Abstract
Background
The effectiveness of glucagon-like peptide 1 receptor agonists (GLP1RA) and sodium–glucose cotransporter 2 inhibitors (SGLT2i) in preventing major adverse cardiac events (MACE) is uncertain for those without pre-existing cardiovascular disease.
Objective
To test the hypothesis that MACE incidence was lower with addition of GLP1RA or SGLT2i compared to dipeptidyl peptidase 4 inhibitors (DPP4i) for primary prevention to baseline therapy.
Design
Retrospective cohort study of US veterans from 2001-2019.
Setting
Veterans 18 years and older receiving care in Veterans Health Administration, with data linkage to Medicare, Medicaid and National Death Index.
Patient Episodes
Addition of GLP1RA; SGLT2i; or DPP4i by veterans who were currently using metformin, sulfonylurea or insulin alone or in combination. Episodes were stratified by history of prior cardiovascular disease.
Measurements
Study outcomes were MACE (acute myocardial infarction, stroke, or cardiovascular death) and heart failure (HF) hospitalization. Cox models compared the outcome between medication groups using pairwise comparisons in a weighted cohort adjusted for covariates.
Results
The cohort included 28,759 GLP1RA vs 28,628 DPP4i and 21,200 SGLT2i vs 21,170 DPP4i weighted pairs. Median age was 67 years and diabetes duration was 8.5 years. GLP1RA was associated with lower MACE and HF vs DPP4i (adjusted Hazard Ratio [aHR] 0.82 [0.72, 0.94]); yielding an adjusted risk difference [aRD] of 3.2 events (1.1, 5.0) per 1,000 person years. SGLT2i was not associated with MACE and HF (aHR 0.91 (0.78, 1.08); aRD 1.28 (−1.12, 3.32) compared with DPP4i.
Limitations
Residual confounding; Use of DPP4i, GLP1RA, SGLT2i as first line therapies were not examined
Conclusions
GLP1RA addition was associated with primary reductions of MACE and HF hospitalization compared with DPP4i use; SGLT2 addition was not associated with primary MACE prevention.
INTRODUCTION
Diabetes is common and confers a high risk for cardiovascular disease (CVD) which remains the leading cause of death (1-4). It is less clear whether CVD onset can be prevented by newer antidiabetic medications (5-10). Since 2008, regulatory guidance for licensure of new antidiabetic medications requires evaluation of both HbA1c and major adverse cardiovascular events (MACE) [myocardial infarction, stroke and cardiovascular death] as study endpoints (11).
There have been multiple pivotal trials of glucagon-like peptide 1 receptor agonists (GLP1RA) or sodium-glucose cotransporter 2 inhibitors (SGLT2i) vs placebo that evaluate MACE outcomes (12-18). These trials individually, along with meta-analyses, demonstrated benefit in MACE risk among those with pre-existing CVD (secondary prevention) (19-21). However, these trials collected little data or were inadequately powered for patients without CVD (primary prevention). Additionally, the dipeptidyl peptidase 4 inhibitors (DPP4i) are regarded as cardio-neutral and they continue to be widely used (5-7,10,22-24 25-27).
Our aim was to test the hypothesis that among patients with diabetes, fatal and nonfatal cardiovascular endpoints was lower with adding either GLP1RA or SGLT2i compared to DPP4i for primary prevention. We conducted confirmatory analyses among the full cohort including those with CVD to evaluate secondary prevention.
METHODS
Study design and Data sources
We assembled a cohort of Veterans Health Administration (VHA) patients with diabetes and a first prescription for an antidiabetic medication between 1/1/2001 and 12/31/2016. Additional cohort follow-up data were obtained through 12/31/2019. VHA data included: demographic, diagnostic, and procedure information from inpatient and outpatient encounters, laboratory results and vital signs from clinical sources. Pharmacy data included medication dispensed, date filled, days supplied, and number of pills/injections dispensed. For Medicare or Medicaid enrollees, we obtained enrollment, claims files, and prescription (Part D) data. We obtained dates and cause of death from vital status and the National Death Index files. The institutional review board of VHA Tennessee Valley Healthcare System approved this study with a waiver of consent. Our retrospective comparative effectiveness study design emulates a controlled trial for each of the comparisons of interest (Supplemental methods).
Diabetes population and episode index date
The source population comprised veterans aged 18 years and older who were regular VHA users, i.e., had an encounter or prescription fill at least once every 365 days for two or more years before cohort entry. We identified patients who newly filled a first antidiabetic prescription (metformin, insulin, or sulfonylurea) without any antidiabetic fill in the 180 days prior. We then identified episodes from this cohort defined by a new fill of one of the following medication classes: GLP1RA; SGLT2i or DPP4i.
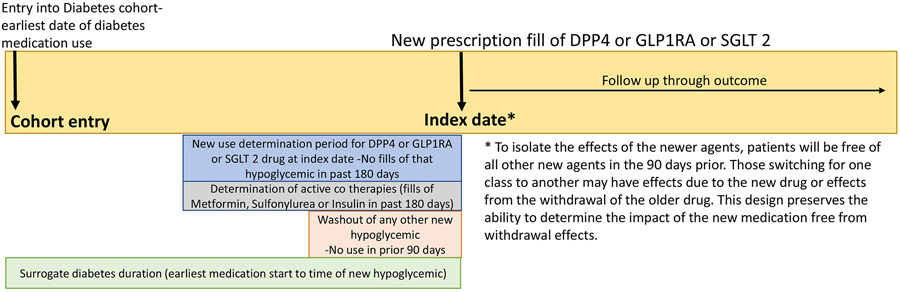
The index date and start of follow-up was the day of GLP1RA; SGLT2i or DPP4i prescription fill. A new episode of use was defined as a new prescription for one of the medications in the study classes (see Exposures, Supplemental Table 1) without use of that medication class in the prior 180 days. We created a wash out period before the index date by restricting use of any other new medication class in the 90 days prior to the index date (Figure 1). This allowed for an evaluation of the new medication without contamination or withdrawal by a different medication class under investigation. For example, a patient who began a new SGLT2i episode would qualify for inclusion as a new episode if there were no fills of SGLT2i in the prior 180 days (new user) and no active days supply of DPP4i or GLP1RA in the prior 90 days. Patients who were on metformin, insulin or sulfonylurea co-therapies (or combinations) in the 180 days before the index date were included. Patients who used all three medications (metformin+ sulfonylurea+ insulin) or another drug class (such as acarbose or thiazolidinedione) were excluded. Patients on dialysis, organ transplantation, or hospice care within the 2 years prior to the index date were excluded.
Figure 1:
Schematic of study entry and index date
All episodes were then stratified by CVD status. Prior CVD was defined as any of the following conditions: myocardial infarction; obstructive coronary disease; peripheral artery disease or revascularization; carotid revascularization and history of stroke or transient ischemic attack in the 720 days prior to the index date.
Exposures
Study exposures included persistent use of: exenatide, liraglutide, semaglutide, dulaglutide, lixisenatide, albiglutide (GLP1RA); empagliflozin, dapagliflozin, canagliflozin (SGLT2i); or alogliptin, linagliptin, saxagliptin, sitagliptin (DPP4i). The index date and start of follow-up was the medication fill date and continued through an outcome or non-persistence of that drug class, defined as 90 days without that drug class or the addition or crossover to a different medication class under investigation. Switching within medication class was allowed. A patient who ended follow-up could re-enter the cohort as a new episode if all entry criteria were satisfied.
Outcomes
The composite outcome was the time to MACE or HF hospitalization. The outcome date was hospital admission date for acute myocardial infarction, ischemic or hemorrhagic stroke, acute heart failure, or cardiovascular death date. The primary discharge diagnosis or underlying cause of death identified each event. We also evaluated each component separately.
We defined acute myocardial infarction by discharge codes 410.x, or I21.x. Stroke hospitalizations included ischemic stroke (433.x1, 434 [excluding 434.x0], 436, or I63.30; I63.40; I63.50; I66.09; I66.19; I66.29; I66.9; I66.9; I67.848; I67.89), intracerebral hemorrhage (431 or I61.x), and subarachnoid hemorrhage (430 or I60.x). When compared to medical record review, these codes demonstrate high positive predictive values (90% acute myocardial infarction; 81% stroke)(28).
HF hospitalization included a primary diagnosis of HF, cardiomyopathy, or hypertensive heart disease with HF (425.X; 428.X; 404.01, 404.03, 404.11, 404.13, 398.91, 402.01, 402.11, 402.91, 404.91, 404.93, I50.2*; I50.3*; I50.9; I42.9; I13.0; I13.2; I09.81; I11.0). HF hospitalization could also be captured with a diagnosis-related group code (127 or 291-293) (29,30). To understand HF hospitalization type, we utilized the natural language processing echocardiogram algorithm developed by Patterson et. al (precision measured for ejection fraction between 0.97 and 1.0) (31). Only echocardiograms conducted within VHA were available to determine HF type based on ejection fraction (reduced <40%; mid-range 40-49%; and preserved ≥50%). The echocardiogram used was the study closest to admission day and up to seven days after admission. If no echocardiogram was obtained, we evaluated echocardiograms up to one year before and closest to admission. If no echocardiogram information was available (no numeric ejection fraction or Medicare claim hospitalization), then HF hospitalization type was considered unknown.
Cardiovascular deaths were identified from death certificates with an underlying cause of death compatible with cardiac death, fatal myocardial infarction, stroke or cardiomyopathy (I00-I78 excluding I30.X [diseases of the pericardium]) or unattended sudden cardiac death (R98, R99, R960, R961). This definition included the Centers for Disease Control and Prevention definition of cardiac death and a validated strategy for identifying sudden cardiac deaths (32).
Covariates
Study covariates were measured up to 720 days before the index date and included: age, sex, race, year, and a surrogate for diabetes duration (years from cohort entry to index date). We accounted for diabetes co-therapies (metformin, sulfonylurea, insulin), and Veterans Integrated Service Network. Each network is a geographic designation for VHA and represents an estimation of geographic variation of diabetes prevalence and care. Physiologic variables were defined as the most recent measure in the two years before index date and included: body mass index, blood pressure, HbA1c, low density lipoprotein, hemoglobin, proteinuria, echocardiogram ejection fraction and creatinine. Creatinine was used to calculate estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration 2021 equation without race adjustment (33,34). Healthcare utilization included: hospitalization, nursing home, number of outpatient visits or medications, Medicare or Medicaid insurance use. We collected data on smoking, co-morbidities and selected medications defined in Supplemental Table 2. We utilized self-reported categorical race from VHA and Medicare in models (35).
Statistical Analyses
The primary analysis used Cox Proportional Hazard models to compare time to MACE and HF events between medication groups using pairwise comparisons in a propensity score weighted cohort in patients without prior CVD. The unit of analysis was the episode of medication use. Censoring criteria included: study end date (December 31, 2019); non persistence (90 days without medication); crossover/addition of diabetes drug in a different class (e.g. SGLT2i user who starts GLP1RA) and the 181st day of no VHA contact (inpatient, outpatient, or pharmacy use).
The propensity score modeled the probability of GLP1RA versus DPP4i or SGLT2i versus DPP4i given baseline covariates noted above and an indicator for imputed covariates. Missing covariates were handled using thirty iterations of chained imputations (36). We used matching weights to balance the distribution of observed baseline values between exposure groups (Supplemental Methods and Supplemental Figures 1-3) (37-39). DPP4i was the reference in each weighted cohort and models adjusted for covariates. For each pairwise comparison, statistical significance for the two-sided p value was set at 0.05 using robust standard errors to account for patients who contributed multiple episodes of medication use. The proportional hazards assumptions were verified through examination of Schoenfeld residuals over time and follow up was truncated at 3.5 years due to sparse data yielding uncertainty in the proportional hazards assumptions (40). The inverse non-parametric Kaplan Meier estimates of the survival function were used to generate the cumulative incidence curves for the time to event in the weighted cohorts with confidence intervals using 5,000 bootstraps (41).
Secondary, Sensitivity, Subgroup analyses, and effect of an unmeasured confounder
We conducted a secondary analysis that compared the incidence of outcomes between SGLT2i and GLP1RA among those without CVD and for the full cohort with and without CVD. GLP1RA users were considered the reference group. The persistence required analysis is most restrictive and requires persistence on medication to evaluate an association on outcomes, because patients must remain on the medication to be analyzed. A sensitivity analysis assumed patients remained in their initial exposure groups with a relaxed adherence requirement; but did not allow cross over to another new medication class. Thus, patients were censored only if they crossed over to another new agent. This analysis is akin to an intention to treat analysis in clinical trials without crossover and increases follow-up time and events but allows misclassification of person time as “time on drug”.
Subgroup analyses report weighted event rates with confidence intervals for the primary prevention cohort as the sample sizes were smaller within subgroups and diagnostic plots suggest that the proportional hazards assumption was violated. Subgroups include: age (≥65, < 65 years), timing of addition (add on as second vs third treatment), baseline estimated glomerular filtration rate (< 60, ≥60 ml/min/1.73m2), and body mass index (<35 kg/m2, ≥35 kg/m2).
We tested the robustness of our findings to unmeasured confounding using an E-value, which quantifies the strength of an unmeasured confounder to render the study results inconclusive (42). That is, if the hypothetical unmeasured confounder could have been adjusted for, it would have shifted the boundary of the effect size's confidence interval to null. All analyses were conducted using R (version 4.1.2) (43).
Role of the Funding source
The VA Clinical Science Research and Development had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
RESULTS
Study cohort and patient characteristics
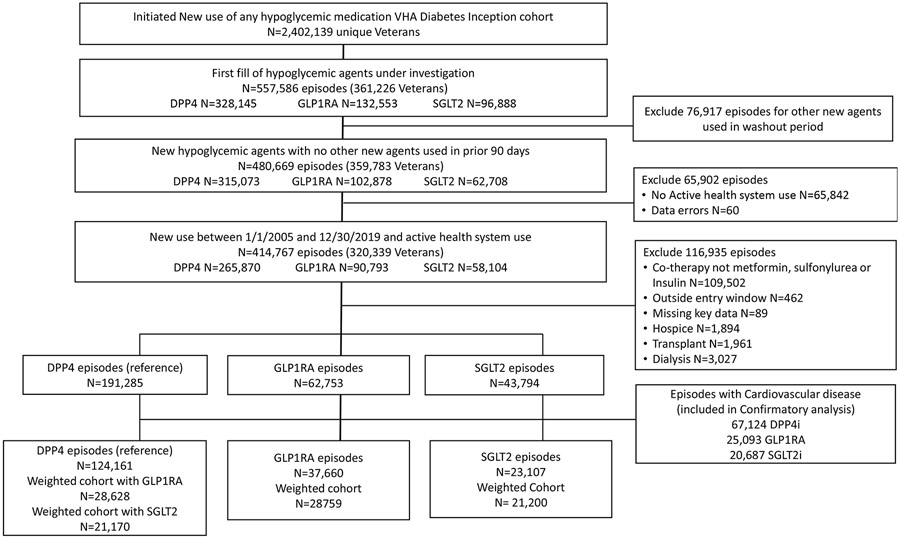
We identified 557,586 new episodes of the three classes under investigation (Figure 2) among 361,226 patients. We excluded 76,917 episodes for concurrent use of another class under evaluation. Additional exclusions were for data errors (n=60); no VHA health system use in the prior 2 years (n=65,842); co- therapy was not metformin, insulin or sulfonylurea (n=109,502); prescription was outside the study time frame (n=462); missing key data (n=89), hospice care (n=1,894), organ transplant (n=1,961); or dialysis use in past 2 years (n=3,027). Episodes identified as having CVD included: 67,124 DPP4i; 25,093 GLP1RA and 20,687 SGLT2i. The unweighted sample included: 124,161 DPP4i episodes (104,575 patients); 37,660 GLP1RA (32,925 patients); and 23,107 SGLT2i (21,690 patients). Patients had on average 1.1 episodes of medication use (11.4% DPP4i; 9.2% GLP1RA; and 5.2% SGLT2i with 2 or more episodes). After propensity score calculation and weighting, the cohort included 28,759 GLP1RA vs 28,628 DPP4i episodes and 21,200 SGLT2i vs 21,170 DPP4i episodes.
Figure 2:
Flow chart of eligible episodes of care and final study sample
The characteristics of the unweighted episodes for those without CVD are described in Supplemental Table 3. After weighting, patient characteristics were similar between each pairwise comparison group (GLP1RA vs DPP4i and SGLT2i vs DPP4i), demonstrating standardized mean differences of less than 0.1 (Table 1, Supplemental Figures 4-5). More than 60% of GLP1RA, SGLT2i or DPP4i episodes were added as a third agent to metformin, insulin and sulfonylurea combination regimens. Within the GLP1RA vs DPP4 weighted cohort, 50% had an HbA1c measured; 61% BMI; and 63% BP measurement within 30 days of the index date; 87% and 89% of had the HbA1c or BP measured within 180 days prior to index date. For the SGLT2 vs DPP4 weighted cohort 56% had HbA1c; 63% BMI; and 67% BP measurement within 30 days of the index date, 88% and 90% of the weighted cohort had the HbA1c and BP measured within 180 days of index date. The characteristics for the weighted cohort with and without cardiovascular disease are reported in Supplemental Table 4.
Table 1:
Patient Characteristics on Index date for those without Cardiovascular Disease
| DPP4i N=28,628 |
GLP1RA N=28,628 |
SMD | DPP4i N=28,628 |
SGLT2i N=28,628 |
SMD | |
|---|---|---|---|---|---|---|
| Age, years* | 66.0 [57.9, 71.0] | 66.2 [57.9, 71.1] | 0.001 | 66.9 [59.1,71.5] | 67.0 [59.1,71.5] | 0.016 |
| Male, (No., %) | 26337 (92.0) | 26488 (92.1) | 0.004 | 19929 (94.1) | 19974 (94.2) | 0.003 |
| Race, (No., %) | 0.005 | 0.004 | ||||
| Other | 1117 ( 4.1) | 1146 ( 4.2) | 773 ( 3.9) | 774 ( 3.9) | ||
| Black | 5905 (21.9) | 5908 (21.8) | 4406 (21.9) | 4446 (22.1) | ||
| White | 19979 (74.0) | 20058 (74.0) | 14900 (74.2) | 14888 (74.0) | ||
| Missing Race (No., %) | 1627 ( 5.7) | 1647 ( 5.7) | 0.002 | 1091 ( 5.2) | 1092 ( 5.2) | <0.001 |
| Medication start to index date, years* | 8.5 (4.8, 12.9) | 8.5 (4.8, 12.9) | 0.004 | 8.8 [5.4, 13.2] | 8.8 [5.3, 13.2] | 0.001 |
| Co-Therapy, (No., %) | 0.005 | 0.005 | ||||
| Metformin | 2915 (10.2) | 2931 (10.2) | 3574 (16.9) | 3552(16.8) | ||
| Sulfonylurea | 1069 ( 3.7) | 1064 ( 3.7) | 1174 ( 5.5) | 1166 ( 5.5) | ||
| Insulin | 6191 (21.6) | 6249 (21.7) | 2802 (13.2) | 2814 (13.3) | ||
| Metformin + Sulfonylurea | 5283 (18.5) | 5271 (18.3) | 6676 (31.5) | 6674 (31.5) | ||
| Metformin + Insulin | 11172 (39.0) | 11253 (39.1) | 6191 (29.2) | 6231 (29.4) | ||
| Sulfonylurea + Insulin | 1997 ( 7.0) | 1991 ( 6.9) | 754 ( 3.6) | 764 ( 3.6) | ||
| Year of cohort entry, (No., %) | 0.098 | 0.054 | ||||
| 2005-2007 | 167 ( 0.6) | 309 ( 1.1) | 0 (0) | 0 (0) | ||
| 2008-2010 | 1067 (3.7) | 883 (3.1) | 0 (0) | 0 (0) | ||
| 2011-2013 | 2340 (8.2) | 2476 (8.6) | 110 ( 0.5) | 59 ( 0.3) | ||
| 2014-2016 | 7223 (25.2) | 7314 (25.4) | 2958 (14.0) | 2966 (13.9) | ||
| 2017-2019 | 17831 (62.3) | 17777 (61.8) | 18103 (85.5) | 18175 (85.7) | ||
| Clinical Variables | ||||||
| Body Mass Index, kg/m2 * | 34.3 [30.4, 38.9] | 34.6 [30.7, 39.2] | 0.039 | 32.8 [29.1,37.2] | 33.0 [29.3, 37.4] | 0.023 |
| Missing BMI Measure (No., %) | 6276 (21.9) | 6264 (21.8) | 0.003 | 4838 (22.9) | 4779 (22.5) | 0.007 |
| Systolic Blood Pressure, mm/Hg * | 133 [123, 143] | 133 [123, 143] | 0.004 | 133 [124, 143] | 133 [123, 143] | 0.003 |
| Diastolic Blood Pressure mm/Hg * | 76 [70, 82] | 76 [70, 83] | 0.001 | 77 [70, 83] | 77 [70, 83] | 0.005 |
| Missing Diastolic Blood Pressure (No., %) | 688 (2.4) | 675 (2.3) | 0.004 | 410 ( 1.9) | 379 ( 1.8) | 0.011 |
| Echocardiogram Ejection Fraction Category (No., %) | 0.019 | 0.018 | ||||
| Normal < 50% | 5044 (17.6) | 5129 (17.8) | 3724 (17.6) | 3756 (17.7) | ||
| Indeterminate 40-49% | 676 (2.4) | 664 (2.3) | 507 (2.4) | 515 (2.4) | ||
| Reduced/Severe < 40% | 630 (2.2) | 652 (2.3) | 519 (2.5) | 551 (2.6) | ||
| Missing/Unavailable | 22277 (77.8) | 22314 (77.6) | 0.005 | 16421(77.6) | 16378 (77.3) | 0.007 |
| Laboratory Variables | ||||||
| HbA1c, % * | 8.4 [7.6, 9.6] | 8.6 [7.6, 9.7] | 0.004 | 8.4 [7.7, 9.4] | 8.5 [7.7, 9.5] | 0.013 |
| Missing HbA1c (No., %) | 2152 (7.5) | 2095 ( 7.3) | 0.009 | 1560 ( 7.4) | 1510 ( 7.1) | 0.01 |
| Hemoglobin, g/dL * | 14.1 [13.1, 15.1] | 14.1 [13.1, 15.1] | 0.005 | 14.3 [13.3, 15.2] | 14.3 [13.3, 15.2] | 0.003 |
| Missing Hemoglobin (No.,%) | 2905 (10.1) | 2902 (10.1) | 0.002 | 1841 ( 8.7) | 1803 ( 8.5) | 0.007 |
| Estimated Glomerular Filtration rate, ml/min/1.73 m2 * | 79.5 [60.5, 95.8] | 79.5 [60.1, 95.7] | 0.002 | 83 [68, 95] | 82 [67, 96] | 0.004 |
| Serum Creatinine mg/dL * | 1.03 [0.89, 1.30] | 1.03 [0.89, 1.30] | 0.001 | 1.00 [0.87, 1.19] | 1.00 [0.87, 1.20] | 0.016 |
| Missing Creatinine (No.,%) | 1905 ( 6.7) | 1893 ( 6.6) | 0.003 | 1241 ( 5.9) | 1172 ( 5.5) | 0.014 |
| Low Density Lipoprotein mg/dL * | 81 [63, 105] | 81 [63, 105] | 0.003 | 81 [62, 105] | 81 [62, 106] | <0.001 |
| Missing Low Density Lipoprotein (No., %) | 1984 (6.9) | 1945 (6.8) | 0.007 | 1239 ( 5.9) | 1175 ( 5.5) | 0.013 |
| Urine protein on urinalysis (No., %) | 0.002 | 0.009 | ||||
| Negative | 11585 (40.5) | 11615 (40.4) | 8851 (41.8) | 8879 (41.9) | ||
| trace or 1+ | 3302 (11.5) | 3321 (11.5) | 2020 ( 9.5) | 2048 ( 9.7) | ||
| 2+ | 1879 ( 6.6) | 1901 ( 6.6) | 1117 ( 5.3) | 1142 ( 5.4) | ||
| 3+/4+/trace to 4+ | 527 ( 1.8) | 529 ( 1.8) | 220 ( 1.0) | 227 ( 1.1) | ||
| Missing Urine protein measure (No., %) | 11335 (39.6) | 11392 (39.6) | 8962 (42.3) | 8904 (42.0) | ||
| Microalbumin to Creatinine ratio (MACR) stage N (No., %) | 0.006 | 0.008 | ||||
| A1 (<30 mg/g- normal to mild increase albuminuria) | 11676 (40.8) | 11769 (40.9) | 9161 (43.3) | 9182 (43.3) | ||
| A2 (30-300 mg/g- moderate increase albuminuria) | 5284 (18.5) | 5348 (18.6) | 3993 (18.9) | 4037 (19.0) | ||
| A3 and positive (>300 mg/g severely increased albuminuria) | 2068 ( 7.2) | 2078 ( 7.2) | 1310 (6.2) | 1334 ( 6.3) | ||
| Missing MACR measure | 9599 (33.5) | 9564 (33.3) | 6706 (31.7) | 6647 (31.4) | ||
| Baseline Comorbidities (No., %) ‡ | ||||||
| Cardiovascular conditions | ||||||
| Congestive Heart Failure | 1366 ( 4.8) | 1383 ( 4.8) | 0.002 | 978 ( 4.6) | 991.4 ( 4.7) | 0.003 |
| Arrhythmias | 1557 ( 5.4) | 1558 ( 5.4) | 0.001 | 950 ( 4.5) | 944.3 ( 4.5) | 0.002 |
| Cardiac valve disease | 392 ( 1.4) | 395 ( 1.4) | <0.001 | 322 ( 1.5) | 322.4 ( 1.5) | <0.001 |
| Pulmonary Conditions | ||||||
| Smoking | 2978 (10.4) | 2987 (10.4) | <0.001 | 1816 ( 8.6) | 1831 ( 8.6) | 0.002 |
| Chronic Obstructive Pulmonary Disease | 3868 (13.5) | 3888 (13.5) | <0.001 | 2691 (12.7) | 2694 (12.7) | <0.001 |
| History of Respiratory failure | 713 ( 2.5) | 712 ( 2.5) | 0.001 | 468 ( 2.2) | 476 ( 2.2) | 0.002 |
| Neuro- Psychiatric Conditions | ||||||
| Serious Mental Illness | 9440 (33.0) | 9532 (33.1) | 0.004 | 6277 (29.6) | 6318 (29.8) | 0.003 |
| Parkinson's | 209 ( 0.7) | 215 ( 0.7) | 0.002 | 149 ( 0.7) | 146 ( 0.7) | 0.002 |
| Retinopathy | 3642 (12.7) | 3666 (12.7) | 0.001 | 2183 (10.3) | 2233 (10.5) | 0.007 |
| Infectious Conditions | ||||||
| History of Sepsis | 494 ( 1.7) | 488 ( 1.7) | 0.002 | 267 ( 1.3) | 263 ( 1.2) | 0.002 |
| History of Pneumonia | 502 ( 1.8) | 499 ( 1.7) | 0.001 | 316 ( 1.5) | 313 ( 1.5) | 0.001 |
| HIV | 130 ( 0.5) | 134 ( 0.5) | 0.002 | 95 ( 0.4) | 97 ( 0.5) | 0.001 |
| Urinary tract infection | 1266 ( 4.4) | 1274 ( 4.4) | <0.001 | 626 ( 3.0) | 625 ( 2.9) | <0.001 |
| Osteomyelitis | 229 ( 0.8) | 222 ( 0.8) | 0.003 | 100 ( 0.5) | 101 ( 0.5) | 0.001 |
| Other serious illness and disabilities | ||||||
| Malignancy | 2729 ( 9.5) | 2761 ( 9.6) | 0.002 | 2119 (10.0) | 2119 (10.0) | 0.001 |
| Liver disease | 1306 ( 4.6) | 1323 ( 4.6) | 0.002 | 1108 (5.2) | 1133 (5.3) | 0.005 |
| History of Kidney disease | 7 ( 0.0) | 7 ( 0.0) | <0.001 | 1 (0.0) | 1 (0.0) | 0.003 |
| Osteoporosis | 197 ( 0.7) | 195 ( 0.7) | 0.001 | 130 ( 0.6) | 128 ( 0.6) | 0.001 |
| Falls | 248 ( 0.9) | 246 ( 0.9) | 0.001 | 197 ( 0.9) | 194 ( 0.9) | 0.002 |
| Fractures | 510 ( 1.8) | 506 ( 1.8) | 0.002 | 323 ( 1.5) | 319 ( 1.5) | 0.002 |
| Amputation | 113 ( 0.4) | 115 ( 0.4) | <0.001 | 56 ( 0.3) | 58 ( 0.3) | 0.001 |
| Use of Medications (No., %) | ||||||
| ACE Inhibitors | 14682 (51.3) | 14739 (51.2) | 0.001 | 11031 (52.1) | 11061 (52.2) | 0.001 |
| Angiotensin Receptor Blockers | 6916 (24.2) | 6960 (24.2) | 0.001 | 4908 (23.2) | 4921 (23.2) | 0.001 |
| Beta Blockers | 10508 (36.7) | 10564 (36.7) | 0.001 | 7697 (36.4) | 7713 (36.4) | <0.001 |
| Calcium Channel Blockers | 8852 (30.9) | 8920 (31.0) | 0.002 | 6385 (30.2) | 6404 (30.2) | 0.001 |
| Thiazide/potassium sparing diuretics | 9886 (34.5) | 9945 (34.6) | 0.001 | 6914 (32.7) | 6947 (32.8) | 0.002 |
| Loop diuretics | 4215 (14.7) | 4218 (14.7) | 0.002 | 2252 (10.6) | 2259 (10.7) | 0.001 |
| Other antihypertensives | 7996 (27.9) | 8020 (27.9) | 0.001 | 5499 (26.0) | 5459 (25.8) | 0.005 |
| Lipid-lowering statins | 22020 (76.9) | 22159 (77.1) | 0.003 | 16680 (78.8) | 16724 (78.9) | 0.002 |
| Non-statin lipid-lowering agents | 4407 (15.4) | 4427 (15.4) | <0.001 | 3019 (14.3) | 3003 (14.2) | 0.003 |
| Anti-arrhythmic digoxin and inotropes | 1387 ( 4.8) | 1397 ( 4.9) | <0.001 | 1190 ( 5.6) | 1177 ( 5.6) | 0.003 |
| Anticoagulants | 2114 ( 7.4) | 2129 ( 7.4) | 0.001 | 1686 ( 8.0) | 1677 ( 7.9) | 0.002 |
| Nitrates | 841 ( 2.9) | 834 ( 2.9) | 0.002 | 617( 2.9) | 616 ( 2.9) | <0.001 |
| Aspirin | 5641 (19.7) | 5723 (19.9) | 0.005 | 4228 (20.0) | 4296 (20.3) | 0.007 |
| Platelet inhibitors | 949 ( 3.3) | 941 ( 3.3) | 0.002 | 857 ( 4.1) | 848 ( 4.0) | 0.003 |
| Antipsychotics | 1853 ( 6.5) | 1879 ( 6.5) | 0.002 | 1284 ( 6.1) | 1287 ( 6.1) | <0.001 |
| Oral Glucocorticoids | 2749 ( 9.6) | 2734 ( 9.5) | 0.003 | 1970 ( 9.3) | 1921 ( 9.1) | 0.008 |
| Indicators of Utilization (No.,%) | ||||||
| Hospitalization within year (Veterans Health) | 2014 (7.0) | 2030 (7.1) | 0.001 | 1247 ( 5.9) | 1284 ( 6.1) | 0.007 |
| Hospitalizations within 30 days (Veterans Health) | 301 (1.1) | 303 (1.1) | <0.001 | 208 ( 1.0) | 216 ( 1.0) | 0.004 |
| Hospitalizations within year (Medicaid/ Medicare) | 1121 (3.9) | 1113 (3.9) | 0.002 | 649 ( 3.1) | 630 ( 3.0) | 0.006 |
| Hospitalizations within 30 days (Medicaid/ Medicare) | 118 (0.4) | 114 (0.4) | 0.002 | 72 ( 0.3) | 73 ( 0.3) | <0.001 |
| Medicaid insurance use in last year | 638 (2.2) | 615 (2.1) | 0.006 | 262 ( 1.2) | 252 ( 1.2) | 0.004 |
| Medicare insurance use in last year | 11733 (41.0) | 11499 (40.0) | 0.02 | 8286 (39.1) | 7886 (37.2) | 0.04 |
| Nursing home encounters | 78 (0.3) | 80 (0.3) | 0.001 | 45 ( 0.2) | 48 ( 0.2) | 0.002 |
| Outpatient visits in last year * | 6.0 [3.0, 10.0] | 6.0 [3.0, 11.0] | 0.012 | 5 [2, 9] | 5 [2, 10] | 0.016 |
| Outpatient Medications§ | 5.0 [3.0, 6.0] | 5.0 [4.0, 6.0] | 0.004 | 4 [3, 6] | 4 [3, 6] | 0.001 |
| Medicare Advantage Use | ∣ 7336 (25.6) | 7271 (25.3) | 0.008 | 5047 (23.8) | 4880 (23.0) | 0.019 |
Median and IQR = Interquartile range
See Supplemental Figures 4 and 5 for the plot of the mean Standardized differences of the pre- matched and matched cohort.
Definitions of co-morbidities in Supplemental Table 2
xcludes Diabetes medications, topical and ophthalmic medications and medical supplies)
The median observed follow-up per episode (truncated at 3.5 years) was: 0.58 years (Interquartile range [IQR] 0.23, 1.36) for GLP1RA vs 0.58 years (IQR 0.25, 1.36) DPP4i. Censoring occurred for the following reasons among GLP1RA and DPP4i episodes: end of study 31.0% vs 27.8%; non persistence 53.2% vs 49.7% and 7.3% vs 11.9% for crossover to another study diabetes drug. Median follow-up was 0.42 years (IQR 0.18, 0.91) for SGLT2i vs 0.47 years (IQR 0.22, 0.98) DPP4i. Censoring occurred for 44.8% vs 45.4% study end; 38.5% vs 37.8% non-persistence of drug and 12.2% vs 10.9% for crossover to another study diabetes drug.
Outcomes: Major Adverse Cardiovascular Events (MACE) or Heart Failure Hospitalization
Exposure GLP1RA vs DPP4i
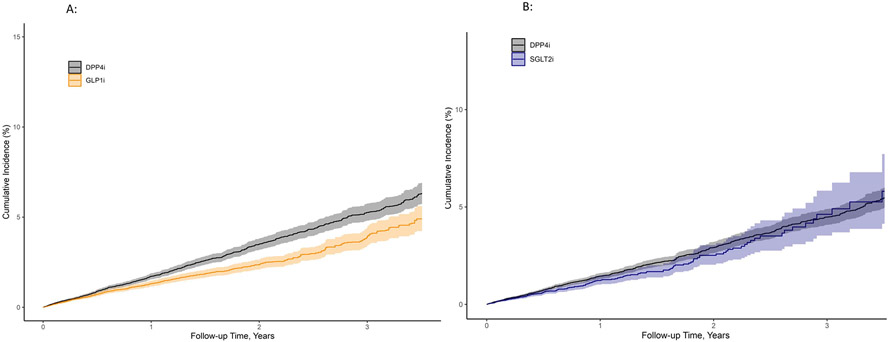
There were 359 composite events among GLP1RA and 482 events in DPP4i users, yielding 13.3 versus 17.8 events per 1,000 person-years of use, respectively; adjusted risk difference (aRD) of 3.2 events (1.1, 5.0) per 1,000 person-years. The matched weighted unadjusted hazard ratio for MACE or HF hospitalization was 0.75 (95% confidence intervals [CI] 0.67, 0.85) and with covariate adjustment the adjusted hazard ratio [aHR] was 0.82 (0.72, 0.94) for use of GLP1RA compared to DPP4i. The cumulative probability of MACE or HF hospitalization at 3.5 years was 1.2% for GLP1RA vs 1.7% for DPP4i (Figure 3A). Results were consistent for each component of the primary outcome but confidence intervals were wide (cardiovascular hospitalizations [aHR of 0.86 (0.70, 1.07)]; cardiovascular deaths [aHR of 0.71 (0.53, 0.94)]; and HF hospitalization [aHR of 0.80 (0.65, 0.99)] (Table 2, Supplemental Figure 6).
Figure 3:
| timePoints | 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 |
| atRiskDPP4 | 28628 | 15476 | 9617 | 6398 | 4227 | 2816 | 1891 | 873 |
| atRiskGLP1 | 28759 | 15534 | 9797 | 6408 | 4156 | 2756 | 1767 | 732 |
| timePoints | 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 |
| atRiskDPP4 | 21170 | 9910 | 5188 | 3019 | 1762 | 1053 | 620 | 257 |
| atRiskSGLT2 | 21200 | 9463 | 4670 | 2680 | 1394 | 748 | 357 | 125 |
Table 2:
MACE and Heart failure event rates and Adjusted Risk difference and Hazard ratios for those without Cardiovascular disease for GLP1RA and SGLT2i vs DPP4i
| DPP4i | GLP1RA | DPP4i | SGLT2i | |
|---|---|---|---|---|
| N at risk in weighted cohort | 28,628 | 28,759 | 21,170 | 21,200 |
| N events primary outcome: | 482 | 359 | 233 | 186 |
| Composite Major Adverse Cardiovascular | ||||
| Events and Heart Failure Hospitalization | ||||
| Person-Years | 27082 | 26921 | 15590 | 14330 |
| Event Rate/1000 person-years (95% CI) | 17.8 (16.3, 19.4) | 13.3 (12.0, 14.8) | 14.9 (13.1, 17.0) | 12.9 (11.2, 14.9) |
| Adjusted Risk Difference (95% CI)† | 3.20 (1.12, 5.01) | 1.28 (−1.12, 3.32) | ||
| Weighted Unadjusted Hazard Ratio (95% CI) | Ref | 0.75 (0.67, 0.85) | Ref | 0.87 (0.74, 1.03) |
| Adjusted Hazard Ratio (95% CI)* | Ref | 0.82 (0.72, 0.94) | Ref | 0.91 (0.78, 1.08) |
| Secondary Outcomes | ||||
| N events cardiovascular hospitalization (Acute Myocardial Infarction, Stroke) | 189 | 140 | 99 | 89 |
| Person-Years | 27217 | 27025 | 15638 | 14358 |
| Event Rate/1000 person-years (95% CI) | 6.9 (6.0, 8.0) | 5.2 (4.4, 6.1) | 6.4 (5.2, 7.7) | 6.2 (5.0, 7.6) |
| Adjusted Risk Difference (95% CI) † | 0.93 (−0.47, 2.06) | .04 (−1.64, 1.36) | ||
| Weighted Unadjusted Hazard Ratio (95% CI) | Ref | 0.75 (0.62, 0.91) | Ref | 0.98 (0.77, 1.25) |
| Adjusted Hazard Ratio (95% CI)* | Ref | 0.87 (0.70, 1.07) | Ref | 0.99 (0.79, 1.26) |
| N events cardiovascular death | 121 | 79 | 61 | 48 |
| Person-Years | 27348 | 27113 | 15700 | 14400 |
| Event Rate/1000 person-years (95% CI) | 4.4 (3.7, 5.3) | 2.9 (2.3, 3.6) | 3.9 (3.0, 4.9) | 3.4 (2.5, 4.5) |
| Adjusted Risk Difference (95% CI) † | 1.29 (0.26, 2.07) | 0.10 (−1.31, 1.12) | ||
| Weighted Unadjusted Hazard Ratio (95% CI) | Ref | 0.66 (0.51, 0.85) | Ref | 0.88 (0.64, 1.22) |
| Adjusted Hazard Ratio (95% CI)* | Ref | 0.71 (0.53, 0.94) | Ref | 0.98 (0.71, 1.34) |
| N events heart failure hospitalization | 204 | 153 | 85 | 56 |
| Person-Years | 27207 | 27007 | 15650 | 14372 |
| Event Rate/1000 person-years (95% CI) | 7.5 (6.5, 8.6) | 5.7 (4.8, 6.6) | 5.4 (4.4, 6.7) | 3.9 (3.0, 5.0) |
| Adjusted Risk Difference (95% CI) † | 1.47 (0.11, 2.59) | 1.27 (−0.13, 2.32) | ||
| Weighted Unadjusted Hazard Ratio (95% CI) | Ref | 0.76 (0.63, 0.91) | Ref | 0.73 (0.54, 0.97) |
| Adjusted Hazard Ratio (95% CI)* | Ref | 0.80 (0.66, 0.99) | Ref | 0.77 (0.57, 1.02) |
| Preserved EF ≥50% | 25 events | 13 events | 10 events | 4 events |
| Event Rate/1000 person-years (95% CI) | 0.9 (0.6, 1.3) | 0.5 (0.3, 0.8) | 0.6 (0.3, 1.2) | 0.3 (0.1, 0.7) |
| Midrange EF 40-50% | 11 events | 7 events | 4 events | 1 event |
| Event Rate/1000 person-years (95% CI) | 0.4 (0.2, 0.7) | 0.3 (0.1, 0.5) | 0.3 (0.1, 0.7) | 0.1 (0.01, 0.4) |
| Reduced EF <40% | 13 events | 11 events | 6 events | 6 events |
| Event Rate/1000 person-years (95% CI) | 0.5 (0.3, 0.8) | 0.4 (0.2, 0.7) | 0.4 (0.2, 0.8) | 0.4 (0.2, 0.9) |
| Unknown EF | 155 events | 122 events | 65 events | 45 events |
| Event Rate/1000 person-years (95% CI) | 5.7 (4.9, 6.7) | 4.5 (3.8, 5.4) | 4.2 (3.3, 5.3) | 3.1 (2.3, 4.2) |
Fully adjusted model uses weighted cohort and adjust for all covariates listed in Table 1 All continuous variables were modeled as restricted cubic splines.
The adjusted rate difference is estimated by multiplying the unadjusted incident rate for DPP4i by the adjusted hazard ratio minus 1. Confidence bounds are calculated using the respective bounds from the hazard ratio
Exposure SGLT2i vs DPP4i
There were 186 composite events among SGLT2i and 233 events in DPP4i users without CVD, yielding 12.9 vs 14.9 events per 1,000 person-years with an aRD of 1.28 events (−1.12, 3.32) per 1,000 person-years. The matched weighted unadjusted HR for MACE or HF hospitalization was 0.87 (0.74, 1.03) and after covariate adjustment 0.91 (0.78, 1.08) for SGLT2i compared to DPP4i. The cumulative probability of MACE or HF hospitalization at 3.5 years was 0.9% for SGLT2i vs 1.1% DPP4i (Figure 3B). Results for each component of the outcome were: cardiovascular hospitalizations aHR of 0.99 (0.79, 1.26); cardiovascular deaths aHR of 0.98 (0.71, 1.34); and HF hospitalization aHR of 0.77 (0.57, 1.02) (Table 2, Supplemental Figure 7).
Additional analyses
In confirmatory analyses for the complete cohort both with and without cardiovascular disease, we demonstrate that results for both GLP1RA and SGLT2i were associated with reduction in both MACE and HF events (Supplemental Table 5 and Supplemental Figure 8). A secondary analysis compared SGLT2i to GLP1RA among the cohort with and without CVD and restricted to the primary prevention cohort demonstrated no statistical differences in the association of MACE or HF hospitalization between groups (Supplemental Table 6A). Sensitivity analysis allowed for reduced medication adherence but no cross over from one class under investigation to another. These results were consistent with main results, but differences were attenuated (Supplemental Table 6B). Subgroup event rates were low but consistent with the main results (Supplemental Table 7 and 8). The E-value for the GLP1RA vs DPP4i analysis was 1.32, meaning a hypothetical confounder would need to have a relative risk of 1.32 between each exposure and the composite MACE and HF outcome to negate findings (See Bias discussion in Supplemental Methods)
DISCUSSION
Among a national cohort of older VHA patients with diabetes but without established CVD, we found that addition of GLP1RA to baseline diabetes therapy was associated with reduced MACE and HF hospitalization events compared with adding DPP4i. These findings were consistent with each outcome component. Adding SGLT2i was not associated with reduced MACE and HF hospitalizations compared with adding DPP4i. However, although not statistically significant, SGLT2i use(over a median follow up 0.42 years, was associated with numerically fewer HF hospitalizations. Among the complete cohort that included patients both with and without CVD, both GLP1RA and SGLT2i were associated with reduced MACE and HF hospitalizations compared with DPP4i users.
These findings have important implications for patient care and advance what we know about use of these medications in primary CVD prevention. Early cardiovascular outcomes trials excluded or had few participants without CVD. Our results expand on the work of a network meta-analysis by Zheng et al. which included over 50 clinical trials. Zheng reported that compared to DPP4i, both SGLT2i and GLP1RA were associated with reductions in mortality and cardiovascular hospitalizations among those with underlying CVD (44). Zelnicker et. al. conducted two meta-analyses of similar clinical trial data and restricted to those without CVD (primary prevention) and both GLP1RA and SGLT2i failed to meet statistical significance vs. placebo for primary CVD prevention (20,45,46).
There are GLP1RA trials that report statistically important reductions in MACE events among primary prevention participants. In both the recent Glycemia Reduction in Type 2 Diabetes trial and the REWIND clinical trial, GLP1RA demonstrated reduced incidence of any CVD (47) and MACE incidence for primary prevention (15). Data from SGLT2i trials have been more heterogeneous. The CREDENCE trial demonstrated reduction in MACE and HF hospitalization (48) while DECLARE TIMI 58 trial found no difference in MACE but a reduction in CV death and HF events among the primary prevention group (14,49).
Our analyses demonstrate consistency with the results of the GLP1RA clinical trials, with larger sample sizes and more real world clinical use among populations who were older, with multiple comorbidities, but without significant CVD. We also report reductions in HF hospitalization outcomes. These findings in aggregate suggest that GLP1RA may have a role in CVD prevention which pertain to all users irrespective of CVD history.
The exact mechanisms of cardio-protection for these two novel medication classes remain relatively unknown. GLP1RAs are postulated to exert their cardioprotective effects via many clinical pathways, including reductions in weight, blood pressure, cholesterol or HBA1c lowering; despite short interval follow up (median 0.58 years; [IQR 0.23, 1.36]) in our cohort, the GLP1RA group still met statistical significance in regards to the primary composite outcome, suggesting there may be alternative explanations for the cardioprotection. Other mechanisms suggest that GLP1RAs may improve endothelial function, vascular responses to ischemia, and platelet function, which could plausibly result in clinically meaningful outcomes in a shorter time course (50-53).
SGLT2i medications are posited to exert cardioprotective effects through indirect systemic effects (diuresis, improved renal function, erythropoiesis), and direct myocardial effects (improved cardiac energy metabolism and inflammation reduction)(54). It is possible our study did not observe statistically significant effects on MACE and HF outcomes, possibly due to the short-observed follow-up (median follow-up was 0.42 years (IQR 0.18, 0.91)). For example, post-hoc analyses of the SGLT2i trials show that sustained and robust efficacy can be observed as early as 28 days after initiation, but primarily among those with pre-existing HF, and it is presumed that these early effects are driven by the diuretic as opposed to myocardial metabolic changes which requires time to manifest (55). In contrast, another cohort comparing SGLT2i to metformin as first line treatment demonstrated SGLT2i use was associated with lower risk of HF but cumulative incidence curves began to separate after about 6 months of use (56).
This study also adds a secondary analysis that compares GLP1RA vs SGLT2i among the complete cohort and the primary prevention population. We are unaware of randomized data directly comparing these two novel classes. In a study by Patorno et. al, no statistical differences in MACE outcomes were found, but results favored SGLT2i when evaluating HF hospitalization (57). Our results are also similar to a Danish study (58) in which cardiovascular outcomes were evaluated after addition of GLP1RA or SGLT2i and found no difference between groups.
There are several study limitations. First, patients were excluded if the initial diabetes therapy did not include metformin, sulfonylurea, or insulin. The American Diabetes Association guidelines continue to recommend that most patients’ treatment includes metformin and lifestyle modifications with risk assessment (CVD, HF, or chronic kidney disease) (59). This differs from the European cardiology and diabetes guidelines which recommend using SGLT2i as first line for those with CVD. This study did not evaluate patients who initiated use of DPP4i, GLP1RA, or SGLT2i, as first line therapy; and it should be noted that most patients added the GLP1RA or SGLT2i onto existing combination regimens (as a third agent). Second, there was a short median follow-up in each weighted cohort; which may have impacted the ability to detect statistical differences between SGLT2i and DPP4i on MACE and HF hospitalization. Third, veterans may not receive all their care at VHA facilities, therefore, misclassification of those without CVD may have occurred and outcomes may have been missed despite the linkage to Medicare and Medicaid data. This also resulted in many patients without echocardiograms. Because of the high proportion of missing echocardiograms, the number and rates for HF type should be interpreted with caution. Fourth, although propensity score weighting and direct covariate adjustment were used to address confounding, there may be residual confounding. An E value of 1.32 indicates that a moderate confounder is needed to negate the study findings. The plausibility of moderate confounders depends on the thoroughness of the covariates. The VHA data merged with Medicare and Medicaid data allowed this study to extensively control for possible confounders. Finally, the study population was mostly white men who based on our data did not have a recorded history of CVD, thus, results may not be generalizable to populations with lower representation in VHA.
In conclusion, this study demonstrates that among a national cohort of VHA patients, adding GLP1RA was associated with primary prevention for MACE and HF vs DPP4i. In contrast, SGLT2i was not associated with primary prevention for MACE and HF although may have been limited by short follow-up time. These findings are hypothesis generating and further evaluation of these medications as part of primary CVD prevention strategy is needed.
Supplementary Material
Acknowledgement:
The authors acknowledge the support for Veterans Affairs/Centers for Medicare & Medicaid Services data provided by the Department of Veterans Affairs, Veterans Affairs Health Services Research and Development Service, , Veterans Affairs Information Resource Center (project numbers SDR 02-237 and 98-004).
Funding
This project was funded by VA Clinical Science Research and Development investigator initiated grant CX000570-10 (Roumie). Drs. Roumie, Hackstadt and Elasy were supported in part by Center for Diabetes Translation Research P30DK092986.
Drs. Roumie, and Hackstadt had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosures None
Statement of Research Reproducibility:
Protocol: Available to interested readers by contacting Dr.Roumie at christianne.roumie@vumc.org
Statistical Code: Available to interested readers by contacting Dr.Roumie at christianne.roumie@vumc.org
Deidentified and Anonymized Data: Available to interested readers by contacting Dr.Roumie at christianne.roumie@vumc.org
REFERENCES
- 1.Xu G, Liu B, Sun Y, Du Y, Snetselaar LG, Hu FB, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018. Sep 4;362:k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, et al. Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation. 2022. Mar;145(9):e722–59. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and Cardiovascular Disease. Circulation. 1999. Sep 7;100(10):1134–46. [DOI] [PubMed] [Google Scholar]
- 4.Matheus AS de M, Tannus LRM, Cobas RA, Palma CCS, Negrato CA, Gomes M de B. Impact of Diabetes on Cardiovascular Disease: An Update. Int J Hypertens. 2013. Mar 4;2013:e653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015. Jul 16;373(3):232–42. [DOI] [PubMed] [Google Scholar]
- 6.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med. 2013. Oct 3;369(14):1317–26. [DOI] [PubMed] [Google Scholar]
- 7.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. N Engl J Med. 2013. Oct 3;369(14):1327–35. [DOI] [PubMed] [Google Scholar]
- 8.A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015. Nov 26;373(22):2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collaboration CTT (CTT). Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. The Lancet. 2010. Nov 13;376(9753):1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA. 2019. Jan 1;321(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidance for Industry. Diabetes Mellit. 2008;8. [Google Scholar]
- 12.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015. Nov 26;373(22):2117–28. [DOI] [PubMed] [Google Scholar]
- 13.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017. Aug 17;377(7):644–57. [DOI] [PubMed] [Google Scholar]
- 14.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019. Jan 24;380(4):347–57. [DOI] [PubMed] [Google Scholar]
- 15.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet. 2019. Jul 13;394(10193):121–30. [DOI] [PubMed] [Google Scholar]
- 16.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016. Jul 28;375(4):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016. Nov 10;375 (19):1834–44. [DOI] [PubMed] [Google Scholar]
- 18.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med. 2020. Oct 8;383(15):1425–35. [DOI] [PubMed] [Google Scholar]
- 19.McGuire DK, Alexander JH, Johansen OE, Perkovic V, Rosenstock J, Cooper ME, et al. Linagliptin Effects on Heart Failure and Related Outcomes in Individuals With Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA. Circulation. 2019. Jan 15;139(3):351–61. [DOI] [PubMed] [Google Scholar]
- 20.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation. 2019. Apr 23;139(17):2022–31. [DOI] [PubMed] [Google Scholar]
- 21.Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021. Oct l;9(10):653–62. [DOI] [PubMed] [Google Scholar]
- 22.Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA. 2019. Sep 24;322(12):1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filion KB, Azoulay L, Platt RW, Dahl M, Dormuth CR, Clemens KK, et al. A Multicenter Observational Study of Incretin-based Drugs and Heart Failure. N Engl J Med. 2016. Mar 24;374(12):1145–54. [DOI] [PubMed] [Google Scholar]
- 24.Toh S, Hampp C, Reichman ME, Graham DJ, Balakrishnan S, Pucino F, et al. Risk for Hospitalized Heart Failure Among New Users of Saxagliptin, Sitagliptin, and Other Antihyperglycemic Drugs. Ann Intern Med. 2016. Jun 7;164(11):705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudsen JS, Baggesen LM, Lajer M, Nurkanovic L, Ustyugova A, Sørensen HT, et al. Changes in SGLT2i and GLP-1RA real-world initiator profiles following cardiovascular outcome trials: A Danish nationwide population-based study. PLOS ONE. 2020. Mar 4;15(3):e0229621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bang C, Mortensen MB, Lauridsen KG, Bruun JM. Trends in antidiabetic drug utilization and expenditure in Denmark: A 22-year nationwide study. Diabetes Obes Metab. 2020. Feb 1;22(2):167–72. [DOI] [PubMed] [Google Scholar]
- 27.Ganz M, Ustyugova A, Nicola Sawalhi -Leckenby, de SS, Zhang L, Gunnarsson E, et al. Utilization of glucose-lowering drugs in patients with t2dm and established cvd in us: a descriptive study using optum clinformatics data. J Am Coll Cardiol. 2020. Mar 24;75(11_Supplement_1):2017–2017. [Google Scholar]
- 28.Niesner K, Murff HJ, Griffin MR, Wasserman B, Greevy R, Grijalva CG, et al. Validation of VA Administrative Data Algorithms for Identifying Cardiovascular Disease Hospitalization. Epidemiology. 2013. Mar;24(2):334–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Presley CA, Min JY, Chipman J, Greevy RA, Grijalva CG, Griffin MR, et al. Validation of an algorithm to identify heart failure hospitalisations in patients with diabetes within the veterans health administration. BMJ Open. 2018. Mar 1;8(3):e020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(S1):129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson OV, Freiberg MS, Skanderson M, Fodeh SJ, Brandt CA, DuVall SL. Unlocking echocardiogram measurements for heart disease research through natural language processing. BMC Cardiovasc Disord. 2017. Jun 12;17(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the Risk of Sudden Cardiac Death. Arch Gen Psychiatry. 2001. Dec 1;58(12):1161–7. [DOI] [PubMed] [Google Scholar]
- 33.Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD. Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J. 2011. Sep l;162(3):548–54. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, Zhang Y (Lucy), Castro AF, Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009. May 5;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis EF, Claggett B, Shah AM, Liu J, Shah SJ, Anand I, et al. Racial Differences in Characteristics and Outcomes of Patients With Heart Failure and Preserved Ejection Fraction in the Treatment of Preserved Cardiac Function Heart Failure Trial. Circ Heart Fail. 2018. Mar;11(3):e004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Buuren S. Flexible Imputation of Missing Data. New York: Chapman and Hall/CRC; 2012. 342 p. [Google Scholar]
- 37.Franklin JM, Eddings W, Austin PC, Stuart EA, Schneeweiss S. Comparing the performance of propensity score methods in healthcare database studies with rare outcomes. Stat Med. 2017. May 30;36(12):1946–63. [DOI] [PubMed] [Google Scholar]
- 38.D’Agostino RB, Rubin DB. Estimating and Using Propensity Scores with Partially Missing Data. J Am Stat Assoc. 2000. Sep 1;95(451):749–59. [Google Scholar]
- 39.Li L, Greene T. A Weighting Analogue to Pair Matching in Propensity Score Analysis. Int J Biostat. 2013. Jul 31;9(2):215–34. [DOI] [PubMed] [Google Scholar]
- 40.GRAMBSCH PM, THERNEAU TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994. Sep 1;81(3):515–26. [Google Scholar]
- 41.Ho DE, Imai K, King G, Stuart EA. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Polit Anal. 2007;15(3):199–236. [Google Scholar]
- 42.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017. Aug 15;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 43.R: The R Project for Statistical Computing [Internet], [cited 2022 Apr 26], Available from: https://www.r-project.org/
- 44.Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, et al. Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA. 2018. Apr 17;319(15):1580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2021. Feb 1;6(2):148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. The Lancet. 2019. Jan 5;393(10166):31–9. [DOI] [PubMed] [Google Scholar]
- 47.Glycemia Reduction in Type 2 Diabetes — Microvascular and Cardiovascular Outcomes. N Engl J Med. 2022. Sep 22;387(12):1075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019. Jun 13;380(24):2295–306. [DOI] [PubMed] [Google Scholar]
- 49.Cahn A, Raz I, Leiter LA, Mosenzon O, Murphy SA, Goodrich EL, et al. Cardiovascular, Renal, and Metabolic Outcomes of Dapagliflozin Versus Placebo in a Primary Cardiovascular Prevention Cohort: Analyses From DECLARE-TIMI 58. Diabetes Care. 2021. May l;44(5):1159–67. [DOI] [PubMed] [Google Scholar]
- 50.Sposito AC, Berwanger O, de Carvalho LSF, Saraiva JFK. GLP-lRAs in type 2 diabetes: mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc Diabetol. 2018. Dec 13;17(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim S, Kim KM, Nauck MA. Glucagon-like Peptide-1 Receptor Agonists and Cardiovascular Events: Class Effects versus Individual Patterns. Trends Endocrinol Metab. 2018. Apr 1;29(4):238–48. [DOI] [PubMed] [Google Scholar]
- 52.Bakbak E, Terenzi DC, Trac JZ, Teoh H, Quan A, Glazer SA, et al. Lessons from bariatric surgery: Can increased GLP-1 enhance vascular repair during cardiometabolic-based chronic disease? Rev Endocr Metab Disord. 2021. Dec l;22(4):1171–88. [DOI] [PubMed] [Google Scholar]
- 53.Zhao X, Wang M, Wen Z, Lu Z, Cui L, Fu C, et al. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front Endocrinol [Internet], 2021. [cited 2022 Nov 16];12. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2021.721135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors. JACC Basic Transl Sci. 2020. Jun;5(6):632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berg DD, Jhund PS, Docherty KF, Murphy SA, Verma S, Inzucchi SE, et al. Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2021. May;6(5):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin H, Schneeweiss S, Glynn RJ, Patorno E. Cardiovascular Outcomes in Patients Initiating First-Line Treatment of Type 2 Diabetes With Sodium–Glucose Cotransporter-2 Inhibitors Versus Metformin. Ann Intern Med. 2022. Jul 19;175(7):927–37. [DOI] [PubMed] [Google Scholar]
- 57.Patorno E, Htoo PT, Glynn RJ, Schneeweiss S, Wexler DJ, Pawar A, et al. Sodium–Glucose Cotransporter-2 Inhibitors Versus Glucagon-like Peptide-1 Receptor Agonists and the Risk for Cardiovascular Outcomes in Routine Care Patients With Diabetes Across Categories of Cardiovascular Disease. Ann Intern Med. 2021. Nov 16;174(11):1528–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thein D, Christiansen MN, Mogensen UM, Bundgaard JS, Rørth R, Madelaire C, et al. Add-on therapy in metformin-treated patients with type 2 diabetes at moderate cardiovascular risk: a nationwide study. Cardiovasc Diabetol. 2020. Jul 6;19(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2021. Dec 16;45(Supplement_1):S125–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.