Abstract
Background/objectives
Pregnancy may potentiate the inherent hypercoagulability of the Fontan circulation, thereby amplifying adverse events. This study sought to evaluate thrombosis and bleeding risk in pregnant women with a Fontan.
Methods
We performed a retrospective observational cohort study across 13 international centres and recorded data on thrombotic and bleeding events, antithrombotic therapies and pre-pregnancy thrombotic risk factors.
Results
We analysed 84 women with Fontan physiology undergoing 108 pregnancies, average gestation 33±5 weeks. The most common antithrombotic therapy in pregnancy was aspirin (ASA, 47 pregnancies (43.5%)). Heparin (unfractionated (UFH) or low molecular weight (LMWH)) was prescribed in 32 pregnancies (30%) and vitamin K antagonist (VKA) in 10 pregnancies (9%). Three pregnancies were complicated by thrombotic events (2.8%). Thirty-eight pregnancies (35%) were complicated by bleeding, of which 5 (13%) were severe. Most bleeds were obstetric, occurring antepartum (45%) and postpartum (42%). The use of therapeutic heparin (OR 15.6, 95% CI 1.88 to 129, p=0.006), VKA (OR 11.7, 95% CI 1.06 to 130, p=0.032) or any combination of anticoagulation medication (OR 13.0, 95% CI 1.13 to 150, p=0.032) were significantly associated with bleeding events, while ASA (OR 5.41, 95% CI 0.73 to 40.4, p=0.067) and prophylactic heparin were not (OR 4.68, 95% CI 0.488 to 44.9, p=0.096).
Conclusions
Current antithrombotic strategies appear effective at attenuating thrombotic risk in pregnant women with a Fontan. However, this comes with high (>30%) bleeding risk, of which 13% are life threatening. Achieving haemostatic balance is challenging in pregnant women with a Fontan, necessitating individualised risk-adjusted counselling and therapeutic approaches that are monitored during the course of pregnancy.
INTRODUCTION
Fontan palliation for functionally univentricular hearts has dramatically improved survival and overall quality of life for affected individuals.1 Fontan surgery connects the vena cavae directly to the pulmonary arteries so that there is no longer a ventricular pump augmenting flow and lowering proximal venous pressures. The cardiac and extra-cardiac complications of this circulation are well described.2 3 Of particular concern is the risk of arterial and venous thrombosis, which has a reported incidence of 17%–33%.4–7 Traditional risk stratification tools have demonstrated poor sensitivity and specificity in this population. Severely complex disease with prior thromboses, ventricular dysfunction, atrial arrhythmia and elevated haematocrit are likely better determinants of thrombotic risk.8
As more women with Fontan physiology approach adulthood, family planning and pregnancy are of increasing interest. In addition to well-described haemodynamic changes, pregnancy is a known hypercoagulable state, attributable to rising circulating coagulation factors and decreased anticoagulant proteins.9 In women with a Fontan circulation, this additional burden may potentiate and contribute to a highly thrombogenic environment.
A thrombus can be catastrophic in univentricular circulations, and prevention with antiplatelet therapy (APT)6 7 and/or anticoagulation medication (ACM) seems warranted. However, the risks associated with bleeding are not benign and may include hypovolemia, haemodynamic instability requiring fluid and blood product administration and/or invasive procedures, and even mortality.
Achieving an optimal balance between effective thrombosis prevention and minimisation of bleeding complication is difficult. However, there are currently insufficient data to help guide the optimal balance of thrombotic prevention versus bleeding risk during pregnancy with antiplatelet or ACM therapy. The aims of this study were therefore to evaluate (1) the risk of thrombosis, (2) the risk of bleeding and (3) the relative benefit and risk of APT and ACM among pregnant women with a Fontan.
METHODS
Study design
This is a retrospective cohort study examining the thrombotic and bleeding outcomes of women with a Fontan circulation undergoing pregnancy. Data on eligible subjects across 13 international sites meeting inclusion criteria were reviewed (2006–2018). Inclusion criteria included prior Fontan palliation, one or more pregnancies carried to ≥20 weeks’ gestation and data available on pregnancy ACM and/or APT use. Data were collected on 84 women who had 108 pregnancies.
Thrombotic risk was defined from previously published risk factors in individuals with a Fontan circulation, including the presence of prior arrhythmia,10 prior history of stroke or transient ischaemic attack,11 history of venous thromboembolism (VTE), cyanosis, left ventricular ejection fraction <40% and presence of an atrio-pulmonary connection.5 For the purposes of this analysis, women with ≥2 risk factors were considered higher risk for thromboembolism.
Data collection
Baseline demographic data were collected and included age, underlying cardiac disease, surgical palliation, cardiac status, New York Heart Association class, ventricular function, documented arrhythmia, thromboembolic event, stroke, hypertension and cardiac medications. For pregnancies that progressed ≥20 weeks’ gestation, detailed intrapartum and postpartum data were collected, including maternal APT/ACM, haematological complications, cardiac complications, obstetric outcomes and neonatal complications.
Haematological complications assessed included both bleeding and thrombotic events. The severity of each event was graded using the NIH/NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.12 Grades were assigned based on collected clinical data on event severity scored from 1 to 5 (table 1).
Table 1.
NIH/NCI Common Criteria for Adverse Events (CTCAE) event severity grades
| Grade | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Symptoms | Asymptomatic or mild | Moderate | Severe (medically significant), disabling | Life threatening | Death |
| Intervention | Nil | Minimal or noninvasive | Requires hospitalisation | Urgent | Nil successful |
For each haematological complication we analysed the type of event, location, severity and treatment. Bleeding events were categorised as follows: antepartum bleeding, postpartum bleeding and non-obstetric bleeding. Thrombotic events were categorised as follows: intracardiac thrombus, deep venous thrombosis (DVT), pulmonary embolus and other.
Obstetric and neonatal outcomes with specific reference to antepartum haemorrhage and postpartum haemorrhage (estimated blood loss >500 mL for vaginal delivery and >1000 mL for caesarean section),13 14 prematurity, birth weight and early neonatal outcomes were assessed.
The terms anticoagulant and ACM were used to reflect use of any medication that was not purely antiplatelet in its anti-thrombotic action. The term antiplatelet therapy/APT was used to reflect drugs that acted primarily to effect platelet inhibition, which in this cohort consisted of ASA only. Antithrombotic therapy was used to indicate any medication active on the haemostatic system. Therapeutic heparin dosing refers to the recommended therapeutic range with or without the addition of drug monitoring. Thrombo-prophylactic dosing reflects a non-treatment regime. When the term ‘heparins’ was used on its own, it included both low molecular weight (LMWH) and unfractionated (UFH).
Data analysis
Statistical analyses were performed using SAS V.9.4 (Cary, NC). Continuous variables were summarised using mean and SD or median and 25th and 75th percentile. Categorical variables were summarised as frequency and proportions. Multiple pregnancies per subject were treated as independent observations when analysing bleeding events, as the intraclass correlation (0.09) and likelihood ratio test (χ2=0.3, df=1, p=0.29) did not support material dependency of bleeding events across pregnancies. In univariate analysis, differences between bleeding and non-bleeding groups were tested using Fisher’s exact test. The differences for categorical variables between risk groups were tested using mixed-effects models treating women as random effect, as the independence test was significant for the risk groups.
Multivariable mixed-effects logistic regression was performed to identify risk factors associated with bleeding events. Pregnancies (nested within women) were modelled as random effects. Trimester, risk category, and APT and ACM were modelled as fixed effects. The Satterthwaite approximation to the denominator df was used to obtain p values for all tests. Dunnett’s adjustment for multiple comparisons with a common reference group was performed to control the type I error rate within tests of a given predictor with multiple levels. All statistical tests were two-sided with a p value of <0.05 considered statistically significant.
Institutional review board approval
This study was approved by the institutional review board at Cincinnati Children’s Medical Center and by the institutional review board or ethics committee at each participating institution. Data sharing agreements were in place with participating sites. No informed consent was required. This study was done without patient involvement. Patients were not invited to comment on the study design, patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document.
RESULTS
Baseline characteristics
A total of 84 women (27±5 years old) with 108 pregnancies met inclusion criteria. Of these, 80 pregnancies (74%) occurred in women at low thrombotic risk and 28 pregnancies (26%) were in women at high thrombotic risk. The most common underlying cardiac diagnoses were tricuspid atresia (21%), double inlet left ventricle (17%) and pulmonary atresia (15%). The most common type of Fontan was lateral tunnel (46%), followed by atrio-pulmonary (32%) and extracardiac (19%). Most women (n=64, 76%) underwent a single pregnancy, with 16 (19%) reporting two pregnancies and 4 (5%) reporting three pregnancies. Pregnancy and fetal outcomes are summarised in table 2. The mean gestational age (GA) at delivery was 33±5 weeks. The mean birth weight was 2040±718 g. Five infants (4.6%) had skeletal or congenital heart defects. Four had congenital heart disease (CHD) and one had a missing nasal bone; however, the mother was not documented to be on warfarin. There were 10 (9.3%) neonatal deaths reported. Of these, 5 were 20–24 weeks GA at delivery, 4 were 25–27 weeks GA and the other was 28–34 weeks GA. No further details surrounding their demise were recorded.
Table 2.
Obstetric and fetal outcomes
| Variable (number used) | Overall (N=108) |
|---|---|
| Gestational age (N=100) | |
| 20–24 | 8 (8.0) |
| 25–27 | 7 (7.0) |
| 28–34 | 30 (30.0) |
| 35–36 | 23 (23.0) |
| 37+ | 32 (32.0) |
| Birth weight (N=84) | 2130 (1620 to 2490) |
| Fetal weight (N=104) | |
| <10th percentile | 24 (23.1) |
| >10th percentile | 84 (80.8) |
| Skeletal or CHD (N=108) | |
| No | 103 (95.4) |
| Yes | 5 (4.6) |
| Neonatal death (N=108) | |
| No | 98 (90.7) |
| Yes | 10 (9.3) |
CHD, congenital heart disease.
The remaining pre-pregnancy characteristics are summarised in table 3. Pre-pregnancy antithrombotic strategies are described in table 4 and were not significantly different between the subgroups retrospectively defined as high or low thrombotic risk.
Table 3.
Pre-pregnancy characteristics
| Variable (n=108) | Number of pregnancies affected |
|---|---|
| AP* Fontan | 34 (31.5%) |
| Arrhythmia | 33 (30.6) |
| VTE†/thrombus | 7 (6.4) |
| Systemic hypertension | 4 (3.7) |
| Cyanosis‡ | 18 (16.7) |
| Baseline saturations | 94 (92.96) |
| NYHA§ class | |
| I | 61 (56.4) |
| II | 22 (20.3) |
| III | 5 (4.6) |
| Ventricular ejection fraction | |
| 0%–20% | 1 (0.9) |
| 20%–40% | 3 (2.7) |
| 40%–60% | 51 (47.2) |
| >60% | 24 (22.2) |
| Medication prior to pregnancy | |
| Cardiac medications | 32 (29.6) |
| Anticoagulation medication | 32 (29.6) |
Values are n (%), median (Q1, Q3).
Atriopulmonary.
Venous thromboembolism.
Cyanosis=clinically visible desaturation.
New York Heart Association function classification.
Table 4.
Antithrombosis strategy by risk factor clustering
| Anticoagulation strategy by trimester | N used | Overall (N=108) | No risk factors (N=47) | 1 risk factor (N=33) | 2+ risk factors (N=28) | P value |
|---|---|---|---|---|---|---|
| Pre-pregnancy | 108 | 0.071 | ||||
| None | 53 (49.1%) | 28 (59.6%) | 16 (48.5%) | 9 (32.1%) | ||
| Aspirin | 23 (21.3%) | 7 (14.9%) | 11 (33.3%) | 5 (17.9%) | ||
| Prophylactic heparin* | 1 (0.9%) | 1 (2.1%) | 0 (0.0%) | 0 (0.0%) | ||
| VKA† | 28 (25.9%) | 10 (21.3%) | 6 (18.2%) | 12 (42.9%) | ||
| Combo‡ | 3 (2.8%) | 1 (2.1%) | 0 (0.0%) | 2 (7.1%) | ||
| Aspirin during pregnancy | 108 | 0.40 | ||||
| No | 54 (50.0%) | 21 (44.7%) | 16 (48.5%) | 17 (60.7%) | ||
| Yes | 54 (50.0%) | 26 (55.3%) | 17 (51.5%) | 11 (39.3%) | ||
| VKA† during pregnancy | 108 | 0.47 | ||||
| No | 93 (86.1%) | 42 (89.4%) | 29 (87.9%) | 22 (78.6%) | ||
| Yes | 15 (13.9%) | 5 (10.6%) | 4 (12.1%) | 6 (21.4%) | ||
| Treatment heparin during pregnancy | 108 | 0.13 | ||||
| NA/unknown | 65 (60.2%) | 30 (63.8%) | 22 (66.7%) | 13 (46.4%) | ||
| Prophylactic heparin* | 21 (19.4%) | 5 (10.6%) | 7 (21.2%) | 9 (32.1%) | ||
| Therapeutic heparin* | 22 (20.4%) | 12 (25.5%) | 4 (12.1%) | 6 (21.4%) | ||
| First trimester | 108 | 0.33 | ||||
| None | 26 (24.1%) | 11 (23.4%) | 10 (30.3%) | 5 (17.9%) | ||
| Aspirin | 47 (43.5%) | 23 (48.9%) | 15 (45.5%) | 9 (32.1%) | ||
| Prophylactic heparin* | 12 (11.1%) | 3 (6.4%) | 3 (9.1%) | 6 (21.4%) | ||
| Therapeutic heparin* | 13 (12.0%) | 6 (12.8%) | 1 (3.0%) | 6 (21.4%) | ||
| VKA† | 6 (5.6%) | 3 (6.4%) | 2 (6.1%) | 1 (3.6%) | ||
| Combo‡ | 4 (3.7%) | 1 (2.1%) | 2 (6.1%) | 1 (3.6%) | ||
| Second trimester | 108 | 0.14 | ||||
| None | 26 (24.1%) | 11 (23.4%) | 11 (33.3%) | 4 (14.3%) | ||
| Aspirin | 38 (35.2%) | 20 (42.6%) | 9 (27.3%) | 9 (32.1%) | ||
| Prophylactic heparin* | 14 (13.0%) | 2 (4.3%) | 7 (21.2%) | 5 (17.9%) | ||
| Therapeutic heparin* | 12 (11.1%) | 8 (17.0%) | 1 (3.0%) | 3 (10.7%) | ||
| VKA† | 8 (7.4%) | 2 (4.3%) | 2 (6.1%) | 4 (14.3%) | ||
| Combo‡ | 10 (9.3%) | 4 (8.5%) | 3 (9.1%) | 3 (10.7%) | ||
| Third trimester | 93 | 0.52 | ||||
| None | 25 (26.9%) | 9 (22.5%) | 11 (35.5%) | 5 (22.7%) | ||
| Aspirin | 31 (33.3%) | 16 (40.0%) | 8 (25.8%) | 7 (31.8%) | ||
| Prophylactic heparin* | 10 (10.8%) | 2 (5.0%) | 4 (12.9%) | 4 (18.2%) | ||
| Therapeutic heparin* | 18 (19.4%) | 11 (27.5%) | 4 (12.9%) | 3 (13.6%) | ||
| VKA† | 3 (3.2%) | 1 (2.5%) | 1 (3.2%) | 1 (4.5%) | ||
| Combo‡ | 6 (6.5%) | 1 (2.5%) | 3 (9.7%) | 2 (9.1%) |
Values are N (%).
Heparin here refers to either unfractionated heparin or low molecular weight heparin.
Vitamin K antagonist.
Combination of anticoagulation medicines.
Anticoagulation and antiplatelet strategies
APT and ACM strategy varied between and within women by trimester (see table 4). ACM was undocumented in 20% of women. Aspirin monotherapy was the most common regimen, used in 46 pregnancies (42.6%). Heparins, the second most common regimen, had greatest prevalence of use during the third trimester in 28 pregnancies (26%). Heparin dosing was split between prophylactic (10 patients (10.8%)) and therapeutic (18 women (19.4%)). Vitamin K antagonist (VKA), the least prescribed therapy, was used most during the second trimester in 10 pregnancies (9.3%) either alone or in combination with heparin (see table 3). The most common indications for VKA use were history of stroke, DVT and arrhythmia. During the third trimester, the most common time for a bleeding event, 31 women (33.3%) were taking ASA, 5 women were taking VKAs (5.4%) and a combination of agents were prescribed in 6 (6.4%). The difference in AT between low-risk and high-risk groups both before and during pregnancy is summarised in table 4.
Bleeding complications
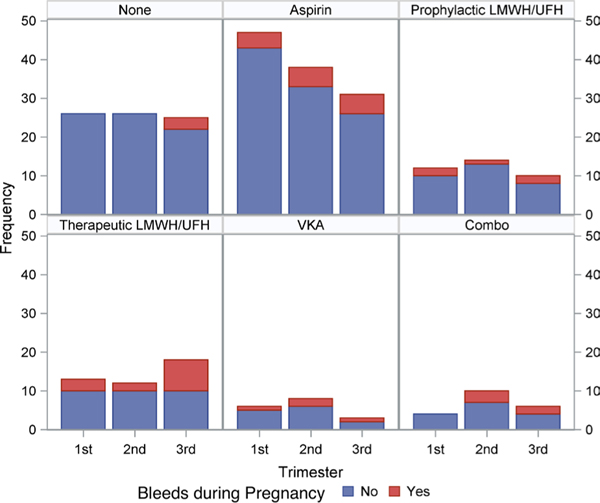
Thirty-eight pregnancies (35.2%) were complicated by bleeding; most were obstetric in origin (33 of the 38, 87%). The third trimester was the most common time for a bleeding event (n=18, 47.6%). Antepartum haemorrhage (n=17, 44.7%) was attributed to subchorionic or placental haematoma in 5 (29.4%) and placental abruption in 2 (11.8%). No cause was specified in the remaining pregnancies. Postpartum haemorrhage (PPH) accounted for 16 bleeding events (42.1%). Five bleeding events (13%) were potentially life threatening. Women with bleeding events in the third trimester were more likely to be on therapeutic LMWH or UFH as compared with women without bleeding events (OR 5.10, 95% CI 1.62 to 16.0, p=0.018). These data are summarised in figure 1 and table 5.
Figure 1.
Anticoagulation strategy and bleeding events during pregnancy. Combo, combination of anticoagulation/antiplatelet medications; LMWH, low molecular weight heparin; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Table 5.
Characteristics of bleeding events during pregnancy
| Trimester | Anticoagulation at time of bleeding event | Severity of bleeding event (CTCAE* grade) | Treatment | |||
|---|---|---|---|---|---|---|
| First (n=10) | Aspirin | 4 | 1 | 8 | Observation/uterotonic | 8 |
| Prophylactic heparin† | 2 | 2 | 1 | medications | 1 | |
| Therapeutic heparin† | 3 | Volume replacement/blood | 1 | |||
| VKA‡ | 1 | transfusion/factor complex | ||||
| Interventional treatment | ||||||
| Second (n=10) (APH n=8, PPH n=2) | Aspirin | 4 | 1 | 6 | Observation/uterotonic | 5 |
| Therapeutic heparin† | 1 | 3 | 2 | medications | 1 | |
| VKA‡ | 2 | Volume replacement/blood | 3 | |||
| Combo§ | 3 | transfusion/factor complex | ||||
| Interventional treatment | ||||||
| Third (n=18) (APH n=4, PPH n=14) | None | 3 | 1 | 9 | Observation/uterotonic | 11 |
| Aspirin | 5 | 2 | 5 | medications | 5 | |
| Prophylactic heparin† | 1 | 4 | 3 | Volume replacement/blood | 1 | |
| Therapeutic heparin† | 6 | transfusion/factor complex | 2 | |||
| VKA‡ | 1 | Interventional treatment | ||||
| Combo§ | 1 | surgery | ||||
| Type of event | ||||||
| Antepartum haemorrhage (n=17) | Aspirin | 6 | 1 | 11 | Observation/uterotonic | 11 |
| Prophylactic heparin† | 3 | 2 | 3 | medications | 2 | |
| Therapeutic heparin† | 3 | 3 | 1 | Volume replacement/blood | 2 | |
| VKA‡ | 3 | transfusion/factor complex | ||||
| Combo§ | 2 | Interventional treatment | ||||
| Postpartum haemorrhage (n=16) | None | 3 | 1 | 10 | Observation/uterotonic | 11 |
| Aspirin | 5 | 2 | 3 | medications | 4 | |
| Prophylactic heparin† | 1 | 4 | 1 | Volume replacement/blood | 2 | |
| Therapeutic heparin† | 5 | transfusion/factor complex | ||||
| VKA‡ | 1 | Interventional treatment | ||||
| Combo§ | 1 | |||||
| Intracranial haemorrhage (n=1) | Combo§ | 1 | III | 1 | Interventional treatment | 1 |
| Other (n=4) | Aspirin | 2 | I | 2 | Observation/uterotonic medications | 2 |
| Therapeutic heparin† | 2 | IV | 2 | Unknown | 2 | |
NIH/NCI Common Terminology Criteria for Adverse Events.
Heparin here refers to either unfractionated heparin or low molecular weight heparin.
Vitamin K antagonist.
Combination of anticoagulation/antiplatelet medications.
APH, antepartum haemorrhage; PPH, postpartum haemorrhage.
Thrombosis complications
Three women had a thrombotic event. The details are summarised in table 6 and the online supplement. Notably, one woman was maintained on ASA and prophylactic LMWH, and the other two were maintained on therapeutic ACM until just before delivery, when it was discontinued.
Table 6.
Patients with thrombotic complications
| Relevant diagnoses | Thrombosis risk factors | Details of thrombotic event | Treatment and outcome | |
|---|---|---|---|---|
| Patient 1 Age 21 years |
Pulmonary atresia+extracardiac Fontan | Prior arrhythmia Pre-pregnancy: ASA |
At 6 weeks’ gestation, an asymptomatic intracardiac thrombus was incidentally noted (CTCAE grade II) | ASA and prophylactic LMWH. Delivered vaginally at 35 weeks after spontaneous preterm labour without complication |
| Patient 2 Age 22 years |
Left atrial isomerism, severe LV dysfunction, moderate to severe AV valve regurgitation | LV dysfunction, prior VTE, cyanosis, saturations 88% Pre-pregnancy: ASA+oral AC |
Hepatic vein thrombosis developed around delivery at 24 weeks’ gestation for umbilical artery flow reversal (CTCAE grade II) | 1st TM: ASA+therapeutic LMWH 2nd TM/post partum—IV UFH – Infant passed away in perinatal period |
| Patient 3 Age 25 years |
Tricuspid atresia+extracardiac Fontan | Mildly reduced ventricular function; chronic hypertension | 3rd pregnancy, delivered via CS at 31 weeks due to PPROM. 24 hours later presented with CV collapse due to PE; CTCAE grade IV |
AC throughout pregnancy: tinzaparin 12 000 IU/day Required ICU management for several days and returned to baseline |
AC, anticoagulation; ASA, acetyls salicylic acid (aspirin); AV, atrioventricular; CS, caesarian section; CV, cardiovascular; ICU, intensive care unit; IV UFH, intravenous unfractionated heparin; LMWH, low molecular weight heparin; LV, left ventricular; PPROM, preterm premature rupture of membranes; TM, trimester; VTE, venous thrombo embolism.
Multivariable analysis
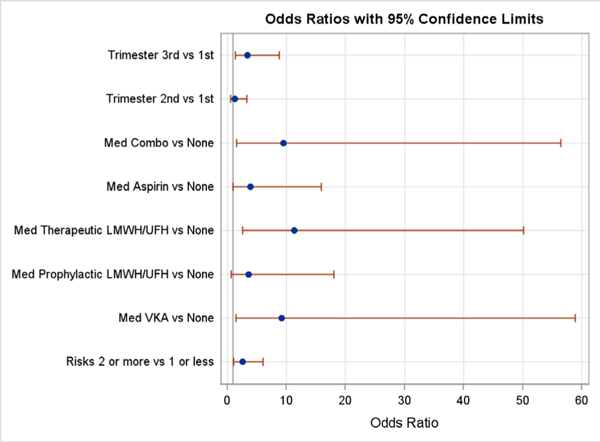
In the multivariable mixed-effects logistic regression models, risk category, trimester and ACM were all significantly associated with bleeding events (p<0.05, figure 2). APT and ACM were combined (ASA, therapeutic heparin, prophylactic heparin, VKA or a combination) and compared with no therapy (neither antiplatelet nor ACM). Women defined as high risk for thrombosis had an increased risk of bleeding in the multivariable model (high-risk OR 2.49, 95% CI 1.05 to 5.89, p=0.038). Bleeding increased in the second trimester by 20%, but this was not statistically significant (OR 1.27, 95% CI 0.436 to 3.69, p=0.62). In contrast, bleeding doubled in the third trimester, achieving statistical significance (OR 3.3, 95% CI 1.39 to 9.63, p=0.025). APT and ACM was significantly associated with bleeding (p=0.032). Compared with no APT or ACM use, the use of therapeutic heparin (OR 15.6, 95% CI 1.88 to 129, p=0.006), VKA (OR 11.7, 95% CI 1.06 to 130, p=0.032) or any combination ACM (OR 13.0, 95% CI 1.13 to 150, p=0.032) were significantly associated with a bleeding event. Aspirin (OR 5.41, 95% CI 0.73 to 40.4, p=0.067) and prophylactic doses of LMWH (OR 4.68, 95% CI 0.488 to 44.9, p=0.096) were not associated with a significantly increased bleeding risk throughout pregnancy (adjusted for both). Since only three women had a thrombotic event during pregnancy, statistical analysis of thrombotic determinants was not pursued.
Figure 2.
Selected factors associated with bleeding events by multivariate logistic regression. Combo, combination of anticoagulation/ antiplatelet medications; LMWH, low molecular weight heparin; UFH, unfractionated heparin; VKA, vitamin K antagonist.
DISCUSSION
To date, this is the only study focusing on bleeding and thrombosis outcomes and risk factors in pregnant women with a Fontan circulation. The results reflect outcomes at 13 international tertiary referral centres. The key finding of this study is that there is high bleeding risk (35%) in women with a Fontan circulation, and this risk is associated with the use of therapeutic ACM as opposed to aspirin. Aspirin and prophylactic LMWH appear safer with respect to bleeding risk. Although variable therapeutic approaches were used in this cohort, the prevalence of thrombosis (2.8%) was not higher than that reported outside of pregnancy in this population. However, it is significantly higher than the prevalence of thrombosis reported in the general pregnant population (0.5–2 per 1000 pregnancies).15
In 28 (25.9%) pregnancies, we observed at least two thrombotic risk factors pre-pregnancy, indicating these women were at high thrombotic risk. Interestingly, we could not demonstrate a statistically greater propensity for these patients to be on ACM as opposed to ASA before or during pregnancy. One patient with thrombosis had one risk factor, while the other two had ≥2 thrombosis risk factors. Thrombotic events for each patient occurred during a period of anticoagulation cessation. No thrombotic event occurred in women with an AP Fontan, despite the understanding that thrombosis risk appears higher with this surgical variant.16 Outside of pregnancy, rates of thromboembolism in Fontan patients range from 4% to 33% and depend on multiple clinical factors.17 In the 2018 update on VTE during pregnancy, the American Society of Haematology recommended that patients with an individual thrombosis risk ≤2% do not need antithrombotic therapy during pregnancy.18 However, this document did not specifically evaluate or make recommendation for patients with CHD. Our observed thrombosis rate was >2%, suggesting that anti-thrombotic therapy is likely warranted during pregnancy.18 Two of the three women with thrombotic events had elevated thrombotic risk based on known risk factors in the published literature. The true impact of ACT in higher risk women is difficult to assess definitively in this study. Women were just as likely to be on ACT as on APT despite being in the higher risk category for thrombosis. However, given the absence of thrombosis in 26 other women with elevated risk, it is tempting to speculate that ACT may have played a beneficial role. The authors believe that those with currently accepted indications for anticoagulation should continue on ACM during pregnancy. In the high thrombosis risk group, therapeutic ACM appears to be an effective strategy. Although most bleeds were minor, 13% of them were severe, underscoring the importance of careful risk evaluation, management and monitoring of those requiring therapeutic ACM. New approaches are necessary to identify those truly at higher thrombosis risk.
Platelet inhibition with ASA monotherapy was the most common anti-thrombotic strategy used during pregnancy. Aspirin use diminished throughout the duration of pregnancy. This appeared to correspond to an increase in LMWH/UFH use as pregnancy progressed. LMWH was the most common ACM agent used. When heparin dosing was recorded, it was split almost equally between prophylactic and therapeutic strategies.
Bleeding complications occurred in more than a third of this cohort. The rate was strongly and independently associated with therapeutic ACM, as opposed to isolated APT or no therapy, and was more likely to occur in the third trimester or immediately postpartum. It is presumably associated with the birth process. These periods also represent the greatest vulnerability to thrombosis. We did not collect data on routine uterotonic medication use and therefore were unable to determine if postpartum bleeds were impacted by underutilisation of uterotonics at delivery. The frequency of bleeding observed was consistent with previously reported rates of bleeding in pregnant Fontan patients19 and remains higher than other patient groups receiving ACM during pregnancy.20–22
The observed antepartum haemorrhage (APH) rate was surprisingly high, accounting for 44.7% of bleeding events, or 15.7% of pregnancies. The reported APH rate in the general population is 3.5%,23 and is largely due to placental abruption or placenta previa, so this finding is concerning. Aspirin does not appear to increase antepartum bleeding in the general population.23 APH rates in patients on therapeutic ACM are scarce and are mainly reported in women with mechanical valves. The largest cohort with APH data found that 17% of women experienced APH.24 This is similar to our reported rate and suggests that our observed APH rates may simply be due to anticoagulation. However, other causes such as abnormal placentation, intrinsic bleeding propensity and/or raised venous pressures cannot be excluded.25
PPH occurred in 14.8% of pregnancies (accounting for 42.1% of all bleeding events). PPH rates for non-CHD patients on ACM are higher than that for patients not receiving ACM.21 For patients on therapeutic LMWH, PPH rates are reported as 30% (vs 18% non-anticoagulated patients) for vaginal delivery and 12% (vs 4% in non-anticoagulated patients) for caesarean section.20 The demonstrated PPH rate is slightly lower than prior reports of PPH in pregnant Fontan patients and similar to PPH rates in non-Fontan patients receiving ACM.26 27
Limitations
Although this study represents the largest pregnant Fontan series evaluating anticoagulation strategy and thrombotic and bleeding outcomes, it is still significantly impacted by the number of women enrolled. Specifically, the small number of thrombosis events makes meaningful analysis difficult. There are inherent deficiencies in the completeness of data available due to the retrospective nature of data collection. Information on ACM and indications for anticoagulation were missing in up to 20% of patients. As some of our data came from databases in which the original chart was unavailable, it was not possible in many cases to obtain this information. Selection bias towards worse outcomes is possible, as all the centres included were tertiary practices. There was no standardised management of anticoagulation for Fontan women during pregnancy across centres. Given the relatively small sample size and heterogenous ACM approaches in this cohort, the observations that aspirin monotherapy and prophylactic LMWH in low-risk patients offer thrombotic protection without higher bleeding risk requires further prospective study to (1) confirm the baseline rate of bleeding and thrombotic events in this cohort and (2) to determine how aspirin and/or prophylactic dose LMWH affects bleeding and thrombotic risk.
CONCLUSIONS
We report results from the largest retrospective analysis of ACM strategy in women with Fontan physiology undergoing pregnancy and delivery. We observed high thrombotic and bleeding risk in a cohort with a variety of ACM regimens. Our data highlight that aspirin monotherapy or prophylactic dose LMWH in low-risk women is reasonable to minimise both thrombotic and bleeding risk. However, ACM, necessary in high-risk women, remains challenging as it is accompanied by a bleeding risk which may be life threatening in up to 13%.
Careful risk stratification for thrombosis as well as anticipated bleeding risk should be emphasised in preconception evaluation and counselling, and should be repeated at regular intervals during the pregnancy, as risk may change. It is especially important to plan anti-thrombotic strategy carefully around the time of delivery in the context of a multidisciplinary team and input from a haematologist with thrombosis expertise may be helpful. In addition, there is a need for the assessment of haemostatic biomarkers that may assist in risk prediction. A number of such biomarkers have already been identified in the non-pregnant state in Fontan subjects,28 29 but their utility to guide management during pregnancy needs further evaluation.
Supplementary Material
Key messages.
What is already known on this subject?
The adult population with a Fontan circulation is increasing, as is the number of these patients who desire safe pregnancy. Pregnancy is a known prothrombotic state and may contribute to a greater risk of thrombosis in patient with a Fontan circulation. There is currently scarce evidence to guide the decision-making for anticoagulation strategies, balancing bleeding and thrombosis risk.
What might this study add?
This study examined anticoagulation strategies and bleeding and thrombosis outcomes in a cohort of Fontan patients undergoing pregnancy. Clinically guided antithrombotic strategies in this cohort resulted in thrombotic events at levels that do not appear higher than in the non-pregnant state. In addition, these observational data have highlighted the significantly increased antepartum and postpartum haemorrhage seen in this cohort. The former may contribute to the high fetal loss rates seen in Fontan women.
How might this impact on clinical practice?
A thorough appreciation of prothrombotic risk factors before and during pregnancy will aid rational employment of anti-thrombotic therapies and mitigate potential over-utilisation of anticoagulation strategies. We believe that ASA may be safe for low-risk patients and presents relatively little bleeding risk. Prophylactic low molecular weight heparin appears to be a good middle ground for those at intermediate risk, although the small numbers in this subgroup make it difficult to apply firm recommendations. Therapeutic anticoagulation medication is clearly necessary for those at highest risk.
Acknowledgements
Sheila Drakeley (Boston Children’s Hospital), and the International Society of Adult Congenital Heart Disease (ISACHD, www.isachd.org) and the AARCC under whose auspices this study was supported. EO currently holds the Bitove Family Professorship of Adult Congenital Heart Disease. Dr Elisa Bradley holds a grant with the following grant number:K08HL148701
Funding
The Heart Institute, Cincinnati Children’s Hospital Medical Center, 333 Burnett Avenue, Cincinnati.
Footnotes
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement
Data is available upon reasonable request and upon agreement of the coathors and individual insitutions
REFERENCES
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971;26:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rychik J, Goldberg DJ. Late consequences of the Fontan operation. Circulation 2014;130:1525–8. [DOI] [PubMed] [Google Scholar]
- 3.van den Bosch AE, Roos-Hesselink JW, Van Domburg R, et al. Long-term outcome and quality of life in adult patients after the Fontan operation. Am J Cardiol 2004;93:1141–5. [DOI] [PubMed] [Google Scholar]
- 4.Coon PD, Rychik J, Novello RT, et al. Thrombus formation after the Fontan operation. Ann Thorac Surg 2001;71:1990–4. [DOI] [PubMed] [Google Scholar]
- 5.Balling G, Vogt M, Kaemmerer H, et al. Intracardiac thrombus formation after the Fontan operation. J Thorac Cardiovasc Surg 2000;119:745–52. [DOI] [PubMed] [Google Scholar]
- 6.Monagle P, Cochrane A, Roberts R, et al. A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the Fontan procedure in children. J Am Coll Cardiol 2011;58:645–51. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs ML, Pourmoghadam KK. Thromboembolism and the role of anticoagulation in the Fontan patient. Pediatr Cardiol 2007;28:457–64. [DOI] [PubMed] [Google Scholar]
- 8.Khairy P, Aboulhosn J, Broberg CS, et al. Anticoagulation therapy in congenital heart disease I and the alliance for adult research in congenital C. thromboprophylaxis for atrial arrhythmias in congenital heart disease: a multicenter study. Int J Cardiol 2016;223:729–35. [DOI] [PubMed] [Google Scholar]
- 9.Brenner B. Haemostatic changes in pregnancy. Thromb Res 2004;114:409–14. [DOI] [PubMed] [Google Scholar]
- 10.Egbe AC, Connolly HM, McLeod CJ, et al. Thrombotic and embolic complications associated with atrial arrhythmia after Fontan operation: role of prophylactic therapy. J Am Coll Cardiol 2016;68:1312–9. [DOI] [PubMed] [Google Scholar]
- 11.McCrindle BW, Manlhiot C, Cochrane A, et al. Factors associated with thrombotic complications after the Fontan procedure. J Am Coll Cardiol 2013;61:346–53. [DOI] [PubMed] [Google Scholar]
- 12.Common terminology criteria for adverse events (CTCAE) version 4.0.
- 13.Committee on practice B-O. practice Bulletin No. 183: postpartum hemorrhage. Obstet Gynecol 2017;130:e168–86. [DOI] [PubMed] [Google Scholar]
- 14.Mousa HA, Blum J, Abou El Senoun G, et al. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev 2014:CD003249. [DOI] [PMC free article] [PubMed]
- 15.James A, Committee on Practice Bulletins—Obstetrics. Practice bulletin no. 123: thromboembolism in pregnancy. Obstet Gynecol 2011;118:718–29. [DOI] [PubMed] [Google Scholar]
- 16.Deshaies C, Hamilton RM, et al. , Alliance for Adult Research in Congenital Cardiology. Thromboembolic risk after atriopulmonary, lateral tunnel, and extracardiac conduit Fontan surgery. J Am Coll Cardiol 2019;74:1071–81. [DOI] [PubMed] [Google Scholar]
- 17.Zentner D, Celermajer DS, Gentles T, et al. Management of people with a Fontan circulation: a cardiac Society of Australia and New Zealand position statement. Heart Lung Circ 2020;29:5–39. [DOI] [PubMed] [Google Scholar]
- 18.Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv 2018;2:3317–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cauldwell M, Von Klemperer K, Uebing A, et al. Why is post-partum haemorrhage more common in women with congenital heart disease? Int J Cardiol 2016;218:285–90. [DOI] [PubMed] [Google Scholar]
- 20.Knol HM, Schultinge L, Veeger NJGM, et al. The risk of postpartum hemorrhage in women using high dose of low-molecular-weight heparins during pregnancy. Thromb Res 2012;130:334–8. [DOI] [PubMed] [Google Scholar]
- 21.Wang EH, Marnoch CA, Khurana R, et al. Haemorrhagic complications of peripartum anticoagulation: a retrospective chart review. Obstet Med 2014;7:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hagen IM, Roos-Hesselink JW, Ruys TPE, et al. Pregnancy in women with a mechanical heart valve: clinical perspective. Circulation 2015;132:132–42. [DOI] [PubMed] [Google Scholar]
- 23.Roberge S, Bujold E, Nicolaides KH. Meta-analysis on the effect of aspirin use for prevention of preeclampsia on placental abruption and antepartum hemorrhage. Am J Obstet Gynecol 2018;218:483–9. [DOI] [PubMed] [Google Scholar]
- 24.McLintock C, McCowan LME, North RA. Maternal complications and pregnancy outcome in women with mechanical prosthetic heart valves treated with enoxaparin. BJOG 2009;116:1585–92. [DOI] [PubMed] [Google Scholar]
- 25.Phillips AL, Cetta F, Kerr SE, et al. The placenta: a site of end-organ damage after Fontan operation. A case series. Int J Cardiol 2019;289:52–5. [DOI] [PubMed] [Google Scholar]
- 26.Cauldwell M, Von Klemperer K, Uebing A, et al. A cohort study of women with a Fontan circulation undergoing preconception counselling. Heart 2016;102:534–40. [DOI] [PubMed] [Google Scholar]
- 27.Gelson E, Curry R, Gatzoulis MA, et al. Maternal cardiac and obstetric performance in consecutive pregnancies in women with heart disease. BJOG 2015;122:1552–9. [DOI] [PubMed] [Google Scholar]
- 28.Chaloupecký V, Svobodová I, Hadacová I, et al. Coagulation profile and liver function in 102 patients after total cavopulmonary connection at mid term follow up. Heart 2005;91:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomkiewicz-Pajak L, Hoffman P, Trojnarska O, et al. Abnormalities in blood coagulation, fibrinolysis, and platelet activation in adult patients after the Fontan procedure. J Thorac Cardiovasc Surg 2014;147:1284–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon reasonable request and upon agreement of the coathors and individual insitutions