Abstract
Reduction of nitrate is an essential, yet challenging chemical task required to manage this relatively inert oxoanion in the environment and biology. We show that thiols, ubiquitous reductants in biology, convert nitrate to nitric oxide at a Cu(II) center under mild conditions. The β-diketiminato complex [Cl2NNF6]Cu(κ2-O2NO) engages in O-atom transfer with various thiols (RSH) to form the corresponding copper(II) nitrite [CuII](κ2-O2N) and sulfenic acid (RSOH). The copper(II) nitrite further reacts with RSH to give S-nitrosothiols RSNO and [CuII]2(μ-OH)2 en route to NO formation via [CuII]-SR intermediates. The gasotransmitter H2S also reduces nitrate at copper(II) to generate NO, providing a lens into NO3−/H2S crosstalk. The interaction of thiols with nitrate at copper(II) releases a cascade of N- and S-based signaling molecules in biology.
Graphical Abstract

INTRODUCTION
An estimated 40% of the human population owes its food to human intervention in the global nitrogen cycle.1,2 Overuse of nitrogenous fertilizers leads to unassimilated nitrate (NO3−) and nitrite (NO2−) runoff resulting in eutrophication, which has a significant impact on the overall climate change.1–3 Various microorganisms partake in the denitrifying process, converting nitrate and nitrite to atmospheric nitrogen. For instance, Thiobacillus denitrificans and thiooxidans generate metabolic energy via chemolithotrophic nitrate reduction using thiosulfate (S2O32−) and sulfide (S2−) to produce dinitrogen (N2) and sulfate (SO42−) as metabolized products.4,5
Nitrate/nitrite and sulfide coexist in diverse biological and geological environments ranging from the human oral and gut microbiomes6,7 to sulfide-rich hydrothermal vents.4,5 In the biological milieu, H2S and thiols are ubiquitous cellular reductants involved in diverse metabolic activity.8–10 While nitrate is the obligate terminal electron acceptor in the denitrifying process, its reduction by thiols/H2S under biological conditions is not well-understood although there are reports on thiol-based reduction of organic nitrates (RONO2).11–13 Moreover, UV-photolysis studies of blood plasma samples containing nitrate and nitrite metabolites show enhanced nitric oxide (NO) formation in the presence of thiols.14 Interestingly, nitrate addition to activated waste sludge or H2S-rich biogas in bioreactors mitigates odorous H2S emissions in waste management and biogas desulfurization.15,16
There are discrete enzymatic machineries that either reduce nitrate to nitrite or nitrite to nitric oxide.17 Mo-based nitrate reductases undergo O-atom transfer (OAT) to the Mo center in reduction of nitrate to nitrite (Figure 1a).18,19 A completely separate class of nitrite reductase enzymes facilitates the 2H+/1e− reduction of nitrite to nitric oxide (Figure 1b).17 Direct reduction of nitrate to nitric oxide, however, is not well-established. Nonetheless, under hypoxic conditions, some nitrate reductases can serve as nitrite reductases with very poor efficiencies.20
Figure 1.

(a) Reduction of nitrate by the nitrate reductase enzyme via OAT; (b) reduction of nitrite by the nitrite reductase enzyme using H+/e−; (c) reduction of nitrate to nitric oxide via nitrite by thiols at copper(II).
Nitrate reduction is challenging due to its low bonding affinity to transition metals coupled with its poor nucleophilicity and kinetic lability toward salt metathesis reactions.21,22 Nitrate reduction at a metal site typically follows one of two mechanistic pathways. Lewis-acid assisted O-atom transfer (OAT) to the metal center (Mn+) resulting in a metal-oxo complex (Mn+2=O, M = Mo, W, Ru, V, or Ce) along with free nitrite represents the pathway followed at Mo-based nitrate reductase enzymes (Figure 1a).18,19,23–29 In a synthetic model, OAT to an iron center also enables the catalytic conversion of nitrate to NO along with formation of FeIII=O species.22 Alternatively, OAT from nitrate may occur directly to a reductant. In some cases, carbon monoxide, phosphines, or the silyl-based Mashima reagent can abstract an O atom from a bound nitrate to give CO2, R3P=O, or TMS-O-TMS.25,30–34 Moreover, intramolecular O-atom transfer between metal-bound nitrate and nitrosyl can form nitrogen dioxide or nitrite.35,36 Other methods include hydrazine-mediated nitrate reduction37 as well as electrocatalytic nitrate reduction using molecular complexes in homogeneous or heterogenized forms.21,38–41
We previously described reduction of nitrite by thiols to nitric oxide and disulfides at the β-diketiminato-supported copper(II) nitrite complex [Cl2NNF6]Cu(κ2-O2N) (1), abbreviated as [CuII](κ2-O2N), and the THF-bound adduct 1-THF.42 Mechanistic studies revealed proton-assisted nucleophilic attack by the thiol RSH at the N atom of the bound nitrite to form the copper(II) hydroxide [CuII]2(μ-OH)2 and S-nitrosothiol (RSNO). The acid–base reaction of thiol RSH with [CuII]2(μ-OH)2 produces the copper(II) thiolate [CuII]-SR that further reacts with RSNO to form the copper(I) disulfide [CuI](RSSR) and NO.42
This work illustrates that both nitrate and nitrate can be reduced at a common copper center by thiols (Figure 1c). Coupling these two pathways allows for the unprecedented reduction of nitrate to nitric oxide via thiols, a class of reductants ubiquitous in biology. Herein, we present an air-stable copper(II) nitrate complex [Cl2NNF6]Cu(κ2-O2NO) (2) that undergoes facile reduction to NO via the copper(II) nitrite [Cl2NNF6] Cu(κ2-O2N) (1). The gasotransmitter H2S also generates NO from nitrate at this copper(II) site.
RESULTS AND DISCUSSION
Synthesis and Characterization of Neutral Copper(II) Nitrate and Anionic Copper(I) Nitrate Complexes.
Oxidation of the β-diketiminato copper(I) complex [Cl2NNF6] Cu(η2-benzene) with AgNO3 in fluorobenzene forms air-stable, greenish-brown [CuII](κ2-O2NO) (2) isolated in 90% yield (Figure 2). The X-ray structure of 2 shows a square planar Cu(II) center with crystallographically imposed symmetric κ2-O1,O2 binding of the nitrate anion (Cu–O1, Cu–O2 = 2.0092(14) Å). The nitrate N3–O1 and N3–O2 bonds (1.2880(19) Å) are markedly longer than the N3–O3 bond (1.200(3) Å) (Figure 2). The bound nitrate N–O stretching frequencies ν(NO2) < νas(NO2) < ν(NO) of 997, 1217, 1562 cm−1, respectively, (Figure S1) follow the trend predicted in the DFT-optimized model of [CuII](κ2-O2NO) (2) (Figure S79) as well as in other complexes featuring symmetric, bidentate nitrate binding.43 Crystallization of 2 from coordinating solvents such as THF and MeCN results in axial ligation to form square pyramidal adducts 2-THF and 2-MeCN that exhibit slightly longer Cu–N and Cu–O distances (Figure 2 and Figures S67–S69).
Figure 2.

Syntheses of [Cl2NNF6]Cu(κ2-O2NO) (2) and {[Cl2NNF6]-Cu(κ1-ONO2)}[PPN] (3). X-ray structures of 2, 2-THF, and 3.
The UV–vis spectrum of 2 in fluorobenzene shows two absorption peaks λmax = 573 nm (260 M−1 cm−1) and λmax =753 nm (74 M−1 cm−1), while 2-THF shows one absorption band at λmax = 673 nm (200 M−1 cm−1) in THF at 22 °C (Figures S2 and S3). The X-band EPR spectrum of 2 recorded at 22 °C in toluene exhibits a four-line pattern centered at giso = 2.105 with Aiso(Cu) = 215 MHz, consistent with a square planar Cu(II) complex (Figure S5).
The cyclic voltammogram of 2 in fluorobenzene with the noncoordinating electrolyte bis(triphenylphosphine)iminium tetrakis(pentafluorophenyl)borate ([PPN]BArF4) (0.1 M) shows a redox event for the CuII/I couple (Epc = −0.08 V, Epa = 0.91 V) vs NHE (Figure S6). The reduced copper(I) nitrate {[CuI](κ1-ONO2)} −[PPN]+ (3) is isolated in 76% yield by the reaction of equimolar bis(triphenylphosphine)iminium nitrate ([PPN]NO3) with [CuI](η2-benzene). The X-ray structure of this monoanionic copper(I) nitrate complex reveals κ1-ONO2 binding to the copper(I) center (Cu–O1, 1.993(2)Å) with an additional possible contact to the nitrate anion (Cu···O2, 2.536(2) Å) (Figure 2).
Reaction of Nitrate with Thiols at Copper(II) and Copper(I).
[CuII](κ2-O2NO) (2) undergoes efficient reduction with 5 equiv of thiol (HSR = HSCPh3, HSAr4-Me, HSCH2Ar4-F, or HSCH2ArH) of varying acid strengths at ambient temperature that converts NO3− to NO (3e− reduction) as well as Cu(II) to Cu(I) (1e− reduction) (Scheme 1a). Net oxidation of 4 equiv of thiol to 2 equiv of disulfide (RSSR) balances this redox transformation that also produces 1 equiv of water. This reaction requires a fifth equiv of thiol to reach completion due to the susceptibility of the resulting copper(I) β-diketiminate complex ([Cl2NNF6]Cu) to undergo a reaction with H-SR to form [Cu-SR]x and free β-diketimine [Cl2NNF6]H (Scheme 1a and Figures S72 and S73). NO (trapped with a cobalt(II) porphyrin)44 and disulfides are isolated in near quantitative yield. The copper(II) oxidation state is critical; the anionic copper(I) nitrate 3 simply releases the nitrate anion upon reaction with HSCPh3 (Scheme 1b).
Scheme 1.
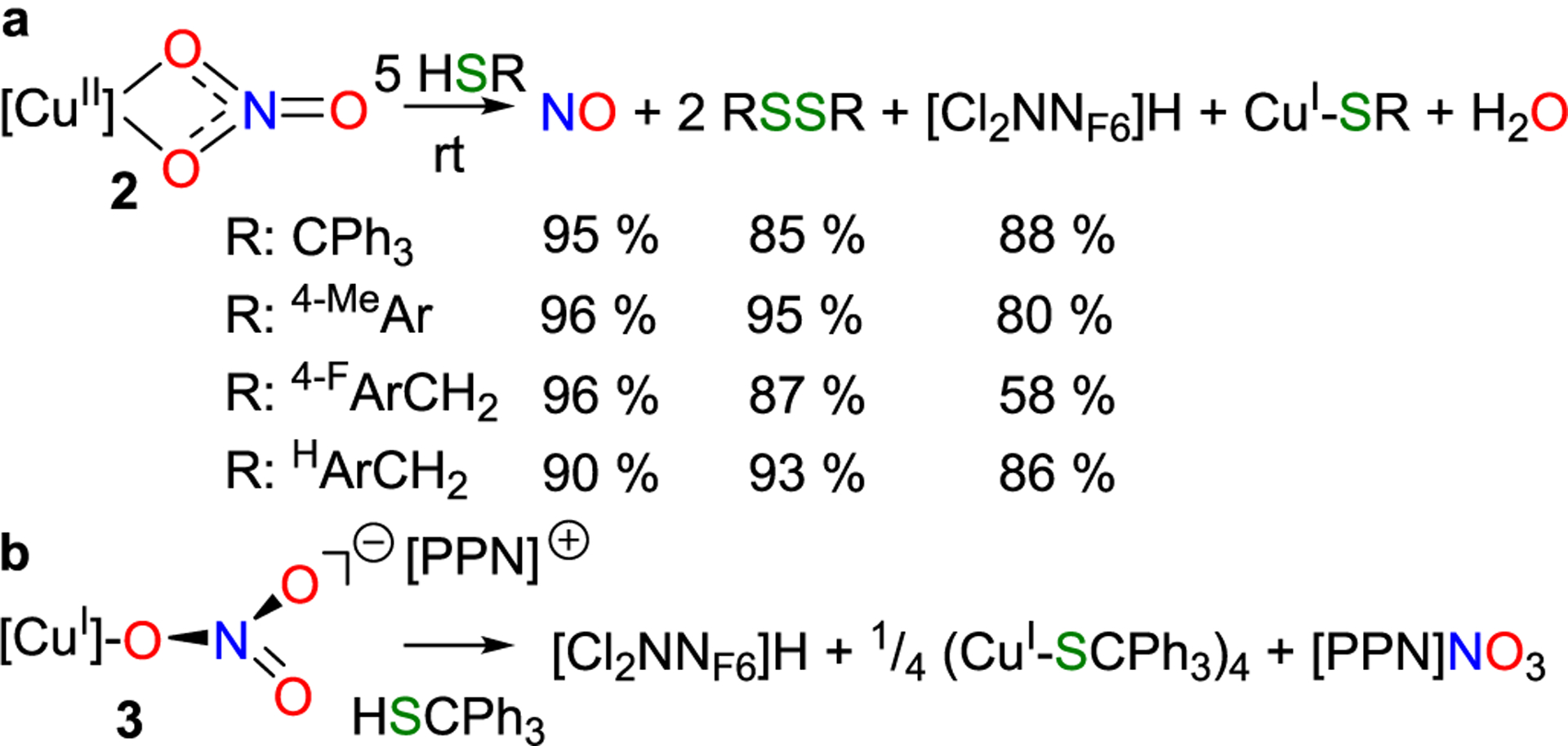
(a) Reaction of HSR with [CuII](κ2-O2NO) (2) Produces NO (b) but Not with {[CuI](κ1-ONO2)}− (3)
Mechanistic Studies: Detection of Intermediates.
UV–vis spectroscopy illuminates several intermediates in the multistep nitrate reduction pathway in the reaction of [CuII](κ2-O2NO) (2) with thiols, provided careful control of conditions. Addition of 2 equiv of HSCPh3 to 2 in CH2Cl2 at rt produces an immediate color change to pale-green signaling formation of the S-nitrosothiol Ph3 CSNO (λ = 556 and 602 nm; Figure S36).42,45 In fluorobenzene at −20 °C, addition of 1 equiv of HSCPh3 to 2 reveals an intense band centered at 763 nm that matches the previously reported copper(II) thiolate [CuII]-SCPh3 (Figures S37–S39).42 Moreover, UV–vis studies in the coordinating solvent THF indicate conversion of copper(II) nitrate 2-THF (λ = 673 nm) to copper(II) nitrite 1-THF (λ = 720 nm)42 upon addition of 1 equiv of HSCPh3 at −60 °C (Figure 3b,c). The crude precipitate formed after addition of 1.5 equiv of HSCPh3 to 2 at −40 °C shows an IR vibrational stretch at (ν(OH)) = 3652 cm−1, consistent with the formation of the copper(II) hydroxide dimer [CuII]2(μ-OH)2 (Figure S41).42
Figure 3.

(a) OAT from 2 by thiols forming 1 and sulfenic acids; trapping of sulfenic acid by dimedone forming the RS-dimedone adduct; (b) OAT from 2-THF by Ph3CSH forming 1-THF; (c) changes in the UV–vis spectra of 2-THF (4 mM in THF at −60 °C) upon adding 1 equiv of Ph3CSH. Inset: overlay of normalized UV–vis spectra of 1-THF and 2-THF at 22 °C in THF.
Observation of the copper(II) nitrite 1 strongly suggests O-atom transfer reactivity from copper(II) nitrate 2 to the reacting thiol RSH. The resulting sulfenic acids RSOH are extremely reactive species quickly consumed by thiol RSH to form disulfides (RSSR) and water.10 Dimedone can out-compete thiols, however, to intercept sulfenic acids RSOH forming a thiol-dimedone adduct (Figure 3a).9,46 The reaction of 2 with 2 equiv of Ph3CSH in the presence of 4 equiv of dimedone at −40 °C in fluorobenzene allows observation of the Ph3 CS-dimedone adduct at m/z = [M + 1] = 415 (Figure S42). This reaction is general across other thiols, 4-FArCH2SH and 4-MeArSH (Figures S43 and S44). In the reaction of 2 with 2 equiv of thiol and 3 equiv of dimedone, we quantify the 4-MeArS-dimedone adduct in 30% yield (Figure 3a and SI Section 18C–E).
These findings support an initial O-atom transfer pathway in nitrate reduction at copper(II) by thiols (Figure 3a). This initial pathway stands in contrast to the proton-assisted nucleophilic attack demonstrated for nitrite that would generate the uncommon RSNO2 intermediate47 precluding the direct formation of the copper(II) nitrite [CuII](κ2-O2N) (1). NO release occurs in the reaction of the observed Snitrosothiol RSNO and copper(II)-thiolate [CuII]-SR that form NO and disulfide RSSR.42,48 For instance, independently synthesized Ph3CSNO and [CuII]-SCPh3 react to form NO (90%) and Ph3CSSCPh3 (84%) (Figures S50 and S51).
Computational Studies.
Guided by experimental findings, we analyzed the reaction of copper(II) nitrate 2 with Ph3CSH by DFT to assess the thermodynamic feasibility of each proposed step (Figure 4 and Figures S75 and S76). The initial step involving OAT from 2 to Ph3CSH enables conversion of nitrate to nitrite at copper(II). The sulfenic acid formed can exist in two tautomers.49 While the formation of 1 and the sulfinyl (Ph3CS(O)H) tautomer is endergonic by ΔG = 10.3 kcal/mol, the sulfenyl (Ph3CSOH) tautomer and 1 formation is modestly exergonic with ΔG = −3.0 kcal/mol (Figure 4a). The thermodynamic stability of the sulfenyl tautomer over the sulfinyl form (ΔG = −13.3 kcal/mol) provides the driving force in OAT from copper(II) nitrate 2 to the thiol Ph3CSH (Figure 4a). Despite the mild exergonicity of the OAT step, the reaction of the sulfenic acid Ph3CSOH with Ph3CSH to give the disulfide Ph3CSSCPh3 and H2O is highly favorable (ΔG = − 25.6 kcal/mol). In contrast, OAT to the related organosulfide Ph3CSMe to form the sulfoxide Ph3CS(O)Me is endergonic (ΔG = +4.5 kcal/mol) (Figure S75c) indicating the importance of a redox-active RS-H moiety over an alkyl group in RS-R for effective OAT. Experimentally, MeSMe does not react with 2 at room temperature (Figures S33 and S34). Ph3CSH further reacts with copper(II) nitrite 1 to form the Snitrosothiol Ph3CSNO and [CuII]-OH. The latter dimerizes to [CuII]2(μ-OH)2 (experimentally observed by IR spectroscopy) rendering the S-nitrosothiol forming step exergonic (ΔG = −10.8 kcal/mol). Indeed, both [CuII]2(μ-OH)2 and Ph3CSNO form in the reaction of [CuII](κ2-O2N) with 1 equiv of Ph3CSH.42 [CuII]2(μ-OH)2 reacts with Ph3CSH to form the observed copper(II) thiolate [CuII]-SCPh3 and water (ΔG = −15.5 kcal/mol). The intermediates Ph3CSNO and [CuII]-SCPh3 further react to form [CuI](RSSR) (R = CPh3) and NO (ΔG = −7.3 kcal/mol) (Figure 4b). While the bulky Ph3CSSCPh3 prevents isolation of the corresponding [CuI] adduct, experimentally, we isolate the [CuI](RSSR) complex (R = HArCH2 (2-BnS2)) from the reaction of [CuII](κ2-O2NO) (2) with benzyl thiol (HArCH2SH) (Figure 5 and Figure S56).
Figure 4.

DFT analysis of (a) OAT by Ph3CSH to form sulfenic acid in two tautomers; (b) individual steps in the reduction of [CuII](κ2-O2NO) (2) with 4 equiv of Ph3CSH. The free energy change (kcal/mol) for each reaction appears in bold and italics. Experimentally observed species are highlighted in rounded boxes.
Figure 5.

X-ray structure of the 2-BnS2 intermediate obtained from the reaction of 2 with HSCH2ArH.
Reaction of Copper(II) Nitrate with H2S.
Importantly, H2S reduces the copper(II) nitrate 2 to give NO (66%), the free β-diketimine ligand [Cl2NNF6]H (71%), and a black amorphous insoluble residue (Scheme 2). Powder XRD studies on the residue show a 92% match with the mineral covellite,50 while XPS studies depict a Cu(I) species having polysulfides (Sn2−) (Figures S61–S63).51 Interestingly, copper-(II) nitrite 1 also produces NO (57%) upon reduction with H2S (Figure S60).
Scheme 2.

H2S Reduction of [CuII](κ2-O2NO) (2) to NO
CONCLUSIONS
While reduction of nitrate and nitrite are carried out by two distinct enzymatic machineries in biology,17 we show that thiols can reduce nitrate to nitrite to nitric oxide at a biologically relevant copper(II) center. Despite a range of abiotic reductants known for nitrate reduction, this work illustrates how biologically ubiquitous thiols can reduce nitrate resulting in a family of N- and S-based signaling molecules.
Nitrate and nitrite reduction by thiols at copper(II) is mechanistically distinct. Nitrate coordination to an electronpoor copper(II) center enables O-atom transfer to a variety of thiols differing in acid strength and size. The presence of a H atom bound to reduced sulfur in thiols RS-H is critical to enable OAT to form the corresponding sulfenic acid RS-OH; thioethers such as Me2S do not undergo OAT under similar conditions. Since this conversion of bound nitrate to nitrite is redox-neutral with respect to the copper(II) centers in [CuII](κ2-O2NO) (2) and [CuII](κ2-O2N) (1), these findings motivate the examination of related thiol reactivity with nitrate bound to biologically relevant redox-inactive metal centers such as zinc. For instance, a zinc nitrite complex forms NO upon reaction with thiols RSH via the intermediacy of the corresponding S-nitrosothiols RSNO.52
A cascade of N- and S-based signaling molecules results upon nitrate reduction by thiols at copper(II). This includes the formation of nitrite bound to copper(II) in 1 along with the sulfenic acid RSOH, S-nitrosothiol RSNO, and disulfide RSSR en route to NO. H2S also reduces copper(II) bound nitrate to NO and forms polysulfides. Informed by mechanistic studies involving thiols, reduction by H2S may release more fleeting intermediates such as HSOH, HSNO, and HSSH. These studies reveal that biologically ubiquitous thiols and H2S reduce nitrate to NO at copper(II) as well as release a cascade of signaling molecules that inform the interconnection, or crosstalk, among signaling pathways involving nitric oxide, thiols, and H2S.8,9,53,54
Supplementary Material
ACKNOWLEDGMENTS
T.H.W. acknowledges funding from the National Institutes of Health (Grant R01GM126205). MSU personnel acknowledge grant NSF-CHE 1919565 for the purchase of an X-ray diffractometer. T.H.W. and P.G. are grateful to Dr. J. L. McCracken for assistance with EPR spectroscopy and to Dr. P. Askeland for XPS measurements. P.G. thanks Dr. S. D. Bhagat for assistance with sulfenyl chloride synthesis.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c00394.
Experimental details; materials and methods; UV–vis, FTIR, EPR, and NMR spectra and X-ray data of new compounds; NO gas determination; computational analyses (PDF)
Accession Codes
CCDC 2157014, 2164468, 2164899, 2164902, 2165202, 2195926, 2195937, and 2204853 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
CCDC 2195937, 2164899, 2164902, 2164468, 2157014, 2165202, 2195926, and 2204853 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.3c00394
The authors declare no competing financial interest.
Contributor Information
Pokhraj Ghosh, Department of Chemistry, Michigan State University, East Lansing, Michigan 48824, United States;; Department of Chemistry, Georgetown University, Washington, D. C. 20057, United States
Molly Stauffer, Department of Chemistry, Michigan State University, East Lansing, Michigan 48824, United States;; Department of Chemistry, Georgetown University, Washington, D. C. 20057, United States
Md Estak Ahmed, Department of Chemistry, Michigan State University, East Lansing, Michigan 48824, United States;; Department of Chemistry, Georgetown University, Washington, D. C. 20057, United States
Jeffery A. Bertke, Department of Chemistry, Georgetown University, Washington, D. C. 20057, United States
Richard J. Staples, Department of Chemistry, Michigan State University, East Lansing, Michigan 48824, United States
Timothy H. Warren, Department of Chemistry, Michigan State University, East Lansing, Michigan 48824, United States; Department of Chemistry, Georgetown University, Washington, D. C. 20057, United States
REFERENCES
- (1).Gruber N; Galloway JN An earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [DOI] [PubMed] [Google Scholar]
- (2).Lehnert N; Musselman BW; Seefeldt LC Grand challenges in the nitrogen cycle. Chem. Soc. Rev 2021, 50, 3640–3646. [DOI] [PubMed] [Google Scholar]
- (3).Zhang X; Ward BB; Sigman DM Global Nitrogen Cycle: Critical enzymes, organisms, and processes for nitrogen budgets and dynamics. Chem. Rev 2020, 120, 5308–5351. [DOI] [PubMed] [Google Scholar]
- (4).Loka Bharathi PA, Sulfur Cycle. Encyclopedia of ecology, Jørgensen SE; Fath BD, Eds. Academic Press: Oxford, 2008; pp. 3424–3431. [Google Scholar]
- (5).Monachon M; Albelda-Berenguer M; Joseph E Biological oxidation of iron sulfides. Adv. Appl. Microbiol 2019, 107, 1–27. [DOI] [PubMed] [Google Scholar]
- (6).Schreiber F; Stief P; Gieseke A; Heisterkamp IM; Verstraete W; de Beer D; Stoodley P Denitrification in human dental plaque. BMC Biol. 2010, 8, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Tiso M; Schechter AN Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS One 2015, 10, No. e0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Cortese-Krott MM; Fernandez BO; Kelm M; Butler AR; Feelisch M On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide 2015, 46, 14–24. [DOI] [PubMed] [Google Scholar]
- (9).Filipovic MR; Zivanovic J; Alvarez B; Banerjee R Chemical biology of H2S signaling through persulfidation. Chem. Rev 2018, 118, 1253–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Poole LB The basics of thiols and cysteines in redox biology and chemistry. Free Radical Biol. Med 2015, 80, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Thatcher GRJ; Nicolescu AC; Bennett BM; Toader V Nitrates and NO release: Contemporary aspects in biological and medicinal chemistry. Free Radical Biol. Med 2004, 37, 1122–1143. [DOI] [PubMed] [Google Scholar]
- (12).Lang BS; Gorren ACF; Oberdorfer G; Wenzl MV; Furdui CM; Poole LB; Mayer B; Gruber K Vascular bioactivation of nitroglycerin by aldehyde dehydrogenase-2: Reaction intermediates revealed by crystallography and mass spectrometry. J. Biol. Chem 2012, 287, 38124–38134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Abrams J Interactions between organic nitrates and thiol groups. Am. J. Med 1991, 91, S106–S112. [DOI] [PubMed] [Google Scholar]
- (14).Kelm M; Feelisch M; Rodriguez J; Gharini P; Hamada S; Rassaf T; Kleinbongard P; Dejam A Thiols enhance NO formation from nitrate photolysis. Free Radical Biol. Med 2003, 35, 1551–1559. [DOI] [PubMed] [Google Scholar]
- (15).Flores-Cortés M; Pérez-Trevilla J; de María Cuervo-López F; Buitrón G; Quijano G H2S oxidation coupled to nitrate reduction in a two-stage bioreactor: Targeting H2S-rich biogas desulfurization. Waste Manage. 2021, 120, 76–84. [DOI] [PubMed] [Google Scholar]
- (16).Fang Y; Du Y; Feng H; Hu L-F; Shen D-S; Long Y-Y Sulfide oxidation and nitrate reduction for potential mitigation of H2S in landfills. Biodegradation 2015, 26, 115–126. [DOI] [PubMed] [Google Scholar]
- (17).Maia LB; Moura JJG How Biology Handles Nitrite. Chem. Rev 2014, 114, 5273–5357. [DOI] [PubMed] [Google Scholar]
- (18).Gonzalez PJ; Rivas MG; Mota CS; Brondino CD; Moura I; Moura JJG Periplasmic nitrate reductases and formate dehydrogenases: Biological control of the chemical properties of Mo and W for fine tuning of reactivity, substrate specificity and metabolic role. Coord. Chem. Rev 2013, 257, 315–331. [Google Scholar]
- (19).Sparacino-Watkins C; Stolz JF; Basu P Nitrate and periplasmic nitrate reductases. Chem. Soc. Rev 2014, 43, 676–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wang J; Keceli G; Cao R; Su J; Mi Z Molybdenum-containing nitrite reductases: Spectroscopic characterization and redox mechanism. Redox Rep. 2017, 22, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ghosh M; Braley SE; Ezhov R; Worster H; Valdez-Moreira JA; Losovyj Y; Jakubikova E; Pushkar YN; Smith JM A spectroscopically observed iron nitrosyl intermediate in the reduction of nitrate by a surface-conjugated electrocatalyst. J. Am. Chem. Soc 2022, 144, 17824–17831. [DOI] [PubMed] [Google Scholar]
- (22).Ford CL; Park YJ; Matson EM; Gordon Z; Fout AR A bioinspired iron catalyst for nitrate and perchlorate reduction. Science 2016, 354, 741–743. [DOI] [PubMed] [Google Scholar]
- (23).Craig JA; Holm RH Reduction of nitrate to nitrite by molybdenum-mediated atom transfer: A nitrate reductase analog reaction system. J. Am. Chem. Soc 1989, 111, 2111–2115. [Google Scholar]
- (24).Jiang J; Holm RH Reaction systems related to dissimilatory nitrate reductase: Nitrate reduction mediated by bis(dithiolene) tungsten complexes. Inorg. Chem 2005, 44, 1068–1072. [DOI] [PubMed] [Google Scholar]
- (25).Majumdar A; Pal K; Sarkar S Chemistry of [Et4N]-[MoIV(SPh)(PPh3)(Mnt)2] as an analogue of dissimilatory nitrate reductase with its inactivation on substitution of thiolate by chloride. J. Am. Chem. Soc 2006, 128, 4196–4197. [DOI] [PubMed] [Google Scholar]
- (26).Man W-L; Lam WWY; Wong W-Y; Lau T-C Oxidation of nitrite by a trans-dioxoruthenium(VI) complex: Direct evidence for reversible oxygen atom transfer. J. Am. Chem. Soc 2006, 128, 14669–14675. [DOI] [PubMed] [Google Scholar]
- (27).Elrod LT; Kim E Lewis acid assisted nitrate reduction with biomimetic molybdenum oxotransferase complex. Inorg. Chem 2018, 57, 2594–2602. [DOI] [PubMed] [Google Scholar]
- (28).Kulbir; Das S; Devi T; Goswami M; Yenuganti M; Bharadwaj P; Ghosh S; Sahoo SC; Kumar P Oxygen atom transfer promoted nitrate to nitric oxide transformation: a step-wise reduction of nitrate → nitrite → nitric oxide. Chem. Sci 2021, 12, 10605–10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Damon PL; Wu G; Kaltsoyannis N; Hayton TW Formation of a Ce(IV) oxo complex via inner sphere nitrate reduction. J. Am. Chem. Soc 2016, 138, 12743–12746. [DOI] [PubMed] [Google Scholar]
- (30).Gwak J; Ahn S; Baik M-H; Lee Y One metal is enough: a nickel complex reduces nitrate anions to nitrogen gas. Chem. Sci 2019, 10, 4767–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Padmanaban S; Choi J; Vazquez-Lima H; Ko D; Yoo D; Gwak J; Cho KB; Lee Y Nickel-catalyzed NO group transfer coupled with NOx conversion. J. Am. Chem. Soc 2022, 144, 4585–4593. [DOI] [PubMed] [Google Scholar]
- (32).Berg JM; Holm RH Model for the active site of oxo-transfer molybdoenzymes: Synthesis, structure, and properties. J. Am. Chem. Soc 1985, 107, 917–925. [Google Scholar]
- (33).Seo J; Cabelof AC; Chen C-H; Caulton KG Selective deoxygenation of nitrate to nitrosyl using trivalent chromium and the Mashima reagent: Reductive silylation. Chem. Sci 2019, 10, 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Beagan DM; Cabelof AC; Pink M; Carta V; Gao X; Caulton KG Nickel-mediated N–N bond formation and N2O liberation via nitrogen oxyanion reduction. Chem. Sci 2021, 12, 10664–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Tsai F-T; Lee Y-C; Chiang M-H; Liaw W-F Nitrateto-nitrite-to-nitric oxide conversion modulated by nitrate-containing {Fe(NO)2}9 dinitrosyl iron complex (DNIC). Inorg. Chem 2013, 52, 464–473. [DOI] [PubMed] [Google Scholar]
- (36).Bhaduri SA; Bratt I; Johnson BFG; Khair A; Segal JA; Walters W; Zuccaro C Some reactions of nitrosyl complexes of nickel, palladium, and platinum. J. Chem. Soc., Dalton Trans 1981, 234–239. [Google Scholar]
- (37).Mondal A; Reddy KP; Som S; Chopra D; Kundu S Nitrate and nitrite reductions at copper(II) sites: Role of noncovalent interactions from second-coordination-sphere. Inorg. Chem 2022, 61, 20337–20345. [DOI] [PubMed] [Google Scholar]
- (38).Partovi S; Xiong Z; Kulesa KM; Smith JM Electrocatalytic reduction of nitrogen oxyanions with a redox-active cobalt macrocycle complex. Inorg. Chem 2022, 61, 9034–9039. [DOI] [PubMed] [Google Scholar]
- (39).Liu J-X; Richards D; Singh N; Goldsmith BR Activity and selectivity trends in electrocatalytic nitrate reduction on transition metals. ACS Catal. 2019, 9, 7052–7064. [Google Scholar]
- (40).Yoshioka T; Iwase K; Nakanishi S; Hashimoto K; Kamiya K Electrocatalytic reduction of nitrate to nitrous oxide by a copper-modified covalent triazine framework. J. Phys. Chem. C 2016, 120, 15729–15734. [Google Scholar]
- (41).Rosca V; Duca M; de Groot MT; Koper MTM Nitrogen cycle electrocatalysis. Chem. Rev 2009, 109, 2209–2244. [DOI] [PubMed] [Google Scholar]
- (42).Kundu S; Kim WY; Bertke JA; Warren TH Copper(II) Activation of nitrite: Nitrosation of nucleophiles and generation of NO by thiols. J. Am. Chem. Soc 2017, 139, 1045–1048. [DOI] [PubMed] [Google Scholar]
- (43).Nakamoto K, Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry. Sixth Edition ed. ed.; A JOHN WILEY & SONS, INC.: Hoboken, New Jersey, 2008, p 1–224. [Google Scholar]
- (44).Stauffer M; Sakhaei Z; Greene C; Ghosh P; Bertke JA; Warren TH Mechanism of O-atom transfer from nitrite: Nitric oxide release at copper(II). Inorg. Chem 2021, 60, 15968–15974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Arulsamy N; Bohle DS; Butt JA; Irvine GJ; Jordan PA; Sagan E Interrelationships between conformational dynamics and the redox chemistry of S-Nitrosothiols. J. Am. Chem. Soc 1999, 121, 7115–7123. [Google Scholar]
- (46).Heinecke J; Ford PC Formation of cysteine sulfenic acid by oxygen atom transfer from nitrite. J. Am. Chem. Soc 2010, 132, 9240–9243. [DOI] [PubMed] [Google Scholar]
- (47).Artz JD; Yang K; Lock J; Sanchez C; Bennett BM; Thatcher GRJ Reactivity of thionitrate esters: putative intermediates in nitrovasodilator activity. Chem. Commun 1996, 927–928. [Google Scholar]
- (48).Zhang S; Celebi-Olcum N; Melzer MM; Houk KN; Warren TH Copper(I) nitrosyls from reaction of copper(II) thiolates with S-nitrosothiols: Mechanism of NO release from RSNOs at Cu. J. Am. Chem. Soc 2013, 135, 16746–16749. [DOI] [PubMed] [Google Scholar]
- (49).Kumar MR; Farmer PJ Trapping reactions of the sulfenyl and sulfinyl tautomers of sulfenic acids. ACS Chem. Biol 2017, 12, 474–478. [DOI] [PubMed] [Google Scholar]
- (50).Mazin II Structural and electronic properties of the two-dimensional superconductor CuS with 11/3-valent copper. Phys. Rev. B 2012, 85, 115133–115138. [Google Scholar]
- (51).Cabrera-German D; García-Valenzuela JA; Martínez-Gil M; Suárez-Campos G; Montiel-González Z; Sotelo-Lerma M; Cota-Leal M Assessing the chemical state of chemically deposited copper sulfide: A quantitative analysis of the X-ray photoelectron spectra of the amorphous-to-covellite transition phases. Appl. Surf. Sci 2019, 481, 281–295. [Google Scholar]
- (52).Cardenas AJP; Abelman R; Warren TH Copper(II) activation of nitrite: Nitrosation of nucleophiles and a generation of NO by thiols. Chem. Commun 2014, 50, 168–170. [Google Scholar]
- (53).Ivanovic-Burmazovic I; Filipovic MR Saying NO to H2S: A story of HNO, HSNO, and SSNO–. Inorg. Chem 2019, 58, 4039–4051. [DOI] [PubMed] [Google Scholar]
- (54).Marcolongo JP; Venancio MF; Rocha WR; Doctorovich F; Olabe JA NO/H2S “crosstalk” reactions: The role of thionitrites (SNO−) and perthionitrites (SSNO−). Inorg. Chem 2019, 58, 14981–14997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


