Abstract
BACKGROUND
Black and Latinx patients bear a disproportionate burden of asthma. Efforts to reduce the disproportionate morbidity have been mostly unsuccessful, and guideline recommendations have not been based on studies in these populations.
METHODS
In this pragmatic, open-label trial, we randomly assigned Black and Latinx adults with moderate-to-severe asthma to use a patient-activated, reliever-triggered inhaled glucocorticoid strategy (beclomethasone dipropionate, 80 μg) plus usual care (intervention) or to continue usual care. Participants had one instructional visit followed by 15 monthly questionnaires. The primary end point was the annualized rate of severe asthma exacerbations. Secondary end points included monthly asthma control as measured with the Asthma Control Test (ACT; range, 5 [poor] to 25 [complete control]), quality of life as measured with the Asthma Symptom Utility Index (ASUI; range, 0 to 1, with lower scores indicating greater impairment), and participant-reported missed days of work, school, or usual activities. Safety was also assessed.
RESULTS
Of 1201 adults (603 Black and 598 Latinx), 600 were assigned to the intervention group and 601 to the usual-care group. The annualized rate of severe asthma exacerbations was 0.69 (95% confidence interval [CI], 0.61 to 0.78) in the intervention group and 0.82 (95% CI, 0.73 to 0.92) in the usual-care group (hazard ratio, 0.85; 95% CI, 0.72 to 0.999; P = 0.048). ACT scores increased by 3.4 points (95% CI, 3.1 to 3.6) in the intervention group and by 2.5 points (95% CI, 2.3 to 2.8) in the usual-care group (difference, 0.9; 95% CI, 0.5 to 1.2); ASUI scores increased by 0.12 points (95% CI, 0.11 to 0.13) and 0.08 points (95% CI, 0.07 to 0.09), respectively (difference, 0.04; 95% CI, 0.02 to 0.05). The annualized rate of missed days was 13.4 in the intervention group and 16.8 in the usual-care group (rate ratio, 0.80; 95% CI, 0.67 to 0.95). Serious adverse events occurred in 12.2% of the participants, with an even distribution between the groups.
CONCLUSIONS
Among Black and Latinx adults with moderate-to-severe asthma, provision of an inhaled glucocorticoid and one-time instruction on its use, added to usual care, led to a lower rate of severe asthma exacerbations. (Funded by the Patient-Centered Outcomes Research Institute and others; PREPARE ClinicalTrials.gov number, NCT02995733.)
In the United States, asthma results in considerable illness, including more than 3300 asthma-attributed deaths in adults each year.1 Annual costs for asthma in adults total more than $67 billion2 and are substantially higher in patients with uncontrolled asthma than in those with controlled asthma.3 Owing to a complex array of factors,4 Black and Latinx populations bear disproportionate asthma morbidity and mortality.1,5 After adjustment for prevalence, the rates of asthma-related emergency department visits and hospitalizations are higher among Black and Latinx persons than among White persons,6–11 and mortality from asthma is twice as high among Black and Latinx persons as among White persons.1,12 Efforts to improve asthma management and reduce this burden have been labor-intensive, expensive, and variably effective.13
Inhaled glucocorticoids are the backbone of asthma-controller therapy. Multiple studies have suggested that in patients with moderate-to-severe asthma, the use of a single inhaler containing a combination of a glucocorticoid and a rapid-onset long-acting β2-agonist (LABA), used as a regular, twice-daily maintenance therapy, plus the use of an as-needed reliever therapy (i.e., a single maintenance and reliever therapy [SMART] strategy), can reduce asthma exacerbations more effectively than the previously more commonly recommended strategy of twice-daily use of the combination product with a short-acting β2-agonist as the reliever. Guideline recommendations have recently been updated to reflect such approaches.14 However, studies supporting the effectiveness of this strategy that have included substantial proportions of Black or Latinx patients have been limited.
Furthermore, few studies in moderate-to-severe asthma have been conducted in real-world U.S. settings in which patients commonly use nebulized β2-agonists as quick-reliever therapy.15,16 The use of nebulizers for quick relief interferes with the supplemental as-needed strategy of SMART, in which patients use combination inhaled glucocorticoid plus LABA instead of a reliever inhaler for acute symptoms, so that inhaled glucocorticoid is delivered each time the patient has symptoms warranting medication use. If patients use nebulizers for relief, they do not receive the intended inhaled glucocorticoid, since they are not using the combination inhaler for relief. Finally, barriers to implementation of the SMART strategy include a caution from the Food and Drug Administration against as-needed use of combination inhaled glucocorticoid plus LABA, the requirement to change the existing controller therapy to the specifically recommended combination inhaled glucocorticoid plus LABA, and varied insurance coverage for this approach.17
Considering the disproportionate burden of asthma in Black and Latinx populations, the difficulties in reducing such morbidity, the paucity of data on the effectiveness of interventions specifically investigated in these populations, and the potential barriers to implementation of current recommendations, we conducted the Person Empowered Asthma Relief (PREPARE) trial. We investigated whether one-time instruction in an approach involving as-needed inhaled glucocorticoid (modified from Calhoun et al.,18 on the basis of feedback from our patient partners and advisors and the results of a pilot study19), added to existing therapy, could improve asthma outcomes in Black and Latinx patients with poorly controlled asthma in a trial with minimal exclusion criteria.
METHODS
TRIAL DESIGN AND OVERSIGHT
We conducted this randomized, open-label, pragmatic trial of the addition of a patient-activated, reliever-triggered inhaled glucocorticoid strategy, which we called PARTICS, to usual care in Black and Latinx patients with moderate-to-severe asthma in the continental United States and Puerto Rico. Full details of the trial protocol, which is available with the full text of this article at NEJM.org, have been published previously.20
The investigators collaborated with Black and Latinx adults with asthma and with caregivers of persons with asthma (patient partners) and additional advisors. The authors designed the trial, gathered the data with assistance from DARTNet Institute and the Asthma Research Center at Brigham and Women’s Hospital (in Boston), and analyzed the data with the assistance of the Duke Clinical Research Institute (in Durham, NC). The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. The first author wrote the first draft of the manuscript. All the authors made the decision to submit the manuscript for publication. There were no restrictive confidentiality agreements.
PATIENT POPULATION
Adults 18 to 75 years of age with clinician-diagnosed asthma who self-identified as Black or Latinx underwent screening at 19 primary care and specialty clinical organizations; the patients had been approached about participation before or at a clinic visit. All the participants provided written informed consent on a form that had been approved by the institutional review board of Partners HealthCare and local institutional review boards.
Key inclusion criteria were a status of being prescribed daily inhaled glucocorticoids, with or without LABA, and having either uncontrolled asthma (Asthma Control Test [ACT] score of ≤19 [indicating asthma that was not well controlled; scores range from 5 to 25, with lower scores indicating less control; minimal clinically important difference, 3]) or at least one participant-reported asthma exacerbation leading to the use of systemic glucocorticoids or overnight hospitalization in the previous year. Former or current smokers were included in the trial, and there were minimal other exclusions.20 Patients who were taking regular systemic glucocorticoids were excluded.
TRIAL PROCEDURES
The trial structure is shown in Figure S1 in the Supplementary Appendix, available at NEJM.org. Site clinicians underwent asthma-treatment training with the Asthma IQ.21 The clinician care that was provided during the trial is referred to here as usual care. During the subsequent 15 months, clinicians were free to modify usual care as necessary.
At the only in-person trial visit, participants were randomly assigned in a 1:1 ratio either to use a patient-activated, reliever-triggered inhaled glucocorticoid strategy plus usual care (intervention) or to continue usual care. Centralized randomization was stratified according to trial site and race and ethnic group. At this visit, participants received instructions, completed questionnaires, and watched a video that was appropriate to the randomization group. All the trial materials were available in English and Spanish.
All the participants received a trial-specific pouch designed to hold two metered-dose inhalers. Participants in the intervention group received an open-label inhaler that administered a metered dose of 80 μg of beclomethasone dipropionate (QVAR [Teva Pharmaceuticals]; the QVAR RediHaler was used after December 2018 owing to discontinuation of the QVAR inhaler). Participants who had been using a nebulized quick-reliever received an additional QVAR inhaler to place with their nebulizer. They were instructed to take one puff of inhaled glucocorticoid for each puff of quick-reliever inhaler and five puffs of inhaled glucocorticoid with each quick-reliever nebulization. Participants could request intervention QVAR refills from the trial pharmacy (AssistRx) by means of a toll-free telephone number.
Participants were followed for up to 15 months by means of monthly surveys, which were administered according to participant preference (Internet, telephone, or mail). They received cash compensation for their visit and for completed surveys. In a subgroup of participants, the fraction of exhaled nitric oxide (FeNO) was measured at baseline, and blood eosinophil counts were obtained at baseline or from records of the previous year.
END POINTS
End points were selected on the basis of input from patient partners and advisors. These end points included exacerbations, owing to their reported disruptive effect; quality of life; and missed days of work, school, or usual activities. In line with these preferences, the primary end point was the annualized rate of American Thoracic Society–defined severe asthma exacerbations (those that led to the use of systemic glucocorticoids for ≥3 days or to an asthma-related hospitalization).22 Severe asthma exacerbations were noted on monthly questionnaires. Central investigators who were unaware of the treatment assignments verified exacerbations by means of site medical records or direct contact with participants.
Prespecified secondary end points included monthly asthma control as measured with the ACT,23–25 preference-based quality of life as measured with the Asthma Symptom Utility Index (ASUI; scores range from 0 to 1, with lower scores indicating greater impairment; minimal clinically important difference, 0.09),26,27 and participant-reported days missed from work, school, or usual activities.28 Data on adverse events that resulted in hospitalization or death were obtained by participant report or site report.
STATISTICAL ANALYSIS
Assuming a severe exacerbation rate of 0.4 exacerbations per participant per year30 and an annualized loss to follow-up of 25%, we calculated that a sample of 1200 participants (600 per group) would provide the trial with 80% power to detect a 23.5% difference in the exacerbation rate. The timing and frequency of severe asthma exacerbations were compared with the use of the Andersen–Gill adaptation of the time-to-event Cox proportional-hazards model, with stratification according to race and ethnic group and with adjustment for baseline characteristics and for a time-dependent covariate to account for the effects of coronavirus disease 2019 on the rate of severe asthma exacerbations.29
All the major between-group treatment comparisons were performed in the intention-to-treat population, which included all the participants who had undergone randomization except for any from a prematurely closed trial site. Details of the analyses of the secondary and exploratory end points are provided in the statistical analysis plan. Statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
A P value of 0.15 or less was prespecified for interaction testing of the effect of the two racial and ethnic groups to permit separate analysis within each group. Missing-data analyses were to be performed if the percent of missing data reached a prespecified threshold of 5%. Results are reported with 95% confidence intervals. The widths of the confidence intervals for secondary, post hoc, and safety analyses have not been adjusted for multiplicity and thus should not be used to infer definitive treatment effects.
RESULTS
POPULATION AND FOLLOW-UP
From November 2017 through March 2021, a total of 1201 participants (603 Black and 598 Latinx) underwent randomization, with 600 participants being assigned to the intervention group and 601 to the usual-care group (Fig. S2). A total of 83.7% of the participants were women (Tables 1 and S1). Approximately half the participants reported health that was fair to poor with multiple coexisting conditions; 68.8% of the participants had obesity, and approximately 20% were current or former smokers. The ACT and ASUI scores indicated poor asthma control and a clinically significant burden of asthma.23,26 A total of 72.2% of the participants reported having had at least one asthma exacerbation that led to the use of systemic glucocorticoids in the previous year. A total of 71.5% of the participants used combination inhaled glucocorticoid plus LABA, and 66.9% used a nebulizer for quick-reliever therapy; 67.5% of those participants (45.2% of the overall population) reported using at least one nebulizer treatment per week. Among the participants with measurements, 30.1% had a FeNO of at least 30 parts per billion, and 26.6% had a blood eosinophil count of at least 300 per cubic millimeter. The characteristics of the participants according to race and ethnic group are shown in Table S2. The representativeness of the participants is discussed in Table S6.
Table 1.
Characteristics of the Participants at Baseline.*
| Characteristic | Intervention Group (N = 600) | Usual-Care Group (N = 601) | Total (N = 1201) |
|---|---|---|---|
| Race and ethnic group — % | |||
| Black | 50.5 | 49.9 | 50.2 |
| Latinx | 49.5 | 50.1 | 49.8 |
| Both Black and Latin† | 6.3 | 4.3 | 5.3 |
| Age — yr | 48.3±13.5 | 47.0±13.9 | 47.7±13.7 |
| Female sex assigned at birth — % | 84.7 | 82.7 | 83.7 |
| Body-mass index‡ | 35.2±9.1 | 35.1±9.5 | 35.1±9.3 |
| Obesity — %‡ | 70.2 | 67.1 | 68.6 |
| Smoking status — %§ | |||
| Current smoker | 11.5 | 12.3 | 11.9 |
| Former smoker | 9.0 | 7.7 | 8.3 |
| Nonsmoker or former smoker in smoking environment — %¶ | 16.0 | 17.8 | 16.9 |
| No. of pack-yr of smoking | 12.4 | 15.8 | 14.1 |
| Maintenance asthma medications — % | |||
| Inhaled glucocorticoid without LABA | 28.5 | 28.1 | 28.3 |
| Combination inhaled glucocorticoid with LABA | 71.3 | 71.7 | 71.5 |
| Long-acting muscarinic antagonist | 10.7 | 13.1 | 11.9 |
| Leukotriene-receptor antagonist | 51.3 | 48.3 | 49.8 |
| Biologic agent∥ | 2.8 | 3.2 | 3.0 |
| Quick-reliever nebulizer use | |||
| Use of quick-reliever nebulizer — % | 68.0 | 65.9 | 66.9 |
| No. of quick-reliever nebulizations per week | 2.7±4.6 | 3.0±4.8 | 2.9±4.7 |
| No. of coexisting conditions — %** | |||
| 0 | 27.7 | 31.8 | 29.7 |
| 1 | 25.0 | 21.0 | 23.0 |
| 2 | 22.2 | 19.8 | 21.0 |
| 3 | 13.5 | 14.3 | 13.9 |
| ≥4 | 11.7 | 13.1 | 12.4 |
| FeNO — ppb | 26.7±27.8 | 30.4±34.9 | 28.6±31.6 |
| Absolute eosinophil count — cells/mm3 | |||
| Mean | 246±229 | 250±247 | 248±238 |
| Median | 188 | 195 | 192 |
| ≥1 Asthma exacerbation in past year — %†† | 73.3 | 71.0 | 72.2 |
| Asthma Control Test score‡‡ | 14.7±4.4 | 14.5±4.5 | 14.6±4.4 |
| Asthma Symptom Utility Index score§§ | 0.67±0.22 | 0.67±0.21 | 0.67±0.21 |
| Medication Adherence Report Scale-5 score¶¶ | 4.2±0.8 | 4.2±0.8 | 4.2±0.8 |
| Low or marginal health literacy — %∥∥ | 16.9 | 16.6 | 16.8 |
| Participant-perceived overall health — % | |||
| Excellent | 2.2 | 1.5 | 1.8 |
| Very good | 11.3 | 10.0 | 10.7 |
| Good | 33.3 | 32.3 | 32.8 |
| Fair | 42.8 | 45.3 | 44.0 |
| Poor | 10.3 | 11.0 | 10.7 |
Plus–minus values are means ±SD. Participants in the intervention group received patient-activated, reliever-triggered inhaled glucocorticoid (beclomethasone dipropionate, 80 μg) added to usual care. All the baseline characteristics listed here were reported by the participant except for fraction of exhaled nitric oxide (FeNO) and absolute eosinophil count. Data on FeNO were missing for 98 participants in the intervention group and for 100 participants in the usual-care group, and data on the absolute eosinophil count were missing for 98 and 107, respectively. Percentages may not total 100 because of rounding. LABA denotes long-acting β2-agonist, and ppb parts per billion.
Participants who identified as both Black and Latinx were classified as Latinx for the purpose of stratification.
The body-mass index is the weight in kilograms divided by the square of the height in meters. Obesity was defined as a body-mass index of 30 or higher.
Current smokers were defined as participants who were currently smoking or had smoked within the previous year. Former smokers were participants who had not smoked in the previous year but had smoked at least 10 pack-years. Nonsmokers were those who had not smoked within the previous year and had smoked less than 10 pack-years.
A smoking environment was defined as others regularly smoking in the participant’s home, work, or car.
Biologic agents included injectable monoclonal antibody therapies targeting IgE, interleukin-5 or the interleukin-5 receptor, or the interleukin-4 receptor.
Coexisting conditions included heart disease, cancer (except skin cancer), stroke, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, human immunodeficiency virus infection or acquired immunodeficiency syndrome, hypertension, depression, and sleep disorder.
Asthma exacerbation in the previous year was defined as a participant-reported emergency department or urgent care visit, hospitalization, or course of systemic glucocorticoids within the 12 months before randomization.
The Asthma Control Test is a participant-administered tool for assessing the level of asthma control.23 Total scores range from 5 to 25, with a score of 20 to 25 indicating well-controlled asthma, a score of 16 to 19 indicating asthma that was not well controlled, and a score of 5 to 15 indicating very poorly controlled asthma.23,24 The minimal clinically important difference is 3 points.25
The Asthma Symptom Utility Index is a participant-administered tool for assessing preference-based quality of life.26 Scores range from 0 (worst possible symptoms) to 1 (no symptoms). The minimal clinically important difference is 0.09.27
The Medication Adherence Report Scale–5 measures participant-reported medication adherence. Mean scores are calculated from five items and range from 1 to 5, with higher scores indicating better adherence.31
Health literacy was assessed with the Brief Health Literacy Scale, which measures participant-reported health literacy and consists of three items. Scoring low or marginal health literacy on any one item classifies a participant as having low or marginal health literacy.32
The median follow-up was 14.9 months in each group. Survey returns did not differ according to treatment group (96.2% of the expected periods in the intervention group and 96.7% of those in the usual-care group).
ADHERENCE TO THE INTERVENTION
Among participants in the intervention group, 81.0% reported using inhaled glucocorticoid with quick-reliever metered-dose inhalers all or most of the time, and 75.7% reported using inhaled glucocorticoid with quick-reliever nebulization all or most of the time. A total of 50.4% of the participants in this group reported using at least four of the instructed five puffs of inhaled glucocorticoid per quick-reliever nebulization.
PRIMARY END POINT
The annualized rate of severe asthma exacerbations was 0.69 (95% confidence interval [CI], 0.61 to 0.78) in the intervention group and 0.82 (95% CI, 0.73 to 0.92) in the usual-care group (hazard ratio, 0.85; 95% CI, 0.72 to 0.999; P = 0.048) (Table 2). This difference was consistent throughout the duration of the trial (Fig. 1). Point estimates from sensitivity analyses of the primary end point were consistent with the overall finding, although not all the results were significant (Table S3). Primary end-point results according to trial site are shown in Figure S3. The percent of missing data (3%) did not reach the prespecified threshold of 5%, so missingdata analyses were not performed.
Table 2.
Primary, Secondary, Post hoc, and Safety Analyses.*
| Analysis | Intervention Group (N = 600) | Usual-Care Group (N = 601) | Between-Group Comparison (95% CI) | P Value |
|---|---|---|---|---|
| Primary analysis: severe asthma exacerbation | ||||
| Total no. of exacerbations | 585 | 680 | ||
| Adjusted annualized rate per participant (95% CI) | 0.69 (0.61–0.78) | 0.82 (0.73–0.92) | Hazard ratio: 0.85 (0.72–0.999) | 0.048 |
| Secondary analyses | ||||
| Asthma Control Test | ||||
| Mean baseline score (95% CI) | 14.7 (14.4–15.1) | 14.5 (14.2–14.9) | ||
| Overall mean postbaseline score (95% CI) | 18.0 (17.7–18.3) | 17.1 (16.7–17.4) | ||
| Least-squares mean change from baseline (95% CI) | 3.4 (3.1–3.6) | 2.5 (2.3–2.8) | Difference: 0.9 (0.5–1.2) | |
| Asthma Symptom Utility Index | ||||
| Baseline mean score (95% CI) | 0.67 (0.65–0.69) | 0.67 (0.65–0.69) | ||
| Overall mean postbaseline score (95% CI) | 0.79 (0.77–0.80) | 0.75 (0.74–0.76) | ||
| Least-squares mean change from baseline (95% CI) | 0.12 (0.11–0.13) | 0.08 (0.07–0.09) | Difference: 0.04 (0.02–0.05) | |
| Annualized no. of days missed from work, school, or usual activities (95% CI) | 13.4 (11.9–15.2) | 16.8 (14.9–18.9) | Rate ratio: 0.80 (0.67–0.95) | |
| Post hoc analysis | ||||
| Annualized no. of months with a reported asthma-related emergency department or urgent care visit (95% CI) | 0.75 (0.65–0.87) | 0.90 (0.77–1.04) | Rate ratio: 0.84 (0.68–1.03) | |
| Safety analysis | ||||
| No. of asthma-related hospitalizations | 70 | 84 | Rate ratio: 0.84 (0.50–1.42) |
Hazard ratio for the primary end point was adjusted for baseline characteristics and for a time-dependent covariate for the effects of coronavirus disease 2019. The widths of the confidence intervals for the secondary, post hoc, and safety analyses have not been adjusted for multiplicity and cannot be used to infer treatment effects.
Figure 1. Mean Cumulative Number of Severe Asthma Exacerbations per Participant over Time, with Adjusted Hazard Ratio.
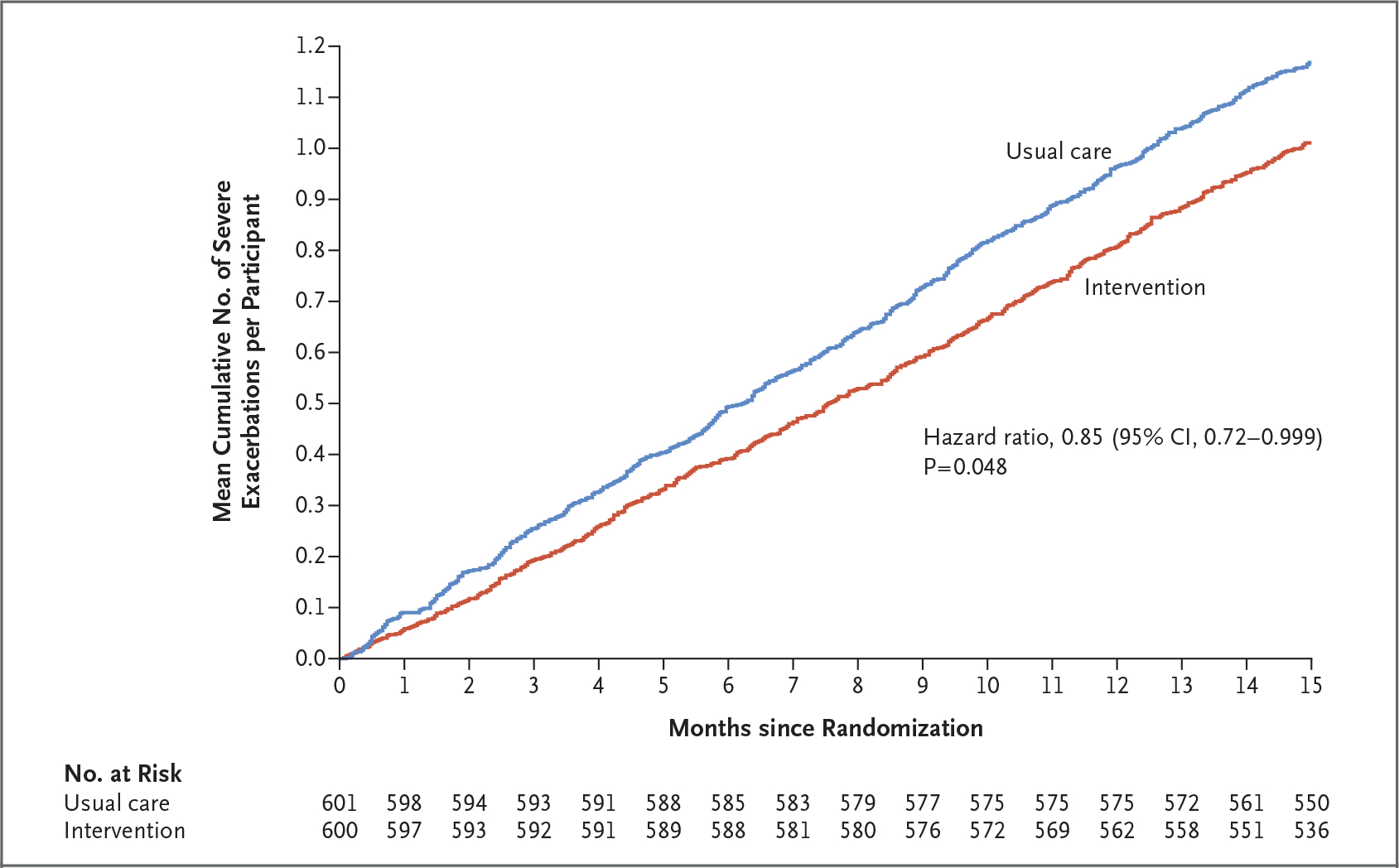
Shown are the mean cumulative numbers of severe asthma exacerbations per participant over time. Participants in the intervention group received patient-activated, reliever-triggered inhaled glucocorticoid in addition to usual care. Differences in treatment-group hazards were compared with the use of the Andersen–Gill model with adjustment for prespecified covariates.
SECONDARY END POINTS
The strategy in the intervention group led to an increase in the ACT scores (minimal clinically important difference, 3 points)25 of 3.4 points, as compared with an increase of 2.5 points in the usual-care group (difference, 0.9 points; 95% CI, 0.5 to 1.2) (Fig. 2A). The intervention strategy also led to an increase in the ASUI score (minimal clinically important difference, 0.09 points)27 of 0.12 points, as compared with an increase of 0.08 points in the usual-care group (difference, 0.04 points; 95% CI, 0.02 to 0.05) (Fig. 2B). Participants in the intervention group had 13.4 annualized days missed of work, school, and usual activities, as compared with 16.8 annualized days missed in the usual-care group (rate ratio, 0.80; 95% CI, 0.67 to 0.95) (Table 2).
Figure 2. Mean Changes from Baseline in Asthma-Related Scores.
The least-squares mean differences between treatment groups in the changes from baseline in asthma-related scores were calculated with the use of a mixed model. The Asthma Control Test (Panel A) is a participant-administered tool for assessing the level of asthma control. Total scores range from 5 to 25, with a score of 20 to 25 indicating well-controlled asthma, a score of 16 to 19 indicating asthma that was not well controlled, and a score of 5 to 15 indicating very poorly controlled asthma; the minimal clinically important difference is 3 points. The Asthma Symptom Utility Index (Panel B) is a participant-administered tool for assessing preference-based quality of life. The summary score is on a continuous scale, ranging from 0 (worst possible symptoms) to 1 (no symptoms); the minimal clinically important difference is 0.09. I bars indicate 95% confidence intervals. The widths of the confidence intervals have not been adjusted for multiplicity and cannot be used to infer treatment effects.
POST HOC AND SAFETY ANALYSES
In a post hoc analysis, participants in the intervention group reported a mean 0.75 months per year in which an emergency department or urgent care visit occurred, as compared with 0.90 months per year in the usual-care group (rate ratio, 0.84; 95% CI, 0.68 to 1.03). In the safety analysis, there were 70 asthma-related hospitalizations in the intervention group and 84 in the usual-care group (rate ratio, 0.84; 95% CI, 0.50 to 1.42).
Post hoc analyses of medication use based on participants’ monthly surveys showed that participants in the intervention group used 1.1 extra inhaler containing inhaled glucocorticoid per year as compared with the usual-care group (8.9 vs. 7.8 inhalers per year). Participants in the intervention group also reported fewer refills of the quick-reliever metered-dose inhaler than those in the usual-care group (4.6 vs. 5.6 refills), as well as fewer months in which they used quick-reliever nebulizer (3.6 vs. 5.4 months) (Table S4).
SUBGROUP ANALYSES
Hazard ratios for severe asthma exacerbations in prespecified subgroups are shown in Figure 3. The hazard ratio for severe exacerbations with the intervention as compared with usual care was 0.77 among Black participants and 0.92 among Latinx participants (P = 0.29 for interaction, which exceeded our prespecified limit for stratified analyses).
Figure 3. Subgroup Analyses of Severe Asthma Exacerbation Outcome.

The forest plot shows the risk of severe asthma exacerbations in selected prespecified subgroups. The widths of the confidence intervals have not been adjusted for multiplicity and cannot be used to infer treatment effects. Race was reported by the participant; those who identified as both Black and Latinx were classified as Latinx. Nonsmokers were those who had not smoked within the previous year and had smoked less than 10 pack-years. The participant’s attitude toward asthma medications was assessed with the Beliefs about Medicines Questionnaire; the “accepting” category indicates that the participant believed the therapy was of high necessity and low concern.33 Depressive status was assessed with the Patient Health Questionnaire–2; scores range from 0 to 6, with a score of 3 or higher indicating depression.34 The Brief Health Literacy Scale measures participant-reported health literacy and consists of three items. If a participant’s response on any item indicated low or marginal health literacy, the participant was considered to have low or marginal health literacy; otherwise, health literacy was considered to be high.32 The body-mass index is the weight in kilograms divided by the square of the height in meters. Participant-reported medication adherence was assessed with the Medication Adherence Report Scale–5 by the calculation of the mean score over the five items. Mean scores range from 1 to 5, with higher scores indicating better adherence.31 FeNO denotes fraction of exhaled nitric oxide, LABA long-acting β2-agonist, and ppb parts per billion.
SERIOUS ADVERSE EVENTS
Serious adverse events occurred in 12.2% of the participants, with an even distribution across the two groups (Table S5). Overall, the most common serious adverse events were asthma (in 7.2% of the participants), infection or infestation (in 1.6%), and cardiac events (in 1.5%), all of which were evenly distributed between the two groups. Hospitalization occurred in 11.8% of the participants in the intervention group and in 11.5% of those in the usual-care group. Three deaths occurred in the intervention group and 4 in the usual-care group. None of the deaths were considered by the investigators to be related to asthma.
DISCUSSION
The disproportionate burden of asthma in underserved populations in the United States persists despite focused interventions.13 In the PREPARE trial, we found that an intervention with a single in-person instruction session led to a 15.4% lower risk of severe asthma exacerbations and also reduced asthma symptoms and the number of days of impairment in Black and Latinx participants with moderate-to-severe asthma. Effects of the patient-activated, reliever-triggered inhaled glucocorticoid (PARTICS) intervention persisted over a period of 15 months and were accompanied by reduced quick-reliever use and a reported mean net difference in the use of glucocorticoid-containing inhalers of only 1.1 inhaler per year, according to a post hoc analysis.
The effects of the intervention were consistent across multiple domains of assessment. In the prespecified secondary analyses, the use of the intervention plus usual care appeared to improve asthma control (as assessed on the ACT) and preference-based quality of life (as assessed on the ASUI) by an amount that exceeded the minimal clinically important difference on each measure25,27 and reduced the number of missed days of work, school, and usual activities. In post hoc analyses, these effects were accompanied by an 18% lower incidence of quick-reliever inhaler refills and by 32% fewer months of quick-reliever nebulizer use with the intervention than with usual care, which is important since the frequency of β2-agonist quick-reliever use has been associated with mortality among patients with asthma.35
The broad entry criteria and design of this trial could have reduced the apparent effectiveness of the patient-activated, reliever-triggered inhaled glucocorticoid strategy. We did not require evidence of bronchodilator responsiveness, we enrolled current and former smokers, and we did not require all participants to have had an exacerbation in the previous year. Application of these criteria to entry would have enriched the trial for a population with a greater response to inhaled glucocorticoids,36–39 a factor that is also suggested by our interaction analysis (Fig. 3). Nonetheless, the reduction in severe exacerbations that we observed (calculated to 0.13 fewer exacerbations per person per year) was similar to the mean reduction in asthma exacerbations (weighted according to sample size and study duration) in the 10 studies cited by the 2020 National Asthma Education and Prevention Program Focused Asthma Updates to support its paradigm-changing recommendation of the SMART strategy for severity steps 3 to 4 (i.e., 0.12 severe exacerbations per patient per year).14 Post hoc calculations suggest that the effect with our intervention was observed with a minimal increase of 30 μg of inhaled glucocorticoid per day.
In contrast to resource-intensive strategies with varied effectiveness in reducing asthma morbidity among Black and Latinx patients, the patient-activated, reliever-triggered inhaled glucocorticoid strategy appears to be an easy-to-implement strategy that improves outcomes. Participants’ existing asthma treatment was not altered. Participant instruction was provided only once with the aid of a Web-based video; participants were sent monthly surveys regarding medication use, asthma control, and asthma exacerbations. We found that participants with low or marginal health literacy benefited from the addition of the trial intervention to usual care (Fig. 3).
Several caveats should be considered in interpreting the trial outcomes. This trial was open-label, and we provided the intervention patient-activated, reliever-triggered inhaled glucocorticoid at no cost, with refills provided by mail on request. However, the mean additional glucocorticoid-containing inhaler use in the intervention group, as compared with the usual-care group, of 1.1 inhaler per year in a post hoc analysis represents a low cost burden to the health care system in light of the potential benefits. Mail-order delivery is likely to be accessible to nearly all patients. Although a placebo effect, as opposed to a trial participation effect, cannot be ruled out, the durability of the effect makes this situation less likely. We did not directly assess adrenal suppression, but given the small mean increase in inhaled glucocorticoid use that was seen in the post hoc analysis and the absence of any major safety signal, clinically significant adverse effects related to inhaled glucocorticoid in this population seem unlikely. Although the lower risk of severe asthma exacerbations with the intervention added to usual care was clinically important, the P value was 0.048.
Finally, although the intervention strategy was effective in a broad population of self-identified Black and Latinx participants, the population included many different ethnic groups (Table S7), which may differ in asthma morbidity and possibly in responsiveness to this treatment strategy. In addition, women were overrepresented in this trial, and thus our findings may not be as generalizable to men. However, women constitute two thirds of adult patients with asthma1 and bear a disproportionate asthma burden, as compared with men.40
The mechanism by which the patient-activated, reliever-triggered inhaled glucocorticoid strategy produces its salutary effect is uncertain. We posit, as has been speculated with the SMART strategy, that it relates to the use of inhaled glucocorticoids early in the course of an asthma worsening.41 It is possible that full implementation of SMART, as recently advocated by the 2020 National Asthma Education and Prevention Program Focused Asthma Updates14 and by the Global Initiative for Asthma, may produce effects that equal or exceed the effects we observed. The SMART strategy offers the advantage of use of a single inhaler for both maintenance and reliever therapy, which potentially simplifies a patient’s regimen. However, as discussed, multiple implementation barriers to SMART exist, including the need to change the patient’s existing asthma therapy. Data on the SMART strategy in Black and Latinx populations or in patients who frequently use nebulized reliever therapy (2.9 times per week in our population) are limited. Use of nebulized reliever therapy can result in a potential failure to trigger extra use of inhaled glucocorticoid as the need for reliever therapy increases. In the United States, patients’ beliefs and preferences16 and insurance reimbursement policies make the discontinuation of nebulizer use unlikely in the near future.17
Reducing disparities in asthma morbidity in Black and Latinx populations has been difficult. In this trial involving an ethnically diverse population of Black and Latinx patients with moderate-to-severe asthma and multiple coexisting conditions, the provision of inhaled glucocorticoid with instructions for use triggered by quick-reliever use (PARTICS), added to existing usual care, led to a lower risk of severe asthma exacerbations. The outcome was observed after a single visit and appeared to be durable. Such a strategy may be easy to implement in populations with disproportionate asthma morbidity, as we continue to assess the effectiveness of additional interventions in diverse populations.
Supplementary Material
Acknowledgments
Supported by a PCORI Award (PCS-1504-30283, to Dr. Israel), the Gloria M. and Anthony C. Simboli Distinguished Chair in Asthma Research award (to Dr. Israel), and by grants from the National Institute of Allergy and Infectious Diseases (K23AI125785, to Dr. Cardet) and the American Lung Association–American Academy of Allergy, Asthma, and Immunology (AI-835475, to Dr. Cardet). QVAR and QVAR RediHaler inhalers were provided free of charge and funding for the AssistRx pharmacy was provided by Teva Pharmaceuticals. NIOX VERO devices for measuring exhaled nitric oxide were provided free of charge by Circassia Pharmaceuticals.
We thank Julia Harder for medical writing assistance (paid through funds from the PCORI grant) with an earlier version of the manuscript; Craig P. Hersh for oversight of patient safety throughout the trial; the advisors listed here for assistance in the development of the trial design and implementation of the trial: Aracelis Diaz, Bridget Hickson, Margie Lorenzi, Kathy Monteiro, Wilfredo Morales-Cosme, Alexander Muniz Ruiz, Addie Perez, Richard Redondo, Marsha Santiago, Joyce Wade, and Mary White (patient partners); Rubin Cohen, Patricia W. Finn, Michael B. Foggs, Robert F. Lemanske, Jr., and Folashade Omole (professional society advisors); Mary Hart, Mario Herrera, and Sharon Schumack (patient advocacy advisors); Juan C. Celedón, Giselle Mosnaim, and Wanda Phipatanakul (expert scientific advisors); Sarah Alwardt, Arif M. Khan, and Troy Trygstad (health policy experts); Tiffany Bendelow, Lauren Bielick, Michelle Campbell Hayes, Erika M. Coleman, Jose Diarte Ortiz, Lynn Fukushima, Nicole P. Grant, Hernidia Guerra, Hilde Heyn, Renita Holmes, Bryonna Jackson, Mary Jo Day, Sylvia Johnson, Tiffani Kaage, Claudia Lechuga, Carese Lee, Brianna M. McQuade, Kathleen Mottus, Melissa Navarro, Grace Ndicu, Angela Nuñez, Pamela Pak, Luzmercy Perez, Matias E. Pollevick, Walter Ramos-Amador, Patricia Rebolledo, Jennifer Rees, Sarah B. Romain, Benjamin J. Rooks, Jasmin Sanchez, Catherine R. Smith, Lindsay E. Shade, Bonnie Telon Sosa, Jeremy Thomas, and Zinnia Valdes (trial site staff); the members of the American Academy of Family Physicians, Asthma Exacerbation Verification Group (Alicia Brooks-Greisen, Ileana Cepeda, Angie Lanigan, Cory B. Lutgen, Elizabeth Staton, and Carolyn Valdez); the members of the Asthma Research Center at Brigham and Women’s Hospital, Supplementary Asthma Exacerbation Verification Group (Eva Fandozzi, Katarina S. Gentile, Meghan N. Le, Allison O’Neill, and Abigail Fairbanks Tulchinsky); Shaddai Amolitos and Gabriela Gaona-Villarreal, of DARTNet Institute, for assistance with the data; Michael Pencina and Karen Chiswell, of Duke Clinical Research Institute, for assistance with the statistical analysis; Donna J. Walsh, of Brigham and Women’s Hospital, for assistance with the project finances; and all the trial participants and their families.
APPENDIX
The authors’ full names and academic degrees are as follows: Elliot Israel, M.D., Juan-Carlos Cardet, M.D., M.P.H., Jennifer K. Carroll, M.D., M.P.H., Anne L. Fuhlbrigge, M.D., Lilin She, Ph.D., Frank W. Rockhold, Ph.D., Nancy E. Maher, M.P.H., Maureen Fagan, D.N.P., F.N.P.-B.C., Victoria E. Forth, M.M.S., P.A.-C., Barbara P. Yawn, M.D., Paulina Arias Hernandez, M.S.W., Jean M. Kruse, B.A., Brian K. Manning, M.P.H., Jacqueline Rodriguez-Louis, M.P.H., M.Ed., Joel B. Shields, M.A., Brianna Ericson, M.P.H., Alex D. Colon-Moya, M.P.H., Suzanne Madison, Ph.D., M.P.H., Tamera Coyne-Beasley, M.D., M.P.H., Gretchen M. Hammer, M.P.H., Barbara M. Kaplan, M.P.H., Cynthia S. Rand, Ph.D., Janet Robles, B.B.A., Opal Thompson, B.S., Michael E. Wechsler, M.D., Juan P. Wisnivesky, M.D., Dr.P.H., M. Diane McKee, M.D., Sunit P. Jariwala, M.D., Elina Jerschow, M.D., Paula J. Busse, M.D., David C. Kaelber, M.D., Ph.D., M.P.H., Sylvette Nazario, M.D., Michelle L. Hernandez, M.D., Andrea J. Apter, M.D., Ku-Lang Chang, M.D., Victor Pinto-Plata, M.D., Paul M. Stranges, Pharm.D., Laura P. Hurley, M.D., M.P.H., Jennifer Trevor, M.D., Thomas B. Casale, M.D., Geoffrey Chupp, M.D., Isaretta L. Riley, M.D., M.P.H., Kartik Shenoy, M.D., Magdalena Pasarica, M.D., Ph.D., Rafael A. Calderon-Candelario, M.D., Hazel Tapp, Ph.D., Ahmet Baydur, M.D., and Wilson D. Pace, M.D.
The authors’ affiliations are as follows: the Divisions of Pulmonary and Critical Care Medicine and Allergy and Immunology, Brigham and Women’s Hospital, Harvard Medical School (E.I., N.E.M., V.E.F., P.A.H., J.M.K., J.R.L., B.E.), and patient partner (O.T.), Boston, and the Lahey Hospital and Medical Center, Burlington (V.P.P.) — all in Massachusetts; the Division of Allergy and Immunology, Department of Internal Medicine, Morsani College of Medicine, University of South Florida, Tampa (J.-C.C., T.B.C.), the Department of Nursing, University of Miami Health System (M.F.), and Miller School of Medicine, University of Miami (R.A.C.C.), Miami, the Department of Community Health and Family Medicine, University of Florida College of Medicine, Gainesville (K.L.C.), and the University of Central Florida College of Medicine, Orlando (M.P.) — all in Florida; the Department of Family Medicine (J.K.C., W.D.P.) and the Division of Pulmonary Sciences and Critical Care Medicine, Department of Medicine (A.L.F.), University of Colorado, Aurora, the Public Leadership Group (G.M.H.), the Department of Medicine, National Jewish Health (M.E.W.), and the Denver Health and Hospital Authority (L.P.H.), Denver — all in Colorado; the American Academy of Family Physicians National Research Network, Leawood, KS (J.K.C., B.K.M., J.B.S.); Duke Clinical Research Institute, Duke University Medical Center, Durham, NC (L.S., F.W.R.); the Department of Family and Community Health, University of Minnesota, Minneapolis (B.P.Y.), and patient partner, St. Paul (S.M.) — both in Minnesota; patient partner, South Jordan, UT (A.D.C.M.); the Division of Adolescent Medicine (T.C.B.) and the Lung Health Center, Department of Medicine, University of Alabama, Birmingham (J.T.); the American Lung Association, Washington, DC (B.M.K.); the Department of Medicine, Johns Hopkins School of Medicine, Baltimore (C.S.R.); patient partner (J.R.), the Divisions of General Internal Medicine and Pulmonary and Critical Care Medicine (J.P.W.) and Clinical Immunology and Allergy (P.J.B.), Icahn School of Medicine at Mount Sinai, and Montefiore Medical Center, (E.J.), Albert Einstein College of Medicine (M.D.M., S.P.J.) — all in New York; the Center for Clinical Informatics Research and Education, the MetroHealth System, and the Departments of Internal Medicine, Pediatrics, and Population and Quantitative Health Sciences, Case Western Reserve University, Cleveland (D.C.K.); the Department of Internal Medicine, Section of Allergy and Immunology, University of Puerto Rico, San Juan (S.N.); the Division of Allergy and Immunology, University of North Carolina School of Medicine, Chapel Hill (M.L.H.), the Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University School of Medicine, Durham (I.L.R.), and the Department of Family Medicine, Atrium Health, Charlotte (H.T.) — all in North Carolina; the Division of Pulmonary, Allergy, and Critical Care Medicine, Perelman School of Medicine, University of Pennsylvania (A.J.A.), and the Temple Lung Center, Lewis Katz School of Medicine at Temple University (K.S.) — both in Philadelphia; the University of Illinois at Chicago College of Pharmacy, Chicago (P.M.S.); the Section of Pulmonary, Critical Care, and Sleep Medicine, Yale School of Medicine, New Haven, CT (G.C.); and the Division of Pulmonay, Critical Care, and Sleep Medicine, Keck School of Medicine, University of Southern California, Los Angeles (A.B.).
Footnotes
All the statements in this article, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or its Methodology Committee.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
Contributor Information
E. Israel, Divisions of Pulmonary and Critical Care Medicine and Allergy and Immunology, Brigham and Women’s Hospital, Harvard Medical School, Massachusetts
J.C. Cardet, Division of Allergy and Immunology, Department of Internal Medicine, Morsani College of Medicine, University of South Florida, Tampa, Florida
J.K. Carroll, Department of Family Medicine, University of Colorado, Aurora American Academy of Family Physicians National Research Network, Leawood, KS.
A.L. Fuhlbrigge, Division of Pulmonary Sciences and Critical Care Medicine, Department of Medicine, University of Colorado, Aurora
L. She, Duke Clinical Research Institute, Duke University Medical Center, Durham, NC, Minnesota
F.W. Rockhold, Duke Clinical Research Institute, Duke University Medical Center, Durham, NC
N.E. Maher, Divisions of Pulmonary and Critical Care Medicine and Allergy and Immunology, Brigham and Women’s Hospital, Harvard Medical School, Massachusetts
M. Fagan, Department of Nursing, University of Miami Health System, Florida
V.E. Forth, Divisions of Pulmonary and Critical Care Medicine and Allergy and Immunology, Brigham and Women’s Hospital, Harvard Medical School, Massachusetts
B.P. Yawn, Department of Family and Community Health, University of Minnesota, Minneapolis, Minnesota
P. Arias Hernandez, Divisions of Pulmonary and Critical Care Medicine and Allergy and Immunology, Brigham and Women’s Hospital, Harvard Medical School, Massachusetts
J.M. Kruse, Divisions of Pulmonary and Critical Care Medicine and Allergy and Immunology, Brigham and Women’s Hospital, Harvard Medical School, Massachusetts
B.K. Manning, American Academy of Family Physicians National Research Network, Leawood, KS American Lung Association, Washington, DC, New York.
J. Rodriguez-Louis, Divisions of Pulmonary and Critical Care Medicine and Allergy and Immunology, Brigham and Women’s Hospital, Harvard Medical School, Massachusetts
J.B. Shields, American Academy of Family Physicians National Research Network, Leawood, KS
B. Ericson, Divisions of Pulmonary and Critical Care Medicine and Allergy and Immunology, Brigham and Women’s Hospital, Harvard Medical School, Massachusetts
A.D. Colon-Moya, patient partner, South Jordan, UT
S. Madison, patient partner, St. Paul, Minnesota
T. Coyne-Beasley, Division of Adolescent Medicine, Department of Medicine, University of Alabama, Birmingham
G.M. Hammer, Public Leadership Group, Denver, Colorado
B.M. Kaplan, American Lung Association, Washington, DC, New York
C.S. Rand, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, New York
J. Robles, patient partner, New York
O. Thompson, patient partner, Boston, Massachusetts
M.E. Wechsler, Department of Medicine, National Jewish Health, Denver, Colorado
J.P. Wisnivesky, Divisions of General Internal Medicine and Pulmonary and Critical Care Medicine, New York
M.D. McKee, Albert Einstein College of Medicine, New York
S.P. Jariwala, Albert Einstein College of Medicine, New York
E. Jerschow, Icahn School of Medicine at Mount Sinai, and Montefiore Medical Center, New York
P.J. Busse, Clinical Immunology and Allergy, New York
D.C. Kaelber, Center for Clinical Informatics Research and Education, the MetroHealth System, and the Departments of Internal Medicine, Pediatrics, and Population and Quantitative Health Sciences, Case Western Reserve University, Cleveland, North Carolina
S. Nazario, Department of Internal Medicine, Section of Allergy and Immunology, University of Puerto Rico, San Juan, North Carolina
M.L. Hernandez, Division of Allergy and Immunology, University of North Carolina School of Medicine, Chapel Hill, North Carolina
A.J. Apter, Division of Pulmonary, Allergy, and Critical Care Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia
K.-L. Chang, Department of Community Health and Family Medicine, University of Florida College of Medicine, Gainesville, Florida
V. Pinto-Plata, Lahey Hospital and Medical Center, Burlington, Massachusetts
P.M. Stranges, University of Illinois at Chicago College of Pharmacy, Chicago
L.P. Hurley, Denver Health and Hospital Authority, Denver, Colorado
J. Trevor, Lung Health Center, Department of Medicine, University of Alabama, Birmingham
T.B. Casale, Division of Allergy and Immunology, Department of Internal Medicine, Morsani College of Medicine, University of South Florida, Tampa, Florida
G. Chupp, Section of Pulmonary, Critical Care, and Sleep Medicine, Yale School of Medicine, New Haven, CT
I.L. Riley, Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University School of Medicine, Durham, North Carolina
K. Shenoy, Temple Lung Center, Lewis Katz School of Medicine at Temple University, Philadelphia
M. Pasarica, University of Central Florida College of Medicine, Orlando, Florida
R.A. Calderon-Candelario, Miller School of Medicine, University of Miami, Florida
H. Tapp, Department of Family Medicine, Atrium Health, Charlotte, North Carolina
A. Baydur, Pulmonay, Critical Care, and Sleep Medicine, Keck School of Medicine, University of Southern California, Los Angeles
W.D. Pace, Department of Family Medicine, University of Colorado, Aurora
References
- 1.Centers for Disease Control and Prevention. Most recent national asthma data (https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm).
- 2.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc 2018;15:348–56. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan SD, Wenzel SE, Bresnahan BW, et al. Association of control and risk of severe asthma-related events in severe or difficult-to-treat asthma patients. Allergy 2007;62:655–60. [DOI] [PubMed] [Google Scholar]
- 4.Leong AB, Ramsey CD, Celedón JC. The challenge of asthma in minority populations. Clin Rev Allergy Immunol 2012;43:156–83. [DOI] [PubMed] [Google Scholar]
- 5.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health 2005;26:89–113. [DOI] [PubMed] [Google Scholar]
- 6.Law HZ, Oraka E, Mannino DM. The role of income in reducing racial and ethnic disparities in emergency room and urgent care center visits for asthma — United States, 2001–2009. J Asthma 2011;48:405–13. [DOI] [PubMed] [Google Scholar]
- 7.Crocker D, Brown C, Moolenaar R, et al. Racial and ethnic disparities in asthma medication usage and health-care utilization: data from the National Asthma Survey. Chest 2009;136:1063–71. [DOI] [PubMed] [Google Scholar]
- 8.Ginde AA, Espinola JA, Camargo CA Jr. Improved overall trends but persistent racial disparities in emergency department visits for acute asthma, 1993–2005. J Allergy Clin Immunol 2008;122:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ash M, Brandt S. Disparities in asthma hospitalization in Massachusetts. Am J Public Health 2006;96:358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta RS, Carrión-Carire V, Weiss KB. The widening black/white gap in asthma hospitalizations and mortality. J Allergy Clin Immunol 2006;117:351–8. [DOI] [PubMed] [Google Scholar]
- 11.Boudreaux ED, Emond SD, Clark S, Camargo CA Jr. Acute asthma among adults presenting to the emergency department: the role of race/ethnicity and socioeconomic status. Chest 2003;124:803–12. [DOI] [PubMed] [Google Scholar]
- 12.Homa DM, Mannino DM, Lara M. Asthma mortality in U.S. Hispanics of Mexican, Puerto Rican, and Cuban heritage, 1990–1995. Am J Respir Crit Care Med 2000;161:504–9. [DOI] [PubMed] [Google Scholar]
- 13.Press VG, Pappalardo AA, Conwell WD, Pincavage AT, Prochaska MH, Arora VM. Interventions to improve outcomes for minority adults with asthma: a systematic review. J Gen Intern Med 2012;27:1001–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol 2020;146:1217–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan JA, Demott M, McCoy JV, Chanmugam A, Rand CS. Nebulized beta2-agonist use in high-risk inner-city adults with asthma. J Asthma 2003;40:367–73. [DOI] [PubMed] [Google Scholar]
- 16.Apter A, Carroll JK, Cardet JC, et al. Nebulizer use by Black and Latinx adults with moderate to severe asthma. J Allergy Clin Immunol Pract 2021. October 18 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Lung Association. Barriers to asthma guidelines-based care coverage (https://www.lung.org/getmedia/8725ddcf-3d4f-4ed1-bb6a-10ebd1f96044/barriers-to-asthma-glbc.pdf.pdf).
- 18.Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA 2012;308:987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardet JC, Busse PJ, Carroll JK, et al. Adherence to adding inhaled corticosteroids to rescue therapy in a pragmatic trial with adults with asthma: a pilot study. Ann Allergy Asthma Immunol 2020(5);124:487–493.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Israel E, Cardet JC, Carroll JK, et al. A randomized, open-label, pragmatic study to assess reliever-triggered inhaled corticosteroid in African American/Black and Hispanic/Latinx adults with asthma: design and methods of the PREPARE trial. Contemp Clin Trials 2021;101:106246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Allergy, Asthma, and Immunology. Asthma IQ: patient management and outcomes (https://education.aaaai.org/AIQMgmtOutcomes).
- 22.Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180:59–99. [DOI] [PubMed] [Google Scholar]
- 23.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59–65. [DOI] [PubMed] [Google Scholar]
- 24.Schatz M, Sorkness CA, Li JT, et al. Asthma control test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006;117:549–56. [DOI] [PubMed] [Google Scholar]
- 25.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the asthma control test. J Allergy Clin Immunol 2009(4);124:719–23.e1. [DOI] [PubMed] [Google Scholar]
- 26.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest 1998;114:998–1007. [DOI] [PubMed] [Google Scholar]
- 27.Bime C, Wei CY, Holbrook JT, Sockrider MM, Revicki DA, Wise RA. Asthma symptom utility index: reliability, validity, responsiveness, and the minimal important difference in adult asthmatic patients. J Allergy Clin Immunol 2012;130:1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Data source with asthma content: national health interview survey (http://www.cdc.gov/asthma/survey/nhis.pdf).
- 29.Salciccioli JD, She L, Tulchinsky A, Rockhold F, Cardet JC, Israel E. Effect of COVID-19 on asthma exacerbation. J Allergy Clin Immunol Pract 2021;9(7):2896–2899.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler ME, Yawn BP, Fuhlbrigge AL, et al. Anticholinergic vs long-acting β-agonist in combination with inhaled corticosteroids in Black adults with asthma: the BELT randomized clinical trial. JAMA 2015;314:1720–30. [DOI] [PubMed] [Google Scholar]
- 31.Chan AHY, Horne R, Hankins M, Chisari C. The medication adherence report scale: a measurement tool for eliciting patients’ reports of nonadherence. Br J Clin Pharmacol 2020;86:1281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haun J, Luther S, Dodd V, Donaldson P. Measurement variation across health literacy assessments: implications for assessment selection in research and practice. J Health Commun 2012;17:Suppl 3:141–59. [DOI] [PubMed] [Google Scholar]
- 33.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1–24. [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284–92. [DOI] [PubMed] [Google Scholar]
- 35.Spitzer WO, Suissa S, Ernst P, et al. The use of β-agonists and the risk of death and near death from asthma. N Engl J Med 1992;326:501–6. [DOI] [PubMed] [Google Scholar]
- 36.Bacharier LB, Guilbert TW, Zeiger RS, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol 2009;123(5):1077–1082.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazarus SC, Chinchilli VM, Rollings NJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med 2007;175:783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramadan AA, Gaffin JM, Israel E, Phipatanakul W. Asthma and corticosteroid responses in childhood and adult asthma. Clin Chest Med 2019;40:163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev 2017;4(4):CD012226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zein JG, Udeh BL, Teague WG, et al. Impact of age and sex on outcomes and hospital cost of acute asthma in the United States, 2011–2012. PLoS One 2016;11(6):e0157301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet 2006;368:744–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



