Abstract
Background
Chronic disease management (CDM) through sustained knowledge translation (KT) interventions ensures long-term, high-quality care. We assessed implementation of KT interventions for supporting CDM and their efficacy when sustained in older adults.
Methods
Design: Systematic review with meta-analysis engaging 17 knowledge users using integrated KT.
Eligibility criteria: Randomized controlled trials (RCTs) including adults (> 65 years old) with chronic disease(s), their caregivers, health and/or policy-decision makers receiving a KT intervention to carry out a CDM intervention for at least 12 months (versus other KT interventions or usual care).
Information sources: We searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials from each database’s inception to March 2020.
Outcome measures: Sustainability, fidelity, adherence of KT interventions for CDM practice, quality of life (QOL) and quality of care (QOC).
Data extraction, risk of bias (ROB) assessment: We screened, abstracted and appraised articles (Effective Practice and Organisation of Care ROB tool) independently and in duplicate. Data synthesis: We performed both random-effects and fixed-effect meta-analyses and estimated mean differences (MDs) for continuous and odds ratios (ORs) for dichotomous data.
Results
We included 158 RCTs (973,074 participants [961,745 patients, 5540 caregivers, 5789 providers]) and 39 companion reports comprising 329 KT interventions, involving patients (43.2%), healthcare providers (20.7%) or both (10.9%). We identified 16 studies described as assessing sustainability in 8.1% interventions, 67 studies as assessing adherence in 35.6% interventions and 20 studies as assessing fidelity in 8.7% of the interventions. Most meta-analyses suggested that KT interventions improved QOL, but imprecisely (36 item Short-Form mental [SF-36 mental]: MD 1.11, 95% confidence interval [CI] [− 1.25, 3.47], 14 RCTs, 5876 participants, I2 = 96%; European QOL-5 dimensions: MD 0.01, 95% CI [− 0.01, 0.02], 15 RCTs, 6628 participants, I2 = 25%; St George’s Respiratory Questionnaire: MD − 2.12, 95% CI [− 3.72, − 0.51] 44 12 RCTs, 2893 participants, I2 = 44%). KT interventions improved QOC (OR 1.55, 95% CI [1.29, 1.85], 12 RCTS, 5271 participants, I2 = 21%).
Conclusions
KT intervention sustainability was infrequently defined and assessed. Sustained KT interventions have the potential to improve QOL and QOC in older adults with CDM. However, their overall efficacy remains uncertain and it varies by effect modifiers, including intervention type, chronic disease number, comorbidities, and participant age.
Systematic review registration
PROSPERO CRD42018084810.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-023-02966-9.
Keywords: Sustainability, Knowledge translation, Chronic disease management, Older adults, Integrated knowledge translation, Patient and public involvement
Summary box
What is already known on this topic
Sustainability of knowledge translation (KT) interventions supporting implementation of chronic disease management (CDM) in older adults (> 65 years) with chronic diseases is vital to ensure long-term, high-quality patient care.
What this study adds
Few RCTs assessed sustainability, fidelity, and adherence of KT interventions for CDM practice for at least 1 year.
Sparce evidence assessing quality of life and care following sustained KT interventions present KT knowledge gaps and analytical challenges.
More studies providing an operational standardized measure of sustained KT interventions are necessary to explore patient outcome heterogeneity and robust conclusions regarding treatments and associated results.
Background
Evidence-based clinical interventions (i.e. early mobilisation in older adults or heart failure medications use) require tailored knowledge translation (KT or implementation) interventions (i.e. patient education or team changes) to optimise use in practice or policy. KT interventions are strategies that facilitate research uptake in practice and policy and include any action or set of actions that target factors that hinder or help someone to use a new practice or evidence-based program [1]. KT interventions are diverse and can focus on patients, caregivers, clinicians, managers and policy makers [2, 3]. Adoption of KT interventions can impact patient care and health system outcomes; however, there is a tendency to return to prior behaviours after initial interventions end [4]. Sustainability of KT interventions is defined as the continued delivery of clinical and KT intervention after its adoption is secured over a period of time (depending on the implementation context), while producing benefits for individuals and systems [5]. Failure to sustain KT interventions can lead to declining patient and health system outcomes and diminish confidence and support for future KT [6, 7].
Adults aged 65 years and older are the largest growing proportion of the global population, and many are affected by chronic diseases [8, 9]. Evidence-based clinical interventions to manage these conditions often include a combination of pharmacological and non-pharmacological interventions. However, to optimise intervention impact, their use needs to be supported at the patient, healthcare provider and health system levels via KT interventions [6]. Sustainability of KT interventions to manage chronic diseases is of paramount importance to ensure long-term, high-quality patient care and optimise health system impact consistently [10–13]. Specifically, optimal chronic disease management (CDM) in older adults requires sustained use of CDM interventions via effective KT interventions [14]. More importantly, it is expected that fostering sustainability will help reduce waste in health by facilitating their effective use. Our previous scoping review on the sustainability of KT interventions to manage CDM in adults included 62 experimental, quasi-experimental and observational studies assessing 13 different types of KT interventions [14]. Evidence showed that 56.1% of the eligible patients received a KT intervention for CDM, and even fewer maintained their use (e.g. 45.4% with diabetes mellitus, 24.7% with atrial fibrillation) over 2 years [15]. Moreover, it remains unclear which KT interventions and their individual components are most effective and sustained to optimise CDM.
The aim of this systematic review and meta-analysis was to describe sustainability of KT to implement a CDM intervention for at least 12 months by engaging 17 knowledge users, including patient partners, throughout using integrated KT. A knowledge user is defined as an individual who is likely to be able to use research results to inform their decisions about health policies, programs and practices (e.g. clinicians, managers, policy makers, patients/families and others) [16, 17]. We aimed to systematically assess the efficacy of sustainability of KT intervention for CDM end-users with comorbid conditions including older patients, their caregivers, health and policy-decision makers on healthcare outcomes (including quality of life [QOL] and quality of care [QOC]) at least 1 year after CDM intervention implementation or the termination of initial funding.
Methods
We registered our protocol with PROSPERO (CRD42018084810) and published it in an open-access journal [18]. Our systematic review follows the PRISMA 2020 [19] and GRIPP-2 [20] reporting guidelines. Our methods are described briefly here (see also Additional file 1: Appendix 1 [3, 14, 18, 21–40]). Any deviations from the protocol are reported in Additional file 1: Appendix 2 [41, 42].
Knowledge user engagement
We enhanced systematic review conduct by employing an integrated KT approach [4] from project onset via established partnerships with 17 knowledge users, including one patient partner (KT), one funder (DAC), one policymaker (AE), 11 international KT researchers (BRH, IDG, JES, JM, JP, LRD, LS, PPG, RCB, WI, TVdW) and four clinicians (BH, FL, HTS, SES). The knowledge users provided input throughout the research process, including formulation of the research question, study protocol, prioritization of outcome measures and interpretation of results based on context relevance [18].
Eligibility criteria, search strategy and selection process
We included randomised controlled trials (RCTs) where the target population for the CDM intervention included patients (at least 65 years old with one or more chronic disease [22]) or their caregiver. End-users of the KT intervention to implement a CDM intervention for at least 12 months included patients aged 65 years and older with at least one chronic disease, their caregivers, clinicians (all disciplines), public health officials, health care managers and policy-makers. RCTs comparing a KT intervention versus other KT interventions or usual care were eligible.
KT interventions were classified using (1) a pre-existing taxonomy developed by the Cochrane Effective Practice and Organisation of Care (EPOC) group and (2) the behaviour change technique (BCT) taxonomy. The primary outcome was sustained implementation of a KT intervention for CDM beyond 1 year after implementation or termination of funding and which KT interventions were used (Additional file 1: Appendix 3 [14]). Secondary outcomes were health-related or disease-specific QOL and process or QOC (Additional file 1: Appendix 4).
We searched the bibliographic databases MEDLINE, EMBASE, and CENTRAL up to March 4, 2020, and developed a grey literature search strategy [21] to seek unpublished studies (Additional file 1: Appendix 5). Reviewers independently and in duplicate screened titles/abstracts in level one and similarly full-text articles in level two. Pairs of reviewers independently abstracted data from each included study. Two pairs of reviewers (ACT, CF, CS, SES) coded each KT intervention within the included studies independently using EPOC and BCT taxonomies [3, 14, 23] (Additional file 1: Appendices 5, 6 and 7 [43–59]).
Within and across study bias assessment
Pairs of reviewers appraised included studies using the EPOC risk of bias (ROB) tool independently [29]. We visually inspected small-study effects and reporting bias using the contour-enhanced funnel plot and Egger’s test when at least ten studies were available [30].
Synthesis
We performed a descriptive analysis for the primary outcome and sustainability of KT interventions and used frequencies and percentages for the a priori defined KT dimensions: sustainability, adherence and fidelity assessment.
We combined study-level data in a meta-analysis using the mean difference (MD) for continuous outcomes (i.e. QOL) and odds ratio (OR) for dichotomous outcomes (i.e. QOC) along with corresponding 95% confidence intervals (95% CI) when at least two studies were available. We performed both random-effects and fixed-effect meta-analysis models using the inverse-variance method. Under the random-effects model, we estimated the overall effect size and its 95% CI using the Hartung–Knapp–Sidik–Jonkman method to handle meta-analyses with few studies [39–41]. In line with recent recommendations, when the estimated heterogeneity was positive (> 0) and at least three studies were included in the meta-analysis, we prioritized the random-effects model, since we expected the studies to be methodologically and clinically different [27, 37]. When the estimated heterogeneity was zero, we prioritized the fixed-effect model since the Hartung–Knapp–Sidik–Jonkman method is considered inadequate [26, 33, 39–41, 60]. When two studies were included and the estimated heterogeneity was positive (> 0), we presented both fixed and random effects findings [37]. We calculated prediction intervals (PIs) for the overall effect under the random-effects model to capture the interval within which we expected the true intervention effect of a new study to fall. We used the restricted maximum likelihood method [29] to estimate the between-study variance τ2 and the Q-profile approach to calculate its 95% CI [32, 36]. We explored potential heterogeneity using predefined meta-regression, subgroup or sensitivity analyses.
Results
Study selection
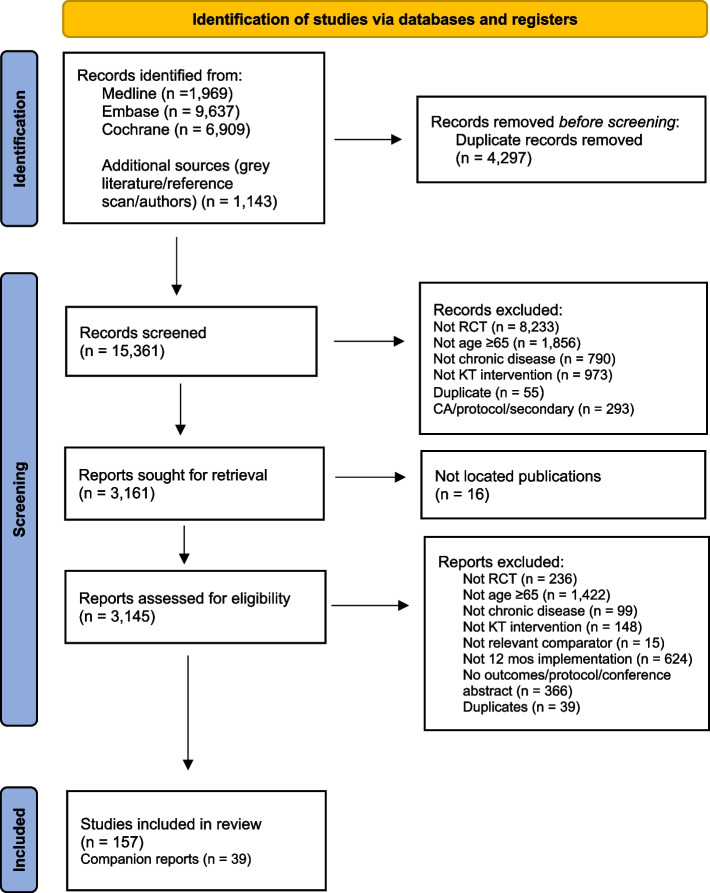
Overall, 157 RCTs (973,074 participants overall [961,745 patients, 5540 caregivers and 5789 providers]) and 39 companion reports were included, after screening 15,361 citations and 3145 full-text articles (Fig. 1). Of the included studies, one was written in non-English language, that was in Chinese [61]. The 157 RCTs included 110 RCTs identified from literature search, 27 RCTs from reference scanning, 19 from other reference scanning in related reviews, protocols, and conference abstracts, and one study from contacted authors (Additional file 1: Appendix 8). Of the 157 RCTs, 51 were cluster-RCTs. A total of 66 of the 197 contacted authors responded to our emails, and 36 provided additional data for analysis.
Fig. 1.
PRISMA flow diagram for identification of eligible studies. Abbreviations: CA conference abstract, KT knowledge translation, mos months, RCT randomised controlled trial. From [19]
Study, patient and intervention characteristics
The included RCTs were published between 1995 and 2020, with most published between 2011 and 2015 (36.9%; Table 1). The studies were largely conducted at multiple sites (82.2%), mainly in Europe (43.9%) and North America (39.5%). The most frequent settings included clinics (38.9%) or home (37.6%), and RCT overall average duration was 36 months (range 12 to 120 months). Most studies were funded through publicly funded or government grants (53.5%), followed by mixed funding (22.9%; Additional file 1: Appendix 9 [43–47, 49, 50, 52–59, 61–200]).
Table 1.
Summary of study characteristics
| Study characteristic | No. (%) of RCTs (N = 157) |
|---|---|
| Year of publication | |
| 1995–2000 | 10 (6.4) |
| 2001–2005 | 25 (15.9) |
| 2006–2010 | 33 (21.0) |
| 2011–2015 | 58 (36.9) |
| 2016–2020 | 31 (19.7) |
| Continent | |
| Europea | 69 (43.9) |
| Australia/New Zealand | 15 (9.6) |
| North America | 62 (39.5) |
| Asia | 9 (5.7) |
| South America | 1 (0.6) |
| Multi-continent | 1 (0.6) |
| Site | |
| Multi-center | 129 (82.2) |
| Single center | 24 (15.3) |
| Not reported | 4 (2.5) |
| Number of arms | |
| Two arms | 145 (92.4) |
| Three arms | 11 (7) |
| Four arms | 1 (0.6) |
| Settingsb | |
| Clinic | 61 (38.9) |
| Home | 59 (37.6) |
| Hospital | 5 (3.2) |
| Community | 5 (3.2) |
| Long-term care | 1 (0.6) |
| Not reportedc | 70 (44.6) |
| Not applicabled | 37 (23.6) |
| Sample size (no. of patients) | |
| 40–200 | 50 (31.8) |
| 201–500 | 49 (31.2) |
| 501–1000 | 16 (10.2) |
| 1001–2000 | 21 (13.4) |
| > 2000 | 19 (12.1) |
| Not involving patientse | 2 (1.3) |
| Sample size (no. of caregivers) | |
| 60–100 | 6 (3.8) |
| 101–300 | 9 (5.7) |
| > 300 | 5 (3.2) |
| Not involving caregivers | 137 (87.3) |
| Sample size (no. of providers) | |
| 9–100 | 17 (10.8) |
| 101–200 | 8 (5.1) |
| 201–900 | 8 (5.1) |
| Not involving providers | 124 (79) |
| Study duration with follow up | |
| 12–19 months | 14 (8.9) |
| 20–29 months | 23 (14.6) |
| 30–39 months | 43 (27.4) |
| 40–49 months | 25 (15.9) |
| > 50 months | 13 (8.3) |
| Not reported | 39 (24.8) |
| Funding | |
| Government | 84 (53.5) |
| Mixedf | 36 (22.9) |
| Commercial | 8 (5.1) |
| Research organization | 8 (5.1) |
| Voluntary organization | 5 (3.2) |
| Charitable trust | 4 (2.5) |
| Not reported | 12 (7.6) |
aThis includes multi-country studies that were all within Europe
bSettings refer to the place of delivery of KT interventions. The number of RCTs exceeds 283 and percent total more than 100% because many studies involved multiple settings. A clinic covers primary or specialty care, hospital covers acute care and community-based health care covers range of primary prevention (including public health) and primary care services within the community (e.g. health promotion and disease prevention, diagnosis/treatment/management of chronic and episodic diseases, rehabilitation support)
cNo reported setting for one or all study arms
dNot applicable refers to studies that have healthcare provider or caregiver KT intervention without a clinical component
eTwo studies involving healthcare providers only
fMixed refers to funding from both industry and governmental organizations/non-governmental organization/research/voluntary
The mean patient age in our review and within the included studies ranged between 65 and 85 years, with cardiovascular diseases being the most frequently reported chronic disease (40.1%; Table 2, Additional file 1: Appendix 10 [19, 43–50, 52–59, 61–170, 172–201]).
Table 2.
Summary of patient characteristics
| Patient characteristic | No. (%) of randomized clinical trials (N = 157) |
|---|---|
| Age, mean, years | |
| 65.0–67.9 | 38 (24.2) |
| 68.0–74.9 | 69 (43.9) |
| 75.0–85.0 | 36 (22.9) |
| Age, median, years | |
| 65.4–83.0 | 7 (4.5) |
| Age, range, years | |
| 60.0–94.0a | 1 (0.6) |
| 65.0–79.0 | 1 (0.6) |
| Not reported | 5 (3.2) |
| % Women | |
| 0–49.9 | 47 (29.9) |
| 50–100 | 49 (31.2) |
| Not reported | 61 (38.9) |
| Chronic diseasesb | |
| Cardiovascular | 63 (40.1) |
| Respiratory | 23 (14.6) |
| Multimorbidity | 21 (13.4) |
| Neurological | 20 (12.7) |
| Diabetes | 15 (9.6) |
| Mental illness | 6 (3.8) |
| Musculoskeletal disorders | 4 (2.5) |
| Hypertension | 3 (1.9) |
| Frailty | 1 (0.6) |
| Chronic kidney disease | 1 (0.6) |
| Co-morbidities reportedc | |
| Yes | 93 (59.2) |
| No | 52 (33.1) |
| Unclear/not reported | 12 (7.6) |
| Comorbidity score reported | |
| No | 111 (70.7) |
| Yes | 46 (29.3) |
aStudy reports on stratified age groups (60–74, 75 years). We included only the 75 age data in this paper
bThe primary condition that is being treated/managed in the trial (e.g. hypertension)
cComorbidities are additional diseases already existing or which occurred during the study that the individuals have along with a primary chronic disease[202, 203]
Overall, 327 KT interventions were identified across all study arms (Additional file 1: Appendix 11), which focused primarily on patients only (42.8%), healthcare providers only (20.8%) and both patients and healthcare providers (11%; Table 3). Most KT interventions were single interventions (42.5%) and were not tailored to end-user type (Table 3). The KT intervention delivery method was not reported in many studies (39.1%), but when reported was frequently in-person (26.3%). Across the 157 RCTs, instruction on performing a behaviour and education targeting patients/caregivers were the most frequently reported BCT and EPOC components (Table 4).
Table 3.
Summary of KT intervention characteristics across study arms
| KT intervention characteristics | No. (%) per study arms in included RCTs (N = 327) |
|---|---|
| Group target | |
| Patients | 140 (42.8) |
| Healthcare providers | 68 (20.8) |
| Patients and healthcare providers | 36 (11) |
| Caregivers | 16 (4.9) |
| Patients and caregivers | 10 (3.1) |
| Patients, caregivers and healthcare providers | 4 (1.2) |
| Caregivers and healthcare providers | 1 (0.3) |
| Not targeted population reported | 52 (15.9) |
| KT intervention complexity | |
| Single | 139 (42.5) |
| Multifactoriala | 60 (18.3) |
| Multiplea | 52 (15.9) |
| Not applicablec | 42 (12.8) |
| Not reported | 34 (10.4) |
| KT intervention delivery | |
| In-person | 86 (26.3) |
| Indirectb | 23 (7) |
| In-person and over telephone | 21 (6.4) |
| In-person or telephone | 9 (2.8) |
| In-person and telemonitoring | 2 (0.6) |
| Telephone | 2 (0.6) |
| Not applicablec | 56 (17.1) |
| Not reported | 128 (39.1) |
| KT intervention duration | |
| 12–14.9 months | 119 (36.4) |
| 15–20.9 months | 26 (8) |
| 21–36 months | 36 (11) |
| Not applicablec | 111 (33.9) |
| Not reported | 35 (10.7) |
| Provider of KT intervention | |
| Physician and/or nurse alone | 110 (33.6) |
| Physician/nurse + clinical staff | 41 (12.5) |
| Clinical staff | 18 (5.5) |
| Non-clinical staff | 8 (2.4) |
| Physician/nurse + non-clinical staff | 1 (0.3) |
| Not applicabled | 99 (30.2) |
| Not reported | 50 (15.3) |
| Tailoring of KT intervention | |
| Not tailored intervention | 190 (58.1) |
| Tailored intervention | 60 (18.3) |
| Not applicablee | 78 (23.9) |
a‘Multiple’ refers to multi-component interventions, where every patient received the same, fixed set of intervention components, whereas and ‘multifactorial’ refers to different sets of intervention components that the patients received, which were tailored to their clinical profile
bIndirect delivery refers to interventions not delivered face-to-face, such as home exercise, medication and self-management
cNot applicable refers to arms that are control group, not receiving a KT intervention
dNot applicable refers to providers delivering KT interventions, which may include treatment arms targeting patients without a clinical component (e.g. receiving educational material, self-management), arms targeting healthcare workers, or arms targeting caregivers. Some interventions without a provider are tailored (e.g. self-management, medication) to while others are not tailored (e.g. web-based education) to a patient’s needs
eNot applicable refers to all arms with healthcare and caregiver population, or all arms targeting patients but without a clinical component (i.e. only have a KT intervention, such as education material)
Table 4.
Summary of KT intervention behaviour change characteristics across studies
| KT intervention characteristics | No. (%) of randomized clinical trials (N = 157) |
|---|---|
| BCTa component (all not tailored) as part of KT intervention (intervention target) | |
| Instruction on how to perform a behaviour (patient) | 80 (50.9) |
| Restructuring the social environment (patient) | 67 (42.7) |
| Instruction on how to perform a behaviour (healthcare provider) | 51 (32.5) |
| Goal setting (outcomes) (patient) | 31 (19.7) |
| Adding objects to the environment (patient) | 24 (15.3) |
| Prompts/cues (healthcare provider) | 22 (14) |
| Self-monitoring of outcome(s) of behaviour (patient) | 22 (14) |
| Problem solving (patient) | 22 (14) |
| Umbrella term—Patient education (patient) | 20 (12.7) |
| Restructuring the social environment (healthcare provider) | 20 (12.7) |
| Goal setting (behaviour) (patient) | 20 (12.7) |
| Prompts/cues (patient) | 19 (12.1) |
| Credible source (healthcare provider) | 16 (10.2) |
| EPOCb component as part of KT intervention (intervention target) | |
| Patient educationc (patients/caregivers) | 110 (70.1) |
| Promotion of self-management (patients/caregivers) | 94 (59.9) |
| Case management (patients/caregivers) | 88 (56.1) |
| Staff education (healthcare providers)c | 74 (47.1) |
| Team changes (healthcare providers) | 51 (32.5) |
| Facilitated relay of information (healthcare providers) | 37 (23.6) |
| Patient reminders (patients/caregivers) | 22 (14.0) |
| Audit and feedback (healthcare providers) | 20 (12.7) |
| Electronic patient registry (healthcare providers) | 20 (12.7) |
| Motivational interview (patients/caregivers) | 20 (12.7) |
aBehaviour change techniques (BCT) taxonomy-based coding
bEffective Practice and Organization of Care (EPOC) taxonomy-based coding
cStaff education and patient education was used as part of control/usual care arms in 24 and 23 studies, respectively
Within-study risk of bias and across-study reporting bias
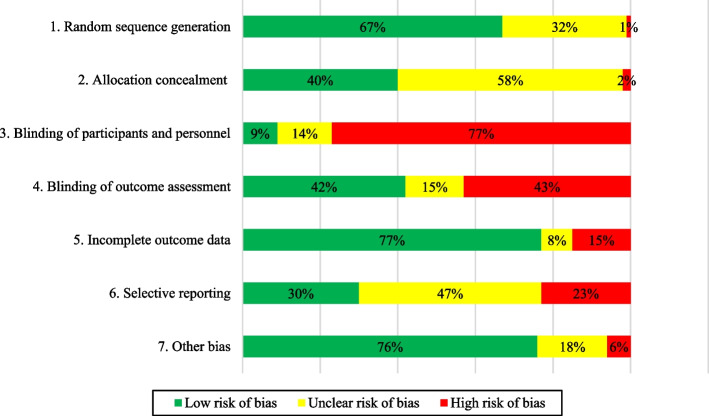
Within-study bias appraisal suggested that low ROB was present for 105 (67%) RCTs for random sequence generation, 63 (40%) RCTs for allocation concealment, 121 (77%) RCTs with incomplete outcome data and 119 (76%) RCTs with ‘other’ bias. Participant and personnel blinding and outcome assessment were judged at high ROB in 121 (77%) and 68 (43%) RCTs, respectively. Selective reporting was of unclear ROB in 74 (47%) RCTs (Additional file 1: Appendix 12 [43–50, 52–59, 61–65, 67–108, 110–201]).
Reporting bias assessment across studies using Egger’s test for each outcome and measurement scale separately suggested no evidence of publication bias or small-study effects (Additional file 1: Appendix 13).
Results of syntheses
Sustainability, fidelity, adherence of KT interventions for CDM practice
Overall, 157 RCTs reported on the primary outcome of sustained implementation of KT intervention for CDM practice. Of these, studies used different terms for sustainability dimensions: 14 studies were described by the authors as assessing sustainability in 25 (8.1%) interventions, 67 studies were described as assessing adherence in 115 (35.6%) interventions and 19 studies were described as assessing fidelity in 27 (8.7%) of the total 327 interventions (Figs. 2, 3 and 4, Additional file 1: Appendix 11). Of the 14 studies, five studies described adherence. Of 67 studies, five were also described by authors as assessing sustainability and 12 described as assessing fidelity. No study reported on all three dimensions. The 36.4% of the 327 KT interventions, representing most of the identified KT interventions, had a duration up to 15 months (Table 3).
Fig. 2.
Cochrane Effective Practice and Organisation of Care (EPOC) risk of bias summary results (n = 157 RCTs)
Fig. 3.
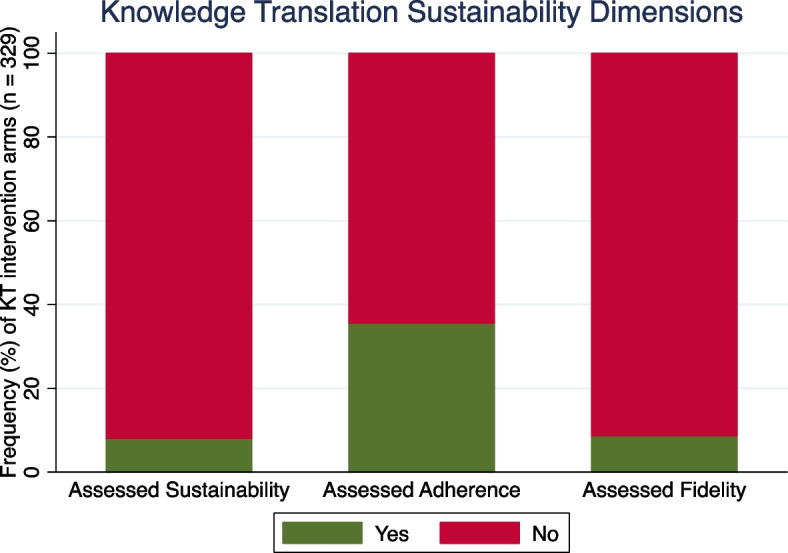
Stacked bar plot of knowledge translation (KT) sustainability dimensions
Fig. 4.
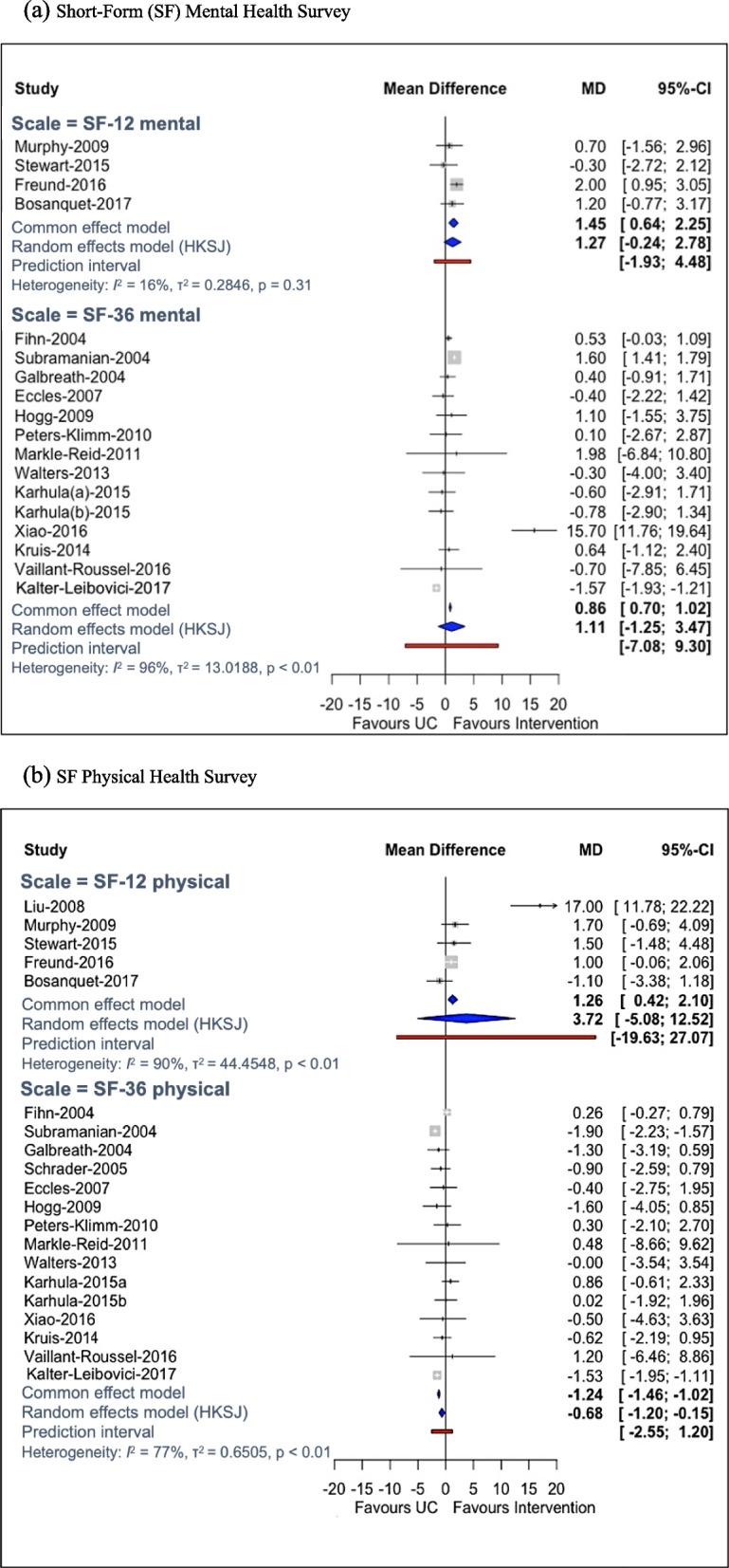
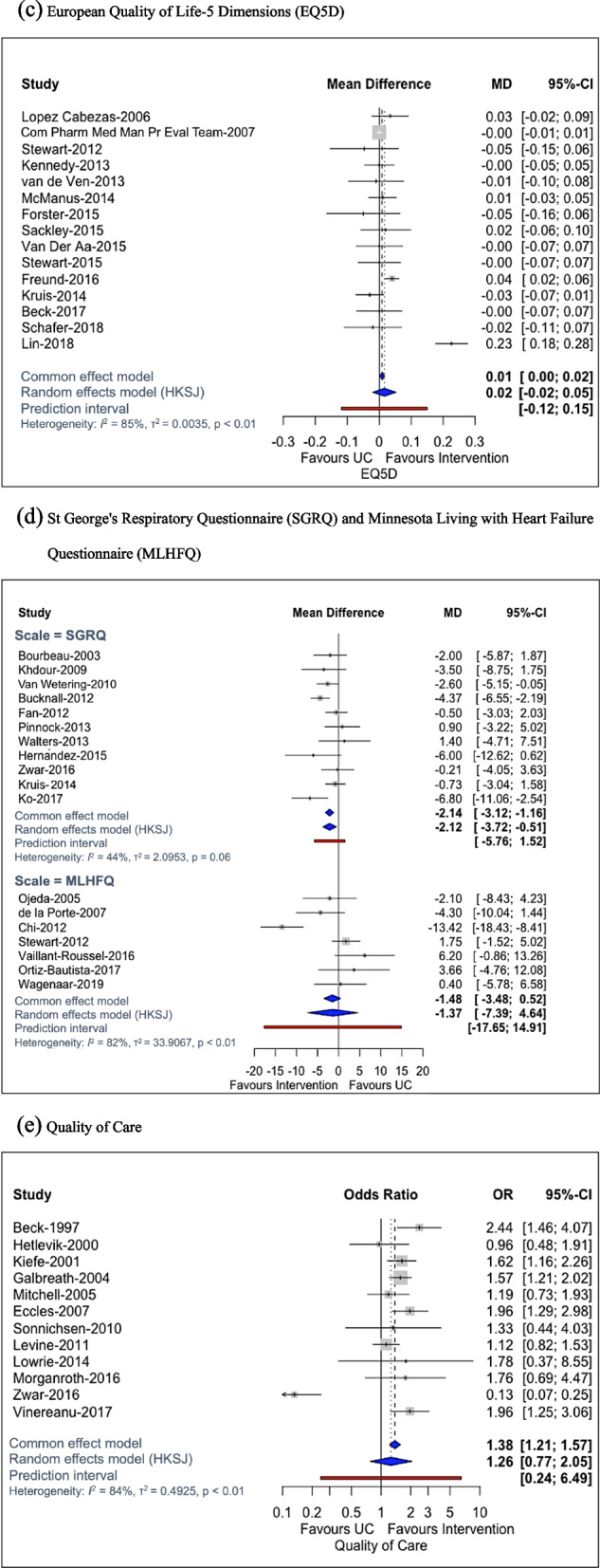
Forest plots for quality of life (a, b, c and d) and care outcomes (e). a Short-Form (SF) Mental Health Survey. b SF Physical Health Survey. c European Quality of Life-5 Dimensions (EQ-5D). d St George’s Respiratory Questionnaire (SGRQ) and Minnesota Living with Heart Failure Questionnaire (MLHFQ). e Quality of care. CI confidence interval, EQ5D European Quality of Life-5 Dimensions, HK Hartung–Knapp–Sidik–Jonkman method, MD mean difference, MLHFQ Minnesota Living with Heart Failure Questionnaire, OR odds ratio, SF Short-Form, SGRQ St George’s Respiratory Questionnaire, UC usual care
Healthcare outcomes with meta-analysis—quality of life (QOL)
QOL was described in 50 studies reporting seven different measurement scales and 49 different interventions, including usual care. Below we present the results of each scale informed by at least 10 studies separately, whereas in Additional file 1: Appendix 14 [103, 137] we show the results with < 10 studies. The individual study results are reported in Additional file 1: Appendix 15 [44, 46, 49, 50, 62, 63, 65, 66, 72, 73, 77, 78, 83, 88, 94, 97, 100, 102–104, 111–113, 123, 124, 126, 128, 129, 134, 136–139, 143, 147, 151, 153, 155–157, 159, 161–163, 170, 176, 177, 180–183, 187–192, 194, 198–200].
Short-form (SF) mental health survey
The 36 item Short-Form mental (SF-36 mental) showed that KT interventions improved QOL compared to usual care, but with imprecise effect estimate and high between-study heterogeneity (MD 1.11, 95% CI [− 1.25, 3.47]; 14 RCTs; 16 interventions plus usual care, 5876 participants; I2 = 96%, τ = 3.60; range of longer follow-up across studies 12–24 months; Additional file 1: Appendix 16). Excluding an outlier from SF-36 mental (MD 0.12, 95% CI [− 0.52, 0.75]; I2 = 95%, τ = 0.98), no differences were observed between groups [199].
For concomitant CDM therapies, results suggested that KT interventions did not improve QOL, but were imprecise (MD − 1.08, 95% CI [− 2.29, 0.12], 4 RCTs, I2 = 8%, τ = 0.59; Additional file 1: Appendices 16–17 [202, 203]). Results were also imprecise in sensitivity analyses restricting to studies with low ROB due to attrition and selective reporting and history of prescription use and to studies with up to 80% male participants (Additional file 1: Appendix 18 [46, 88, 136, 137, 155, 181, 199, 200]). Results showed no differences when a different number of chronic diseases or comorbidities were included or for different duration of KT sustainability (12 months vs > 12 months). A home setting was associated with the highest KT intervention effect among all groups, yet with wide uncertainty and heterogeneity (MD 3.44, 95% CI [− 5.26, 12.13], 5 RCTs, I2 = 95%, τ = 6.78). Meta-regression suggested that publication year and mean participant age after excluding an outlier had no important impact on the KT intervention effect (Additional file 1: Appendix 19). Baseline low QOL was an important factor for higher KT intervention efficacy, but results were informed by 10 RCTs and driven by an outlier [199].
Short-Form (SF) physical health survey
The SF-36 physical scale meta-analysis suggested that KT interventions were not associated with improvement in QOL, yet the 95% PI suggested that new evidence may change results (15 RCTs, 5678 participants, 14 interventions plus usual care; MD − 0.68, 95% CI [− 1.20, − 0.15], 95% PI [− 2.55, 1.98]; I2 = 77%, τ = 0.81; range of longer follow-up across studies 12–24 months). Results were in agreement when excluding studies with imputed SDs.
Inconclusive results were provided with sensitivity analyses restricting to studies with concomitant CDM therapies; these results aligned with the overall meta-analysis and according to PIs, restricting to studies with up to 80% male, low ROB due to attrition and selective reporting. History of patient prescription use studies suggested that KT interventions do not improve QOL (MD − 1.41, 95% CI [− 1.81, − 1.02], 8 RCTs, I2 = 0%, τ = 0.29). No differences in results were observed when a different number of comorbidities were included and when the follow-up time changed from 12 months to longer. For studies in the home setting, usual care was better (MD − 1.51, 95% CI [− 1.91, − 1.10], five RCTs, I2 = 0%, τ = 0.00). Similarly, 13 RCTs favoured usual care when a single chronic disease was present (MD − 0.81, 95% CI [− 1.38, − 0.24], I2 = 50%, τ = 0.76). Meta-regression suggested that publication year, QOL baseline, and mean participant age had no significant impact on the magnitude of KT intervention effect (Additional file 1: Appendix 19).
European quality of life-5 dimensions (EQ-5D)
The EQ-5D scale was assessed in 15 studies of 15 interventions plus usual care (6628 participants). KT interventions only marginally improved QOL, but the result was imprecise (MD 0.02, 95% CI [− 0.02, 0.05]; I2 = 85%, τ = 0.06; range of longer follow-up across studies 12–24 months; all studies included male participant proportions < 80%). Results were insignificant (MD 0.01, 95% CI [− 0.01, 0.02]; I2 = 25%, τ = 0.02) with an excluded outlier [61].
Restricting to studies with low ROB due to attrition and selective reporting, history of prescription use and concomitant CDM therapy, results suggested no clear differences between KT interventions and usual care. Similarly, no differences were observed among the different subgroups of time in KT intervention sustainability, study settings, number of chronic diseases and comorbidities. Publication year, QOL baseline and mean participant age did not impact KT intervention effect on QOL (Additional file 1: Appendix 19).
St George's Respiratory Questionnaire (SGRQ)
The SGRQ scale was reported in 12 studies of nine interventions plus usual care. Meta-analysis of 11 studies (2893 participants) comparing any intervention vs usual care showed that KT interventions improved QOL (MD − 2.12, 95% CI [− 3.72, − 0.51]; I2 = 44%, τ = 1.45; range of longer follow-up across studies 12–24 months, single chronic disease across all studies).
Results were in agreement with primary meta-analysis when restricting to studies with up to 80% male participant proportions, a history of prescription use and concomitant CDM therapy. Similar results were observed for sensitivity analysis restricting to studies with low ROB due to attrition and selective reporting, yet KT intervention effects were imprecise (Additional file 1: Appendix 18 [46, 88, 136, 137, 155, 181, 199, 200]). No major differences were observed across subgroups of a different number of comorbidities, time in sustainability of KT interventions and settings. Publication year did not impact the KT intervention effect. The effect increased with mean participant age, suggesting that KT interventions improve QOL more effectively in older people (regression coefficient: MD = − 0.60, 95% CI [− 1.15, − 0.06]; I2 = 14%, τ = 0.74; Additional file 1: Appendix 19).
Quality of care (QOC)
QOC was reported in 14 RCTs comparing 16 interventions plus usual care. Meta-analysis of 12 RCTs (5271 participants) comparing any intervention vs usual care showed that KT interventions improved QOC; nonetheless, this result was surrounded with high uncertainty and between-study heterogeneity (OR 1.26, 95%CI [0.77, 2.05]; I2 = 84%, τ = 0.70; range of longer follow-up across studies 12–18 months). Excluding an outlier [200], results suggested KT interventions improved statistically significantly QOC compared to usual care (OR 1.55, 95% CI [1.29, 1.85]; I2 = 21%, τ = 0.15). The combination of team, case management and patient education interventions was associated with the largest effect compared with usual care, but evidence derived from a single study (OR 2.44, 95% CI [1.46, 4.07]) [65].
Results agreed with primary meta-analysis in studies with low ROB due to selective reporting but were imprecise when restricting to studies with up to 80% male participants, low ROB due to attrition and history of prescription use. No major differences were observed across subgroups of study settings and time in sustainability of KT interventions (Additional file 1: Appendices 16–17 [202, 203]). The number of chronic diseases statistically significantly modified the QOC effect of KT interventions vs usual care (p = 0.04), but a single study [65] informed the group of at least one chronic disease vs the 11 RCTs in the one chronic disease group (one or more chronic diseases: OR 2.44, 95% CI [1.46, 4.07], one RCT; one chronic disease: OR 1.18, 95% CI [0.70, 1.98], 11 RCTs, I2 = 85%, τ = 0.71). QOC improved with KT interventions when four or more comorbidities were included (four comorbidities: OR 1.62, 95% CI (1.16, 2.26), one RCT; five or more comorbidities: OR 1.63, 95% CI (1.12, 2.37), 5 RCTs, I2 = 52%, τ = 0.23). KT interventions improved QOC with concomitant CDM therapies, but results may change when a new study becomes available (OR 1.94, 95% CI (1.45, 2.59), 95% PI (0.29, 12.77), 3 RCTs, I2 = 0%, τ = 0.00). Publication year and QOC baseline did not impact QOC (Additional file 1: Appendix 19).
Discussion
Sustainability of KT interventions is critical to ensuring long-term, high QOL and consistent care for patients. This is very important in older adults with many chronic diseases. Overall, few trials evaluated the dimensions of sustainability of any KT intervention with data points on assessing adherence being the most frequently reported dimension. For trials that reported on QOL, sustained KT interventions were on average helpful. But, evidence was imprecise at improving CDM intervention outcomes. Our results should be interpreted with caution, since individual study effects were small, imprecise and heterogeneous. In most studies, there was a high risk of bias detected for participant and personnel blinding and outcome assessment. Also, KT interventions improved on average QOC, but uncertainty around the point estimate was high. PIs suggested that results were robust with low heterogeneity when an outlier was excluded. Varied KT intervention effect may be explained by the number of chronic diseases and comorbidities that are present. Efficacy tends to improve as the number of chronic diseases and comorbidities accumulate, with concomitant CDM therapies, history of prescription use and in older adults. Also, we expect that people with a greater number and severity of complex conditions may require different doses and combinations of interventions. However, there was insufficient evidence to make definite conclusions or explore the heterogeneity in varying population and intervention characteristics that could modify the intervention effect (e.g. dose or duration).
Our findings showed that long-term, maintained implementation of KT interventions was rarely defined and infrequently assessed, suggesting fundamental gaps in knowledge. This finding aligns with the conceptual analysis done by Proctor and colleagues [6], which described sustainability as one of the most significant KT research gaps. Similar findings were also observed by others given the evolving nature of healthcare. Specifically, there is overlap across sustainability, adaptation and fidelity concepts [204], and sustainability is a dynamic concept with anticipated adaptation of KT interventions [10].
Our 2016 scoping review on the sustainability of KT interventions in CDM provided an overview of all available studies, irrespective of their study design, and described their results narratively. In the scoping review, we identified 62 studies assessing sustainability of 13 KT interventions; most studies focused on patient-level interventions [14]. In the present systematic review and in contrast to the scoping review, we assessed a more focused research question. We examined the impact of sustainable KT interventions on health outcomes, included RCTs, and performed a meta-analysis of the RCT findings. In this systematic review, we found substantial publication growth, and while most interventions were similarly intended for patients, they were not tailored for patient use. Stirman and colleagues identified 125 studies in their systematic review of public health and clinical intervention sustainability; half were quantitative studies and few reported rigorous evaluation methods [7]. The authors noted a limitation that there is insufficient intervention or outcome details to inform what interventions are effective in which contexts [7].
Two frequent KT challenges in the majority of studies included in this review are a lack of a clear definition of sustainability and the scarcity of evidence assessing QOL and QOC in KT interventions. We defined KT sustainability in this study as clinical and KT interventions continuing to be delivered beyond a certain period of time. Ideally, sustainability studies should specify whether the relevant outcomes are sustained, which is difficult to report given the short duration of grant funding. Researchers and implementers should consider other sustainability aspects, including capacity to sustain implementation. Our findings can be used by knowledge users (e.g. patients, clinicians, policy-makers) regarding the sustainability of KT interventions for CDM. Initial implementation strategies may need to be modified over-time to facilitate the intervention’s sustainability, as inducing behavioural changes in patients for extended periods of time may be difficult.
Prolonged implementation of effective clinical CDM interventions through sustainable KT interventions has the potential to optimise QOL and QOC in older adults with chronic diseases. More studies are necessary to assess the efficacy of individual KT interventions and their separate components in a network meta-analysis [18]. Future work could build on our study by addressing this research gap and relevant KT intervention costs. We anticipate that these results will help to explore sustainable KT interventions development for CDM in older adults and outline how to tailor interventions. In particular, our unique review provides a more granular look at KT intervention components and behaviour change strategies.
Strengths of our study include that we followed the Cochrane Handbook methods for systematic reviews [26]. Reviewers worked in pairs and independently for screening, data abstraction and risk of bias appraisal. We reported the results using the PRISMA 2020 statement [19]. To our knowledge, this is the first study assessing the KT intervention efficacy in a systematic review with meta-analysis of RCTs. We used novel approaches to engage knowledge users and integrate their views and values in this research [4]. We used different taxonomies (EPOC and BCT) to code KT interventions, allowing researchers to use our results to build their interventions to optimise future studies [23].
Our study has some limitations to be considered. First, due to the small number of studies, we were unable to compare the efficacy of different KT interventions. High heterogeneity might be due to varied KT interventions combined in a single group. Initially, we aimed to perform a network meta-analysis to compare multiple KT interventions and produce a ranked order of their KT sustainability efficacy; however, the available evidence did not permit this. Based on the network meta-analysis results, we planned to perform an economic analysis of the interventions identified as effective. Moving forward, we plan to update our systematic review and conduct a network meta-analysis to examine the impact of different sustained KT interventions in older adults with comorbid conditions and determine which approaches are most successful and cost-effective. We will explore how different KT intervention types are linked to CDM practice. Second, the scarcity of available data is a limitation in that many KT interventions were informed by only a few studies and patients. This could affect our ability to detect differences in effects due to reduced statistical power. Also, demographic variables that may explain heterogeneity, such as age categories, living with or without a partner, were not available in the original studies. Third, our literature search is about 3 years old and new relevant studies may be available [205]. However, institutional COVID-19 lockdowns, remote work and logistical difficulties in coordinating a geographically dispersed team have resulted in extended time taken to gather, analyze, organize and present this data—excessive financial cost and lost personnel make updating this review non-feasible at present.
Conclusions
Detailed assessment of KT intervention sustainability and understanding which are the most effective intervention components remain important research gaps. The overall efficacy of KT interventions regarding supporting a better QOL and QOC remains uncertain. Our results should be interpreted with caution due to small, imprecise and heterogeneous observed study effects with high risk of bias in participant and personnel blinding and outcome assessment. Also, KT intervention efficacy may vary depending on the intervention type, number of chronic diseases, comorbidities and participant age, among other effect modifiers. For example, the number of chronic diseases and patient comorbidities may account for varying KT intervention effect, with a tendency to observe improved KT intervention efficacy as health issues accumulated. However, it is important to note that the relationship between these factors and KT intervention efficacy is complex and requires careful interpretation. Addressing specific outcome effect modifiers can be exploited by tailoring KT interventions in future studies.
Supplementary Information
Additional file 1: Appendix 1. Systematic Review Methods. Appendix 2. Protocol Deviations Summary Sheet. Appendix 3. Delphi Results. Appendix 4. KT sustainability outcome definitions. Appendix 5. Search Strategy for MEDLINE. Appendix 6. Coding Guide for KT Intervention Components Using EPOC Taxonomy Coding. Appendix 7. Coding Guides for Clinical Intervention Components using BCT coding. Appendix 8. List of Included Studies. Appendix 9. Individual Study Characteristics. Appendix 10. Individual Patient Characteristics. Appendix 11. Sustainability of KT Interventions Summarized Results. Appendix 12. Cochrane Effective Practice and Organisation of Care (EPOC) Risk of Bias Results. Appendix 13. Contour-Enhanced Funnel Plots. Appendix 14. Additional Analysis Results. Appendix 15. Individual Study Results. Appendix 16. Meta-analysis Results of All Interventions vs Usual Care. Appendix 17. Subgroup Analyses of All KT Interventions vs Usual Care. Appendix 18. Sensitivity Analyses of All KT Interventions vs Usual Care. Appendix 19. Meta-regression for Each Outcome/Scale Comparing Any KT Intervention vs Usual Care.
Acknowledgements
We thank Erin Lillie, Chantelle Lachance and Roberta Cardoso for screening some of the citations and full-text articles. We thank Amruta Radhakrishnan and Krystle Amog for assisting with data cleaning. We thank Raman Brar for screening citations and full-text articles, assisting with data abstraction and cleaning, and appraising study quality. We thank Julia Moore for providing feedback on the protocol and participating in the Delphi process, as well as Chantelle C. Lachance, Lee Fairclough, Nicole Beben, and the late Sumit Majumdar for providing feedback at an early stage of the protocol. We also thank Andreea Manea, Bronwyn Barker, Christine Mehling, Mame Awa Lajante, and Negin Pak from the Plain Language Working Group at the Knowledge Translation Program at St. Michael’s Hospital, Unity Health Toronto, for providing edits to our manuscript. Finally, we thank Becky Skidmore for peer reviewing the literature search and Faryal Khan and Brahmleen Kaur for formatting the paper.
Abbreviations
- BCT
Behaviour change technique
- CDM
Chronic disease management
- CI
Confidence interval
- CIHR
Canadian Institutes of Health Research
- EPOC
Cochrane Effective Practice and Organisation of Care
- EQ-5D
European Quality of Life-5 Dimensions
- GP
General practitioner
- KT
Knowledge translation
- MD
Mean difference
- MLHFQ
Minnesota Living with Heart Failure Questionnaire
- OR
Odds ratio
- PI
Prediction intervals
- PRESS
Peer Review of Electronic Search Strategies
- QOC
Quality of care
- QOL
Quality of life
- RCTs
Randomized controlled trials
- ROB
Risk of bias
- SD
Standard deviation
- SF
Short-form
- SGRQ
St. George's Respiratory Questionnaire
Authors’ contributions
AAV helped conceive the study, analyzed the data, interpreted the results, and wrote the first draft of the manuscript. SES conceptualised the study, designed the study, obtained funding, helped write the article, and is the guarantor of the review. ACT conceptualised the study, designed the study, helped obtain funding for the study, and helped write the article. VN screened citations, and full-text articles, appraised quality, resolved discrepancies, and edited the manuscript. CS provided methodological support and edited the manuscript. YL, PR, HM, PK, MG, and FY screened citations and/or full-text articles, abstracted data, and/or appraised quality and edited the manuscript. BH, LS, IDG, JP, FL, BRH, KT, JM, RCB, TVdW participated in the Delphi rounds. AE, BH, BRH, CF, DAC, FL, HTS, IDG, JES, JM, JP, LRD, KT, LS, PPG, RCB, TVdW, WI helped conceive the study and edited the manuscript. All authors read and approved the final manuscript.
Authors’ Twitter handles
The Twitter handles for the following authors AAV, ACT, and CF are @AVeroniki, @ATricco, and @christine_fahim, respectively.
Funding
This work was funded by a Canadian Institutes of Health Research (CIHR) Foundation Grant [No 154334]. The funders had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript. SES is funded by a Tier 1 Canada Research Chair in Knowledge Translation and Quality of Care, the Mary Trimmer Chair in Geriatric Medicine, and a Foundation Grant (Canadian Institutes of Health Research). ACT holds a Tier 2 Canada Research Chair in Knowledge Synthesis.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional file. The full list of excluded studies from this review at level 1 (titles/abstracts) or level 2 (full-texts) will be made available upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Patient involvement and dissemination
This work involved one patient partner to ensure patient perspectives are integrated in our research question. The patient partner was involved from the outset when defining the research question, and we plan to meet regularly in the update of this review to support dissemination of the results. The research question was developed based on the patient’s concerns and priorities. The patient was not involved in the interpretation of results and writing of the article because she retired at that time and was unable to continue due to personal circumstances. The results will be disseminated to the lay audience through a press release, social media, through the SPOR-EA website and through public presentations.
Transparency declaration
The senior author (SES) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Copyright/license for publication
The corresponding author (SES) has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide license to the publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to (i) publish, reproduce, distribute, display and store the contribution, (ii) translate the contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the contribution, (iii) create any other derivative work(s) based on the contribution, (iv) to exploit all subsidiary rights in the contribution, (v) the inclusion of electronic links from the contribution to third party material wherever it may be located; and (vi) license any third party to do any or all of the above.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Glasgow RE, Chambers D. Developing robust, sustainable, implementation systems using rigorous, rapid and relevant science. Clin Transl Sci. 2012;5(1):48–55. doi: 10.1111/j.1752-8062.2011.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. doi: 10.1186/s13012-015-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(EPOC) EPaOoC. EPOC Taxonomy [Available from: http://epoc.cochrane.org/epoc-taxonomy.
- 4.Straus SE, Tetroe J, Graham ID. Knowledge translation in health care: moving from evidence to practice. 2. Chichester: John Wiley & Sons, Ltd.; 2013. [Google Scholar]
- 5.Moore JE, Mascarenhas A, Bain J, Straus SE. Developing a comprehensive definition of sustainability. Implement Sci. 2017;12(1):110. doi: 10.1186/s13012-017-0637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proctor E, Luke D, Calhoun A, McMillen C, Brownson R, McCrary S, et al. Sustainability of evidence-based healthcare: research agenda, methodological advances, and infrastructure support. Implement Sci. 2015;10:88. doi: 10.1186/s13012-015-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiltsey Stirman S, Kimberly J, Cook N, Calloway A, Castro F, Charns M. The sustainability of new programs and innovations: a review of the empirical literature and recommendations for future research. Implement Sci. 2012;7:17. doi: 10.1186/1748-5908-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. JAMA. 1996;276(18):1473–1479. doi: 10.1001/jama.1996.03540180029029. [DOI] [PubMed] [Google Scholar]
- 9.Wodchis WP, Austin PC, Henry DA. A 3-year study of high-cost users of health care. CMAJ. 2016;188(3):182–8. doi: 10.1503/cmaj.150064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci. 2013;8:117. doi: 10.1186/1748-5908-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle C, Howe C, Woodcock T, Myron R, Phekoo K, McNicholas C, et al. Making change last: applying the NHS institute for innovation and improvement sustainability model to healthcare improvement. Implement Sci. 2013;8:127. doi: 10.1186/1748-5908-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schell SF, Luke DA, Schooley MW, Elliott MB, Herbers SH, Mueller NB, et al. Public health program capacity for sustainability: a new framework. Implement Sci. 2013;8:15. doi: 10.1186/1748-5908-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson DD. A framework for implementing sustainable oral health promotion interventions. J Public Health Dent. 2011;71(Suppl 1):S84–94. [PubMed] [Google Scholar]
- 14.Tricco AC, Ashoor HM, Cardoso R, MacDonald H, Cogo E, Kastner M, et al. Sustainability of knowledge translation interventions in healthcare decision-making: a scoping review. Implement Sci. 2016;11:55. doi: 10.1186/s13012-016-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 16.Research CIoH. Knowledge User Engagement 2016 [Available from: https://cihr-irsc.gc.ca/e/49505.html.
- 17.Graham ID, Kothari A, McCutcheon C, Integrated Knowledge Translation Research Network Project L Moving knowledge into action for more effective practice, programmes and policy: protocol for a research programme on integrated knowledge translation. Implement Sci. 2018;13(1):22. doi: 10.1186/s13012-017-0700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tricco AC, Moore JE, Beben N, Brownson RC, Chambers DA, Dolovich LR, et al. Sustaining knowledge translation interventions for chronic disease management in older adults: protocol for a systematic review and network meta-analysis. Syst Rev. 2018;7(1):140. doi: 10.1186/s13643-018-0808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C. PRISMA 2020 statement: an updated guideline for reporting systematic reviews. MetaArXiv. 2020;2020:2020. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. doi: 10.1136/bmj.j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CADTH. Grey matters: A tool for searching health-related grey literature. Ottawa2022 [Available from: https://greymatters.cadth.ca.
- 22.WHO. Noncommunicable diseases 2022 [Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
- 23.Presseau J, Ivers NM, Newham JJ, Knittle K, Danko KJ, Grimshaw JM. Using a behaviour change techniques taxonomy to identify active ingredients within trials of implementation interventions for diabetes care. Implement Sci. 2015;10:55. doi: 10.1186/s13012-015-0248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins J, Li T, Deeks J, editors. Cochrane Handbook for Systematic Reviews of Interventions. version 6.3 ed. Cochrane: (updated February 2022); 2022.
- 25.Cochrane EPOC Risk of Bias tool [Available from: http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/Risk%20of%20Bias%2005-01-2009.doc.
- 26.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions. version 6.1 ed. Higgins J, Li T, Deeks J, editors. Cochrane: (updated September 2020); 2020.
- 27.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichard P, Pihl M, Rosenqvist U, Sule J. Complications in IDDM are caused by elevated blood glucose level: the Stockholm Diabetes Intervention Study (SDIS) at 10-year follow up. Diabetologia. 1996;39(12):1483–1488. doi: 10.1007/s001250050602. [DOI] [PubMed] [Google Scholar]
- 29.Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7(1):55–79. doi: 10.1002/jrsm.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 31.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 32.Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Stat Med. 2007;26(1):37–52. doi: 10.1002/sim.2514. [DOI] [PubMed] [Google Scholar]
- 33.Rover C, Knapp G, Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. 2015;15:99. doi: 10.1186/s12874-015-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarzer G. meta: An R package for meta-analysis. R news. 2007;7(3):40–45. [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.Veroniki AA. Random-effects meta-analysis methods in RevMan. 2022. [Google Scholar]
- 38.Veroniki AA, Jackson D, Viechtbauer W, Bender R, Knapp G, Kuss O, et al. Recommendations for quantifying the uncertainty in the summary intervention effect and estimating the between-study heterogeneity variance in random-effects meta-analysis. In: Chandler J, McKenzie J, Boutron I, Welch V, editors.: Cochrane Methods. Cochrane Database of Systematic Reviews; 2015.
- 39.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20(24):3875–3889. doi: 10.1002/sim.1009. [DOI] [PubMed] [Google Scholar]
- 40.Sidik K, Jonkman JN. A simple confidence interval for meta-analysis. Stat Med. 2002;21(21):3153–3159. doi: 10.1002/sim.1262. [DOI] [PubMed] [Google Scholar]
- 41.Veroniki AA, Jackson D, Bender R, Kuss O, Langan D, Higgins JPT, et al. Methods to calculate uncertainty in the estimated overall effect size from a random-effects meta-analysis. Res Synth Methods. 2019;10(1):23–43. doi: 10.1002/jrsm.1319. [DOI] [PubMed] [Google Scholar]
- 42.Sadeghirad B, Foroutan F, Zoratti MJ, Busse JW, Brignardello-Petersen R, Guyatt G, et al. Theory and practice of Bayesian and frequentist frameworks for network meta-analysis. BMJ Evid Based Med. 2022. [DOI] [PubMed]
- 43.Chen S, Conwell Y, He J, Lu N, Wu J. Depression care management for adults older than 60 years in primary care clinics in urban China: a cluster-randomised trial. Lancet Psychiat. 2015;2(4):332–339. doi: 10.1016/S2215-0366(15)00002-4. [DOI] [PubMed] [Google Scholar]
- 44.de Fine ON, Beck-Nielsen H, Andreasen AH, Hørder M, Pedersen PA. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. BMJ. 2001;323(7319):970. doi: 10.1136/bmj.323.7319.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dracup K, Moser DK, Pelter MM, Nesbitt TS, Southard J, Paul SM, et al. Randomized, controlled trial to improve self-care in patients with heart failure living in rural areas. Circulation. 2014;130(3):256–264. doi: 10.1161/CIRCULATIONAHA.113.003542. [DOI] [PubMed] [Google Scholar]
- 46.Eccles MP, Whitty PM, Speed C, Steen IN, Vanoli A, Hawthorne GC, et al. A pragmatic cluster randomised controlled trial of a Diabetes REcall And Management system: the DREAM trial. Implement Sci. 2007;2(1):1–12. doi: 10.1186/1748-5908-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goderis G, Borgermans L, Grol R, Van Den Broeke C, Boland B, Verbeke G, et al. Start improving the quality of care for people with type 2 diabetes through a general practice support program: a cluster randomized trial. Diabetes Res Clin Pract. 2010;88(1):56–64. doi: 10.1016/j.diabres.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Heisler M, Hofer TP, Schmittdiel JA, Selby JV, Klamerus ML, Bosworth HB, et al. Improving blood pressure control through a clinical pharmacist outreach program in patients with diabetes mellitus in 2 high-performing health systems: the adherence and intensification of medications cluster randomized, controlled pragmatic trial. Circulation. 2012;125(23):2863–2872. doi: 10.1161/CIRCULATIONAHA.111.089169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ. 2013;346:f2882. doi: 10.1136/bmj.f2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiefe CI, Allison JJ, Williams OD, Person SD, Weaver MT, Weissman NW. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285(22):2871–2879. doi: 10.1001/jama.285.22.2871. [DOI] [PubMed] [Google Scholar]
- 51.Miller W, Rollnick S. Rollnick S. Motivational interviewing: preparing people for change, edn. New York Guilford. 2002. [Google Scholar]
- 52.Moy ML, Martinez CH, Kadri R, Roman P, Holleman RG, Kim HM, et al. Long-term effects of an internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial. J Med Internet Res. 2016;18(8):e5622. doi: 10.2196/jmir.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olaiya MT, Kim J, Nelson MR, Srikanth VK, Bladin CF, Gerraty RP, et al. Effectiveness of a shared team approach between nurses and doctors for improved risk factor management in survivors of stroke: a cluster randomized controlled trial. Eur J Neurol. 2017;24(7):920–928. doi: 10.1111/ene.13306. [DOI] [PubMed] [Google Scholar]
- 54.Palacio AM, Uribe C, Hazel-Fernandez L, Li H, Tamariz LJ, Garay SD, et al. Can phone-based motivational interviewing improve medication adherence to antiplatelet medications after a coronary stent among racial minorities? A randomized trial. J Gen Intern Med. 2015;30(4):469–475. doi: 10.1007/s11606-014-3139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schraeder C, Fraser C, Clark I, Newcomer R, Stoll J, Krock C, et al. The effect of primary care management on lipids testing and LDL-C control of elderly patients with comorbidities. Prof Case Manag. 2009;14(2):84–95. doi: 10.1097/NCM.0b013e31819e01fb. [DOI] [PubMed] [Google Scholar]
- 56.Shea S, Weinstock RS, Starren J, Teresi J, Palmas W, Field L, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc. 2006;13(1):40–51. doi: 10.1197/jamia.M1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tjia J, Field T, Mazor K, Lemay CA, Kanaan AO, Donovan JL, et al. Dissemination of evidence-based antipsychotic prescribing guidelines to nursing homes: a cluster randomized trial. J Am Geriatr Soc. 2015;63(7):1289–1298. doi: 10.1111/jgs.13488. [DOI] [PubMed] [Google Scholar]
- 58.Trofimov EE, Poskrebysheva A, Gurskaya A, Novikova A. Therapeutic education for patients with heart failure: effect on medication adherence and clinical outcomes. Eur J Heart Fail; 2015.
- 59.Vickrey B, Mittman B, Connor K, Pearson M, Della Penna R, Ganiats T, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med. 2006;145(10):713–726. doi: 10.7326/0003-4819-145-10-200611210-00004. [DOI] [PubMed] [Google Scholar]
- 60.Bender R, Friede T, Koch A, Kuss O, Schlattmann P, Schwarzer G, et al. Methods for evidence synthesis in the case of very few studies. Res Synth Methods. 2018;9(3):382–392. doi: 10.1002/jrsm.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Zhou Q, Zou F, Wu L, Chen H, Liu Z. Effectiveness of systematic self-management education on blood sugar level of patients in the community with type 2 diabetes. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37(4):355–358. doi: 10.3969/j.issn.1672-7347.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Ansari M, Shlipak MG, Heidenreich PA, Van Ostaeyen D, Pohl EC, Browner WS, et al. Improving guideline adherence: a randomized trial evaluating strategies to increase β-blocker use in heart failure. Circulation. 2003;107(22):2799–2804. doi: 10.1161/01.CIR.0000070952.08969.5B. [DOI] [PubMed] [Google Scholar]
- 63.Baker R, Fraser RC, Stone M, Lambert P, Stevenson K, Shiels C. Randomised controlled trial of the impact of guidelines, prioritized review criteria and feedback on implementation of recommendations for angina and asthma. Br J Gen Pract. 2003;53(489):284–291. [PMC free article] [PubMed] [Google Scholar]
- 64.Batchelor FA, Hill KD, Mackintosh SF, Said CM, Whitehead CH. Effects of a multifactorial falls prevention program for people with stroke returning home after rehabilitation: a randomized controlled trial. Arch Phys Med Rehabil. 2012;93(9):1648–1655. doi: 10.1016/j.apmr.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 65.Beck A, Scott J, Williams P, Robertson B, Jackson D, Gade G, et al. A randomized trial of group outpatient visits for chronically ill older HMO members: the Cooperative Health Care Clinic. J Am Geriatr Soc. 1997;45(5):543–549. doi: 10.1111/j.1532-5415.1997.tb03085.x. [DOI] [PubMed] [Google Scholar]
- 66.Beck CA, Beran DB, Biglan KM, Boyd CM, Dorsey ER, Schmidt PN, et al. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89(11):1152–1161. doi: 10.1212/WNL.0000000000004357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bekelman DB, Plomondon ME, Carey EP, Sullivan MD, Nelson KM, Hattler B, et al. Primary results of the patient-centered disease management (PCDM) for heart failure study: a randomized clinical trial. JAMA Intern Med. 2015;175(5):725–732. doi: 10.1001/jamainternmed.2015.0315. [DOI] [PubMed] [Google Scholar]
- 68.Benzo R, Vickers K, Novotny PJ, Tucker S, Hoult J, Neuenfeldt P, et al. Health coaching and chronic obstructive pulmonary disease rehospitalization. A randomized study. Am J Respir Crit Care Med. 2016;194(6):672–80. doi: 10.1164/rccm.201512-2503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA, Murdoch DR, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323(7315):715–718. doi: 10.1136/bmj.323.7315.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bohingamu Mudiyanselage S, Stevens J, Watts JJ, Toscano J, Kotowicz MA, Steinfort CL, et al. Personalised telehealth intervention for chronic disease management: a pilot randomised controlled trial. J Telemed Telecare. 2019;25(6):343–352. doi: 10.1177/1357633X18775850. [DOI] [PubMed] [Google Scholar]
- 71.Böhm M, Drexler H, Oswald H, Rybak K, Bosch R, Butter C, et al. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J. 2016;37(41):3154–3163. doi: 10.1093/eurheartj/ehw099. [DOI] [PubMed] [Google Scholar]
- 72.Bosanquet K, Adamson J, Atherton K, Bailey D, Baxter C, Beresford-Dent J, et al. CollAborative care for Screen-Positive EldeRs with major depression (CASPER plus): a multicentred randomised controlled trial of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2017;21(67):1. doi: 10.3310/hta21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupré A, Bégin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163(5):585–591. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 74.Boyne JJ, Vrijhoef HJ, Crijns HJ, De Weerd G, Kragten J, Gorgels AP, et al. Tailored telemonitoring in patients with heart failure: results of a multicentre randomized controlled trial. Eur J Heart Fail. 2012;14(7):791–801. doi: 10.1093/eurjhf/hfs058. [DOI] [PubMed] [Google Scholar]
- 75.Bruce ML, Raue PJ, Reilly CF, Greenberg RL, Meyers BS, Banerjee S, et al. Clinical effectiveness of integrating depression care management into medicare home health: the Depression CAREPATH Randomized trial. JAMA Intern Med. 2015;175(1):55–64. doi: 10.1001/jamainternmed.2014.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruce ML, Ten Have TR, Reynolds CF, III, Katz II, Schulberg HC, Mulsant BH, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 77.Bucknall C, Miller G, Lloyd S, Cleland J, McCluskey S, Cotton M, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060. doi: 10.1136/bmj.e1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Team CPMMPE The MEDMAN study: a randomized controlled trial of community pharmacy-led medicines management for patients with coronary heart disease. Family Practice. 2007;24(2):189–200. doi: 10.1093/fampra/cml075. [DOI] [PubMed] [Google Scholar]
- 79.Burns R, Nichols LO, Graney MJ, Cloar FT. Impact of continued geriatric outpatient management on health outcomes of older veterans. Arch Intern Med. 1995;155(12):1313–1318. doi: 10.1001/archinte.1995.00430120103012. [DOI] [PubMed] [Google Scholar]
- 80.Burns R, Nichols LO, Martindale-Adams J, Graney MJ, Lummus A. Primary care interventions for dementia caregivers: 2-year outcomes from the REACH study. Gerontologist. 2003;43(4):547–555. doi: 10.1093/geront/43.4.547. [DOI] [PubMed] [Google Scholar]
- 81.Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 82.Campbell NC, Ritchie L, Thain J, Deans H, Rawles J, Squair J. Secondary prevention in coronary heart disease: a randomised trial of nurse led clinics in primary care. Heart. 1998;80(5):447–452. doi: 10.1136/hrt.80.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chi C, Chen H. Daily-based self-management for non-hospitalised heart failure patients improve prognosis. Heart. 2012;98(Suppl 2):E231–E232. doi: 10.1136/heartjnl-2012-302920o.2. [DOI] [Google Scholar]
- 84.Ciaschini PM, Straus SE, Dolovich LR, Goeree RA, Leung KM, Woods CR, et al. Community based intervention to optimize osteoporosis management: randomized controlled trial. BMC Geriatr. 2010;10(1):1–7. doi: 10.1186/1471-2318-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cleveringa FG, Gorter KJ, Van Den Donk M, Rutten GE. Combined task delegation, computerized decision support, and feedback improve cardiovascular risk for type 2 diabetic patients: a cluster randomized trial in primary care. Diabetes Care. 2008;31(12):2273–2275. doi: 10.2337/dc08-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coleman E, Grothaus L, Sandhu N, Wagner E. Chronic care clinics: a randomized controlled trial of a new model of primary care for frail older adults. J Am Geriatr Soc. 1999;47(7):775–783. doi: 10.1111/j.1532-5415.1999.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 87.Coull AJ, Taylor VH, Elton R, Murdoch PS, Hargreaves AD. A randomised controlled trial of senior Lay Health Mentoring in older people with ischaemic heart disease: The Braveheart Project. Age Ageing. 2004;33(4):348–354. doi: 10.1093/ageing/afh098. [DOI] [PubMed] [Google Scholar]
- 88.de la Porte PW, Lok DJ, van Veldhuisen DJ, van Wijngaarden J, Cornel JH, Zuithoff NP, et al. Added value of a physician-and-nurse-directed heart failure clinic: results from the Deventer-Alkmaar heart failure study. Heart. 2007;93(7):819–825. doi: 10.1136/hrt.2006.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Lusignana S, Gallagher H, Jones S, Chan T, Van Vlymen J, Tahir A, et al. Audit-based education lowers systolic blood pressure in chronic kidney disease: the Quality Improvement in CKD (QICKD) trial results. Kidney Int. 2013;84(3):609–620. doi: 10.1038/ki.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DeBusk RF, Miller NH, Parker KM, Bandura A, Kraemer HC, Cher DJ, et al. Care management for low-risk patients with heart failure: a randomized, controlled trial. Ann Intern Med. 2004;141(8):606–613. doi: 10.7326/0003-4819-141-8-200410190-00008. [DOI] [PubMed] [Google Scholar]
- 91.Del Sindaco D, Pulignano G, Minardi G, Apostoli A, Guerrieri L, Rotoloni M, et al. Two-year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure. J Cardiovasc Med. 2007;8(5):324–329. doi: 10.2459/JCM.0b013e32801164cb. [DOI] [PubMed] [Google Scholar]
- 92.DeVore AD, Cox M, Heidenreich PA, Fonarow GC, Yancy CW, Eapen ZJ, et al. Cluster-randomized trial of personalized site performance feedback in get with the guidelines-heart failure. Circ Cardiovasc Qual Outcomes. 2015;8(4):421–427. doi: 10.1161/CIRCOUTCOMES.114.001333. [DOI] [PubMed] [Google Scholar]
- 93.Döpp CM, Graff MJ, Teerenstra S, OldeRikkert MG, Nijhuis–van der Sanden MW, Vernooij-Dassen MJ. Effectiveness of a training package for implementing a community-based occupational therapy program in dementia: a cluster randomized controlled trial. Clin Rehabil. 2015;29(10):974–86. doi: 10.1177/0269215514564699. [DOI] [PubMed] [Google Scholar]
- 94.Dunagan WC, Littenberg B, Ewald GA, Jones CA, Emery VB, Waterman BM, et al. Randomized trial of a nurse-administered, telephone-based disease management program for patients with heart failure. J Card Fail. 2005;11(5):358–365. doi: 10.1016/j.cardfail.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 95.Eckert K, Schrader G, Wilkinson D, Askew D, Dick M, Wade T, et al. Detection and management of depression in patients with chronic heart disease: the TAKE heart in primary care cluster randomised controlled trial. Heart Lung Circ. 2010;19:S240–S241. doi: 10.1016/j.hlc.2010.06.584. [DOI] [Google Scholar]
- 96.Ell K, Unützer J, Aranda M, Gibbs NE, Lee P-J, Xie B. Managing depression in home health care: a randomized clinical trial. Home Health Care Serv Q. 2007;26(3):81–104. doi: 10.1300/J027v26n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156(10):673–683. doi: 10.7326/0003-4819-156-10-201205150-00003. [DOI] [PubMed] [Google Scholar]
- 98.Federman AD, O’Conor R, Mindlis I, Hoy-Rosas J, Hauser D, Lurio J, et al. Effect of a self-management support intervention on asthma outcomes in older adults: the SAMBA study randomized clinical trial. JAMA Intern Med. 2019;179(8):1113–1121. doi: 10.1001/jamainternmed.2019.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fihn SD, Bucher JB, McDonell M, Diehr P, Rumsfeld JS, Doak M, et al. Collaborative care intervention for stable ischemic heart disease. Arch Intern Med. 2011;171(16):1471–1479. doi: 10.1001/archinternmed.2011.372. [DOI] [PubMed] [Google Scholar]
- 100.Fihn SD, McDonell MB, Diehr P, Anderson SM, Bradley KA, Au DH, et al. Effects of sustained audit/feedback on self-reported health status of primary care patients. Am J Med. 2004;116(4):241–248. doi: 10.1016/j.amjmed.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 101.Forster A, Young J. Specialist nurse support for patients with stroke in the community: a randomised controlled trial. BMJ. 1996;312(7047):1642–1646. doi: 10.1136/bmj.312.7047.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Forster A, Young J, Chapman K, Nixon J, Patel A, Holloway I, et al. Cluster randomized controlled trial: clinical and cost-effectiveness of a system of longer-term stroke care. Stroke. 2015;46(8):2212–2219. doi: 10.1161/STROKEAHA.115.008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Freund T, Peters-Klimm F, Boyd CM, Mahler C, Gensichen J, Erler A, et al. Medical assistant–based care management for high-risk patients in small primary care practices: a cluster randomized clinical trial. Ann Intern Med. 2016;164(5):323–330. doi: 10.7326/M14-2403. [DOI] [PubMed] [Google Scholar]
- 104.Galbreath AD, Krasuski RA, Smith B, Stajduhar KC, Kwan MD, Ellis R, et al. Long-term healthcare and cost outcomes of disease management in a large, randomized, community-based population with heart failure. Circulation. 2004;110(23):3518–3526. doi: 10.1161/01.CIR.0000148957.62328.89. [DOI] [PubMed] [Google Scholar]
- 105.Gallagher RA, Miller C, Cronan TA, Groessl E. Gender differences in participation and responsiveness to a health intervention for older Americans. J Womens Health. 1997;25(3):63–81. doi: 10.1300/J013v25n03_05. [DOI] [PubMed] [Google Scholar]
- 106.Gaugler JE, Roth DL, Haley WE, Mittelman MS. Can counseling and support reduce Alzheimer’s caregivers’ burden and depressive symptoms during the transition to institutionalization? Results from the NYU caregiver intervention study. J Am Geriatr Soc. 2008;56(3):421. doi: 10.1111/j.1532-5415.2007.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gellis ZD, Kenaley B, McGinty J, Bardelli E, Davitt J, Ten Have T. Outcomes of a telehealth intervention for homebound older adults with heart or chronic respiratory failure: a randomized controlled trial. Gerontologist. 2012;52(4):541–552. doi: 10.1093/geront/gnr134. [DOI] [PubMed] [Google Scholar]
- 108.Graven C, Brock K, Hill KD, Cotton S, Joubert L. First year after stroke: an integrated approach focusing on participation goals aiming to reduce depressive symptoms. Stroke. 2016;47(11):2820–2827. doi: 10.1161/STROKEAHA.116.013081. [DOI] [PubMed] [Google Scholar]
- 109.Haerter M, Dirmaier J, Dwinger S, Kriston L, Herbarth L, Siegmund-Schultze E, et al. Effectiveness of telephone-based health coaching for patients with chronic conditions: a randomised controlled trial. PLoS ONE. 2016;11(9):e0161269. doi: 10.1371/journal.pone.0161269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hendriks JM, De Wit R, Crijns HJ, Vrijhoef HJ, Prins MH, Pisters R, et al. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. 2012;33(21):2692–9. doi: 10.1093/eurheartj/ehs071. [DOI] [PubMed] [Google Scholar]
- 111.Hernández C, Alonso A, Garcia-Aymerich J, Serra I, Marti D, Rodriguez-Roisin R, et al. Effectiveness of community-based integrated care in frail COPD patients: a randomised controlled trial. NPJ Prim Care Respir Med. 2015;25(1):1–6. doi: 10.1038/npjpcrm.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hetlevik I, Holmen J, Krüger Ø, Kristensen P, Iversen H, Furuseth K. Implementing clinical guidelines in the treatment of diabetes mellitus in general practice: evaluation of effort, process, and patient outcome related to implementation of a computer-based decision support system. Int J Technol Assess Health Care. 2000;16(1):210–227. doi: 10.1017/S0266462300161185. [DOI] [PubMed] [Google Scholar]
- 113.Hogg W, Lemelin J, Dahrouge S, Liddy C, Armstrong CD, Legault F, et al. Randomized controlled trial of anticipatory and preventive multidisciplinary team care: for complex patients in a community-based primary care setting. Can Fam Physician. 2009;55(12):e76–e85. [PMC free article] [PubMed] [Google Scholar]
- 114.Holbrook A, Pullenayegum E, Thabane L, Troyan S, Foster G, Keshavjee K, et al. Shared electronic vascular risk decision support in primary care: Computerization of Medical Practices for the Enhancement of Therapeutic Effectiveness (COMPETE III) randomized trial. Arch Intern Med. 2011;171(19):1736–1744. doi: 10.1001/archinternmed.2011.471. [DOI] [PubMed] [Google Scholar]
- 115.Holm T, Lassen J, Husted S, Christensen P, Heickendorff L. A randomized controlled trial of shared care versus routine care for patients receiving oral anticoagulant therapy. J Intern Med. 2002;252(4):322–331. doi: 10.1046/j.1365-2796.2002.01039.x. [DOI] [PubMed] [Google Scholar]
- 116.Holt TA, Thorogood M, Griffiths F, Munday S, Friede T, Stables D. Automated electronic reminders to facilitate primary cardiovascular disease prevention: randomised controlled trial. Br J Gen Pract. 2010;60(573):e137–e143. doi: 10.3399/bjgp10X483904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hughes S, Weaver F, Giobbie-Hurder A, Manheim L, Henderson W, Kubal J, et al. Department of veterans affairs cooperative study group on home-based primary care effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA. 2000;284(22):2877–2885. doi: 10.1001/jama.284.22.2877. [DOI] [PubMed] [Google Scholar]
- 118.Tremont G, Duncan Davis J, Bishop DS, Fortinsky RH. Telephone-delivered psychosocial intervention reduces burden in dementia caregivers. Dementia. 2008;7(4):503–520. doi: 10.1177/1471301208096632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hunger M, Kirchberger I, Holle R, Seidl H, Kuch B, Wende R, et al. Does nurse-based case management for aged myocardial infarction patients improve risk factors, physical functioning and mental health? The KORINNA trial. Eur J Prev Cardiol. 2015;22(4):442–450. doi: 10.1177/2047487314524682. [DOI] [PubMed] [Google Scholar]
- 120.Irewall A-L, Ögren J, Bergström L, Laurell K, Söderström L, Mooe T. Nurse-led, telephone-based, secondary preventive follow-up after stroke or transient ischemic attack improves blood pressure and LDL cholesterol: results from the first 12 months of the randomized, controlled NAILED stroke risk factor trial. PLoS ONE. 2015;10(10):e0139997. doi: 10.1371/journal.pone.0139997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jaarsma T, Van Der Wal M, Lesman-Leegte I, Luttik M, Hogenhuis J, Veeger N, et al. Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Investigators. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Arch Intern Med. 2008;168(3):316–24. doi: 10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]
- 122.Joling KJ, van Marwijk HW, Smit F, van der Horst HE, Scheltens P, van de Ven PM, et al. Does a family meetings intervention prevent depression and anxiety in family caregivers of dementia patients? A randomized trial. PLoS ONE. 2012;7(1):e30936. doi: 10.1371/journal.pone.0030936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kalter-Leibovici O, Freimark D, Freedman LS, Kaufman G, Ziv A, Murad H, et al. Disease management in the treatment of patients with chronic heart failure who have universal access to health care: a randomized controlled trial. BMC Med. 2017;15(1):1–13. doi: 10.1186/s12916-017-0855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karhula T, Vuorinen A-L, Rääpysjärvi K, Pakanen M, Itkonen P, Tepponen M, et al. Telemonitoring and mobile phone-based health coaching among Finnish diabetic and heart disease patients: randomized controlled trial. J Med Internet Res. 2015;17(6):e4059. doi: 10.2196/jmir.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kennedy CC, Ioannidis G, Thabane L, Adachi JD, Marr S, Giangregorio LM, et al. Successful knowledge translation intervention in long-term care: final results from the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Trials. 2015;16(1):1–11. doi: 10.1186/s13063-015-0720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol. 2009;68(4):588–598. doi: 10.1111/j.1365-2125.2009.03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim KB, Han H-R, Huh B, Nguyen T, Lee H, Kim MT. The effect of a community-based self-help multimodal behavioral intervention in Korean American seniors with high blood pressure. Am J Hypertens. 2014;27(9):1199–1208. doi: 10.1093/ajh/hpu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ko FW, Cheung N, Rainer TH, Lum C, Wong I, Hui DS. Comprehensive care programme for patients with chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2017;72(2):122–128. doi: 10.1136/thoraxjnl-2016-208396. [DOI] [PubMed] [Google Scholar]
- 129.Kruis AL, Boland MR, Assendelft WJ, Gussekloo J, Tsiachristas A, Stijnen T, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ. 2014;349:g5392. doi: 10.1136/bmj.g5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krum H, Forbes A, Yallop J, Driscoll A, Croucher J, Chan B, et al. Telephone support to rural and remote patients with heart failure: the Chronic Heart Failure Assessment by Telephone (CHAT) study. Cardiovasc Ther. 2013;31(4):230–237. doi: 10.1111/1755-5922.12009. [DOI] [PubMed] [Google Scholar]
- 131.Kurz A, Wagenpfeil S, Hallauer J, Schneider-Schelte H, Jansen S, Group AS Evaluation of a brief educational program for dementia carers: the AENEAS study. Int J Geriatr Psychiatry. 2010;25(8):861–9. doi: 10.1002/gps.2428. [DOI] [PubMed] [Google Scholar]
- 132.Leveille SG, Wagner EH, Davis C, Grothaus L, Wallace J, LoGerfo M, et al. Preventing disability and managing chronic illness in frail older adults: a randomized trial of a community-based partnership with primary care. J Am Geriatr Soc. 1998;46(10):1191–1198. doi: 10.1111/j.1532-5415.1998.tb04533.x. [DOI] [PubMed] [Google Scholar]
- 133.Leventhal ME, Denhaerynck K, Brunner-La Rocca HP, Burnand B, Conca-Zeller A, Bernasconi A, et al. Swiss Interdisciplinary Management Programme for Heart Failure (SWIM-HF): a randomised controlled trial study of an outpatient inter-professional management programme for heart failure patients in Switzerland. Swiss Med Wkly. 2011;141(0910). [DOI] [PubMed]
- 134.Levine DA, Funkhouser EM, Houston TK, Gerald JK, Johnson-Roe N, Allison JJ, et al. Improving care after myocardial infarction using a 2-year internet-delivered intervention: the Department of Veterans Affairs myocardial infarction–plus cluster-randomized trial. Arch Intern Med. 2011;171(21):1910–1917. doi: 10.1001/archinternmed.2011.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Licskai C, Ferrone M, Malus N, Stitt L, O'Callahan T, Roberts Z, et al. COPD collaborative self-management in primary care: A randomized controlled trial. Eur Respiratory Soc. 2016.
- 136.Lin H-W, Lin C-H, Chang C-K, Chou C-Y, Yu I-W, Lin C-C, et al. Economic outcomes of pharmacist-physician medication therapy management for polypharmacy elderly: a prospective, randomized, controlled trial. J Formos Med Assoc. 2018;117(3):235–243. doi: 10.1016/j.jfma.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 137.Liu W, Wang C, Lin H, Lin S, Lee K, Lo Y, et al. Efficacy of a cell phone-based exercise programme for COPD. Eur Respir J. 2008;32(3):651–659. doi: 10.1183/09031936.00104407. [DOI] [PubMed] [Google Scholar]
- 138.López Cabezas C, Falces Salvador C, Cubí Quadrada D, Arnau Bartés A, Ylla Bore M, Muro Perea N, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farm Hosp. 2006;30(6):328–342. doi: 10.1016/S1130-6343(06)74004-1. [DOI] [PubMed] [Google Scholar]
- 139.Lowrie R, Lloyd SM, McConnachie A, Morrison J. A cluster randomised controlled trial of a pharmacist-led collaborative intervention to improve statin prescribing and attainment of cholesterol targets in primary care. PLoS ONE. 2014;9(11):e113370. doi: 10.1371/journal.pone.0113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Machline-Carrion MJ, Soares RM, Damiani LP, Campos VB, Sampaio B, Fonseca FH, et al. Effect of a multifaceted quality improvement intervention on the prescription of evidence-based treatment in patients at high cardiovascular risk in Brazil: the bridge cardiovascular prevention cluster randomized clinical trial. JAMA Cardiol. 2019;4(5):408–417. doi: 10.1001/jamacardio.2019.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mahoney DF, Tarlow BJ, Jones RN. Effects of an automated telephone support system on caregiver burden and anxiety: findings from the REACH for TLC intervention study. Gerontologist. 2003;43(4):556–567. doi: 10.1093/geront/43.4.556. [DOI] [PubMed] [Google Scholar]
- 142.Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2008;149(12):869–878. doi: 10.7326/0003-4819-149-12-200812160-00006. [DOI] [PubMed] [Google Scholar]
- 143.Markle-Reid M, Orridge C, Weir R, Browne G, Gafni A, Lewis M, et al. Interprofessional stroke rehabilitation for stroke survivors using home care. Can J Neurol Sci. 2011;38(2):317–334. doi: 10.1017/S0317167100011537. [DOI] [PubMed] [Google Scholar]
- 144.Markun S, Dishy A, Neuner-Jehle S, Rosemann T, Frei A. The chronic care for wet age related macular degeneration (CHARMED) study: a randomized controlled trial. PLoS ONE. 2015;10(11):e0143085. doi: 10.1371/journal.pone.0143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McCluskey A, Ada L, Kelly PJ, Middleton S, Goodall S, Grimshaw JM, et al. A behavior change program to increase outings delivered during therapy to stroke survivors by community rehabilitation teams: The Out-and-About trial. Int J Stroke. 2016;11(4):425–437. doi: 10.1177/1747493016632246. [DOI] [PubMed] [Google Scholar]
- 146.McElrath M, Myers J, Chan K, Fonda H. Exercise adherence in the elderly: experience with abdominal aortic aneurysm simple treatment and prevention. J Vasc Nurs. 2017;35(1):12–20. doi: 10.1016/j.jvn.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 147.McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312(8):799–808. doi: 10.1001/jama.2014.10057. [DOI] [PubMed] [Google Scholar]
- 148.Meeuwsen EJ, Melis RJ, Van Der Aa GC, Golüke-Willemse GA, De Leest BJ, Van Raak FH, et al. Effectiveness of dementia follow-up care by memory clinics or general practitioners: randomised controlled trial. BMJ. 2012;344. [DOI] [PMC free article] [PubMed]
- 149.Meisinger C, Stollenwerk B, Kirchberger I, Seidl H, Wende R, Kuch B, et al. Effects of a nurse-based case management compared to usual care among aged patients with myocardial infarction: results from the randomized controlled KORINNA study. BMC Geriatr. 2013;13(1):1–10. doi: 10.1186/1471-2318-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mejhert M, Kahan T, Persson H, Edner M. Limited long term effects of a management programme for heart failure. Heart. 2004;90(9):1010–1015. doi: 10.1136/hrt.2003.014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mitchell E, Sullivan F, Grimshaw JM, Donnan PT, Watt G. Improving management of hypertension in general practice: a randomised controlled trial of feedback derived from electronic patient data. Br J Gen Pract. 2005;55(511):94–101. [PMC free article] [PubMed] [Google Scholar]
- 152.Moher M, Yudkin P, Wright L, Turner R, Fuller A, Schofield T, et al. Cluster randomised controlled trial to compare three methods of promoting secondary prevention of coronary heart disease in primary care. BMJ. 2001;322(7298):1338. doi: 10.1136/bmj.322.7298.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morganroth M, Pape G, Rozenfeld Y, Heffner JE. Multidisciplinary COPD disease management program: impact on clinical outcomes. Postgrad Med. 2016;128(2):239–249. doi: 10.1080/00325481.2016.1129259. [DOI] [PubMed] [Google Scholar]
- 154.Moriyama M, Nakano M, Kuroe Y, Nin K, Niitani M, Nakaya T. Efficacy of a self-management education program for people with type 2 diabetes: results of a 12 month trial. Jpn J Nurs Sci. 2009;6(1):51–63. doi: 10.1111/j.1742-7924.2009.00120.x. [DOI] [PubMed] [Google Scholar]
- 155.Murphy AW, Cupples M, Smith S, Byrne M, Byrne M, Newell J. Effect of tailored practice and patient care plans on secondary prevention of heart disease in general practice: cluster randomised controlled trial. BMJ. 2009;339. [DOI] [PMC free article] [PubMed]
- 156.Nguyen HQ, Donesky D, Reinke LF, Wolpin S, Chyall L, Benditt JO, et al. Internet-based dyspnea self-management support for patients with chronic obstructive pulmonary disease. J Pain Symptom Manag. 2013;46(1):43–55. doi: 10.1016/j.jpainsymman.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ojeda S, Anguita M, Delgado M, Atienza F, Rus C, Granados AL, et al. Short-and long-term results of a programme for the prevention of readmissions and mortality in patients with heart failure: are effects maintained after stopping the programme? Eur J Heart Fail. 2005;7(5):921–926. doi: 10.1016/j.ejheart.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 158.Olson KL, Delate T, Rasmussen J, Humphries TL, Merenich JA. Outcomes of patients discharged from pharmacy-managed cardiovascular disease management. Am J Manag Care. 2009;15(8):497–503. [PubMed] [Google Scholar]
- 159.Ortiz-Bautista C, Diaz M, Delgado-Nicolas M, Moran-Fernandez L, De Juan-Baguda J, Ponz I, et al., editors. Evaluation of a nurse-led cross intervention program in heart failure. Eur J Heart Fail; 2017: WILEY 111 RIVER ST, HOBOKEN 07030–5774, NJ USA.
- 160.Ostwald SK, Godwin KM, Cron SG, Kelley CP, Hersch G, Davis S. Home-based psychoeducational and mailed information programs for stroke-caregiving dyads post-discharge: a randomized trial. Disabil Rehabil. 2014;36(1):55–62. doi: 10.3109/09638288.2013.777806. [DOI] [PubMed] [Google Scholar]
- 161.Peters-Klimm F, Campbell S, Hermann K, Kunz CU, Müller-Tasch T, Szecsenyi J. Case management for patients with chronic systolic heart failure in primary care: the HICMan exploratory randomised controlled trial. Trials. 2010;11(1):1–14. doi: 10.1186/1745-6215-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Piette JD, Striplin D, Marinec N, Chen J, Trivedi RB, Aron DC, et al. A mobile health intervention supporting heart failure patients and their informal caregivers: a randomized comparative effectiveness trial. J Med Internet Res. 2015;17(6):e4550. doi: 10.2196/jmir.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pinnock H, Hanley J, McCloughan L, Todd A, Krishan A, Lewis S, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ. 2013;347. [DOI] [PMC free article] [PubMed]
- 164.Pols AD, Van Dijk SE, Bosmans JE, Hoekstra T, Van Marwijk HW, Van Tulder MW, et al. Effectiveness of a stepped-care intervention to prevent major depression in patients with type 2 diabetes mellitus and/or coronary heart disease and subthreshold depression: a pragmatic cluster randomized controlled trial. PLoS ONE. 2017;12(8):e0181023. doi: 10.1371/journal.pone.0181023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J. 2004;34(11):608–614. doi: 10.1111/j.1445-5994.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- 166.Reiber GE, Au D, McDonell M, Fihn SD. Diabetes quality improvement in Department of Veterans Affairs Ambulatory Care Clinics: a group-randomized clinical trial. Diabetes Care. 2004;27(suppl_2):b61–b8. doi: 10.2337/diacare.27.suppl_2.B61. [DOI] [PubMed] [Google Scholar]
- 167.Rovner BW, Casten RJ, Piersol CV, White N, Kelley M, Leiby BE. Improving glycemic control in African Americans with diabetes and mild cognitive impairment. J Am Geriatr Soc. 2020;68(5):1015–1022. doi: 10.1111/jgs.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rubenstein LZ, Alessi CA, Josephson KR, Trinidad Hoyl M, Harker JO, Pietruszka FM. A randomized trial of a screening, case finding, and referral system for older veterans in primary care. J Am Geriatr Soc. 2007;55(2):166–174. doi: 10.1111/j.1532-5415.2007.01044.x. [DOI] [PubMed] [Google Scholar]
- 169.Saal S, Becker C, Lorenz S, Schubert M, Kuss O, Stang A, et al. Effect of a stroke support service in Germany: a randomized trial. Top Stroke Rehabil. 2015;22(6):429–436. doi: 10.1179/1074935714Z.0000000047. [DOI] [PubMed] [Google Scholar]
- 170.Sackley CM, Walker MF, Burton CR, Watkins CL, Mant J, Roalfe AK, et al. An occupational therapy intervention for residents with stroke related disabilities in UK care homes (OTCH): cluster randomised controlled trial. BMJ. 2015;350. [DOI] [PMC free article] [PubMed]
- 171.Salinero-Fort MA, Pau EC-dS, Arrieta-Blanco FJ, Abanades-Herranz JC, Martín-Madrazo C, Rodés-Soldevila B, et al. Effectiveness of PRECEDE model for health education on changes and level of control of HbA1c, blood pressure, lipids, and body mass index in patients with type 2 diabetes mellitus. BMC Public Health. 2011;11(1):1–9. doi: 10.1186/1471-2458-11-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Salisbury C, Man M-S, Bower P, Guthrie B, Chaplin K, Gaunt DM, et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet. 2018;392(10141):41–50. doi: 10.1016/S0140-6736(18)31308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Samus QM, Johnston D, Black BS, Hess E, Lyman C, Vavilikolanu A, et al. A multidimensional home-based care coordination intervention for elders with memory disorders: the maximizing independence at home (MIND) pilot randomized trial. Am J Geriatr Psychiatry. 2014;22(4):398–414. doi: 10.1016/j.jagp.2013.12.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sánchez-Nieto JM, Andújar-Espinosa R, Bernabeu-Mora R, Hu C, Gálvez-Martínez B, Carrillo-Alcaraz A, et al. Efficacy of a self-management plan in exacerbations for patients with advanced COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1939. doi: 10.2147/COPD.S104728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sarkadi A, Rosenqvist U. Experience-based group education in type 2 diabetes: a randomised controlled trial. Patient Educ Couns. 2004;53(3):291–298. doi: 10.1016/j.pec.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 176.Schäfer I, Kaduszkiewicz H, Mellert C, Löffler C, Mortsiefer A, Ernst A, et al. Narrative medicine-based intervention in primary care to reduce polypharmacy: results from the cluster-randomised controlled trial MultiCare AGENDA. BMJ Open. 2018;8(1):e017653. doi: 10.1136/bmjopen-2017-017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schrader G, Cheok F, Hordacre AL, Marker J, Wade V. Effect of psychiatry liaison with general practitioners on depression severity in recently hospitalised cardiac patients: a randomised controlled trial. Med J Aust. 2005;182(6):272–276. doi: 10.5694/j.1326-5377.2005.tb06699.x. [DOI] [PubMed] [Google Scholar]
- 178.Smith BJ, Appleton S, Bennett P, Roberts G, Fante PD, Adam R, et al. The effect of a respiratory home nurse intervention in patients with chronic obstructive pulmonary disease (COPD) Aust N Z J Med. 1999;29(5):718–725. doi: 10.1111/j.1445-5994.1999.tb01621.x. [DOI] [PubMed] [Google Scholar]
- 179.Solomon DH, Iversen MD, Avorn J, Gleeson T, Brookhart MA, Patrick AR, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. 2012;172(6):477–483. doi: 10.1001/archinternmed.2011.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sönnichsen AC, Winkler H, Flamm M, Panisch S, Kowatsch P, Klima G, et al. The effectiveness of the Austrian disease management programme for type 2 diabetes: a cluster-randomised controlled trial. BMC Fam Pract. 2010;11(1):1–10. doi: 10.1186/1471-2296-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stewart S, Ball J, Horowitz JD, Marwick TH, Mahadevan G, Wong C, et al. Standard versus atrial fibrillation-specific management strategy (SAFETY) to reduce recurrent admission and prolong survival: pragmatic, multicentre, randomised controlled trial. Lancet. 2015;385(9970):775–784. doi: 10.1016/S0140-6736(14)61992-9. [DOI] [PubMed] [Google Scholar]
- 182.Stewart S, Carrington MJ, Marwick TH, Davidson PM, Macdonald P, Horowitz JD, et al. Impact of home versus clinic-based management of chronic heart failure: the WHICH?(Which Heart Failure Intervention Is Most Cost-Effective & Consumer Friendly in Reducing Hospital Care) multicenter, randomized trial. J Am Coll Cardiol. 2012;60(14):1239–1248. doi: 10.1016/j.jacc.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 183.Subramanian U, Fihn SD, Weinberger M, Plue L, Smith FE, Udris EM, et al. A controlled trial of including symptom data in computer-based care suggestions for managing patients with chronic heart failure. Am J Med. 2004;116(6):375–384. doi: 10.1016/j.amjmed.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 184.Suominen MH, Puranen T, Jyväkorpi S, Eloniemi-Sulkava U, Kautiainen H, Siljamäki-Ojansuu U, et al. Nutritional guidance improves nutrient intake and quality of life, and may prevent falls in aged persons with Alzheimer disease living with a spouse (NuAD trial) J Nutr Health Aging. 2015;19(9):901–907. doi: 10.1007/s12603-015-0558-0. [DOI] [PubMed] [Google Scholar]
- 185.Tomita MR, Tsai B-M, Fisher NM, Kumar NA, Wilding G, Stanton K, et al. Effects of multidisciplinary Internet-based program on management of heart failure. J Multidiscip Healthc. 2009;2:13. doi: 10.2147/jmdh.s4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Trento M, Gamba S, Gentile L, Grassi G, Miselli V, Morone G, et al. Rethink Organization to iMprove Education and Outcomes (ROMEO): a multicenter randomized trial of lifestyle intervention by group care to manage type 2 diabetes. Diabetes Care. 2010;33(4):745–747. doi: 10.2337/dc09-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vaillant-Roussel H, Laporte C, Pereira B, De Rosa M, Eschalier B, Vorilhon C, et al. Impact of patient education on chronic heart failure in primary care (ETIC): a cluster randomised trial. BMC Fam Pract. 2016;17(1):1–13. doi: 10.1186/s12875-016-0473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.van de Ven G, Draskovic I, Adang EM, Donders R, Zuidema SU, Koopmans RT, et al. Effects of dementia-care mapping on residents and staff of care homes: a pragmatic cluster-randomised controlled trial. PLoS ONE. 2013;8(7):e67325. doi: 10.1371/journal.pone.0067325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.van der Aa HP, van Rens GH, Comijs HC, Margrain TH, Gallindo-Garre F, Twisk JW, et al. Stepped care for depression and anxiety in visually impaired older adults: multicentre randomised controlled trial. BMJ. 2015;351. [DOI] [PMC free article] [PubMed]
- 190.van Wetering CR, Hoogendoorn M, Mol S, Rutten-van Mölken M, Schols A. Short-and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax. 2010;65(1):7–13. doi: 10.1136/thx.2009.118620. [DOI] [PubMed] [Google Scholar]
- 191.Vinereanu D, Lopes RD, Bahit MC, Xavier D, Jiang J, Al-Khalidi HR, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. The Lancet. 2017;390(10104):1737–1746. doi: 10.1016/S0140-6736(17)32165-7. [DOI] [PubMed] [Google Scholar]
- 192.Wagenaar KP, Broekhuizen BD, Jaarsma T, Kok I, Mosterd A, Willems FF, et al. Effectiveness of the European Society of Cardiology/Heart Failure Association website ‘heartfailurematters.org’and an e-health adjusted care pathway in patients with stable heart failure: results of the ‘e-Vita HF’randomized controlled trial. Eur J Heart Failure. 2019;21(2):238–46. doi: 10.1002/ejhf.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Waldorff F, Buss D, Eckermann A, Rasmussen M, Keiding N, Rishøj S, et al. Efficacy of psychosocial intervention in patients with mild Alzheimer’s disease: the multicentre, rater blinded, randomised Danish Alzheimer Intervention Study (DAISY). Bmj. 2012;345. [DOI] [PMC free article] [PubMed]
- 194.Walters J, Cameron-Tucker H, Wills K, Schüz N, Scott J, Robinson A, et al. Effects of telephone health mentoring in community-recruited chronic obstructive pulmonary disease on self-management capacity, quality of life and psychological morbidity: a randomised controlled trial. BMJ Open. 2013;3(9):e003097. doi: 10.1136/bmjopen-2013-003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Whittle J, Schapira MM, Fletcher KE, Hayes A, Morzinski J, Laud P, et al. A randomized trial of peer-delivered self-management support for hypertension. Am J Hypertens. 2014;27(11):1416–1423. doi: 10.1093/ajh/hpu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wilcock J, Iliffe S, Griffin M, Jain P, Thuné-Boyle I, Lefford F, et al. Tailored educational intervention for primary care to improve the management of dementia: the EVIDEM-ED cluster randomized controlled trial. Trials. 2013;14(1):1–10. doi: 10.1186/1745-6215-14-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wright LK, Litaker M, Laraia MT, DeAndrade S. Continuum of care for Alzheimer's disease: a nurse education and counseling program. Issues Ment Health Nurs. 2001;22(3):231–252. doi: 10.1080/01612840152053084. [DOI] [PubMed] [Google Scholar]
- 198.Xi F, Wang Z, Qi Y, Brightwell R, Roberts P, Stewart A, et al. Long-term effect of respiratory training for chronic obstructive pulmonary disease patients at an outpatient clinic: a randomised controlled trial. Clin Transl Med. 2015;4(1):1–7. doi: 10.1186/s40169-015-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xiao LD, De Bellis A, Kyriazopoulos H, Draper B, Ullah S. The effect of a personalized dementia care intervention for caregivers from Australian minority groups. Am J Alzheimers Dis Other Demen. 2016;31(1):57–67. doi: 10.1177/1533317515578256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zwar NA, Bunker JM, Reddel HK, Dennis SM, Middleton S, van Schayck OC, et al. Early intervention for chronic obstructive pulmonary disease by practice nurse and GP teams: a cluster randomized trial. Fam Pract. 2016;33(6):663–670. doi: 10.1093/fampra/cmw077. [DOI] [PubMed] [Google Scholar]
- 201.Fortinsky RH, Kulldorff M, Kleppinger A, Kenyon-Pesce L. Dementia care consultation for family caregivers: collaborative model linking an Alzheimer's association chapter with primary care physicians. Aging Ment Health. 2009;13(2):162–170. doi: 10.1080/13607860902746160. [DOI] [PubMed] [Google Scholar]
- 202.Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 1970;23(7):455–468. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 203.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7(4):357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shelton RC, Cooper BR, Stirman SW. The sustainability of evidence-based interventions and practices in public health and health care. Annu Rev Public Health. 2018;39:55–76. doi: 10.1146/annurev-publhealth-040617-014731. [DOI] [PubMed] [Google Scholar]
- 205.Stokes G, Sutcliffe K, Thomas J. Is a one-size-fits-all '12-month rule' appropriate when it comes to the last search date in systematic reviews?. BMJ Evid Based Med. 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. Systematic Review Methods. Appendix 2. Protocol Deviations Summary Sheet. Appendix 3. Delphi Results. Appendix 4. KT sustainability outcome definitions. Appendix 5. Search Strategy for MEDLINE. Appendix 6. Coding Guide for KT Intervention Components Using EPOC Taxonomy Coding. Appendix 7. Coding Guides for Clinical Intervention Components using BCT coding. Appendix 8. List of Included Studies. Appendix 9. Individual Study Characteristics. Appendix 10. Individual Patient Characteristics. Appendix 11. Sustainability of KT Interventions Summarized Results. Appendix 12. Cochrane Effective Practice and Organisation of Care (EPOC) Risk of Bias Results. Appendix 13. Contour-Enhanced Funnel Plots. Appendix 14. Additional Analysis Results. Appendix 15. Individual Study Results. Appendix 16. Meta-analysis Results of All Interventions vs Usual Care. Appendix 17. Subgroup Analyses of All KT Interventions vs Usual Care. Appendix 18. Sensitivity Analyses of All KT Interventions vs Usual Care. Appendix 19. Meta-regression for Each Outcome/Scale Comparing Any KT Intervention vs Usual Care.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional file. The full list of excluded studies from this review at level 1 (titles/abstracts) or level 2 (full-texts) will be made available upon request.