Abstract
Objectives:
To examine the effect of muscle fiber recruitment patterns on muscle oxygen utilization during treadmill walking in a group of individuals who have incomplete spinal cord injury.
Methods:
5 participants with motor incomplete spinal cord injury (Age; 42.2±18.8 years, Male; n=4) completed an over ground locomotor training program. Muscle utilization/oxygenation and activation of the medial gastrocnemius were measured by near infrared spectroscopy and surface electromyography pre- and post-over ground locomotor training during two separate treadmill walking bouts at self-selected speeds. Outcomes were changes in deoxygenation hemoglobin/myoglobin concentrations, and the change in median power of the power spectrum of the electromyography after training.
Results:
A significant increase in median power of the power spectrum of the electromyography signal was observed during both bouts of treadmill walking, 6-minute walking bout and longer fatiguing bout (49% p= 0.047 and 48% p= 0.035, respectively) post-over ground locomotor training. There was no significant change in muscle utilization/oxygenation post-over ground locomotor training. There was no significant effect of median power of the power spectrum on deoxygenation hemoglobin/myoglobin during either of the walking bouts.
Conclusions:
The main finding of the current study was that median power of the power spectrum significantly increased following 12 weeks of over ground locomotor training, with no significant change in deoxygenation hemoglobin/myoglobin. The recruitment of more and/or larger motor units was seen in conjunction with no changes in muscle oxygen utilization for the same walking task.
Keywords: Incomplete Spinal Cord Injury, Electromyography, Over-ground locomotor training, muscle oxygenation
Introduction
Traumatic injury to the spinal cord often results in disabling injury, the severity of which is determined by the vertebral level of the lesion and the degree to which the lesion transects the spinal cord. Generally, the higher the injury on the spinal cord the greater the level of dysfunction, (1–3) which manifests as alterations in skeletal muscle innervation,(4) deficits in skeletal muscle oxygenation (1,5) and dysregulation of the cardiovascular and pulmonary systems. (6) As a result of reduced activation, muscles of individuals with spinal cord injury (SCI) tend to atrophy neurogenically due to sporadic arrival and, low or negligible amplitude or frequency of stimulatory impulses arrival at the motor end plates. (7) This maladaptation of skeletal muscle innervation may also adversely affect the orderly recruitment of motor units, which could in turn blunt the oxidative metabolic response during physical activity. (8)
In addition to gross morphological maladaptation’s (8,9) chronically altered central motor function (10) may lead to histological alterations in which fatigue resistant (type I and the more oxidative of the type IIA ) muscle fibers take on the characteristics of more fatigable fibers (nonoxidative type IIA and Type IIX) thus reducing the oxidative capacity of the muscle overall. (8) One of the key characteristics of type II muscle fibers in general is a propensity to rely more heavily on the glycolytic pathway for the production of adenosine triphosphate, which is associated with reduced ability to sustain the contraction rate during continuous muscle activity, diminished oxidative phosphorylation capacity, and alterations in the orderly recruitment of the fibers. In the intact neuromuscular system, the recruitment of muscle fibers follows a well-defined pattern of recruitment based on the size of the motor units, in which the smaller motor units with lower action potential thresholds are typically recruited first in a contraction. As more force is required, the larger motor units are recruited in a graded, step wise fashion. An altered motor unit recruitment pattern has been observed in SCI (4,11) in which the larger more fatigable motor units, generally associated with type II fibers, are recruited early and often during movement. Therefore, the outcome of inadequate muscular innervation in SCI appears to be a shift in the muscle characteristics to less fatigue resistant type II fibers, accompanied by an alteration in recruitment pattern, which leads to larger type II fibers being deployed early in the contraction.
Previously, it has been suggested that muscle oxygen utilization may be diminished following incomplete SCI compared to individuals who do not have spinal cord injury. (12) A mechanism for this diminution has not been delineated and the relative contributions of neurogenic reduction in the oxidative metabolic pathway versus alterations in motor unit recruitment in those with incomplete SCI have not been clarified. We hypothesized that a change in the electromyography (EMG) power-frequency spectrum would affect change in muscle oxygen utilization during treadmill walking in a group of individuals who have incomplete SCI before and after a novel 12-week locomotor training (OLT) program.
Materials & Methods
This study was approved by the George Mason Institutional Review Board (#618911). We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. Prior to participation, written informed consent was voluntarily given by each of the participants following verbal explanation of the study protocol and all risks associated with testing and training. Participants were recruited from the Greater Washington DC metropolitan region. Study inclusion and exclusion criteria are included in table 1. After meeting inclusion criteria, ruling out all exclusion criteria and providing consent, participants were asked to refrain from any strenuous physical activity 24 hours prior to initial testing, and to abstain from completing any other structured training protocol during the OLT program.
Table 1:
Inclusion and exclusion criteria
| Inclusion | Exclusion |
|---|---|
| i) Age 18 or older. ii) at least 6-months post SCI iii) classified by the American Spinal Cord Association Impairment Scale (AIS) as either AIS C or D, iv) able to stand with minimal assistance, voluntarily initiate at least one step with or without the use of an assistive device. v) safely walk on a treadmill at a minimum speed of at least 0.5mph |
i) had a complete SCI or were graded as AIS A or B. ii) any spasms, contractures or other orthopedic complications that would prevent walking on the treadmill safely iii) any history of ischemic heart disease, known cardiovascular, pulmonary, or metabolic diseases. iv) HIV infection or use of antiretroviral therapy, v) severe psychiatric disease. |
During the first study visit, participants performed treadmill walking tests during which gross muscle activation, muscle fiber recruitment, and muscle oxygenation were indexed using surface EMG and near infrared spectroscopy (NIRS). Participants’ height (SECA 213, Medical Measuring Systems and Scales, Hamburg, Germany) to the closest cm and weight (Tanita BC-418, IL, USA) in kgs were recorded prior to all tests.
Prior to the treadmill walking test, a total arterial occlusion, muscle oxygenation test of the gastrocnemius muscle of the left leg was performed using NIRS for determination of individuals physiological ranges of muscle oxygen desaturation. Following the arterial occlusion test, a constant work rate test was performed using a motor driven treadmill (Trackmaster, FullVison Inc, Newton, KS, USA). The constant work rate test included two separate bouts of treadmill walking at a speed that was individually selected by each of the participants, following the 12-weeks of OLT the same self-selected walking speeds were used for post testing, in order to match work-rate. Duration of the bouts differed, the first bout was six minutes long (exercise bout 1) while the second bout (exercise bout 2) was sustained to volitional exhaustion or until 30-minutes had elapsed. The self-selected speed was determined by participants’ own assessments of their ability to safely maintain the treadmill belt speed during 6 minutes of walking. The test began with 3 minutes of quiet baseline rest, followed by exercise bout 1. After a 6-minute rest period, participants were again asked to walk at their self-selected speed for exercise bout 2. Volitional exhaustion was determined by the participants’ indication that she or he must stop walking due to feelings of fatigue or tiredness. To ensure the safety of the participants, the study personnel ended the test if the gait mechanics of the participants were severely diminished resulting in the inability to maintain proper form during walking. Criteria for determination of a breakdown of gait mechanics included excessive toe drag, inability to stay towards the front of the treadmill belt and/or inability to lift their foot through the swing phase. Participants were permitted to use the handrails of the treadmill for balance, however, they were asked to apply as little weight as possible on the handrails during walking.
Skeletal muscle oxygenation was measured by NIRS (Oxymon MK-III, Artinis Medical Systems, The Netherlands) in which muscle tissue absorption of refracted light emitted from a near infrared photon emitter was measured relative to wavelengths associated with oxygenated (O2Hb/Mb) and deoxygenated (HHb/Mb) hemoglobin and myoglobin concentrations. A built-in algorithm using the modified Beer-Lambert Law was used to analyze the NIRS signals. The primary variables measured by NIRS are the relative changes in the concentration of O2Hb/Mb and HHb/Mb during occlusion, reperfusion, and during exercise. Total hemoglobin/myoglobin (totHb/Mb), calculated as the sum of O2Hb/Mb and HHb/Mb and tissue saturation index, an absolute measure of oxygenated hemoglobin/myoglobin, were recorded during the same time points. Calibration of the NIRS system was conducted per manufacture’s instruction prior to all testing.
The belly of the left medial gastrocnemius was used for NIRS analysis, as the gastrocnemius is a primary muscle used for ambulation (13) and is located superficially. The muscle belly was identified by manual palpation and marked with a pen for reference. Distance of the muscle belly from the knee joint was recorded to allow for similar placement between pre- and post-OLT testing. The areas around the light emitting and receiving optodes were shaved and cleaned using isopropyl alcohol wipes, and NIRS optodes were fixed to the skin using double sided adhesive tape. Further, an athletic wrap was used to hold the optodes in place to minimize movement artifact during the treadmill walking bouts.
The lower limb occlusion test involved placing a pressure cuff around the thigh of the participants left leg, above the knee joint. Using a rapid inflator system (E20 Rapid Cuff Inflator, Hokanson, Bellevue, WA, USA) the cuff was inflated and maintained at ~220 mm Hg to ensure that venous and arterial blood flow to the left gastrocnemius was completely occluded. The cuff remained inflated until total desaturation of the muscle was complete (approximately 7 minutes). The point of total desaturation was chosen as the observance of a clear plateau in the O2Hb/Mb, HHb/Mb concentrations and tissue saturation index. The cuff was then rapidly deflated and the time course of muscle reperfusion was measured to account for reactive hyperemia. The NIRS optode remained in place during the constant work rate test to allow for continuous measurements throughout both exercise bouts.
EMG was recorded from the gastrocnemius during treadmill walking. The area where the EMG electrodes were positioned was shaved and cleaned using isopropyl alcohol wipes. Bipolar surface electrodes (Ag/AgCL self-adhesive dual electrodes) were longitudinally positioned over the belly of the muscle in line with the approximate muscle fiber direction. EMG signals were collected using a Noraxon (TeleMyo DTS, Noraxon, Scottsdale, AZ, USA) system at a sampling rate of 1500 Hz. Individual EMG units were fixed to the muscle using tape, which reduced movement artifact during data collection. Once all electrodes were in place, a participant’s maximal voluntary contraction (MVC) for gastrocnemius was measured. Briefly, in a standing position, the participants maximally contracted gastrocnemius for 3 seconds by completing a calf raise followed by complete relaxation, a total of 2 times. EMG data was collected during the entire six minutes of the first exercise bout. During the second bout, EMG data were collected during the first 20 seconds of each exercising minute.
A detailed description of the OLT program can be found elsewhere (14). Briefly, the program is a performance-based intervention founded on the principles of adaption and motor learning, consisting of 24 sessions over 12 weeks. Participants underwent two 90-minute sessions twice a week separated by at least 48 hours. The 24 sessions were reduced to three blocks of 8 sessions focused on four uniplanar (forward) days and four multiplanar days (one session each of backwards walking, lateral stepping, crossover stepping and rotational stepping). During each individual training participants were taken through five distinct segments: joint mobility, volitional neuromuscular activation, task-isolation, task-integration, and activity rehearsal. Progressive overload was achieved by varying the movement complexity, resistance, velocity and volume of the specific task within the session. During OLT, participants performed all activity under full volitional control using only minimal assistance from walkers or walking aids.
A custom Matlab script (Mathworks, Natick, MA, USA) was written to analyze all collected NIRS data. All raw NIRS data was collected at 1000Hz and was subsequently time-averaged into 1 second bins to reduce the noise of the raw signal. The averaged 1-second data was then separated into individual data sets for each of the time points, namely occlusion, reperfusion, exercise bout 1 and exercise bout 2. The change in gastrocnemius oxygenation capacity was determined as the amplitude of change in HHb/Mb during occlusion and the amplitude of change in O2Hb/Mb and total Hb/Mb concentration during reperfusion. During exercise, the NIRS signal was broken down into set periods of time to allow comparison with the collected EMG signal, explained in detail below. The time periods chosen were the 5th to the 15th second of every minute for all NIRS variables. An average value for HHb/Mb and O2Hb/Mb during each of the 10 second time epochs was calculated, and a physiological calibration was used to normalize the averaged NIRS signals to those found during occlusion and reperfusion. The nadir of the O2Hb/Mb signal during occlusion can be defined as 0% muscle oxygen saturation, while 100% muscle saturation occurs at the zenith of the signal during reactive hyperemia (reperfusion). (15)
The EMG data was processed using custom written Matlab scripts (Mathworks, Natick, MA, USA). Raw EMG data was refined in each of two ways. The first procedure involved creating a linear envelope of data collected from the MVC, and the treadmill walking bouts. EMG data was band pass filtered using a 5th order Butterworth filter to remove any low and high frequency values that were outside the biological norms of 5 – 600Hz. The filtered EMG signals were full wave rectified, which converted all signals to one single polarity frequency. A low-pass filter was applied to the rectified signal to create a linear envelope of the EMG data. Rectified EMG data was broken into specific epochs of time (the 5th to the 15th second of each recorded minute) for further analysis. The data from each time epoch was then ensemble-averaged allowing for the calculation of the mean and standard deviation. The amplitude of activation during walking was normalized against the activation levels found during the MVC. The root mean square (RMS), which is an indication of overall muscle activation, was found during the MVC and the two walking bouts. A higher RMS generally is seen in conjunction with increased muscle activity. (16) All values from the walking bouts were normalized to the MVC, meaning a normalized RMS value was generated for each minute the participants walked on the treadmill.
The second EMG procedure consisted of finding the power spectral density (PSD) of the EMG signal. The PSD of a signal refers to the distribution of power per unit time of the signal and is said to represent the conduction velocity of motor units. (17) The raw EMG data was segmented in the same manner as previously explained to create a window of analysis for each minute of treadmill walking. Using a fast-Fourier transformation with a window size of 50 ms the PSD of each individual time epoch was found. The median frequency of the PSD (PSDMF) was calculated as the variable of interest, higher power spectrum density median frequency is suggestive of larger fiber types (18), which are generally Type II muscle fibers.
All group data are reported as mean ± standard deviation. Data was analyzed at two time points, pre and post the 12-week OLT program using a paired sample Student’s t-test. Statistical significance was set at p ≤ 0.05 for two-tailed hypotheses. The effect size of OLT was determined using Cohen’s D. A mixed-effects model was used to assess the interaction between muscle activation (RMS and PSDMF) and muscle HHb/Mb during each of the treadmill walking bouts. The use of mixed effects models allows for flexibility in the analyses of grouped data, particularly when multiple measurements are taken per subject over many time points, as the random effect of the model assumes that each participant has a different baseline for PSDMF. Statistical analyses were performed using Stata version 15.1 (STATA, College Station, TX, USA).
Results
Seven participants were enrolled in the study and completed OLT. Two of the 7 participants were excluded from the analyses because their EMG data was unusable. The data was deemed unusable because the percent difference between RMS obtained during MVC and standing rest was −17% and 1% respectively, indicating there was inadequate activation of the gastrocnemius, which would imply that these individuals could not initiate a muscular contraction. Therefore, these observations indicated that the SCI eliminated volitional activation of the left gastrocnemius in these participants at baseline. Table 2 represents demographic information for study participants included in the analyses.
Table 2.
Participant Characteristics (N=5)
| Characteristic | Value |
|---|---|
|
| |
| Sex | |
|
| |
| Male | 4 |
| Female | 1 |
|
| |
| Age (y) | |
|
| |
| Mean ± SD | 42.2±18.8 |
| Range | 20–67 |
|
| |
| AIS grade | |
|
| |
| C | 4 |
| D | 1 |
|
| |
| Level of Injury | |
|
| |
| Cervical | 3 |
| Lumbar | 2 |
|
| |
| Time since injury (months) | 7–49 |
Values are number of participants or as otherwise indicated.
Treadmill walking speed for exercise bout 1 and bout 2 averaged 0.86 ± 0.3 mph. There was no significant difference in walking duration during exercise bout 2 between pre- and post-OLT (pre = 1387 seconds and post = 1192 seconds). There were no significant changes in gastrocnemius O2Hb/Mb, HHb/Mb, or totHb/Mb concentrations obtained during NIRS arterial occlusion and reperfusion following OLT (table 3). There was no change in the time course of muscle oxygen desaturation or reperfusion during the arterial occlusion test following OLT. A change in muscle oxygenation amplitude was not observed in either the 6-minute walk (exercise bout 1) or the fatiguing walk (exercise bout 2) as a result of OLT (table 4 & figure 2). A significant increase in PSDMF of the EMG signal was observed during both treadmill walking bouts (table 4 & figure 3). A restricted likelihood ratio test indicated that the differences in slopes for the effect of PSDMF impacted the outcome for the pre- and post-exercise bout 1 mixed effect models, (p=0.0381 and p=0.0345, respectively). The results of the mixed effect models indicated that PSDMF did not have a significant influence on the outcome of HHb/Mb in either of the two exercise bouts for both pre and post OLT. During the shorter exercise bout (bout 1), there was no significant interaction between RMS and HHb/Mb pre-OLT. However, following OLT, there was a significant inverse relationship between RMS and HHb/Mb, such that RMS increased with a decrease in HHb/Mb. Therefore, RMS significantly influenced HHb/Mb during exercise bout 2 for both pre and post OLT.
Table 3.
NIRS values during occlusion and reperfusion
| Variable | Pre | Post | |
|---|---|---|---|
| Occlusion | O2Hb/Mb (a.u.) | 19.26 (4.09) | 19.89 (2.7) |
| HHb/Mb (a.u.) | 18.73 (3.18) | 17.84 (1.73) | |
| totHb/Mb (a.u.) | 37.35 (6.58) | 36.83 (3.77) | |
| Reperfusion | O2Hb/Mb (a.u.) | 28.87 (5.01) | 28.45 (4.19) |
| HHb/Mb (a.u.) | 23.44 (3.8) | 22.66 (2.17) | |
| totHb/Mb (a.u.) | 51.62 (7.99) | 49.7 (5.36) |
Values are means (SD). O2 Hb/Mb oxygenated hemoglobin, HHb/Mb deoxygenated hemoglobin, totHb/Mb total hemoglobin. a.u. arbitrary units
Table 4.
NIRS and EMG estimates pre and post OLT.
| Variable | Pre | Post | |
|---|---|---|---|
| Exercise Bout 1 | O2Hb/Mb (a.u.) | 18.89 (8.57) | 12.96 (4.4) |
| HHb/Mb (a.u.) | 14.9 (4.42) | 24.07 (4.6) | |
| RMS (%) | 87 (20.5) | 70.49 (14.98) | |
| PSDMF (Hz) | 57.12 (24.15) | 85.3 (20.19) * | |
| Exercise Bout 2 | |||
| O2Hb/Mb (a.u.) | 19.54 (6.76) | 15.46 (6.91) | |
| HHb/Mb (a.u.) | 14.12 (4.27) | 18.48 (2.1) | |
| RMS (%) | 92.09 (25.7) | 78.78 (17.79) | |
| PSDMF (Hz) | 50.54 (22.78) | 75.13 (21.83) * | |
Values are means (SD). O2Hb/Mb oxygenated hemoglobin, HHb/Mb deoxygenated heamoglobin, RMS root mean squared normalized to MVC, PSDMF power spectral density median frequency, Hz hertz.
Significant difference (p < 0.05)
Figure 2:
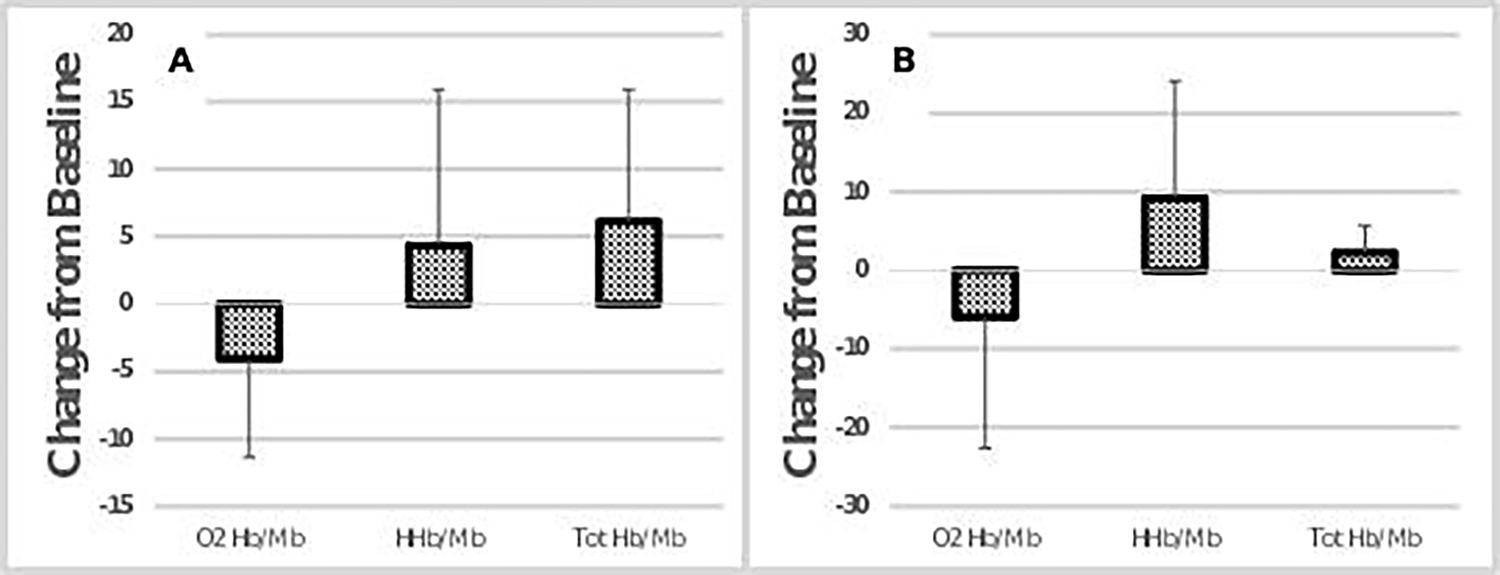
Absolute change score during post testing compared to pre OLT for NIRS variables during Exercise bout 1 (A) and Exercise bout 2 (B). No significant differences from pre to post values. Error bars represent standard deviation.
Figure 3:
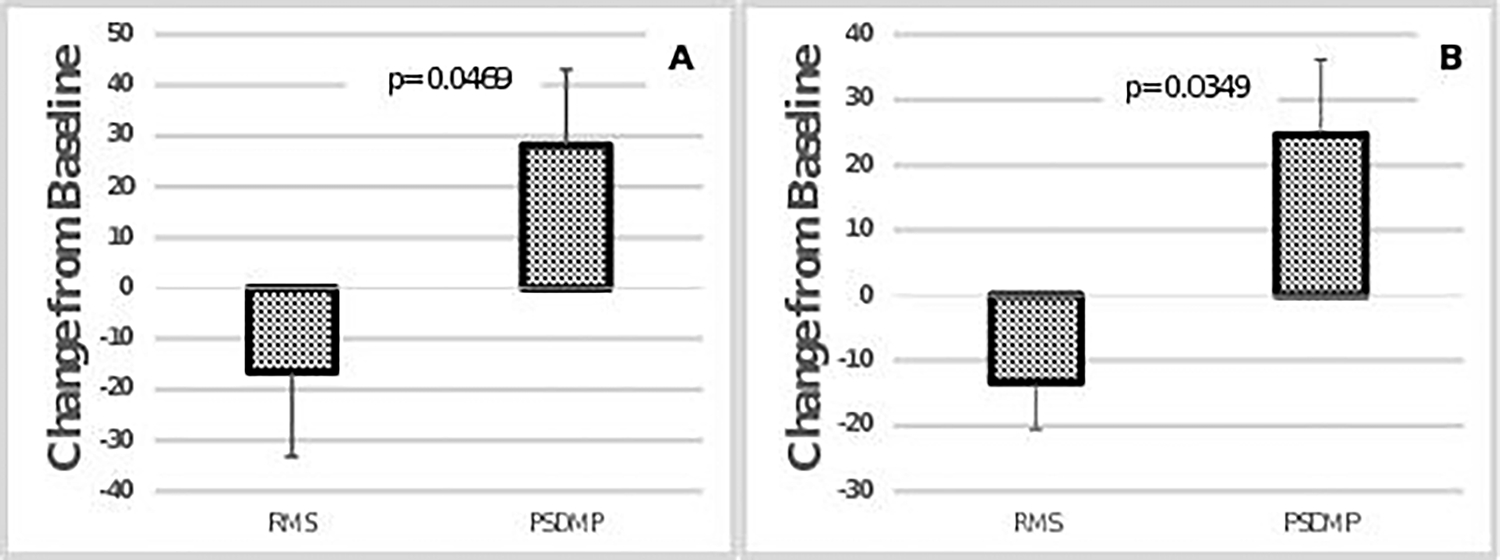
Absolute change score during post testing compared to pre OLT for EMG exercise bout 1, (A) and exercise bout 2, (B). P-values indicate difference between pre and post OLT. Error bars represent standard deviation.
Discussion
Findings of this study suggested that larger muscle fibers of increased force generating capacity and/or a larger number of motor units may have been recruited during similar exercise intensities without adversely affecting gastrocnemius utilization/oxygenation after OLT. In individuals with incomplete SCI, muscle denervation tends to result in a shift toward glycolytic state characteristics associated with Type II fibers, leading to decreased exercise endurance and increased physical activity intolerance. (9,19) Gastrocnemius median power frequency during both exercise bouts increased following OLT (by 49% and 48% respectively). This increase might well have been related to recruitment of larger motor units suggestive of increased Type II fiber recruitment, (20,21) since the inference can then be made that a higher EMG power frequency could be associated with these larger Type II motor units. Therefore, one possible explanation of the shift observed in our cohort is a more prominent recruitment of these Type II fibers post intervention. Since neurogenically-mediated changes in fiber characteristics are typically toward the more glycolytic metabolic state, and since there was no change in gastrocnemius utilization/oxygenation, the findings of this study may suggest that not only were a greater amount of motor units recruited following OLT, the metabolic function in terms of oxygen utilization did not change. Based on mixed effects models, PSDMF did not have a significant influence on muscle oxygenation during the two bouts of treadmill walking in incomplete SCI, either before or after OLT. This observation may provide additional evidence that a larger number or larger sized motor units were recruited following the OLT regimen given the concomitance of an increase in median power frequency without a change in muscle utilization/oxygenation.
In those who are healthy with an intact spinal cord, training status is known to influence the interaction between muscle oxygenation and fiber recruitment patterns. (22) Boone et al. (2015) (22) examined the breakpoints of muscle deoxygenation and EMG activation during continuous incremental exercise. It was noted that breakpoints for both variables occurred at a later stage of the ramp protocol when the individual was more aerobically fit. Higher percentages of Type I fibers is related to greater aerobic capacity. (23) The results of the current study highlight the fact that there was no change in muscle utilization/oxygenation. It is unclear whether enzymatic or oxidative changes actually occurred in the muscles, as tissue samples were not obtained. It has been previously documented that Type IIa muscle fibers are distinctive in their ability to favor ATP production through both oxidative and glycolytic pathways depending on the level and type of their conditioning. (24) While it is possible that muscle fibers can improve oxidative ability and result in the recruitment of more oxidatively efficient fibers, muscle biopsies were not taken in the current study and any changes with respect to adaptations can only be speculated.
Previous studies have demonstrated an increase in power spectral characteristics with an increase in force production, (25,26) which is consistent with the theory of recruitment of greater numbers of Type II fibers. Suzuki et al. (27) showed surface EMG to be a strong predictor of motor unit action potential amplitude, which is then related to motor unit size. However, it should be noted that these two studies (26,27) determined fiber type composition from EMG data collected during a different activity from the present study. Both studies used a static isometric knee extension exercise with minimal movement about the joints, (26,27) while the current study assessed EMG activity patterns during dynamic treadmill walking with large degrees of freedom around multiple joints. Moreover, muscle fiber length and muscle fiber shortening, both of which would be held constant in isometric contractions, vary considerably during a dynamic activity such as walking, and are factors that could affect EMG interpretation. (28)
This study had several limitations. First, we cannot assume that our sample is representative of the incomplete SCI population in general. The convenience sample and the inability to account for variance in the variables of interest in the general incomplete SCI population limit our ability to generalize these findings beyond the current sample, despite the use of the mixed-effects models to enable using multiple data points from each participant in the sample. Conversely, the heterogeneity of the group in terms of injury level and severity may also have affected the results, as the level of the injury is understood to have a large influence on the level of dysfunction. (1,29) A large effect size for a pre- to post-OLT increase in muscle oxygenation (HHb/Mb; effect size = 0.9) was observed in the current study. Thus, it appears that the study was underpowered for detecting significant changes in some of the muscle oxygenation variables in the face of wide group heterogeneity, even when the effect size is large. A larger participant pool could possibly have yielded more complete group convergences and better interactions between HHb/Mb and EMG variables. While steps were taken to replicate the electrode placement for EMG and NIRS analysis, a slight deviation in electrode placement can affect collected signals from pre to post testing.
The current OLT program did not appear to influence the interaction between HHb/Mb and the EMG variables. One possible explanation is that the OLT program was not designed to focus solely on improving gross muscle activation or muscle oxygenation. The core principle of the OLT program was to create a training framework whereby the many systems involved in walking are integrated into the program. (14) Thereby, the 12-week OLT program used may have concealed specific changes to muscle activation as the overall OLT program integrated many physiological systems. A program specific to aerobic training, such as treadmill walking, may have yielded a more targeted adaptation. Finally, a muscle biopsy could have given a clearer sense of the specific fiber type associated with the gastrocnemius. Future studies should aim to utilize both EMG and muscle biopsy in the identification of specific patterns fiber type recruitment following OLT.
Conclusions
The results of the present study suggest larger and/or more muscle fibers were recruited during treadmill walking at a self-selected pace, following a novel OLT regimen in these participants with incomplete SCI. The increase in median power combined with no change in muscle utilization/oxygenation suggests a possible increase in the number of fibers recruited during similar workloads following OLT. While the small sample size limits generalization of these results, this OLT program may have improved gastrocnemius function in these participants with incomplete SCI.
Figure 1:
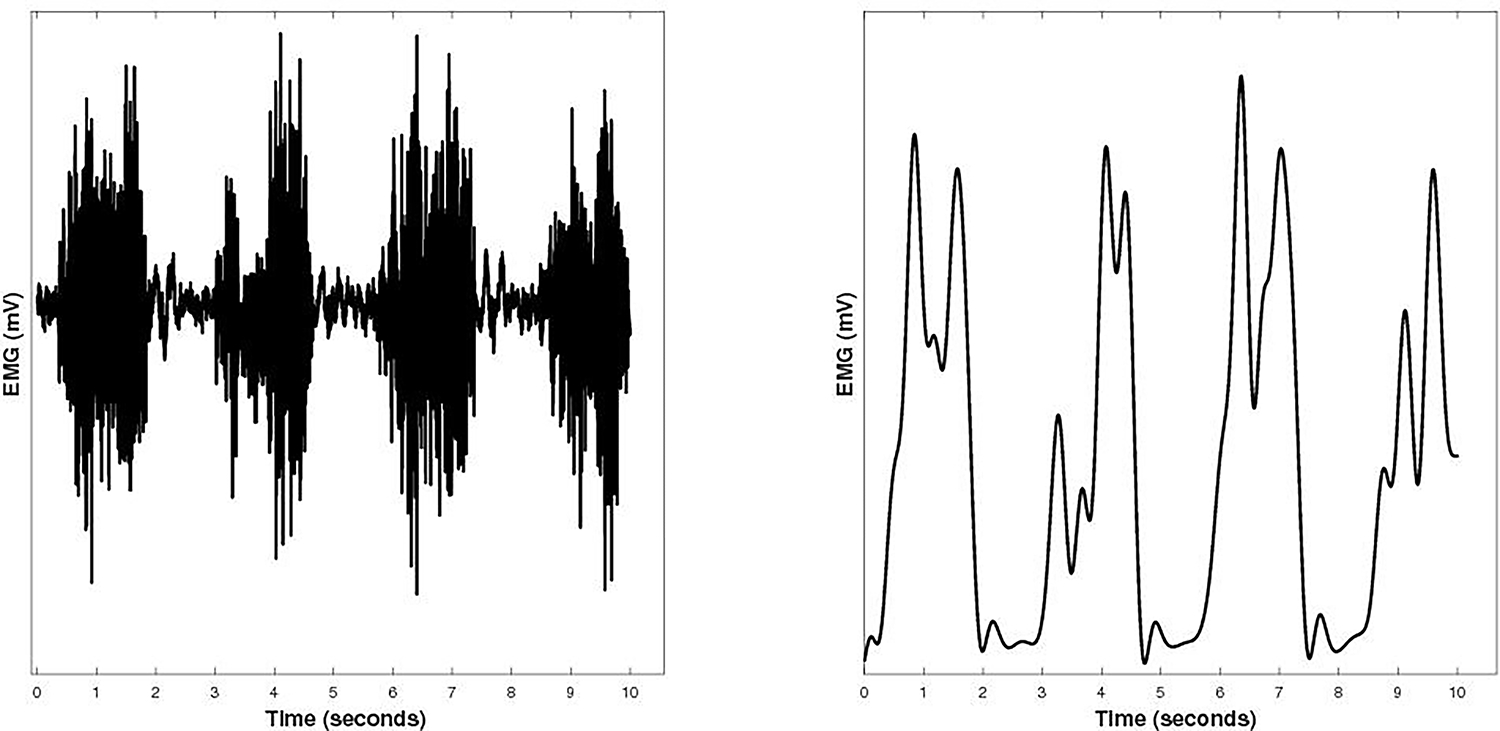
Depicts a 10 second EMG signal from the left gastrocnemius of a representative spinal cord injury subject during exercise bout 1 of a constant work rate test. The left side represents the raw unfiltered EMG signal, while on the right shows the EMG signal following the creation of the linear envelope.
Footnotes
No potential conflict of interest was reported by the authors.
The authors report no declarations of interest
REFERENCES
- 1.Wouda MF, Wejden L, Lundgaard E, Strøm V. Energetic and cardiovascular responses to treadmill walking and stationary cycling in subjects with incomplete spinal cord injury. Spinal Cord. 2016. Jan;54(1):51–6. [DOI] [PubMed] [Google Scholar]
- 2.Fukuoka Y, Endo M, Kagawa H, Itoh M, Nakanishi R. Kinetics and steady-state of VO2 responses to arm exercise in trained spinal cord injury humans. Spinal Cord. 2002. Dec;40(12):631–8. [DOI] [PubMed] [Google Scholar]
- 3.Myers JN, Hsu L, Hadley D, Lee MY, Kiratli BJ. Post-exercise heart rate recovery in individuals with spinal cord injury. Spinal Cord. 2010. Aug;48(8):639–44. [DOI] [PubMed] [Google Scholar]
- 4.Jayaraman A, Liu M, Ye F, Walter G, Vandenborne K. Regenerative responses in slow- and fast-twitch muscles following moderate contusion spinal cord injury and locomotor training. Eur J Appl Physiol. 2013. Jan;113(1):191–200. [DOI] [PubMed] [Google Scholar]
- 5.Pépin A, Norman KE, Barbeau H. Treadmill walking in incomplete spinal-cord-injured subjects: 1. Adaptation to changes in speed. Spinal Cord. 2003. May;41(5):257–70. [DOI] [PubMed] [Google Scholar]
- 6.Machač S, Radvanský J, Kolář P, Kříž J. Cardiovascular response to peak voluntary exercise in males with cervical spinal cord injury. J Spinal Cord Med. 2016. Jul;39(4):412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrest GF, Sisto SA, Barbeau H, Kirshblum SC, Wilen J, Bond Q, et al. Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete AIS-B spinal cord injury. J Spinal Cord Med. 2008. Nov;31(5):509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields RK. Muscular, skeletal, and neural adaptations following spinal cord injury. J Orthop Sports Phys Ther. 2002. Feb;32(2):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah PK, Stevens JE, Gregory CM, Pathare NC, Jayaraman A, Bickel SC, et al. Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil. 2006. Jun;87(6):772–8. [DOI] [PubMed] [Google Scholar]
- 10.Ng AV, Miller RG, Gelinas D, Kent-Braun JA. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle Nerve. 2004. Jun 1;29(6):843–52. [DOI] [PubMed] [Google Scholar]
- 11.Price M Energy Expenditure and Metabolism during Exercise in Persons with a Spinal Cord Injury. Sports Med Auckl. 2010. Aug;40(8):681–96. [DOI] [PubMed] [Google Scholar]
- 12.Gollie JM, Herrick JE, Keyser RE, Chin LMK, Collins JP, Shields RK, et al. Fatigability, oxygen uptake kinetics and muscle deoxygenation in incomplete spinal cord injury during treadmill walking. Eur J Appl Physiol. 2017. Oct;117(10):1989–2000. [DOI] [PubMed] [Google Scholar]
- 13.Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol. 2004. Apr 1;556(1):267–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gollie JM, Guccione AA. Overground Locomotor Training in Spinal Cord Injury: A Performance-Based Framework. Top Spinal Cord Inj Rehabil. 2017;23(3):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erickson M, Ryan T, Young H-J, McCully K. Near-infrared assessments of skeletal muscle oxidative capacity in persons with spinal cord injury. Eur J Appl Physiol. 2013. Sep;113(9):2275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda TY, Echeimberg JO, Pompeu JE, Lucareli PRG, Garbelotti S, Gimenes RO, et al. Root Mean Square value of the electromyographic signal in the isometric torque of the quadriceps, hamstrings and brachial biceps muscles in female subjects. J Appl Res. 2010. Feb;10(1):32–9. [Google Scholar]
- 17.Farina D, Fosci M, Merletti R. Motor unit recruitment strategies investigated by surface EMG variables. J Appl Physiol Bethesda Md 1985. 2002. Jan;92(1):235–47. [DOI] [PubMed] [Google Scholar]
- 18.Kupa EJ, Roy SH, Kandarian SC, Luca CJD. Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J Appl Physiol. 1995. Jul 1;79(1):23–32. [DOI] [PubMed] [Google Scholar]
- 19.Qin W, Bauman WA, Cardozo C. Bone and muscle loss after spinal cord injury: organ interactions. Ann N Y Acad Sci. 2010. Nov;1211:66–84. [DOI] [PubMed] [Google Scholar]
- 20.Backus SI, Tomlinson DP, Vanadurongwan B, Lenhoff MW, Cordasco FA, Chehab EL, et al. A Spectral Analysis of Rotator Cuff Musculature Electromyographic Activity: Surface and Indwelling. HSS J. 2011. Feb;7(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomonow M, Baten C, Smit J, Baratta R, Hermens H, D’Ambrosia R, et al. Electromyogram power spectra frequencies associated with motor unit recruitment strategies. J Appl Physiol Bethesda Md 1985. 1990. Mar;68(3):1177–85. [DOI] [PubMed] [Google Scholar]
- 22.Boone J, Barstow TJ, Celie B, Prieur F, Bourgois J. The interrelationship between muscle oxygenation, muscle activation, and pulmonary oxygen uptake to incremental ramp exercise: influence of aerobic fitness. Appl Physiol Nutr Metab. 2015. Oct 6;41(1):55–62. [DOI] [PubMed] [Google Scholar]
- 23.Barstow TJ, Jones AM, Nguyen PH, Casaburi R. Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol. 1996. Oct 1;81(4):1642–50. [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Mac Gabhann F, Popel AS. Effects of fiber type and size on the heterogeneity of oxygen distribution in exercising skeletal muscle. Plos One. 2012;7(9):e44375–e44375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerdle B, Eriksson NE, Brundin L. The behaviour of the mean power frequency of the surface electromyogram in biceps brachii with increasing force and during fatigue. With special regard to the electrode distance. Electromyogr Clin Neurophysiol. 1990. Dec;30(8):483–9. [PubMed] [Google Scholar]
- 26.Karlsson S, Gerdle B. Mean frequency and signal amplitude of the surface EMG of the quadriceps muscles increase with increasing torque — a study using the continuous wavelet transform. J Electromyogr Kinesiol. 2001. Apr 1;11(2):131–40. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Conwit RA, Stashuk D, Santarsiero L, Jeffrey Metter E. Relationships between surface-detected EMG signals and motor unit activation: Med Sci Sports Exerc. 2002. Sep;34(9):1509–17. [DOI] [PubMed] [Google Scholar]
- 28.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004. Apr;96(4):1486–95. [DOI] [PubMed] [Google Scholar]
- 29.Sedlock DA, Schneider DA, Gass E, Gass G. Excess post-exercise oxygen consumption in spinal cord-injured men. Eur J Appl Physiol. 2004. Sep 2;93(1–2):231–6. [DOI] [PubMed] [Google Scholar]


