Abstract
Background and aims
Preterm birth has been linked with an increased risk of cardiovascular (CV) disease from childhood into adolescence and early adulthood. In this study, we aimed to investigate differences in CV health profiles between former term- and preterm-born infants in a cohort of Tyrolean adolescents.
Methods
The Early Vascular Aging (EVA)-Tyrol study is a population-based non-randomized controlled trial, which prospectively enrolled 14- to 19-year-old adolescents in North Tyrol, Austria and South Tyrol, Italy between 2015 and 2018. Metrics of CV health (body mass index (BMI), systolic (SBP) and diastolic blood pressure (DBP), smoking, physical activity, dietary patterns, total cholesterol and fasting blood glucose) were assessed and compared between former term- and preterm-born girls and boys.
Results
In total, 1,491 study participants (59.5% female, mean age 16.5 years) were included in the present analysis. SBP and DBP were significantly higher in former preterm-born adolescents (mean gestational age 34.6 ± 2.4 weeks) compared to term-born controls (p < 0.01). In the multivariate regression analysis these findings remained significant after adjustment for potential confounders in all models. No differences were found in all other CV health metrics. The number of participants meeting criteria for all seven health metrics to be in an ideal range was generally very low with 1.5% in former term born vs. 0.9% in former preterm born adolescents (p = 0.583).
Conclusions
Preterm birth is associated with elevated SBP and DBP in adolescence, which was even confirmed for former late preterm-born adolescents in our cohort. Our findings underscore the importance of promoting healthy lifestyles in former term- as well as preterm-born adolescents. In addition, we advise early screening for hypertension and long-term follow-up in the group of preterm-born individuals.
Keywords: Preterm birth, Cardiovascular health, Health metrics, Risk factors, Adolescents, Health promotion
Introduction
Preterm birth is defined as a live birth that occurs before 37 completed weeks of pregnancy [1]. About 15 million children are born prematurely worldwide every year, which corresponds to a preterm birth rate of about 11%. Rates vary by the countries´ standard of living from approximately 9% in Europe to the highest rates of over 13% in low-income countries in Asia and sub-Saharan Africa [1, 2]. According to the World Health Organization prematurity and related complications are the leading causes of mortality and morbidity in children under five years of age worldwide [3, 4]. However, due to advances in perinatal care, survival rates and survival without major morbidity have continuously improved over the past 20 years in high- and middle-income countries [5, 6]. With an increasing number of former preterm infants reaching adolescence and adulthood, new health challenges arise [7] which affect the respiratory tract, the endocrine system, and the psycho-motoric development [8–10]. In addition, prematurity has been linked to altered CV health profiles at a pre-school age [11], as well as in early adolescence and adulthood [12–14] resulting in an increased risk of CV and cardiometabolic disease [15, 16]. It has been shown that young adults born preterm are less physically active in sports in their leisure time [17, 18] or consume a more unhealthy diet than their term-born peers [19]. As these differences are modifiable by appropriate lifestyle changes, it is of great interest to identify subgroups at risk for CV and cardiometabolic disease to plan targeted intervention programs and health promotions accordingly [20]. Furthermore, there is evidence that preterm birth is associated with a higher total fat mass and more abdominal fat, which may have detrimental effects on cardiometabolic and CV health [15]. Although manifest CV and cardiometabolic disease is a rare condition in youth, its antecedents may develop early in life. There is evidence that vascular alterations like thickening of arterial vessel wall already starts in the first decades of life and is associated with classical risk factors (i.e., high BMI, elevated SBP, hypercholesterolemia or smoking) [21–23].
The aim of the present study was to investigate differences in CV health profiles between former term and preterm infants in a cohort of Tyrolean adolescents. To the best of our knowledge, we are the first to investigate the impact of preterm birth on the seven CV health metrics defined by the American Heart Association (AHA) [20] in Central Europe.
Methods
Study population and design
The present study was conducted as part of the EVA-Tyrol study, a population-based, non-randomized controlled trial at the Department of Pediatrics II at the Medical University of Innsbruck. EVA-Tyrol is aiming to assess the prevalence of CV health profiles and the efficacy of a health promotion intervention on CV risk factors and health behaviors in a cohort of adolescents in the whole federal province of Tyrol (Austria) with a population of about 745,000 inhabitants and in Bruneck, a city in the autonomous province of Bolzano/South Tyrol (Italy) with about 80,000 inhabitants. Local schools and big companies were contacted and pupils and apprentices with a target age between 14 and 19 years were invited to take part in this study. Whole school classes were recruited thus some older students or apprentices, who wanted to participate were also included. 2273 persons did express their interest in participation in the EVA Tyrol study, yet 171 did either not sign the informed consent or signed the informed consent but did not show up on the examination day. For the latter group no study specific measures were performed. All participants provided a written informed consent or if the participants had not attained the age of 18 years, a parent or legal guardian additionally provided written consent.
The study was designed with an intervention group, which received a baseline examination and was invited to a follow-up examination after receiving a health intervention, and a control group. For this exploratory data analysis, information from both the baseline and the control group without health intervention was used to achieve a representative cohort of the adolescent population, in the following they are referred to as the EVA-cohort. A detailed description of the study protocol has been previously published [24]. The study was performed in accordance with the Declaration of Helsinki, ethical approval was granted from the review board of the Medical University of Innsbruck, Austria (approval number AN 2015–0005 345/4.13) and all participants and legal representatives signed informed consent for participation. The study is registered at www.clinical.trials.gov(NCT number 03929692).
The present study represents a subanalysis of the EVA-Tyrol study and highlights data on the impact of preterm birth on cardiovascular health.
Perinatal characteristics
All participating adolescents were asked bringing the so-called “mother–child booklet” with them on the day of examination. Within this official medical document health data about pregnancy, birth and regular pediatric examinations during the first 5 years of life are documented in Austria and Italy. We registered infant data on perinatal characteristics, namely gestational age (GA) and birth weight. To account for gender- and GA-specific differences, birth weight z-scores were calculated for every subject using the Fenton 2013 reference dataset [25]. Small for gestational age (SGA) was defined as birth weight < 10th percentile for GA and sex. Preterm birth was defined as being born < 37 completed weeks of gestation. Definition of early preterm was birth before 32 completed weeks of gestation, moderately preterm was defined as GA between 32–34 weeks of gestation and late preterm between 34–37 weeks of gestation.
CV health was assessed by the concept of the seven so called CV health metrics defined by the AHA including three health factors (SBP and DBP, total cholesterol and fasting blood glucose) and four health behaviors (non-smoking, BMI, regular physical activity and favorable dietary patterns) [20].
Anthropometry
For anthropometric measurements study participants wore light indoor clothes without shoes. Weight was assessed using calibrated medical precision scales and height was determined using a Harpenden stadiometer (Holtain, Crymych, United Kingdom). BMI was calculated as body weight in kilograms divided by the square of height in meters and converted to percentiles according to reference data by Kromeyer-Hauschild et al. [26]. SBP and DBP were computed as the mean of 3 independent measurements on the left and right upper arm in a sitting position and recorded after at least 5 min at rest by an automated oscillometric device (OMRON M4-I, Omron Healthcare Co., Lake Forest, Illinois, USA). Z-scores and percentiles for SBP and DBP were calculated based on a reference data set [27].
Assessment of lifestyle risk factors
Behavioral risk factors, such as smoking status and physical activity, were assessed in a standardized medical interview conducted by trained medical staff in attendance of a specialist in pediatrics, compiled and adapted from the questionnaires from the Atherosclerosis Risk-Factors in Male Youngsters, Atherosclerosis Risk-Factors in Female Youngsters, and Bruneck studies [21, 22, 28]. Individuals were categorized as smokers if they smoked at least one cigarette per week on a regular basis. Physical activity was recorded as participation in moderate or vigorous intensity sports (e.g., leading to an increase in heart rate and/or sweating) as average amount of minutes per day. Dietary habits were evaluated according to a score based on the Dietary Approaches to Stop Hypertension- (DASH-) diet consisting of five favorable components [29]. For meeting the criteria one point is scored for each of the following components: four to five servings of fruit and vegetable per day; at least two fish meals per week; at least three servings of whole-grain products per day, each about 30 g; less than 1.5 g of salt per day and a maximum of one liter of sugar-sweetened beverages per week, i.e., not more than 450 kcal per week, respectively.
Blood sample collection and laboratory measurements
Blood samples were taken early in the morning after an overnight fasting of at least eight hours and were immediately stored in cooling boxes (at approximately 4 °C). After rapid transport to the ISO-certified Central Institute for Medical and Chemistry Laboratory Diagnosis at Medical University of Innsbruck serum glucose was assessed by a hexokinase method (Cobas 8000, Roche Diagnostics, Rotkreuz, Switzerland). Total cholesterol was determined by standard enzymatic colorimetric assays (Cobas 8000, Roche Diagnostics, Rotkreuz, Switzerland).
Assessment of socio-economic status
The Family Affluence Score (FAS) was used to determine the socio-economic status. Study participants were asked about four status items (including owning a car in the family, having an own bedroom, going on vacations and number of computers in the household) and classified into the categories high, middle and low affluence [30]. Low FAS occurred in less than 1% of cases in our cohort so that it was combined with the middle affluence category for this analysis.
Classification of education
After nine years of compulsory school attendance, the Austrian education system offers three different educational pathways. In secondary high schools adolescents receive general academic education, secondary vocational schools provide general education as well as occupation-specific knowledge or adolescents may be trained as apprentices within companies.
Statistical analysis
Statistical analysis was performed using SPSS version 27.0 for Windows (IBM Corporation, Armonk, New York). Data of the study characteristics at baseline and follow-up are shown as mean ± SD or median (interquartile ranges) and categorical variables as count (percentages). Differences in CV health metrics were determined using t-test or Mann–Whitney-U-test (depending on data distribution) and Pearson χ2- test (for categorical variables). To assess the relationship between prematurity and CV health metrics in the preterm and the term group binary logistic regression with progressive adjustment was used to estimate. A stepwise approach was used with regard to covariates and potential confounders. Model A was unadjusted. Parameters included in the model were SPB or DBP, sex and age at examination in Model B, SPB or DBP, sex, age at examination, BMI, smoking status, physical activity and diet in Model C, SPB or DBP, sex, age at examination, BMI, smoking status, physical activity, diet and SGA in Model D and SPB or DBP, sex, age at examination, BMI, smoking status, physical activity, diet, SGA, fasting blood glucose and total cholesterol in Model E. P-values of less than 0.05 were considered statistically significant.
Results
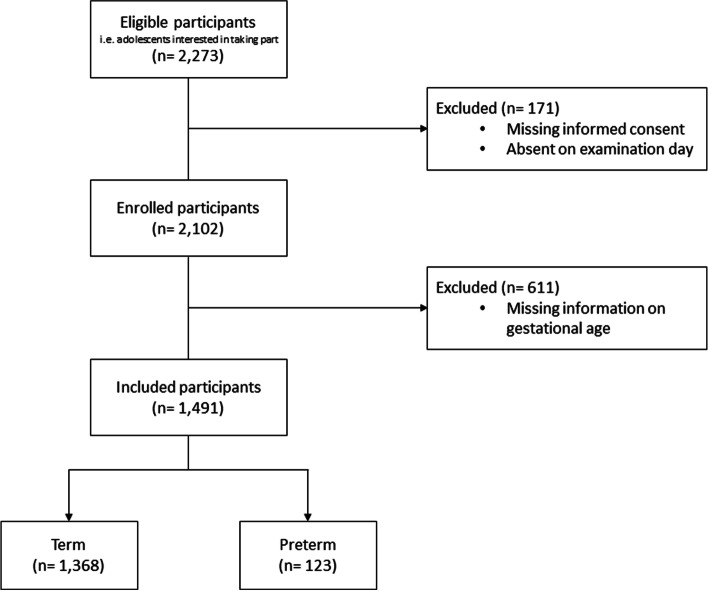
A total number of 2,273 adolescents was assessed for eligibility, of which 171 were excluded due to missing informed consent or absence on examination day. Thus, 2,102 adolescents were included for the following investigation forming the EVA-cohort, with 1,573 participants in the baseline cohort and 529 adolescents who did not undergo a health intervention. Information on GA was missing in 611 cases leaving 1,491 young adults for further analysis of differences between former term- and preterm-born infants (Fig. 1).
Fig. 1.
Flow chart for inclusion of study participants
Characteristics of the study cohort are displayed in Table 1.
Table 1.
Characteristics of the study population
| Parameter | Total population n = 1491 |
Term n = 1368 (91.8%) |
Preterm n = 123 (8.2%) |
P-value* |
|---|---|---|---|---|
| Age at examination, years | 16.3 ± 1.1 | 16.4 ± 1.1 | 16.2 ± 1.1 | 0.177a |
| Sex, female (%) | 887 (59.5) | 819 (59.9) | 68 (55.3) | 0.338b |
| FAS-Score, high (%) | 984 (66.0) | 906 (66.2) | 78 (63.4) | 0.523b |
| Education (%) | ||||
| High school | 507 (34.0) | 468 (34.2) | 39 (31.7) | 0.575b |
| Vocational school | 820 (55.0) | 746 (54.5) | 74 (60.2) | 0.229b |
| Trainee | 164 (11.0) | 154 (11.3) | 10 (8.1) | 0.288b |
| Mean gestational age, weeks | 39.4 ± 2.0 | 39.8 ± 1.2 | 34.6 ± 2.4 | < 0.01c |
| Non-smokers (%) | 1086 (72.8) | 997 (72.9) | 89 (72.4) | 0.831b |
| Healthy diet score, components (n) | 2.0 ± 1.1 | 2.0 ± 1.1 | 2.1 ± 1.1 | 0.495a |
| Physical activity (min/day) | 53.0 (30–60) | 53.1 (25–60) | 52.6 (30–60) | 0.362c |
| BMI (kg/m2) | 21.8 ± 3.5 | 21.8 ± 3.5 | 21.5 ± 3.3 | 0.510c |
| SBP (mmHg) | 122.3 11.5 | 122.1 ± 11.5 | 124.5 ± 11.7 | 0.024c |
| z-score Systolic BP | 0.6 ± 1.1 | 0.6 ± 1.1 | 0.8 ± 1.0 | 0.014c |
| DBP (mmHg) | 71.3 ± 7.6 | 71.1 ± 7.6 | 72.9 ± 7.6 | 0.026c |
| z-score Diastolic BP | 0.2 ± 1.1 | 0.2 ± 1.1 | 0.5 ± 1.1 | 0.01c |
| Total cholesterol (mg/dl) | 160.0 (138.0–177.5) | 160.0 (139.0–178.0) | 157.6 (137.0–175.0) | 0.277c |
| Fasting blood glucose (mg/dl) | 76.7 ± 9.4 | 76.7 ± 9.4 | 77.2 ± 9.0 | 0.571a |
Values are displayed as n (%), mean ± SD, median (IQR) or %. BMI Body Mass Index, DBP diastolic blood pressure, SBP systolic blood pressure, FAS Family Affluence Score, *P values are derived from aStudent ´s t-test, bPearson-χ 2- test and cMann-Whitney-U-Test
Adolescents were categorized into the term (n = 1,368, 91.8%) and the preterm (n = 123, 8.2%) group with a mean gestational age of 39.8 (± 1.2) for the term and 34.6 (± 2.4) weeks for the preterm group, respectively. The distribution of girls and boys in the groups did not differ significantly (p = 0.322). No significant differences regarding socioeconomic status (p = 0.523) and level of education (p = 0.394) between the two groups of former term- and preterm-born infants were observed. In the total population, SBP (p = 0.024) and DBP (p = 0.026) were significantly higher in former preterm-born adolescents compared to term-born controls. This was also shown for SBP z-scores (p = 0.014) and DBP z-scores (p = 0.01). No differences were found in all other CV health metrics. In the multivariate binary logistic regression model higher SBP and DBP values in the preterm compared to the term population remained significant in all models that adjusted for potential confounding factors. After adjustment for sex, age at examination, BMI, smoking status, physical activity, diet, SGA, fasting blood glucose and total cholesterol a beta coefficient of -0.028 (Std. error 0.01), OR = 0.972 (95% CI: 0.953, 0.991) for SBP and a beta coefficient of -0.039 (Std. error 0.013), OR = 0.961 (95% CI: 0.937, 0.987) for DBP was observed. The results of multivariate testing are listed in Tables 2 and 3.
Table 2.
Binary logistic regression on the effect of prematurity on systolic blood pressure
| Model | Parameter | β | Std. Error | p-value | Exp (B) | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Model A | Systolic blood pressure | -0.018 | 0.008 | 0.028 | 0.982 | 0.967 – 0.998 |
| Model B | Systolic blood pressure | -0.019 | 0.009 | 0.032 | 0.981 | 0.964 – 0.998 |
| Sex | -0.002 | 0.209 | 0.991 | 0.998 | 0.663 – 1.502 | |
| Age | 0.139 | 0.088 | 0.114 | 1.149 | 0.967 – 1.365 | |
| Model C | Systolic blood pressure | -0.025 | 0.009 | 0.009 | 0.975 | 0.957 – 0.994 |
| Sex | -0.048 | 0.22 | 0.828 | 0.953 | 0.619 – 1.468 | |
| Age | 0.134 | 0.092 | 0.145 | 1.143 | 0.955 – 1.369 | |
| BMI | 0.052 | 0.031 | 0.095 | 1.054 | 0.991 – 1.121 | |
| Smoking | -0.17 | 0.225 | 0.451 | 0.844 | 0.543 – 1.312 | |
| Physical activity | 0.002 | 0.003 | 0.42 | 1.002 | 0.997 – 1.007 | |
| Diet | -0.71 | 0.09 | 0.427 | 0.931 | 0.781 – 1.11 | |
| Model D | Systolic blood pressure | -0.025 | 0.009 | 0.009 | 0.976 | 0.958 – 0.994 |
| Sex | -0.053 | 0.22 | 0.81 | 0.949 | 0.616 – 1.461 | |
| Age | 0.136 | 0.092 | 0.14 | 1.145 | 0.957 – 1.372 | |
| BMI | 0.054 | 0.032 | 0.089 | 1.055 | 0.992 – 1.122 | |
| Smoking | -0.168 | 0.225 | 0.455 | 0.845 | 0.544 – 1.313 | |
| Physical activity | 0.002 | 0.003 | 0.407 | 1.002 | 0.997 – 1.007 | |
| Diet | -0.71 | 0.09 | 0.428 | 0.931 | 0.781 – 1.11 | |
| SGA | -0.241 | 0.345 | 0.485 | 0.786 | 0.4 – 1.545 | |
| Model E | Systolic blood pressure | -0.028 | 0.01 | 0.004 | 0.972 | 0.953 – 0.991 |
| Sex | 0.026 | 0.248 | 0.918 | 1.026 | 0.631 – 1.668 | |
| Age | 0.071 | 0.093 | 0.447 | 1.073 | 0.894 – 1.289 | |
| BMI | 0.68 | 0.033 | 0.041 | 1.071 | 1.003 – 1.143 | |
| Smoking | -0.189 | 0.233 | 0.417 | 0.827 | 0.524 – 1.308 | |
| Physical activity | 0.002 | 0.003 | 0.462 | 1.002 | 0.997 – 1.007 | |
| Diet | -0.1 | 0.093 | 0.282 | 0.905 | 0.754 – 1.086 | |
| SGA | -0.222 | 0.363 | 0.541 | 0.801 | 0.393 – 1.632 | |
| Fasting blood Glucose | -0.003 | 0.011 | 0.751 | 0.997 | 0.975 – 1.018 | |
| Total cholesterol | 0.003 | 0.004 | 0.399 | 1.003 | 0.996 – 1.01 |
p-values are derived from multivariate binary logistic regression with the dependent variable being born term or preterm, β Beta Coefficient, BMI Body Mass Index, Exp (B) Odds Ratio, SGA Small for gestational age, Std. Error Standard Error
Table 3.
Binary logistic regression on the effect of prematurity on diastolic blood pressure
| Model | Parameter | β | Std. Error | p-value | Exp (B) | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Model A | Diastolic blood pressure | -0.03 | 0.012 | 0.013 | 0.97 | 0.947 – 0.994 |
| Model B | Diastolic blood pressure | -0.031 | 0.012 | 0.01 | 0.969 | 0.946 – 0.993 |
| Sex | -0.211 | 0.192 | 0.272 | 0.81 | 0.557 – 1.179 | |
| Age | 0.128 | 0.088 | 0.145 | 1.136 | 0.957 – 1.349 | |
| Model C | Diastolic blood pressure | -0.034 | 0.013 | 0.007 | 0.966 | 0.943 – 0.991 |
| Sex | -0.293 | 0.203 | 0.148 | 0.746 | 0.501 – 1.11 | |
| Age | 0.122 | 0.092 | 0.182 | 1.13 | 0.944 – 1.352 | |
| BMI | 0.038 | 0.029 | 0.197 | 1.038 | 0.981 – 1.1 | |
| Smoking | -0.172 | 0.225 | 0.446 | 0.842 | 0.542 – 1.309 | |
| Physical activity | 0.001 | 0.003 | 0.648 | 1.001 | 0.996 – 1.006 | |
| Diet | -0.08 | 0.09 | 0.372 | 0.923 | 0.774 – 1.1 | |
| Model D | Diastolic blood pressure | -0.034 | 0.013 | 0.007 | 0.966 | 0.943 – 0.991 |
| Sex | -0.298 | 0.203 | 0.141 | 0.742 | 0.499 – 1.104 | |
| Age | 0.125 | 0.092 | 0.174 | 1.133 | 0.946 – 1.356 | |
| BMI | 0.04 | 0.029 | 0.178 | 1.04 | 0.982 – 1.102 | |
| Smoking | -0.17 | 0.225 | 0.449 | 0.843 | 0.543 – 1.311 | |
| Physical activity | 0.001 | 0.003 | 0.631 | 1.001 | 0.996 – 1.006 | |
| Diet | -0.08 | 0.09 | 0.374 | 0.923 | 0.775 – 1.101 | |
| SGA | -0.264 | 0.346 | 0.444 | 0.768 | 0.39 – 1.511 | |
| Model E | Diastolic blood pressure | -0.039 | 0.013 | 0.003 | 0.961 | 0.937 – 0.987 |
| Sex | -0.252 | 0.229 | 0.272 | 0.777 | 0.496 – 1.218 | |
| Age | 0.057 | 0.093 | 0.538 | 1.059 | 0.883 – 1.271 | |
| BMI | 0.051 | 0.031 | 0.101 | 1.052 | 0.99 – 1.118 | |
| Smoking | -0.191 | 0.234 | 0.413 | 0.826 | 0.523 – 1.306 | |
| Physical activity | 0.001 | 0.003 | 0.717 | 1.001 | 0.996 – 1.006 | |
| Diet | -0.107 | 0.093 | 0.248 | 0.898 | 0.748 – 1.078 | |
| SGA | -0.243 | 0.364 | 0.505 | 0.784 | 0.384 – 1.602 | |
| Fasting blood Glucose | -0.002 | 0.011 | 0.864 | 0.998 | 0.977 – 1.02 | |
| Total cholesterol | 0.004 | 0.004 | 0.295 | 1.004 | 0.997 – 1.011 |
p-values are derived from multivariate binary logistic regression with the dependent variable being born term or preterm, β Regression Coefficient B, BMI Body Mass Index, Exp (B) Odds Ratio, SGA Small for gestational age, Std. Error Standard Error
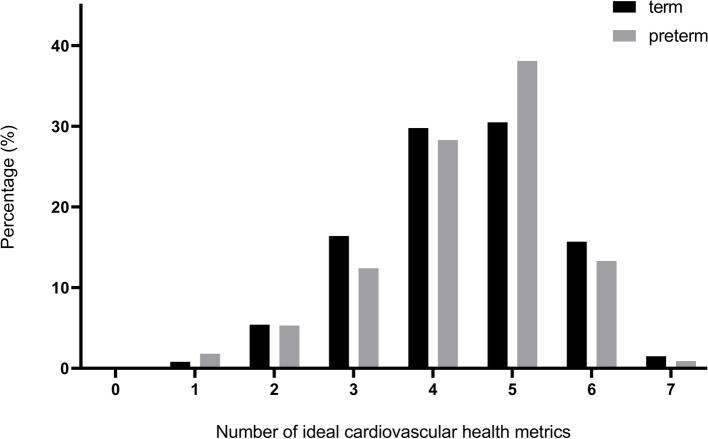
Figure 2 shows the number of health metrics in which participants were able to achieve the ideal range. Only in 1.5% of former term-born adolescents and 0.9% and former preterm-born adolescents all seven CVH determinants were in an ideal range. There was no significant difference in the distribution of health metrics classified as ideal between former term- and preterm-born infants (p = 0.583).
Fig. 2.
Prevalence of ideal cardiovascular health determinants in former term- and preterm-born adolescents. Displays the percentages of adolescents by number of ideal cardiovascular health metrics according to being born term or preterm
Discussion
Although preterm birth rates in developed high-income countries have stagnated or declined over the past 1.5 decades [31], prematurity remains a substantial health determining factor in the pediatric population. Medical advances have led to an increasing number of prematurely born children reaching adolescence and adulthood without major damage [5, 6] and a growing body of literature now focusses on long-term implications of preterm birth. Morbidity and mortality in preterm-born adolescents are increased and the associated costs are a socioeconomic issue [32]. In this regard, a key aspect is the evidence of an increased risk of CV disease for former preterm-born individuals [11–14, 16]. To achieve the AHA´s goals of reducing CV disease, myocardial infarction, and stroke, it is necessary to identify markers of increased CV risk in order to organize effective targeted preventive measures [20]. Therefore, the aim of the present study was to assess CV health metrics (SBP and DBP, BMI, smoking, physical activity, dietary patterns, total cholesterol and fasting blood glucose) and to determine differences between former term- and preterm-born adolescents from North- and South Tyrol, a region in the high-income countries Austria and Italy. Results of our study confirm the findings of previous observations but further underscore the impact of prematurity on CV risk.
Regarding blood pressure, SBP as well as DBP were significantly higher in former preterm-born adolescents compared to term-born controls after adjustment for sex, age at examination, BMI, smoking status, physical activity, diet, being SGA, fasting blood glucose and total cholesterol in the multivariate logistic regression model. This finding is corroborated by several meta-analyses, which have also demonstrated an increased risk for elevated blood pressure in the preterm population [13, 33, 34] and has also been described for former very preterm-born children (mean gestational age 29.3 weeks) at preschool age and for late preterms (mean gestational age 34.8 weeks) in adolescence by our study group previously [11, 35].
Although these differences are small and within normal ranges for this age group, there is evidence that elevated blood pressure levels associated with preterm birth worsen with increasing age [36] and may be responsible for an increased risk of CV diseases in later life [13, 16].
In addition to prenatal factors, such as preeclampsia, gestational diabetes, obesity, intrauterine growth retardation, which lead to adverse fetal programming, there is evidence that a number of other causes play a role in the development of elevated blood pressure in former preterm infants during the postnatal course [13, 33, 37], for instance impaired nephrogenesis, extra uterine growth restriction or forced weight gain caused by high calorie nutrition [13].
Regarding all other health metrics, we could not identify any impact of preterm birth in our cohort. Altered lipid profiles [38] as well as pathologic glucose metabolism [39] have been identified as potential cardiometabolic risk factors in former preterm-born adolescents. However, findings are inconsistent and a relation to the extent of prematurity with the highest risk in the extremely preterm-born ones has been found [40]. Several studies have reported an impact of prematurity on physical activity in later life with extremely preterm ones being less active than their term-born peers [17, 18]. A mean gestational age of 34 weeks in our cohort may explain why no significant difference between former preterm-born and term-born adolescents regarding lipid and glucose metabolism and physical activity was found. In addition, sports are very much promoted in the Tyrol region, which means that also at-risk groups such as former premature individuals can be motivated by the number of activities offered. In addition, the impact of preterm birth was also shown for dietary habits with less healthy dietary quality and behaviors in young adults born prematurely [19, 41]. We could also not show any difference regarding dietary habits according to the DASH-score in the current study, but it has to be added that the rate of healthy diet in our cohort is generally very low with more than 30% categorized as poor in both the preterm as well as in the term group. In general, our results show that there is potential for improvement in the prevalence of ideal CV health in both groups in our cohort, because only 0.9% of former preterm-born and 1.5% of term-born teenagers met ideal criteria for all seven health metrics defined by the AHA. For example, the proportion of smokers in both groups is to be regarded as relatively high at about 30%. This highlights the need of specialized health interventional programs in adolescence in order to sustain improvements in CV health behavior that persist into adulthood.
Strengths and limitations
One of the main strengths of our study is the large and homogenous study cohort, which includes adolescents from all types of schools and apprentices of the same age from the entire study region. Furthermore, we investigated a broad spectrum of CV health parameters and data was collected prospectively by a small and stable study team within approximately three years.
The small number of former preterm-born adolescents compared with term-born individuals is a limitation, which must be mentioned. The rate of former preterm born adolescents in our cohort, however, corresponds to the rate of preterm birth in Austria [42]. Unfortunately, 611 individuals had to be excluded from analysis because of missing information on GA. The main reasons were either that the mother–child booklet was no longer available or lack of consent of the mother. Due to the small number of preterm infants and consequently the low number of former early preterm (< 32 weeks of gestation, n = 12) and moderate preterm (32–34 weeks of gestation, n = 21) adolescents further sub-group analyses could not be performed. According to recent literature hereditary factors may play an important role in developing hypertension [43]. Unfortunately, no data were available on the blood pressure levels of the participants´ parents in our study. Furthermore, measurement of body impedance was not part of the study protocol thus we cannot draw any conclusions about the differences in body fat composition between former preterm- and term-born adolescents.
Implications and conclusion
The results of our study underscore the impact of prematurity on CV risk. In our cohort, SBP and DBP were significantly higher in former preterm-born than in term-born adolescents. It should be emphasized that elevated blood pressure levels were even found in adolescents born late preterm. Therefore, early screening for hypertension and long-term follow-up in individuals with a history of preterm birth is warranted. The low number of adolescents meeting the criteria for ideal CV health metrics highlights the need for promotion of a healthy lifestyle in both former preterm- and term-born individuals.
Acknowledgements
None to declare
Early Vascular Ageing (EVA) Study Group
Mandy, Asare2
Manuela Bock-Bartl, MSc6
Maximilian Bohl, MD2
Christina Schreiner, MD1
Gregor Brössner, MD2
Tatjana Heisinger1
Julia Klingenschmid, MD1
Martina Kothmayer1
Julia Marxer, MD1
Raimund Pechlaner, MD2
Maximilian Pircher1
Carmen Reiter1
Stefan Kiechl, MD2,6
Bernhard Winder, MD1,6
1Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria
2Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
6VASCage, Centre on Clinical Stroke Research , Innsbruck, Tyrol, Austria
Abbreviations
- AHA
American Heart Association
- BMI
Body Mass Index
- CV
Cardiovascular
- DASH
Dietary Approaches to Stop Hypertension
- DBP
Diastolic Blood Pressure
- EVA
Early Vascular Ageing
- FAS
Family Affluence Score
- GA
Gestational Age
- SBP
Systolic Blood Pressure
- SGA
Small for Gestational Age
Authors’ contributions
CH and J-PN These authors takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. CH, J-PN, NG, AS, BB, KS, SZK, RG, EG, MK and UKK. These authors acquired the data, critically revised the manuscript for key intellectual content and approved the final manuscript. MK and UKK are the corresponding authors and contributed equally to the elaboration of the study protocol, they are principal investigators in Tyrol (Austria) and are EVA-Tyrol’s project directors. All authors read and approved the final manuscript.
Funding
Acknowledgement of grant support: The EVA-Tyrol study is financially supported by the excellence initiative (Competence Centers for Excellent Technologies—COMET) of the Austrian Research Promotion Agency FFG: “Research Center of Excellence in Vascular Ageing—Tyrol, VASCage” (K project number 843536) and by the excellence initiative VASCage (Centre for Promoting Vascular Health in the Ageing Community, project number 868624) of the Austrian Research Promotion Agency FFG (COMET program), both funded by the Austrian Ministry for Transport, Innovation and Technology, the Austrian Ministry for Digital and Economic Affairs and the federal states Tyrol (via Standortagentur), Salzburg and Vienna (via Vienna Business Agency).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki, ethical approval was approved by the Institutional Review board of the Medical University of Innsbruck, Austria (approval number AN 2015–0005 345/4.13, first registered 29/04/2019). The study is registered at www.clinical.trials.gov (NCT number 03929692).
All study participants provided written informed consent. In case they were younger than the age of 18, a written informed consent was signed by the parents or the legal guardian.
Consent for publication
Not applicable.
Competing interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael Knoflach, Email: michael.knoflach@i-med.ac.at.
Ursula Kiechl-Kohlendorfer, Email: ursula.kohlendorfer@i-med.ac.at.
Early Vascular Ageing (EVA) Study Group:
Mandy Asare, Manuela Bock-Bartl, Maximilian Bohl, Christina Schreiner, Gregor Brössner, Tatjana Heisinger, Julia Klingenschmid, Martina Kothmayer, Julia Marxer, Raimund Pechlaner, Maximilian Pircher, Carmen Reiter, Stefan Kiechl, and Bernhard Winder
References
- 1.Vogel JP, Chawanpaiboon S, Moller A-B, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12. [DOI] [PubMed] [Google Scholar]
- 2.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walani SR. Global burden of preterm birth. Int J Gynaecol Obstet. 2020;150(1):31–3. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Apremont I, Marshall G, Musalem C, Mariani G, Musante G, Bancalari A, et al. Trends in perinatal practices and neonatal outcomes of very low birth weight infants during a 16-year period at NEOCOSUR centers. J Pediatr. 2020;225:44–50.e1. [DOI] [PubMed] [Google Scholar]
- 7.Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10 Suppl 1(Suppl 1):S2. [DOI] [PMC free article] [PubMed]
- 8.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–9. [DOI] [PubMed] [Google Scholar]
- 9.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. 2017;106(9):1409–37. [DOI] [PubMed] [Google Scholar]
- 10.Crump C. Preterm birth and mortality in adulthood: a systematic review. J Perinatol. 2020;40(6):833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posod A, Odri Komazec I, Kager K, Pupp Peglow U, Griesmaier E, Schermer E, et al. Former very preterm infants show an unfavorable cardiovascular risk profile at a Preschool Age. PLoS One. 2016;11(12): e0168162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sipola-Leppänen M, Vääräsmäki M, Tikanmäki M, Hovi P, Miettola S, Ruokonen A, et al. Cardiovascular risk factors in adolescents born preterm. Pediatrics. 2014;134(4):e1072–81. [DOI] [PubMed] [Google Scholar]
- 13.Andraweera PH, Condon B, Collett G, Gentilcore S, Lassi ZS. Cardiovascular risk factors in those born preterm - systematic review and meta-analysis. J Dev Orig Health Dis. 2021;12(4):539–54. [DOI] [PubMed] [Google Scholar]
- 14.Cheong JLY, Haikerwal A, Wark JD, Irving L, Garland SM, Patton GC, et al. Cardiovascular Health Profile at Age 25 Years in Adults Born Extremely Preterm or Extremely Low Birthweight. Hypertension. 2020;76(6):1838–46. [DOI] [PubMed] [Google Scholar]
- 15.Markopoulou P, Papanikolaou E, Analytis A, Zoumakis E, Siahanidou T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta-analysis. J Pediatr. 2019;210:69–80.e5. [DOI] [PubMed] [Google Scholar]
- 16.Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of preterm birth with risk of Ischemic heart disease in adulthood. JAMA Pediatr. 2019;173(8):736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaseva N, Wehkalampi K, Strang-Karlsson S, Salonen M, Pesonen AK, Räikkönen K, et al. Lower conditioning leisure-time physical activity in young adults born preterm at very low birth weight. PLoS One. 2012;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kajantie E, Strang-Karlsson S, Hovi P, Räikkönen K, Pesonen AK, Heinonen K, et al. Adults born at very low birth weight exercise less than their peers born at term. J Pediatr. 2010;157(4):610–6, 6.e1. [DOI] [PubMed]
- 19.Sharafi M, Duffy VB, Miller RJ, Winchester SB, Huedo-Medina TB, Sullivan MC. Dietary behaviors of adults born prematurely may explain future risk for cardiovascular disease. Appetite. 2016;99:157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 21.Knoflach M, Kiechl S, Kind M, Said M, Sief R, Gisinger M, et al. Cardiovascular risk factors and atherosclerosis in young males: ARMY study (Atherosclerosis Risk-Factors in Male Youngsters). Circulation. 2003;108(9):1064–9. [DOI] [PubMed] [Google Scholar]
- 22.Knoflach M, Kiechl S, Penz D, Zangerle A, Schmidauer C, Rossmann A, et al. Cardiovascular risk factors and atherosclerosis in young women: atherosclerosis risk factors in female youngsters (ARFY study). Stroke. 2009;40(4):1063–9. [DOI] [PubMed] [Google Scholar]
- 23.Staudt A, Stock K, Gande N, Bernar B, Hochmayr C, Pechlaner R, et al. Impact of lifestyle and cardiovascular risk factors on early atherosclerosis in a large cohort of healthy adolescents: The Early Vascular Ageing (EVA)-Tyrol Study. Atherosclerosis. 2020;305:26–33. [DOI] [PubMed] [Google Scholar]
- 24.Bernar B, Gande N, Stock KA, Staudt A, Pechlaner R, Geiger R, et al. The Tyrolean early vascular ageing-study (EVA-Tyrol): study protocol for a non-randomized controlled trial : Effect of a cardiovascular health promotion program in youth, a prospective cohort study. BMC Cardiovasc Disord. 2020;20(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kromeyer-Hauschild K, Wabitsch M, Kunze D, Geller F, Geiß H-C, Hesse V, et al. Perzentile für den Body-mass-Index für das Kindes-und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschrift kinderheilkunde. 2001;149(8):807–18. [Google Scholar]
- 27.Neuhauser H, Schienkiewitz A, Rosario AS, Dortschy R, Kurth B-M. Referenzperzentile für anthropometrische Maßzahlen und Blutdruck aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS). 2013.
- 28.Kiechl S, Willeit J. The natural course of atherosclerosis: Part I: incidence and progression. Arterioscler Thromb Vasc Biol. 1999;19(6):1484–90. [DOI] [PubMed] [Google Scholar]
- 29.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117–24. [DOI] [PubMed] [Google Scholar]
- 30.Boyce W, Torsheim T, Currie C, Zambon A. The family affluence scale as a measure of national wealth: validation of an adolescent self-report measure. Soc Indic Res. 2006;78(3):473–87. [Google Scholar]
- 31.Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol. 2017;41(7):387–91. [DOI] [PubMed] [Google Scholar]
- 32.Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. 2016;21(2):68–73. [DOI] [PubMed] [Google Scholar]
- 33.Parkinson JRC, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics. 2013;131(4):e1240–63. [DOI] [PubMed] [Google Scholar]
- 34.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59(2):226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stock K, Schmid A, Griesmaier E, Gande N, Hochmayr C, Knoflach M, et al. The impact of being born preterm or small for gestational age on early vascular aging in adolescents. J Pediatr. 2018;201:49–54.e1. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Srinivasan SR, Berenson GS. Amplification of the association between birthweight and blood pressure with age: the Bogalusa Heart Study. J Hypertens. 2010;28(10):2046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andraweera PH, Lassi ZS. Cardiovascular risk factors in offspring of preeclamptic pregnancies—systematic review and meta-analysis. J Pediatr. 2019;208:104–13.e6. [DOI] [PubMed] [Google Scholar]
- 38.Crump C, Sundquist J, Sundquist K. Association of preterm birth with lipid disorders in early adulthood: A Swedish cohort study. PLoS Med. 2019;16(10): e1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia. 2020;63(3):508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sipola-Leppänen M, Kajantie E. Should we assess cardiovascular risk in young adults born preterm? Curr Opin Lipidol. 2015;26(4):282–7. [DOI] [PubMed] [Google Scholar]
- 41.Kaseva N, Wehkalampi K, Hemiö K, Hovi P, Järvenpää AL, Andersson S, et al. Diet and nutrient intake in young adults born preterm at very low birth weight. J Pediatr. 2013;163(1):43–8. [DOI] [PubMed] [Google Scholar]
- 42.Austria S. Statistik der natürlichen Bevölkerungsbewegung. Erstellt am 01.07.2022. – Lebendgeborene von Müttern mit österreichischem Wohnsitz, Geburtsort im Inland. 2022 [28.09.2022]. Available from: https://www.statistik.at/statistiken/bevoelkerung-und-soziales/bevoelkerung/geburten/medizinische-und-sozialmedizinische-merkmale-von-geborenen. Accessed 28 Sept 2022.
- 43.Seidel E, Scholl UI. Genetic mechanisms of human hypertension and their implications for blood pressure physiology. Physiol Genomics. 2017;49(11):630–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.