Abstract
This study reports the first example of using a dual catalytic system with copper(II) acetate and 2-hydroxynicotinaldehyde to achieve transient C(sp2)–H sulfonylation of benzylamines with sulfinate salts via a dynamically formed imine directing group. Manganese(IV) oxide was identified as an effective oxidant and base. Computational density functional theory investigations suggest that the transient directing group lowers the energy barrier for an acetate-mediated, turnover-limiting C–H activation step and subsequent combination of the cupracycle with a RSO2 radical.
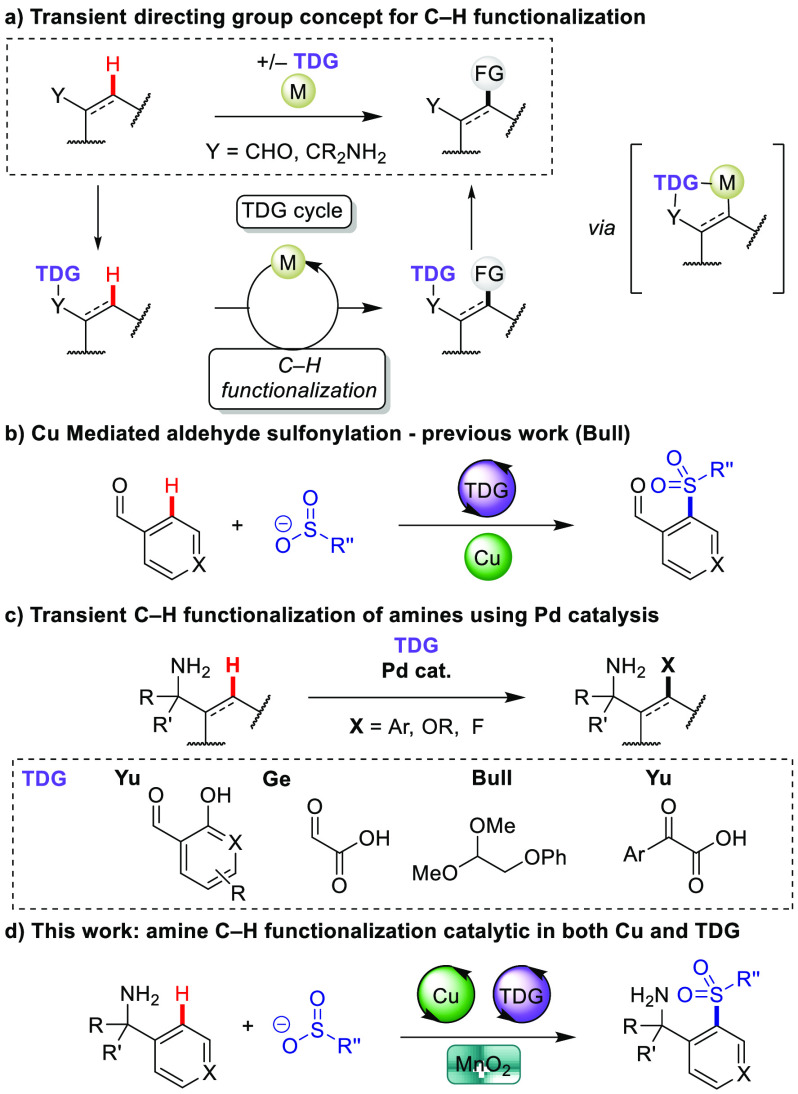
Developments in the functionalization of C–H bonds continue to streamline synthetic routes to medicinal compounds and materials.1 Transient C–H functionalization, involving an in situ formed transient directing group (TDG) from common functionality, presents additional opportunities for efficient synthesis by avoiding steps for directing group installation and removal (Figure 1a).2 Pioneering work by Jun et al.3 and Yu and co-workers4 established the potential for aldehydic C–H and benzylic C–H functionalization, respectively, with imine directing groups. Subsequent developments have enabled palladium-catalyzed C–H functionalization of benzaldehydes and aliphatic aldehydes,5 with fewer examples on amines.6−15 These approaches directly reveal useful functionality for further derivatization. To date, palladium and other precious metal catalysts have been employed almost exclusively. Given the increasing price and undesirable toxicity profile of Pd, the development of new methods relying on cheap and readily available base metals is crucial to sustainable synthesis. We recently reported the first example of copper-mediated transient C–H functionalization in the sulfonylation of benzaldehydes with sulfinate salts. β-Alanine was used as a catalytic TDG (Figure 1b).16
Figure 1.
Concept of C–H functionalization using transient directing groups and copper-catalyzed transient C–H functionalization.
Amine functionalities feature in countless pharmaceutically active compounds and fine chemicals17 and present additional challenges for C–H functionalization as a result of coordinative poisoning of metal catalysts. There are reports of free amine-directed C(sp2)–H functionalization;18 however, robust amide and sulfonamide directing groups have been used most commonly.19,20 Karmakar and Samanta reported the palladium-catalyzed C–H sulfonylation of benzylamines with sulfinate salts using picolinamide as a directing group.21
All prior reports using TDGs with amine substrates have involved palladium catalysis (Figure 1c).2 Notably, Yu and co-workers developed 2-hydroxynicotinaldehyde as a powerful TDG for Pd-catalyzed C(sp3)–H arylation,6 oxygenation,7 and fluorination8 of amines. The use of this TDG for Pd-catalyzed C–H arylation of amines was also described by Kameneka and co-workers for alkyl and benzylamines.9 Other catalytic TDGs for amine functionalization include glyoxylic acid developed by Liu and Ge,10 aryl keto acids for δ-arylation,11 and acetal ethers.12 There are no examples of C–S bond formation on amine substrates using TDGs. Given the value of sulfones in medicinal chemistry,22 we envisaged the direct C–H sulfonylation of amine precursors to form valuable amino sulfone building blocks. Here, we report a dual copper/TDG-catalyzed C(sp2)–H sulfonylation of benzylamines using MnO2 as the terminal oxidant. This represents the first C–S-bond-forming transient C–H functionalization methodology for amines and the first example of sub-stoichiometric copper salt being used with a TDG. Computational studies reveal the mechanistic features of the reaction.
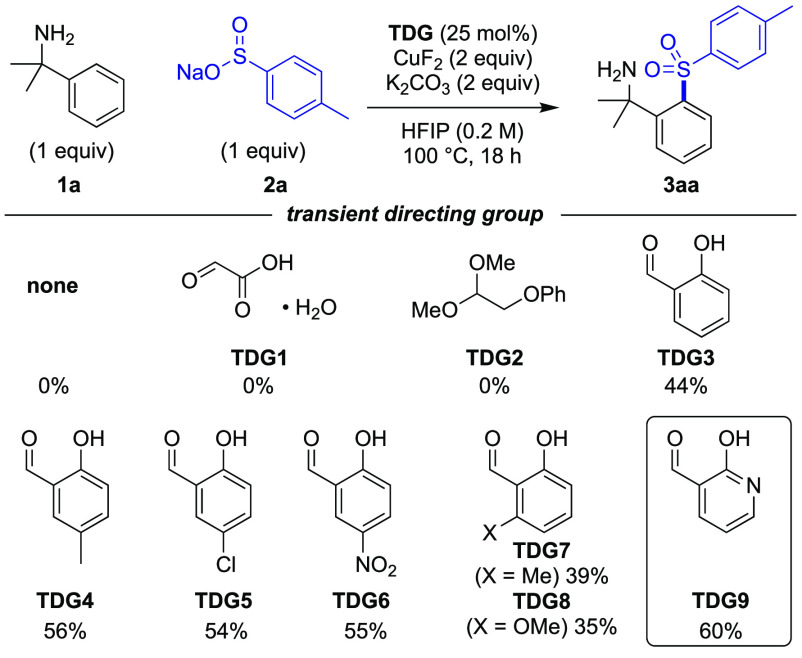
We first examined different aldehydes to function as the TDG (Scheme 1). A catalytic TDG (25 mol %) along with a stoichiometric quantity of copper fluoride was employed. Hexafluoro-2-propanol (HFIP) was used as the solvent at 100 °C in the presence of K2CO3, with 1 equiv of p-tolylSO2Na. No reaction was observed in the absence of a TDG nor using glyoxalic acid TDG1 or 2-phenoxyacetaldehyde dimethyl acetal TDG2. Pleasingly, salicaldehyde (TDG3) afforded compound 3aa in a 44% yield. 5-Substituted salicaldehydes (TDG4–TDG6) gave similar improved yields (54–56%), whereas 6-substituted derivatives were less effective (TDG7 and TDG8). 2-Hydroxynicotinaldehyde (TDG9) was the most effective TDG, affording compound 3aa in a 60% yield.
Scheme 1. Optimization of the TDG.
Reactions were performed on a 0.2 mmol scale. Yields were determined by 1H nuclear magnetic resonance (NMR) using 1,3,5-trimethoxybenzene as an internal standard.
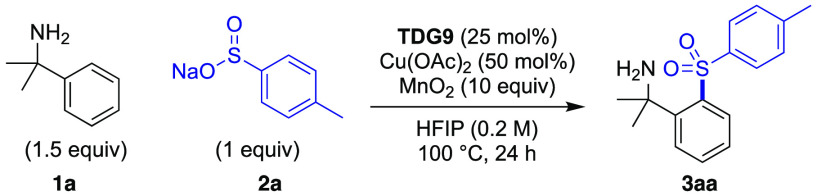
Encouraged by these results, different copper sources, co-oxidants, TDG loading, concentration, and base were investigated to develop a catalytic process.23 Inexpensive and readily available copper(II) acetate in combination with MnO2 was identified as an effective catalyst system. Furthermore, MnO2 acts as both a base and oxidant, avoiding the need for an additional base. Under these optimized conditions, compound 3aa was isolated in 68% yield (entry 1 in Table 1).24 Cu(OAc)2 was critical for the coupling, with one turnover observed in the absence of an oxidant (entries 2 and 3).24 In the absence of TDG9, a low but non-zero yield was obtained (entry 4). Testing other TDGs under the catalytic conditions showed the same trend as that using stoichiometric copper (entries 5–7). Changing the oxidant to K2S2O8 was detrimental (entry 8). The addition of K2CO3 gave no change in the isolated yield (entry 9). The addition of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) as a radical trap fully suppressed the reaction, suggesting a radical reaction pathway in operation (entry 10). Notably, the formation of sulfonamide was never observed, despite the potential for direct coupling with the amine moiety.25
Table 1. Control Reactions Describing Deviation from Standard Conditions Using Catalytic Copper and TDGa.
| entry | deviation from standard conditions | yield of compound 3aa (%)b |
|---|---|---|
| 1 | none | 67 (68) |
| 2 | no [Cu] | 0 |
| 3 | no MnO2 | 17 |
| 4 | no TDG | 15 |
| 5 | using TDG1 | 5 |
| 6 | using TDG2 | 0 |
| 7 | using TDG3 | 43 |
| 8 | K2S2O8 as an oxidant (2–10 equiv) | 11–21 |
| 9 | + K2CO3 (2 equiv) | 70 (68) |
| 10 | + TEMPO (1 equiv) | 0 |
Reactions were performed on a 0.2 mmol scale with respect to the sulfinate salt.
Yields were determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. Isolated yields are in parentheses. The starting material is volatile; therefore, recovered starting material was not reliably determined.
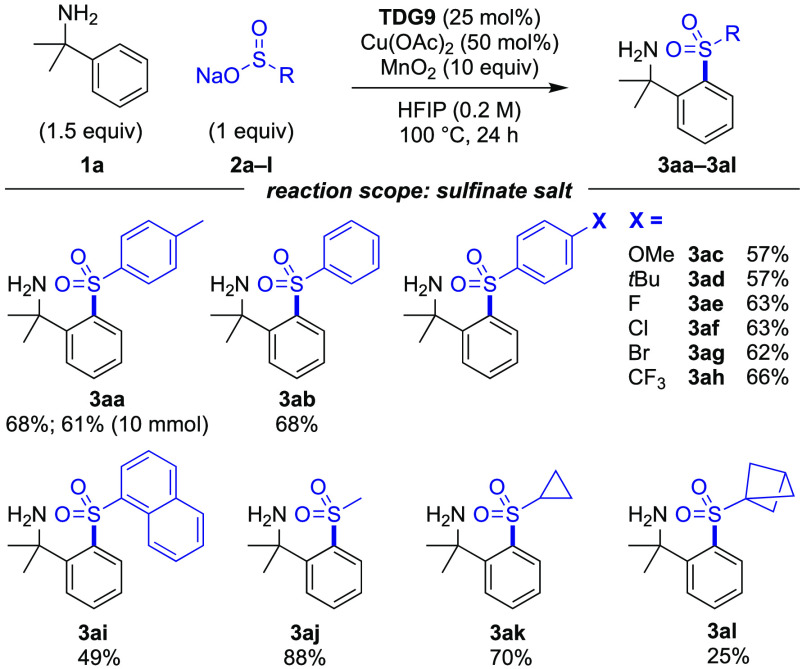
The reaction scope varying the sulfinate salt was then investigated (Scheme 2). Compound 3aa was obtained in a 68% yield, which was readily scaled affording the product in a 61% yield (1.76 g). Aryl sulfinates bearing electron-neutral (H), electron-rich (OMe and tBu), or electron-poor (CF3 and halogens) substituents all gave good yields with a slight preference for the electron-poor sulfinate salts (3ab–3ah). More sterically hindered naphthyl-substituted example 3ai was less effective. Methyl and cyclopropyl sulfinate salts were both highly effective, affording compounds 3aj and 3ak in 88 and 70% yields, respectively. The reaction with the bicyclo[1.1.1]pentane (BCP) sulfinate salt was also successful in generating sulfone 3al.
Scheme 2. Reaction Scope Varying the Sulfinate Salt.
Reactions were performed on a 0.2 mmol scale, with isolated yields reported.
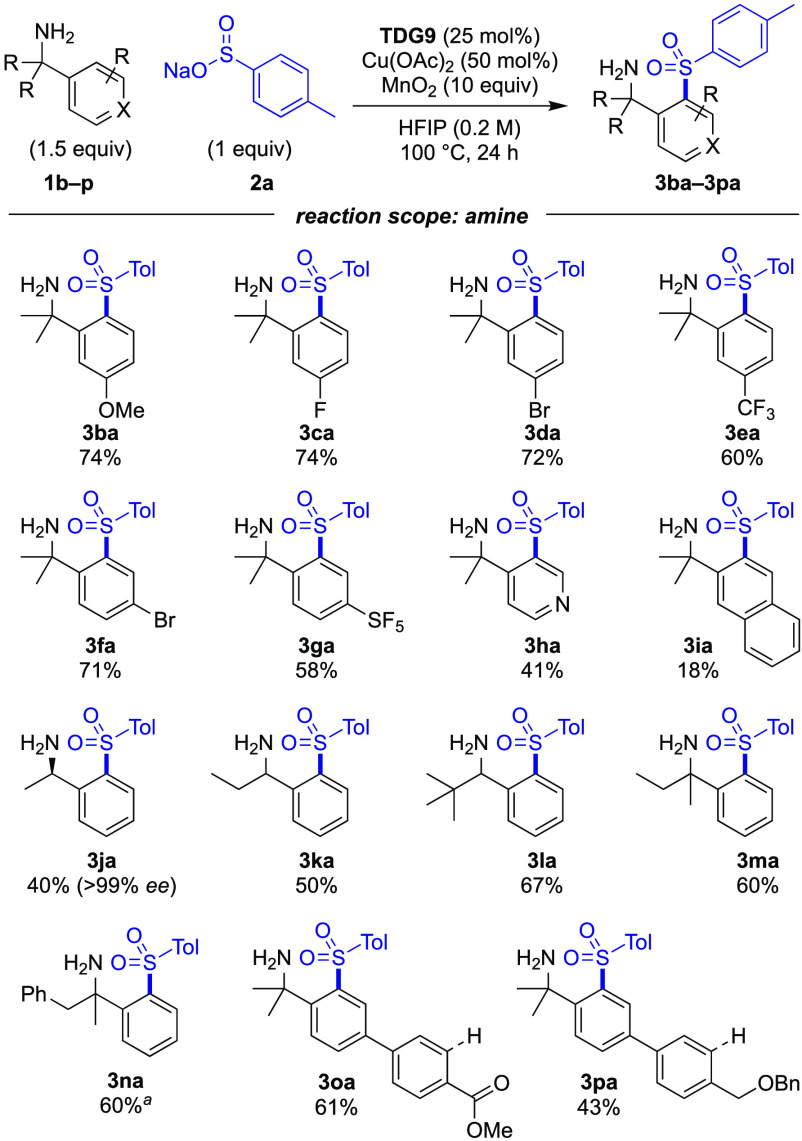
Next, the benzylamine component was investigated (Scheme 3). Initially, we varied the substituent in the 3 position (para to the C–H bond being functionalized), whereby substrates with electron-rich and electron-poor substituents reacted effectively (3ba–3ea), with slightly improved yields for the 3-OMe (3ba) and 3-F-phenyl (3ca) derivatives. Changing the bromo substituent from the 3 to 4 position gave sulfonyl amine 3fa in a 71% yield. The pentafluorosulfanyl (SF5) group is increasingly of interest in medicinal chemistry, and SF5-substituted benzylamine was effectively sulfonylated to give amine 3ga in a 58% yield. The functionalization of the more challenging pyridyl-containing substrate 3ha was also realized, despite the presence of the additional coordinating moiety.
Scheme 3. Reaction Scope Varying the Amine.
Contains 6% inseparable starting material.
Reactions were performed on a 0.2 mmol scale, with isolated yields reported.
2-Naphthyl derivative 3ia was formed with a selective reaction at the 3 position. A range of α-alkylbenzylamines were also converted to the sulfonylated products 3ja, 3ka, and 3la in good yields. Sulfonylation of enantioenriched amine retained the chirality of the starting amine [>99% enantiomeric excess (ee)]. However, unsubstituted benzylamines were unsuccessful. Ethyl- and benzyl-substituted amines were sulfonylated exclusively at the ortho position to give sulfones 3ma and 3na in a 60% yield. In the benzyl-substituted example, no sulfonylation of the more distal aryl group was observed. Biaryl substrates 3oa and 3pa possessing functional groups capable of directing ortho-metalation were sulfonylated exclusively at the ortho position to the amine without any sulfonylation adjacent to either the ester or the ether functionality. Furthermore, both the methyl ester and benzyl groups remained intact under these conditions. In all cases, only monosulfonylation was observed.
The amine products generated in this transient process were directly available for further derivatization (Scheme 4). To illustrate this, sulfone 3aa was readily acetylated with acetyl chloride to form amide 4 and was converted to aminooxetane 5 using an oxetane sulfonyl fluoride reagent in a defluorosulfonylative process.26 Reductive alkylation and nucleophilic aromatic substitution (SNAr) reactions, as commonly employed in medicinal chemistry programs, were also readily demonstrated to provide alkyl amine 6 and aryl amine 7.
Scheme 4. Derivatization of the Amine Functionality.
Reactions were performed on a 0.20 mmol scale, with isolated yields reported. Reaction conditions: (i) AcCl, NEt3, CH2Cl2, room temperature, 18 h; (ii) 3-(4-methoxyphenyl)-3-oxetanesulfonyl fluoride, K2CO3, MeCN, 80 °C, 1 h; (iii) cyclohexanecarboxaldehyde, NaBH(OAc)3, DCE, room temperature, 24 h; and (iv) methyl 4-fluoro-3-nitrobenzoate, iPrOH, 100 °C, 4 h.
To provide insight into the reaction mechanism, a competition kinetic isotope effect (KIE) experiment gave a preferential reaction of the protic substrate [H/D of 3.88]. Similarly, H/D exchange was not observed in the product or recovered starting materials when running the reaction with either a deuterated substrate or the protic substrate in d2-HFIP.27 These results were suggestive of an irreversible C–H functionalization process under the reaction conditions, that is, the turnover-limiting step.
We then investigated elementary steps through density functional theory (DFT) calculations (Figure S1 of the Supporting Information).28−30,23 The C–H activation to form a cupracycle was calculated to proceed via a 5-coordinate inner sphere transition state (TS-3/4), in which an axial acetate ligand mediates C–H activation, with a barrier of 24.1 kcal mol–1. Natural bond orbital (NBO) analysis of the transition state revealed a charge distribution and geometry similar to those of an arenium ion, in addition to a significant stabilizing effect from donation from the C–Cπ system into empty orbitals on Cu. This is indictive of a Wheland-like transition state for C–H activation, consistent with our previous mechanistic studies on the copper-mediated transient C–H functionalization of aldehydes.31 Noticeably the energy barrier of the concerted metalation–deprotonation (CMD) step is significantly lowered in the presence of the TDG when compared to the free amine-directed reaction (TDG, +24.1 kcal mol–1; free amine, +32.2 kcal mol–1; see the Supporting Information). The influence of the TDG is therefore in the provision of improved ligation properties to promote the CMD.
Association of the sulfinyl radical to the copper center occurs in a barrierless process (Int-5–Int-7; Figure S1 of the Supporting Information).32 Oxidation of the sulfinate salt to the radical was calculated to occur readily by a single-electron transfer (SET) process, mediated by copper acetate.33 Cyclic voltammetry (CV) studies indicate that the sulfinate salt can be oxidized in the redox window of the reaction (CVs in HFIP versus Fc/Fc+: MeSO2Na, Epa = +1.02 V; TolSO2Na, Epa = +1.06 V). Reductive elimination forms the C–S bond and a CuI species, with a barrier of +21.2 kcal mol–1.
In summary, C–H sulfonylation of benzylamines has been achieved using both catalytic copper acetate and a catalytic aldehyde TDG to form γ-sulfonyl amines. Earth abundant and cheap manganese dioxide was used as a stoichiometric oxidant and base. Selective reactivity was maintained in the presence of other coordinating functionalities, and the sulfonyl amine products were readily diversified. A significant role of the TDG is to lower the barrier for C–H activation and formation of the cupracycle through an inner sphere CMD step, involving a Wheland-type intermediate.
Acknowledgments
The authors gratefully acknowledge The Royal Society [University Research Fellowship, UF140161 and URF\R\201019 (to James A. Bull), URF Appointed Grant RG150444, and URF Enhancement Grant RGF\EA\180031]. The authors gratefully acknowledge Dr. Andreas Phanopoulos (Department of Chemistry, Imperial College London) for guidance in DFT computational methods.
Data Availability Statement
Raw and processed characterization data for all novel compounds and Cartesian coordinates from computed structures can be found at the Imperial College London Research Data Repository: 10.14469/hpc/12033. A version of this manuscript was deposited on the preprint repository ChemRxiv.34 The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c01783.
Optimization reactions, deuteration experiments, KIE experiments, unsuccessful substrates, details of computational studies, and experimental procedures and characterization data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Yamaguchi J.; Yamaguchi A. D.; Itami K. C–H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; b He J.; Wasa M.; Chan K. S. L.; Shao Q.; Yu J. Q. Palladium-Catalyzed Transformations of Alkyl C–H Bonds. Chem. Rev. 2017, 117, 8754–8786. 10.1021/acs.chemrev.6b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lam N. Y. S.; Wu K.; Yu J. Q. Advancing the Logic of Chemical Synthesis: C–H Activation as Strategic and Tactical Disconnections for C–C Bond Construction. Angew. Chem., Int. Ed. 2021, 60, 15767–15790. 10.1002/anie.202011901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews of transient C–H functionalization, see; a Gandeepan P.; Ackermann L. Transient Directing Groups for Transformative C–H Activation by Synergistic Metal Catalysis. Chem. 2018, 4, 199–222. 10.1016/j.chempr.2017.11.002. [DOI] [Google Scholar]; b St John-Campbell S.; Bull J. A. Transient Imines as “Next Generation” Directing Groups for the Catalytic Functionalisation of C–H Bonds in a Single Operation. Org. Biomol. Chem. 2018, 16, 4582–4595. 10.1039/C8OB00926K. [DOI] [PubMed] [Google Scholar]; c Niu B.; Yang K.; Lawrence B.; Ge H. Transient Ligand-Enabled Transition Metal-Catalyzed C–H Functionalization. ChemSusChem 2019, 12, 2955–2969. 10.1002/cssc.201900151. [DOI] [PubMed] [Google Scholar]; d Higham J. I.; Bull J. A. Transient Imine Directing Groups for the C–H Functionalisation of Aldehydes, Ketones and Amines: An Update 2018–2020. Org. Biomol. Chem. 2020, 18, 7291–7315. 10.1039/D0OB01587C. [DOI] [PubMed] [Google Scholar]; e Goswami N.; Bhattacharya T.; Maiti D. Transient directing ligands for selective metal-catalysed C–H activation. Nat. Chem. Rev. 2021, 5, 646–659. 10.1038/s41570-021-00311-3. [DOI] [PubMed] [Google Scholar]
- Jun C. H.; Lee D. Y.; Hong J. B. Hydroacylation of 1-Alkene with Heteroaromatic Aldehyde by Rh(I) and Additives. Tetrahedron Lett. 1997, 38, 6673–6676. 10.1016/S0040-4039(97)01562-1. [DOI] [Google Scholar]
- Zhang F.-L.; Hong K.; Li T.-J.; Park H.; Yu J.-Q. Functionalization of C(sp3)–H Bonds Using a Transient Directing Group. Science 2016, 351, 252–256. 10.1126/science.aad7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For leading references, see; a Yang K.; Li Q.; Liu Y.; Li G.; Ge H. Catalytic C–H Arylation of Aliphatic Aldehydes Enabled by a Transient Ligand. J. Am. Chem. Soc. 2016, 138, 12775–12778. 10.1021/jacs.6b08478. [DOI] [PubMed] [Google Scholar]; b St John-Campbell S.; White A. J. P.; Bull J. A. Single Operation Palladium Catalysed C(sp3)–H Functionalisation of Tertiary Aldehydes: Investigations into Transient Imine Directing Groups. Chem. Sci. 2017, 8, 4840–4847. 10.1039/C7SC01218G. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Li B.; Lawrence B.; Li G.; Ge H. Ligand-Controlled Direct γ-C–H Arylation of Aldehydes. Angew. Chem., Int. Ed. 2020, 59, 3078–3082. 10.1002/anie.201913126. [DOI] [PubMed] [Google Scholar]; d Liu X.-H.; Park H.; Hu J.-H.; Hu Y.; Zhang Q.-L.; Wang B.-L.; Sun B.; Yeung K.-S.; Zhang F.-L.; Yu J.-Q. Diverse ortho-C(sp2)–H Functionalization of Benzaldehydes Using Transient Directing Groups. J. Am. Chem. Soc. 2017, 139, 888–896. 10.1021/jacs.6b11188. [DOI] [PubMed] [Google Scholar]; e Chen X. Y.; Sorensen E. J. Pd-Catalyzed, Ortho C–H Methylation and Fluorination of Benzaldehydes Using Orthanilic Acids as Transient Directing Groups. J. Am. Chem. Soc. 2018, 140, 2789–2792. 10.1021/jacs.8b00048. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Chen Y. Q.; Liu T.; Eastgate M. D.; Yu J. Q. Pd-Catalyzed γ-C(sp3)–H Arylation of Free Amines Using a Transient Directing Group. J. Am. Chem. Soc. 2016, 138, 14554–14557. 10.1021/jacs.6b09653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Q.; Wu Y.; Wang Z.; Qiao J. X.; Yu J. Q. Transient Directing Group Enabled Pd-Catalyzed γ-C(sp3)–H Oxygenation of Alkyl Amines. ACS Catal. 2020, 10, 5657–5662. 10.1021/acscatal.0c01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Q.; Singh S.; Wu Y.; Wang Z.; Hao W.; Verma P.; Qiao J. X.; Sunoj R. B.; Yu J. Q. Pd-Catalyzed γ-C(sp3)–H Fluorination of Free Amines. J. Am. Chem. Soc. 2020, 142, 9966–9974. 10.1021/jacs.9b13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.; Wang C.; Bannister T. D.; Kamenecka T. M. Site-Selective γ-C(sp3)–H and γ-C(sp2)–H Arylation of Free Amino Esters Promoted by a Catalytic Transient Directing Group. Chem. - Eur. J. 2018, 24, 9535–9541. 10.1002/chem.201802465. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Ge H. Site-Selective C–H Arylation of Primary Aliphatic Amines Enabled by a Catalytic Transient Directing Group. Nat. Chem. 2017, 9, 26–32. 10.1038/nchem.2606. [DOI] [Google Scholar]
- Chen Y.-Q.; Wang Z.; Wu Y.; Wisniewski S. R.; Qiao J. X.; Ewing W. R.; Eastgate M. D.; Yu J.-Q. Overcoming the Limitations of γ- and δ-C–H Arylation of Amines through Ligand Development. J. Am. Chem. Soc. 2018, 140, 17884–17894. 10.1021/jacs.8b07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John-Campbell S.; Ou A. K.; Bull J. A. Palladium-Catalyzed C(sp3)–H Arylation of Primary Amines Using a Catalytic Alkyl Acetal to Form a Transient Directing Group. Chem. - Eur. J. 2018, 24, 17838–17843. 10.1002/chem.201804515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reports using CO2 as a transient directing group, see; a Kapoor M.; Chand-Thakuri P.; Young M. C. Carbon Dioxide-Mediated C(sp2)–H Arylation of Primary and Secondary Benzylamines. J. Am. Chem. Soc. 2019, 141, 7980–7989. 10.1021/jacs.9b03375. [DOI] [PubMed] [Google Scholar]; b Kapoor M.; Liu D.; Young M. C. Carbon Dioxide-Mediated C(sp3)–H Arylation of Amine Substrates. J. Am. Chem. Soc. 2018, 140, 6818–6822. 10.1021/jacs.8b05061. [DOI] [PubMed] [Google Scholar]
- Yada A.; Liao W.; Sato Y.; Murakami M. Buttressing Salicylaldehydes: A Multipurpose Directing Group for C(sp3)–H Bond Activation. Angew. Chem., Int. Ed. 2017, 56, 1073–1076. 10.1002/anie.201610666. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Young M. C.; Wang C.; Magness D. M.; Dong G. Catalytic C(sp3)–H Arylation of Free Primary Amines with an Exo Directing Group Generated In Situ. Angew. Chem., Int. Ed. 2016, 55, 9084–9087. 10.1002/anie.201604268. [DOI] [PubMed] [Google Scholar]
- Higham J. I.; Bull J. A. Amine-Catalyzed Copper-Mediated C–H Sulfonylation of Benzaldehydes via a Transient Imine Directing Group. Angew. Chem., Int. Ed. 2022, 61, e202202933 10.1002/anie.202202933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- For reports of free amine-directed C–H functionalization, see; a Lazareva A.; Daugulis O. Direct Palladium-Catalyzed Ortho-Arylation of Benzylamines. Org. Lett. 2006, 8, 5211–5213. 10.1021/ol061919b. [DOI] [PubMed] [Google Scholar]; b López B.; Rodriguez A.; Santos D.; Albert J.; Ariza X.; Garcia J.; Granell J. Preparation of Benzolactams by Pd(II)-Catalyzed Carbonylation of N-Unprotected Arylethylamines. Chem. Commun. 2011, 47, 1054–1056. 10.1039/C0CC03478A. [DOI] [PubMed] [Google Scholar]; c Mancinelli A.; Alamillo C.; Albert J.; Ariza X.; Etxabe H.; Farràs J.; Garcia J.; Granell J.; Quijada F. J. Preparation of Substituted Tetrahydroisoquinolines by Pd(II)-Catalyzed NH2-Directed Insertion of Michael Acceptors into C–H Bonds Followed by NH2-Conjugated Addition. Organometallics 2017, 36, 911–919. 10.1021/acs.organomet.6b00944. [DOI] [Google Scholar]; d Chand-Thakuri P.; Alahakoon I.; Liu D.; Kapoor M.; Kennedy J. F.; Jenkins K. W.; Rabon A. M.; Young M. C. Native Amine-Directed ortho-C–H Halogenation and Acetoxylation/Condensation of Benzylamines. Synthesis 2022, 54, 341–354. 10.1055/a-1625-9095. [DOI] [Google Scholar]
- Zaitsev V. G.; Shabashov D.; Daugulis O. Highly Regioselective Arylation of sp3 C–H Bonds Catalyzed by Palladium Acetate. J. Am. Chem. Soc. 2005, 127, 13154–13155. 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]
- For reviews detailing the development of amine C–H functionalization, see; a He G.; Wang B.; Nack W. A.; Chen G. Syntheses and Transformations of α-Amino Acids via Palladium-Catalyzed Auxiliary-Directed sp3 C–H Functionalization. Acc. Chem. Res. 2016, 49, 635–645. 10.1021/acs.accounts.6b00022. [DOI] [PubMed] [Google Scholar]; b Noisier A. F. M.; Brimble M. A. C–H Functionalization in the Synthesis of Amino Acids and Peptides. Chem. Rev. 2014, 114, 8775–8806. 10.1021/cr500200x. [DOI] [PubMed] [Google Scholar]; c Sambiagio C.; Schönbauer D.; Blieck R.; Dao-Huy T.; Pototschnig G.; Schaaf P.; Wiesinger T.; Zia M. F.; Wencel-Delord J.; Besset T.; Maes B. U. W.; Schnürch M. A Comprehensive Overview of Directing Groups Applied in Metal-Catalysed C–H Functionalisation Chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. 10.1039/C8CS00201K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar U.; Samanta R. Pd(II)-Catalyzed Direct Sulfonylation of Benzylamines Using Sodium Sulfinates. J. Org. Chem. 2019, 84, 2850–2861. 10.1021/acs.joc.8b03098. [DOI] [PubMed] [Google Scholar]
- a Feng M.; Tang B.; Liang S. H.; Jiang X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. 10.2174/1568026615666150915111741. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ilardi E. A.; Vitaku E.; Njardarson J. T. Data-Mining for Sulfur and Fluorine: An Evaluation of Pharmaceuticals to Reveal Opportunities for Drug Design and Discovery. J. Med. Chem. 2014, 57, 2832–2842. 10.1021/jm401375q. [DOI] [PubMed] [Google Scholar]
- For the optimization of reaction parameters, see pages S5–S12 of the Supporting Information. For full details on the computational investigations, calculated reaction profile, and investigations into the nature of C–H activation, see pages S17–S25 of the Supporting Information.
- No unreacted amine was observed, suggesting a background decomposition over the course of the reaction.
- A total of 2 equiv of Cu is needed to produce 1 equiv of product by the mechanism proposed; hence, 50 mol % [Cu] is effectively 25 mol % with respect to the amount of product formed by one turnover. Therefore, just under one turnover of Cu is observed in the absence of the oxidant.
- a Tang X.; Huang L.; Qi C.; Wu X.; Wu W.; Jiang H. Copper-Catalyzed Sulfonamides Formation from Sodium Sulfinates and Amines. Chem. Commun. 2013, 49, 6102–6104. 10.1039/c3cc41249k. [DOI] [PubMed] [Google Scholar]; b Lam L. Y.; Chan K. H.; Ma C. Copper-Catalyzed Synthesis of Functionalized Aryl Sulfonamides from Sodium Sulfinates in Green Solvents. J. Org. Chem. 2022, 87, 8802–8810. 10.1021/acs.joc.2c00777. [DOI] [PubMed] [Google Scholar]
- a Rojas J. J.; Croft R. A.; Sterling A. J.; Briggs E. L.; Antermite D.; Schmitt D. C.; Blagojevic L.; Haycock P.; White A. J. P.; Duarte F.; Choi C.; Mousseau J. J.; Bull J. A. Amino-Oxetanes as Amide Isosteres by an Alternative Defluorosulfonylative Coupling of Sulfonyl Fluorides. Nat. Chem. 2022, 14, 160–169. 10.1038/s41557-021-00856-2. [DOI] [PubMed] [Google Scholar]; b Rojas J. J.; Bull J. A. 3-(4-Methoxyphenyl)-3-oxetanesulfonyl Fluoride. Encycl. Reagents Org. Synth. 2023, 10.1002/047084289X.rn02501. [DOI] [Google Scholar]
- In the absence of sulfinate salt, the same experiments that gave low levels of ortho-deuteration indicative of potential reversibility, consistent with the energy barriers observed by DFT, do not occur under the productive reaction conditions (see pages S13–S15 of the Supporting Information).
- The mechanism was interrogated using a ωB97X-D functional (ref (30)) with the following basis sets applied to specific atoms: C and H, 6-31+g(d,p); N, O, and S, 6-311+g(d); H deprotonated in CMD and H bound to TDG–O, 6-31++G(d,3pd); and Cu, SDD pseudo-potential.
- a Chai J.-D.; Head-Gordon M. Systematic Optimization of Long-Range Corrected Hybrid Density Functionals. J. Chem. Phys. 2008, 128, 084106 10.1063/1.2834918. [DOI] [PubMed] [Google Scholar]; b Alipour M.; Fallahzadeh P. First Principles Optimally Tuned Range-Separated Density Functional Theory for Prediction of Phosphorus–Hydrogen Spin–Spin Coupling Constants. Phys. Chem. Chem. Phys. 2016, 18, 18431–18440. 10.1039/C6CP02648F. [DOI] [PubMed] [Google Scholar]
- For prior computational studies of amide-directed copper-mediated C–H functionalization, see; a Chen C.; Hao Y.; Zhang T.-Y.; Pan J.-L.; Ding J.; Xiang H.-Y.; Wang M.; Ding T.-M.; Duan A.; Zhang S.-Y. Computational and Experimental Studies on Copper-Mediated Selective Cascade C–H/N–H Annulation of Electron-Deficient Acrylamide with Arynes. Chem. Commun. 2019, 55, 755–758. 10.1039/C8CC08708C. [DOI] [PubMed] [Google Scholar]; b Kim H.; Heo J.; Kim J.; Baik M.-H.; Chang S. Copper-Mediated Amination of Aryl C–H Bonds with the Direct Use of Aqueous Ammonia via a Disproportionation Pathway. J. Am. Chem. Soc. 2018, 140, 14350–14356. 10.1021/jacs.8b08826. [DOI] [PubMed] [Google Scholar]; c Yang Y.; Cao F.; Yao L.; Shi T.; Tang B.; Kuninobu Y.; Wang Z. C–N and C–O Bond Formation in Copper-Catalyzed/Mediated sp3 C–H Activation: Mechanistic Studies from Experimental and Computational Aspects. J. Org. Chem. 2020, 85, 9713–9726. 10.1021/acs.joc.0c01038. [DOI] [PubMed] [Google Scholar]; d Wootton T. L.; Porter J. A.; Grewal K. S.; Chirila P. G.; Forbes S.; Coles S. J.; Horton P. N.; Hamilton A.; Whiteoak C. J. Merging Cu-Catalysed C–H Functionalisation and Intramolecular Annulations: Computational and Experimental Studies on an Expedient Construction of Complex Fused Heterocycles. Org. Chem. Front. 2020, 7, 1235–1242. 10.1039/D0QO00283F. [DOI] [Google Scholar]
- An alternative pathway with coordination of the sulfinate anion to the copper center followed by oxidation was also computationally a low-energy pathway. The formation of the radical intermediates is consistent with the observed TEMPO inhibition.
- Reddy R. J.; Kumari A. H. Synthesis and Applications of Sodium Sulfinates (RSO2Na): A Powerful Building Block for the Synthesis of Organosulfur Compounds. RSC Adv. 2021, 11, 9130–9221. 10.1039/D0RA09759D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham J. I.; Ma T.-K.; Bull J. A. Copper Catalyzed C–H Sulfonylation of Benzylamines with a Catalytic Transient Directing Group. ChemRxiv 2023, 10.26434/chemrxiv-2023-mj0wm. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw and processed characterization data for all novel compounds and Cartesian coordinates from computed structures can be found at the Imperial College London Research Data Repository: 10.14469/hpc/12033. A version of this manuscript was deposited on the preprint repository ChemRxiv.34 The data underlying this study are available in the published article and its Supporting Information.