derivative, consisting of a fused planar pyrimidine-imidazole ring system. As a substructure of guanosine, it plays an outstanding role in the propagation of genetic information in living organisms by RNA and DNA (Figure 1A).2 Moreover, guanine is a part of several cofactors, for example, cGMP or GDP. Numerous diseases, e.g., cancer and K-RAS, are associated with malfunctioning guanine-dependent proteins and/or guanine catabolism.3 Several approved drugs are based on guanine moieties such as the anti-herpes simplex acyclovir or the antineoplastic 8-azaguanine (Figure 1B).4 Guanine derivatives are typically synthetically accessed by a sequential multistep synthesis from heterocyclic guanine precursors.5 Thus, there is an urgent need for convergent short and diverse syntheses of novel guanine derivatives. Analysis of the guanine binding interaction in DNA, RNA, and proteins reveals that key pharmacophoric elements include a flat heterocyclic 5–6 ring system and a hydrogen-bonding triade HBA–HBD–HBD (HBA = hydrogen bond acceptor; HBD = hydrogen bond donor) of an acceptor carbonyl-O, an adjacent NH, and an exocyclic amino group (Figure 1C). Therefore, a guanine bioisostere should be composed of a flat heterocyclic ring system incorporating the essential HBA–HBD–HBD triade. Based on our interest in multicomponent reaction (MCR) chemistry, we reasoned that a generalized scaffold obeying the pharmacophore requirements of guanine would be accessible by an unprecedented atypical Groebke-Blackburn-Bienaymé reaction (GBB-3CR) of a heterocyclic amidine, an aldehyde, and an isocyanide (Figure 1C).6
Figure 1.
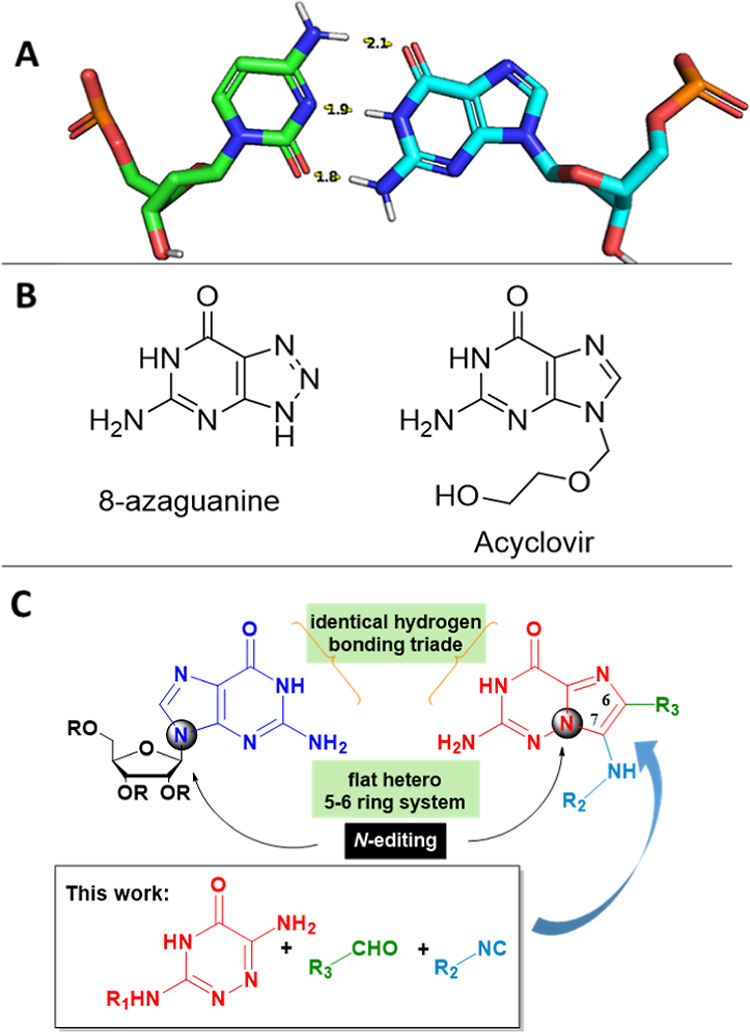
Nature of guanine. (A) Watson–Crick base pairing involving cytosine and guanine. (B) Guanine moiety-containing drugs. (C) Design of a guanine isosteric scaffold by N-editing and its multicomponent reaction synthesis.
In the spirit of the emerging research area ‘single-atom skeletal editing’, the imidazo-N-9 of the purine would shift into the next bridgehead 4-position. The resulting scaffold indeed would closely resemble guanine: the key hydrogen-bonding triade is identical, the scaffold consists of a flat hetero 5–6 ring system, and the chemistry would allow substitution at the 6 and 7 positions.
The 7-position corresponds to the (deoxy)ribose position of guanine and biologically relevant derivatives. Due to the logic of the herein used chemistry, a bridgehead N is shifted in the new scaffold, which corresponds to the neighboring 9-position in guanine. In principle, the new heterocyclic ring system could result in a differential distribution of tautomeric microspecies which is important for biological activity. Indeed, analyzing the tautomeric microspecies using the ChemAxon Tautomerizer in water at room temperature revealed that the major species is identical with the guanine major tautomer (Supporting Information).7 Interestingly, the major tautomer species is present over a broad pH range from 2.5 until 9. Taken together, our design and the predicted properties made us confident to investigate and optimize the GBB-3CR reaction to access a new class of guanine bioisosteres.
Results and Discussion
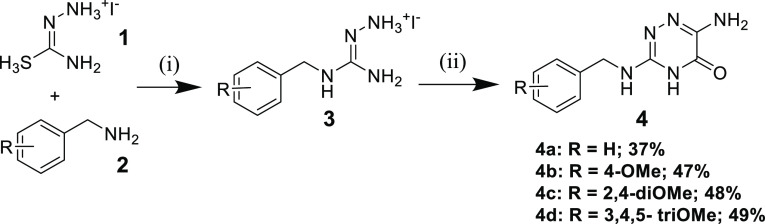
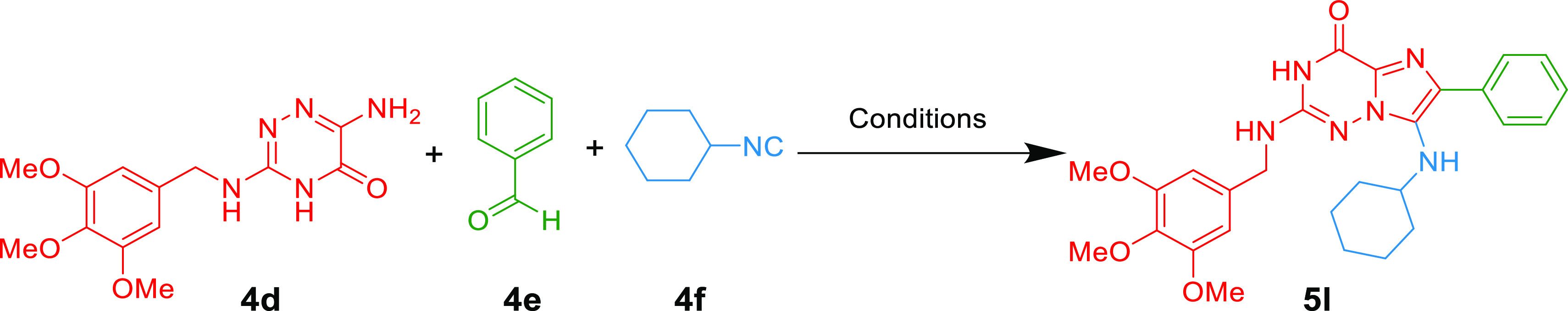
Here we reported the synthesis of novel 4-aza-9-deaza-guanine isosteres by a one-pot two-step protocol combining the GBB-3CR and an acid-assisted deprotection reaction, resulting in a library of diverse analogues bearing imidazo[2,1-f][1,2,4]triazin-4(3H)-one scaffold. First, we synthesized 1-amino-3-benzylguanidine hydroiodide (3) from hydrazinecarbothioamide hydroiodide (1) and benzylamine (2).8 The cyclization of 3 with ethyl 2-amino-2-thioxoacetate (4) provided the four building blocks 1,2,4-triazin-5-one 4a–4d with different benzyl protecting groups in 37–49% yields (Scheme 1).
Scheme 1. Synthesis of 1,2,4-Triazin-5(4H)-ones.
Reaction conditions: (i) 2-propanol, 40 °C, then r.t., 2 d; (ii) ethyl 2-amino-2-thioxoacetate, 75 °C, 2.5 h, then ice water, 16 h.
To optimize the reaction condition for the construction of the GBB intermediates, 6-amino-3-((3,4,5-trimethoxybenzyl)amino)-1,2,4-triazin-5-one (4d), benzaldehyde (4e), and cyclohexyl isocyanide (4f) were selected for a model reaction (Table 1). Initially, 4d, 4e, and 4f were combined sequentially in methanol (0.5 M) at 100 °C at room temperature for 12 h in the presence of 0.2 equimolar Sc(OTf)3, which gave the GBB product 5l in a moderate yield (45%, entry 1). Decreasing the amount of catalyst to 0.1 equimolar slightly improved the yield from 45 to 49%. Microwave irradiation promoted the reaction when heating the system at 100 °C for 2 h only with 0.1 equimolar Sc(OTf)3 (entry 4, 60%) rather than 0.2 equimolar Sc(OTf)3 (entry 3, 39%). Both shortening (entry 5, 51%) and prolonging (entry 6, 44%) the reaction time are detrimental to the reaction. Conventional heating (entry 7, 49%) offered no advantage in increasing the yield. Some other popular solvents for the GBB reactions were also screened in this work, but higher yields could not be obtained with polar solvents such as EtOH or nonpolar solvents such as toluene and acetonitrile. Catalyst variations such as La(OTf)3, Gd(OTf)3, HClO4, and AcOH failed to further improve the yield, only realizing lower yields from 20 to 38%.
Table 1. Optimization of the GBB-3CR Conditionsa.
| entry | catalyst | solvent | temperature ( °C) | time | yields (%) |
|---|---|---|---|---|---|
| 1 | Sc(OTf)3(0.2) | MeOH | r.t. | 12 h | 45 |
| 2 | Sc(OTf)3(0.1) | MeOH | r.t. | 12 h | 49 |
| 3 | Sc(OTf)3(0.2) | MeOH | 100 | 2 hb | 39 |
| 4 | Sc(OTf)3(0.1) | MeOH | 100 | 2 hb | 60 |
| 5 | Sc(OTf)3(0.1) | MeOH | 100 | 1 hb | 51 |
| 6 | Sc(OTf)3(0.1) | MeOH | 100 | 4 hb | 44 |
| 7 | Sc(OTf)3(0.1) | MeOH | 100 | 2 hc | 49 |
| 8 | Sc(OTf)3(0.1) | EtOH | 100 | 2 hb | 53 |
| 9 | Sc(OTf)3(0.1) | toluene | 100 | 2 hb | 44 |
| 10 | Sc(OTf)3(0.1) | MeCN | 100 | 2 hb | 28 |
| 11 | La(OTf)3 | MeOH | 100 | 2 hb | 38 |
| 12 | Gd(OTf)3 | MeOH | 100 | 2 hb | 35 |
| 13 | HClO4(0.15) | MeOH | r.t. | 12 h | 37 |
| 14 | HClO4(0.15) | MeOH | 100 | 2 hb | 34 |
| 15 | AcOH(2) | MeOH | r.t. | 12 h | 20 |
Reaction conditions: 4d (0.5 mmol), 4e (0.6 mmol), 4f (0.6 mmol), catalyst (10 or 20 mmol %), and solvent (2 mL).
Microwave condition.
Conventional heating using aluminum heating blocks.
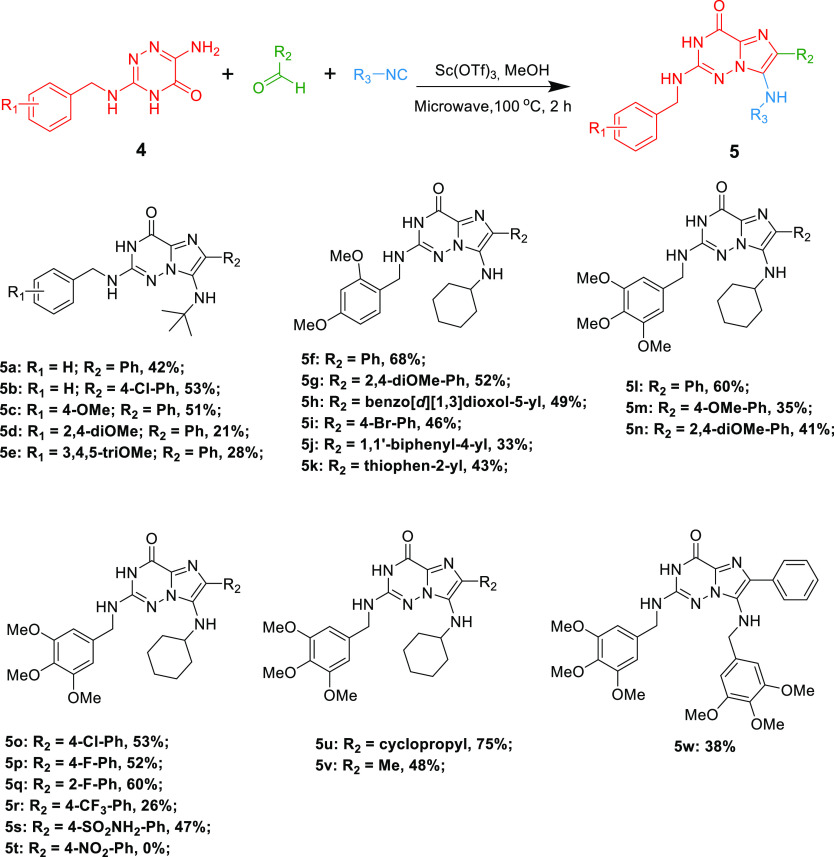
With optimal reaction conditions in hand, the scope of the 6-amino-1,2,4-triazin-5-ones, aldehydes, and isocyanides was explored (Scheme 2). Tert-butyl isocyanide and cyclohexyl isocyanide were first introduced in the reaction to exploit cleavability of R3 in the next step. With tert-butyl isocyanide, the relevant GBB products (5a–5e) could be achieved in 21–53% yields.
Scheme 2. Variation of the 2-Amidines, Aldehydes, and Isocyanides.
Employing a benzyl-protecting group (R1 = H) in the amidine 4, the GBB compounds 5a and 5b could be obtained smoothly in moderate 42 and 50% yields, respectively. 4-chlorobenzaldehyde and benzaldehyde performed similarly (5b vs 5c, 53 vs 51%). Introducing para-methoxy group in R1 yielded a moderate 51%. On replacing R1 with 2,4-dimethoxy (5d, 21%) or 3,4,5-trimethoxy (5e, 28%) group, the yields decreased. Running the reaction with cyclohexyl isocyanide, almost all GBB products could be realized successfully, except with 4-NO2-benzaldehyde (5t, 0%). With the 2,4-dimethoxy group in the R1 part, both electron-donating group and electron-withdrawing group in R2 are tolerated, achieving final products in medium yields, from 33 to 68%. Generally, electron-donating groups (5f–5h, 5j) in R2 are more active than electron-withdrawing groups (5i, 5k). When replacing 2,4-dimethoxy group with 3,4,5-trimethoxy group in R1, the GBB cyclization compounds could also be readily obtained with 26–75% yields. Overall, benzaldehydes with electron-withdrawing groups (5o–5q and 5s, 47–60% yields) could achieve higher yields than electron-donating-group-substituted benzaldehydes (5l–5n, 35–60% yields), with two exceptions, 4-CF3-benzaldehyde (5r, 26%) and the aforementioned 4-NO2-benzaldehyde (5s, 0%). Interestingly, aliphatic aldehydes like cyclopropane carbaldehyde (5u, 75%) and acetaldehyde (5v, 48%) resulted also in the desired products in a medium to good yield. Moreover, the aromatic 3,4,5-trimethoxybenzyl isocyanide was well tolerated, providing the GBB intermediate 5w in a 38% yield.
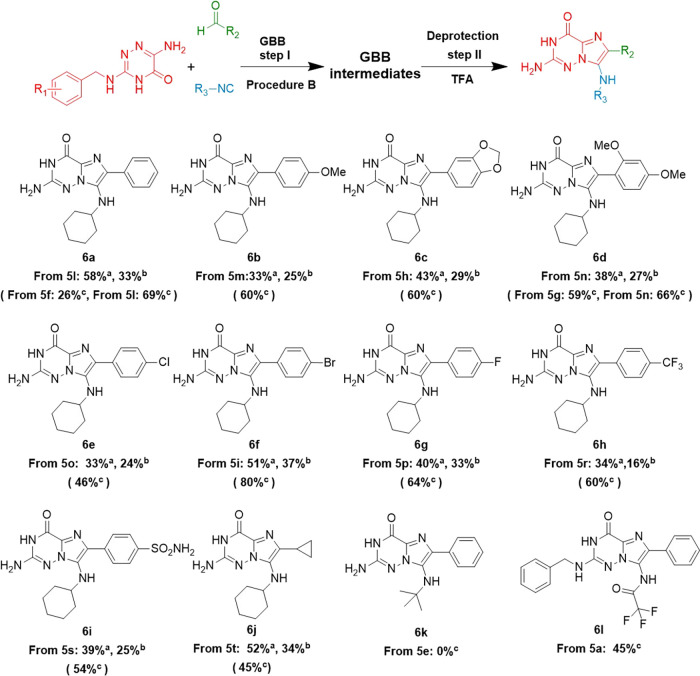
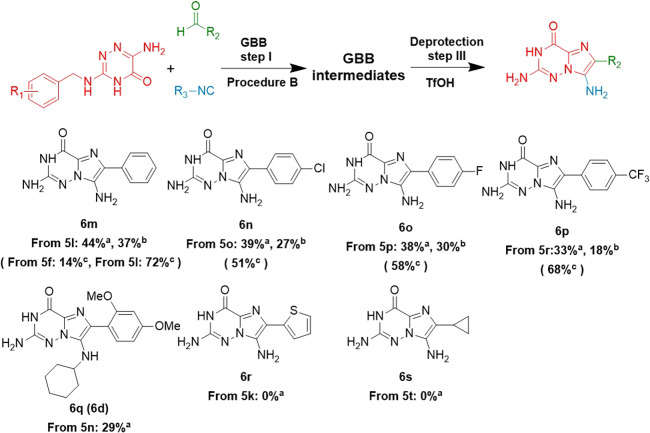
Next, in order to produce the target 4-aza-9-deaza-guanine isosteres, we needed to deprotect the benzyl groups in the previously obtained GBB-3CR intermediates in the following step. For this, we screened 20 deprotection conditions (Table S1, Supporting Information) and found that both trifluoroacetic acid (TFA)9 and trifluoromethansulfonic acid (TfOH)10 could cleave the benzyl groups, but the final deprotected products induced by those two acids are slightly different. While TFA can only deprotect the benzyl group (Scheme 3), the superacid TfOH cleaves simultaneously the benzyl group and the R3 group (Scheme 4).
Scheme 3. One-Pot Benzyl Deprotection with TFA.
Reaction conditions of Step II: Ugi reaction crude (0.2–0.5 mmol), 0.1 M TFA, 80 °C, 12 h, conventional heating; ayields from the one-pot procedure without isolation of GBB intermediates; btotal yields calculated over the two-step procedure with isolated GBB intermediates; cyields from only the deprotection step II with purified GBB intermediates.
Scheme 4. One-Pot Double Deprotection with TfOH.
Reaction conditions of Step III: Ugi reaction crude (0.1–0.5 mmol), 0.1 M TfOH, 55 °C, 4 h, conventional heating. ayields from the one-pot procedure without isolation of GBB intermediates; btotal yields calculated over the two-step procedure with isolated GBB intermediates; cyields from only the deprotection step III with purified GBB intermediates.
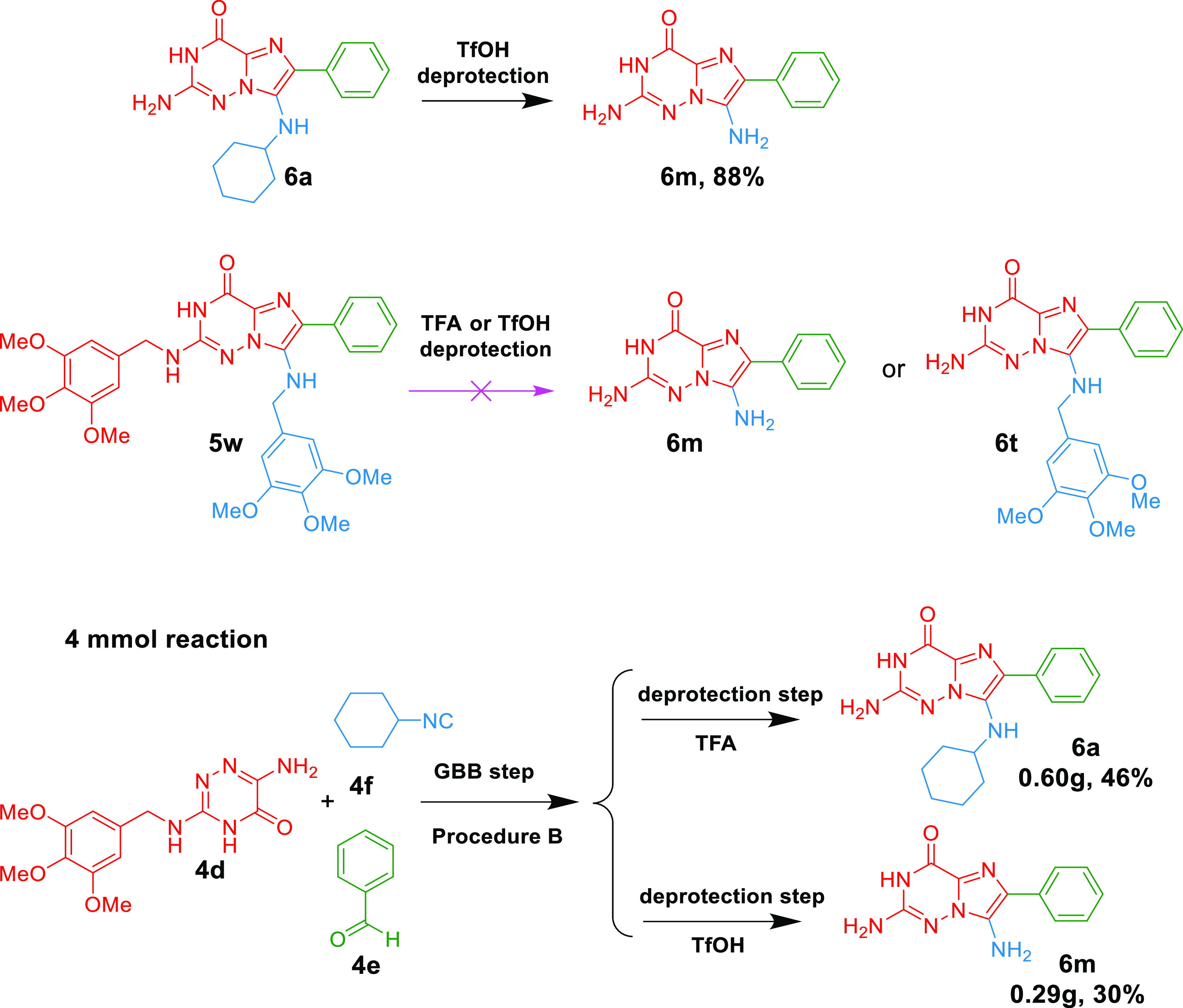
In the TFA-assisted one-pot deprotection, we found that the deprotected products 6a–6j formed well when R3 was a cyclohexyl group, achieving yields from 33 to 58%. GBB-3CR intermediates derived from electron-donating-groups- (6a–6d) and electron-withdrawing-groups- (6e–6i) substituted benzaldehydes both proceeded successfully in the one-pot benzyl deprotection step. It is noteworthy that the one-pot yields of all deprotected products 6a–6j are on average 14% higher than those generated by the two separate steps procedure. Moreover, we also ran the deprotection reactions with all purified GBB-3CR intermediates and summarized the yields in Scheme 3. It is noteworthy that the yield of 6a is much higher when generated from 5l (69%) than 5f (26%), and 5n (66%) can obtain 6d in a higher yield than 5g (59%) as well. Those two examples suggest that the 3,4,5-trimethoxy group in R1 is easier to cleave than the 2,4-dimethoxy substituent. In addition, on replacing R3 with the tert-butyl group, the GBB-3CR intermediate 5e failed to provide the target compound 6k, while 5a gave the unexpected trifluoroacetylation product 6l in a 45% yield.
The TfOH-promoted one-pot double deprotection reactions worked well with the benzaldehydes-derived GBB intermediates, affording products 6m–6p in 33–44% yields. The yields of 6m–6p achieved by the one-pot method were found to be superior to those from the two separate steps procedure by an average of 11%. Surprisingly, the GBB product 5n generated from 2,4-dimethoxy benzaldehyde provided the mono-deprotected compound 6q (29%) rather than the double-deprotected product. The distinct yields difference from single-step deprotection in the synthesis of 6m from 5f (14%) or 5l (72%) demonstrated that the 3,4,5-trimethoxy group in R1 is easier to deprotect TfOH-assisted, in accordance with its higher ring electron density. However, GBB-3CR intermediates constructed with thiophene-2-carbaldehyde (5k) and cyclopropane carbaldehyde (5t) did not afford the desired products 6r or 6s. This may be due to instability of the thiophene and cyclopropane rings in TfOH.
To further fortify the usefulness of our new synthesis, we carried out the control experiment and scale–up reaction (Scheme 5). It turned out that the mono-deprotected compound 6a could be further deprotected in the presence of TfOH to provide 6m in an 88% yield, which indicated that TfOH could not only achieve double deprotection of benzyl and cyclohexyl groups but also cleave the cyclohexyl alone. Our attempt to figure out whether TFA or TfOH could simultaneously cleave two benzyl groups failed; compound 5w could not yield either double-deprotected 6m or single-deprotected 6t. The scale-up reactions of our one-pot procedures were performed on a 4 mmol scale, providing 6a and 6m in 46 and 30% yields, respectively. The D2O exchange NMR experiments of 6a and 6m were done to prove the mono- or double-deprotection (Figures S3 and S4, Supporting Information).
Scheme 5. Control Experiment and Scale-Up Reaction.
The X-ray crystal structures of 4d (Supporting Information) and 5l were obtained, demonstrating the solid-state structures of the scaffold. Interestingly, the G-analogue 5l exhibits a trifurcated hydrogen-bonding pattern in a circular tetrameric macrocyclic conformation (Figure 2). This closely mimics the G-binding pattern found in all GTP/GDP-protein structures, suggesting that our heterocyclic G-mimic indeed could act as a bioisosteric G-mimic.
Figure 2.
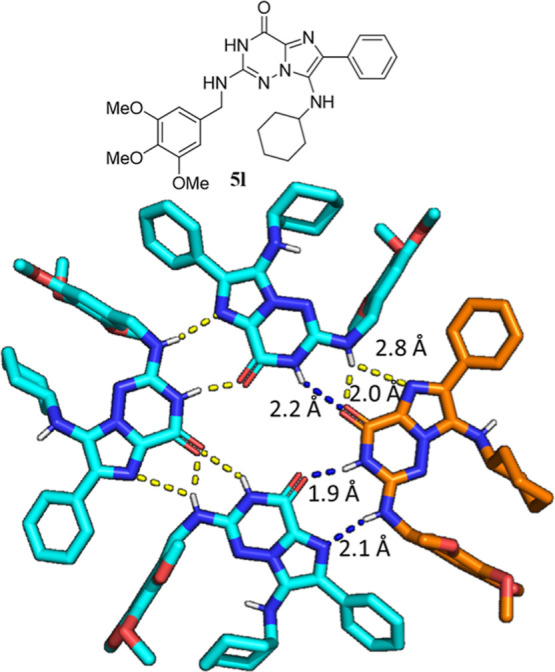
X-ray structure of G-analogue 5l (CCDC 2190420) in solid state. 2D structure and 3D structure of the tetrameric macrocyclic assembly exhibiting a dense hydrogen-bonding network (dotted lines). For clarity, one molecule is shown in golden sticks, including the important trifurcated hydrogen-bonding pattern (blue dotted lines).
In summary, an innovative GBB-3CR-based one-pot two-step synthesis of novel 4-aza-9-deaza-guanine isosteres has been developed using a ‘single-atom skeletal editing’ strategy. Generally, most of our two-step syntheses are not high yielding, still they are superior to previous multistep synthesis protocols and will help to enrich the toolbox of guanine isosteres by an unprecedented new member scaffold. Combining GBB-3CR reaction and subsequent TFA- or TfOH-assisted deprotection reaction, mono-deprotected guanine isosteres and double deprotected guanine isosteres can be achieved, separately. Having the same hydrogen-binding pattern as guanine, our 9-deaza-guanine isosteres may also form similar interactions with biological receptors. Currently, biological evaluation of our G-analogues is ongoing and will be reported in due course.
Experimental Section
General Information
Reagents were available from commercial suppliers and used without any purification unless otherwise noted. All isocyanides were made in-house via the Ugi procedure.11 Other reagents were purchased from Sigma-Aldrich, ABCR, Acros, Fluorochem, AK Scientific, Combiblocks, or A2B and were used without further purification. Nuclear magnetic resonance spectra were recorded on a Bruker Avance 500 spectrometer. Chemical shifts for 1H NMR were reported relative to TMS (δ 0 ppm) or internal solvent peak (CDCl3 δ 7.26 ppm, CD3OD δ 3.31 ppm, or D2O δ 4.79 ppm), and coupling constants were in hertz (Hz). The following abbreviations were used for spin multiplicity: s = singlet, d = doublet, t = triplet, dt = double triplet, ddd = doublet of double doublet, m = multiplet, and br = broad. Chemical shifts for 13C NMR were reported in ppm relative to the solvent peak (CDCl3 δ 77.23 ppm, DMSO δ 39.52 ppm, and CD3OD δ 49.00 ppm). Flash chromatography was performed on a Grace Reveleris X2 system using Grace Reveleris Silica columns (12 g), and a gradient of petroleum ether/ethyl acetate (0–100%) or dichloromethane/methanol (0–20%) was applied. Thin layer chromatography was performed on Fluka precoated silica gel plates (0.20 mm thick, particle size 25 μm). Mass spectra were measured on a Waters Investigator Supercritical Fluid Chromatograph with a 3100 MS Detector (ESI) using a solvent system of methanol and CO2 on a Viridis silica gel column (4.6 × 250 mm, 5 μm particle size) and reported as (m/z). High-resolution mass spectra (HRMS) were recorded using an LTQ-Orbitrap-XL (Thermo Fisher Scientific; ESI pos. mode) at a resolution of 60,000@m/z 400. All microwave irradiation reactions were carried out in a Biotage Initiator microwave synthesizer. Melting points were obtained on a melting point apparatus and were uncorrected. The yields given refer to chromatographically purified compounds unless otherwise stated.
General Experimental Procedure and Characterization
General Procedure A: Synthesis of 1,2,4-Triazin-5(4H)-ones (4a–4d)
Hydrazinecarbothioamide hydroiodide (20 mmol, 1.0 equiv) was suspended in 20 mL of 2-propanol (20 mL, 1.0 M), then benzylamine (21 mmol, 1.05 equiv) was added. The reaction was heated at 40 °C for 10 h, and then the reaction mixture was kept stirring at room temperature for 2 more days. The solid was filtered and the solvents were evaporated in vacuum. The remaining crude was recrystallized with DCM and diethyl ether to give white solid amino-3-benzylguanidine hydroiodide. To a mixture of 1-amino-3-benzylguanidine hydroiodide (5.0 mmol, 1.0 equiv) and K2CO3 (5.1 mmol, 1.02 equiv) in DMSO (10 mL, 0.5 M), ethyl-2-amino-2-thioxo-acetate (5.5 mmol, 1.1 equiv) was added and kept for 2.5 h at 75 °C in an oil bath. After heating, the gray mixture was poured under vigorous stirring into 70 mL of ice water and stirred for another 18 h to yield a yellow crystalline precipitate. The precipitate was filtration and washed with water (3 × 20 mL) and then ethylacetate/ether (1:1, 3 × 20 mL). The solid was recrystallized from methanol or purified by silica chromatography to give 4a–4d.
General Procedure B
Corresponding 6-amino-3-(benzylamino)-1,2,4-triazin-5(4H)-one (0.2–1 mmol, 1.0 equiv) and aldehyde (0.24–1.4 mmol, 1.2 equiv) were dissolved in MeOH (0.8–4 mL, 0.25 M) in a microwave tube, the mixture was stirred at room temperature for 10 min, then isocyanide (0.24–1.4 mmol, 1.2 equiv) and Sc(OTf)3 (10 mmol %, 0.1 equiv) were added, and the tube was sealed. Then the mixture was heated at 100 °C under microwave in a sealed tube for 2 h. During the reaction, the temperature was monitored by the temperature–time profile on the screen of the microwave machine. After the reaction, the mixture was purified by silica gel column chromatography (MeOH/DCM = 1–5%) to give compounds 5a–5u.
General Procedure C: Benzyl Deprotection with TFA
GBB intermediate (0.2–0.5 mmol) was dissolved in TFA (2–5 mL, 0.1 M). The reaction mixture was stirred at 80 °C overnight in a sealed tube. After reaction, the reaction mixture was diluted with 20 mL of DCM, and then the solvents were removed under reduced pressure. Then, the residue was diluted with EA (50 mL) and washed with sat·NaHCO3 (50 mL × 3). Then the organic layer was dried over MgSO4, filtered, and the solvent was removed under reduced pressure. Then the crude compound was purified by silica gel column chromatography (MeOH/DCM = 2–10%) to get the deprotected products 6a–6l.
General Procedure D: Deprotection with TfOH
GBB intermediate (0.1–0.5 mmol) was treated with triflic acid (1–5 mL, 0.1 M), then heated at 55 °C for 4 h. After the reaction, the mixture was quenched with water and neutralized with sat.NaHCO3. The aqueous layer was extracted with EA and the combined organic layer was washed with brine, dried, and concentrated under vacuum. Then the crude compounds were purified by silica gel column chromatography (MeOH/DCM = 2–10%) to get the deprotected products 6m–6q.
General Procedure E: One-Pot Synthesis
First, GBB reactions were carried out according to procedure B; after the reaction, the solvent was removed directly and the reaction mixture underwent in situ deprotection reaction following procedure C or D. Then the crude compounds were purified by silica gel column chromatography (MeOH/DCM = 2–10%) to get the deprotected products 6m–6q.
6-Amino-3-(benzylamino)-1,2,4-triazin-5(4H)-one (4a)
It was synthesized according to general procedure A on a 5 mmol scale and isolated using 1–3% MeOH/dichloromethane (v/v) to afford 4a (406 mg, 37%) as a white solid. mp 219–221 °C. Rf = 0.46 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO): δ 11.05 (s, 1H), 7.28 (ddd, J = 26.5, 14.6, 7.4 Hz, 5H), 7.07–6.95 (m, 1H), 5.79 (s, 2H), and 4.38 (d, J = 6.3 Hz, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 159.5, 154.0, 146.7, 139.5, 128.3, 127.0, 126.8, and 43.0. HRMS (ESI) m/z: [M + H]+ calcd for C10H12ON5, 218.1036; found, 218.1036.
6-Amino-3-((4-methoxybenzyl)amino)-1,2,4-triazin-5(4H)-one (4b)
It was synthesized according to general procedure A on a 5 mmol scale and isolated using 1–3% MeOH/dichloromethane (v/v) to afford 4b (442 mg, 47%) as a light brown solid. mp 224–226 °C. Rf = 0.40 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.01 (s, 1H), 7.22 (d, J = 8.2 Hz, 2H), 6.97 (s, 1H), 6.88 (d, J = 8.3 Hz, 2H), 5.78 (s, 2H), 4.30 (d, J = 5.9 Hz, 2H), and 3.72 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 159.5, 158.3, 153.9, 146.6, 131.2, 129.0, 128.5, 113.7 (d, J = 15.8 Hz), 55.0, and 42.5. HRMS (ESI) m/z: [M + H]+ calcd for C11H14O2N5, 248.1142; found, 248.1142.
6-Amino-3-((2,4-dimethoxybenzyl)amino)-1,2,4-triazin-5(4H)-one (4c)
It was synthesized according to general procedure A on a 5 mmol scale and isolated using 1–3% MeOH/dichloromethane (v/v) to afford 4c (402 mg, 48%) as a yellow solid. mp 218–220 °C. Rf = 0.36 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.90 (s, 1H), 7.07 (d, J = 8.4 Hz, 1H), 6.63 (s, 1H), 6.56 (s, 1H), 6.48 (d, J = 8.2 Hz, 1H), 5.77 (s, 2H), 4.25 (d, J = 6.0 Hz, 2H), 3.80 (s, 3H), and 3.73 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 159.9, 159.5, 157.8, 154.1, 146.6, 128.6 (d, J = 11.3 Hz), 118.8, 104.2 (d, J = 19.9 Hz), 98.3 (d, J = 21.5 Hz), 55.4 (d, J = 16.6 Hz), 55.2 (d, J = 13.4 Hz), and 38.5. HRMS (ESI) m/z: [M + H]+ calcd for C12H16O3N5, 278.1248; found, 278.1247.
6-Amino-3-((3,4,5-trimethoxybenzyl)amino)-1,2,4-triazin-5(4H)-one (4d)
It was synthesized according to general procedure A on a 3 mmol scale and isolated using 1–3% MeOH/dichloromethane (v/v) to afford (753 mg, 49%) as a yellow solid. mp 210–212 °C. Rf = 0.23 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.03 (s, 1H), 6.99 (s, 1H), 6.64 (s, 2H), 5.80 (s, 2H), 4.30 (d, J = 6.3 Hz, 2H), 3.74 (s, 6H), and 3.62 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 159.9, 154.4, 153.3, 147.2, 136.8, 135.4, 104.6 (d, J = 22.2 Hz), 60.5, 56.3 (d, J = 16.0 Hz), and 43.8. HRMS (ESI) m/z: [M + H]+ calcd C13H18O4N5, 308.1353; found, 308.1353.
2-(Benzylamino)-7-(tert-butylamino)-6-phenylimidazo[2,1-f][1,2,4]triazin-4(3H)-one (5a)
It was synthesized according to general procedure B on a 0.375 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5a (61 mg, 42%) as a white solid. mp 246–248 °C. Rf = 0.23 (3% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.18 (s, 1H), 8.09 (d, J = 7.7 Hz, 2H), 7.36 (dt, J = 24.4, 7.6 Hz, 6H), 7.24 (dt, J = 15.0, 7.4 Hz, 2H), 6.56 (t, J = 5.8 Hz, 1H), 4.47 (d, J = 6.1 Hz, 2H), 3.89 (s, 1H), and 0.96 (s, 9H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.2, 148.2, 139.0, 135.0, 134.7, 129.8, 128.3, 127.9, 127.3, 126.9, 126.7, 126.6, 126.5, 55.9, and 30.1. HRMS (ESI) m/z: [M + H]+ calcd for C22H25ON6, 389.2084; found, 389.2083.
2-(Benzylamino)-7-(tert-butylamino)-6-(4-chlorophenyl)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5b)
It was synthesized according to general procedure B on a 0.25 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5b (55 mg, 53%) as a white solid. mp 258–260 °C. Rf = 0.32 (3% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.27 (s, 1H), 8.11 (d, J = 8.2 Hz, 2H), 7.48–7.17 (m, 7H), 6.61 (s, 1H), 4.47 (s, 2H), 3.94 (s, 1H), and 0.96 (s, 9H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.8, 148.8, 139.5, 134.4, 134.0, 131.5, 130.4, 128.8, 128.5, 128.4, 127.8, 127.4, 127.3, 56.5, 44.7, and 30.5 (d, J = 8.3 Hz). HRMS (ESI) m/z: [M + H]+ calcd for C22H24ON6Cl, 423.1695; found, 423.1692.
7-(tert-Butylamino)-2-((4-methoxybenzyl)amino)-6-phenylimidazo[2,1-f][1,2,4]triazin-4(3H)-one (5c)
It was synthesized according to general procedure B on a 1 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5c (213 mg, 51%) as a white solid. mp 246–248 °C. Rf = 0.82 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.24 (s, 1H), 8.11 (d, J = 7.2 Hz, 2H), 7.42–7.30 (m, 4H), 7.23 (t, J = 7.4 Hz, 1H), 6.91 (d, J = 8.6 Hz, 2H), 6.58 (s, 1H), 4.40 (d, J = 5.7 Hz, 2H), 3.93 (s, 1H), 3.74 (s, 3H), and 1.01 (s, 9H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 158.4, 152.6, 148.4, 135.1, 134.7, 130.9, 129.7, 128.8, 127.9, 126.7, 126.5, 126.4, 113.7, 56.0, 55.1 (d, J = 12.4 Hz), 43.8, and 30.2. HRMS (ESI) m/z: [M + H]+ calcd for C23H27O2N6, 419.2190; found, 419.2187.
7-(tert-Butylamino)-6-phenyl-2-((2,4-dimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5d)
It was synthesized according to general procedure B on a 1 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5d (94 mg, 21%) as a yellow solid. mp 236–238 °C. Rf = 0.50 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 1H NMR (500 MHz, DMSO-d6): δ 11.00 (s, 1H), 8.13–8.08 (m, 2H), 7.36 (t, J = 7.8 Hz, 2H), 7.26–7.19 (m, 2H), 6.59 (d, J = 2.4 Hz, 1H), 6.48 (dd, J = 8.3, 2.4 Hz, 1H), 6.25 (t, J = 5.7 Hz, 1H), 4.36 (s, 2H), 3.98 (s, 1H), 3.82 (s, 3H), 3.74 (s, 3H), and 1.03 (s, 9H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.1, 158.1, 152.2, 148.2, 135.1, 134.8, 129.9, 129.7, 127.9, 126.7, 126.6, 126.5 (d, J = 4.3 Hz), 118.3, 104.3 (d, J = 20.2 Hz), 98.4 (d, J = 21.1 Hz), 56.1, 56.0, 55.5 (d, J = 17.4 Hz), 55.2 (d, J = 13.7 Hz), and 30.2. HRMS (ESI) m/z: [M + H]+ calcd for C24H29O3N6, 449.2296; found, 449.2294.
7-(tert-Butylamino)-6-phenyl-2-((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5e)
It was synthesized according to general procedure B on a 1 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5e (133 mg, 28%) as a light yellow solid. mp 231–233 °C. Rf = 0.37 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.15 (s, 1H), 8.09 (dd, J = 8.2, 1.1 Hz, 2H), 7.36 (t, J = 7.8 Hz, 2H), 7.22 (t, J = 7.4 Hz, 1H), 6.70 (s, 2H), 6.52 (t, J = 5.6 Hz, 1H), 4.39 (d, J = 5.7 Hz, 2H), 3.92 (s, 1H), 3.76 (s, 6H), 3.62 (s, 3H), and 0.97 (s, 9H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.9, 152.2, 148.1, 136.4, 135.0, 134.8, 134.7, 129.8, 127.9, 126.7, 126.5 (d, J = 4.8 Hz), 104.6, 104.5, 60.0, 56.0, 55.9 (d, J = 18.1 Hz), 44.6, and 30.1. HRMS (ESI) m/z: [M + H]+ calcd for C25H31O4N6, 479.2401; found, 479.2399.
7-(Cyclohexylamino)-2-((2,4-dimethoxybenzyl)amino)-6-phenylimidazo[2,1-f][1,2,4]triazin-4(3H)-one (5f)
It was synthesized according to general procedure B on a 1 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5f (322 mg, 68%) as a light yellow solid. mp 190–192 °C. Rf = 0.35 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.91 (s, 1H), 7.99–7.91 (m, 2H), 7.39 (t, J = 7.8 Hz, 2H), 7.26 (d, J = 8.4 Hz, 1H), 7.23 (d, J = 7.4 Hz, 1H), 6.59 (d, J = 2.4 Hz, 1H), 6.47 (dd, J = 2.4, 8.4 Hz, 1H), 6.26 (s, 1H), 4.38–4.35 (m, 2H), 4.34 (s, 1H), 3.84 (s, 3H), 3.74 (s, 3H), 3.13–3.02 (m, 1H), 1.71 (d, J = 10.4 Hz, 2H), 1.64–1.58 (m, 2H), 1.46 (d, J = 9.5 Hz, 1H), 1.21 (d, J = 11.7 Hz, 2H), and 1.12–0.99 (m, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 170.4, 160.0, 158.1, 151.9, 148.2, 134.6, 131.9, 129.6, 129.3, 128.4, 126.4, 125.6, 125.4, 118.3, 98.4 (d, J = 21.5 Hz), 59.8, 55.6, 55.2 (d, J = 13.3 Hz), 33.5, 24.4, 20.8 (d, J = 11.9 Hz), and 14.1 (d, J = 13.3 Hz). HRMS (ESI) m/z: [M + H]+ calcd for C26H31O3N6, 475.2452; found, 475.2451.
7-(Cyclohexylamino)-2-((2,4-dimethoxybenzyl)amino)-6-(2,4-dimethoxyphenyl)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5g)
It was synthesized according to general procedure B on a 1 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5g (277 mg, 52%) as a yellow solid. mp 176–178 °C. Rf = 0.38 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.70 (s, 1H), 7.36 (d, J = 8.4 Hz, 1H), 7.21 (d, J = 8.4 Hz, 1H), 6.64–6.58 (m, 3H), 6.48 (dd, J = 2.4, 8.4 Hz, 1H), 6.15 (t, J = 5.9 Hz, 1H), 4.31 (d, J = 5.8 Hz, 2H), 4.26 (d, J = 9.9 Hz, 1H), 3.83 (s, 3H), 3.80 (s, 3H), 3.79 (s, 3H), 3.74 (s, 3H), 3.28–3.21 (m, 1H), 1.64 (q, J = 4.6 Hz, 2H), 1.51 (s, 2H), 1.41 (d, J = 11.7 Hz, 1H), and 1.00 (t, J = 9.8 Hz, 5H). 13C {1H} NMR (126 MHz, DMSO-d6): δ 160.1, 160.0, 158.0, 156.9, 151.6, 147.8, 132.6, 131.5, 129.2, 125.3, 124.4, 118.4, 116.4, 105.5, 105.3, 98.5, 98.3, 55.5, 55.3, 55.2, 55.1, 52.2, 33.4, 25.3, 24.3, and 24.1. HRMS (ESI) m/z: [M + H]+ calcd for C28H35O5N6, 535.2663; found, 535.2661.
6-(Benzo[d][1,3]dioxol-5-yl)-7-(cyclohexylamino)-2-((2,4-dimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5h)
It was synthesized according to general procedure B on a 1 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5h (253 mg, 49%) as a yellow solid. mp 183–185 °C. Rf = 0.44 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.88 (s, 1H), 7.50–7.45 (m, 2H), 7.25 (d, J = 8.4 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 6.59 (d, J = 2.4 Hz, 1H), 6.46 (dd, J = 2.4, 8.3 Hz, 1H), 6.24 (t, J = 5.8 Hz, 1H), 6.02 (s, 2H), 4.33 (d, J = 6.0 Hz, 2H), 4.30 (d, J = 8.8 Hz, 1H), 3.83 (s, 3H), 3.74 (s, 3H), 3.05 (d, J = 9.0 Hz, 1H), 1.70 (d, J = 11.5 Hz, 2H), 1.62 (d, J = 12.0 Hz, 2H), 1.46 (s, 1H), 1.21 (d, J = 22.5 Hz, 3H), and 1.09 (d, J = 7.7 Hz, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.0, 158.1, 151.8, 148.2, 147.3, 145.8, 131.1, 129.6 (d, J = 14.2 Hz), 128.8, 125.1, 119.1 (d, J = 19.2 Hz), 118.3, 108.3 (d, J = 12.8 Hz), 105.9 (d, J = 19.7 Hz), 104.2 (d, J = 24.7 Hz), 100.8, 98.3, 80.5, 55.5 (d, J = 20.2 Hz), 55.3, 55.1, 33.5, 28.3, 25.3, and 24.3 (d, J = 23.4 Hz). HRMS (ESI) m/z: [M + H]+ calcd for C27H31O5N6, 519.235; found, 519.2347.
6-(4-Bromophenyl)-7-(cyclohexylamino)-2-((2,4-dimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5i)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5i (127 mg, 46%) as a yellow solid. mp 180–183 °C. Rf = 0.46 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.97 (s, 1H), 7.91 (d, J = 8.7 Hz, 2H), 7.58 (d, J = 8.7 Hz, 2H), 7.26 (d, J = 8.4 Hz, 1H), 6.59 (d, J = 2.4 Hz, 1H), 6.46 (dd, J = 2.5, 8.4 Hz, 1H), 6.28 (t, J = 6.1 Hz, 1H), 4.43 (d, J = 8.8 Hz, 1H), 4.34 (d, J = 5.8 Hz, 2H), 3.83 (s, 3H), 3.73 (s, 3H), 3.03 (d, J = 9.0 Hz, 1H), 1.70 (d, J = 10.1 Hz, 2H), 1.62 (s, 2H), 1.46 (s, 1H), 1.21 (d, J = 12.8 Hz, 3H), and 1.10–1.06 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.0, 158.1, 151.9, 148.2, 133.8, 132.1, 131.4, 131.2, 129.6, 128.5, 127.4, 127.3, 125.7, 119.2, 118.3, 98.3, 68.5, 55.8, 55.6, 55.3, 33.5, 29.6, and 24.5. HRMS (ESI) m/z: [M + H]+ calcd for C26H30O3N6Br, 553.1557; found, 553.1555.
6-([1,1′-Biphenyl]-4-yl)-7-(cyclohexylamino)-2-((2,4-dimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5j)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5j (91 mg, 33%) as a yellow solid. mp 169–171 °C. Rf = 0.48 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.00 (s, 1H), 8.06 (d, J = 8.5 Hz, 2H), 7.75–7.70 (m, 4H), 7.46 (t, J = 7.8 Hz, 2H), 7.35 (t, J = 7.3 Hz, 1H), 7.27 (d, J = 8.4 Hz, 1H), 6.60 (d, J = 2.4 Hz, 1H), 6.47 (dd, J = 2.4, 8.4 Hz, 1H), 6.31 (t, J = 6.0 Hz, 1H), 4.44 (d, J = 9.0 Hz, 1H), 4.35 (d, J = 5.8 Hz, 2H), 3.84 (s, 3H), 3.74 (s, 3H), 3.16–3.06 (m, 1H), 1.74 (d, J = 11.5 Hz, 2H), 1.65–1.59 (m, 2H), 1.47 (s, 1H), 1.24 (d, J = 14.8 Hz, 3H), and 1.09 (d, J = 4.1 Hz, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.0, 158.1, 151.9, 148.2, 139.8, 137.8, 133.7, 132.1, 129.6, 129.1, 126.6, 126.5, 126.4, 126.3, 125.9, 125.6, 118.3, 104.1, 98.4 (d, J = 21.1 Hz), 68.5, 65.0, 55.8, 55.3, 33.6, 29.6, and 24.4. HRMS (ESI) m/z: [M + H]+ calcd for C32H35O3N6, 551.2765; found, 551.2762.
7-(Cyclohexylamino)-2-((2,4-dimethoxybenzyl)amino)-6-(thiophen-2-yl)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5k)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5k (103 mg, 43%) as a white solid. mp 186–189 °C. Rf = 0.41 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.97 (s, 1H), 7.41 (ddd, J = 1.3, 4.4, 9.3 Hz, 2H), 7.24 (d, J = 8.4 Hz, 1H), 7.08 (dd, J = 3.6, 5.0 Hz, 1H), 6.59 (d, J = 2.4 Hz, 1H), 6.46 (dd, J = 2.4, 8.3 Hz, 1H), 6.29 (t, J = 5.4 Hz, 1H), 4.42 (d, J = 8.7 Hz, 1H), 4.33 (d, J = 5.8 Hz, 2H), 3.83 (s, 3H), 3.73 (s, 3H), 3.25–3.17 (m, 1H), 1.75 (d, J = 12.1 Hz, 2H), 1.64 (s, 2H), 1.49 (s, 1H), 1.29–1.20 (m, 3H), and 1.12–1.09 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.0, 158.1, 151.7, 148.2, 137.8, 130.6, 129.5, 127.7 (d, J = 5.5 Hz), 126.6, 124.0 (d, J = 14.7 Hz), 122.2 (d, J = 15.6 Hz), 118.3, 104.2 (d, J = 28.9 Hz), 98.4 (d, J = 25.2 Hz), 68.5, 55.8, 55.2 (d, J = 15.6 Hz), 54.8, 33.7, 29.6 (d, J = 9.6 Hz), and 24.5. HRMS (ESI) m/z: [M + H]+ calcd for C24H29O3N6S, 481.2016; found, 481.2015.
7-(Cyclohexylamino)-6-phenyl-2-((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5l)
It was synthesized according to general procedure B on a 1 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5l (302 mg, 60%) as a white solid. mp 224–226 °C. Rf = 0.43 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.09 (s, 1H), 7.93 (dd, J = 8.3, 1.3 Hz, 2H), 7.39 (t, J = 7.9 Hz, 2H), 7.32–7.16 (m, 1H), 6.72 (s, 2H), 6.55 (s, 1H), 4.39 (d, J = 5.8 Hz, 2H), 4.33 (d, J = 9.3 Hz, 1H), 3.77 (s, 6H), 3.62 (s, 3H), 3.07–2.99 (m, 1H), 1.68 (d, J = 10.2 Hz, 2H), 1.56 (d, J = 13.1 Hz, 2H), 1.41 (d, J = 9.3 Hz, 1H), 1.15 (q, J = 11.3, 10.4 Hz, 2H), and 1.09–0.94 (m, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.9, 152.1, 148.2, 136.4, 134.8, 134.5, 131.9, 129.1, 128.3, 126.4, 125.5, 125.4, 104.5, 59.9, 56.4, 55.9, 55.8, 55.3, 55.1, 44.5, 33.4, 25.2, and 24.5. HRMS (ESI) m/z: [M + H]+ calcd for C27H33O4N6, 505.2558; found, 505.2557.
7-(Cyclohexylamino)-6-(4-methoxyphenyl)-2-((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5m)
It was synthesized according to general procedure B on a 1 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5m (187 mg, 35%) as white solid. mp 239–241 °C. Rf = 0.76 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.03 (s, 1H), 7.87 (d, J = 8.8 Hz, 2H), 6.97 (d, J = 8.8 Hz, 2H), 6.72 (s, 2H), 6.53 (s, 1H), 4.38 (d, J = 6.0 Hz, 2H), 4.23 (d, J = 9.1 Hz, 1H), 3.77 (s, 9H), 3.62 (s, 3H), 3.01 (d, J = 9.3 Hz, 1H), 1.73–1.62 (m, 2H), 1.56 (d, J = 8.5 Hz, 2H), 1.42 (d, J = 8.7 Hz, 1H), 1.14 (q, J = 8.4, 9.9 Hz, 2H), and 1.07–0.96 (m, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 157.9, 152.9, 151.9, 148.2, 136.4, 134.8, 131.0, 129.6, 127.1, 126.7, 125.1, 113.8, 104.5, 60.0, 56.4, 55.8 (d, J = 18.3 Hz), 55.4 (d, J = 31.2 Hz), 55.0 (d, J = 16.5 Hz), 44.5, 33.6, and 24.2. HRMS (ESI) m/z: [M + H]+ calcd for C28H35O5N6, 535.2663; found, 535.2658.
7-(Cyclohexylamino)-6-(2,4-dimethoxyphenyl)-2-((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5n)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5n (116 mg, 41%) as a white solid. mp 202–204 °C. Rf = 0.30 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 7.39 (d, J = 8.4 Hz, 1H), 6.67 (s, 2H), 6.65–6.60 (m, 2H), 6.58 (s, 2H), 5.12 (s, 2H), 4.20 (d, J = 10.1 Hz, 1H), 3.80 (d, J = 1.6 Hz, 6H), 3.72 (s, 6H), 3.63 (s, 3H), 3.29–3.19 (m, 1H), 1.65 (d, J = 10.7 Hz, 2H), 1.50 (d, J = 5.2 Hz, 2H), 1.42 (d, J = 10.9 Hz, 1H), and 1.09–0.93 (m, 5H). 13C {1H} NMR (126 MHz, DMSO-d6): δ 160.2, 157.1, 152.9, 152.1, 149.4, 136.8, 132.2, 131.7, 126.6, 124.2, 116.4, 105.5, 105.3, 104.8 (d, J = 15.1 Hz), 98.3, 60.0, 56.4, 55.9 (d, J = 12.8 Hz), 55.4 (t, J = 22.5 Hz), 52.1, 43.6, 33.3, 25.2, and 24.3. HRMS (ESI) m/z: [M + H]+ calcd for C29H37O6N6, 565.2769; found, 565.2765.
6-(4-Chlorophenyl)-7-(cyclohexylamino)-2-((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5o)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5o (143 mg, 53%) as a light yellow solid. mp 248–250 °C. Rf = 0.33 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6) δ 11.25 (s, 1H), 7.96 (d, J = 8.2 Hz, 2H), 7.45 (d, J = 8.2 Hz, 2H), 6.72 (s, 2H), 6.68 (d, J = 7.3 Hz, 1H), 4.39 (d, J = 6.1 Hz, 2H), 4.36 (s, 1H), 3.76 (s, 6H), 3.62 (s, 3H), 3.00 (q, J = 9.2, 8.6 Hz, 1H), 1.67 (d, J = 12.4 Hz, 2H), 1.59–1.53 (m, 2H), 1.46–1.39 (m, 1H), 1.15 (q, J = 11.3 Hz, 2H), and 1.07–0.98 (m, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.9, 152.5, 148.6, 136.4, 134.8, 133.5, 132.0, 130.6, 128.4, 128.2, 127.0, 125.8, 104.5, 59.9, 55.9, 55.3, 44.5, 33.42, 25.22, and 24.35. HRMS (ESI) m/z: [M + H]+ calcd for C27H32O4N6Cl, 539.2168; found, 539.2165.
7-(Cyclohexylamino)-6-(4-fluorophenyl)-2-((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5p)
It was synthesized according to general procedure B on a 1 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5p (271 mg, 52%) as a yellow solid. mp 242–244 °C. Rf = 0.34 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.20 (s, 1H), 7.97 (dd, J = 5.5, 9.0 Hz, 2H), 7.23 (t, J = 8.9 Hz, 2H), 6.72 (s, 2H), 6.71–6.63 (m, 1H), 4.39 (d, J = 5.7 Hz, 2H), 4.33 (d, J = 8.8 Hz, 1H), 3.76 (s, 6H), 3.62 (s, 3H), 3.05–2.90 (m, 1H), 1.67 (d, J = 9.8 Hz, 2H), 1.56 (d, J = 13.1 Hz, 2H), 1.41 (s, 1H), 1.20 (d, J = 23.3 Hz, 3H), and 1.03 (d, J = 8.4 Hz, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.9 (d, J = 243.3 Hz), 152.9, 152.3, 148.5, 136.4, 134.8, 131.5, 131.1 (d, J = 2.8 Hz), 128.6, 127.3 (d, J = 10.5 Hz), 125.6, 115.2 (d, J = 21.1 Hz), 104.5 (d, J = 13.3 Hz), 68.5, 59.9 (d, J = 6.0 Hz), 55.9, 55.8 (d, J = 10.1 Hz), 44.5, 29.6, and 24.4 (d, J = 29.4 Hz). HRMS (ESI) m/z: [M + H]+ calcd for C27H32O4N6F, 523.2464; found, 523.246.
7-(Cyclohexylamino)-6-(2-fluorophenyl)-2-((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5q)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5q (156 mg, 60%) as a yellow solid. mp 239–241 °C. Rf = 0.28 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.03 (s, 1H), 7.58 (d, J = 1.9 Hz, 1H), 7.45–7.33 (m, 1H), 7.31–7.19 (m, 2H), 6.73 (s, 2H), 6.53 (t, J = 5.8 Hz, 1H), 4.47–4.42 (m, 1H), 4.38 (d, J = 5.8 Hz, 2H), 3.76 (s, 6H), 3.62 (s, 3H), 3.18–3.06 (m, 1H), 1.63 (d, J = 8.8 Hz, 2H), 1.50 (d, J = 13.4 Hz, 2H), 1.37 (d, J = 3.8 Hz, 1H), 1.12–0.97 (m, 3H), and 0.91 (t, J = 12.5 Hz, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 159.3 (d, J = 246.1 Hz), 152.9, 151.9, 148.1, 136.4, 134.7, 133.1, 131.1 (d, J = 15.5 Hz), 129.2 (d, J = 8.3 Hz), 125.1, 124.3, 122.6 (d, J = 14.7 Hz), 121.6, 115.5, 104.6 (d, J = 13.3 Hz), 59.9 (d, J = 3.7 Hz), 55.9, 55.8, 52.9, 44.5, 33.2, 25.2, and 24.3. HRMS (ESI) m/z: [M + H]+ calcd for C27H32O4N6F, 523.2452; found, 523.2454.
7-(Cyclohexylamino)-6-(4-(trifluoromethyl)phenyl)-2-((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5r)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5r (74 mg, 26%) as yellow solid. mp 253–255 °C. Rf = 0.68 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.21 (s, 1H), 8.15 (d, J = 8.5 Hz, 2H), 7.74 (d, J = 8.7 Hz, 2H), 6.72 (s, 2H), 6.61 (t, J = 6.0 Hz, 1H), 4.51 (d, J = 9.0 Hz, 1H), 4.40 (d, J = 5.7 Hz, 2H), 3.77 (s, 6H), 3.61 (s, 3H), 3.08–2.97 (m, 1H), 1.76–1.64 (m, 2H), 1.60–1.51 (m, 2H), 1.41 (s, 1H), 1.17 (q, J = 11.3, 12.1 Hz, 2H), and 1.03 (t, J = 11.8 Hz, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.9, 152.2, 148.3, 138.6, 136.4, 134.8, 133.0, 127.7, 126.4, 126.2, 126.1, 125.6, 125.4, 125.2, 123.5, 104.5, 104.4, 60.5, 59.9, 59.4, 55.9, 55.8, 55.5, 55.3, 44.5, 33.5, 25.3, and 24.3. HRMS (ESI) m/z: [M + H]+ calcd for C28H32O4N6F3, 573.2432; found, 573.2428.
4-(7-(Cyclohexylamino)-4-oxo-2-((3,4,5-trimethoxybenzyl)amino)-3,4-dihydroimidazo[2,1-f][1,2,4]triazin-6-yl)benzenesulfonamide (5s)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5s (138 mg, 47%) as yellow solid. mp 268–270 °C. Rf = 0.38 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.22 (s, 1H), 8.12 (d, J = 8.2 Hz, 2H), 7.85 (d, J = 8.3 Hz, 2H), 7.34 (s, 2H), 6.73 (s, 2H), 6.66–6.61 (m, 1H), 4.50 (d, J = 9.1 Hz, 1H), 4.41 (d, J = 5.7 Hz, 2H), 3.78 (s, 6H), 3.63 (s, 3H), 3.04 (tt, J = 9.8, 6.1 Hz, 1H), 1.71 (d, J = 12.8 Hz, 2H), 1.58 (d, J = 11.3 Hz, 2H), 1.47–1.40 (m, 1H), 1.19 (q, J = 11.2, 10.7 Hz, 2H), and 1.06 (dt, J = 20.6, 12.2 Hz, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.9, 152.2, 148.3, 141.4, 137.8, 136.4, 134.7, 133.0, 127.8, 126.2, 125.3, 104.6, 104.5, 79.2, 79.2, 60.0, 55.9, 55.8, 55.4, 44.5, 33.5, 25.2, and 24.4. HRMS (ESI) m/z: [M + H]+ calcd for C27H34N7O6S, 584.2216; found, 584.2213.
7-(Cyclohexylamino)-6-cyclopropyl-2-((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5u)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5u (176 mg, 75%) as a yellow solid. mp 253–255 °C. Rf = 0.25 (3% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.81 (s, 1H), 6.70 (s, 2H), 6.43 (t, J = 5.8 Hz, 1H), 4.35 (d, J = 9.0 Hz, 1H), 4.32 (d, J = 5.8 Hz, 2H), 3.75 (s, 6H), 3.61 (s, 3H), 3.32 (s, 1H), 1.92 (ddd, J = 13.2, 8.3, 5.0 Hz, 1H), 1.76 (d, J = 9.1 Hz, 2H), 1.62 (d, J = 4.7 Hz, 2H), 1.47 (d, J = 7.9 Hz, 1H), 1.13 (td, J = 17.0, 14.9, 7.5 Hz, 5H), 0.78 (ddd, J = 8.0, 5.9, 3.5 Hz, 2H), and 0.73 (dt, J = 5.0, 2.7 Hz, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.8, 151.4, 147.9, 136.4, 134.8, 132.1, 131.7, 123.3, 104.6, 60.0, 59.9, 56.4, 55.9, 55.8, 55.3, 54.2, 44.5, 33.6, 24.4, and 7.2. HRMS (ESI) m/z: [M + H]+ calcd for C24H33N6O4, 469.2547; found, 469.2548.
7-(Cyclohexylamino)-6-methyl-2-((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5v)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5v (103 mg, 48%) as a gray solid. mp 167–169 °C. Rf = 0.52 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.78 (s, 1H), 6.70 (s, 2H), 6.38 (s, 1H), 4.33 (d, J = 5.8 Hz, 2H), 4.25 (d, J = 8.5 Hz, 1H), 3.76 (s, 6H), 3.62 (s, 3H), 3.21 (s, 1H), 2.15 (s, 3H), 1.72 (s, 2H), 1.61 (s, 2H), 1.49 (s, 1H), and 1.12 (d, J = 8.2 Hz, 5H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.8, 151.4, 147.9, 136.5, 134.7, 131.8, 126.3, 123.4, 104.6, 60.4, 56.5, 56.1 (d, J = 56.4 Hz), 55.5 (d, J = 56.8 Hz), 53.8 (d, J = 16.5 Hz), 44.4, 33.8, and 24.3 (d, J = 32.5 Hz). HRMS (ESI) m/z: [M + H]+ calcd for C21H31O4N6, 431.2401; found, 431.2405.
6-Phenyl-2,7-bis((3,4,5-trimethoxybenzyl)amino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (5w)
It was synthesized according to general procedure B on a 0.5 mmol scale and isolated using 3–5% MeOH/dichloromethane (v/v) to afford 5w (114 mg, 38%) as a yellow solid. mp 267–269 °C. Rf = 0.44 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.06 (s, 1H), 7.82–7.76 (m, 2H), 7.39 (t, J = 7.6 Hz, 2H), 7.25 (t, J = 7.4 Hz, 1H), 6.76 (s, 2H), 6.56 (t, J = 5.8 Hz, 1H), 6.38 (s, 2H), 5.58 (t, J = 7.0 Hz, 1H), 4.40 (d, J = 5.8 Hz, 2H), 4.29 (d, J = 6.9 Hz, 2H), 3.75 (s, 6H), 3.60 (s, 3H), 3.53 (s, 3H), and 3.50 (s, 6H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.8, 152.5, 151.9, 148.1, 136.5, 136.1, 135.9, 134.6, 134.4, 132.1, 129.0, 128.3, 126.3 (d, J = 28.6 Hz), 126.0, 125.2, 104.9, 104.3 (d, J = 23.9 Hz), 59.9, 55.9, 55.7, 55.4 (d, J = 12.9 Hz), 49.0, and 44.6. HRMS (ESI) m/z: [M + H]+ calcd for C31H35O6N7, 603.2491; found, 603.2489.
2-Amino-7-(cyclohexylamino)-6-phenylimidazo[2,1-f][1,2,4]triazin-4(3H)-one (6a)
It was synthesized according to general procedure C on a 0.5 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6a (from 5f, 42 mg, 26%; from 5l, 112 mg, 69%) as a white solid. mp 274–276 °C. Rf = 0.14 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.06 (s, 1H), 7.96 (d, J = 8.0 Hz, 2H), 7.40 (t, J = 7.7 Hz, 2H), 7.23 (t, J = 7.5 Hz, 1H), 6.14 (s, 2H), 4.21 (d, J = 8.8 Hz, 1H), 3.11 (t, J = 9.8 Hz, 1H), 1.79–1.68 (m, 2H), 1.68–1.56 (m, 2H), 1.47 (s, 1H), 1.20 (q, J = 7.1, 8.8 Hz, 2H), and 1.08 (dd, J = 7.7, 20.7 Hz, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.2, 149.6, 134.6, 131.3, 129.8, 128.3, 126.4, 125.7, 125.6, 54.8, 33.44 (t, J = 54.4 Hz), 25.3, and 24.41 (d, J = 26.6 Hz). HRMS (ESI) m/z: [M + H]+ calcd for C17H21ON6, 325.1771; found, 325.1769.
2-Amino-7-(cyclohexylamino)-6-(4-methoxyphenyl)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (6b)
It was synthesized according to general procedure C on a 0.2 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6b (42 mg, 60%) as a white solid. mp 282–284 °C. Rf = 0.25 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.98 (s, 1H), 7.90 (d, J = 9.0 Hz, 2H), 6.97 (d, J = 9.0 Hz, 2H), 6.11 (s, 2H), 4.13 (d, J = 8.5 Hz, 1H), 3.78 (s, 3H), 3.12–3.01 (m, 1H), 1.73 (d, J = 12.8 Hz, 2H), 1.64 (s, 2H), 1.48 (s, 1H), and 1.20–1.09 (m, 5H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 158.0, 152.0, 149.4, 130.4, 130.3, 127.1, 126.9 (d, J = 17.2 Hz), 125.3, 113.7, 55.6, 55.0 (t, J = 21.9, 20.0 Hz), 33.4, 25.4, and 24.5. HRMS (ESI) m/z: [M + H]+ calcd for C18H23O2N6, 355.1877; found, 355.1877.
2-Amino-6-(benzo[d][1,3]dioxol-5-yl)-7-(cyclohexylamino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (6c)
It was synthesized according to general procedure C on a 0.2 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6c (44 mg, 60%) as a brown solid. mp 250–252 °C. Rf = 0.17 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.90 (s, 1H), 7.50 (d, J = 12.6 Hz, 2H), 6.95 (d, J = 8.2 Hz, 1H), 6.15 (s, 2H), 6.03 (s, 2H), 4.16 (d, J = 8.4 Hz, 1H), 3.08 (d, J = 8.5 Hz, 1H), 1.73 (d, J = 13.7 Hz, 2H), 1.67–1.60 (m, 2H), 1.49 (s, 1H), and 1.23–1.09 (m, 5H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.1, 149.5, 147.3, 145.9, 130.5, 130.2, 128.8, 125.4, 119.2 (d, J = 19.2 Hz), 108.3, 106.0 (d, J = 21.5 Hz), 100.9, 54.9, 33.4, 25.5, and 24.3. HRMS (ESI) m/z: [M + H]+ calcd for C18H21O3N6, 369.167; found, 369.1669.
2-Amino-7-(cyclohexylamino)-6-(2,4-dimethoxyphenyl)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (6d)
It was synthesized according to general procedure C on a 0.2 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6d (from 5g, 45 mg, 59%; from 5n, 51 mg, 66%) as a brown solid. mp 298–300 °C. Rf = 0.12 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.90 (s, 1H), 7.34 (d, J = 8.4 Hz, 1H), 6.72–6.53 (m, 2H), 6.08 (s, 2H), 4.16 (d, J = 10.1 Hz, 1H), 3.80 (s, 3H), 3.79 (s, 3H), 3.16 (s, 1H), 1.69–1.59 (m, 2H), 1.49 (s, 2H), 1.40 (s, 1H), and 0.99 (dt, J = 9.1, 21.9 Hz, 5H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.2, 157.1, 151.9, 149.2, 132.2, 131.6, 125.8, 124.7, 116.5, 105.3 (d, J = 22.9 Hz), 98.4 (d, J = 19.2 Hz), 55.5, 55.2, 52.0, 33.3 (d, J = 21.5 Hz), 25.3 (d, J = 17.1 Hz), and 24.2 (d, J = 25.2 Hz). HRMS (ESI) m/z: [M + H]+ calcd for C19H25O3N6, 385.1983; found, 385.1982.
2-Amino-6-(4-chlorophenyl)-7-(cyclohexylamino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (6e)
It was synthesized according to general procedure C on a 0.3 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6e (49 mg, 46%) as a white solid. mp 296–298 °C. Rf = 0.26 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.13 (s, 1H), 7.99 (d, J = 8.7 Hz, 2H), 7.45 (d, J = 8.7 Hz, 2H), 6.17 (s, 2H), 4.26 (d, J = 8.4 Hz, 1H), 3.06 (d, J = 8.5 Hz, 1H), 1.74 (d, J = 12.5 Hz, 2H), 1.63 (s, 2H), 1.48 (s, 1H), 1.25–1.16 (m, 2H), and 1.10 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.1, 149.5, 133.5, 131.4, 130.8, 129.0, 128.3 (d, J = 8.9 Hz, 1H), 127.5–126.9 (m), 126.0, 55.1, 33.4, 25.4, and 244 (d, J = 20.7 Hz, 1H),. HRMS (ESI) m/z: [M + H]+ calcd for C17H20ON6Cl, 359.1382; found, 359.1382.
2-Amino-6-(4-bromophenyl)-7-(cyclohexylamino)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (6f)
It was synthesized according to general procedure C on a 0.2 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6f (64 mg, 80%) as a light yellow solid. mp 295–297 °C. Rf = 0.24 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.11 (s, 1H), 7.95 (d, J = 8.7 Hz, 2H), 7.60 (d, J = 8.5 Hz, 2H), 6.17 (s, 2H), 4.27 (d, J = 8.4 Hz, 1H), 1.75 (d, J = 12.3 Hz, 2H), 1.64 (s, 2H), 1.50 (s, 1H), and 1.24–1.11 (m, 5H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.1, 149.5, 133.8, 131.5, 131.2 (d, J = 23.7 Hz), 129.1, 127.5 (d, J = 19.2 Hz), 126.0, 119.3, 55.1, 33.4, 25.4, and 24.4. HRMS (ESI) m/z: [M + H]+ calcd for C17H20ON6Br, 403.0876; found, 403.0874.
2-Amino-7-(cyclohexylamino)-6-(4-fluorophenyl)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (6g)
It was synthesized according to general procedure C on a 0.3 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6g (66 mg, 64%) as a white solid. mp > 300 °C. Rf = 0.28 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.06 (s, 1H), 8.00 (dd, J = 5.7, 9.0 Hz, 2H), 7.23 (t, J = 8.9 Hz, 2H), 6.14 (s, 2H), 4.22 (d, J = 8.4 Hz, 1H), 3.16–2.99 (m, 1H), 1.74 (d, J = 13.9 Hz, 2H), 1.63 (t, J = 4.9 Hz, 2H), 1.48 (s, 1H), 1.23–1.16 (m, 2H), and 1.14–1.03 (m, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.9 (d, J = 243.3 Hz), 152.2, 149.5, 131.1 (d, J = 2.9 Hz), 130.9, 129.4, 127.5 (d, J = 5.8 Hz), 125.7, 115.1 (d, J = 21.5 Hz), 54.9, 33.3, 25.9, and 24.2. HRMS (ESI) m/z: [M + H]+ calcd for C17H20ON6F, 343.1677; found, 343.1676.
2-Amino-7-(cyclohexylamino)-6-(4-(trifluoromethyl)phenyl)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (6h)
It was synthesized according to general procedure C on a 0.2 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6h (47 mg, 60%) as a white solid. mp > 300 °C. Rf = 0.57 (20% Acetone/Dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.16 (s, 1H), 8.19 (d, J = 8.4 Hz, 2H), 7.75 (d, J = 8.7 Hz, 2H), 6.22 (s, 2H), 4.37 (d, J = 8.5 Hz, 1H), 3.11 (t, J = 9.2 Hz, 1H), 1.79–1.73 (m, 2H), 1.67–1.60 (m, 2H), 1.49 (s, 1H), and 1.17 (d, J = 52.8 Hz, 5H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.3, 149.7, 138.6, 132.3, 128.5, 126.4, 126.2, 125.7 (dd, J = 16.4, 23.6 Hz), 125.3 (d, J = 28.0 Hz), 123.5, 54.9, 33.4, 25.4, and 24.3. HRMS (ESI) m/z: [M + H]+ calcd for C18H20ON6F3, 393.1645; found, 393.1645.
4-(2-Amino-7-(cyclohexylamino)-4-oxo-3,4-dihydroimidazo[2,1-f][1,2,4]triazin-6-yl)benzenesulfonamide (6i)
It was synthesized according to general procedure C on a 0.2 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6i (44 mg, 54%) as a white solid. mp 177–180 °C. Rf = 0.18 (7% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.13 (s, 1H), 8.14 (d, J = 8.1 Hz, 2H), 7.86–7.81 (m, 2H), 7.33 (s, 2H), 6.19 (s, 2H), 4.37 (d, J = 8.5 Hz, 1H), 3.13–3.04 (m, 1H), 1.76 (d, J = 12.6 Hz, 2H), 1.67–1.60 (m, 2H), 1.48 (s, 1H), 1.21 (dd, J = 16.0, 7.3 Hz, 2H), and 1.12 (d, J = 8.4 Hz, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.3, 149.6, 141.4, 137.9, 132.4, 128.6, 126.4, 125.9, 125.8, 125.4, 55.3, 33.4, 25.4, 24.5, and 24.3. HRMS (ESI) m/z: [M + H]+ calcd for C17H22O3N7S, 404.1430; found, 404.1427.
2-Amino-7-(cyclohexylamino)-6-cyclopropylimidazo[2,1-f][1,2,4]triazin-4(3H)-one (6j)
It was synthesized according to general procedure C on a 0.5 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6j (65 mg, 45%) as a white solid. mp 127–130 °C. Rf = 0.11 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.77 (s, 1H), 6.00 (s, 2H), 4.18 (d, J = 8.8 Hz, 1H), 3.31 (s, 1H), 1.93 (ddd, J = 5.1, 8.2, 13.2 Hz, 1H), 1.82 (d, J = 9.8 Hz, 2H), 1.72–1.63 (m, 2H), 1.54 (d, J = 12.5 Hz, 1H), 1.24–1.13 (m, 5H), 0.79 (dt, J = 2.7, 8.2 Hz, 2H), and 0.75 (dt, J = 2.5, 4.9 Hz, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 151.5, 149.2, 132.5, 131.4, 123.6, 55.8, 55.0, 54.2, 33.6, 24.9 (d, J = 121.0 Hz), 8.0 (d, J = 17.9 Hz), and 7.2. HRMS (ESI) m/z: [M + H]+ calcd for C14H21ON6, 289.1771; found, 289.1770.
N-(2-(Benzylamino)-4-oxo-6-phenyl-3,4-dihydroimidazo[2,1-f][1,2,4]triazin-7-yl)-2,2,2-trifluoroacetamide (6l)
It was synthesized according to general procedure C on a 0.5 mmol scale and isolated using 3–7% MeOH/dichloromethane (v/v) to afford 6l (96 mg, 45%) as a white solid. mp 248–250 °C. Rf = 0.16 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 11.77 (s, 1H), 11.60 (s, 1H), 7.81–7.76 (m, 2H), 7.46 (t, J = 7.7 Hz, 2H), 7.38–7.34 (m, 3H), 7.31 (dd, J = 6.5, 8.4 Hz, 2H), 7.28–7.22 (m, 1H), 6.81 (t, J = 5.9 Hz, 1H), and 4.35 (d, J = 5.8 Hz, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 156.5 (q, J = 37.3 Hz), 152.2, 149.1, 138.7, 135.9, 132.5, 128.9, 128.8 (d, J = 7.7 Hz),128.3, 127.9, 127.2, 125.9, 125.7, 117.0, 114.7, and 44.3. HRMS (ESI) m/z: [M + H]+ calcd for C20H16O2N6F3, 429.1281; found, 429.1279.
2,7-Diamino-6-phenylimidazo[2,1-f][1,2,4]triazin-4(3H)-one (6m)
It was synthesized according to general procedure D on a 0.2 mmol scale and isolated using 3–10% MeOH/dichloromethane (v/v) to afford 6m (from 5f, 17 mg, 14%; from 5l, 87 mg, 72%) as a gray solid. mp 260–262 °C. Rf = 0.13 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.90 (s, 1H), 7.83 (d, J = 7.2 Hz, 2H), 7.57–7.28 (m, 2H), 7.25–7.08 (m, 1H), 6.10 (s, 2H), and 5.29 (s, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.0, 149.5, 134.7, 132.2, 128.4, 125.6, 124.9 (d, J = 16.0 Hz)., 123.9, and 123.7. HRMS (ESI) m/z: [M + H]+ calcd for C11H11ON6, 243.0989; found, 243.0988.
2,7-Diamino-6-(4-chlorophenyl)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (6n)
It was synthesized according to general procedure D on a 0.2 mmol scale and isolated using 3–10% MeOH/dichloromethane (v/v) to afford 6n (28 mg, 51%) as a dark green solid. mp 312–314 °C. Rf = 0.25 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.90 (s, 1H), 7.91–7.75 (m, 2H), 7.51–7.31 (m, 2H), 6.09 (s, 2H), and 5.36 (s, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 151.9, 149.4, 133.6, 132.4, 129.8, 128.3, 126.44 (d, J = 12.8 Hz), 124.1, and 122.5. HRMS (ESI) m/z: [M + H]+ calcd for C11H10 ON6Cl, 277.0599; found, 277.0597.
2,7-Diamino-6-(4-fluorophenyl)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (6o)
It was synthesized according to general procedure D on a 0.1 mmol scale and isolated using 3–10% MeOH/dichloromethane (v/v) to afford 6o (15 mg, 58%) as a brown solid. mp 273–275 °C. Rf = 0.11 (5% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.92 (s, 1H), 7.88–7.81 (m, 2H), 7.21 (t, J = 8.9 Hz, 2H), 6.10 (s, 2H), and 5.28 (s, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.5 (d, J = 242.4 Hz), 152.0, 149.5, 131.9, 131.2 (d, J = 3.2 Hz), 126.7 (d, J = 7.0 Hz), 123.9, 123.0, and 115.2 (d, J = 21.1 Hz). HRMS (ESI) m/z: [M + H]+ calcd for C11H10ON6F, 261.0895; found, 261.0892.
2,7-Diamino-6-(4-(trifluoromethyl)phenyl)imidazo[2,1-f][1,2,4]triazin-4(3H)-one (6p)
It was synthesized according to general procedure D on a 0.2 mmol scale and isolated using 3–10% MeOH/dichloromethane (v/v) to afford 6p (42 mg, 68%) as a dark green solid. mp >300 °C. Rf = 0.62 (10% MeOH/dichloromethane). 1H NMR (500 MHz, DMSO-d6): δ 10.92 (s, 1H), 8.03 (d, J = 8.5 Hz, 2H), 7.70 (d, J = 8.5 Hz, 2H), 6.13 (s, 2H), and 5.50 (s, 2H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 152.1, 149.5, 138.7, 133.4, 125.6 (d, J = 27.0 Hz), 125.2 (dd, J = 7.2, 13.9 Hz), 124.9 (d, J = 8.2 Hz), 124.6, 123.5, and 121.8. HRMS (ESI) m/z: [M + H]+ calcd for C12H10ON6F3, 311.0863; found, 311.0862.
Acknowledgments
We thank Marcel de Vries (University of Groningen) for his help in HRMS analysis. Xin Li acknowledges the China Scholarship Council for support.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c00467.
Experimental procedure and 1H and 13C{1H} NMR spectra for all compounds along with the X-ray crystallographic data for 4d and 5l (PDF)
Author Contributions
Alexander Dömling and Xin Li conceptualized the study and designed the methodology. Xin Li, Marina Diguele Romero, and Sona Tcaturian performed the experiments. Xin Li analyzed the data. Katarzyna Kurpiewska performed the crystallographic studies. Alexander Dömling and Xin Li wrote the manuscript. Alexander Dömling received the funding. All authors approved the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Unger B. Das Guanin und seine Verbindungen” (Guanine and its compounds). Ann. Chem. Pharm. 1846, 59, 58–68. 10.1002/jlac.18460590108. [DOI] [Google Scholar]
- a Yadav P.; Owiti N.; Kim N. The role of topoisomerase I in suppressing genome instability associated with a highly transcribed guanine-rich sequence is not restricted to preventing RNA: DNA hybrid accumulation. Nucleic Acids Res. 2016, 44, 718–729. 10.1093/nar/gkv1152. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Doluca O.; Withers J. M.; Filichev V. V. Molecular engineering of guanine-rich sequences: Z-DNA, DNA triplexes, and G-quadruplexes. Chem. Rev. 2013, 113, 3044–3083. 10.1021/cr300225q. [DOI] [PubMed] [Google Scholar]
- a Syrovatkina V.; Alegre K. O.; Dey R.; Huang X. Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. 10.1016/j.jmb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Colicelli J. Human RAS superfamily proteins and related GTPases. Sci. STKE 2004, 2004, Re13. 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Huang Z.; Xie N.; Illes P.; Di Virgilio F.; Ulrich H.; Semyanov A.; Verkhratsky A.; Sperlagh B.; Yu S.-G.; Huang C.; Tang Y. From purines to purinergic signalling: molecular functions and human diseases. Sig. Transduct. Target Ther. 2021, 6, 162. 10.1038/s41392-021-00553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a OʼBrien J. J.; Campoli-Richards D. M. Acyclovir. Drugs. 1989, 37, 233–309. 10.2165/00003495-198937030-00002. [DOI] [PubMed] [Google Scholar]; b Wood A. J.; Whitley R. J.; Gnann J. W. Acyclovir: a decade later. N. Engl. J. Med. 1992, 327, 782–789. 10.1056/nejm199209103271108. [DOI] [PubMed] [Google Scholar]; c Parks R.; Agarwal K.. 8-Azaguanine. Antineoplastic and Immunosuppressive Agents; Springer, 1975; pp 458–467. [Google Scholar]; d Nelson J. A.; Carpenter J. W.; Rose L. M.; Adamson D. J. Mechanisms of action of 6-thioguanine, 6-mercaptopurine, and 8-azaguanine. Cancer Res. 1975, 35, 2872. [PubMed] [Google Scholar]
- a Sessler J. L.; Magda D.; Furuta H. Synthesis and binding properties of monomeric and dimeric guanine and cytosine amine derivatives. J. Org. Chem. 1992, 57, 818–826. 10.1021/jo00029a008. [DOI] [Google Scholar]; b Yu K. L.; Bronson J. J.; Yang H.; Patick A.; Alam M.; Brankovan V.; Datema R.; Hitchcock M. J.; Martin J. C. Synthesis and antiviral activity of methyl derivatives of 9-[2-(phosphonomethoxy) ethyl] guanine. J. Med. Chem. 1992, 35, 2958–2969. 10.1021/jm00094a005. [DOI] [PubMed] [Google Scholar]
- Boltjes A.; Dömling A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019, 2019, 7007–7049. 10.1002/ejoc.201901124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin Was Used for Drawing, Displaying and Characterizing Chemical Structures, Substructures and Reactions, Marvin n.n.n (version number), 201n (insert year of version release), ChemAxon; (http://www.chemaxon.com).; b Stack D. E. Identifying the tautomeric form of a deoxyguanosine-estrogen quinone intermediate. Metabolites 2015, 5, 475–488. 10.3390/metabo5030475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Geffken D.; Köllner M. A. Synthesis of 2-Amino-Imidazo [1, 2-b] [1, 2, 4] Triazine-3, 6, 7 (5H)-Triones. Heterocycl. Commun. 2010, 16, 145–150. 10.1515/hc.2010.16.2-3.145. [DOI] [Google Scholar]; b Geffken D.; Köllner M. A. Synthese und Eigenschaften 3,6-diaminosubstituierter 1,2,4-Triazin-5(2H)-one/Synthesis and Properties of 3,6-Diamino-substituted 1,2,4-Triazin-5(2H)-ones. Zeitschrift für Naturforschung B 2010, 65, 571–577. 10.1515/znb-2010-0506. [DOI] [Google Scholar]
- a Patterson J. R.; Graves A. P.; Stoy P.; Cheung M.; Desai T. A.; Fries H.; Gatto G. J.; Holt D. A.; Shewchuk L.; Totoritis R.; Wang L. P.; Kallander L. S. Identification of Diarylurea Inhibitors of the Cardiac-Specific Kinase TNNI3K by Designing Selectivity Against VEGFR2, p38α, and B-Raf. J. Med. Chem. 2021, 64, 15651–15670. 10.1021/acs.jmedchem.1c00700. [DOI] [PubMed] [Google Scholar]; b Rickmeier J.; Ritter T. Site-Specific Deoxyfluorination of Small Peptides with [18F]Fluoride. Angew. Chem., Int. Ed. 2018, 57, 14207–14211. 10.1002/anie.201807983. [DOI] [PubMed] [Google Scholar]; c Mackman R. L.; Mish M.; Chin G.; Perry J. K.; Appleby T.; Aktoudianakis V.; Metobo S.; Pyun P.; Niu C.; Daffis S.; Yu H.; Zheng J.; Villasenor A. G.; Zablocki J.; Chamberlain J.; Jin H. L.; Lee G.; Suekawa-Pirrone K.; Santos R.; Delaney W. E.; Fletcher S. P. Discovery of GS-9688 (Selgantolimod) as a Potent and Selective Oral Toll-Like Receptor 8 Agonist for the Treatment of Chronic Hepatitis B. J. Med. Chem. 2020, 63, 10188–10203. 10.1021/acs.jmedchem.0c00100. [DOI] [PubMed] [Google Scholar]
- a Tam J. P.; Heath W. F.; Merrifield R. B. Mechanisms for the removal of benzyl protecting groups in synthetic peptides by trifluoromethanesulfonic acid-trifluoroacetic acid-dimethyl sulfide. J. Am. Chem. Soc. 1986, 108, 5242–5251. 10.1021/ja00277a031. [DOI] [Google Scholar]; b Jung M. E.; Koch P. Mild, selective deprotection of PMB ethers with triflic acid/1, 3-dimethoxybenzene. Tetrahedron Lett. 2011, 52, 6051–6054. 10.1016/j.tetlet.2011.08.102. [DOI] [Google Scholar]; c Javorskis T.; Orentas E. Chemoselective Deprotection of Sulfonamides Under Acidic Conditions: Scope, Sulfonyl Group Migration, and Synthetic Applications. J. Org. Chem. 2017, 82, 13423–13439. 10.1021/acs.joc.7b02507. [DOI] [PubMed] [Google Scholar]
- Patil P.; Ahmadian-Moghaddam M.; Dömling A. Isocyanide 2.0. Green Chem. 2020, 22, 6902–6911. 10.1039/d0gc02722g. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.