Abstract
Analysis by two-dimensional gel electrophoresis revealed that Mycobacterium avium expresses several proteins unique to an intracellular infection. One abundant protein with an apparent molecular mass of 50 kDa was isolated, and the N-terminal sequence was determined. It matches a sequence in the M. tuberculosis database (Sanger) with similarity to the enzyme isocitrate lyase of both Corynebacterium glutamicum and Rhodococcus fascians. Only marginal similarity was observed between this open reading frame (ORF) (termed icl) and a second distinct ORF (named aceA) which exhibits a low similarity to other isocitrate lyases. Both ORFs can be found as distinct genes in the various mycobacterial databases recently published. Isocitrate lyase is a key enzyme in the glyoxylate cycle and is essential as an anapleurotic enzyme for growth on acetate and certain fatty acids as carbon source. In this study we express and purify Icl, as well as AceA proteins, and show that both exhibit isocitrate lyase activity. Various known inhibitors for isocitrate lyase were effective. Furthermore, we present evidence that in both M. avium and M. tuberculosis the production and activity of the isocitrate lyase is enhanced under minimal growth conditions when supplemented with acetate or palmitate.
Most microorganisms growing on acetate or fatty acids as the sole carbon source employ the glyoxylate bypass for the biosynthesis of cellular material. The key enzymes of this bypass are isocitrate lyase and malate synthase (18). The former cleaves isocitrate to succinate and glyoxylate, and the latter condenses glyoxylate with acetyl coenzyme A (acetyl-CoA) to yield malate. The glyoxylate bypass circumvents the loss of two carbon dioxides of the tricarboxylic acid cycle (TCA cycle), thereby permitting net incorporation of carbon into cellular structures during growth on acetate. In addition, even during operation of the TCA cycle, many fatty acids are partially metabolized to acetyl-CoA, thus requiring the presence of isocitrate lyase (for a review, see reference 37).
Isocitrate lyase competes with the TCA cycle enzyme isocitrate dehydrogenase for their common substrate isocitrate. By changing the total cellular activity of either of the two enzymes and/or by changing their affinities toward isocitrate, control of carbon flux between the two cycles is achieved (22). In Escherichia coli growth on acetate leads to a decrease in NADP+-dependent isocitrate dehydrogenase activity caused by the reversible phosphorylation of isocitrate dehydrogenase. The corresponding isocitrate dehydrogenase-kinase is encoded in the same operon as the isocitrate lyase and the malate synthase. The reduction in isocitrate dehydrogenase activity redirects isocitrate into the glyoxylate cycle through the activity of isocitrate lyase. The phosphorylation-dephosphorylation of isocitrate dehydrogenase is believed to regulate entry of the substrate into the glyoxylate bypass (26, 39). In addition, E. coli isocitrate lyase is inhibited by several metabolites, e.g., succinate, 3-phosphoglycerate, or phosphoenolpyruvate, leading to a more subtle control of the carbon flux (23, 30).
In mycobacteria, isocitrate lyase activity has been reported to increase continuously with the age of the culture in Mycobacterium tuberculosis H37Rv (25) but not in M. tuberculosis H37Ra or M. smegmatis (34). Other studies report enhanced glyoxylate cycle enzyme activity under low oxygen tension (41) or when the mycobacteria are grown in the presence of acetate (15).
Previous studies in our laboratory employing two-dimensional (2-D) sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE analysis of M. avium have identified a 50-kDa polypeptide, the expression of which was markedly upregulated upon infection of macrophages (35). N-terminal sequencing of the protein showed 15 amino acid residues, 13 of which were identical to the aceA (Rv 0467) sequence in M. tuberculosis databases. Upon examination of the databases, we found another open reading frame (ORF) that is identified as an isocitrate lyase, namely, aceA (4). In H37Rv, this ORF has a single-base-pair overlap resulting in two ORFs (aceAa/Rv1915 and aceAb/Rv1916; Sanger database), while it is read as one continuous ORF in CSU93 (MT1966; The Institute for Genomic Research database). For ease of reference and in light of the current findings, we refer to Rv0467 as icl (for isocitrate lyase) to distinguish it from the latter ORF, which we refer to as aceA.
To investigate whether both genes gave rise to active enzymes, we cloned the ORFs based on the DNA sequence of CSU93 and overexpressed the proteins in Escherichia coli. The proteins were purified by the 6His-tag attached to the N terminus. Enzyme assays with the purified proteins indicated that both function as isocitrate lyases.
We also demonstrate here at both the protein and enzyme levels that the expression of isocitrate lyase in M. avium and M. tuberculosis increases when acetate or palmitate are the limiting carbon sources and that cultivation in the presence of succinate suppresses isocitrate lyase expression. Preliminary experiments with a polyclonal antibody raised against recombinant AceA indicate that it is expressed under similar conditions as Icl only in M. tuberculosis CSU93 and not in M. tuberculosis H37Rv. In addition, the differences in Km and Vmax between the two enzymes suggest that AceA fulfills a role subordinate to Icl in the processing of isocitrate.
MATERIALS AND METHODS
Materials.
All chemicals were obtained from Sigma Chemical Company, St. Louis, Mo., and were of the highest purity. MTS was purchased from Promega, Madison, Wis., and the lactate dehydrogenase was obtained from Calbiochem-Novabiochem Corp., La Jolla, Calif.
Organisms and growth conditions.
Experiments were conducted with M. avium 101, M. tuberculosis CSU93, and M. tuberculosis H37Rv. The media used included Middlebrook 7H9 medium (Difco) containing 10% (vol/vol) OADC enrichment medium and 0.05% Tween 80. In addition, a modified Dubos medium was employed as a defined minimal medium, containing (per liter) 2 g of asparagine, 1 g of KH2PO4, 2.5 g of Na2HPO4, 10 mg of MgSO4 · 7H2O, 50 mg of ferric ammonium citrate, 0.5 mg of CaCl2, 0.1 mg of ZnSO4, 0.1 mg of CuSO4, and 0.05% Tween 80. For M. tuberculosis cultures, 0.5 g of Casitone (Difco) was added. The pH of the medium was 6.6. When required, carbon sources were added to a final concentration of 10 mM, with the exception of acetate, which was added to a maximal final concentration of 3 mM to M. avium cultures (see also reference 8). Nonaerated cultures were grown as a 30-ml culture in a 25-cm2 flask without stirring. Aeration of cultures was achieved with a 50-mm Teflon-coated magnetic stirring bar in a 500-ml Erlenmeyer flask containing 100 ml of medium at a stir rate of 80 rpm.
E. coli HB101 harboring the two plasmids (see below) was grown in Luria-Bertani medium (32) with ampicillin (50 μg/ml) and kanamycin (50 μg/ml).
Preparation of cell extracts.
Mycobacterial cultures were grown in the appropriate medium. Cells were harvested, washed three times with PBST (phosphate buffered saline plus 0.05% Tween 80), and resuspended in MOPS buffer (50 mM MOPS [morpholinepropane sulfonate], pH 6.8; 5 mM MgCl2, 5 mM l-cysteine, 1 mM EDTA) supplemented with protease inhibitors (tosyl-l-lysine chloromethyl ketone, 100 μg/ml; pepstatine A, 50 μg/ml; leupeptine, 50 μg/ml; trans-epoxysuccinyl-l-leucylamido(4-guanidino)-butane, 50 μg/ml). The cells were disrupted with a Bead-Beater (Biospec, Bartlesville, Okla.). The supernatant was harvested after centrifugation for 30 min at 300,000 × g. This cell extract was frozen in liquid nitrogen and stored at −80°C.
Cloning and expression of the Icl protein.
A 1.3-kb DNA fragment containing the putative icl gene was amplified by PCR by using the following oligonucleotide primers: 5′-AGC GCA TAT GTC TGT CGT CGG-3′ and 5′-GTC GGA TCC AGA CTA GTG GAA CTG G-3′ and M. tuberculosis CSU93 DNA as template. PCR amplification conditions were as recommended by the manufacturer for fragments smaller than 5 kb with a Perkin-Elmer 480 thermocycler with Advantage GC cDNA polymerase (Clontech). The amplified DNA was digested with NdeI and BamHI and inserted into p6HisF-11d (kindly provided by Cheng-Ming Chiang, The Rockefeller University, New York, N.Y. [3]). For overexpression, E. coli HB101(pGP1-2) cells carrying the recombinant p6HisF-11d(icl) plasmid were grown to exponential phase and treated according to the method of Tabor and Richardson (36). Briefly, cells containing the plasmids were grown exponentially at 30°C in Luria-Bertani medium (32) in the presence of the required antibiotics. Overexpression of Icl was induced by shifting the temperature to 42°C for 20 min. After induction, the temperature was shifted down to 37°C for an additional 90 min, cells were harvested by centrifugation at 4°C, and cell pellets were frozen in liquid nitrogen.
Cloning of the AceA protein.
The aceA gene was amplified by using primers 5′-TAG CAC ATA TGG CCA TCG CCG AAA CG-3′ and 5′-GAC TGG ATC CTA CGC CTC CTT CGT GAT-3′, with CSU93 DNA as the template. PCR amplification, cloning, and expression conditions were essentially as described for the Icl protein.
Purification of Icl and AceA.
Cell lysates were prepared by resuspending the frozen cell pellets of a 500-ml culture [HB101(pGP1-2)] with the respective plasmid carrying icl or aceA in 30 ml of buffer 1 {50 mM NaH2PO4, 20 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 100 mM NaCl, 10 mM imidazole; pH 8} with protease inhibitors (tosyl-l-lysine chloromethyl ketone, 100 μg/ml; pepstatine A, 50 μg/ml; leupeptine, 50 μg/ml; trans-epoxysuccinyl-l-leucylamido(4-guanidino)-butane, 50 μg/ml) and by sonicating the suspension on ice for 5 min in intervals with 30-s breaks (Biosonik III; Bronwil Scientific, Rochester, N.Y.). Cell debris and membranes were removed by centrifugation at 300,000 × g for 30 min. This cell extract was applied to 3 ml of a metal affinity resin (Talon; Clontech, Palo Alto, Calif.) equilibrated with buffer 1. After sample application, the column was washed with 30 ml of buffer 1, followed by a wash with 30 ml of buffer 2 (same as buffer 1 but with a pH of 5.6 and no imidazole). The His-tagged proteins were eluted from the resin with buffer 3 (same as buffer 1 plus 5% glycerol, 1 mM β-mercaptoethanol, and 300 mM imidazole). Fractions containing isocitrate lyase activity were pooled and frozen in liquid nitrogen.
Assay of isocitrate lyase.
To determine the cleavage reaction of the isocitrate lyase, the glyoxylate formed during catalysis was reduced to glycolate by lactate dehydrogenase (LDH) with the concomitant oxidation of NADH (40). By using 96-well plates, 50 μl (65 μl if purified protein was examined) of MOPS buffer containing 2 U of LDH was mixed with 20 μl of cell extract (5 μl of purified protein). Then, 0.2 mM NADH was added, and the mixture was preincubated for 5 min at 37°C. After the addition of 2 mM threo d-(s) isocitrate, the mixture was incubated for 20 min at 37°C. The reaction was stopped with 4 M urea, and the decrease of the NADH was determined by adding the formazan dye MTS (MTS/PMS ratio of 100:1) at a final concentration of 0.2 mg/ml and reading the plate at 490 nm. Activities lower than 5 nmol/min/mg were considered to be below the sensitivity of the assay.
Determination of Michaelis and inhibition constants.
The Km and Vmax values were determined by using a double-reciprocal Lineweaver-Burke plot. Ki values were determined from linear replots of Lineweaver-Burke slopes versus inhibitor concentrations (33).
Determination of pH optimum.
Isocitrate lyase assays were performed as described above, but different buffers were used in place of 50 mM MOPS (pH 6.8). These included morpholineethanesulfonic acid-NaOH at pHs 5.5, 6, and 6.5; MOPS-NaOH at pHs 6.5, 7, and 7.5; Tricine-HCl at pHs 7.5, 8, and 8.5; and Tris-HCl at pHs 8.5 and 9.
Metal ion activity studies.
The purified proteins were dialyzed against Mg2+-free buffer. Isocitrate lyase assays were performed either with different metal ions or as a combination with MgCl2 at a concentration of 5 mM. The metals used were MnCl2, CoCl2, FeCl2, CaCl2, BaCl2, NiCl2, ZnCl2, CuCl2, and HgCl2.
Protein determination.
Protein content was determined by the dye-binding method of Bradford (2).
Electrophoresis.
SDS-PAGE was performed in 9% gels at 150 V by the method of Laemmli (19) by using a LKB 2050 Midget Electrophoresis Unit. 2-D electrophoresis was performed as described previously (35).
Production of polyclonal antibodies to Icl and AceA.
Antibodies against the N-terminal peptide of Icl and an internal fragment of AceA (ESDDKLANVIFQPIQDRR) were produced in New Zealand White rabbits after coupling of the peptide to ovalbumin. Antibodies against AceA were also produced by injecting the purified, recombinant protein into New Zealand White rabbits. The polyclonal antibodies were affinity purified by using the respective antigen coupled to CNBr-activated Sepharose 4B.
RESULTS
N-terminal amino acid sequence.
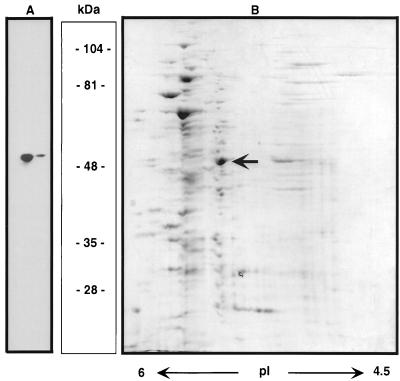
As reported previously (35), 2-D SDS-PAGE analysis of established (8- to 12-day) M. avium infections in murine bone marrow-derived macrophages revealed several polypeptides that were upregulated in expression when compared to bacteria grown in Middlebrook medium. The most notable of these was a protein with an apparent molecular mass of 50 kDa and an isoelectric point of approximately 5.0. This protein was transferred onto polyvinylidene difluoride membranes, and the N-terminal sequence was determined. The first 15 amino acids were found to be the following: MSVVGTPKSAEIQKD. A polyclonal antibody raised against a synthetic peptide corresponding to this amino acid sequence reacted specifically with a protein of 50 kDa in immunoblots of M. avium grown on acetate (Fig. 1). The same antibody recognized a single band of comparable mobility in SDS-PAGE-separated extracts from M. tuberculosis grown in macrophages and M. leprae isolated from SCID mice (M. leprae was kindly provided by J. Krahenbuhl, Louisiana State University, Carville) (data not shown).
FIG. 1.
2-D PAGE of cell extracts from acetate-grown M. avium cultures. A total of 30 μg of crude protein extracts was applied to the gel. The Icl is indicated by an arrow. (A) Immunoblot, probed with anti-Icl antibody. (B) Coomassie blue staining. The figure also indicates the specificity of the antibody.
Comparison of the amino acid sequence of the isocitrate lyase from M. tuberculosis with those from other organisms.
The N-terminal sequence obtained from the 50-kDa protein matched a sequence of 428 amino acids in the mycobacterial genome databases (assembled by the Sanger Center, as well as by TIGR) encoded by a gene which we termed icl for isocitrate lyase. Further general databank searches with this ORF revealed closest similarity to the isocitrate lyases from Corynebacterium glutamicum and Rhodococcus fascians with identities to the M. tuberculosis sequence of 79 and 80%, respectively.
These searches also revealed a second ORF in M. tuberculosis with similarity to isocitrate lyase, although its level of identity with enzymes from E. coli (35%) and M. tuberculosis Icl (37%) is lower. In M. tuberculosis H37Rv this second isocitrate lyase gene (named aceA) consists of two overlapping ORFs (aceAa and aceAb) that share a single base pair at the 3′ end of aceAa and the 5′ end of aceAb. However, in M. tuberculosis CSU93 aceA appears to be a single ORF.
The recent progress of the sequencing projects from M. avium, M. tuberculosis, and M. leprae show that both the icl and the aceA ORFs exist as two distinct genes present in single copies in all of those strains, with a sequence identity of ca. 80% between species.
Expression and purification of recombinant Icl and AceA from M. tuberculosis CSU93 in E. coli.
To confirm that the Icl and AceA are active isocitrate lyases, we expressed the two ORFs of M. tuberculosis (CSU93) in E. coli by using a pET-based vector. The ORFs were amplified by PCR, ligated to p6HisF-11d vector DNA, and cloned into E. coli HB101. The recombinant proteins were purified to approximately 90% in a one-step procedure by using a metal-affinity resin. Figure 2 shows a Coomassie blue-stained gel of the purified proteins and the corresponding Western blots. Recombinant Icl reacted with the antibody generated against the N-terminal amino acid sequence characterized in Fig. 1. The high-molecular-weight bands appear to be multimers formed in E. coli. We did not observe these bands on analysis of extracts from Mycobacterium spp. AceA reacted with an antipeptide antibody generated against an internal sequence of the aceA ORF. These blots confirm the identity of the recombinant enzymes and their corresponding ORFs.
FIG. 2.
SDS-PAGE of recombinant Icl and AceA. Approximately 2 μg of purified protein were applied to each lane. (A) Coomassie blue-stained SDS-PAGE gels. (B) Immunoblots with anti-Icl or anti-AceA peptide antibodies, diluted 1:5,000, respectively. The additional protein bands that also react with the antibody constitute multimers of Icl.
Characteristics of the purified Icl.
The specific activity of the purified enzyme for isocitrate was 1.3 μmol/min/mg of protein. The Km of the purified recombinant Icl for threo D-(s) isocitrate was determined to be 145 μM by using a Hanes-Woolf plot.
The inhibition of Icl activity by several compounds, known to be effective against various isocitrate lyases, was examined. Itaconate, itaconic anhydride, bromopyruvate, and 3-nitropropionate were found to be the most potent inhibitors, with inhibition constants of 120, 190, 120, and 3 μM, respectively. Interestingly, at saturating substrate conditions succinate did not reveal any inhibitory effect on the recombinant enzyme. Oxalate and malate were also shown to inhibit the activity to approximately 50% at 5 mM inhibitor concentration. 3-Phosphoglycerate, 6-phosphogluconate, fructose-1,6-bisphosphate, and malonic acid had no inhibitory effect. Most of the effectors can be classified as structural analogs of the reaction products succinate (itaconate, itaconic anhydride, and 3-nitropropionate) or glyoxylate (3-bromopyruvate and oxalate).
In the absence of divalent cations, only negligible activity was measured for the purified Icl, whereas addition of Mg2+ or Mn2+ supported enzyme activity. Mg2+ at 5 mM was found to be the most effective cation. Mn2+ was able to replace Mg2+, yielding 39% of the activity obtained with Mg2+. Co2+, Fe2+, Ca2+, Ba2+, Ni2+, Cd2+, Zn2+, Cu2+, and Hg2+ were not able to support significant Icl activity. A variety of metal ion combinations were studied for their ability to inhibit Icl activity. The cations were present in the assay mixture in an equimolar concentration with magnesium. Ca2+ and Ba2+ had no inhibitory effect. Mn2+ and Zn2+ both reduced the activity to almost 25%, while all other ions inhibited activity below the sensitivity of the assay. It is known for the isocitrate lyases of C. glutamicum and Acinetobacter calcoaceticus that Mn2+ can partially substitute for Mg2+ (12, 30).
The Icl activity was assayed for its pH dependence. Four different buffers were used in the pH range of 5.5 to 9. These buffers were used through overlapping pH values to control for possible effects of the different buffer components on Icl activity. The optimal pH for the assay of Icl activity was found to be 6.8 with a MOPS buffer.
Enzyme activity was stable for several months when the purified protein was frozen in liquid nitrogen and stored at −80°C. Even upon thawing and storage at 4°C, only minimal activity was lost after 2 months. Addition of glycerol and the presence of a reducing agent in the purification buffer appeared to be necessary to retain activity of the purified recombinant protein.
Characteristics of the purified AceA.
Recombinant AceA also showed isocitrate lyase activity after purification, the Km of the recombinant protein for threo D-(s) isocitrate was determined to be 1.3 mM by using a Tricine-HCl buffer at pH 7.5 (which was established as the optimal assay buffer for this protein). This Km is approximately 10-fold higher than that of Icl. The Vmax of the purified enzyme for threo D-(s) isocitrate was 0.41 μmol/min/mg, ca. three times slower than Icl. The inhibitor profile of AceA was similar to that of the Icl protein. Itaconate, itaconic anhydride, bromopyruvate, and 3-nitropropionate were effective with Ki values of 220, 480, 710, and 110 μM, respectively. Succinate inhibited the activity to approximately 50% at a 5 mM inhibitor concentration. The purified enzyme proved to be very unstable, losing its activity within a couple of hours on ice. Neither the addition of glycerol nor bovine serum albumin improved its stability.
Effects of carbon sources on Icl and AceA induction.
In most organisms, the expression of isocitrate lyase activity is dependent on the carbon source in the growth medium. To elucidate whether the induction of Icl and AceA expression and activity in M. avium and M. tuberculosis is also dependent on nutrients, crude bacterial extracts were assayed for both isocitrate lyase activity and expression.
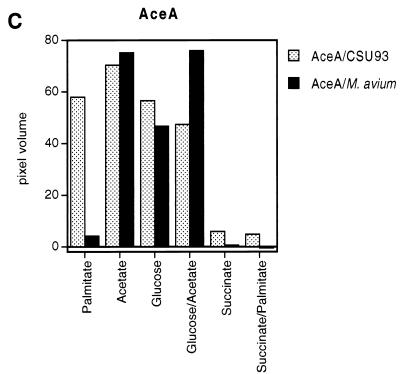
The specific total activity of the isocitrate lyase in M. avium varied widely depending on the primary carbon source (Table 1). The lowest level of induction was observed when cells were grown on succinate or glucose, with specific activities of 12.3 ± 0.35 and 97.6 ± 0.2 nmol/min/mg of protein, respectively. Growth on acetate or on palmitate led to high levels of induction (439.6 ± 0.2 and 1,193.4 ± 0.2 nmol/min/mg of protein, respectively). We also determined whether either acetate or palmitate could still upregulate isocitrate lyase activity when the cultures were given an alternate carbon source. M. avium cells were harvested in mid-log phase to avoid depletion of any of the carbon sources. When cultures were grown on palmitate with glucose, the levels of enzyme activity were about half that of acetate-grown cultures (217.5 ± 0.51 nmol/min/mg of protein). Less activity was demonstrated with M. avium cultivated on acetate plus glucose at 84.7 ± 0.35 nmol/min/mg of protein. Growth of M. avium on a combination of succinate and acetate or on succinate and palmitate in minimal medium, however, did not result in any upregulation of the enzyme. Thus, the presence of an alternate carbon source does not completely repress induction of the isocitrate lyase unless succinate is present. Because the total isocitrate lyase activity is the product of two discrete enzymes, we examined the relative levels of Icl and AceA protein by immunoblotting comparable amounts of SDS-PAGE separated extracts from bacilli grown under differing conditions (Fig. 3). It was found that the activity levels correlated with the relative amount of Icl protein detected in immunoblots. Expression of AceA in M. avium is apparently only upregulated by acetate and not by palmitate, while succinate, again, repressed expression of this enzyme completely. The data indicate strongly that the primary regulation of carbon flux into the glyoxylate pathway is at the level of isocitrate lyase expression. The pronounced effect of succinate on the Icl and AceA production is likely attributable to feedback inhibition since succinate is one of the products of isocitrate lyase activity.
TABLE 1.
Isocitrate lyase activity in M. avium grown on various carbon substratesa
| Carbon source | Mean enzyme sp act (nmol/min/mg of protein) ± SD |
|---|---|
| Palmitate | 1,193.4 ± 0.2 |
| Acetate | 439.6 ± 0.2 |
| Glucose | 97.6 ± 0.2 |
| Succinate | 12.3 ± 0.35 |
| Acetate-glucose | 84.7 ± 0.35 |
| Acetate-succinate | 17 ± 0.5 |
| Palmitate-glucose | 217.5 ± 0.51 |
| Palmitate-succinate | ≤5 |
| Middlebrook medium | 120.5 ± 0.5 |
Cell extracts were made from cultures grown on the indicated carbon source and assayed for enzyme activity. The values represent the mean of at least three independent experiments.
FIG. 3.
Effects of carbon source on expression of Icl and AceA in M. avium and M. tuberculosis. The mycobacterial cultures were grown in minimal medium with the carbon sources indicated. Cells were harvested, and cell extracts were prepared. A total of 2 μg of total bacterial extract was applied to each lane. Proteins were transferred to a nitrocellulose membrane. (A) The extracts were probed with either an anti-Icl peptide antibody or an anti-AceA antibody raised against the recombinant protein. Antibodies were diluted 1:10,000, respectively. Lanes: 1, minimal medium (MM) plus palmitate; 2, MM plus acetate; 3, MM plus glucose; 4, MM plus glucose and acetate; 5 MM plus succinate; 6, MM plus succinate and palmitate. Quantification of the Icl (B) and AceA (C) amounts. All membranes were exposed to Amersham Hyperfilm MP, and the volumes (i.e., the sum of pixel values within an object) were quantitated by densitometry (FluorImager SI; Molecular Dynamics). The carbon sources used for each culture and the respective strains are indicated.
We also examined the levels of expression of Icl and AceA in M. tuberculosis CSU93 and H37Rv under different culture conditions. However, M. tuberculosis is more fastidious than M. avium and would not grow on minimal medium that was not supplemented with Casamino Acids in addition to acetate or palmitate, although the latter carbon sources were limiting for growth. As a result we do not know whether the activity levels obtained were maximal. By according to glucose-grown bacteria an arbitrary value, we found that palmitate induced isocitrate lyase activity (threefold) to a greater extent than acetate (twofold) and that succinate repressed the enzyme activity below levels of detection. Glucose, on the other hand, could not repress expression of activity when given together with acetate or palmitate (these remained two- to threefold). Once again these data were substantiated by immunoblots with anti-Icl and anti-AceA antibodies (Fig. 3). In contrast to M. avium, AceA production in strain CSU93 increased when the cells were grown on palmitate. Interestingly, when grown under the same conditions, only strain CSU93 and not H37Rv expressed levels of AceA detectable by immunoblot (not shown). The apparent lack of expression of this enzyme in H37Rv may indicate that the ORF(s) Rv1915 and Rv1916 do not give rise to a product.
Our attempts to measure malate synthase activity in crude mycobacterial extracts were unsuccessful. However, preliminary studies with an antipeptide antibody against the malate synthase from M. tuberculosis CSU93 showed that under the conditions examined no differential expression of protein could be observed (not shown).
DISCUSSION
Isocitrate lyase and the glyoxylate bypass are required by microorganisms for growth on the two-carbon compound acetate. In addition, many fatty acids are metabolized, at least partially, to acetyl-CoA, which is mainly catabolized via the TCA cycle. However, with each turn of the cycle two carbon atoms are lost as CO2. The glyoxylate pathway circumvents the loss of carbon dioxide and so facilitates a net gain of carbon into cellular structures. It acts as a link in the formation of carbohydrates from fatty acids and occurs in seedlings rich in oil such as castor bean (Ricinus communis) (16). It is therefore not surprising that the enzymes of this cycle are present in mycobacteria, which are renowned for their lipid content.
By searching the mycobacterial databases with the amino terminus of a protein which is abundant in intracellular M. avium, we were able to align the sequence with an ORF (Rv0467, named icl) which showed high similarity to an isocitrate lyase of C. glutamicum and R. fascians (28, 38). The latter organisms, as well as mycobacteria, belong to the Actinomycetes, and high similarity between equivalent proteins of these groups is not unusual (13, 14). Interestingly, the mycobacterial databases contain another ORF (aceA) which shows some homology to isocitrate lyases. In H37Rv this ORF contains two genes, aceAa (Rv1915) and aceAb (Rv1916). The two genes overlap by one nucleotide according to the published database of the Sanger Center. This arrangement is in contrast to the highly similar ORF in CSU93 (MT1966; TIGR database), as well as a closely related ORF from M. leprae (7), which both show a single, continuous reading frame.
Polyclonal antibodies, generated against both a synthetic peptide from the predicted amino acid sequence of AceA and the recombinant protein itself, indicated that the enzyme is expressed as a protein of approximately 67 kDa in M. tuberculosis CSU93 under the same conditions as Icl. In H37Rv, however, this gene appears to be a pseudogene, since AceA is not expressed. The high Km value of the purified, recombinant enzyme (1.3 mM versus 145 μM for Icl) and its lower Vmax (0.45 versus 1.3 μmol/min/mg), together with its absence in H37Rv, lead us to speculate that the protein might assume a subordinate role in M. tuberculosis. AceA may only be needed when the level of isocitrate rises above a certain threshold. The intracellular concentration of isocitrate has not been calculated for Mycobacterium spp.; however, in Pseudomonas nautica the intracellular isocitrate concentration did not exceed 300 μM (31), which (if applicable for M. tuberculosis) is considerably below the Km for AceA. Since the Km for threo D-(s) isocitrate in the cytoplasmic extracts of M. avium grown on palmitate corresponds to the Km for the recombinant, purified Icl, we conclude that under normal conditions AceA does not play a major role in the glyoxylate pathway of mycobacteria.
Affinity-purified, antipeptide antibody directed against the Icl protein, in conjunction with enzyme assays, demonstrated that Icl expression can be upregulated by the growth of both M. avium and M. tuberculosis in minimal medium supplemented with either acetate or palmitate. However, a low level of Icl expression was consistently observed irrespective of the carbon source. This was more marked in M. tuberculosis, where the Icl appeared to be particularly responsive to oxygen availability since cultures grown under aeration produced less enzyme than comparable cultures grown for 1 to 2 weeks without disturbance (not shown). This correlates with the observations of Wayne et al. (41), who described a transient increase of isocitrate lyase activity in M. tuberculosis cells during adaptation to microaerophilic conditions. Our analysis also reveals that isocitrate lyase activity is elevated in bacteria when grown on other carbon sources in the presence palmitate and acetate. A similar situation has been described for C. glutamicum, where isocitrate lyase activity is also high when the organism is grown on various carbon sources together with acetate. This contrasts with other microorganisms, such as E. coli (17), Rhodobacter capsulatus (1), and Bradyrhyzobium japonicum (10), that express isocitrate lyase when acetate or fatty acids provide the sole carbon source but do not express the enzyme in cultures grown on any other substrate, irrespective of the presence or absence of acetate.
Obviously the arrangement of the genes of the glyoxylate shunt pathway within the genomes of organisms can have a fundamental influence on the regulation and routing of carbon flux. In E. coli, the isocitrate lyase gene aceA and the malate synthase A gene aceB are organized as an operon together with aceK (isocitrate dehydrogenase kinase-phosphatase [IDHP]) (5). IDHP activates and deactivates isocitrate dehydrogenase by phosphorylation and dephosphorylation, thereby regulating the flux of isocitrate into the glyoxylate cycle (20, 21). A repressor protein, IclR (24) that in turn is positively regulated by FadR, a fatty acid degradation-synthesis regulator (11), regulates the expression of this operon. This cascade of regulation limits expression of the ace operon to growth on acetate and fatty acids as the sole carbon source. If another preferred substrate is present (e.g., glucose or pyruvate), expression of the glyoxylate bypass enzymes is repressed (6, 18).
Analyses of the databases for M. tuberculosis H37Rv and CSU93 reveal another arrangement: the genes encoding malate synthase and isocitrate lyase are separated by several thousand base pairs (4). The malate synthase gene shows very high homology to the corresponding C. glutamicum enzyme (29). However, both of these genes show weak identity with aceB, which encodes malate synthase A from E. coli, which is associated with the glyoxylate cycle. In contrast, the malate synthase genes of both M. tuberculosis and C. glutamicum are much more closely related (>55% identity) to glcB, which encodes malate synthase G, part of the glycolate utilization pathway of E. coli (27).
While the sequence similarity between the enzymes of the glyoxylate cycle of M. tuberculosis and C. glutamicum is very high, the gene organization appears to be quite different, suggesting that the cycle may respond to different regulatory pathways. In M. avium, the isocitrate lyase is clearly upregulated when the cells utilize acetate as the sole carbon source. Growth on palmitate increases this enzyme activity an additional twofold. Furthermore, the amount of Icl expressed was elevated when M. avium were grown intracellularly. However, it should be noted that we found the levels of expression of Icl by intracellular M. avium to be extremely variable, and we suspect that the intracellular environment per se was only part of the stimulus for Icl upregulation. In a recent series of experiments conducted by Graham and Clark-Curtiss employing differential gene expression, these authors also detected the expression of Icl but not AceA in intracellular M. tuberculosis H37Rv (9). One might speculate that the intracellular environment of M. avium and M. tuberculosis, the mycobacterium-containing phagosome, is one of restricted nutrients and that host lipids and other long-chain fatty acids may be one of the more abundant carbon sources. This, in combination with a microaerophilic environment, could lead to an upregulation of the glyoxylate cycle as an anapleurotic sequence for replenishing the pool of substrates used for biosynthetic processes. Our current studies are aimed at delineating the environmental stimuli responsible for mobilization of the glyoxylate pathway.
ACKNOWLEDGMENTS
This work was supported by grants AI33348, A143702, and HL55936 from the U.S. Public Health Services. K.H. is supported by a grant from Glaxo-Wellcome Action TB. A.M. is supported in part by OTKA 25326 from the Hungarian Science Fund.
REFERENCES
- 1.Blasco R, Cárdenas J, Castillo F. Regulation of isocitrate lyase in Rhodobacter capsulatus E1F1. Curr Microbiol. 1991;22:73–76. [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Chiang C-M, Roeder R G. Cloning of an intrinsic human TFIID subunit that interacts with multiple transcriptional activators. Science. 1995;267:531–536. doi: 10.1126/science.7824954. [DOI] [PubMed] [Google Scholar]
- 4.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 5.Cortay J C, Bleicher F, Duclos B, Cenatiempo Y, Gautier C, Prato J L, Cozzone A J. Utilization of acetate in Escherichia coli: structured organization and differential expression of the ace operon. Biochimie. 1989;77:1043–1049. doi: 10.1016/0300-9084(89)90109-0. [DOI] [PubMed] [Google Scholar]
- 6.Cronan J E, LaPorte D C. Tricarboxylic acid cycle and glyoxylate bypass. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 206–216. [Google Scholar]
- 7.Fishi H, Cole S T. The Mycobacterium leprae genome: systematic sequence analysis identifies key catabolic enzymes, ATP-dependent transport systems and a novel polA locus associated with genomic variability. Mol Microbiol. 1995;16:909–919. doi: 10.1111/j.1365-2958.1995.tb02317.x. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Flores R, Kansal R G, Tamez-Guerra R, Mehta R T. Growth promotion of Mycobacterium avium-M. intracellulare complex by enterobacterial acetate. Am J Trop Med Hyg. 1996;55:610–616. doi: 10.4269/ajtmh.1996.55.610. [DOI] [PubMed] [Google Scholar]
- 9.Graham J E, Clark-Curtiss J E. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS) Proc Natl Acad Sci. 1999;96:11554–11559. doi: 10.1073/pnas.96.20.11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green L S, Karr D B, Emerich D W. Isocitrate dehydrogenase and glyoxylate cycle enzyme activities in Bradyrhizobium japonicum under various growth conditions. Arch Microbiol. 1998;169:445–451. doi: 10.1007/s002030050595. [DOI] [PubMed] [Google Scholar]
- 11.Gui L, Sunnarborg A, LaPorte D C. Regulated expression of a repressor protein: FadR activates IclR. J Bacteriol. 1996;178:4704–4709. doi: 10.1128/jb.178.15.4704-4709.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyt J C, Johnson K E, Reeves H C. Purification and characterization of Acinetobacter calcoaceticus isocitrate lyase. J Bacteriol. 1991;173:6844–6848. doi: 10.1128/jb.173.21.6844-6848.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jager W, Peters-Wendisch P G, Kalinowski J, Puhler A. A Corynebacterium glutamicum gene encoding a two-domain protein similar to biotin carboxylases and biotin-carboxyl-carrier proteins. Arch Microbiol. 1996;166:72–82. doi: 10.1007/s002030050359. [DOI] [PubMed] [Google Scholar]
- 14.Joliff G, Mathieu L, Hahn V, Bayan N, Duchiron F, Renaud M, Shechter E, Leblon G. Cloning and nucleotide sequence of the csp1 gene encoding PS1, one of the two major secreted proteins of Corynebacterium glutamicum: the deduced N-terminal region of PS1 is similar to the Mycobacterium antigen 85 complex. Mol Microbiol. 1992;6:2349–2362. doi: 10.1111/j.1365-2958.1992.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 15.Kannan K B, Katoch V M, Bharadwaj V P, Sharma V D, Datta A K, Shivannavar C T. Metabolic studies on mycobacteria. II. Glyoxylate by-pass (TCA cycle) enzymes of slow and fast growing mycobacteria. Indian J Lepr. 1985;57:542–548. [PubMed] [Google Scholar]
- 16.Kornberg H L, Beevers H. A mechanism of conversion of fat to carbohydrate in castor beans. Nature. 1957;180:35–36. doi: 10.1038/180035a0. [DOI] [PubMed] [Google Scholar]
- 17.Kornberg H L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966;99:1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornberg H L. Anapleurotic sequences and their role in metabolism. Essays Biochem. 1966;2:1–31. [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.LaPorte D C, Koshland D E., Jr A protein with kinase and phosphatase activities involved in regulation of tricarboxylic acid cycle. Nature. 1982;300:458–460. doi: 10.1038/300458a0. [DOI] [PubMed] [Google Scholar]
- 21.LaPorte D C, Walsh K, Koshland D E., Jr The branchpoint effect: ultrasensitivity and subsensitivity to metabolic control. J Biol Chem. 1984;259:14068–14075. [PubMed] [Google Scholar]
- 22.LaPorte D C, Thorsness P E, Koshland D E., Jr Compensatory phosphorylation of isocitrate dehydrogenase, a mechanism for adaptation to the intracellular environment. J Biol Chem. 1985;260:10563–10568. [PubMed] [Google Scholar]
- 23.MacKintosh C, Nimmo H G. Purification and regulatory properties of isocitrate lyase from Escherichia coli ML308. Biochem J. 1988;250:25–31. doi: 10.1042/bj2500025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maloy S R, Nunn W D. Genetic regulation of the glyoxylate shunt in Escherichia coli K-12. J Bacteriol. 1982;149:173–180. doi: 10.1128/jb.149.1.173-180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy P S, Sirsi M, Ramakrishnan T. Effects of age on the enzymes of tricarboxylic acid and related cycles in Mycobacterium tuberculosis H37Rv. Am Rev Respir Dis. 1973;108:689–690. doi: 10.1164/arrd.1973.108.3.689. [DOI] [PubMed] [Google Scholar]
- 26.Nimmo H G, Borthwick A C, El-Mansi E M T, Holms W H, MacKintosh C, Nimmo G A. The branchpoint between the citric acid cycle and the glyoxylate bypass in Escherichia coli. Biochem Soc Symp. 1987;54:93–101. [PubMed] [Google Scholar]
- 27.Pellicer M-T, Badía J, Aguilar J, Baldomà L. glc locus of Escherichia coli: characterization of genes encoding the subunits of glycolate oxidase and the glc regulator protein. J Bacteriol. 1996;178:2051–2059. doi: 10.1128/jb.178.7.2051-2059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinscheid D J, Eikmanns B J, Sahm H. Characterization of the isocitrate lyase gene from Corynebacterium glutamicum and biochemical analysis of the enzyme. J Bacteriol. 1994;176:3474–3483. doi: 10.1128/jb.176.12.3474-3483.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinscheid D J, Eikmanns B J, Sahm H. Malate synthase from Corynebacterium glutamicum: sequence analysis of the gene and biochemical characterization of the enzyme. Microbiology. 1994;140:3099–3108. doi: 10.1099/13500872-140-11-3099. [DOI] [PubMed] [Google Scholar]
- 30.Robertson E F, Reeves H C. Purification and characterization of isocitrate lyase from E. coli. Curr Microbiol. 1987;14:347–350. [Google Scholar]
- 31.Roy S O, Packard T T. NADP-isocitrate dehydrogenase from Pseudomonas nautica: kinetic constant determination and carbon limitation effects on the pool of intracellular substrates. Appl Environ Microbiol. 1998;64:4958–4964. doi: 10.1128/aem.64.12.4958-4964.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Segel I H. Enzyme kinetics. New York, N.Y: Wiley Interscience; 1975. [Google Scholar]
- 34.Seshadri R, Murthy P S, Venkitasubramanian T A. Isocitrate lyase in Mycobacteria. Indian J Biochem Biophys. 1976;13:95–96. [PubMed] [Google Scholar]
- 35.Sturgill-Koszycki S, Haddix P L, Russell D G. The interaction between Mycobacterium and the macrophage analyzed by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1997;18:1558–1565. doi: 10.1002/elps.1150181411. [DOI] [PubMed] [Google Scholar]
- 36.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanni P, Giachetti E, Pinzauti G, McFadden B. Comparative structure, function and regulation of isocitrate lyase, an important assimilatory enzyme. Comp Biochem Physiol. 1990;95B:431–458. doi: 10.1016/0305-0491(90)90002-b. [DOI] [PubMed] [Google Scholar]
- 38.Vereecke D, Villarroel R, Van Montagu M, Desomer J. Cloning and sequence analysis of the gene encoding isocitrate lyase from Rhodococcus fascians. Gene. 1994;145:109–114. doi: 10.1016/0378-1119(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 39.Walsh K, Koshland D E., Jr Determination of flux through the branch point of two metabolic cycles: the tricarboxylic acid cycle and the glyoxylate shunt. J Biol Chem. 1984;259:9646–9654. [PubMed] [Google Scholar]
- 40.Warren W A. Catalysis of both oxidation and reduction of glyoxylate by pig heart lactate dehydrogenase isoenzyme 1. J Biol Chem. 1970;245:1675–1681. [PubMed] [Google Scholar]
- 41.Wayne L G, Lin K-Y. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 1982;37:1042–1049. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]