Abstract
Objectives
In this experimental study, we aimed to investigate the effectiveness of oral pirfenidone (PFD) treatment on preventing tendon adhesion and tendon healing in rats.
Materials and methods
A total of 21 rats were assigned into three groups including seven rats in each group. In Group 1 (sham group), no surgical procedure was performed. In Group 2 (control group), tendon repair was performed following right achillotomy. In Group 3 (treatment group), the rats also underwent tendon repair after right achillotomy. Additionally, 30 mg/kg of oral PFD was initiated from the postoperative Day 1 and administered via gavage for 28 days. At the end of the study, tendon healing and fibrosis levels in the tendon repair site were compared macroscopically, histopathologically, and immunohistochemically among the groups.
Results
Macroscopically, moderate and severe adhesions were observed in four and three rats, respectively in the control group, while no adhesion was found in four rats and filmy adhesions were observed in three rats in the treatment group (p<0.01). Microscopically, there was moderate adhesions in three rats and severe adhesions in four rats in the control group, while three rats had no adhesions and four rats had slight adhesions in the treatment group (p<0.01). Microscopically, tendon healing was good in six rats and fair in one rat in the control group, while five rats showed excellent tendon healing and two rats showed good tendon healing in the treatment group (p<0.01). Immunohistochemically, expressions of collagen I (p<0.01), collagen III (p<0.001), vascular endothelial growth factor (VEGF) (p<0.001), and proliferating cell nuclear antigen (PCNA) (p<0.001) significantly decreased in the treatment group compared to the control group.
Conclusion
Our study results indicated that PFD decreased collagen synthesis and prevented the formation of peritendinous adhesion in rats; however, it did not impair tendon healing.
Keywords: Pirfenidone, rats, tendon adhesion, tendon healing.
Introduction
Peritendinous adhesion is one of the most important factors that decrease treatment success after tendon repair. Peritendinous adhesions are the fibrous bands that form between the injured tendon and the surrounding tissues and limit the motion of the tendon. They are particularly observed after tendon repairs in the hand. Although improvements in tendon repair techniques and postoperative rehabilitation programs have reduced adhesion rates in tendon surgery, a significant adhesion rate still continues.[1,2]
Peritendinous adhesions are associated with functional losses, temporary or permanent workforce losses, psychological complications due to defects in daily life and financial problems. To date, many studies in the literature have addressed the prevention of tendon adhesions. Various chemical (5-fluorouracil, tranexamic acid) and biological (amniotic membrane) materials have been used to prevent adhesions in tendon repair.[3-5] Currently, there is no product that is routinely used for the prevention of tendon adhesions.
Tendon healing occurs with extrinsic and intrinsic mechanisms. In the extrinsic mechanism, fibroblasts, inflammatory cells and veins migrate from the surrounding sheath and peritendinous tissue. In the intrinsic mechanism, active tenocytes originating from the epitenon and endotenon and intratendinous vascularization play a role in healing.[6] Extrinsic healing is responsible for adhesion formation via the increased intensity of fibroblasts and inflammatory cells, accumulation of excessive and disorganized collagen, and intense water content. On the other hand, intrinsic healing is responsible for reorganization of collagen fibers and maintenance of collagen fibrillar continuity.[7,8]
Pirfenidone (PFD) is a drug with anti-fibrotic and anti-inflammatory properties and is currently used in the treatment of idiopathic pulmonary fibrosis. A number of cell-based studies have shown that PFD reduces fibroblast proliferation and inhibits collagen production by inhibiting the production of fibrogenic mediators such as transforming growth factor-beta (TGF-β).[9-11] Pirfenidone has also been shown to reduce the production of inflammatory mediators such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1 beta (IL-1β) in both cultured cells and isolated human peripheral blood mononuclear cells.[12]
Previous studies have suggested that PFD can prevent the formation of fibrotic lesions and inhibit the formation of fibrosis after tissue injuries. Therefore, it has been used experimentally to prevent abdominal adhesions, epidural fibrosis, and keloid formation.[13-15] In the present study, we aimed to investigate the effectiveness of oral PFD treatment on preventing tendon adhesion and tendon healing in rats.
Patients and Methods
In the study, 21 adult rats weighing between 200 and 250 g were used. The animals were kept at optimal laboratory conditions for 12-h light and 12-h dark cycle at 22°C room temperature. In addition, the animals were given access to standard laboratory pellet and water ad libitum.
Experimental groups
The animals were randomly selected and divided into three groups including seven rats in each group.
Group 1 (sham group): No surgical procedure was performed to the rats in this group and no drug was administered.
Group 2 (control group): Right achillotomy and primary tendon repair were performed to the rats in this group.
Group 3 (treatment group): Right achillotomy and primary tendon repair were performed to the rats in this group. Additionally, 30 mg/kg of oral PFD[16] was initiated via gavage method from Postoperative Day 1 and was administered via gavage for 28 days.
Surgical technique
All the invasive procedures were implemented under general anesthesia. General anesthesia was achieved by intraperitoneal administration of 50 mg/kg of ketamine hydrochloride (10% Alfamine® Atafen, Izmir, Türkiye) and 10 mg/kg of xylazine (2% Rompun® Bayer, Istanbul, Türkiye). After the rats were evaluated regarding deep anesthesia applying finger gripping test, the right legs of the rats were shaved and the right lower extremity was stained with povidone-iodine 10% (Betadix; Naturel Medical, Istanbul, Türkiye) to create a sterile surgical site. The rats were positioned appropriately, and sterile staining and covering were applied. Following skin incision, the right Achilles tendons of the rats were exposed and paratenon was opened. The Achilles tendon was cut transversely with full thickness 0.5 cm upward from calcaneus using a size No. 15 surgical blade. The proximal and distal ends of the cut tendon were repaired end-to-end with the modified Kessler suture technique (a core suture) using 4-0 round polydioxanone (PDS) material. Then, tendon repair was strengthened with epitendinous suture technique using 5-0 round polypropylene suture material, and surgery was terminated with skin suturing. During the postoperative period, no splinting was performed to protect the repaired tendons due to technical difficulty. Cefazolin sodium 15 mg/kg (Eqizolin; Tum Ekip Pharmaceuticals AS, Istanbul, Türkiye) was administered intraperitoneally for three days in the postoperative period to reduce the risk for wound site infection in all the rats.
On postoperative Day 28, all subjects were given anesthesia via intraperitoneal administration of 50 mg/kg of ketamine hydrochloride and 10 mg/kg of xylazine. A surgical entry was made through the previous incision site and macroscopic examination of the tendon repair and fibrosis levels in the tendon repair site was performed. Then, the right Achilles tendons of the rats were totally detached for histopathological and immunohistochemical examination. At the end of the procedure, all the rats were sacrificed with cervical dislocation.
Macroscopic examination
Semi-quantitative grading system was used to analyze the severity and extent of the peritendinous adhesion based on the surgical findings. This grading system was divided to five levels. Grade 1, no adhesion; Grade 2, filmy adhesion (separable with blunt dissection); Grade 3, slight (separable with sharp dissection); Grade 4, moderate (adhesion field of 35 to 60%); and Grade 5, severe (adhesion field >60%).[11] All macroscopic examinations were performed by a researcher who was blind to the group allocation.
Histopathological examination
The detached tendon tissue was fixed in 10% formaldehyde solution and prepared as paraffin block after routine histological tissue monitoring stages. The 5 µm sagittal sections in thickness taken from the paraffin blocks were stained with hematoxylin and eosin (H&E) staining method to examine the probable pathological changes in the tissue and masson-trichrome (MT) staining method to assess the collagen fiber rate.
Histologically, tendon healing grading system used by Turner et al.[12] was utilized to assess the level of tendon healing, while the grading system described by Tang et al.[17] was used to assess the severity of adhesion formation in the peritendinous field (Tables I and II). An experienced histopathologist carried out the histological examination as a blind observer without having any information about tendons.
Table 1. Criteria described by Turner et al.[12] for microscopic evaluation of tendon healing grading system.
| Histological tendon healing grading system | ||
| Grade | Points | Description of tendon healing |
| Excellent | 0 | Reestablishment of tendon continuity with smooth epitenon |
| Good | 1 | Apposition of wound margins with regular intratendinous collagen and epitenon disorganization, but no significant adhesions to the epitenon |
| Fair | 2 | Apposition of wound margins with irregular intratendinous collagen, often interrupted by adhesions to the epitenon. |
| Poor | 3 | Complete deterioration of the repair site due to adhesions |
Table 2. Criteria described by Tang et al.[17] for microscopic evaluation of peritendinous adhesion grading system.
| Points features of adhesion | |
| Quantity | |
| 0 | No apparent adhesions |
| 1 | A number of scattered filaments |
| 2 | A large number of filaments |
| 3 | Countless filaments |
| Quality | |
| 0 | No apparent adhesions |
| 1 | Regular, elongated, fine, and filamentous |
| 2 | Irregular, mixed, shortened, and filamentous |
| 3 | Dense, not filamentous |
| Grading of adhesions | |
| 0 | None |
| 1-2 | Slight |
| 3-4 | Moderate |
| 5-6 | Severe |
Immunohistochemical analysis
Expressions of collagen I and collagen III in the granulation tissue were determined by immunohistochemical analysis. In addition, proliferating cell nuclear antigen (PCNA) expression to determine fibroblast cell proliferation, which plays an important role in tendon healing and vascular endothelial growth factor (VEGF) expression to determine angiogenesis density were evaluated. The sections taken for immunohistochemical analysis were deparaffinized and rehydrated. The slides were exposed to 3% H2O2 to prevent endogenous peroxidase activity. These were heated in citrate buffer (pH: 6.0) twice to avoid antigen masking in nucleus and incubated in Ultra-V-Block and, then, incubated with collagen I (Bioss, bs-10423R, 1:300), collagen III (Bioss, bs-0948R, 1:300), PCNA (Santacruz, sc-25280, 1:100), and VEGF (Bioss, bs-0279R, 1:300) primary antibodies at +4°C for overnight. Then, the sections were washed with phosphate-buffered saline (PBS) and incubated in biotinylated secondary antibody and streptavidin-peroxidase, respectively. After the sections were stained with chromogen diaminobenzidine and counterstaining Mayer’s hematoxylin, they were examined under a light microscope. They were analyzed and photographed using cellSens software imaging systems (Olympus, Tokyo, Japan) in a light microscope (Olympus BX53, Tokyo, Japan). For immunohistochemical evaluation, it was scored according to intensity indices of the brown color.
Statistical analysis
the SAS version 9.4 software (SAS Institute, NC, USA). Descriptive data were expressed in mean ± standard deviation (SD) or number and frequency, where applicable. The Shapiro-Wilk test was used for the assumption of normality of the data. Immunohistochemical differences among the groups were evaluated using one-way analysis of variance (ANOVA) and the Duncan’s post-hoc test. The MannWhitney U test was used to analyze the differences between two groups for histological assessment and macroscopic adhesion. A p value of <0.05 was considered statistically significant.
Results
Macroscopic examination of the adhesions
In the control group, moderate and severe adhesions were observed in four and three rats, respectively. In the treatment group, no adhesion was found in four rats, while filmy adhesions were observed in three rats (Figure 1, Table III). There was a statistically significant difference in the macroscopic examination of the adhesions between the control and treatment groups (p<0.01).
Figure 1. The macroscopic view of tendon adhesion. (a) Control group, (b) treatment group.
Table 3. Macroscopic adhesion results of the groups.
| Macroscopic adhesion* | |||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Control | 0 | 0 | 0 | 4 | 3 |
| Treatment | 4 | 3 | 0 | 0 | 0 |
| * p<0.01. | |||||
Histopathological findings
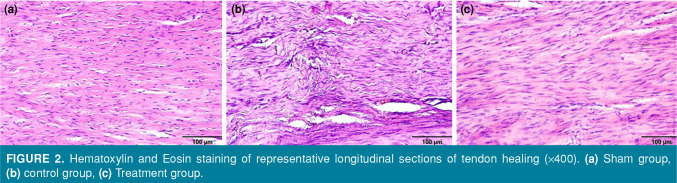
Sagittal sections stained by H&E and MT revealed that collagen fibers increased in the control group and showed a disorganized pattern, whereas collagen fibers decreased in the treatment group and collagen fiber integrity showed a more regular formation (Figure 2). Histologically, the healing level of the tendons and severity of peritendinous adhesions were evaluated based on the groups (Figure 3, Table IV). The treatment group was statistically significant in terms of tendon healing and tendon adhesions compared to the control group (p<0.01).
Figure 2. Hematoxylin and Eosin staining of representative longitudinal sections of tendon healing (x400). (a) Sham group, (b) control group, (c) Treatment group.
Figure 3. Hematoxylin and Eosin staining of representative longitudinal sections of tendon adhesion (x100). (a) Sham group, (b) control group, (c) treatment group.
Table 4. Histological results of tendon healing and tendon adhesion.
| Histological assessment | ||||||||
| Tendon healing* | Tendon adhesion** | |||||||
| Excellent | Good | Fair | Poor | None | Slight | Moderate | Severe | |
| Control | 0 | 6 | 1 | 0 | 0 | 0 | 3 | 4 |
| Treatment | 5 | 2 | 0 | 0 | 3 | 4 | 0 | 0 |
| * p<0.01; ** p<0.01. | ||||||||
Immunohistochemical findings
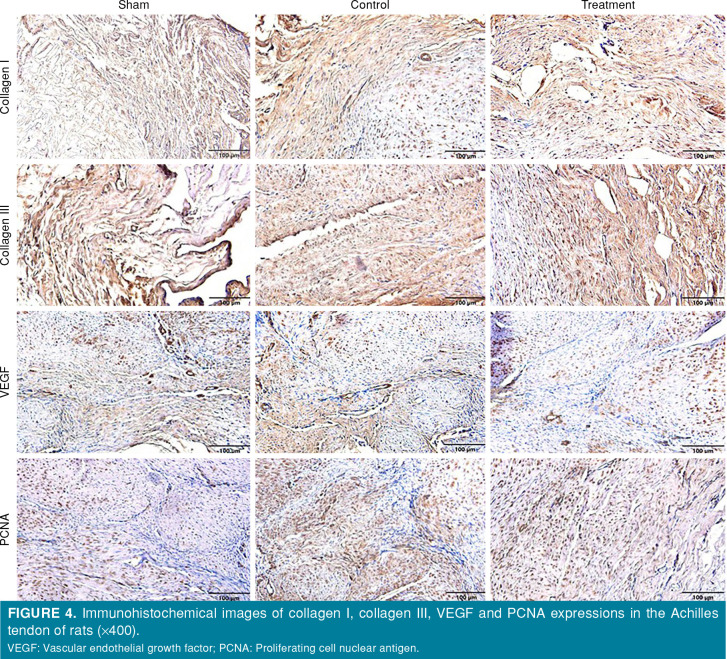
The highest value in terms of collagen I was in the sham group and the lowest value was in the treatment group (p<0.05). In terms of collagen III, VEGF and PCNA, the highest value was observed in the control group, while the lowest value was obtained in the sham group (p<0.05). The difference among the groups was significant for collagen I (p<0.01), collagen III (p<0.001), VEGF (p<0.001), andPCNA (p<0.001) (Figure 4, Table V).
Figure 4. Immunohistochemical images of collagen I, collagen III, VEGF and PCNA expressions in the Achilles tendon of rats (x400). VEGF: Vascular endothelial growth factor; PCNA: Proliferating cell nuclear antigen.
Table 5. ANOVA and post-hoc (Duncan) test results for collagen I, collagen III, VEGF, and PCNA.
| Groups | Collagen I | Collagen III | VEGF | PCNA |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | |
| Sham | 108.20±12.48a | 25.20±5.68c | 8.60±2.70c | 21.20±6.18c |
| Control | 88.20±14.03b | 7720±15.11a | 29.60±5.41a | 95.60±13.69a |
| Treatment | 68.80±7.66c | 58.60±12.16b | 17.60±5.18b | 51.40±11.85b |
| p | * | ** | ** | ** |
| ANOVA: Analysis of variance; VEGF: Vascular endothelial growth factor; PCNA: Proliferating cell nuclear antigen; * p<0.01 ** p<0.001; a, b, c: Values in the same column without a common superscript differ significantly (p<0.05). | ||||
Discussion
In this preliminary study, which was carried out by creating a tendon injury model in rats, the effects of PFD on the prevention of tendon adhesions and tendon healing were investigated. The findings of the study showed that PFD was effective in preventing peritendinous adhesions macroscopically, histopathologically and immunohistochemically, and did not impair tendon healing.
Tendon adhesions are the fibrous bands which are formed between the injured tendon and the surrounding tissues, and limit the motion of the tendon. These adhesions occur as a result of excessive and irregular accumulation of collagen. The TGF-β is the main cytokine responsible for fibrosis formation. The increased TGF-β expression in systemic sclerosis, hypertrophic scar tissue after burn, and in keloid has been associated with high collagen I, III, and VI production. On the contrary, the inhibition of TGF-β expression decreases accumulation of collagen and cicatrix.[18,19]
In the macroscopic and microscopic examination in this study, we observed that peritendinous adhesions were statistically significantly less in the PFD group. This can be attributed to the anti-fibrotic activity of PFD. Previous studies have shown that PFD can prevent the formation of fibrotic lesions and can inhibit fibrosis formation after tissue injuries. It exerts this anti-fibrotic activity by inhibiting both fibroblast proliferation and differentiation of fibroblasts into myofibroblasts via TGF-β and reducing collagen synthesis.[20] Bayhan et al.[13] experimentally showed that PFD could be used as an effective agent in prevention of postoperative intra-abdominal adhesions and inflammation. Besides, Shi et al.[14] showed in a rat laminectomy model that PFD reduced the formation of epidural fibrosis by inhibiting fibroblast proliferation and suppressing collagen synthesis.
In another study including 19 patients, PFD reduced scar tissue formation and increased epithelialization in the donor site from which split-thickness skin graft was taken.[21] Also, PFD was shown to suppress the contraction of keloid fibroblasts in an in vitro scar contraction model and, therefore, it could be used as a therapeutic for keloid lesions.[15] Chan et al.[22] documented in a study in which they experimentally created cartilage damage in a rat knee that PFD both prevented joint fibrosis and also demonstrated protective effect on cartilage and bone.
Ideal agents and materials used in tendon repair are expected to prevent tendon adhesions without impairing tendon healing. In this study, we observed histologically that collagen fiber density decreased, but the collagen fiber integrity had a more regular and organized formation in the PFD treatment group compared to the control group. In a previous study, the alignment and organization of collagen fibrils required for tendon healing were more important than the amount of collagen.[23] Around the fourth week after injury, proliferation of fibroblasts of intrinsic origin increases. These fibroblasts (tenocytes) mostly originate from endotenon and actively remodel collagen.[6] In the current study, PFD reduced collagen synthesis and provided collagen maturation. Based on these findings, it can be speculated that PFD does not impair intrinsic tendon healing.[24]
Another important finding of this study was that collagen I and collagen III expressions were lower in the PFD group than in the control group. Collagen I and collagen III are seen intensely in fibrosis and scar tissue. Fibroblasts, which migrate to the damaged tendon area around the fifth day after injury, begin to synthesize collagen and continue to increase until the remodeling phase. When TGF-β is applied to the healing tendon, the expression of collagen I and III is induced.[25] We believe that the reason for the low collagen expression in this study is that PFD inhibits fibroblast proliferation and the production of fibrogenic mediators, such as TGF-β, during the repair phase of tendon healing.
In the current study, we also observed that PCNA expression was lower in the treatment group compared to the control group. The proliferative phase of tendon healing is hypercellular and growth factors such as platelet-derived growth factor, insulin-like growth factor, TGF-β, and basic fibroblast growth factor regulate fibroblast proliferation.[6] We consider that the reason for low PCNA expression in the current study may be due to the anti-proliferative activity of PFD, which acts by inhibiting growth factors, particularly TGF-β.
Another finding of this study was that VEGF expression was lower in the treatment group compared to the control group. Angiogenesis is crucial for facilitating tendon healing, such as delivering oxygen and nutrients to the damaged tendon area, removing waste products, and controlling immune responses. The VEGF is one of the most vital angiogenic factors that regulate blood vessel formation in tendon healing. It plays a prominent role in the inflammatory and proliferative phases of tendon healing. Its overproduction has been associated with fibrosis.[26] Pirfenidone has been shown to have an antiangiogenic effect by inhibiting VEGF expression during the wound healing process after glaucoma surgery.[27] The low expression of VEGF in the current study supports the anti-angiogenetic activity of PFD.
Extrinsic healing is more dominant than intrinsic healing in the inflammatory, proliferative, and reparative phases of tendon healing after tendon injury. The extrinsic mechanism is responsible for tendon adhesions. Fibroblast proliferation and excessive synthesis of collagen should be inhibited to prevent tendon adhesions. Anti-proliferative, antifibrotic, and anti-inflammatory activity of PFD can suppress the extrinsic mechanism, reduce tendon adhesions, and provide tendon healing without disturbing the intrinsic mechanism in rats.
The lack of biomechanical analyses is the main limitation to this study. The application of biomechanical tests which measures the strength of the tendon is as important as histological evaluation of tendon healing. Another limitation of this study is that the effects of different doses of PFD on tendon adhesion and tendon repair were unable to be examined. However, to the best of our knowledge, this is to first study to investigate the effectiveness of PDF on tendon adhesion and tendon repair in rats. Further studies should be conducted to evaluate varying doses and efficacy of PFD.
In conclusion, in this experimental study, PFD decreased collagen synthesis by inhibiting fibroblast proliferation and prevented the formation of peritendinous adhesion; however, it did not impair tendon healing in rats. The results of this study suggest that PFD can be a potential therapeutic drug for prevention of tendon adhesions. Nevertheless, there are further clinical studies to confirm these findings.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea/concept, design/control/ supervision, writing the article: T.T.; Critical review: N.G.; Histological assesment/ analysis and/or interpretation: F.A.; Literature review: A.T.; Analysis and/or interpretation: M.A.G.; Surgical intervention/data collection and/or processing: U.İ., M.A.U.; Statistical assesment/analysis and/or interpretation: S.A.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Wang F, Zhang R, Liu S, Ruan H, Xu J, Kang Q. Severe spaghetti wrist injury: Should we expand the terminology from wrist to proximal forearm. Jt Dis Relat Surg. 2022;33:273–284. doi: 10.52312/jdrs.2022.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulos N, Bozentka DJ. Management of complications of flexor tendon injuries. Hand Clin. 2015;31:293–299. doi: 10.1016/j.hcl.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Nazifi O, Stuart AL, Nikkhah D. The use of 5-fluorouracil in the prevention of tendon adhesions: A systematic review. Animal Model Exp Med. 2020;3:87–92. doi: 10.1002/ame2.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zukawa M, Okabe M, Osada R, Makino H, Nogami M, Seki S, et al. Effect of hyperdry amniotic membrane in preventing tendon adhesion in a rabbit model. J Orthop Sci. 2022;27:707–712. doi: 10.1016/j.jos.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Sarı A, Dinçel YM, Karabağ S, Çetin MÜ. Histopathological and immunohistochemical investigation of the local and systemic effects of tranexamic acid on the healing of the Achilles tendon in rats. Jt Dis Relat Surg. 2021;32:152–161. doi: 10.5606/ehc.2021.76301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller S, Todorov A, Heisterbach P, Majewski M. In: Achilles tendon. Cretnik A, editor. London: InTech; 2011. Tendon healing with growth factors; pp. 43–62. [Google Scholar]
- 7.Ataker Y, Ece SC, Güdemez E. Fleksör tendon tamiri sonrası rehabilitasyon. Türkiye Klinikleri Dergisi Ortopedi ve Travmatoloji. 2011;4:31–42. [Google Scholar]
- 8.James R, Kesturu G, Balian G, Chhabra AB. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: Treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. e3-19Am J Respir Crit Care Med. 2015;192 doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 11.Hsu SH, Dai LG, Hung YM, Dai NT. Evaluation and characterization of waterborne biodegradable polyurethane films for the prevention of tendon postoperative adhesion. Int J Nanomedicine. 2018;13:5485–5497. doi: 10.2147/IJN.S169825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner JB, Corazzini RL, Butler TJ, Garlick DS, Rinker BD. Evaluating adhesion reduction efficacy of type I/III collagen membrane and collagen-GAG resorbable matrix in primary flexor tendon repair in a chicken model. Hand (N Y) 2015;10:482–488. doi: 10.1007/s11552-014-9715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayhan Z, Zeren S, Kocak FE, Kocak C, Akcılar R, Kargı E, et al. Antiadhesive and anti-inflammatory effects of pirfenidone in postoperative intra-abdominal adhesion in an experimental rat model. J Surg Res. 2016;201:348–355. doi: 10.1016/j.jss.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Shi K, Wang F, Xia J, Zuo B, Wang Z, Cao X. Pirfenidone inhibits epidural scar fibroblast proliferation and differentiation by regulating TGF-β1-induced Smaddependent and -independent pathways. Am J Transl Res. 2019;11:1593–1604. [PMC free article] [PubMed] [Google Scholar]
- 15.Saito M, Yamazaki M, Maeda T, Matsumura H, Setoguchi Y, Tsuboi R. Pirfenidone suppresses keloid fibroblastembedded collagen gel contraction. Arch Dermatol Res. 2012;304:217–222. doi: 10.1007/s00403-011-1184-2. [DOI] [PubMed] [Google Scholar]
- 16.Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A, et al. Antifibrotic action of pirfenidone and prednisolone: Different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008;590:400–408. doi: 10.1016/j.ejphar.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 17.Tang JB, Shi D, Zhang QG. Biomechanical and histologic evaluation of tendon sheath management. J Hand Surg Am. 1996;21:900–908. doi: 10.1016/S0363-5023(96)80212-7. [DOI] [PubMed] [Google Scholar]
- 18.Ghahary A, Shen YJ, Scott PG, Gong Y, Tredget EE. Enhanced expression of mRNA for transforming growth factor-beta, type I and type III procollagen in human post-burn hypertrophic scar tissues. J Lab Clin Med. 1993;122:465–473. [PubMed] [Google Scholar]
- 19.Peltonen J, Hsiao LL, Jaakkola S, Sollberg S, Aumailley M, Timpl R, et al. Activation of collagen gene expression in keloids: Co-localization of type I and VI collagen and transforming growth factor-beta 1 mRNA. J Invest Dermatol. 1991;97:240–248. doi: 10.1111/1523-1747.ep12480289. [DOI] [PubMed] [Google Scholar]
- 20.Stahnke T, Kowtharapu BS, Stachs O, Schmitz KP, Wurm J, Wree A, et al. Suppression of TGF-β pathway by pirfenidone decreases extracellular matrix deposition in ocular fibroblasts in vitro. e0172592PLoS One. 2017;12 doi: 10.1371/journal.pone.0172592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mecott-Rivera GÁ, Aguilar-Baqueiro JA, Bracho S, Miranda-Maldonado I, Franco-Márquez R, Castro-Govea Y, et al. Pirfenidone increases the epithelialization rate of skin graft donor sites. Burns. 2018;44:2051–2058. doi: 10.1016/j.burns.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Chan DD, Li J, Luo W, Predescu DN, Cole BJ, Plaas A. Pirfenidone reduces subchondral bone loss and fibrosis after murine knee cartilage injury. J Orthop Res. 2018;36:365–376. doi: 10.1002/jor.23635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 2013;99:203–222. doi: 10.1002/bdrc.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atik OŞ. Which articles do the editors prefer to publish. Jt Dis Relat Surg. 2022;33:1–2. doi: 10.52312/jdrs.2022.57903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou Y, Mao Z, Wei X, Lin L, Chen L, Wang H, et al. Effects of transforming growth factor-beta1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix Biol. 2009;28:324–335. doi: 10.1016/j.matbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Chu M. Differential roles of VEGF: Relevance to tissue fibrosis. J Cell Biochem. 2019;120:10945–10951. doi: 10.1002/jcb.28489. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Yang Y, Guo X, Liu L, Wu K, Yu M. The Antiangiogenesis effect of pirfenidone in wound healing in vitro. J Ocul Pharmacol Ther. 2017;33:693–703. doi: 10.1089/jop.2017.0007. [DOI] [PubMed] [Google Scholar]