ABSTRACT
Shewanella algae are gram-negative bacteria commonly found in aquatic environments. Infections caused by this agent are rarely documented; however, they are increasingly reported, mainly in countries with warm to temperate climates. Herein, we present a case of a 46-year-old immunocompetent woman with acute cellulitis and S. algae bacteremia (the first isolation culture performed at our hospital). To better understand the epidemiology, clinical outcomes, and treatment possibilities for S. algae bacteremia, we searched literature for similar cases; however, we did not find any cases of infections caused by this microorganism reported in Portugal or the Azores.
Keywords: Shewanella algae, Bacteremia, Cellulitis
INTRODUCTION
Shewanella algae are saprophytic gram-negative bacilli normally found in aquatic environments (fresh water, saltwater, and sewage), as well as in raw fish1-3. Infections caused by this agent are rarely documented; however, they are increasingly reported2,4,5, with a broad spectrum of manifestations, including hepatobiliary, cutaneous, soft-tissue, respiratory, and gastrointestinal infections, along with more severe cases of sepsis and bacteremia. Moreover, endocarditis and nervous system involvement are reported5,6. Multiple predisposing factors are associated with Shewanella infections, including geographic factors (warm climates and exposure to aquatic environments) and individual risk factors (peripheral vascular disease [PVD], obesity, diabetes mellitus, hepatobiliary disease, and chronic kidney disease, especially in hemodialysis patients, neoplasms, or other immunodeficiency states). Cutaneous infections may progress to ulceration even in healthy patients.2,5-7. To the best of our knowledge, this is the first report on acute cellulitis with Shewanella algae bacteremia in Portugal, as reviewed in databases such as the Cochrane Library, LILACS, SciELO, MEDLINE, PubMed, and PubMed Central.
CASE REPORT
A 46-year-old woman with a history of arterial hypertension, morbid obesity (body mass index: 58 kg/m2), PVD, and allergy to penicillin and contrast products presented to our emergency department (ED) with inflammatory signs in the left leg for 1 week, associated with fever (maximum temperature: 39.5 °C) within the last 2 days. When asked about the epidemiological context, she denied a history of leg trauma, ingestion of shellfish or undercooked food, or exposure to aquatic environments in the previous days. Moreover, approximately 1 month ago, she presented with inflammatory signs in the same leg, without previous trauma, and was treated with amoxicillin/clavulanic acid for probable cellulitis. However, she only received the prescribed antibiotic for 3 days because she experienced an allergic reaction (an edema of the face and tongue and dyspnea); thus, the antibiotic was suspended. These skin lesions were improved with the use of antibiotic wound dressings.
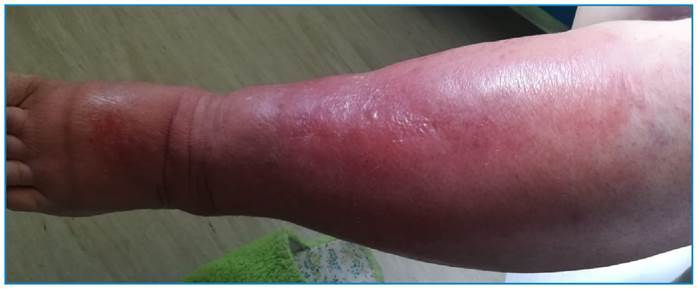
Upon admission to the ED, she was alert, oriented and cooperative, eupneic on room air, hemodynamically normal, and febrile (temperature: 38.2 °C). The left lower limb (LLL) showed an exuberant edema (Godet grade III/IV) extending up to the knee/lower area of the ipsilateral thigh, associated with marked poorly circumscribed erythema and heat, especially on the anterior surface of the leg (Figure 1). Moreover, the dorsum of the foot showed an exuberant transudative edema with interdigital fissures and no apparent superinfection. Results of the remaining objective examinations were insignificant. Analytically, she showed mild leukocytosis of 12,240/µL, neutrophilia of 1,120/µL, and C-reactive protein (CRP) levels of 11.7 mg/dL. Renal function and liver enzyme levels were within normal limits. Assuming cellulitis of the LLL, we collected two pairs of blood cultures, and the patient was empirically administered with levofloxacin. In the first 48 h, inflammatory signs were markedly worsened, and the CRP level increased to 40 mg/dL; thus, we escalated to meropenem. The blood cultures isolated S. algae, and the susceptibility tests showed sensitivity to gentamicin, ciprofloxacin, piperacillin/tazobactam, and meropenem, with no defined pattern of resistance. The result of the human immunodeficiency virus 1/2 test was negative. The inflammatory signs in the left leg slowly regressed; however, the pain and edema were worsened. Peripheral venous ultrasonography on the LLL revealed normal permeability of the superficial and deep venous systems. Ultrasonography of the soft tissues revealed thickening and increased echogenicity of the skin and subcutaneous tissue, with no evidence of complications, such as abscesses or compartmental syndrome.
FIGURE 1: Inflammatory signs on the left lower limb upon admission - an edema (Godet grade III/IV) extending up to the knee, associated with erythema and heat, especially on the anterior surface of the leg.
Considering the poor clinical and analytical evolution, we decided not to de-escalate the antibiotics and added empirical coverage for Staphylococcus aureus, initially with vancomycin (suspended after the onset of skin rash and pruritus) and later on, with linezolid. A complete clinical and analytical resolution was achieved with meropenem in 14 days and with linezolid in 7 days.
DISCUSSION
Infections caused by S. algae have been poorly described. However, soft tissue infection is a common clinical manifestation, and chronic ulcers are one of the main underlying comorbidities4,8. Our patient presented with a soft tissue infection, a very common condition, and S. algae bacteremia (the first culture isolation performed at our hospital). This report presented a unique case because of the rarity of isolation, as the patient was an immunocompetent woman whose only risk factors were obesity and PVD without any identifiable preceding exposures. The initial clinical presentation was not atypical; the inflammatory signs of the LLL, its location and distribution, and the remaining clinical history were not suggestive of atypical pathogens. Thus, we cannot determine the point of entry with certainty. Although the patient presented with interdigital fissures, she denied having been exposed to contaminated water. However, the increasing incidence of these microorganisms requires further consideration.
The only isolated microorganism was S. algae. We cannot exclude the possibility that there was no co-infection or over-infection with S. aureus or that clinical resolution did not occur as a result of the antibiotic coverage of this organism. However, we can claim that the prolongation of the duration of administering meropenem was also a factor in the clinical improvement. Existing literature has reported more severe cases that require a longer period of receiving antibiotic therapy5-7.
There are currently no guidelines for treating Shewanella infections. Based on the available data, S. algae are resistant to first- and second-generation cephalosporins and penicillin, which represent the first-line treatment for soft-tissue and skin infections. Resistance to quinolones and carbapenems has already been reported4,5,7. The lack of guidelines and knowledge of susceptibility studies promotes the varying usage of antibiotics across centers and their prolonged use in treating the infection6,7,9, which may contribute to the growingly reported resistance.
To the best of our knowledge, S. algae cellulitis or bacteremia has not been previously reported in Portugal, specifically in the Azorean Islands. These regions favor infections caused by S. algae because of their climatic conditions (hot and humid climate)1. Thus, this case highlights S. algae as a potentially emerging pathogen, even in patients without underlying comorbidities, emphasizing the need for greater case reporting to better optimize clinical decisions.
ACKNOWLEDGMENTS
The authors of the manuscript have no acknowledgments.
Footnotes
Financial Support: The authors received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.1. Holt HM, Gahrn-Hansen B, Bruun B. Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clin Microbiol Infect. 2005;11(5):347-52. [DOI] [PubMed]; Holt HM, Gahrn-Hansen B, Bruun B. Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clin Microbiol Infect. 2005;11(5):347–352. doi: 10.1111/j.1469-0691.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 2.2. Janda JM. Shewanella: A Marine Pathogen as an Emerging Cause of Human Disease. Clin Microbiol News. 2014;36(4):25-9.; Janda JM. Shewanella: A Marine Pathogen as an Emerging Cause of Human Disease. Clin Microbiol News. 2014;36(4):25–29. [Google Scholar]
- 3.3. Sharma KK, Kalawat U. Emerging Infections: Shewanella - A Series of Five Cases. J Lab Physicians. 2010;2(2):61-5. [DOI] [PMC free article] [PubMed]; Sharma KK, Kalawat U. Emerging Infections: Shewanella - A Series of Five Cases. J Lab Physicians. 2010;2(2):61–65. doi: 10.4103/0974-2727.72150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.4. Jampala S, Meera P, Vivek V, Kavitha DR. Skin and soft tissue infections due to Shewanella algae - An emerging pathogen. J Clin Diagn Res. 2015;9(2):16-20. [DOI] [PMC free article] [PubMed]; Jampala S, Meera P, Vivek V, Kavitha DR. Skin and soft tissue infections due to Shewanella algae - An emerging pathogen. J Clin Diagn Res. 2015;9(2):16–20. doi: 10.7860/JCDR/2015/12152.5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.5. Clément LF, Gallet C, Perron J, Lesueur A. Dermohypodermite bactérienne aiguë et septicémie à Shewanella alga chez un homme immunocompétent. Ann Dermatol Venereol. 2004;131(12):1095-7. [DOI] [PubMed]; Clément LF, Gallet C, Perron J, Lesueur A. Dermohypodermite bactérienne aiguë et septicémie à Shewanella alga chez un homme immunocompétent. Ann Dermatol Venereol. 2004;131(12):1095–1097. doi: 10.1016/s0151-9638(04)93848-3. [DOI] [PubMed] [Google Scholar]
- 6.6. Brugnaro P, Morelli E, Ebo F, Rosini G, Cattelan F, Petrucci A et al. The first italian case report of leg ulcer and sepsis caused by shewanella algae in a immunocompetent patient. Infez Med. 2019;27(2):179-82. [PubMed]; Brugnaro P, Morelli E, Ebo F, Rosini G, Cattelan F, Petrucci A, et al. The first italian case report of leg ulcer and sepsis caused by shewanella algae in a immunocompetent patient. Infez Med. 2019;27(2):179–182. [PubMed] [Google Scholar]
- 7.7. Latif A, Kapoor V, Vivekanandan R, Reddy JT. A rare case of Shewanella septicemia: Risk factors, environmental associations and management. BMJ Case Rep. 2019;12(9):1-2. [DOI] [PMC free article] [PubMed]; Latif A, Kapoor V, Vivekanandan R, Reddy JT. A rare case of Shewanella septicemia: Risk factors, environmental associations and management. BMJ Case Rep. 2019;12(9):1–2. doi: 10.1136/bcr-2019-230252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.8. Tsai MS, You HL, Tang YF, Liu JW. Shewanella soft tissue infection: case report and literature review. Int J Infect Dis. 2008;12(6):e119-24. [DOI] [PubMed]; Tsai MS, You HL, Tang YF, Liu JW. Shewanella soft tissue infection: case report and literature review. Int J Infect Dis. 2008;12(6):e119-24. doi: 10.1016/j.ijid.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 9.9. Cimmino T, Olaitan AO, Rolain JM. Whole genome sequence to decipher the resistome of Shewanella algae, a multidrug-resistant bacterium responsible for pneumonia, Marseille, France. Expert Rev Anti Infect Ther. 2016;14(2):269-75. [DOI] [PubMed]; Cimmino T, Olaitan AO, Rolain JM. Whole genome sequence to decipher the resistome of Shewanella algae, a multidrug-resistant bacterium responsible for pneumonia, Marseille, France. Expert Rev Anti Infect Ther. 2016;14(2):269–275. doi: 10.1586/14787210.2016.1106936. [DOI] [PubMed] [Google Scholar]


