ABSTRACT
There is a consensus that the antifungal repertoire for the treatment of cryptococcal infections is limited. Standard treatment involves the administration of an antifungal drug derived from natural sources (i.e., amphotericin B) and two other drugs developed synthetically (i.e., flucytosine and fluconazole). Despite treatment, the mortality rates associated with fungal cryptococcosis are high. Amphotericin B and flucytosine are toxic, require intravenous administration, and are usually unavailable in low-income countries because of their high cost. However, fluconazole is cost-effective, widely available, and harmless with regard to its side effects. However, fluconazole is a fungistatic agent that has contributed considerably to the increase in fungal resistance and frequent relapses in patients with cryptococcal meningitis. Therefore, there is an unquestionable need to identify new alternatives or adjuvants to conventional drugs for the treatment of cryptococcosis. A potential antifungal agent should be able to kill cryptococci and “bypass” the virulence mechanism of the yeast. Furthermore, it should have fungicidal action, low toxicity, high selectivity, easily penetrate the central nervous system, and widely available. In this review, we describe cryptococcosis, its conventional therapy, and failures arising from the use of drugs traditionally considered to be the reference standard. Additionally, we present the approaches used for the discovery of new drugs to counteract cryptococcosis, ranging from the conventional screening of natural products to the inclusion of structural modifications to optimize anticryptococcal activity, as well as drug repositioning and combined therapies.
Keywords: Cryptococcosis, Therapeutic failures, Anticryptococcal drug development
INTRODUCTION
Cryptococcosis, a potentially fatal fungal infection in immunosuppressed patients, especially in those infected with human immunodeficiency virus (HIV), is caused by the inhalation of encapsulated yeasts belonging to the Cryptococcus neoformans and Cryptococcus gattii species complex1. It is associated with high mortality in low- and middle-income countries, and causes approximately 181,000 deaths annually2-3. Sub-Saharan Africa reports the highest number of cases, with approximately 720,000 cases per year, followed by Southeast Asia and Latin America, which are the second and third regions most affected by cryptococcal meningitis3-4.
Results of antifungal therapies for cryptococcosis are limited. Depending on an individual’s immune status, disease severity, and availability of antifungals, the standard treatment is based only on amphotericin B, fluconazole, and flucytosine5-6. Owing to its relatively low cost, high oral bioavailability, and low toxicity profile, fluconazole is often used to replace amphotericin B and flucytosine in resource-limited settings. However, resistant fungi and persistent therapeutic failure have been observed in patients with cryptococcosis undergoing prolonged therapy with fluconazole7. In addition, the limited antifungal arsenal, serious adverse effects of amphotericin B and flucytosine, and intrinsic resistance of C. neoformans to echinocandins, the only new broadly available class of tantifungal drugs developed in decades, have stimulated new studies in search of better antifungal agents to treat cryptococcosis8-10.
Drugs can be discovered in natural products that, since antiquity, have been an important source of attractive bioactive compounds for drug development or can be produced through full or partial synthesis11. However, despite advances in molecular techniques and medicinal chemistry, the development of new drugs remains slow and expensive. In addition, several drug candidates are barred during the transition from the preclinical to the clinical stage, with 89% failing due to toxicity12. Thus, the reuse of drugs, that is, the definition of new therapeutic indications for substances already approved by the Food and Drug Administration, has attracted considerable attention. Another used approach is combining antifungal agents with other drugs, thus improving the activity of traditional antifungals due to their associated action on more than one target10.
This review aims to provide an overview of the scientific evidence available for cryptococcosis in general, current treatment options, therapeutic failures, and methodologies for obtaining new anticryptococcal drugs, for example, by bioprospecting natural products and structural modifications. In addition, it aims to address potential drugs, or drug combinations, which are undergoing preclinical and clinical investigations for drug repurposing and combined therapy.
CRYPTOCOCCOSIS
Cryptococcosis or cryptococcal infection is a life-threatening fungal disease caused by the inhalation of encapsulated yeasts (Figure 1) belonging to the C. neoformans and C. gattii species complex1-13. With the evolution of molecular biology techniques and the use of different genotyping methods, it has become possible to assign these species to eight main genotypes: VNI, VNII, VNIII, and VNIV for C. neoformans and VGI, VGII, VGIII, and VGIV for C. gatti14-17. Recently, a fifth genotype (VGV) has been described in the C. gattii species complex18.
FIGURE 1: Micromorphological characteristic of Cryptococcus spp. Direct exam, prepared with Indian ink (400×).
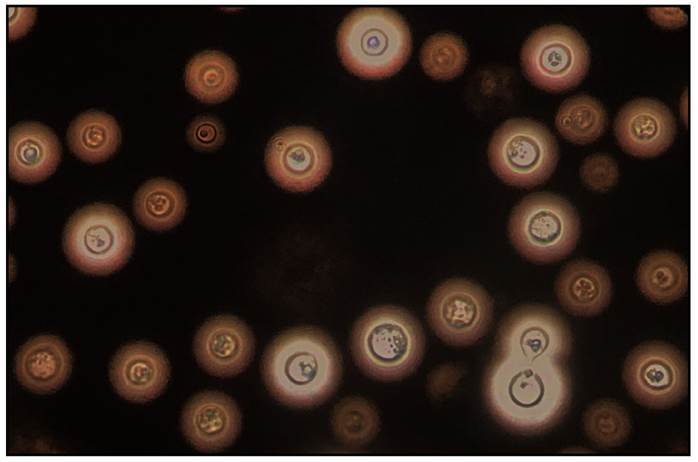
The causative agent is widely distributed in the natural environment, commonly in feces and birds nest, but mainly in pigeons, dead organic matter, bark, leaves, and fruit trees17. Cryptococcus spp. are globally distributed, and until 1955, prior to the availability of antifungals especially amphotericin, cryptococcosis was inevitably fatal19. Today, mortality remains high, particularly in the endemic regions of sub-Saharan Africa, a setting where access to healthcare is limited and the number of HIV infected individuals is high20-21. In developed countries, the observed drop in mortality rate can be explained by early diagnosis and wide availability of antiretroviral therapy22.
Cryptococcosis occurs predominantly in immunocompromised patients and is a major cause of morbidity and mortality in these individuals, especially in those infected with HIV21-23. Individuals with diabetes and lupus erythematosus, transplant recipients, patients using immunosuppressive therapies, and patients with malignant neoplasms are also frequently affected with cryptococcosis, thus becoming a worldwide concern5-7. Cryptococcal infection also manifests in immunocompetent patients, and the signs and symptoms of infection are often nonspecific. This lack of specificity often leads to a delay in diagnosis and initiation of appropriate treatment, which in turn may lead to a severe clinical course and rapid death, even in patients without HIV24. In addition, delayed diagnosis can lead to additional morbidities such as stroke, blindness, deafness, neurological impairment, and cognitive dysfunction25.
The primary manifestation, pulmonary cryptococcosis, can range from mild colonization of the lungs to severe lung infection5-6. At this stage, yeast can be spontaneously eliminated or remain in a non-replicative state for months or even years in immunocompetent hosts26-27. However, in cases of impaired immunity, yeasts are reactivated and disseminated via the blood to various organs, especially the brain and meninges, leading to cryptococcal meningitis. The latter is the most common and severe clinical manifestation of cryptococcosis, primarily affecting immunosuppressed patients, particularly those with depleted or defective CD4+ T cells5-25-28. The infection also involves other sites such as the skin, skeletal system, digestive tract, and prostate; though uncommon this is well-documented in the literature 18-29-30.
CONVENTIONAL THERAPY
Depending on the individual’s immune status, site of infection, disease severity and drug availability, several therapeutic regimens can be considered for the treatment of cryptococcosis5-28-31. Although adapted to the infection severity and state of the host’s immunity, the World Health Organization (WHO) recommends the treatment of cryptococcal infections using a three-stage therapeutic strategy: induction, consolidation, and maintenance. The standard therapy is limited to the use of the following drugs: amphotericin B, flucytosine, and fluconazole28. In summary, amphotericin B, alone or in combination with flucytosine, is employed as an initial induction therapy, and fluconazole is suggested for the consolidation and maintenance therapy28-32-33.
Among the three drugs available, amphotericin B is the oldest antifungal drug for systemic use. It acts by binding to ergosterol in fungal cell membranes, forming pores that allow the leakage of cell contents, such as K+, Na+, H+, and Cl− ions, which consecutively leads to apoptosis34-35. Despite being considered as one of the systemic antifungals with the broadest fungicidal activity, the use of amphotericin B has some limitations that are mainly associated with its nephrotoxicity36. Lipid formulations of amphotericin B with reduced toxicity have been developed; however, although liposomal amphotericin B has an improved safety profile and greater efficacy than conventional amphotericin B7, the cost of these lipid formulations continues to be a barrier for the treatment of cryptococcosis in resource-limited countries37.
The synthetic drug flucytosine, which was first evaluated as an antitumor agent38, is recommended by WHO; however, it is mainly available in resource-rich countries. The drug is efficient for the treatment of cryptococcosis when combined with amphotericin B 39-40. However, its use as a single antifungal agent is discouraged owing to its significant adverse effects, in particular, hepatotoxicity, myelotoxicity, and resistance when used in monotherapy, thereby compromising therapeutic success8-41-43.
Fluconazole is one of the best-known antifungal drugs for the systemic treatment of a broad spectrum of fungal infections. Azoles constitute a class of synthetic antifungals with fungistatic activity, and fluconazole, in particular, has been in clinical use since the 1980s44. In cryptococcosis therapy, the main advantage of fluconazole is its lack of severe nephrotoxic effects. Furthermore, they are frequently used to replace amphotericin B or flucytosine when their availability is limited33. However, because the duration of therapy is long, significant resistance is often reported in this antifungal class7.
WHO has recently published new strategies and guidelines for the management of patients with cryptococcosis28. These protocols were established in association with a clinical trial carried out by Jarvis and colleagues31 that recommend the use of liposomal amphotericin B as a first-line treatment for cryptococcal meningitis. It was administered as a single dose on day one, followed by 14 days of flucytosine and fluconazole administration. The study revealed that this treatment scheme considerably improved survival rates, reduced neurological impairment, and decreased adverse events in patients with infection. The WHO stresses the importance of early diagnosis and treatment of cryptococcosis, together with recommendations of closely monitoring patients during and after treatment to avoid relapses.
In summary, access to only the antifungal drugs available for the standard treatment of cryptococcosis remains insufficient, especially in resource-poor countries, where a high incidence of cryptococcal meningitis is observed7-23. In addition, increased fungal resistance to azoles, difficulty in administering and monitoring the adverse effects of amphotericin B and flucytosine, and their high costs remain important challenges in medical practice, even in resource-rich countries.
THERAPEUTIC FAILURES
This phenomenon of antimicrobial resistance results in serious restrictions on the available options for cryptococcosis clinical treatment. Common antifungal resistance mechanisms include a decrease in the effective drug concentration, alterations or overexpression of drug targets, and metabolic deviations45. Thus, therapeutic failure in cryptococcosis may be related to both host factors and the existence of strains of Cryptococcus spp. that develop resistance to antifungal drugs46.
Extrapolations from previous studies on other fungal species may improve our understanding of the resistance mechanisms employed by C. neoformans7 for which research is scarce. Reports of Cryptococcus spp. being resistant to amphotericin B are relatively rare; however, this phenomenon is already a concern47. The mechanisms that confer resistance to polyenes are related to mutations in ergosterol biosynthesis pathway genes, resulting in reduced binding of amphotericin B and/or inactivation of the drug, leading to fungal resistance48-49. The mechanisms of flucytosine resistance in Cryptococcus spp. remain unresolved and further investigation is needed to define them7. Approximately 10% of fungal isolates, even in the absence of previous drug exposure, show primary resistance to flucytosine50. In the case of infections with C. neoformans in particular, monotherapy with flucytosine is discouraged because of the rapid and frequent appearance of resistant isolates51.
In the 1990s, especially in patients with HIV, the indiscriminate use of fluconazole resulted in the emergence of drug-resistant Cryptococcus spp. strains among susceptible populations52-54. Azole resistance is a relatively common event in recurrent episodes of cryptococcal meningitis33-55. The molecular basis of this resistance in Cryptococcus spp. is poorly resolved; however, overexpression of the AFR1 gene that codes for the azole efflux pump and point mutations in the ERG11 gene, that is, the gene encoding lanosterol 14α-demethylase as the target enzyme of azoles, have been associated with alterations in susceptibility to fluconazole in C. neoformans7-56-59.
Resistance to fluconazole in Cryptococcus spp. may also be associated with heteroresistance, an adaptive mode of resistance against azoles60. This phenomenon refers to the heterogeneous susceptibility of a microorganism population to fluconazole, meaning that some clones are resistant whereas others are susceptible61. Resistant subpopulations gradually adapt to increasing drug concentrations. However, this acquired resistance to high concentrations of fluconazole can be lost during repeated passages in drug-free media and the clones return to their original level of heteroresistance60-62.
The rise of heteroresistance in isolates of the C. neoformans species complex against fluconazole has been identified as one of the causes of cryptococcosis63. Heteroresistance may explain treatment failure in some patients, even when they are treated with the appropriate choices and concentrations of antifungal drugs61. Furthermore, current antifungal susceptibility testing algorithms have not been designed to detect heteroresistance; accordingly, unreliable susceptibility testing results are expected in the case of infections with heteroresistant Cryptococcus spp. strains62-64-66.
BIOPROSPECTING OF NATURAL PRODUCTS WITH ANTIFUNGAL ACTIVITY
Historically, nature has been an important source of therapeutic molecules. Currently, secondary metabolites of natural products produced by plants, microorganisms, marine animals, and other aquatic systems comprise approximately half of all pharmaceutical products on the market67-68. This reveals an immeasurable source of opportunities in the area of scientific and technological research on natural products, and prospecting new drugs from biodiversity remains one of the main choices for the identification of new drugs69-70.
Bioprospecting of anticryptococcal drugs is commonly performed using classic or virtual (computational) cell screening. In the course of these screening approaches, bioproducts obtained from natural sources, such as plants, fungi, bacteria, insects, animals, and marine organisms71-72, were initially tested using bioassays that assess antifungal activity10. The disk diffusion assay is the most commonly used qualitative method for initial screening of antifungal activity73. The second most common method is the broth microdilution method, which is described by the Clinical and Laboratory Standards Institute (CLSI; document M-27 A4) or the European Committee on Antimicrobial Susceptibility Testing (document EDef 7.3.1), and is used to quantitatively determine the minimum inhibitory concentration (MIC) of substances with antimicrobial effects against pathogenic yeasts74-75.
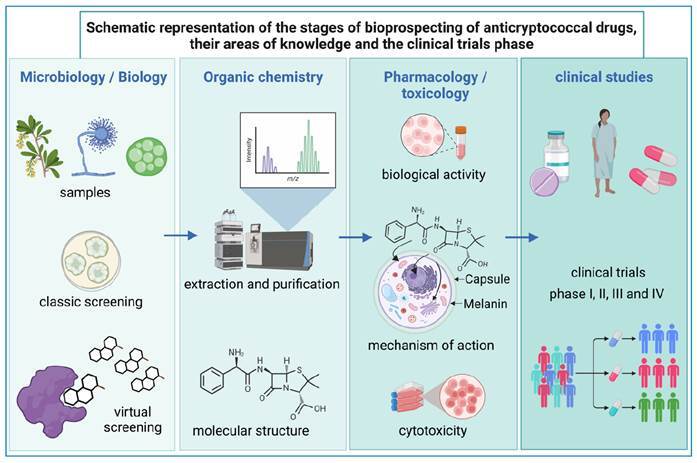
Once the antifungal potential is identified, the bioproducts are subjected to extraction, isolation, and identification steps, which include different techniques capable of detecting the presence of compounds and then characterizing them76. In summary, the discovery of natural products with antifungal activity generally comprises: 1) classic or virtual cell screening; 2) extraction, isolation of compounds and structural characterization by thin layer chromatography, variations of chromatography associated with mass spectrometry, analysis of carbon 13 nuclear magnetic resonance, and hydrogen nuclear magnetic resonance analysis; 3) pharmacological studies to determine the mode of action; 4) toxicological studies to delineate the substance’s safety; 5) preclinical trials and, if successful; 6) clinical and marketing studies (Figure 2).
FIGURE 2: Bioprospecting steps for anticryptococcal drugs, their areas of knowledge, and the clinical trials phase. Created with BioRender.com.
Several new natural products from fungi, bacteria, insects, sponges, algae, and plants have proven to be effective alternatives with the potential to form new drugs that can be effectively used against strains of C. neoformans and gattii76-77. In recent years, marine sponges and algae have emerged as important sources of new natural products with antifungal activity78; however, plants and fungi are still the most productive sources of antifungal compounds with anticryptococcal activity, including phenols, flavonoids, terpenoids, alkaloids, and peptides, as the main chemical classes represented in these plants77.
Natural products are important sources of therapeutic drugs. However, it is generally accepted that the drug discovery and development processes are time- and resource-intensive. Thus, in recent years, both computational and experimental techniques have played important roles and represent complementary approaches76. For a complete review of computer-aided drug design and virtual screening for lead molecules in the discovery of new drugs against Cryptococcus spp., the comprehensive work by Manjunath and Skariyachan (2018) should be consulted79. Table 1 summarizes the lead molecules selected from natural sources with antifungal activity against Cryptococcus spp. that have been identified in recent years.
TABLE 1: Lead molecules selected from natural sources with antifungal activity against Cryptococcus spp. that have been identified in recent years.
| Source | Natural source | Compound/ chemical class | Reference |
|---|---|---|---|
| Plant | Ocimum basilicum (Linnaeus) | Sesquiterpenes | (80) |
| Lafoensia pacari (St-Hilaire) | Punicalagin (tannins) | (81) | |
| Thymus vulgaris (Linnaeus) | Terpenoids | (82) | |
| Xylosma prockia (Turcz) | Phenolic metabolites | (83) | |
| Uvaria comperei (Le Thomas) | Alkaloid and flavonoids | (84) | |
| Gentiana crassicaulis (Duthie ex Burkill) | Bisphosphocholines | (85) | |
| Chromolaena odorata (Linnaeus) | Flavonoids | (86) | |
| Cistus ladanifer (Linnaeus) | Terpenoids | (87) | |
| Hypoxis daylily (Linnaeus) | Benzoylcyclopropane derivatives | (88) | |
| Annona mucosa (Jacquin) | Liriodenine | (89) | |
| Verbesina turbacensis (Kunth) | Hydroxycinnamic esters | (90) | |
| Fungus | Pestalotiopsis sp. | Pestalactams | (91) |
| Auxarthron / Pseudauxarthron | Phenalenones and cyclic tetrapeptides | (92) | |
| Ruby discosia | Chaetoglobosins | (93) | |
| Preussia typharum | Macrolides | (94) | |
| Aspergillus terreus | Terrestrial | (95) | |
| Sodiomyces alkalinus | Hydrophobins | (96) | |
| Animal | Hippospongia sp. | Sesquiterpene quinones | (97) |
| Plakortis zyggompha and Plakortis halichondrioides | Plakinic acid and plakortides | (98) | |
| Tetrigone melanoleuca and Tetragonula laeviceps | Propolis | (99) | |
| Meccus pallidipennis and Rhodnius prolixus | Peptides | (100) | |
| Bacterium | Streptomyces clavuligerus | Ibomycin | (101) |
STRUCTURAL MODIFICATION
The first step in the design of new anticryptococcal drugs using structural modification is the use of a well-defined chemical substance with previously characterized biological activity102. The next step involves the techniques required to derive new analogs, homologues, or structural congeners with improved pharmacological properties. For this purpose, general processes of simplification and molecular association have been applied102-104. In summary, the final product was designed by the partial molecular modification of the prototype compound with the inclusion or exclusion of chemical structures that favor greater potency, stability, and safety characteristics than the original compound68.
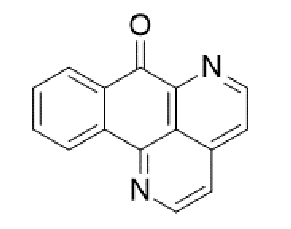
Substituted derivatives of terpenoids, quinones, naphthoquinones and coumaric acid are among the compounds with antifungal properties whose derivatives have been extensively studied in recent years for their anticryptococcal activity105-110. Recently, derivatives of sampagin, an alkaloid extracted from the stem bark of Cananga odorata Lamarck, have been shown to mediate potent antifungal activity against C. neoformans and gattii species110. In this study, a series of tricyclic isoxazole derivatives with excellent anticryptococcal activities were identified by structural simplification and alteration of the sample skeleton. The derived compound (Table 2) showed a high degree of inhibitory activity against C. neoformans, with an MIC80 value of 0.031 μg/mL. This activity was more potent than that of substances such as fluconazole and voriconazole. Furthermore, the substance showed potent inhibitory effects against important virulence factors, such as biofilm activity, melanin production, and urease activity of yeasts110.
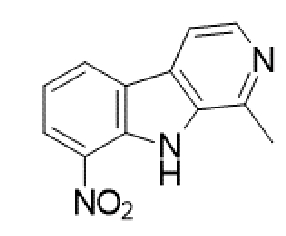
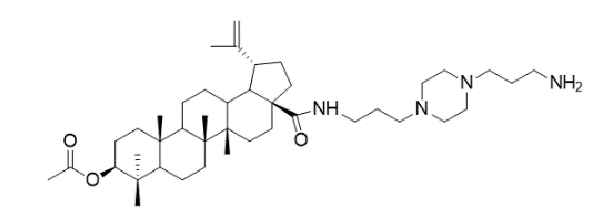
TABLE 2: Chemical structure of substituted derivatives with noteworthy activity against Cryptococcus neoformans and Cryptococcus gattii strains obtained by applying molecular modification.
| Starting material (prototype) | Derivative with increased activity | Reference |
|---|---|---|
 |
 |
|
| 109 | ||
| 2-hydroxynaphthalene-1,4-dione | 1 H -cyclopenta[ b ]naphtho[2,3- d ]furan-5,10(3a H,10b H )-dione | |
 |
 |
108 |
| 9 H -pyrido[3,4- b ]indole | 1-methyl-8-nitro-9 H -pyrido[3,4- b ]indole | |
 |
 |
111 |
| Betulinic acid | ( 1R,3a S,5a S,5b R,9 S,1(1a R )-3a-((3-(4-(3aminopropyl)piperazin-1-yl)propryl)carbamoyl)5a,5b,8,8, 11a-pentamethyl-1-(prop-1-em-2-yl)-icosahydro-1 H -cyclopenta[ a ]chrysen-9-yl acetate | |
 |
 |
110 |
| 7 H -naphtho[1,2,3- ij ][2,7]naphthyridin-7-one | 3-ethylthieno[3',2':4,5]benzo[1,2 - d ]isoxazole-4,8-dione | |
 |
 |
112 |
| 5,7-dihydroxy-2-methyl-4 H -chromen-4-one | ( E )-2-(5-hydroxy-2-methyl-4-methylene-4,6,9,10-tetrahydrooxocino[3,2- g ]chromen-8-yl)ethyl acetate | |
 |
 |
113 |
| ( S )-6-isopeopyl-3-methylcyclohex-2-enone | 3-hydroxy-2-isopropyl-5-methylcyclohexa-2,5,-diene-1,4-dione | |
 |
 |
114 |
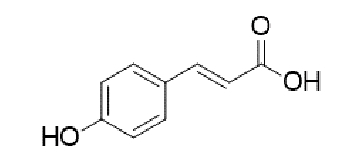
| ( E )-3-(4-hydroxyphenyl)acrylic acid | ( E )-ethyl 3-(4-hydroxyphenyl)acrylate | |
 |
 |
105 |
| 5-methyl-5 H -indolo[3,2- b ]quinolin-11(10 H )-one | 5,10-dimethyl-5 H -indolo[3,2- b ]quinolin-11(10 H )-one |
Structures were designed using Chemdraw 19.0
Despite the considerable efforts invested in the search for antifungals, several new compounds that were screened or obtained by structural modification and demonstrated antifungal activity against Cryptococcus spp. remain poorly investigated77. However, there is hope that some will progress into useful antifungal agents owing to molecular modifications. Moreover, in the next step, such new drugs with anticryptococcal activity will hopefully advance to clinical trials.
DRUG REPURPOSING
To accelerate the development of new antifungal agents, drugs developed for other therapeutic purposes can be repurposed if they also show antifungal activity2. Wemuth was an early advocate of screening approved drugs for new therapeutic indications and coined the term systematic optimization of side-activities (SOSA), which has become well known as a drug repositioning strategy115.
The repositioning of drugs has few advantages, namely: 1) pharmacological, pharmacokinetic and safety data in humans have already been previously established in preclinical and human trials, 2) the clinical use of a drug already available on the market is immediate, and 3) reduction in research costs associated with the expansion of the therapeutic indication8-115-116. Therefore, expanding the applicability of a drug to other diseases is a promising approach that has been successfully used in recent years to identify drugs with antifungal activity37.
In recent years, a series of drugs developed for other therapeutic purposes have demonstrated antifungal activity against Cryptococcus spp.117-130. The most notable examples of repurposed pharmaceutical compounds for cryptococcal meningitis that have reached the clinical trial stage involve the drugs sertraline and tamoxifen2-117. Tamoxifen has not shown any benefit in eliminating Cryptococcus spp. from the cerebrospinal fluid, and the sertraline study had to be terminated early due to serious adverse effects116-117. It is important to note that repurposed drugs are not optimized for indications other than those on the leaflet. Therefore, their pharmacokinetic properties and efficacy often need to be improved if off-label applications are desired. Considering this observation, an alternative approach to repurposing is the optimization of a compound or drug for its secondary effect, also known as SOSA115. For a comprehensive review of this approach, please refer to the recent work of Donlin and Meyers (2022)118.
COMBINATION THERAPY
Compared with the discovery of antibiotics, the discovery of antifungal agents is much more difficult. A common explanation for this finding is that fungus, similar to its human host, is a eukaryotic organism. This phylogenetic relatedness hinders the development of effective antifungal agents that are nontoxic to human cells130. This problem is evident within the Cryptococcus genus because of the pathogenicity, virulence, and resistance mechanisms that these fungi have developed6. In this context, combining different drugs for antifungal therapy is a feasible strategy to increase the efficacy of antifungals, decrease and/or avoid toxicity, and prevent fungal resistance.
The commonly used mode of assessing the combined effects of the two substances is the checkerboard test131-133. This method is based on the broth microdilution technique, in line with document M7-A4 of the CLSI74. Table 3 summarizes published drug combination studies of amphotericin B and fluconazole against Cryptococcus spp. In summary, the presented combinations are associated with improved activity of conventional antifungal agents owing to the combined action of more than one target, as well as reduced toxicity, because small amounts of one or both drugs can be used in combination12. An example of this is flucytosine, which seems to be of little use when used on its own for cryptococcosis therapy but has been reported to act synergistically in combination with amphotericin B. Therefore, additional benefits for the treatment of cryptococcal meningitis are observed when this drug is used in combination8. Consequently, combined antifungal therapy using flucytosine and amphotericin B has been used for at least four decades. However, as mentioned previously, the adverse effects, high cost, and unavailability of flucytosine in resource-poor countries still negatively interfere with the treatment of cryptococcal meningitis25-39.
TABLE 3: Studies assessing combinations of drugs or bioactive compounds with promising antifungal activity against Cryptococcus spp.
| Combination | Screening | Reference |
|---|---|---|
| Coumaric acid analogues + amphotericin B | Checkerboard assays | 114 |
| Artovastatin + fluconazole | Checkerboard assays | 120 |
| Curcumin + amphotericin B | Checkerboard assays | 134 |
| Dicyclomine + fluconazole | Virtual library | 135 |
| Duloxetine + fluconazole | Checkerboard assays | 136 |
| Erythromycin + amphotericin B | Virtual library | 37 |
| Fluoxetine + amphotericin B | Checkerboard assays | 137 |
| Glimepiride+ amphotericin B | Virtual library | 37 |
| Lactoferrin + amphotericin B | Checkerboard assays | 138 |
| Minocycline + fluconazole | Checkerboard assays | 10 |
| N-acetylcysteine + amphotericin B | Checkerboard assays | 139 |
| Simvastatin + amphotericin B | Checkerboard assays | 140 |
| Tamoxifen + amphotericin B | Checkerboard assays | 117 |
| Triclosan + fluconazole | Checkerboard assays | 141 |
| Pedalitin + amphotericin B | Checkerboard assays | 142 |
| α -Cyperone + fluconazole | Checkerboard assays | 143 |
There is some hope on the horizon, with the new antifungals fosmanogepix and opelconazole, which are in the advanced stages of clinical development and exhibit antifungal activity against Cryptococcus spp. However, the available antifungal therapies for this infection remain limited. The adverse effects and high costs of the combined amphotericin B and flucytosine therapy, as well as the emerging resistance of C. neoformans and C. gattii to fluconazole, pose considerable challenges to clinical treatment. To overcome these problems, the use of drugs and combination therapies has attracted considerable attention in recent years. These methodologies have been increasingly applied because they are associated with a fast and economical mode of searching for new antifungal agents with antifungal activity against cryptococci. In parallel, research on the bioprospecting of natural products and studies, including planned structural modifications of bioactive molecules, continues in research laboratories. These combined efforts have fueled the ongoing hope of identifying a successful new antifungal agent, either by screening or targeted modifications of pre-existing molecules.
ACKNOWLEDGMENTS
The authors would like to thank Matthew Miller for the critical and stylistic review of the manuscript. We would like to thank Fundação de Amparo à Pesquisa do Estado do Amazonas, Conselho Nacional de Desenvolvimento Científico e Tecnológico and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
Funding Statement
Financial Support: We would like to thank Fundação de Amparo à Pesquisa do State of Amazonas (FAPEAM) for the funding of the research by Naira Sulany Oliveira de Sousa through the granting of the POSGRAD UEA 2021 scholarship. The authors also would like to recognize funding received from Fundação de Amparo à Pesquisa do Estado do Amazonas (Public Notice N. 001/2017 −PPSUS), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq.
Footnotes
Financial Support: We would like to thank Fundação de Amparo à Pesquisa do State of Amazonas (FAPEAM) for the funding of the research by Naira Sulany Oliveira de Sousa through the granting of the POSGRAD UEA 2021 scholarship. The authors also would like to recognize funding received from Fundação de Amparo à Pesquisa do Estado do Amazonas (Public Notice N. 001/2017 −PPSUS), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq.
REFERENCES
- 1.1. Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, et al. The Case for Adopting the “Species Complex” Nomenclature for the Etiologic Agents of Cryptococcosis. Msphere. 2017;2(1):1-7. [DOI] [PMC free article] [PubMed]; Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, et al. The Case for Adopting the “Species Complex” Nomenclature for the Etiologic Agents of Cryptococcosis. Msphere. 2017;2(1):1–7. doi: 10.1128/mSphere.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2. Iyer KR, Revie NM, Fu C, Robbins N, Cowen LE. Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat Rev Microbiol. 2021;19(7):454-66. [DOI] [PMC free article] [PubMed]; Iyer KR, Revie NM, Fu C, Robbins N, Cowen LE. Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat Rev Microbiol. 2021;19(7):454–466. doi: 10.1038/s41579-021-00511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.3. Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Physiol Behav. 2017;176(10):139-48. [DOI] [PMC free article] [PubMed]; Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Physiol Behav. 2017;176(10):139–148. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.4. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. 2009;23(4):525-30. [DOI] [PubMed]; Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 5.5. Soares EA, Lazera MS, Wanke B, Faria M De, Soares EA, Coutinho ZF. Mortality by cryptococcosis in Brazil from 2000 to 2012 : A descriptive epidemiological study. PLoS Negl Trop Dis. 2019;13(7):1-17. [DOI] [PMC free article] [PubMed]; Soares EA, Lazera MS, Wanke B, De Faria M, Soares EA, Coutinho ZF. Mortality by cryptococcosis in Brazil from 2000 to 2012 : A descriptive epidemiological study. PLoS Negl Trop Dis. 2019;13(7):1–17. doi: 10.1371/journal.pntd.0007569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.6. Zavala S, Baddley JW. Cryptococcosis. Semin Respir Crit Care. 2020;41(1):69-79. [DOI] [PubMed]; Zavala S, Baddley JW. Cryptococcosis. Semin Respir Crit Care. 2020;41(1):69–79. doi: 10.1055/s-0039-3400280. [DOI] [PubMed] [Google Scholar]
- 7.7. Zaragoza O. Basic principles of the virulence of Cryptococcus. Virulence. 2019;10(1):490-501. [DOI] [PMC free article] [PubMed]; Zaragoza O. Basic principles of the virulence of Cryptococcus. Virulence. 2019;10(1):490–501. doi: 10.1080/21505594.2019.1614383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.8. Bermas A, Geddes-McAlister J. Combatting the evolution of antifungal resistance in Cryptococcus neoformans. Mol Microbiol. 2020;114(5):721-34. [DOI] [PubMed]; Bermas A, Geddes-McAlister J. Combatting the evolution of antifungal resistance in Cryptococcus neoformans. Mol Microbiol. 2020;114(5):721–734. doi: 10.1111/mmi.14565. [DOI] [PubMed] [Google Scholar]
- 9.9. Spadari C de C, Wirth F, Lopes LB, Ishida K. New approaches for cryptococcosis treatment. Microorganisms. 2020;8(4):1-15. [DOI] [PMC free article] [PubMed]; Spadari C de C, Wirth F, Lopes LB, Ishida K. New approaches for cryptococcosis treatment. Microorganisms. 2020;8(4):1–15. doi: 10.3390/microorganisms8040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.10. Kong Q, Cao Z, Lv N, Zhang H, Liu Y, Hu L, et al. Minocycline and fluconazole have a synergistic effect Against Cryptococcus neoformans Both in vitro and in vivo. Front Microbiol. 2020;11(05):1-11. [DOI] [PMC free article] [PubMed]; Kong Q, Cao Z, Lv N, Zhang H, Liu Y, Hu L, et al. Minocycline and fluconazole have a synergistic effect Against Cryptococcus neoformans Both in vitro and in vivo. Front Microbiol. 2020;11(05):1–11. doi: 10.3389/fmicb.2020.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.11. Katz L, Baltz RH. Natural product discovery: past, present, and future. J Ind Microbiol Biotechnol. 2016;43(2-3):155-76. [DOI] [PubMed]; Katz L, Baltz RH. Natural product discovery: past, present, and future. J Ind Microbiol Biotechnol. 2016;43(2-3):155–176. doi: 10.1007/s10295-015-1723-5. [DOI] [PubMed] [Google Scholar]
- 12.12. Van Norman GA. Limitations of animal studies for predicting toxicity in clinical trials: Is it time to rethink our current approach?. JACC Basic to Transl Sci. 2019;4(7):845-54. [DOI] [PMC free article] [PubMed]; Van Norman GA. Limitations of animal studies for predicting toxicity in clinical trials: Is it time to rethink our current approach? JACC Basic to Transl Sci. 2019;4(7):845–854. doi: 10.1016/j.jacbts.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.13. Chen YC, Chang TY, Liu JW, Chen FJ, Chien CC, Lee CH, et al. Increasing trend of fluconazole-non-susceptible Cryptococcus neoformans in patients with invasive cryptococcosis: A 12-year longitudinal study. BMC Infect Dis. 2015;15(1):1-7. [DOI] [PMC free article] [PubMed]; Chen YC, Chang TY, Liu JW, Chen FJ, Chien CC, Lee CH, et al. Increasing trend of fluconazole-non-susceptible Cryptococcus neoformans in patients with invasive cryptococcosis: A 12-year longitudinal study. BMC Infect Dis. 2015;15(1):1–7. doi: 10.1186/s12879-015-1023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.14. Firacative C, Lizarazo J, Illnait-Zaragozí MT, Castañeda E, Arechavala A, Córdoba S, et al. The status of cryptococcosis in latin America. Mem Inst Oswaldo Cruz. 2018;113(7):1-23. [DOI] [PMC free article] [PubMed]; Firacative C, Lizarazo J, Illnait-Zaragozí MT, Castañeda E, Arechavala A, Córdoba S, et al. The status of cryptococcosis in latin America. Mem Inst Oswaldo Cruz. 2018;113(7):1–23. doi: 10.1590/0074-02760170554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.15. Meyer W, Trilles L. Genotyping of the Cryptococcus neoformans/C. gattii species complex. Australian Biochemist. 2010;41(1):12-16.; Meyer W, Trilles L. Genotyping of the Cryptococcus neoformans/C. gattii species complex. Australian Biochemist. 2010;41(1):12–16. [Google Scholar]
- 16.16. Meyer W, Aanensen DM, Boekhout T, Cogliati M, Diaz MR, Esposto MC, et al. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med Mycol. 2009;47(6):561-70. [DOI] [PMC free article] [PubMed]; Meyer W, Aanensen DM, Boekhout T, Cogliati M, Diaz MR, Esposto MC, et al. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med Mycol. 2009;47(6):561–570. doi: 10.1080/13693780902953886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.17. Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, et al. The case for adopting the “Species Complex” nomenclature for the etiologic agents of Cryptococcosis. Msphere. 2017;2(1):1-7. [DOI] [PMC free article] [PubMed]; Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, et al. The case for adopting the “Species Complex” nomenclature for the etiologic agents of Cryptococcosis. Msphere. 2017;2(1):1–7. doi: 10.1128/mSphere.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.18. Farrer RA, Chang M, Davis MJ, Dorp L Van, Yang D, Shea T, et al. A New Lineage of Cryptococcus gattii (VGV) discovered in the Central Zambezian Miombo Woodlands. Ecol Evol Sci. 2019;10(6):e02306-19. [DOI] [PMC free article] [PubMed]; Farrer RA, Chang M, Davis MJ, Van Dorp L, Yang D, Shea T, et al. A New Lineage of Cryptococcus gattii (VGV) discovered in the Central Zambezian Miombo Woodlands. Ecol Evol Sci. 2019;10(6):e02306-19. doi: 10.1128/mBio.02306-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.19. Negroni R. Cryptococcosis. Clin Dermatol. 2012;30(6):599-609. [DOI] [PubMed]; Negroni R. Cryptococcosis. Clin Dermatol. 2012;30(6):599–609. doi: 10.1016/j.clindermatol.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 20.20. Reis-Filho JB dos, Neves AC, Zymberg ST, Oliveira R de MC de. O líquido cefalorraquiano inicial nas meningencefalites por Cryptococcus neoformans. Rev Inst Med Trop S Paulo. 1985;27(4):173-8. [DOI] [PubMed]; Reis-Filho JB dos, Neves AC, Zymberg ST, Oliveira R de MC de. O líquido cefalorraquiano inicial nas meningencefalites por Cryptococcus neoformans. Rev Inst Med Trop S Paulo. 1985;27(4):173–178. doi: 10.1590/s0036-46651985000400003. [DOI] [PubMed] [Google Scholar]
- 21.21. Siddiqi OK, Ghebremichael M, Dang X, Atadzhanov M, Kaonga P, Khoury MN, et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected zambian adults. Clin Infect Dis. 2014;58(12):1771-7. [DOI] [PMC free article] [PubMed]; Siddiqi OK, Ghebremichael M, Dang X, Atadzhanov M, Kaonga P, Khoury MN, et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected zambian adults. Clin Infect Dis. 2014;58(12):1771–1777. doi: 10.1093/cid/ciu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.22. Hurtado JC, Castillo P, Fernandes F, Navarro M, Lovane L, Casas I, et al. Mortality due to Cryptococcus neoformans and Cryptococcus gattii in low-income settings: an autopsy study. Sci Rep. 2019;9(1):1-10. [DOI] [PMC free article] [PubMed]; Hurtado JC, Castillo P, Fernandes F, Navarro M, Lovane L, Casas I, et al. Mortality due to Cryptococcus neoformans and Cryptococcus gattii in low-income settings: an autopsy study. Sci Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-43941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.23. Miot J, Leong T, Takuva S, Parrish A, Dawood H. Cost-effectiveness analysis of flucytosine as induction therapy in the treatment of cryptococcal meningitis in HIV-infected adults in South Africa. BMC Health Serv Res. 2021;21(1):1-11. [DOI] [PMC free article] [PubMed]; Miot J, Leong T, Takuva S, Parrish A, Dawood H. Cost-effectiveness analysis of flucytosine as induction therapy in the treatment of cryptococcal meningitis in HIV-infected adults in South Africa. BMC Health Serv Res. 2021;21(1):1–11. doi: 10.1186/s12913-021-06268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.24. Pinheiro SB, Sousa ES, Cortez ACA, da Silva Rocha DF, Menescal LSF, Chagas VS, et al. Cryptococcal meningitis in non-HIV patients in the State of Amazonas, Northern Brazil. Brazilian J Microbiol. 2020;52(1):279-88. [DOI] [PMC free article] [PubMed]; Pinheiro SB, Sousa ES, Cortez ACA, da Silva Rocha DF, Menescal LSF, Chagas VS, et al. Cryptococcal meningitis in non-HIV patients in the State of Amazonas, Northern Brazil. Brazilian J Microbiol. 2020;52(1):279–288. doi: 10.1007/s42770-020-00383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.25. Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLoS One. 2013;8(3):e60431 [DOI] [PMC free article] [PubMed]; Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLoS One. 2013;8(3):e60431 . doi: 10.1371/journal.pone.0060431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.26. Bielska E, May RC. What makes Cryptococcus gattii a pathogen? FEMS Yeast Res. 2016;16(1):1-12. [DOI] [PubMed]; Bielska E, May RC. What makes Cryptococcus gattii a pathogen? FEMS Yeast Res. 2016;16(1):1–12. doi: 10.1093/femsyr/fov106. [DOI] [PubMed] [Google Scholar]
- 27.27. Hommel B, Sturny-Leclère A, Volant S, Veluppillai N, Duchateau M, Yu CH, et al. Cryptococcus neoformans resists to drastic conditions by switching to viable but non-culturable cell phenotype. Plos Pathog. 2019;15(9):e1008070. [DOI] [PMC free article] [PubMed]; Hommel B, Sturny-Leclère A, Volant S, Veluppillai N, Duchateau M, Yu CH, et al. Cryptococcus neoformans resists to drastic conditions by switching to viable but non-culturable cell phenotype. Plos Pathog. 2019;15(9):e1008070. doi: 10.1371/journal.ppat.1007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.28. World Health Organization (WHO). Guidelines for diagnosing, preventing and managing cryptococcal disease among adults, adolescents and children living with HIV. Geneva: WHO; 2022.64p. [PubMed]; World Health Organization (WHO) Guidelines for diagnosing, preventing and managing cryptococcal disease among adults, adolescents and children living with HIV. Geneva: WHO; 2022. 64p. [PubMed] [Google Scholar]
- 29.29. Alvarez M, Chipana CT, Suarez F. Proctocolitis by cryptococcus in an immunocompetent patient: first report in Peru. Rev Gastroenterol Peru. 2019;39(3):288-91. [PubMed]; Alvarez M, Chipana CT, Suarez F. Proctocolitis by cryptococcus in an immunocompetent patient: first report in Peru. Rev Gastroenterol Peru. 2019;39(3):288–291. [PubMed] [Google Scholar]
- 30.30. Tan GSE, Singh R, Chong TYR, Su PQ, Lee JSS, Wong KJH, et al. Severe primary cutaneous Cryptococcus gattii causing ulcerative cellulitis in an immunocompetent patient. Lancet Infect Dis. 2019;19(10):1148-49. [DOI] [PubMed]; Tan GSE, Singh R, Chong TYR, Su PQ, Lee JSS, Wong KJH, et al. Severe primary cutaneous Cryptococcus gattii causing ulcerative cellulitis in an immunocompetent patient. Lancet Infect Dis. 2019;19(10):1148–1149. doi: 10.1016/S1473-3099(19)30409-8. [DOI] [PubMed] [Google Scholar]
- 31.31. Jarvis JN, Lawrence DS, Meya DB, Kagimu E, Kasibante J, Mpoza E, Rutakingirwa MK, et al. Single-dose lipossomal amphotericin B treatment for cryptococcal meningitis. N Engl J Med. 2022;386(12):1109-20. [DOI] [PMC free article] [PubMed]; Jarvis JN, Lawrence DS, Meya DB, Kagimu E, Kasibante J, Mpoza E, Rutakingirwa MK, et al. Single-dose lipossomal amphotericin B treatment for cryptococcal meningitis. N Engl J Med. 2022;386(12):1109–1120. doi: 10.1056/NEJMoa2111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.32. Eileen K, Maziarz MD, John R Perfect M. Cryptococcosis. Intraocular Inflamm. 2016;30(1):1277-83.; Eileen K, Maziarz MD, John R Perfect M. Cryptococcosis. Intraocular Inflamm. 2016;30(1):1277–1283. [Google Scholar]
- 33.33. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis . 2010;50(3):291-322. [DOI] [PMC free article] [PubMed]; Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.34. Ellis D. Amphotericin B: Spectrum and resistance. J Antimicrob Chemother. 2002;49(SUPL. S1):7-10. [DOI] [PubMed]; Ellis D. Amphotericin B: Spectrum and resistance. J Antimicrob Chemother. 2002;49(SUPL. S1):7–10. doi: 10.1093/jac/49.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 35.35. Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci USA. 2012;109(7):2234-9. [DOI] [PMC free article] [PubMed]; Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci USA. 2012;109(7):2234–2239. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.36. Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. 2009;26(4):223-7. [DOI] [PubMed]; Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. 2009;26(4):223–227. doi: 10.1016/j.riam.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 37.37. Rossi SA, De Oliveira HC, Agreda-Mellon D, Lucio J, Soares Mendes-Giannini MJ, García-Cambero JP, et al. Identification of off-patent drugs that show synergism with amphotericin B or that present antifungal action against Cryptococcus neoformans and Candida spp. Antimicrob Agents Chemother. 2020;64(4):1-16. [DOI] [PMC free article] [PubMed]; Rossi SA, De Oliveira HC, Agreda-Mellon D, Lucio J, Soares Mendes-Giannini MJ, García-Cambero JP, et al. Identification of off-patent drugs that show synergism with amphotericin B or that present antifungal action against Cryptococcus neoformans and Candida spp. Antimicrob Agents Chemother. 2020;64(4):1–16. doi: 10.1128/AAC.01921-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.38. Montgomery JA, Hewson K. Synthesis of potential anticancer agents. X. 2-Fluoroadenosine. J Am Chem Soc. 1957;79(16):4559-60.; Montgomery JA, Hewson K. Synthesis of potential anticancer agents. X. 2-Fluoroadenosine. J Am Chem Soc. 1957;79(16):4559–4560. [Google Scholar]
- 39.39. Bennett JE, Dismukes WE, Duma RJ, Medoff G, Sande MA, Gallis H, et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N Engl J Med . 1979:301(3):126-31. [DOI] [PubMed]; Bennett JE, Dismukes WE, Duma RJ, Medoff G, Sande MA, Gallis H, et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N Engl J Med. 1979;301(3):126–131. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 40.40. Dromer F, Bernede-Bauduin C, Guillemot D, Lortholary O. Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PLoS One . 2008;3(8):e2870. [DOI] [PMC free article] [PubMed]; Dromer F, Bernede-Bauduin C, Guillemot D, Lortholary O. Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PLoS One. 2008;3(8):e2870. doi: 10.1371/journal.pone.0002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.41. Hope WW, Tabernero L, Denning DW, Anderson MJ. Molecular mechanisms of primary resistance to flucytosine in Candida albicans. Antimicrob Agents Chemother . 2004;48(11):4377-86. [DOI] [PMC free article] [PubMed]; Hope WW, Tabernero L, Denning DW, Anderson MJ. Molecular mechanisms of primary resistance to flucytosine in Candida albicans. Antimicrob Agents Chemother. 2004;48(11):4377–4386. doi: 10.1128/AAC.48.11.4377-4386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.42. Billmyre RB, Applen Clancey S, Li LX, Doering TL, Heitman J. 5-fluorocytosine resistance is associated with hypermutation and alterations in capsule biosynthesis inCryptococcus. Nat Commun. 2020;11(1):1-9. [DOI] [PMC free article] [PubMed]; Billmyre RB, Applen Clancey S, Li LX, Doering TL, Heitman J. 5-fluorocytosine resistance is associated with hypermutation and alterations in capsule biosynthesis in Cryptococcus. Nat Commun. 2020;11(1):1–9. doi: 10.1038/s41467-019-13890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.43. Vidal JE, de Albuquerque Moraes C, de Siqueira REB, Miranda NFB, Marcusso R, Boulware DR, et al. HIV-associated cryptococcal meningitis patients treated with Amphotericin B deoxycholate plus flucytosine under routine care conditions in a referral center in São Paulo, Brazil. Mycopathologia. 2021;186(1):93-102. [DOI] [PubMed]; Vidal JE, de Albuquerque Moraes C, de Siqueira REB, Miranda NFB, Marcusso R, Boulware DR, et al. HIV-associated cryptococcal meningitis patients treated with Amphotericin B deoxycholate plus flucytosine under routine care conditions in a referral center in São Paulo, Brazil. Mycopathologia. 2021;186(1):93–102. doi: 10.1007/s11046-020-00512-2. [DOI] [PubMed] [Google Scholar]
- 44.44. Richardson k, Copper K, Marriott MS, Tarbit MH, Troke PF, Whittle PJ. Discovery of Fluconazole, a Novel Antifungal Agent. Rev Infect Dis. 1990;12(3):267-71. [DOI] [PubMed]; k Richardson, Copper K, Marriott MS, Tarbit MH, Troke PF, Whittle PJ. Discovery of Fluconazole, a Novel Antifungal Agent. Rev Infect Dis. 1990;12(3):267–271. doi: 10.1093/clinids/12.supplement_3.s267. [DOI] [PubMed] [Google Scholar]
- 45.45. Sanglard D. Emerging threats in antifungal-resistant fungal pathogens. Front Med. 2016;3:1-10. [DOI] [PMC free article] [PubMed]; Sanglard D. Emerging threats in antifungal-resistant fungal pathogens. Front Med. 2016;3:1–10. doi: 10.3389/fmed.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.46. Gulshan K, Moye-Rowley WS. Multidrug resistance in fungi. Eukaryot Cell. 2007;6(11):1933-42. [DOI] [PMC free article] [PubMed]; Gulshan K, Moye-Rowley WS. Multidrug resistance in fungi. Eukaryot Cell. 2007;6(11):1933–1942. doi: 10.1128/EC.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.47. Howard-Jones AR, Sparks R, Pham D, Halliday C, Beardsley J, Chen SC. Pulmonary cryptococcosis. J Fungi. 2022;8(11):1-19. [DOI] [PMC free article] [PubMed]; Howard-Jones AR, Sparks R, Pham D, Halliday C, Beardsley J, Chen SC. Pulmonary cryptococcosis. J Fungi. 2022;8(11):1–19. doi: 10.3390/jof8111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.48. Kelly SL, Lamb DC, Taylor M, Corran AJ, Baldwin BC, Powderly WG. Resistance to amphotericin B associated with defective sterol Δ8→7 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS Microbiol Lett. 1994;122(1-2):39-42. [DOI] [PubMed]; Kelly SL, Lamb DC, Taylor M, Corran AJ, Baldwin BC, Powderly WG. Resistance to amphotericin B associated with defective sterol Δ8→7 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS Microbiol Lett. 1994;122(1-2):39–42. doi: 10.1111/j.1574-6968.1994.tb07140.x. [DOI] [PubMed] [Google Scholar]
- 49.49. Carolus H, Pierson S, Lagrou K, Van Dijck P. Amphotericin b and other polyenes-discovery, clinical use, mode of action and drug resistance. J Fungi. 2020;6(4):1-20. [DOI] [PMC free article] [PubMed]; Carolus H, Pierson S, Lagrou K, Van Dijck P. Amphotericin b and other polyenes-discovery, clinical use, mode of action and drug resistance. J Fungi. 2020;6(4):1–20. doi: 10.3390/jof6040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.50. Scorzoni L, de Paula e Silva ACA, Marcos CM, Assato PA, de Melo WCMA, de Oliveira HC, et al. Antifungal therapy: New advances in the understanding and treatment of mycosis. Front Microbiol. 2017;8(1):1-23. [DOI] [PMC free article] [PubMed]; Scorzoni L, Paula de, Silva ACA, Marcos CM, Assato PA, de Melo WCMA, de Oliveira HC, et al. Antifungal therapy: New advances in the understanding and treatment of mycosis. Front Microbiol. 2017;8(1):1–23. doi: 10.3389/fmicb.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.51. Chang YC, Lamichhane AK, Cai H, Walter PJ, Bennett JE, Kwon-Chung KJ. Moderate levels of 5-fluorocytosine cause the emergence of high frequency resistance in cryptococci. Nat Commun. 2021;12(1):1-13. [DOI] [PMC free article] [PubMed]; Chang YC, Lamichhane AK, Cai H, Walter PJ, Bennett JE, Kwon-Chung KJ. Moderate levels of 5-fluorocytosine cause the emergence of high frequency resistance in cryptococci. Nat Commun. 2021;12(1):1–13. doi: 10.1038/s41467-021-23745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.52. Venkateswarlu K, Taylor M, Manning NJ, Rinaldi MG. Fluconazole Tolerance in Clinical Isolates of Cryptococcus neoformans. Antimicrob Agents Chemother . 1997;41(4):748-51. [DOI] [PMC free article] [PubMed]; Venkateswarlu K, Taylor M, Manning NJ, Rinaldi MG. Fluconazole Tolerance in Clinical Isolates of Cryptococcus neoformans. Antimicrob Agents Chemother. 1997;41(4):748–751. doi: 10.1128/aac.41.4.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.53. Peetermans W, Bobbaers H, Verhaegen J, Vandepitte J. Fluconazole-resistant Cryptococcus neoformans var gattii in an AIDS patient. Acta Clin Belg. 1993;48(6):405-9. [DOI] [PubMed]; Peetermans W, Bobbaers H, Verhaegen J, Vandepitte J. Fluconazole-resistant Cryptococcus neoformans var gattii in an AIDS patient. Acta Clin Belg. 1993;48(6):405–409. doi: 10.1080/17843286.1993.11718338. [DOI] [PubMed] [Google Scholar]
- 54.54. Bongomin F, Oladele RO, Gago S, Moore CB, Richardson MD. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus specie. Mycoses. 2018;61(5):290-7. [DOI] [PubMed]; Bongomin F, Oladele RO, Gago S, Moore CB, Richardson MD. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus specie. Mycoses. 2018;61(5):290–297. doi: 10.1111/myc.12747. [DOI] [PubMed] [Google Scholar]
- 55.55. Loyse A, Dromer F, Day J, Lortholary O, Harrison TS. Flucytosine and cryptococcosis: Time to urgently address the worldwide accessibility of a 50-year-old antifungal. J Antimicrob Chemother. 2013;68(11):2435-44. [DOI] [PMC free article] [PubMed]; Loyse A, Dromer F, Day J, Lortholary O, Harrison TS. Flucytosine and cryptococcosis: Time to urgently address the worldwide accessibility of a 50-year-old antifungal. J Antimicrob Chemother. 2013;68(11):2435–2444. doi: 10.1093/jac/dkt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.56. Perfect JR, Dismukes WE, Dromer F, Goldman DL, John R, Hamill RJ, et al. Cryptococcosis. Proc Natl Acad Sci USA . 2012;30(1 SUPPL.):S3-13.; Perfect JR, Dismukes WE, Dromer F, Goldman DL, John R, Hamill RJ, et al. Cryptococcosis. Proc Natl Acad Sci USA. 2012;30(1 SUPPL.):S3–13. [Google Scholar]
- 57.57. Rodero L, Mellado E, Rodriguez AC, Salve A, Guelfand L, Cahn P, et al. G484S Amino Acid Substitution in Lanosterol 14-α Demethylase (ERG11) is related to fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate. Antimicrob Agents Chemother . 2003;47(11):3653-6. [DOI] [PMC free article] [PubMed]; Rodero L, Mellado E, Rodriguez AC, Salve A, Guelfand L, Cahn P, et al. G484S Amino Acid Substitution in Lanosterol 14-α Demethylase (ERG11) is related to fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate. Antimicrob Agents Chemother. 2003;47(11):3653–3656. doi: 10.1128/AAC.47.11.3653-3656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.58. Sanguinetti M, Posteraro B, La Sorda M, Torelli R, Fiori B, Santangelo R, et al. Role of AFR1, an ABC transporter-encoding gene, in the in vivo response to fluconazole and virulence of Cryptococcus neoformans. Infect Immun. 2006;74(2):1352-9. [DOI] [PMC free article] [PubMed]; Sanguinetti M, Posteraro B, La Sorda M, Torelli R, Fiori B, Santangelo R, et al. Role of AFR1, an ABC transporter-encoding gene, in the in vivo response to fluconazole and virulence of Cryptococcus neoformans. Infect Immun. 2006;74(2):1352–1359. doi: 10.1128/IAI.74.2.1352-1359.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.59. Chang M, Sionov E, Lamichhane AK, Kwon-chung KJ, Chang YC. Roles of Three Cryptococcus neoformans and Cryptococcus gattii efflux pump-coding genes in response to drug treatment. Antimicrob Agents Chemother . 2018;62(4):1-14. [DOI] [PMC free article] [PubMed]; Chang M, Sionov E, Lamichhane AK, Kwon-chung KJ, Chang YC. Roles of Three Cryptococcus neoformans and Cryptococcus gattii efflux pump-coding genes in response to drug treatment. Antimicrob Agents Chemother. 2018;62(4):1–14. doi: 10.1128/AAC.01751-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.60. Sionov E, Lee H, Chang YC, Kwon-Chung KJ. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog. 2010;6(4):1-13. [DOI] [PMC free article] [PubMed]; Sionov E, Lee H, Chang YC, Kwon-Chung KJ. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog. 2010;6(4):1–13. doi: 10.1371/journal.ppat.1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.61. Ferreira GF, Santos DA. Heteroresistance and fungi. Mycoses. 2017;60(9):562-8. [DOI] [PubMed]; Ferreira GF, Santos DA. Heteroresistance and fungi. Mycoses. 2017;60(9):562–568. doi: 10.1111/myc.12639. [DOI] [PubMed] [Google Scholar]
- 62.62. Brukner I, Oughton M. A fundamental change in antibiotic susceptibility testing would better prevent therapeutic failure: from individual to population-based analysis. Front Microbiol. 2020;11:1-3. [DOI] [PMC free article] [PubMed]; Brukner I, Oughton M. A fundamental change in antibiotic susceptibility testing would better prevent therapeutic failure: from individual to population-based analysis. Front Microbiol. 2020;11:1–3. doi: 10.3389/fmicb.2020.01820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.63. Varma A, Kwon-Chung KJ. Heteroresistance of Cryptococcus gattii to fluconazole. Antimicrob Agents Chemother . 2010;54(6):2303-11. [DOI] [PMC free article] [PubMed]; Varma A, Kwon-Chung KJ. Heteroresistance of Cryptococcus gattii to fluconazole. Antimicrob Agents Chemother. 2010;54(6):2303–2311. doi: 10.1128/AAC.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.64. Badali H, Wiederhold NP. Antifungal Resistance Testing and Implications for Management. Curr Fungal Infect Rep. 2019;13(4):274-83.; Badali H, Wiederhold NP. Antifungal Resistance Testing and Implications for Management. Curr Fungal Infect Rep. 2019;13(4):274–283. [Google Scholar]
- 65.65. de Sousa ESO, Cortez ACA, de Souza Carvalho Melhem M, Frickmann H, de Souza JVB. Factors influencing susceptibility testing of antifungal drugs: a critical review of document M27-A4 from the Clinical and Laboratory Standards Institute (CLSI). Brazilian J Microbiol . 2020;51(4):1791-800. [DOI] [PMC free article] [PubMed]; de Sousa ESO, Cortez ACA, de Souza Carvalho Melhem M, Frickmann H, de Souza JVB. Factors influencing susceptibility testing of antifungal drugs: a critical review of document M27-A4 from the Clinical and Laboratory Standards Institute (CLSI) Brazilian J Microbiol. 2020;51(4):1791–1800. doi: 10.1007/s42770-020-00354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.66. Moreira IDMB, Cortez ACA, De Souza ÉS, Pinheiro SB, De Souza Oliveira JG, Sadahiro A, et al. Investigation of fluconazole heteroresistance in clinical and environmental isolates of Cryptococcus neoformans complex and Cryptococcus gattii complex in the state of Amazonas, Brazil. Med Mycol. 2022;60(3):1-9. [DOI] [PubMed]; Moreira IDMB, Cortez ACA, De Souza ÉS, Pinheiro SB, De Souza Oliveira JG, Sadahiro A, et al. Investigation of fluconazole heteroresistance in clinical and environmental isolates of Cryptococcus neoformans complex and Cryptococcus gattii complex in the state of Amazonas, Brazil. Med Mycol. 2022;60(3):1–9. doi: 10.1093/mmy/myac005. [DOI] [PubMed] [Google Scholar]
- 67.67. Pereira DG. Importância do metabolismo no planejamento de fármacos. Quim Nova. 2007;30(1):171-7.; Pereira DG. Importância do metabolismo no planejamento de fármacos. Quim Nova. 2007;30(1):171–177. [Google Scholar]
- 68.68. Wright GD. Unlocking the potential of natural products in drug discovery. Microb Biotechnol. 2019;12(1):55-7. [DOI] [PMC free article] [PubMed]; Wright GD. Unlocking the potential of natural products in drug discovery. Microb Biotechnol. 2019;12(1):55–57. doi: 10.1111/1751-7915.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.69. Ramírez-Rendon D, Passari AK, Ruiz-Villafán B, Rodríguez-Sanoja R, Sánchez S, Demain AL. Impact of novel microbial secondary metabolites on the pharma industry. Appl Microbiol Biotechnol. 2022;106(5-6):1855-78. [DOI] [PMC free article] [PubMed]; Ramírez-Rendon D, Passari AK, Ruiz-Villafán B, Rodríguez-Sanoja R, Sánchez S, Demain AL. Impact of novel microbial secondary metabolites on the pharma industry. Appl Microbiol Biotechnol. 2022;106(5-6):1855–1878. doi: 10.1007/s00253-022-11821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.70. Calixto JB. The role of natural products in modern drug discovery. Biological Sciences. 2019;91(Suppl 3):e20190105. [DOI] [PubMed]; Calixto JB. The role of natural products in modern drug discovery. Biological Sciences. 2019;91(Suppl 3):e20190105. doi: 10.1590/0001-3765201920190105. [DOI] [PubMed] [Google Scholar]
- 71.71. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770-803. [DOI] [PubMed]; Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 72.72. El-Naggar HA, Bashar MAE, Rady I, El-Wetidy MS, Suleiman WB, Al-Otibi FO, et al. Two red sea sponge extracts (Negombata magnifica and Callyspongia siphonella) induced anticancer and antimicrobial activity. Appl Sci. 2022;12(3):1-23.; El-Naggar HA, Bashar MAE, Rady I, El-Wetidy MS, Suleiman WB, Al-Otibi FO, et al. Two red sea sponge extracts (Negombata magnifica and Callyspongia siphonella) induced anticancer and antimicrobial activity. Appl Sci. 2022;12(3):1–23. [Google Scholar]
- 73.73. CLSI. M44-A2: Method for antifungal disk diffusion susceptibility testing of yeasts. Clin Lab Stand Institute. 2009;29(17).; CLSI M44-A2: Method for antifungal disk diffusion susceptibility testing of yeasts. Clin Lab Stand Institute. 2009;29(17) [Google Scholar]
- 74.74. CLSI. M27-A4. Reference method for broth dilution antifungal susceptibility testing of yeasts. Clin Lab Stand Institute . 2017;4th ed.; CLSI M27-A4. Reference method for broth dilution antifungal susceptibility testing of yeasts. Clin Lab Stand Institute. (4th ed) 2017 [Google Scholar]
- 75.75. Def EE. Susceptibility testing of yeasts. Clin Microbiol Infect. 1997;3(1):14-6.; Def EE. Susceptibility testing of yeasts. Clin Microbiol Infect. 1997;3(1):14–16. [Google Scholar]
- 76.76. Trivella DBB, Bruder MCP, Oliveira FCB, Porcaro R, Rustiguel JK, Ribeiro LB, et al. Descoberta de fármacos a partir de produtos naturais e a abordagem molecular power house (MPH). Rev Fitos. 2022;16(Supl. 2):176-92.; Trivella DBB, Bruder MCP, Oliveira FCB, Porcaro R, Rustiguel JK, Ribeiro LB, et al. Descoberta de fármacos a partir de produtos naturais e a abordagem molecular power house (MPH) Rev Fitos. 2022;16(Supl. 2):176–192. [Google Scholar]
- 77.77. Aldholmi M, Marchand P, Ourliac-Garnier I, Le Pape P, Ganesan A. A decade of antifungal leads from natural products: 2010-2019. Pharmaceuticals. 2019;12(4):2010-9. [DOI] [PMC free article] [PubMed]; Aldholmi M, Marchand P, Ourliac-Garnier I, Le Pape P, Ganesan A. A decade of antifungal leads from natural products: 2010-2019. Pharmaceuticals. 2019;12(4):2010–2019. doi: 10.3390/ph12040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.78. Ribeiro R, Pinto E, Fernandes C, Sousa E. Marine cyclic peptides: antimicrobial activity and synthetic strategies. Mar Drugs. 2022;20(6):2-51. [DOI] [PMC free article] [PubMed]; Ribeiro R, Pinto E, Fernandes C, Sousa E. Marine cyclic peptides: antimicrobial activity and synthetic strategies. Mar Drugs. 2022;20(6):2–51. doi: 10.3390/md20060397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.79. Manjunath M, Skariyachan S. Screening of natural lead molecules against putative molecular targets of drug-resistant cryptococcus spp: an insight from computer-aided molecular design. Curr Top Med Chem. 2019;18(31):2681-701. [DOI] [PubMed]; Manjunath M, Skariyachan S. Screening of natural lead molecules against putative molecular targets of drug-resistant cryptococcus spp: an insight from computer-aided molecular design. Curr Top Med Chem. 2019;18(31):2681–2701. doi: 10.2174/1568026619666190119145434. [DOI] [PubMed] [Google Scholar]
- 80.80. Cardoso NNR, Alviano CS, Blank AF, Arrigoni-Blank M de F, Romanos MT V, Cunha MML, et al. Anti-cryptococcal activity of ethanol crude extract and hexane fraction from Ocimum basilicum var. Maria bonita: Mechanisms of action and synergism with amphotericin B and Ocimum basilicum essential oil. Pharm Biol. 2017;55(1):1380-8. [DOI] [PMC free article] [PubMed]; Cardoso NNR, Alviano CS, Blank AF, Arrigoni-Blank M de F, Romanos MT V, Cunha MML, et al. Anti-cryptococcal activity of ethanol crude extract and hexane fraction from Ocimum basilicum var. Maria bonita: Mechanisms of action and synergism with amphotericin B and Ocimum basilicum essential oil. Pharm Biol. 2017;55(1):1380–1388. doi: 10.1080/13880209.2017.1302483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.81. Silva TC, de Ávila RI, Zara ALSA, Santos AS, Ataídes F, Freitas VAQ, et al. Punicalagin triggers ergosterol biosynthesis disruption and cell cycle arrest in Cryptococcus gattii and Candida albicans: Action mechanisms of punicalagin against yeasts. Brazilian J Microbiol . 2020;51(4):1719-27. [DOI] [PMC free article] [PubMed]; Silva TC, de Ávila RI, Zara ALSA, Santos AS, Ataídes F, Freitas VAQ, et al. Punicalagin triggers ergosterol biosynthesis disruption and cell cycle arrest in Cryptococcus gattii and Candida albicans : Action mechanisms of punicalagin against yeasts. Brazilian J Microbiol. 2020;51(4):1719–1727. doi: 10.1007/s42770-020-00364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.82. Teixeira AP de C, Nóbrega R de O, Lima E de O, Araújo W de O, Lima I de O. Antifungal activity study of the monoterpene thymol against Cryptococcus neoformans. Nat Prod Res. 2018;34(18):2630-3. [DOI] [PubMed]; Teixeira AP de C, Nóbrega R de O, Lima E de O, Araújo W de O, Lima I de O. Antifungal activity study of the monoterpene thymol against Cryptococcus neoformans. Nat Prod Res. 2018;34(18):2630–2633. doi: 10.1080/14786419.2018.1547296. [DOI] [PubMed] [Google Scholar]
- 83.83. Folly MLC, Ferreira GF, Salvador MR, Sathler AA, da Silva GF, Santos JCB, et al. Evaluation of in vitro antifungal activity of Xylosma prockia (Turcz.) Turcz. (Salicaceae) leaves against Cryptococcus spp. Front Microbiol. 2020;10(2)1-13. [DOI] [PMC free article] [PubMed]; Folly MLC, Ferreira GF, Salvador MR, Sathler AA, da Silva GF, Santos JCB, et al. Evaluation of in vitro antifungal activity of Xylosma prockia (Turcz.) Turcz. (Salicaceae) leaves against Cryptococcus spp. Front Microbiol. 2020;10(2):1–13. doi: 10.3389/fmicb.2019.03114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.84. Kayo MT, Simo MK, Tagatsing Fotsing M, Talla E, Laurent S, Elst L Vander, et al. Antifungal potential of extracts, fractions and compounds from Uvaria comperei (Annonaceae) and Oxyanthus unilocularis (Rubiaceae). Nat Prod Res . 2020;35(24):5732-6. [DOI] [PubMed]; Kayo MT, Simo MK, Tagatsing Fotsing M, Talla E, Laurent S, Vander Elst L, et al. Antifungal potential of extracts, fractions and compounds from Uvaria comperei (Annonaceae) and Oxyanthus unilocularis (Rubiaceae) Nat Prod Res. 2020;35(24):5732–5736. doi: 10.1080/14786419.2020.1828409. [DOI] [PubMed] [Google Scholar]
- 85.85. Ren S, Deng K, Qiu S, Wang M, Avula B, Tripathi SK, et al. Identification of antifungal bisphosphocholines from medicinal Gentiana species. J Nat Prod . 2020;83(10):3207-11. [DOI] [PubMed]; Ren S, Deng K, Qiu S, Wang M, Avula B, Tripathi SK, et al. Identification of antifungal bisphosphocholines from medicinal Gentiana species. J Nat Prod. 2020;83(10):3207–3211. doi: 10.1021/acs.jnatprod.0c00584. [DOI] [PubMed] [Google Scholar]
- 86.86. Omokhua-Uyi AG, Abdalla MA, Leonard CM, Aro A, Uyi OO, Van Staden J, et al. Flavonoids isolated from the South African weed Chromolaena odorata (Asteraceae) have pharmacological activity against uropathogens. BMC Complement Med Ther. 2020;20(1):1-15. [DOI] [PMC free article] [PubMed]; Omokhua-Uyi AG, Abdalla MA, Leonard CM, Aro A, Uyi OO, Van Staden J, et al. Flavonoids isolated from the South African weed Chromolaena odorata (Asteraceae) have pharmacological activity against uropathogens. BMC Complement Med Ther. 2020;20(1):1–15. doi: 10.1186/s12906-020-03024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.87. El Karkouri J, Bouhrim M, Al Kamaly OM, Mechchate H, Kchibale A, Adadi I, et al. Chemical composition, antibacterial and antifungal activity of the essential oil from Cistus ladanifer L. Plants. 2021;10(10):1-16. [DOI] [PMC free article] [PubMed]; El Karkouri J, Bouhrim M, Al Kamaly OM, Mechchate H, Kchibale A, Adadi I, et al. Chemical composition, antibacterial and antifungal activity of the essential oil from Cistus ladanifer L. Plants. 2021;10(10):1–16. doi: 10.3390/plants10102068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.88. Zulfiqar F, Pandey P, Tripathi SK, Ali Z, Chittiboyina AG, Khan IA. Benzoylcyclopropane derivatives from Hypoxis hemerocallidea corms. Planta Med. 2021:685-92. [DOI] [PMC free article] [PubMed]; Zulfiqar F, Pandey P, Tripathi SK, Ali Z, Chittiboyina AG, Khan IA. Benzoylcyclopropane derivatives from Hypoxis hemerocallidea corms. Planta Med. 2021:685–692. doi: 10.1055/a-1540-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.89. Levorato-Vinche AD, Melhem M de SC, Bonfietti LX, de-la-Cruz-Chacón I, Boaro CSF, Fabro AT, et al. Antifungal activity of liriodenine on clinical strains of Cryptococcus neoformans and Cryptococcus gattii species complexes. J Venom Anim Toxins Incl Trop Dis. 2022;28(9):1-11. [DOI] [PMC free article] [PubMed]; Levorato-Vinche AD, Melhem M de SC, Bonfietti LX, de-la-Cruz-Chacón I, Boaro CSF, Fabro AT, et al. Antifungal activity of liriodenine on clinical strains of Cryptococcus neoformans and Cryptococcus gattii species complexes. J Venom Anim Toxins Incl Trop Dis. 2022;28(9):1–11. doi: 10.1590/1678-9199-JVATITD-2022-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.90. Powers CN, Mayo JA, Moriarity DM, Vogler B, Setzer WN, McFeeters RL. Identification of Anticryptococcal bornyl compounds from Verbesina turbacensis and their structure-activity relationships. Planta Med. 2022;88:1341-47. [DOI] [PubMed]; Powers CN, Mayo JA, Moriarity DM, Vogler B, Setzer WN, McFeeters RL. Identification of Anticryptococcal bornyl compounds from Verbesina turbacensis and their structure-activity relationships. Planta Med. 2022;88:1341–1347. doi: 10.1055/a-1792-3214. [DOI] [PubMed] [Google Scholar]
- 91.91. Beattie KD, Ellwood N, Kumar R, Yang X, Healy PC, Choomuenwai V, et al. Antibacterial and antifungal screening of natural products sourced from Australian fungi and characterisation of pestalactams D-F. Phytochemistry. 2016;124:79-85. [DOI] [PubMed]; Beattie KD, Ellwood N, Kumar R, Yang X, Healy PC, Choomuenwai V, et al. Antibacterial and antifungal screening of natural products sourced from Australian fungi and characterisation of pestalactams D-F. Phytochemistry. 2016;124:79–85. doi: 10.1016/j.phytochem.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 92.92. Li Y, Yue Q, Jayanetti DR, Swenson DC, Bartholomeusz GA, An Z, et al. Anti-Cryptococcus Phenalenones and cyclic tetrapeptides from Auxarthron pseudauxarthron. J Nat Prod . 2017;80(7):2101-9. [DOI] [PMC free article] [PubMed]; Li Y, Yue Q, Jayanetti DR, Swenson DC, Bartholomeusz GA, An Z, et al. Anti-Cryptococcus Phenalenones and cyclic tetrapeptides from Auxarthron pseudauxarthron. J Nat Prod. 2017;80(7):2101–2109. doi: 10.1021/acs.jnatprod.7b00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.93. Perlatti B, Nichols CB, Lan N, Wiemann P, Harvey CJB, Alspaugh JA, et al. Identification of the Antifungal Metabolite chaetoglobosin p from Discosia rubi using a cryptococcus neoformans inhibition assay: insights into mode of action and biosynthesis. Front Microbiol. 2020;11:1766. [DOI] [PMC free article] [PubMed]; Perlatti B, Nichols CB, Lan N, Wiemann P, Harvey CJB, Alspaugh JA, et al. Identification of the Antifungal Metabolite chaetoglobosin p from Discosia rubi using a cryptococcus neoformans inhibition assay: insights into mode of action and biosynthesis. Front Microbiol. 2020;11:1766–1766. doi: 10.3389/fmicb.2020.01766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.94. Perlatti B, Lan N, Xiang M, Earp CE, Spraker JE, Harvey CJB, et al. Anti-cryptococcal activity of preussolides A and B, phosphoethanolamine-substituted 24-membered macrolides, and leptosin C from coprophilous isolates of Preussia typharum. J Ind Microbiol Biotechnol . 2021;48(9-10). [DOI] [PMC free article] [PubMed]; Perlatti B, Lan N, Xiang M, Earp CE, Spraker JE, Harvey CJB, et al. Anti-cryptococcal activity of preussolides A and B, phosphoethanolamine-substituted 24-membered macrolides, and leptosin C from coprophilous isolates of Preussia typharum. J Ind Microbiol Biotechnol. 2021;48:9–10. doi: 10.1093/jimb/kuab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.95. Cadelis M, Grey A, van de Pas S, Geese S, Weir BS, Copp B, et al. Terrien, a metabolite made by Aspergillus terreus, has activity against Cryptococcus neoformans. PeerJ. 2022;10:e14239. [DOI] [PMC free article] [PubMed]; Cadelis M, Grey A, van de Pas S, Geese S, Weir BS, Copp B, et al. Terrien, a metabolite made by Aspergillus terreus, has activity against Cryptococcus neoformans. PeerJ. 2022;10:e14239. doi: 10.7717/peerj.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.96. Kuvarina AE, Rogozhin EA, Sykonnikov MA, Timofeeva AV, Serebryakova MV, Fedorova NV, et al. Isolation and characterization of a novel hydrophobin, Sa-HFB1, with antifungal activity from an alkaliphilic fungus, Sodiomyces alkalinus. J Fungi. 2022;8(7):659. [DOI] [PMC free article] [PubMed]; Kuvarina AE, Rogozhin EA, Sykonnikov MA, Timofeeva AV, Serebryakova MV, Fedorova NV, et al. Isolation and characterization of a novel hydrophobin, Sa-HFB1, with antifungal activity from an alkaliphilic fungus, Sodiomyces alkalinus. J Fungi. 2022;8(7):659–659. doi: 10.3390/jof8070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.97. Kumar R, Subramani R, Aalbersberg W. Three bioactive sesquiterpene quinones from the Fijian marine sponge of the genus Hippospongia. Nat Prod Res . 2013;27(16):1488-91. [DOI] [PubMed]; Kumar R, Subramani R, Aalbersberg W. Three bioactive sesquiterpene quinones from the Fijian marine sponge of the genus Hippospongia. Nat Prod Res. 2013;27(16):1488–1491. doi: 10.1080/14786419.2012.722086. [DOI] [PubMed] [Google Scholar]
- 98.98. Jamison MT, Dalisay DS, Molinski TF. Peroxide Natural Products from Plakortis zyggompha and the Sponge Association Plakortis halichondrioides-Xestospongia deweerdtae: Antifungal Activity against Cryptococcus gattii. J Nat Prod . 2016;79(3):555-63. [DOI] [PubMed]; Jamison MT, Dalisay DS, Molinski TF. Peroxide Natural Products from Plakortis zyggompha and the Sponge Association Plakortis halichondrioides-Xestospongia deweerdtae: Antifungal Activity against Cryptococcus gattii. J Nat Prod. 2016;79(3):555–563. doi: 10.1021/acs.jnatprod.5b00951. [DOI] [PubMed] [Google Scholar]
- 99.99. Thammasit P, Iadnut A, Mamoon K, Khacha-ananda S, Chupradit K, Tayapiwatana C, et al. A potential of propolis on major virulence factors of Cryptococcus neoformans. Microb Pathog. 2018;123:296-303. [DOI] [PubMed]; Thammasit P, Iadnut A, Mamoon K, Khacha-ananda S, Chupradit K, Tayapiwatana C, et al. A potential of propolis on major virulence factors of Cryptococcus neoformans. Microb Pathog. 2018;123:296–303. doi: 10.1016/j.micpath.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 100.100. Menezes-Silva L, da Silva CJ, de Faria LC, Pizzolante BC, Andrade-Silva LE, da Silva MV, et al. Hemolymph of triatomines presents fungistatic activity against Cryptococcus neoformans and improves macrophage function through MCP-I/TNF-α increase. J Venom Anim Toxins Incl Trop Dis . 2022;28:e20210124. [DOI] [PMC free article] [PubMed]; Menezes-Silva L, da Silva CJ, de Faria LC, Pizzolante BC, Andrade-Silva LE, da Silva MV, et al. Hemolymph of triatomines presents fungistatic activity against Cryptococcus neoformans and improves macrophage function through MCP-I/TNF-α increase. J Venom Anim Toxins Incl Trop Dis. 2022;28:e20210124. doi: 10.1590/1678-9199-JVATITD-2021-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.101. Robbins N, Spitzer M, Wang W, Waglechner N, Patel DJ, O’Brien JS, et al. Discovery of ibomycin, a complex macrolactone that exerts antifungal activity by impeding endocytic trafficking and membrane function. Cell Chem Biol. 2016;23(11):1383-94. [DOI] [PubMed]; Robbins N, Spitzer M, Wang W, Waglechner N, Patel DJ, O’Brien JS, et al. Discovery of ibomycin, a complex macrolactone that exerts antifungal activity by impeding endocytic trafficking and membrane function. Cell Chem Biol. 2016;23(11):1383–1394. doi: 10.1016/j.chembiol.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 102.102. Beattie SR, Krysan DJ. Antifungal drug screening: thinking outside the box to identify novel antifungal scaffolds. Current Opinion in Microbiology. 2020;57(10):1-6. [DOI] [PMC free article] [PubMed]; Beattie SR, Krysan DJ. Antifungal drug screening: thinking outside the box to identify novel antifungal scaffolds. Current Opinion in Microbiology. 2020;57(10):1–6. doi: 10.1016/j.mib.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.103. Jiang Z, Liu N, Hu D, Dong G, Miao Z, Yao J, et al. The discovery of novel antifungal scaffolds by structural simplification of the natural product sampangine. Chem Commun. 2015;51(78):14648-51. [DOI] [PubMed]; Jiang Z, Liu N, Hu D, Dong G, Miao Z, Yao J, et al. The discovery of novel antifungal scaffolds by structural simplification of the natural product sampangine. Chem Commun. 2015;51(78):14648–14651. doi: 10.1039/c5cc05699c. [DOI] [PubMed] [Google Scholar]
- 104.104. Barreiro EJ, Fraga CAM. Química Medicinal: As Bases Moleculares da Ação dos fármacos. 3ª edição. São Paulo: Artmed; 2014. 608 p.; Barreiro EJ, Fraga CAM. Química Medicinal: As Bases Moleculares da Ação dos fármacos. 3ª edição. São Paulo: Artmed; 2014. 608 p [Google Scholar]
- 105.105. Oliveira MS, Chaves OS, Cordeiro LV, Gomes ANP, Fernandes DA, Telles YCF, et al. Indoquinoline alkaloids from Sida rhombifolia (L.) (Malvaceae) and antimicrobial evaluation of Cryptolepinone derivatives. J Braz Chem Soc. 2022;00(00):1-8.; Oliveira MS, Chaves OS, Cordeiro LV, Gomes ANP, Fernandes DA, Telles YCF, et al. Indoquinoline alkaloids from Sida rhombifolia (L.) (Malvaceae) and antimicrobial evaluation of Cryptolepinone derivatives. J Braz Chem Soc. 2022;00(00):1–8. [Google Scholar]
- 106.106. Freire CPV, Ferreira SB, De Oliveira NSM, Matsuura ABJ, Gama IL, Da Silva FDC, et al. Synthesis and biological evaluation of substituted α- And β-2,3-dihydrofuran naphthoquinones as potent anticandidal agents. Medchemcomm. 2010;1(3):229-32.; Freire CPV, Ferreira SB, De Oliveira NSM, Matsuura ABJ, Gama IL, Da Silva FDC, et al. Synthesis and biological evaluation of substituted α- And β-2,3-dihydrofuran naphthoquinones as potent anticandidal agents. Medchemcomm. 2010;1(3):229–232. [Google Scholar]
- 107.107. Jiang Z, Liu N, Dong G, Jiang Y, Liu Y, He X, et al. Scaffold hopping of sampangine: Discovery of potent antifungal lead compound against Aspergillus fumigatus and Cryptococcus neoformans. Bioorganic Med Chem Lett. 2014;24(17):4090-4. [DOI] [PubMed]; Jiang Z, Liu N, Dong G, Jiang Y, Liu Y, He X, et al. Scaffold hopping of sampangine: Discovery of potent antifungal lead compound against Aspergillus fumigatus and Cryptococcus neoformans. Bioorganic Med Chem Lett. 2014;24(17):4090–4094. doi: 10.1016/j.bmcl.2014.07.064. [DOI] [PubMed] [Google Scholar]
- 108.108. Cruz KS, Lima ES, Silva MDJA Da, Souza ES De, Montoia A, Pohlit AM, et al. Screening and antifungal activity of a β-carboline derivative against cryptococcus neoformans and C. gattii. Int J Microbiol. 2019;2019:7157845. [DOI] [PMC free article] [PubMed]; Cruz KS, Lima ES, Da Silva MDJA, De Souza ES, Montoia A, Pohlit AM, et al. Screening and antifungal activity of a β-carboline derivative against cryptococcus neoformans and C. gattii. Int J Microbiol. 2019;2019:7157845–7157845. doi: 10.1155/2019/7157845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.109. Ferreira M do PSBC, Cardoso MF do C, da Silva F de C, Ferreira VF, Lima ES, Souza JVB. Antifungal activity of synthetic naphthoquinones against dermatophytes and opportunistic fungi: Preliminary mechanism-of-action tests. Ann Clin Microbiol Antimicrob. 2014;13(1):1-6. [DOI] [PMC free article] [PubMed]; Ferreira M do PSBC, Cardoso MF do C, da Silva F de C, Ferreira VF, Lima ES, Souza JVB. Antifungal activity of synthetic naphthoquinones against dermatophytes and opportunistic fungi: Preliminary mechanism-of-action tests. Ann Clin Microbiol Antimicrob. 2014;13(1):1–6. doi: 10.1186/1476-0711-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.110. Li Z, Liu N, Tu J, Ji C, Han G, Wang Y, et al. Discovery of novel simplified isoxazole derivatives of sampangine as potent anti-cryptococcal agents. Bioorganic Med Chem. 2019;27(5):832-40. [DOI] [PubMed]; Li Z, Liu N, Tu J, Ji C, Han G, Wang Y, et al. Discovery of novel simplified isoxazole derivatives of sampangine as potent anti-cryptococcal agents. Bioorganic Med Chem. 2019;27(5):832–840. doi: 10.1016/j.bmc.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 111.111. Krummenauer ME, Lopes W, Garcia AWA, Schrank A, Gnoatto SCB, Kawano DF, et al. A highly active triterpene derivative capable | of biofilm damage to control cryptococcus spp. Biomolecules. 2019;9(12):1-13. [DOI] [PMC free article] [PubMed]; Krummenauer ME, Lopes W, Garcia AWA, Schrank A, Gnoatto SCB, Kawano DF, et al. A highly active triterpene derivative capable | of biofilm damage to control cryptococcus spp. Biomolecules. 2019;9(12):1–13. doi: 10.3390/biom9120831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.112. Malefo MS, Ramadwa TE, Famuyide IM, McGaw LJ, Eloff JN, Sonopo MS, et al. Synthesis and antifungal activity of chromones and benzoxepines from the leaves of Ptaeroxylon obliquum. J Nat Prod . 2020;83(8):2508-17. [DOI] [PubMed]; Malefo MS, Ramadwa TE, Famuyide IM, McGaw LJ, Eloff JN, Sonopo MS, et al. Synthesis and antifungal activity of chromones and benzoxepines from the leaves of Ptaeroxylon obliquum. J Nat Prod. 2020;83(8):2508–2517. doi: 10.1021/acs.jnatprod.0c00587. [DOI] [PubMed] [Google Scholar]
- 113.113. Masila VM, Ndakala AJ, Byamukama R, Midiwo JO, Kamau RW, Wang M, et al. Synthesis, structural assignments and antiinfective activities of 3-O-benzyl-carvotacetone and 3-hydroxy-2-isopropyl-5-methyl-p-benzoquinone. Nat Prod Res . 2021;35(21):3599-607. [DOI] [PubMed]; Masila VM, Ndakala AJ, Byamukama R, Midiwo JO, Kamau RW, Wang M, et al. Synthesis, structural assignments and antiinfective activities of 3-O-benzyl-carvotacetone and 3-hydroxy-2-isopropyl-5-methyl-p-benzoquinone. Nat Prod Res. 2021;35(21):3599–3607. doi: 10.1080/14786419.2020.1716346. [DOI] [PubMed] [Google Scholar]
- 114.114. Oliveira L, Ferrarini M, dos Santos AP, Varela MT, Corrêa ITS, Tempone AG, et al. Coumaric acid analogues inhibit growth and melanin biosynthesis in Cryptococcus neoformans and potentialize amphotericin B antifungal activity. Eur J Pharm Sci. 2020;153:105473. [DOI] [PubMed]; Oliveira L, Ferrarini M, dos Santos AP, Varela MT, Corrêa ITS, Tempone AG, et al. Coumaric acid analogues inhibit growth and melanin biosynthesis in Cryptococcus neoformans and potentialize amphotericin B antifungal activity. Eur J Pharm Sci. 2020;153:105473–105473. doi: 10.1016/j.ejps.2020.105473. [DOI] [PubMed] [Google Scholar]
- 115.115. Wermuth CG. Selective optimization of side activities: The SOSA approach. Drug Discov Today. 2006;11(3/4):160-4. [DOI] [PubMed]; Wermuth CG. Selective optimization of side activities: The SOSA approach. Drug Discov Today. 2006;11(3/4):160–164. doi: 10.1016/S1359-6446(05)03686-X. [DOI] [PubMed] [Google Scholar]
- 116.116. Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: Progress, challenges and recommendations. Nat Rev Drug Discov. 2018;18(1):41-58. [DOI] [PubMed]; Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: Progress, challenges and recommendations. Nat Rev Drug Discov. 2018;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 117.117. Hai TP, Van AD, Ngan NTT, Nhat LTH, Lan NPH, Vinh Chau N V, et al. The combination of tamoxifen with amphotericin B, but not with fluconazole, has synergistic activity against the majority of clinical isolates of Cryptococcus neoformans. Mycoses. 2019;62(9):818-25. [DOI] [PMC free article] [PubMed]; Hai TP, Van AD, Ngan NTT, Nhat LTH, Lan NPH, Vinh Chau N V, et al. The combination of tamoxifen with amphotericin B, but not with fluconazole, has synergistic activity against the majority of clinical isolates of Cryptococcus neoformans. Mycoses. 2019;62(9):818–825. doi: 10.1111/myc.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.118. Donlin MJ, Meyers MJ. Repurposing and optimization of drugs for discovery of novel antifungals. Drug Discov Today . 2022;27(7):2008-14. [DOI] [PMC free article] [PubMed]; Donlin MJ, Meyers MJ. Repurposing and optimization of drugs for discovery of novel antifungals. Drug Discov Today. 2022;27(7):2008–2014. doi: 10.1016/j.drudis.2022.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.119. Ogundeji AO, Pohl CH, Sebolai OM. Repurposing of aspirin and ibuprofen as candidate anti-Cryptococcus drugs. Antimicrob Agents Chemother . 2016;60(8):4799-808. [DOI] [PMC free article] [PubMed]; Ogundeji AO, Pohl CH, Sebolai OM. Repurposing of aspirin and ibuprofen as candidate anti-Cryptococcus drugs. Antimicrob Agents Chemother. 2016;60(8):4799–4808. doi: 10.1128/AAC.02810-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.120. Ribeiro N de Q, Costa MC, Magalhães TFF, Carneiro HCS, Oliveira LV, Fontes ACL, et al. Atorvastatin as a promising anticryptococcal agent. Int J Antimicrob Agents. 2017;49(6):695-702. [DOI] [PubMed]; Ribeiro N de Q, Costa MC, Magalhães TFF, Carneiro HCS, Oliveira LV, Fontes ACL, et al. Atorvastatin as a promising anticryptococcal agent. Int J Antimicrob Agents. 2017;49(6):695–702. doi: 10.1016/j.ijantimicag.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 121.121. Cheng Zhen, Hui Lu, Yuang-ying jiang FY. P092 Otilonium bromide is a potente antifungal agent against fluconazole - and flucytosine - resistant Cryptococcus neoformans strains. Medical Mycology. 2022; 60(9):72-92.; Zhen Cheng, Lu Hui, Yuang-ying jiang FY. P092 Otilonium bromide is a potente antifungal agent against fluconazole - and flucytosine - resistant Cryptococcus neoformans strains. Medical Mycology. 2022;60(9):72–92. [Google Scholar]
- 122.122. Brilhante RSN, Silva JAT, Dos Santos Araújo G, Pereira VS, Gotay WJP, De Oliveira JS, et al. Darunavir inhibits Cryptococcus neoformans/Cryptococcus gattii species complex growth and increases the susceptibility of biofilms to antifungal drugs. J Med Microbiol. 2020;69(6):830-7. [DOI] [PubMed]; Brilhante RSN, Silva JAT, Dos Santos Araújo G, Pereira VS, Gotay WJP, De Oliveira JS, et al. Darunavir inhibits Cryptococcus neoformans/Cryptococcus gattii species complex growth and increases the susceptibility of biofilms to antifungal drugs. J Med Microbiol. 2020;69(6):830–837. doi: 10.1099/jmm.0.001194. [DOI] [PubMed] [Google Scholar]
- 123.123. Truong M, Monahan LG, Carter DA, Charles IG. Repurposing drugs to fast-track therapeutic agents for the treatment of cryptococcosis. PeerJ. 2018;2018(5):1-18. [DOI] [PMC free article] [PubMed]; Truong M, Monahan LG, Carter DA, Charles IG. Repurposing drugs to fast-track therapeutic agents for the treatment of cryptococcosis. PeerJ. 2018;2018(5):1–18. doi: 10.7717/peerj.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.124. de Oliveira HC, Joffe LS, Simon KS, Castelli RF, Reis FCG, Bryan AM, et al. Fenbendazole controls in vitro growth, virulence potential, and animal infection in the Cryptococcus model. Antimicrob Agents Chemother . 2020;64(6):e00286-20. [DOI] [PMC free article] [PubMed]; de Oliveira HC, Joffe LS, Simon KS, Castelli RF, Reis FCG, Bryan AM, et al. Fenbendazole controls in vitro growth, virulence potential, and animal infection in the Cryptococcus model. Antimicrob Agents Chemother. 2020;64(6):e00286-20. doi: 10.1128/AAC.00286-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.125. Joffe LS, Schneider R, Lopes W, Azevedo R, Staats CC, Kmetzsch L, et al. The anti-helminthic compound mebendazole has multiple antifungal effects against Cryptococcus neoformans. Front Microbiol. 2017;8:535. [DOI] [PMC free article] [PubMed]; Joffe LS, Schneider R, Lopes W, Azevedo R, Staats CC, Kmetzsch L, et al. The anti-helminthic compound mebendazole has multiple antifungal effects against Cryptococcus neoformans. Front Microbiol. 2017;8:535–535. doi: 10.3389/fmicb.2017.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.126. De Castro Spadari C, Vila T, Rozental S, Ishida K. Miltefosine has a postantifungal effect and induces apoptosis in cryptococcus yeasts. Antimicrob Agents Chemother . 2018;62(8):e00312-18. [DOI] [PMC free article] [PubMed]; De Castro Spadari C, Vila T, Rozental S, Ishida K. Miltefosine has a postantifungal effect and induces apoptosis in cryptococcus yeasts. Antimicrob Agents Chemother. 2018;62(8):e00312-18. doi: 10.1128/AAC.00312-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.127. Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother . 2012;56(7):3758-66. [DOI] [PMC free article] [PubMed]; Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother. 2012;56(7):3758–3766. doi: 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.128. Breuer MR, Dasgupta A, Vasselli JG, Lin X, Shaw BD, Sachs MS. The antidepressant sertraline induces the formation of supersized lipid droplets in the human pathogen Cryptococcus neoformans. J Fungi. 2022;8(6):642. [DOI] [PMC free article] [PubMed]; Breuer MR, Dasgupta A, Vasselli JG, Lin X, Shaw BD, Sachs MS. The antidepressant sertraline induces the formation of supersized lipid droplets in the human pathogen Cryptococcus neoformans. J Fungi. 2022;8(6):642–642. doi: 10.3390/jof8060642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.129. Dolan K, Montgomery S, Buchheit B, DiDone L, Wellington M, Krysan DJ. Antifungal activity of tamoxifen: In vitro and in vivo activities and mechanistic characterization. Antimicrob Agents Chemother . 2009;53(8):3337-46. [DOI] [PMC free article] [PubMed]; Dolan K, Montgomery S, Buchheit B, DiDone L, Wellington M, Krysan DJ. Antifungal activity of tamoxifen: In vitro and in vivo activities and mechanistic characterization. Antimicrob Agents Chemother. 2009;53(8):3337–3346. doi: 10.1128/AAC.01564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.130. Butts A, Krysan DJ. Antifungal Drug Discovery: something old and something new. PLoS Pathog . 2012;8(9):9-11. [DOI] [PMC free article] [PubMed]; Butts A, Krysan DJ. Antifungal Drug Discovery: something old and something new. PLoS Pathog. 2012;8(9):9–11. doi: 10.1371/journal.ppat.1002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.131. Jung EH, Meyers DJ, Bosch J, Casadevall A. Novel antifungal compounds discovered in medicines for malaria venture’s malaria box. Msphere. 2018;3(2):1-12. [DOI] [PMC free article] [PubMed]; Jung EH, Meyers DJ, Bosch J, Casadevall A. Novel antifungal compounds discovered in medicines for malaria venture’s malaria box. Msphere. 2018;3(2):1–12. doi: 10.1128/mSphere.00537-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.132. Bonapace CR, Bosso JA, Friedrich L V, White RL. Comparison of methods of interpretation of checkerboard synergy testing. Diagn Microbiol Infect Dis. 2002;44(4):363-6. [DOI] [PubMed]; Bonapace CR, Bosso JA, Friedrich L V, White RL. Comparison of methods of interpretation of checkerboard synergy testing. Diagn Microbiol Infect Dis. 2002;44(4):363–366. doi: 10.1016/s0732-8893(02)00473-x. [DOI] [PubMed] [Google Scholar]
- 133.133. Livengood SJ, Drew RH, Perfect JR. Combination therapy for invasive fungal infections. Curr Fungal Infect Rep. 2020;14(1):40-9.; Livengood SJ, Drew RH, Perfect JR. Combination therapy for invasive fungal infections. Curr Fungal Infect Rep. 2020;14(1):40–49. [Google Scholar]
- 134.134. da Silva DL, Magalhães TFF, dos Santos JRA, de Paula TP, Modolo L V, de Fátima A, et al. Curcumin enhances the activity of fluconazole against Cryptococcus gattii-induced cryptococcosis infection in mice. J Appl Microbiol. 2016;120(1):41-8. [DOI] [PubMed]; da Silva DL, Magalhães TFF, dos Santos JRA, de Paula TP, Modolo L V, de Fátima A, et al. Curcumin enhances the activity of fluconazole against Cryptococcus gattii-induced cryptococcosis infection in mice. J Appl Microbiol. 2016;120(1):41–48. doi: 10.1111/jam.12966. [DOI] [PubMed] [Google Scholar]
- 135.135. Wambaugh MA, Denham ST, Ayala M, Brammer B, Stonhill MA, Brown JCS. Synergistic and antagonistic drug interactions in the treatment of systemic fungal infections. Elife. 2020;9:e54160. [DOI] [PMC free article] [PubMed]; Wambaugh MA, Denham ST, Ayala M, Brammer B, Stonhill MA, Brown JCS. Synergistic and antagonistic drug interactions in the treatment of systemic fungal infections. Elife. 2020;9:e54160. doi: 10.7554/eLife.54160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.136. Menezes RT, Pereira TC, Junqueira JC, Oliveira LD, Scorzoni L. Synergistic combination of duloxetine hydrochloride and fluconazole reduces the cell growth and capsule size of Cryptococcus neoformans. An Acad Bras Cienc. 2022;94(2):1-7. [DOI] [PubMed]; Menezes RT, Pereira TC, Junqueira JC, Oliveira LD, Scorzoni L. Synergistic combination of duloxetine hydrochloride and fluconazole reduces the cell growth and capsule size of Cryptococcus neoformans. An Acad Bras Cienc. 2022;94(2):1–7. doi: 10.1590/0001-3765202220211021. [DOI] [PubMed] [Google Scholar]
- 137.137. Pereira TC, De Menezes RT, De Oliveira HC, De Oliveira LD, Scorzoni L. In vitro synergistic effects of fluoxetine and paroxetine in combination with amphotericin B against Cryptococcus neoformans. Pathog Dis. 2021;79(2):1-9. [DOI] [PubMed]; Pereira TC, De Menezes RT, De Oliveira HC, De Oliveira LD, Scorzoni L. In vitro synergistic effects of fluoxetine and paroxetine in combination with amphotericin B against Cryptococcus neoformans. Pathog Dis. 2021;79(2):1–9. doi: 10.1093/femspd/ftab001. [DOI] [PubMed] [Google Scholar]
- 138.138. Fernandes KE, Weeks K, Carter DA. Lactoferrin is broadly active against yeasts and highly synergistic with amphotericin B. Antimicrob Agents Chemother . 2020;64(5):1-22. [DOI] [PMC free article] [PubMed]; Fernandes KE, Weeks K, Carter DA. Lactoferrin is broadly active against yeasts and highly synergistic with amphotericin B. Antimicrob Agents Chemother. 2020;64(5):1–22. doi: 10.1128/AAC.02284-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.139. Ferreira Magalhães TF, Costa MC, Holanda RA, Ferreira GF, Dutra Carvalho VS, Cota Freitas GJ, et al. N-acetylcysteine reduces amphotericin B deoxycholate nephrotoxicity and improves the outcome of murine cryptococcosis. Med Mycol. 2021;58(6):835-44. [DOI] [PubMed]; Ferreira Magalhães TF, Costa MC, Holanda RA, Ferreira GF, Dutra Carvalho VS, Cota Freitas GJ, et al. N-acetylcysteine reduces amphotericin B deoxycholate nephrotoxicity and improves the outcome of murine cryptococcosis. Med Mycol. 2021;58(6):835–844. doi: 10.1093/mmy/myz129. [DOI] [PubMed] [Google Scholar]
- 140.140. Silva THS, Araújo CV, Santos KM da C, Alves NDS, Gomes THS, E Silva AKF, et al. Synergic effect of simvastatin in combination with amphotericin b against environmental strains of cryptococcus neoformans from northeastern brazil: A prospective experimental study. Sao Paulo Med. J 2020;138(1):40-6. [DOI] [PMC free article] [PubMed]; Silva THS, Araújo CV, Santos KM da C, Alves NDS, Gomes THS, E Silva AKF, et al. Synergic effect of simvastatin in combination with amphotericin b against environmental strains of cryptococcus neoformans from northeastern brazil: A prospective experimental study. Sao Paulo Med. J. 2020;138(1):40–46. doi: 10.1590/1516-3180.2019.0107.R2.16092019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.141. Movahed E, Yi Tan GM, Munusamy K, Yeow TC, Tay ST, Wong WF, et al. Triclosan demonstrates synergic effect with amphotericin B and fluconazole and induces apoptosis-like cell death in Cryptococcus neoformans. Front Microbiol. 2016;7:360. [DOI] [PMC free article] [PubMed]; Movahed E, Yi Tan GM, Munusamy K, Yeow TC, Tay ST, Wong WF, et al. Triclosan demonstrates synergic effect with amphotericin B and fluconazole and induces apoptosis-like cell death in Cryptococcus neoformans. Front Microbiol. 2016;7:360–360. doi: 10.3389/fmicb.2016.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.142. Sangalli-Leite F, Scorzoni L, Alves de Paula e Silva AC, da Silva J de F, de Oliveira HC, de Lacorte Singulani J, et al. Synergistic effect of pedalitin and amphotericin B against Cryptococcus neoformans by in vitro and in vivo evaluation. Int J Antimicrob Agents . 2016;48(5):504-11. [DOI] [PubMed]; Sangalli-Leite F, Scorzoni L, Paula Alves de, Silva AC, da Silva J de F, de Oliveira HC, de Lacorte Singulani J, et al. Synergistic effect of pedalitin and amphotericin B against Cryptococcus neoformans by in vitro and in vivo evaluation. Int J Antimicrob Agents. 2016;48(5):504–511. doi: 10.1016/j.ijantimicag.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 143.143. Horn C, Vediyappan G. Anticapsular and antifungal activity of α-cyperone. Antibiotics. 2021;10(1):1-10. [DOI] [PMC free article] [PubMed]; Horn C, Vediyappan G. Anticapsular and antifungal activity of α-cyperone. Antibiotics. 2021;10(1):1–10. doi: 10.3390/antibiotics10010051. [DOI] [PMC free article] [PubMed] [Google Scholar]


