Abstract
Objectives
This study aims to investigate whether the medial cortical ratio (MCR) is associated with fixation failure in patients undergoing plate-screw fixation due to proximal humeral fracture.
Patients and methods
Between May 2011 and October 2020, a total of 93 patients (25 males, 68 females; mean age: 74.2±5.3 years; range, 65 to 92 years) who were operated for a proximal humeral fracture were retrospectively analyzed. The patients were divided into two groups during follow-up: patients with fixation failure (n=18) and patients with intact fixation (n=75). After radiological analysis, the MCR and fracture type were recorded. The effects of MCR and fracture type on the development of failure were evaluated.
Results
The mean follow-up was 41.3±4.7 (range, 18 to 66) months. There was no significant correlation between fixation failure and MCR in either group (p=0.535). However, the risk of fixation failure increased by seven-fold in patients with MCR greater than 0.09 compared to the other patients. In addition, the fracture type was significantly more severe in the failed fixation group than the intact fixation group (p<0.001).
Conclusion
Surgical treatment of proximal humeral fractures in elderly patients still remains a challenge for surgeons due to the high failure rate. In elderly patients scheduled for surgery to treat a proximal humerus fracture, a reverse shoulder arthroplasty may be a reasonable choice to avoid reoperation due to fixation failure in elderly with three-part or more fragmented fractures and high MCR.
Keywords: Elderly, failure, fixation, medial cortical ratio, proximal humerus.
Introduction
Proximal humeral fractures are common fractures in the osteoporotic population over 65 years of age.[1] Although most of these patients are treated non-operatively, complex fractures, such as fracturedislocations, head-split fractures, varus displacement, and complete head-shaft displacement, often have poor outcomes and require surgical fixation.[2]
Surgical treatment options include percutaneous nailing, locking plate-screw application, and arthroplasty, and the complexity of the fracture determines the surgical method.[3] Although the treatment of proximal humeral fractures has evolved in recent years toward preservation of the humeral head, osteoporosis-related bone loss in the humeral head causes complications, such as poor fixation and screw cut-out or screw penetration due to impaired fracture healing.[1] Despite the importance of bone density, patients often do not undergo whole-body or operative site-directed bone mineral densitometry prior to surgery. In addition, limited mobilization due to the fracture makes it difficult to measure bone mineral density (BMD) before surgery and prevents bone quality from guiding surgical decision-making.[4]
Since Barnett and Nordin,[5] followed by Virtama and Telkka[6] reported that cortical thickness determination could be used as a predictor of bone mineralization, cortical thickness measurements have been used to estimate osteoporotic changes in the bone. Considering the high risk of insufficiency after fixation in osteoporotic patients, the medial cortical ratio (MCR) may be associated with insufficiency and indirectly affect the choice of surgical method.[7,8] However, to our current knowledge, there are few studies in the literature investigating this approach.
In the present study, we aimed to investigate whether MCR was associated with fixation failure in whom a plate-screw was used due to a proximal humeral fracture. By clarifying this relationship, we aimed to determine whether the MCR measured before surgery could be used to select the appropriate surgical method and to identify the relationship between the fracture type according to the Neer classification[2] and the development of fixation failure.
Patients and Methods
This single-center, retrospective study was conducted at Başkent University Faculty of Medicine, Department of Orthopedics and Traumatology between May 2011 and October 2020. A total of 139 patients with a diagnosis of proximal humeral fracture during the study period were screened. Patients older than 65 years with an isolated traumatic proximal humeral fracture and subsequent plate-screw fixation with a proximal humeral internal locking system (PHILOS™; DePuy Synthes, Raynham, MA, USA) with at least two years of follow-up were included. Patients without displaced fractures (n=10), open fractures (n=1), pathological fractures (n=3), multiple trauma (n=7), patients who underwent shoulder arthroplasty (n=6), patients with missing data (n=7), and patients with poor reduction quality based on the intact measurements (e.g., varus malalignment, low headshaft angle value, lack of medial cortical support after surgery) (n=12) were excluded from the study. Finally, a total of 93 patients (25 males, 68 females; mean age: 74.2±5.3 years; range, 65 to 92 years) who met the study criteria were recruited.
Data included demographic information, such as age and sex, follow-up period, fracture type according to Neer classification, and follow-up radiographs, which were retrieved from the computed tomography database and medical records. Radiological follow-up was performed at 1.5 months, three months, six months, and 12 months in the first year after surgery and annually thereafter. Radiographic examination was performed in patients who developed abnormal symptoms, such as sudden onset of increased pain and limitation of motion, during clinical follow-up after surgery, regardless of the pre-specified schedule.
Radiological analyses and determination of MCR
Radiological images of all patients were reviewed using the Picture Archiving Communications System (PACS). At least one set of follow-up radiographs was available for each patient. All radiographs were evaluated by two independent observers with at least 10 years of experience in orthopedic surgery.
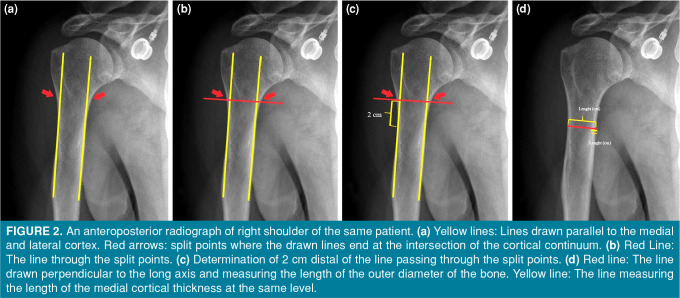
First, the preoperative radiographs were evaluated, and the fracture type was recorded according to the Neer classification and the MCR according to Newton et al.[9] (Figure 1). The MCR was measured using the digital ruler available at PACS. The measurement was taken on the unaffected extremity, 20 mm distal to the most proximal level of the humeral diaphysis, where the endosteal borders of the lateral and medial cortices were parallel. The total diameter of the diaphysis and the thickness of the medial cortex were measured at this point and used to determine the MCR (Figure 2). The patients’ follow-up radiographs were reviewed, and those in whom fracture fixation failed were identified. Radiological failure was defined according to the definition of Krappinger et al.[10] as any type of postoperative relative movement between the implant and the humeral head or shaft. We defined failure based on radiographs, if any of the following was present: displacement of the fracture components, screw cut-out, or a change in the neck-shaft angle, as described by Newton et al.[9] Therefore, the patients were divided into two groups during follow-up: patients in whom fixation failed (Group F) and patients in whom fixation did not fail (Group N). After radiological analysis, the MCR and fracture type were assessed. The effects of MCR and fracture type on the development of failure were evaluated.
Figure 1. An anteroposterior radiograph of left shoulder of a patient (Neer type 3 fracture).

Figure 2. An anteroposterior radiograph of right shoulder of the same patient. (a) Yellow lines: Lines drawn parallel to the medial and lateral cortex. Red arrows: split points where the drawn lines end at the intersection of the cortical continuum. (b) Red Line: The line through the split points. (c) Determination of 2 cm distal of the line passing through the split points. (d) Red line: The line drawn perpendicular to the long axis and measuring the length of the outer diameter of the bone. Yellow line: The line measuring the length of the medial cortical thickness at the same level.
Statistical analysis
Statistical analysis was performed using the IBM SPSS version 23.0 software (IBM Corp., Armonk, NY, USA). Continuous variables were presented in mean ± standard deviation (SD), while categorical variables were presented in number and frequency. The Shapiro-Wilk test was used to determine the normality distribution of the data. As the numerical values of the study groups were normally distributed, comparison of numerical measurements was performed with a two-sample t-test (Student t-test) for two independent groups. The chi-square test was used to compare ratios between the groups. Univariate and multivariate analyses were also performed to examine risk factors for fixation failure; binary logistic regression analysis was used. Receiver operating characteristic (ROC) analysis was used to determine the cut-off values of numeric scores. A p value of <0. 05 was considered statistically significant.
Results
The mean follow-up was 41.3±4.7 (range, 18 to 66) months. During follow-up, fixation failure occurred in 18 of 93 (19%) patients who underwent radiographic evaluation. There was no significant difference in the MCR of the patients with failed surgical fixation and those with intact fixation (p=0.535). The fracture type of patients in the fixation failure group was significantly more severe than in the group without fixation failure (p<0.001). Additionally, the relationship between the MCR and fracture type was evaluated between the groups. Although the fracture type worsened as the MCR value decreased in the groups, there was no statistically significant relationship (p=0.054) (Table I).
Table 1. Demographic and radiological data of patients.
| Group F | Group N | ||||||
| n | % | Mean±SD | n | % | Mean±SD | p | |
| Age (year) | 77.7±6.3 | 71.0±5.8 | 0.724† | ||||
| Sex | 0.924* | ||||||
| Male | 5 | 27.8 | 20 | 26.7 | |||
| Female | 13 | 72.2 | 55 | 73.3 | |||
| MCR | 0.12±0.03378 | 0.15±0.03894 | 0.535† | ||||
| Fracture type | <0.001* | ||||||
| Type 1 | 0 | 0 | 2 | 2.9 | |||
| Type 2 | 0 | 0 | 42 | 60 | |||
| Type 3 | 14 | 78.8 | 24 | 34.3 | |||
| Type 4 | 4 | 22.2 | 2 | 2.9 | |||
| SD: Standard deviation; MCR: Medial cortical ratio. | |||||||
The univariate analysis revealed that the risk of fixation failure increased 0.1-fold per year in patients older than 73 years (r=0.238, p<0.001). In addition, fixation failure increased by seven-fold in patients with an MCR of >0.09 compared to other patients. In the multivariate analysis, both age and MCR were found to be significant risk factors for the development of fixation failure (r=0.232, p<0.001) (Table II).
Table 2. Analysis of risk factors for development of failure.
| Univariate | p | Multivariate | p | |||
| OR | 95% CI | OR | 95% CI | |||
| Age | 1.173 | 1.073-1.282 | <0.001 | |||
| Sex | 0.945 | 0.299-2.990 | 0.924 | |||
| MCR >0.09 | 7 | 1.841-26.613 | 0.004 | 7.653 | 1.685-34.753 | 0.008 |
| Age >73 (year) | 0.143 | 0.043-0.479 | 0.002 | 0.135 | 0.037-0.492 | 0.002 |
| OR: Odds ratio; CI: Confidence interval; MCR: Medial cortical ratio. | ||||||
Discussion
In the present study, we investigated the relationship between the MCR and fixation failure in patients with proximal humerus fracture treated with a plate-screw fixation. The type of fracture was the most important factor in implant failure in elderly patients who underwent open reduction and internal fixation (ORIF) after proximal humerus fracture. After surgical fixation, age of the patient and MCR were significant risk factors for fixation failure.
It has been well documented that individuals aged 65 years and older are at a higher risk of proximal humerus fractures due to osteoporosis. The fracture rate in women is up to 2.5 times higher than in men.[11,12] Several factors have been identified in the literature that are associated with implant failure after fixation of proximal humeral fractures with locking plates. Among 48 patients with a mean age of 61±15 years Adıyeke et al.[3] found that 33 patients were 53 years or older and 52% of them (n=11) who developed insufficiency were aged between 56 and 75 years. Krappinger et al.[10] also reported that 6 of 34 patients who had proximal humerus fracture were treated with locking plate-screw, and the risk of failure increased after age 63 years. In contrast, Hepp et al.[13] analyzed data from 113 patients with an average age of 66 years to examine factors associated with reoperation after fracture fixation. Twenty-two (50%) of 44 patients with an average age of 60 years required reoperation due to implant failure or insufficiency at the implant-bone interface. The failure rates reported in these studies after fixation with a locking plate-screw were 22.4%, 17.6%, and 19.4%.[3,10,13] In this study, the majority of participants were female, consistent with the literature. However, the mean age of our patients was higher than in other studies. Fixation failure occurred in only 19.3% of patients, despite their older age. This may be due to the fact that we did not include patients who had poor reduction after surgery. Considering age as a risk factor for the development of failure, we also found that in patients older than 73 years, the risk of developing a fracture increased by 0.1-fold. Our results support the findings of Krappinger et al.[10] reporting that advanced age increased the fracture risk.
Although the gold-standard method in the diagnosis of osteoporosis is dual-energy X-ray absorptiometry (DXA), the device is not available in every center, and it is costly to use. In addition, in acute situations such as osteoporotic fractures, access to devices is limited and local assessment of BMD is almost impossible. The use of cortical thicknesses in different anatomical regions to predict BMD was first described by Barnett and Nordin[5] and this result was supported by the study of Virtama et al.[6] in the following years. As a result, the researchers thought that this method could be an easily accessible, low-cost, simple, and effective complementary method for estimating BMD. The interest in studies measuring BMD with the measurement of cortical thickness and the ratios formulated from it until today is increasing day by day (Table III).[14-23] One of these ratios that has been revealed is the MCR. The MCR was first introduced by Newton et al.[9] in 2016. In the study conducted by Skedros et al.,[24] the MCR significantly decreased (p<0.001) in patients older than 60 years (0.12) compared to patients younger than 60 years old (0.16). In addition, a correlation between the MCR and DXA-based BMD measurements was also observed.
Table 3. Literature studies showing the relationship between cortical thickness measured in different anatomical regions and bone mineral densitometry.
| Study | Year | Anatomic region | Measurement type | Critical value for low BMI |
| Tingart et al.[21] | 2003 | Proximal humerus | CCT | CI <4 mm |
| Sah et al.[18] | 2007 | Femur diaphysis | CTI | CTI <0.40 |
| Rodriguez-Soto et al.[17] | 2010 | Femoral neck | CTI | Unspecified |
| Spross et al.[20] | 2015 | Proximal humerus | DTI | DTI <1.4 |
| Webber et al.[22] | 2015 | Distal radius | BCT | BCT <5 mm |
| Baumgärtner et al.[14] | 2015 | Proximal femur | CTI | Unspecified |
| Patterson et al.[16] | 2016 | Distal tibia | CBT (AP and Lat) | CT (AP) <3.5 mm |
| He et al.[15] | 2018 | Distal femur | CBT and DFCI | CBT <4.4 mm and DFCI <1.10 mm |
| Schmidutz et al.[19] | 2021 | Distal radius | CI | Unspecified |
| Yoshii et al.[23] | 2021 | 3rd metacarpal bone | CTR | Unspecified |
| BMI: Body mass index; CCT: Combine cortical thickness; CI: Cortical index; CTI: Cortical thickness index; DTI: Deltoid tuberosity index; BCT: Bicortical thickness; CBT: Cortical bone thickness; AP: Anterior posterior; Lat: Lateral; DFCI: Distal femoral cortical index; CT: Cortical thickness; CTR: Cortical thickness ratio. | ||||
It is usually accepted that osteopenia of the humeral head increases with age and that a decrease in BMD is a major cause of fixation failure after osteosynthesis for proximal humerus fractures.[13] A relationship between medial cortical thickness and BMD was first described by Tingart et al.[21] in the proximal humerus. Further studies confirmed this finding and even suggested that median cortical thickness and, thus, MCR could be used to clinically exclude osteoporosis.[7,24] However, the results of our study showed no statistically significant correlation between MCR and the development of fixation failure. This result may be due to the small sample size with disabilities in the patient group compared to the control group. In addition, this result may be related to the fact that our study population included only patients older than 65 years with osteopenia or osteoporosis. When we examined whether MCR was a risk factor for the development of fixation failure, an MCR greater than 0.09 was found to be significantly associated with a seven-fold increased risk of fixation failure.[25] This finding indicates that the MCR cut-off value of 0.09 can be used in future studies in evaluating the association between MCR and fixation failure.[25]
Currently, ORIF is the preferred surgical method of treating proximal humerus fractures. However, recent studies have shown that the preferred surgical treatment method in elderly patients has tended to shift toward reverse shoulder replacement surgery over the past decade. The most likely reason for this trend is that patients undergoing ORIF are at a higher risk for reoperation, primarily due to fracture fixation failure and complications, than patients undergoing reverse shoulder arthroplasty (RSA).[26] One of the most important reasons for the high rate of fixation failure (19.4% in our study and 17.6 to 22.4% in the literature) after fixation of proximal humerus fracture with locking-plate is the decreased bone quality with increasing age. Decreased bone quality with age causes complex fractures even with low-energy trauma and, therefore, Neer type 3 and type 4 fractures are more common in these patients. In their study, Barlow et al.[27] evaluated 131 proximal humerus fractures in patients over 60 years of age, and they found the rate of failure development in Neer 3-part fractures to be 39%, while this rate increased to 45% in 4-part fractures. The most common cause of failure in these patients was collapse due to humeral head avascular necrosis, followed by intra-articular screw penetration, implant failure, post-traumatic arthritis, and rotator cuff insufficiency, respectively. Solberg et al.[28] also examined surgical results in Neer 3 and 4 fragment fractures in elderly patients. The two most common causes of failure development were avascular necrosis and screw penetration. In this study, varus malalignment developed during or after surgery. In another study by Schumaier and Grawe,[11] approximately 20% of proximal humeral fractures were Neer 3-4-part fractures. In the surgical treatment of comminuted fractures in elderly osteoporotic patients, bone quality is often insufficient for fixation. In a study by Adıyeke et al.,[3] 84% of patients who experienced failure after fixation with a plate-screw had 3-4-part fractures, while in the study of Newton et al.,[9] this rate was 93%. All patients who developed fixation failure in our study had Neer 3-4-part fractures, consistent with the literature. These findings indicate that locking plate-screw systems may be inadequate in the presence of poor bone quality, despite adequate fracture reduction and further developments in implant technology. In this study, we showed that MCR and age, which are the main indicators of bone quality, could predict the risk of fixation failure. Therefore, we suggest that shoulder arthroplasty is the preferred option for surgical treatment of patients with advanced age and high MCR.
Nonetheless, this study has several limitations. First, the study has a single-center, retrospective design. Second, and more importantly, BMD measurements were unable to be obtained in all patients included in the study. Therefore, we could not assess whether there was a correlation between MCR and BMD. In addition, the sample size of the group in which fixation failure occurred is small.
In conclusion, surgical treatment of proximal humerus fractures due to osteopenia and osteoporosis in elderly patients still remains a challenge for surgeons, despite ongoing developments in the implant technology. Reverse shoulder arthroplasty may be a viable alternative to avoid repeated surgeries due to fixation failure in elderly patients with threepart or more fragmented fractures and high MCR who are scheduled for surgery to treat proximal humerus fractures. However, further studies are needed to draw more reliable conclusions on this subject.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea and concept, data collection and processing, literature review, writing the article: A.M.; Study design, data collection, writing the article, materials: E.K.Ş.; Control and supervision, critical review, references and fundings: B.H.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Wallace MJ, Bledsoe G, Moed BR, Israel HA, Kaar SG. Relationship of cortical thickness of the proximal humerus and pullout strength of a locked plate and screw construct. J Orthop Trauma. 2012;26:222–225. doi: 10.1097/BOT.0b013e31822421f7. [DOI] [PubMed] [Google Scholar]
- 2.Murray IR, Amin AK, White TO, Robinson CM. Proximal humeral fractures: Current concepts in classification, treatment and outcomes. J Bone Joint Surg [Br] 2011;93:1–11. doi: 10.1302/0301-620X.93B1.25702. [DOI] [PubMed] [Google Scholar]
- 3.Adıyeke L, Geçer A, Bulut O. Comparison of effective factors in loss of reduction after locking plate-screw treatment in humerus proximal fractures. Ulus Travma Acil Cerrahi Derg. 2022;28:1008–1015. doi: 10.14744/tjtes.2022.28742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panagiotopoulou VC, Varga P, Richards RG, Gueorguiev B, Giannoudis PV. Late screw-related complications in locking plating of proximal humerus fractures: A systematic review. Injury. 2019;50:2176–2195. doi: 10.1016/j.injury.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Barnett E, Nordin BE. The radiological diagnosis of osteoporosis: A new approach. Clin Radiol. 1960;11:166–174. doi: 10.1016/s0009-9260(60)80012-8. [DOI] [PubMed] [Google Scholar]
- 6.Virtama P, Telkka A. Cortical thickness as an estimate of mineral content of human humerous and femur. Br J Radiol. 1962;35:632–633. doi: 10.1259/0007-1285-35-417-632. [DOI] [PubMed] [Google Scholar]
- 7.Mather J, MacDermid JC, Faber KJ, Athwal GS. Proximal humerus cortical bone thickness correlates with bone mineral density and can clinically rule out osteoporosis. J Shoulder Elbow Surg. 2013;22:732–738. doi: 10.1016/j.jse.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Skedros JG, Knight AN, Pitts TC, O'Rourke PJ, Burkhead WZ. Radiographic morphometry and densitometry predict strength of cadaveric proximal humeri more reliably than age and DXA scan density. J Orthop Res. 2016;34:331–341. doi: 10.1002/jor.22994. [DOI] [PubMed] [Google Scholar]
- 9.Newton AW, Selvaratnam V, Pydah SK, Nixon MF. Simple radiographic assessment of bone quality is associated with loss of surgical fixation in patients with proximal humeral fractures. Injury. 2016;47:904–908. doi: 10.1016/j.injury.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42:1283–1288. doi: 10.1016/j.injury.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Schumaier A, Grawe B. Proximal humerus fractures: Evaluation and management in the elderly patient. Geriatr Orthop Surg Rehabil. 2018;9:2151458517750516–2151458517750516. doi: 10.1177/2151458517750516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Rüden C, Augat P. Failure of fracture fixation in osteoporotic bone. S3-10Injury. 2016;47 Suppl 2 doi: 10.1016/S0020-1383(16)47002-6. [DOI] [PubMed] [Google Scholar]
- 13.Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129:1251–1259. doi: 10.1007/s00402-009-0889-6. [DOI] [PubMed] [Google Scholar]
- 14.Baumgärtner R, Heeren N, Quast D, Babst R, Brunner A. Is the cortical thickness index a valid parameter to assess bone mineral density in geriatric patients with hip fractures. Arch Orthop Trauma Surg. 2015;135:805–810. doi: 10.1007/s00402-015-2202-1. [DOI] [PubMed] [Google Scholar]
- 15.He QF, Sun H, Shu LY, Zhu Y, Xie XT, Zhan Y, et al. Radiographic predictors for bone mineral loss: Cortical thickness and index of the distal femur. Bone Joint Res. 2018;7:468–475. doi: 10.1302/2046-3758.77.BJR-2017-0332.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson J, Rungprai C, Den Hartog T, Gao Y, Amendola A, Phisitkul P, et al. Cortical bone thickness of the distal part of the tibia predicts bone mineral density. J Bone Joint Surg Am. 2016;98:751–760. doi: 10.2106/JBJS.15.00795. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Soto AE, Fritscher KD, Schuler B, Issever AS, Roth T, Kamelger F, et al. Texture analysis, bone mineral density, and cortical thickness of the proximal femur: Fracture risk prediction. J Comput Assist Tomogr. 2010;34:949–957. doi: 10.1097/RCT.0b013e3181ec05e4. [DOI] [PubMed] [Google Scholar]
- 18.Sah AP, Thornhill TS, LeBoff MS, Glowacki J. Correlation of plain radiographic indices of the hip with quantitative bone mineral density. Osteoporos Int. 2007;18:1119–1126. doi: 10.1007/s00198-007-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidutz F, Schopf C, Yan SG, Ahrend MD, Ihle C, Sprecher C. Cortical bone thickness of the distal radius predicts the local bone mineral density. Bone Joint Res. 2021;10:820–829. doi: 10.1302/2046-3758.1012.BJR-2020-0271.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spross C, Kaestle N, Benninger E, Fornaro J, Erhardt J, Zdravkovic V, et al. Deltoid Tuberosity Index: A simple radiographic tool to assess local bone quality in proximal humerus fractures. Clin Orthop Relat Res. 2015;473:3038–3045. doi: 10.1007/s11999-015-4322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tingart MJ, Apreleva M, von Stechow D, Zurakowski D, Warner JJ. The cortical thickness of the proximal humeral diaphysis predicts bone mineral density of the proximal humerus. J Bone Joint Surg [Br] 2003;85:611–617. doi: 10.1302/0301-620x.85b4.12843. [DOI] [PubMed] [Google Scholar]
- 22.Webber T, Patel SP, Pensak M, Fajolu O, Rozental TD, Wolf JM. Correlation between distal radial cortical thickness and bone mineral density. J Hand Surg Am. 2015;40:493–499. doi: 10.1016/j.jhsa.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Yoshii I, Sawada N, Chijiwa T, Kokei S. Usefulness of cortical thickness ratio of the third metacarpal bone for prediction of major osteoporotic fractures. Bone Rep. 2021;16:101162–101162. doi: 10.1016/j.bonr.2021.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skedros JG, Mears CS, Burkhead WZ. Ultimate fracture load of cadaver proximal humeri correlates more strongly with mean combined cortical thickness than with areal cortical index, DEXA density, or canal-to-calcar ratio. Bone Joint Res. 2017;6:1–7. doi: 10.1302/2046-3758.61.BJR2016-0145.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atik OŞ. Which articles do the editors prefer to publish. Jt Dis Relat Surg. 2022;33:1–2. doi: 10.52312/jdrs.2022.57903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alrabaa RG, Ma G, Truong NM, Lansdown DA, Feeley BT, Zhang AL, et al. Trends in surgical treatment of proximal humeral fractures and analysis of postoperative complications over a decade in 384,158 patients. e22JB JS Open Access. 2022;7 doi: 10.2106/JBJS.OA.22.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlow JD, Logli AL, Steinmann SP, Sems SA, Cross WW, Yuan BJ, et al. Locking plate fixation of proximal humerus fractures in patients older than 60 years continues to be associated with a high complication rate. J Shoulder Elbow Surg. 2020;29:1689–1694. doi: 10.1016/j.jse.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: The effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23:113–119. doi: 10.1097/BOT.0b013e31819344bf. [DOI] [PubMed] [Google Scholar]