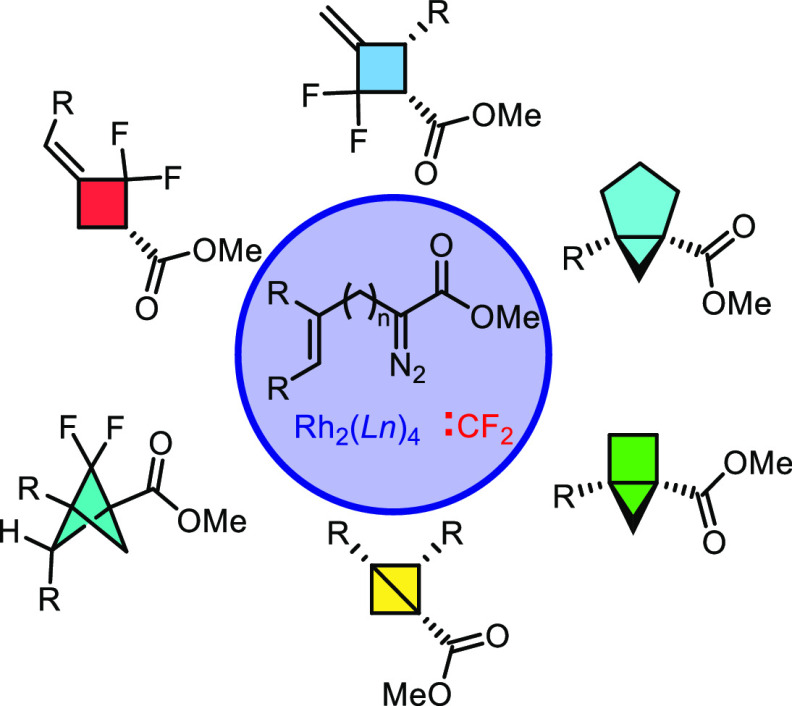
Abstract
Rapid access to 2,2-difluorobicylco[1.1.1]pentanes is enabled from an α-allyldiazoacetate precursor in a one-pot process through cyclopropanation to afford a 3-aryl bicyclo[1.1.0]butane, followed by reaction with difluorocarbene in the same reaction flask. The modular synthesis of these diazo compounds affords novel 2,2-difluorobicyclo[1.1.1]pentanes that were inaccessible through previously reported methods. The reactions of chiral 2-arylbicyclo[1.1.0]butanes in the same manner generate altogether different products with high asymmetric induction, methylene-difluorocyclobutanes. Larger ring systems including bicyclo[3.1.0]hexanes are also rapidly furnished due to the modular nature of the diazo starting material.
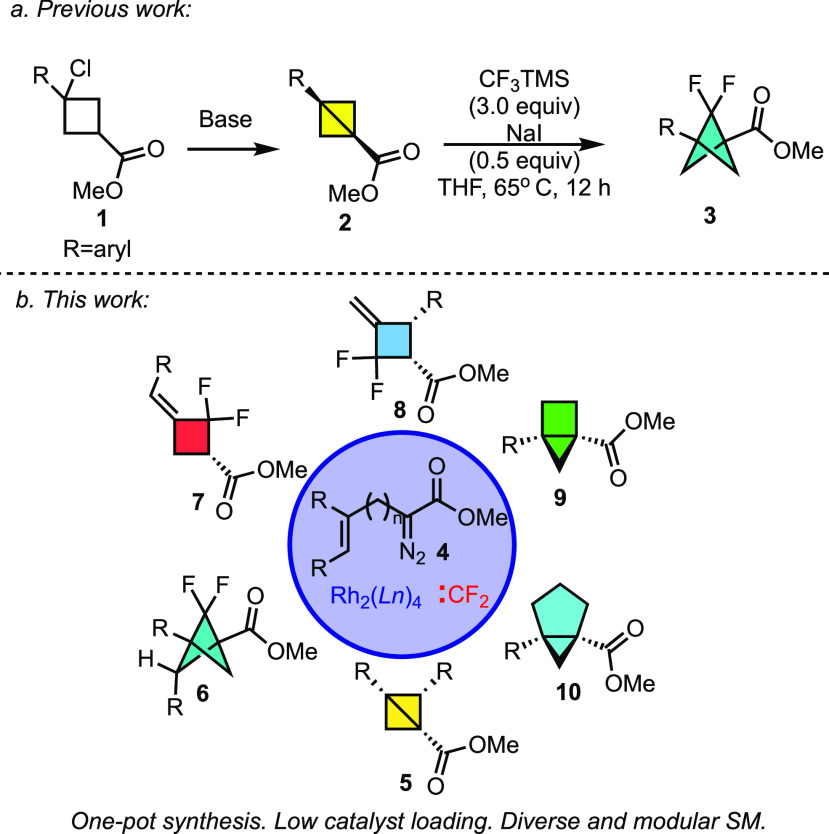
2,2-Difluorobicylco[1.1.1]pentanes have generated considerable interest in recent years due to their role as phenyl bioisosteres.1 Inclusion of these groups in the place of phenyl rings can serve to eliminate metabolic liabilities in drug molecules and on occasion can even provide substances with greater potency than the corresponding aryl analogues.1a,2 Several effective methods have recently emerged for generating 2-substituted bicyclo[1.1.1]pentanes.1d,3 A particularly innovative method is the difluorocarbene-induced ring-expansion of 3-arylbicylco[1.1.0]butanes 2 to form difluorobicyclo[1.1.1]pentanes 3 (Scheme 1a).1a,1b,3h While this route is practical for the exploration of several substrates, the scope of the transformation is limited due to the synthetic accessibility of bicyclo[1.1.0]butane precursors.1a,1b Introduction of additional functionality at the other methylene positions of the difluorobicyclo[1.1.1]pentane using this ring expansion methodology has so far been limited to deuterium.3h We envisioned that a wider array of derivatives would be accessible by combining intramolecular cyclopropanation of diazo compounds 4 with difluorocarbene ring expansion (Scheme 1b). These efforts resulted in the formation of the desired bicyclo[1.1.0]butanes 5 and difluorobicyclo[1.1.1]pentanes 6. Additionally, an alternative difluorocarbene reaction was discovered to form difluorocyclobutanes 7 and 8. The intramolecular cyclopropanation could also be applied to furnish extended strained ring systems, including bicyclo[1.1.0]pentanes 9 and bicyclo[1.1.0]hexanes 10.
Scheme 1. Modular Diazo Starting Material Provides Rapid Access to a Diverse Series of Molecules.
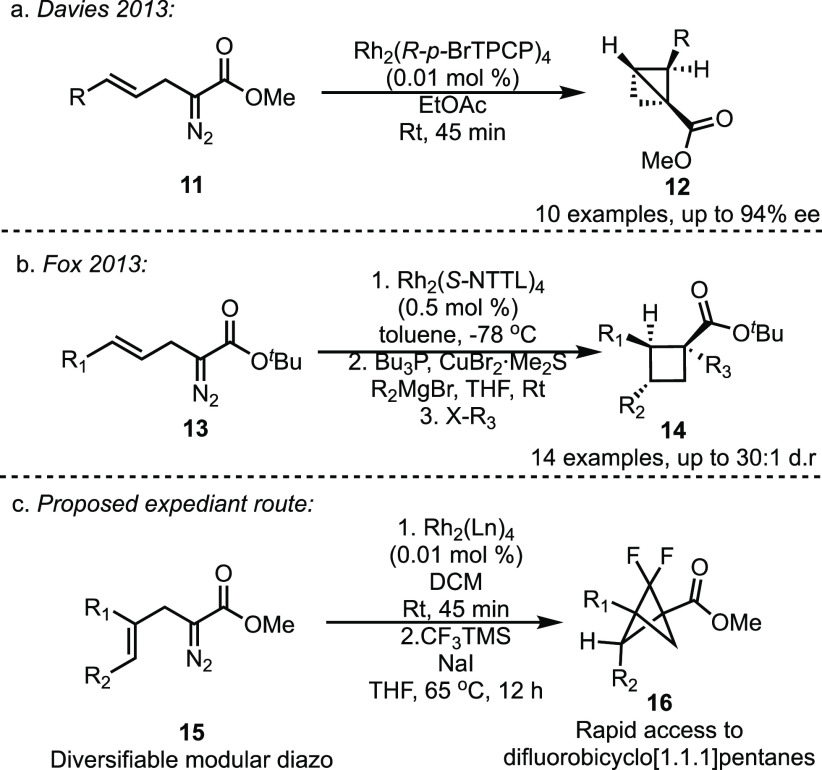
The impetus for this study was a method we had previously reported for the asymmetric synthesis of 2-arylbicyclo[1.1.0]butanes 12 through the intramolecular cyclopropanation of α-allyldiazoacetates 11 in the presence of a chiral dirhodium tetracarboxylate catalyst (Scheme 2a).4 This method generated a series of 2-arylbicyclo[1.1.0]butanes with high yields and enantioselectivity. Soon thereafter Fox independently reported the synthesis of highly functionalized cyclobutanes 14 from α-allyldiazoacetates 13 in a one-pot process, highlighting the potential to telescope this reaction with further derivatization (Scheme 2b).5 The previous intramolecular cyclopropanation had been limited to the formation of 2-arylbicyclo[1.1.0]butanes,4,5 but we envisioned that this reaction could be extended to a wider range of bicyclo[1.1.0]butanes by using the appropriate α-allyldiazoacetates 15 (Scheme 2c). Given the mild conditions of the intramolecular cyclopropanation, we suspected that the intramolecular cyclopropanation could be telescoped to include a reaction with difluorocarbene, thereby generating a variety difluorobicyclo[1.1.1]pentanes 16 in a one-pot process without the need to isolate the intermediate bicyclo[1.1.0]butanes.5 The development of this approach and the discovery of some unexpected transformations are described herein.
Scheme 2. One-Pot Synthesis of Diverse Carbocycles.
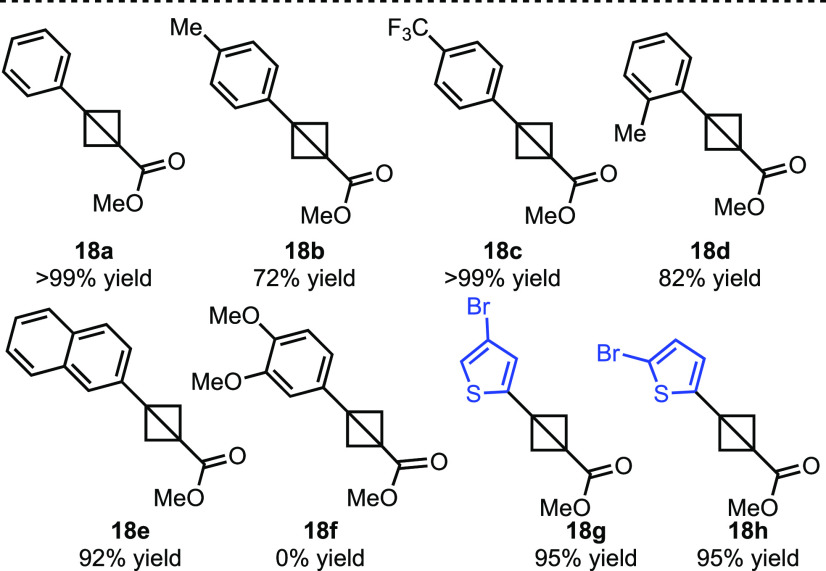
The first stage of the project examined whether the intramolecular cyclopropanation could offer a simple entry to 3-arylbicyclo[1.1.0]butanes, the substrates that had been previously used for the synthesis of difluorobicyclo[1.1.1]pentanes.1a,1b A series of 3-substituted α-allyldiazoacetates 17 were prepared via a two-step process from readily accessible allyl-bromo-α-methylstyrene derivatives (see Supporting Information for details). The bromide was first displaced with methyl acetoacetate, followed by a deacylative diazo transfer to afford a diverse series of diazo compounds, 17a–h. These precursors were then reacted in the presence of a dirhodium catalyst to afford 3-arylbicyclo[1.1.0]butanes 18 in high yield (Table 1). It is noteworthy that compound 18d includes an ortho-substituted arene and compounds 18g and 18h feature thiophene heterocycles. None of these compounds have been prepared using the previously reported routes to access 3-arylbicyclo[1.1.0]butanes.1a Electron-rich arenes were not compatible with this method, as can be seen with compound 17f. The problem here appears to be the instability of the product which degrades rapidly upon forming. These substrates were shown to be incompatible in previous reports.1a,1b Weakly electron-donating substituents are tolerated, however, and compounds 18b, 18d, and 18e, are readily prepared.
Table 1. Synthesis of Bicyclo[1.1.0]butanes 18.
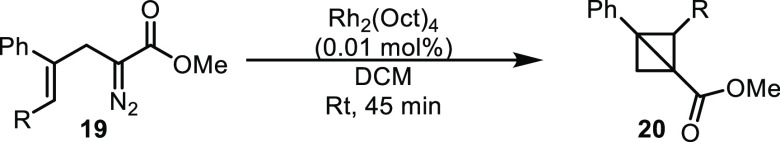
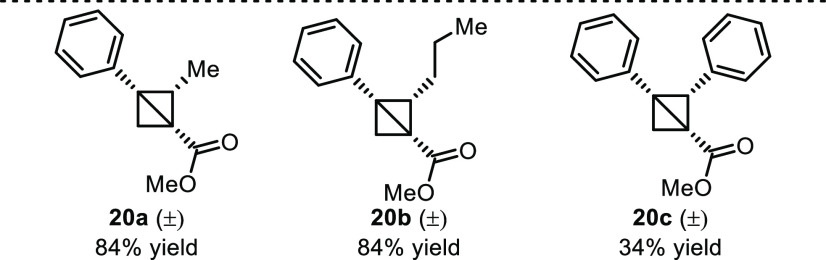
These 3-aryl substituted compounds were achiral, but if a group could be installed at the 2-position, a chiral trisubstituted difluorobicyclo[1.1.1]pentane could be generated.1c,2b,4 By using the more highly substituted diazoacetates 19, 2-substituted 3-phenylbicyclo[1.1.0]butanes 20 were also prepared via this method (Table 2). Compounds 20a and 20b were generated in excellent yield; however, the formation of compound 20c was less effective. To see if any chiral information could be generated in the synthesis of products 20a–c, an exhaustive screen of chiral catalysts was performed (see Supporting Information for details). Unfortunately, the most effective catalyst, Rh2(R-NTTL)4, was able to afford the product with only moderate levels of enantioselectivity (up to 50% ee) regardless of the conditions used.5 As a result, the further reactions of 20a–c were examined with racemic substrates generated by the intramolecular cyclopropanation with Rh2(Oct)4 as catalyst.
Table 2. Synthesis of Trisubstituted Bicyclo[1.1.0]butanes 20.
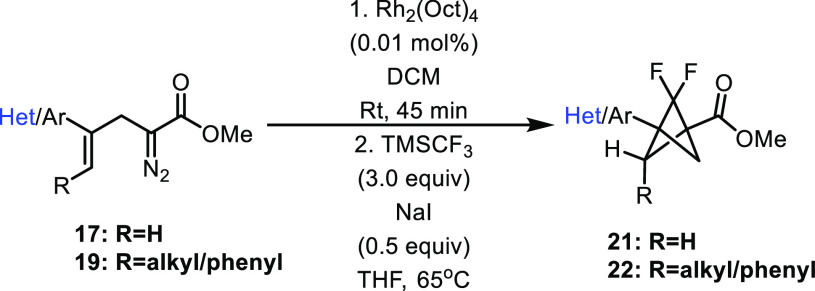
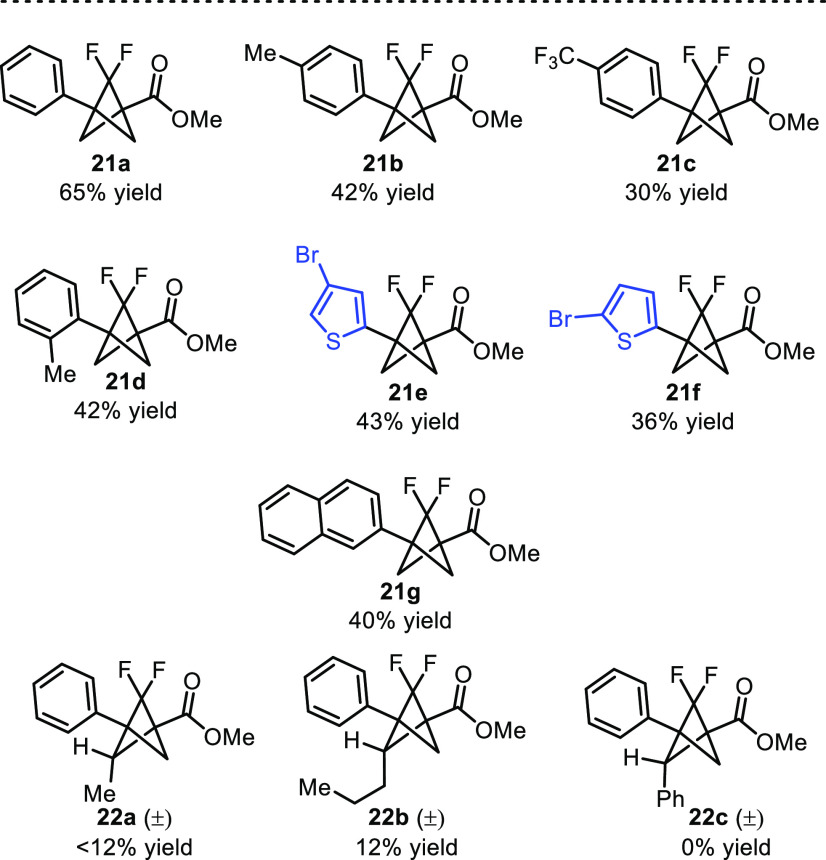
With this method in hand, we wanted to evaluate the efficacy of telescoping the transformation to include difluorocarbene insertion to generate 2,2-difluorobicyclo[1.1.1]pentanes. Previous methods have required isolation of the bicyclo[1.1.0]butane by column chromatography before conducting difluorocarbene insertion, but we reasoned that the small amount of dirhodium catalyst (0.01 mol %) and the lack of unreacted starting material obviated the need for isolation of the product. A solvent exchange was needed, however, as the intramolecular cyclopropanation is less competent in THF as a solvent, and difluorocarbene insertion requires THF to achieve optimal results. The results of the reaction of the diazo compounds 17 and 19, first with Rh2(Oct)4 followed by a solvent switch and treatment with NaI and CF3TMS, are reported in Table 3.1a,1b 3-Aryl-bicyclo[1.1.0]butanes were generally effective in the ring-expansion with difluorocarbene, and several 3-aryl-2,2-difluorobicyclo[1.1.1]pentanes 21 could be synthesized by this method, including the novel derivatives, 21d, 21e, and 21f.1a,1b Unfortunately, the 2,3-disubstituted derivatives 19a–c were less effective in these sequential reactions. 20a and 20b afforded the desired 2,2-difluorobicyclo[1.1.1]pentane products 22a and 22b but in low yield, whereas 20c gave none of the desired product. Interestingly, 22a and 22b were generated as single diastereomers which suggests that the addition of difluorocarbene to the bicyclo[1.1.0]butane is controlled by the orientation of the substituents set during the intramolecular cyclopropanation.1d,3h,6−8
Table 3. One-Pot Synthesis of 2,2-Difluorobicyclo[1.1.1]pentanes.
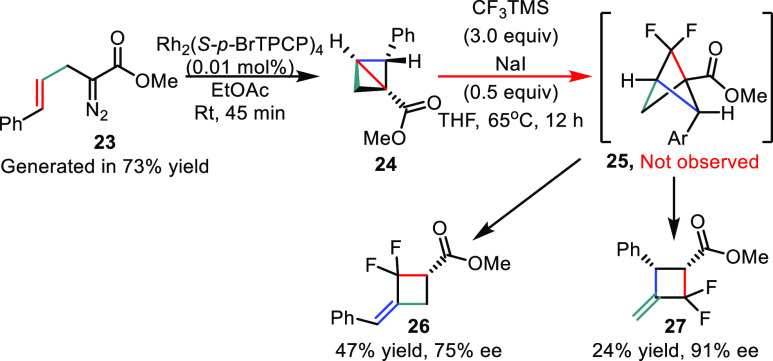
We were curious to see if the chiral 2-arylbicyclo[1.1.0]butanes we had previously reported5 would similarly generate difluorobicyclo[1.1.1]pentanes, analogous to the 3-aryl-substituted system. To evaluate this possibility, α-allyl diazoacetates were prepared by the literature method4 and subjected to the one-pot difluorocarbene reaction optimized for difluorobicyclo[1.1.1]pentane generation (Scheme 3). In the first step the diazo compound 23 was reacted in the presence of Rh2(S-p-BrTPCP)4 (0.01 mol %) as catalyst9 and ethyl acetate (EtOAc) as solvent, the combination which was shown to afford the highest asymmetric induction in our previous study (Scheme 4).4 Again conversion to the desired bicyclo[1.1.0]butane product 24 was monitored by FTIR, and the reaction was complete after 45 min.4 The crude reaction mixture was concentrated in vacuo to remove the solvent, then dissolved in THF, and reacted with CF3TMS (3 equiv) at 65 °C in the presence of NaI (0.5 equiv) overnight. Upon completion of the reaction, the products were isolated and analyzed by 1H and 19F NMR. We observed that no difluorobicyclo[1.1.1]pentane 25 was generated. Instead, the reaction yielded a 2:1 mixture of methylene difluorocyclobutenes 26 and 27 (2:1 ratio).10 Furthermore, 26 and 27 were obtained with high enantioselectivity: compound 26 was isolated in 75% ee, and 27 was isolated in 91% ee. This type of reaction occurs with all 2-arylbicyclo[1.1.0]butanes we examined, but the products are generally quite unstable and could not be isolated in pure form.
Scheme 3. Formation of Methylene-Difluorocyclobutenes 26 and 27.
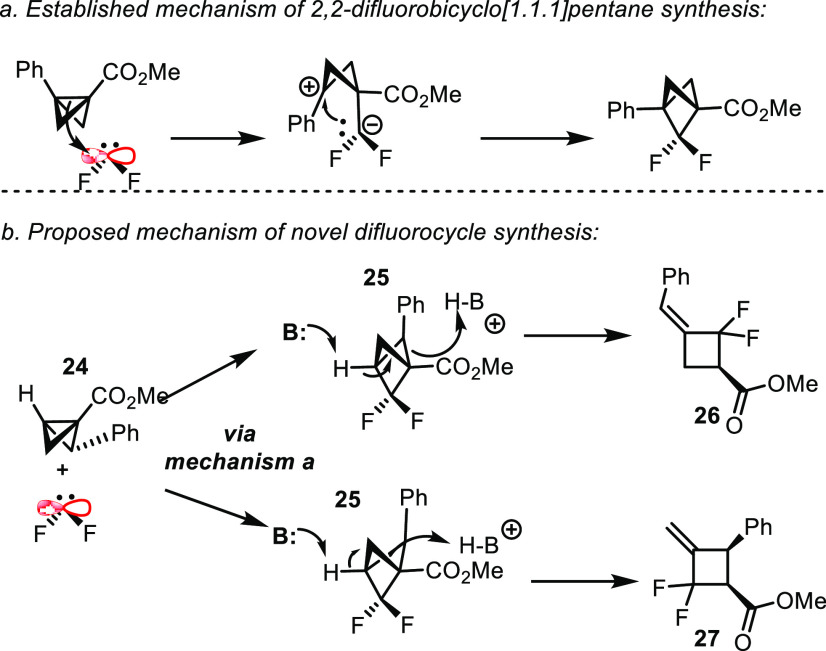
Scheme 4. Proposed Mechanism of Difluorocarbene Reaction with Bicyclo[1.1.0]butanes.
A reasonable mechanism to explain the role of substituents on the product outcome from the reaction of bicyclo[1.1.0]butane 24 with difluorocarbene is shown in Scheme 4.11 Previously, it has been proposed that the insertion of difluorocarbene is a stepwise process beginning with attack of the difluorocarbene on the central C1–C3 bond of the bicyclo[1.1.0]butane, followed by recombination of the resultant zwitterion to form a 2,2-difluorobicyclo[1.1.1]pentane.1a,1b,3h,12 In the case of 3-substituted bicyclo[1.1.1]pentanes 21a–g and 22a–b, the products are stable and isolable,1a,1b and this was confirmed in our studies (Table 3). In contrast, for the 3-unsubstituted bicyclo[1.1.1]pentanes, we propose that the difluorobicyclo[1.1.1]pentane 25 is formed as expected, but they are unstable and rearrange under the reaction conditions. The methine C3 proton of 25 is acidic due to its proximity to the difluoromethylene group and the geometry of the bicyclo[1.1.1]pentane moiety. It is well-known that deprotonation and radical reactivity of the bicyclo[1.1.1]pentane methine proceeds easily under mild conditions, and it appears that the catalytic iodide present in the reaction mixture is sufficiently basic to catalyze this process.3f,13 The resultant anion then forms an alkene with either of the adjacent carbons, and the C1–C2/C4 bond is protonated by the resultant conjugate acid. This opens the caged compound resulting in the formation of isomeric difluorocyclobutenes 26 and 27 (Scheme 4). Attempts to trap out difluorobicyclo[1.1.1]pentane intermediate 25 by skipping workup or running the reaction for less time invariably failed, suggesting that the decomposition of this intermediate is fast. The weakest C–C bond (C1–C2) is the most easily cleaved during alkene formation, which explains the 2:1 product distribution in favor of 26 (Scheme 3). Interestingly only one alkene isomer of 26 was obtained in the reaction which indicates that the addition of difluorocarbene to the bicyclo[1.1.0]butane intermediate was diastereoselective as was observed in the trisubstituted bicyclo[1.1.0]butanes 22a and 22b.3h
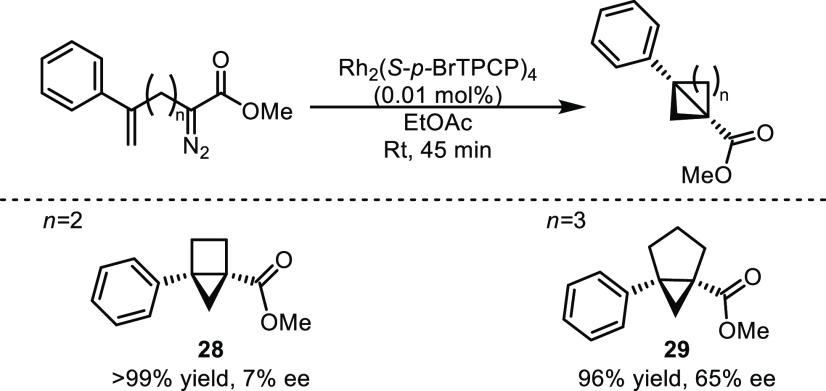
The modularity of the diazo compound starting material has broader potential. Extending the alkene-diazo linker should allow facile access to expanded ring systems including bicyclo[2.1.0]pentanes and bicyclo[3.1.0]hexanes which have thus far been unexplored as substrates for the difluorocarbene reaction but could generate novel interesting caged architectures, difluorobicyclo[2.1.1]hexane and difluorobicyclo[3.1.1]heptane.11,14 Similar caged compounds have recently garnered significant interest as meta- and ortho-substituted phenyl-bioisosteres for medicinal applications.14f,14g,15 To this effect, diazo compounds were synthesized, and the intramolecular cyclopropanation was performed to generate the novel bicyclo[2.1.0]pentane 28 and bicyclo[3.1.0]hexane 29. Both products were afforded in excellent yield, highlighting the robustness of this chemistry despite the modular design of the diazo compound precursors (Scheme 5). Interestingly bicylco[3.1.0]hexane 29 was afforded in high enantioselectivity (65% ee) through the use of the chiral catalyst Rh2(S-p-BrTPCP)4 and ethyl acetate as solvent (Scheme 3). Surprisingly, neither of these expanded ring systems were able to successfully react with difluorocarbene. Bicyclo[2.1.0]pentane 28 did not generate the desired 2,2-difluorobicyclo[2.1.1]hexane and instead formed a complex mixture of products. 3-Phenyl bicyclo[3.1.0]hexane 29 was also unreactive under the standard conditions. It is likely that 29 does not contain sufficient ring strain across the C1–C3 bond to engage with difluorocarbene. More forcing conditions (higher temperature, longer reaction times, alternative difluorocarbene sources) also did not afford difluorocarbene insertion product in either case.3h
Scheme 5. Expanded Ring Synthesis.
In this work, we have developed a novel approach to synthesizing 3-arylbicyclo[1.1.0]butanes and more substituted analogues via a modular α-allyldiazoacetate precursor. The novel method for synthesizing 3-arylbicyclo[1.1.0]butanes is more tolerant of various functionality and generally proceeds with very high yield at low catalyst loading (0.01 mol %), enabling the synthesis of bicyclo[1.1.0]butanes that were unsuccessful by previously reported methods. It was also possible to generate a series of difluorobicyclo[1.1.1]pentanes from the α-allyl diazoacetate in a one-pot process with yields comparable to those of previous reports. We were also able to explore difluorocarbene insertion of 2-arylbicyclo[1.1.0]butanes and determine the structure of the unusual products generated as well as propose a mechanism for their formation, although the compounds proved unstable. Future work will be conducted to determine the scope of transformations accessible to these highly substituted bicyclo[1.1.0]butanes and improve the asymmetric induction. Additionally, diversification of the methylenedifluorocyclobutene products will be explored to afford stable and isolable highly substituted difluorocyclobutane products with high asymmetric induction. Finally, the novel bicyclo[3.1.0]hexane and bicyclo[2.1.0]pentane products will be diversified. Our hope is that new methods for the generation of difluorocarbene may allow these products to be transformed into the complex caged compounds initially targeted in this work.
Acknowledgments
This work was supported by the National Science Foundation (CHE-1956154 and the CCI Centre for Selective C–H Functionalization (CHE-1700982)). Instrumentation used in this work was supported by the National Science Foundation (CHE 1531620 and CHE 1626172). Constructive discussions within the Catalysis Innovation Consortium facilitated this study. We thank Joshua K. Sailer, Emory University for assistance with some of the compound characterization.
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c01664.
Complete experimental procedures and compound characterization (PDF)
The authors declare the following competing financial interest(s): H.M.L.D. is a named inventor on a patent entitled, Dirhodium Catalyst Compositions and Synthetic Processes Related Thereto (US 8,974,428, issued March 10, 2015). The other authors have no competing financial interests.
Supplementary Material
References
- a Bychek R. M.; Hutskalova V.; Bas Y. P.; Zaporozhets O. A.; Zozulya S.; Levterov V. V.; Mykhailiuk P. K. Difluoro-substituted bicyclo [1.1.1] pentanes for medicinal chemistry: Design, synthesis, and characterization. J. Org. Chem. 2019, 84, 15106–15117. 10.1021/acs.joc.9b01947. [DOI] [PubMed] [Google Scholar]; b Ma X.; Sloman D. L.; Han Y.; Bennett D. J. A selective synthesis of 2,2-difluorobicyclo [1.1.1] pentane analogues: “BCP-F2. Org. Lett. 2019, 21, 7199–7203. 10.1021/acs.orglett.9b02026. [DOI] [PubMed] [Google Scholar]; c Rentería-Gómez A.; Lee W.; Yin S.; Davis M.; Gogoi A. R.; Gutierrez O. General and practical route to diverse 1-(difluoro)alkyl-3-arylbicyclo[1.1.1]pentanes enabled by an Fe-catalyzed multicomponent radical cross-coupling reaction. ACS Catal. 2022, 12, 11547–11556. 10.1021/acscatal.2c03498. [DOI] [Google Scholar]; d Turkowska J.; Durka J.; Gryko D. Strain release–an old tool for new transformations. Chem. Commun. 2020, 56, 5718–5734. 10.1039/D0CC01771J. [DOI] [PubMed] [Google Scholar]; e Bychek R.; Mykhailiuk P. K. A practical and scalable approach to fluoro-substituted Bicyclo [1.1.1] pentanes. Angew. Chem., Int. Ed. 2022, 61, e202205103 10.1002/anie.202205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Makarov I. S.; Brocklehurst C. E.; Karaghiosoff K.; Koch G.; Knochel P. Synthesis of bicyclo[1.1.1]pentane bioisosteres of internal alkynes and para-disubstituted benzenes from [1.1.1]propellane. Angew. Chem., Int. Ed. 2017, 56, 12774–12777. 10.1002/anie.201706799. [DOI] [PubMed] [Google Scholar]; b Zhao J.-X.; Chang Y.-X.; He C.; Burke B. J.; Collins M. R.; Del Bel M.; Elleraas J.; Gallego G. M.; Montgomery T. P.; Mousseau J. J. 1, 2-Difunctionalized bicyclo[1.1.1] pentanes: Long–sought-after mimetics for ortho/meta-substituted arenes. Proc. Nat. Acad. Sci. 2021, 118, e2108881118 10.1073/pnas.2108881118. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Alnajjar R.; Mohamed N.; Kawafi N. Bicyclo[1.1.1]pentane as phenyl substituent in atorvastatin drug to improve physicochemical properties: drug-likeness, DFT, pharmacokinetics, docking, and molecular dynamic simulation. J. MoL. Struct. 2021, 1230, 129628 10.1016/j.molstruc.2020.129628. [DOI] [Google Scholar]
- a Yang Y.; Tsien J.; Hughes J. M.; Peters B. K.; Merchant R. R.; Qin T. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 2021, 13, 950–955. 10.1038/s41557-021-00786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Anderson J. M.; Measom N. D.; Murphy J. A.; Poole D. L. Bridge functionalisation of bicyclo[1.1.1]pentane derivatives. Angew. Chem., Int. Ed. 2021, 60, 24754–24769. 10.1002/anie.202106352. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ma X.; Nhat Pham L. Selected topics in the syntheses of bicyclo[1.1.1]pentane (BCP) analogues. Asian J. Org. Chem. 2020, 9, 8–22. 10.1002/ajoc.201900589. [DOI] [Google Scholar]; d Nugent J.; Arroniz C.; Shire B. R.; Sterling A. J.; Pickford H. D.; Wong M. L.; Mansfield S. J.; Caputo D. F.; Owen B.; Mousseau J. J. A general route to bicyclo[1.1.1]pentanes through photoredox catalysis. ACS Catal. 2019, 9, 9568–9574. 10.1021/acscatal.9b03190. [DOI] [Google Scholar]; e Wong M. L.; Sterling A. J.; Mousseau J. J.; Duarte F.; Anderson E. A. Direct catalytic asymmetric synthesis of α-chiral bicyclo[1.1.1]pentanes. Nat. Commun. 2021, 12, 1–9. 10.1038/s41467-021-21936-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Yen-Pon E.; Li L.; Levitre G.; Majhi J.; McClain E. J.; Voight E. A.; Crane E. A.; Molander G. A. On-DNA hydroalkylation to introduce diverse bicyclo[1.1.1]pentanes and abundant alkyls via halogen atom transfer. J. Am. Chem. Soc. 2022, 144, 12184–12191. 10.1021/jacs.2c03025. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Kaleta J.; Roncevic I.; Cisarova I.; Dracinsky M.; Solinova V.; Kasicka V.; Michl J. Bridge-chlorinated bicyclo[1.1.1]pentane-1, 3-dicarboxylic acids. J. Org. Chem. 2019, 84, 2448–2461. 10.1021/acs.joc.8b02780. [DOI] [PubMed] [Google Scholar]; h McNamee R. E.; Thompson A. L.; Anderson E. A. Synthesis and applications of polysubstituted bicyclo[1.1.0]butanes. J. Am. Chem. Soc. 2021, 143, 21246–21251. 10.1021/jacs.1c11244. [DOI] [PubMed] [Google Scholar]; i Zhao J. X.; Chang Y. X.; He C.; Burke B. J.; Collins M. R.; Del Bel M.; Elleraas J.; Gallego G. M.; Montgomery T. P.; Mousseau J. J.; Nair S. K.; Perry M. A.; Spangler J. E.; Vantourout J. C.; Baran P. S. 1,2-Difunctionalized bicyclo 1.1.1 pentanes: Long-sought-after mimetics for ortho/ meta-substituted arenes. Proc. Nat. Acad. Sci. (USA) 2021, 118, e2108881118 10.1073/pnas.2108881118. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Anderson J. M.; Measom N. D.; Murphy J. A.; Poole D. L. Bridge heteroarylation of bicyclo[1.1.1]pentane derivatives. Org. Lett. 2023, 25, 2053–2057. 10.1021/acs.orglett.3c00412. [DOI] [PubMed] [Google Scholar]; k Garry O. L.; Heilmann M.; Chen J.; Liang Y.; Zhang X.; Ma X.; Yeung C. S.; Bennett D. J.; MacMillan D. W. C. Rapid access to 2-substituted bicyclo[1.1.1]pentanes. J. Am. Chem. Soc. 2023, 145, 3092–3100. 10.1021/jacs.2c12163. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Ma X.; Han Y.; Bennett D. J. Selective Synthesis of 1-dialkylamino-2-alkylbicyclo-[1.1.1]pentanes. Org. Lett. 2020, 22, 9133–9138. 10.1021/acs.orglett.0c03612. [DOI] [PubMed] [Google Scholar]
- Qin C.; Davies H. M. L. Enantioselective synthesis of 2-arylbicyclo[1.1.0]butane carboxylates. Org. Lett. 2013, 15, 310–313. 10.1021/ol303217s. [DOI] [PubMed] [Google Scholar]
- Panish R.; Chintala S. R.; Boruta D. T.; Fang Y.; Taylor M. T.; Fox J. M. Enantioselective synthesis of cyclobutanes via sequential Rh-catalyzed bicyclobutanation/Cu-catalyzed homoconjugate addition. J. Am. Chem. Soc. 2013, 135, 9283–9286. 10.1021/ja403811t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bennett S. H.; Fawcett A.; Denton E. H.; Biberger T.; Fasano V.; Winter N.; Aggarwal V. K. Difunctionalization of C–C σ-bonds enabled by the reaction of bicyclo[1.1.0]butyl boronate complexes with electrophiles: reaction development, scope, and stereochemical origins. J. Am. Chem. Soc. 2020, 142, 16766–16775. 10.1021/jacs.0c07357. [DOI] [PubMed] [Google Scholar]; b Lai W.; Zhong K.; Liu S.; Liu S.; Chen H.; Ni H.; Zeng Z.; Zhao Z.; Lan Y.; Bai R. How strain-release determines chemoselectivity: A mechanistic study of rhodium-catalyzed bicyclo[1.1.0]butane activation. J. Phys. Chem. Lett. 2022, 13, 7694–7701. 10.1021/acs.jpclett.2c01528. [DOI] [PubMed] [Google Scholar]
- a Zhang Z.; Yu W.; Wu C.; Wang C.; Zhang Y.; Wang J. Reaction of diazo compounds with difluorocarbene: an efficient approach towards 1,1-difluoroolefins. Angew. Chem., Int. Ed. 2016, 55, 273–277. 10.1002/anie.201509711. [DOI] [PubMed] [Google Scholar]; b Zheng J.; Cai J.; Lin J.-H.; Guo Y.; Xiao J.-C. Synthesis and decarboxylative Wittig reaction of difluoromethylene phosphobetaine. Chem. Commun. 2013, 49, 7513–7515. 10.1039/c3cc44271c. [DOI] [PubMed] [Google Scholar]
- Jiang Q.; Liang Y.; Zhang Y.; Zhao X. Chalcogenide-catalyzed intermolecular electrophilic thio- and halofunctionalization of gem-difluoroalkenes: construction of diverse difluoroalkyl sulfides and halides. Org. Lett. 2020, 22, 7581–7587. 10.1021/acs.orglett.0c02784. [DOI] [PubMed] [Google Scholar]
- Qin C.; Boyarskikh V.; Hansen J. H.; Hardcastle K. I.; Musaev D. G.; Davies H. M. L. D2-Symmetric dirhodium catalyst derived from a 1,2,2-triarylcyclopropanecarboxylate ligand: design, synthesis and application. J. Am. Chem. Soc. 2011, 133, 19198–19204. 10.1021/ja2074104. [DOI] [PubMed] [Google Scholar]
- a Chernykh A. V.; Melnykov K. P.; Tolmacheva N. A.; Kondratov I. S.; Radchenko D. S.; Daniliuc C. G.; Volochnyuk D. M.; Ryabukhin S. V.; Kuchkovska Y. O.; Grygorenko O. O. Last of the gem-difluorocycloalkanes: synthesis and characterization of 2,2-difluorocyclobutyl-substituted building blocks. J. Org. Chem. 2019, 84, 8487–8496. 10.1021/acs.joc.9b00719. [DOI] [PubMed] [Google Scholar]; b Chang J.; Xu C.; Gao J.; Gao F.; Zhu D.; Wang M. Me3SiCF2Br-self-assisted Domino reaction: Catalytic synthesis of α,α-difluorocyclopentanones from methylvinylketones. Org. Lett. 2017, 19, 1850–1853. 10.1021/acs.orglett.7b00611. [DOI] [PubMed] [Google Scholar]; c Shen Q.; Hammond G. B. Regiospecific synthesis of bicyclo- and heterobicyclo- gem-difluorocyclobutenes using functionalized fluoroallenes and a novel Mo-catalyzed intramolecular [2 + 2] cycloaddition reaction. J. Am. Chem. Soc. 2002, 124, 6534–6535. 10.1021/ja0263893. [DOI] [PubMed] [Google Scholar]; d Grygorenko O. O.; Melnykov K. P.; Holovach S.; Demchuk O. Fluorinated cycloalkyl building blocks for drug discovery. ChemMedChem. 2022, 17, e202200365 10.1002/cmdc.202200365. [DOI] [PubMed] [Google Scholar]; e Remete A. M.; Nonn M.; Fustero S.; Haukka M.; Fülöp F.; Kiss L. Fluorine-containing functionalized cyclopentene scaffolds through ring contraction and deoxofluorination of various substituted cyclohexenes. Eur. J. Org. Chem. 2018, 2018, 3735–3742. 10.1002/ejoc.201800057. [DOI] [Google Scholar]
- Semeno V. V.; Vasylchenko V. O.; Vashchenko B. V.; Lutsenko D. O.; Iminov R. T.; Volovenko O. B.; Grygorenko O. O. Building the housane: diastereoselective synthesis and characterization of bicyclo [2.1.0] pentane carboxylic acids. J. Org. Chem. 2020, 85, 2321–2337. 10.1021/acs.joc.9b03044. [DOI] [PubMed] [Google Scholar]
- García-Domínguez A.; West T. H.; Primozic J. J.; Grant K. M.; Johnston C. P.; Cumming G. G.; Leach A. G.; Lloyd-Jones G. C. Difluorocarbene generation from TMSCF3: kinetics and mechanism of NaI-mediated and Si-induced anionic chain reactions. J. Am. Chem. Soc. 2020, 142, 14649–14663. 10.1021/jacs.0c06751. [DOI] [PubMed] [Google Scholar]
- Garlets Z. J.; Sanders J. N.; Malik H.; Gampe C.; Houk K.; Davies H. M. L. Enantioselective C–H functionalization of bicyclo[1.1.1]pentanes. Nat. Catal. 2020, 3, 351–357. 10.1038/s41929-019-0417-1. [DOI] [Google Scholar]
- a Ye L.; Gu Q.-S.; Tian Y.; Meng X.; Chen G.-C.; Liu X.-Y. Radical asymmetric intramolecular α-cyclopropanation of aldehydes towards bicyclo[3.1.0]hexanes containing vicinal all-carbon quaternary stereocenters. Nat. Commun. 2018, 9, 1–13. 10.1038/s41467-017-02231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kobayashi T.; Suemasa A.; Igawa A.; Ide S.; Fukuda H.; Abe H.; Arisawa M.; Minami M.; Shuto S. Conformationally restricted GABA with bicyclo[3.1.0]hexane backbone as the first highly selective BGT-1 inhibitor. ACS Med. Chem. Lett. 2014, 5, 889–893. 10.1021/ml500134k. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kim H. S.; Ohno M.; Xu B.; Kim H. O.; Choi Y.; Ji X. D.; Maddileti S.; Marquez V. E.; Harden T. K.; Jacobson K. A. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists. J. Med. Chem. 2003, 46, 4974–4987. 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Wiberg K. B.; Kass S. R.; Bishop K. III Electrophilic cleavage of cyclopropanes. Acetolysis of bicyclo[2.1.0]pentane and bicyclo[3.1.0]hexane. J. Am. Chem. Soc. 1985, 107, 996–1002. 10.1021/ja00290a041. [DOI] [Google Scholar]; e Jung M.; Lindsay V. N. One-pot synthesis of strain-release reagents from methyl sulfones. J. Am. Chem. Soc. 2022, 144, 4764–4769. 10.1021/jacs.2c00923. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Denisenko A.; Garbuz P.; Shishkina S. V.; Voloshchuk N. M.; Mykhailiuk P. K. Saturated bioisosteres of ortho-substituted benzenes. Angew. Chem. 2020, 132, 20696–20702. 10.1002/ange.202004183. [DOI] [PubMed] [Google Scholar]; g Mykhailiuk P. K. Saturated bioisosteres of benzene: where to go next?. Org. Biomol. Chem. 2019, 17, 2839–2849. 10.1039/C8OB02812E. [DOI] [PubMed] [Google Scholar]
- a Herter L.; Koutsopetras I.; Turelli L.; Fessard T.; Salomé C. Preparation of new bicyclo[2.1.1]hexane compact modules: an opening towards novel sp3-rich chemical space. Org. Biomol. Chem. 2022, 20, 9108–9111. 10.1039/D2OB01669A. [DOI] [PubMed] [Google Scholar]; b Rigotti T.; Bach T. Bicyclo[2.1.1]hexanes by visible light-driven intramolecular crossed [2 + 2] photocycloadditions. Org. Lett. 2022, 24, 8821–8825. 10.1021/acs.orglett.2c03606. [DOI] [PubMed] [Google Scholar]; c Levterov V. V.; Michurin O.; Borysko P. O.; Zozulya S.; Sadkova I. V.; Tolmachev A. A.; Mykhailiuk P. K. Photochemical in-flow synthesis of 2,4-methanopyrrolidines: pyrrolidine analogues with improved water solubility and reduced lipophilicity. J. Org. Chem. 2018, 83, 14350–14361. 10.1021/acs.joc.8b02071. [DOI] [PubMed] [Google Scholar]; d Levterov V. V.; Panasyuk Y.; Pivnytska V. O.; Mykhailiuk P. K. Water-soluble non-classical benzene mimetics. Angew. Chem., Int. Ed. 2020, 59, 7161–7167. 10.1002/anie.202000548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.