Abstract
Ferroptosis is a form of programmed cell death characterized by elevated intracellular ferrous ion levels and increased lipid peroxidation. Since its discovery and characterization in 2012, considerable progress has been made in understanding the regulatory mechanisms and pathophysiological functions of ferroptosis. Recent findings suggest that numerous organ injuries (e.g., ischemia/reperfusion injury) and degenerative pathologies (e.g., aortic dissection and neurodegenerative disease) are driven by ferroptosis. Conversely, insufficient ferroptosis has been linked to tumorigenesis. Furthermore, a recent study revealed the effect of ferroptosis on hematopoietic stem cells under physiological conditions. The regulatory mechanisms of ferroptosis identified to date include mainly iron metabolism, such as iron transport and ferritinophagy, and redox systems, such as glutathione peroxidase 4 (GPX4)-glutathione (GSH), ferroptosis-suppressor-protein 1 (FSP1)-CoQ10, FSP1-vitamin K (VK), dihydroorotate dehydrogenase (DHODH)-CoQ, and GTP cyclohydrolase 1 (GCH1)-tetrahydrobiopterin (BH4). Recently, an increasing number of studies have demonstrated the important regulatory role played by epigenetic mechanisms, especially DNA, RNA, and protein methylation, in ferroptosis. In this review, we provide a critical analysis of the molecular mechanisms and regulatory networks of ferroptosis identified to date, with a focus on the regulatory role of DNA, RNA, and protein methylation. Furthermore, we discuss some debated findings and unanswered questions that should be the foci of future research in this field.
Keywords: Ferroptosis, DNA methylation, RNA m6A methylation, Protein methylation, Histone methylation, Disulfidptosis
Introduction
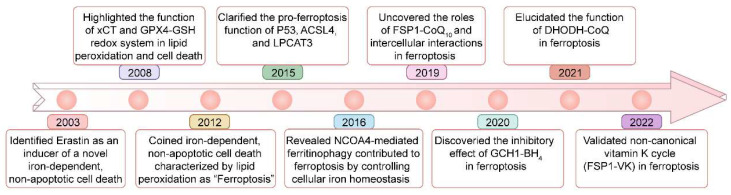
Ferroptosis is regulated cell death driven by iron overload-triggered lipid peroxidation and is involved in a wide range of diseases, including cancers, cardiovascular diseases, degenerative diseases, infectious diseases, autoimmune diseases, and ischemia/reperfusion injury1-7. Although first defined in 2012, as early as 2003, when high-throughput screening revealed compounds with the ability to kill engineered tumorigenic cells, this nonapoptotic cell death, now known as ferroptosis, was initially characterized and shown to be induced by Erastin1, 8, 9 (Figure 1). Five years later (in 2008), the importance of ferrous iron, the system xc- (xCT) controlled cystine/cysteine redox cycle, and the glutathione peroxidase 4 (GPX4)-glutathione (GSH) redox system in lipid peroxidation and non-apoptotic cell death was highlighted by different research groups10-12. In 2012, ferrostatin-1, the first inhibitor of ferroptosis, was discovered by Brent R Stockwell and his colleagues1. Since then, considerable advances have been made in understanding the pathological functions of ferroptosis, such as its roles in tumorigenesis and degenerative diseases13-15, but its precise physiological function remained unknown. Recently, Zhao et al. demonstrated that histone deubiquitinase MYSM1 (Myb-like, SWIRM and MPN domains 1) deficiency reduced the translation of ferroptosis-protective mRNAs, resulting in increased ferroptosis of human hematopoietic stem cells (HSCs), and HSC population maintenance was fully restored by the ferroptosis inhibitors (e.g., deferoxamine (DFO), ferrostatin-1 (Fer-1), and vitamin E)16. This study revealed the specific vulnerability of HSCs to ferroptosis and a unique physiological role played by ferroptosis in human hematopoiesis.
Figure 1.
Key milestones in the study of ferroptosis over time.
In recent years, many key molecular mechanisms of ferroptosis have been identified, including GPX4-GSH, ferroptosis-suppressor-protein 1 (FSP1)-CoQ10, GTP cyclohydrolase 1 (GCH1)-tetrahydrobiopterin (BH4), dihydroorotate dehydrogenase (DHODH)-CoQ, and FSP1-vitamin K (VK)8, 17-19 (Figure 1). Additionally, epigenetic mechanisms such as DNA methylation, RNA methylation, protein methylation, and noncoding RNAs have gained increased attention. For instance, DNA methylation of key ferroptosis-related genes, such as GPX4, FSP1 and NFE2-like BZIP transcription factor 2 (NRF2) genes, has been shown to affect the expression of these genes, which participate in the regulation of ferroptosis20-22. Furthermore, prediction models combining RNA m6A modification regulators with ferroptosis-related genes have been established to effectively reflect the progression and prognosis of tumors23, and the RNA m6A modification can also regulate the fate of ferroptosis-related mRNAs, thereby controlling their protein expression3, 24. Targeting protein methylation factors has been shown to improve the prognosis of related diseases by regulating ferroptosis. For example, we recently found that the histone methyltransferase inhibitor BRD4770 can delay the pathological progression of aortic dissection by inhibiting ferroptosis4. There have been major advances in our understanding of the mechanisms related to DNA, RNA, and protein methylation that govern ferroptosis. Therefore, it is necessary to summarize and review the research progress in this field.
Many reviews have summarized and discussed in detail the regulatory mechanisms, biology, and roles of ferroptosis in disease2, 8, 13, 25-29, and readers interested in a more in-depth understanding can consult the relevant literature. In the present review, we focus on the effects of DNA methylation, the RNA m6A modification, and protein methylation and the underlying mechanisms of these effects on ferroptosis.
An overview of ferroptosis
Lipid peroxidation
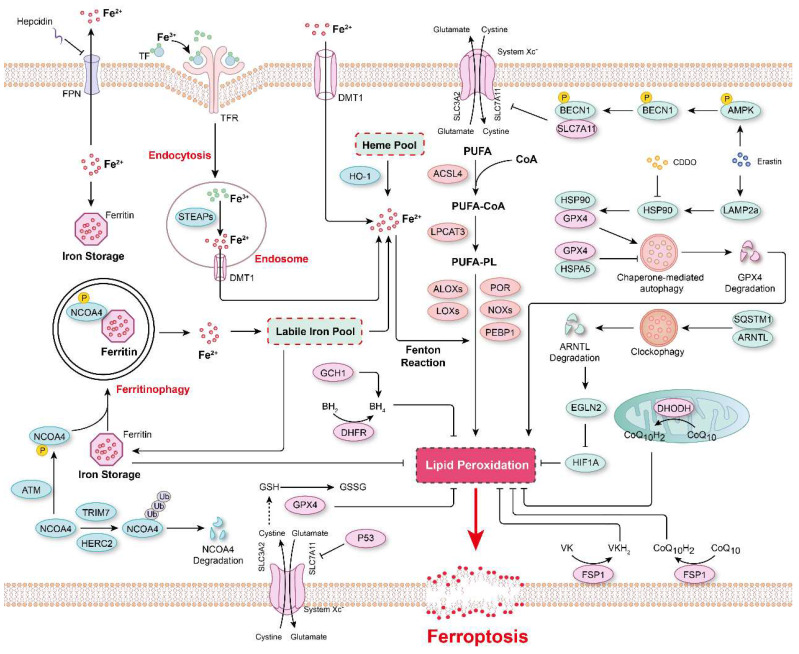
Ferroptosis is a form of programmed cell death regulated by multiple cellular metabolic pathways, such as iron metabolism, redox homeostasis, and mitochondrial activity, and executed by phospholipid hydroperoxides (PLOOHs), a form of lipid-based reactive oxygen species (ROS)1, 2, 13. However, the subcellular membrane lipid peroxidation that is essential for ferroptosis has remained uncharacterized for many years. Recently, von Krusenstiern et al. demonstrated that various subcellular membranes undergo lipid peroxidation during ferroptosis, and the resulting lipid peroxides accumulate initially in the endoplasmic reticulum (ER) membrane and later in the plasma membrane30. Their results indicated that the ER membrane is a key site of lipid peroxidation during ferroptosis30. Moreover, at least two membrane-remodeling enzymes, lysophosphatidylcholine acyltransferase 3 (LPCAT3) and acyl-CoA synthetase long-chain family member 4 (ACSL4), have been reported to contribute to lipid peroxidation31, 32. Genetic loss or pharmacological inhibition of ACSL4 leads to a marked shift from the incorporation of long-chain polyunsaturated fatty acid (PUFA) tails to that of short-chain and monounsaturated fatty acyl (MUFA) tails into phospholipids to prevent ferroptosis31, 33, 34. Therefore, increasing the content of MUFAs in cells, such as that mediated via ACSL3-dependent enrichment of membranes with MUFAs, stearoyl-CoA desaturase 1 (SCD1)-mediated cellular MUFA production, and supplementation with exogenous MUFAs, has been reported to inhibit ferroptosis35-37. Furthermore, certain lipoxygenases (LOXs) have been found to be involved in ferroptosis because of their ability to directly oxygenate PUFAs and PUFA-containing lipids in biological membranes36, 38, 39 (Figure 2). For example, arachidonate 12-lipoxygenase, 12S type (ALOX12) inactivation abrogated p53-mediated ferroptosis in cancer cells, and ALOX12 also nullified p53-dependent inhibition of tumor growth in xenograft model mice40. In a myocardial ischemia/reperfusion (I/R) injury model, arachidonic acid 15-lipoxygenase 1 (ALOX15) was found to be the primary mediator of ischemia-induced PUFA-phospholipid peroxidation, triggering ferroptosis and exacerbating myocardial damage41. In summary, these studies indicated that the composition of phospholipids in biological membranes, especially the degree of unsaturation lipids in bilayer membranes, is key for determining the vulnerability of cells to ferroptosis.
Figure 2.
The major mechanisms regulating ferroptosis. The mechanisms underlying lipid peroxidation, regulatory signaling, intracellular iron storage/release and import/export, and ferritinophagy in ferroptosis. The major pathways that regulate ferroptosis include glutathione peroxidase 4 (GPX4)-glutathione (GSH), ferroptosis-suppressor-protein 1 (FSP1)-CoQ10, FSP1-vitamin K (VK), dihydroorotate dehydrogenase (DHODH)-CoQ, and GTP cyclohydrolase 1 (GCH1)-tetrahydrobiopterin (BH4).
Iron in ferroptosis
Lipid peroxidation in biological membranes is mediated by the nonenzymatic, iron-dependent Fenton reaction13, 42. Both ferrous and ferric ions are involved in the conversion of PLOOHs to the free radicals PLO• and PLOO•, which trigger damaging peroxidation chain reactions13, 43. Therefore, it is critical to maintain cellular iron homeostasis, and intracellular iron storage/release and import/export are intricately regulated (Figure 2). In mammals, iron is mostly present in the form of heme, and heme oxygenase-1 (HO-1) catalyzes the degradation of heme into biliverdin, iron, and carbon monoxide44, 45. Ferric ions bind to transferrin (Tf), which is recognized by its receptor TfR and then imported into cells through TfR-mediated endocytosis13, 46, 47. In addition, the metal transporters DMT1 (Divalent metal transporter 1) and SLC39A14 (Solute carrier family 39 member 14) are important channels for iron import2, 13, 48. In contrast, Ferroportin (FPN, also known as SLC40A1) is a ferrous iron Fe(II) transporter that is critical for the release of Fe(II) from cells49, 50. Systemic iron homeostasis is regulated by hepcidin, a regulatory peptide produced mainly in the liver, and it binds to FPN, leading to the internalization and degradation of FPN50, 51. Furthermore, a prevalent FPN Q248H mutation reduces sensitivity of cells to physiological concentrations of hepcidin and prevents hepcidin-induced degradation of FPN in humans52, 53. Numerous cellular processes regulate the sensitivity of cells toward ferroptosis by altering the intracellular labile iron content, and the iron chelator deferoxamine (DFO) can completely inhibit ferroptosis13, 54, 55. Ferritin, a spherical iron storage protein in cells, is composed of a combination of 24 subunits of heavy-chain ferritin (ferritin-H) and light-chain ferritin (ferritin-L), which bind and sequester intracellular iron in the form of ferric ions13, 56, 57. Overexpression of ferritin reduces the content of intracellular labile iron and prevents lipid peroxidation and ferroptosis57, 58.
Interestingly, the protein level of ferritin is regulated via a biological process named ferritinophagy59 (Figure 2). Nuclear receptor coactivator 4 (NCOA4) functions as a cargo receptor that mediates the delivery of iron-filled ferritin-H and ferritin-L to lysosomes via autophagy for breakdown, resulting in the release of iron59. An excessive rate of ferritinophagy can lead to intracellular iron overload and subsequent lipid peroxidation and ferroptosis60, 61. The Ser/Thr protein kinase ATM phosphorylates NCOA4 to enhance its interaction with ferritin, thereby increasing the ferritinophagy rate and the level of intracellular labile free iron, resulting in ferroptosis62. In contrast, tripartite motif-containing protein 7 (TRIM7) directly binds to and ubiquitinates NCOA4 in a K48-linked polyubiquitylation manner, facilitating the degradation of NCOA4 and reducing the ferritinophagy and ferroptosis of human glioblastoma cells63. Similarly, after iron repletion, the E3 ubiquitin ligase HERC2 (HECT and RLD domain-containing E3 ubiquitin protein ligase 2) interacts with NCOA4 and induces its proteasomal degradation, rendering HERC2 a negative regulator of ferritinophagy60.
Autophagy in ferroptosis
In addition to ferritinophagy, upregulated microtubule-associated protein 1 light chain 3 II (LC3 II) expression and increased autophagic flux are observed during ferroptosis64, 65. Moreover, autophagy inhibitors (e.g., bafilomycin A1 and chloroquine) or knockout of autophagy-related gene 3 (ATG3) and ATG13, two core contributors to autophagy, reduced cell sensitivity to ferroptosis66. In Erastin-induced ferroptosis, the expression of lysosome-associated membrane protein 2a (LAMP2a) is increased, which promotes heat shock protein 90 (HSP90)-dependent autophagy, which in turn mediates the degradation of GPX467. Inhibiting HSP90 function with 2-amino-5-chloro-N, 3-dimethylbenzamide (CDDO) blocks GPX4 degradation and prevents cells from undergoing ferroptosis67. Additionally, the molecular chaperone heat shock protein family A member 5 (HSPA5) interacts with GPX4 and blocks GPX4 degradation, thus decreasing the sensitivity of cells to ferroptosis68. Similarly, after ferroptosis stimulation, phosphorylated AMP-activated protein kinase (AMPK) mediates BECN1 phosphorylation, leading to the formation of the BECN1-SLC7A11 complex, which blocks System Xc- activity and aggravates ferroptosis69. Recently, a novel selective autophagy process, termed clockophagy, has been described to mediate ferroptosis through the ARNTL-EGLN2-HIF1A signaling pathway70. The core circadian clock protein ARNTL regulates the expression of the transcription factor HIF1A, inhibiting ferroptosis by suppressing the transcription of EGLN271. The activation of clockophagy selectively degrades ARNTL through cargo receptor sequestosome 1 (SQSTM1), destabilizing HIF1A protein, and ultimately exacerbating lipid peroxidation and ferroptosis71. Furthermore, lipophagy, a biological process involved in the autophagic degradation of intracellular lipid droplets, has been implicated in ferroptosis72. Thus, some scholars have proposed that ferroptosis is an autophagy-dependent form of programmed cell death73, 74 (Figure 2). Nevertheless, more evidence is necessary to confirm a causal relationship between ferroptosis and autophagy and to indicate whether the factors in these processes show synergistic relationships. Furthermore, in any causal relationship that might be established, its dependence on specific treatments will need to be determined.
Major pathways and defense systems in ferroptosis regulation
Lipid peroxidation has been identified as a key driver of ferroptosis; thus, the redox systems play a critical role in this process. More than 80-90% of total ROS are reportedly generated by mitochondrial electron chain Complexes I and III, and NADPH after oxidation by NOXs (NADPH oxidases) is another important source of O2•- and H2O2 producing-proteins in signal transduction75, 76. Hydrogen peroxide is converted into highly toxic hydroxyl free radicals in the presence of ferrous ions via the Fenton reaction76. Multiple peroxidase system enzymes, such as glutathione peroxidase (GPX), catalase, NADH peroxidase, thioredoxin (TRX), and peroxiredoxin (PRX), are involved in converting H2O2 into H2O75, 77. Additionally, ubiquinone (also known as coenzyme Q) is a ubiquitous lipid-soluble redox cofactor that primarily transfers electrons and protons across the inner mitochondrial membrane78. Therefore, any disorder in these redox/defense systems accelerates lipid peroxidation and facilitates the onset of ferroptosis (Figure 2).
GPX4-GSH was the first known redox system to be implicated in the regulation of ferroptosis79. By using targeted metabolomic profiling, Yang et al. demonstrated that depletion of glutathione causes the inactivation of GPXs in response to ferroptosis inducers. Furthermore, GPX4 overexpression inhibits the lethality of 12 ferroptosis inducers, which indicates that GPX4 is a central regulator of ferroptosis79. Several GPX4 inhibitors, including RSL3, ML162 and ML210, have been shown to augment ferroptosis in many cell types and cell lines80-82. In both RSL3- and ML162-resistant cells, ACSL4 and LPCAT3 have been shown to be significantly enriched for gene trap insertions, indicating that ACSL4 and LPCAT3 are indispensable for the execution of GPX4 inhibition-induced ferroptosis83. The xCT complex, composed of solute carrier family 7 member 11 (SLC7A11) and SLC3A2, mediates the exchange of cystine and glutamate across the plasma membrane, promoting GSH synthesis84. Therefore, cystine deficiency or the inhibition of xCT function leads to impaired GSH synthesis and promotes ferroptosis3, 4, 85-88. P53, a tumor suppressor, has been reported to inhibit cystine uptake and sensitize cells to ferroptosis by downregulating the expression of SLC7A1188. SLC7A11 binds directly to ALOX12 and inhibits its lipoxygenase activity40, which offsets P53-mediated ferroptosis induced by ROS stress and reverses P53-dependent tumor growth suppression in xenograft models40. In contrast, cancer cells with high levels of SLC7A11 (SLC7A11high) have been observed to accumulate intracellular cystine when glucose levels are low, draining the cellular NADPH pool and leading to the aberrant accumulation of intracellular disulfides and subsequently to disulfidptosis, a previously uncharacterized form of cell death that differs from apoptosis and ferroptosis89, 90. Further investigation revealed that disulfidptosis is inhibited by inactivation of the WAVE regulatory complex, while it is facilitated by the constitutive activation of Rac90. These studies demonstrated that maintaining cystine homeostasis within cells is essential for cell survival, as cystine deficiency causes ferroptosis, and excessive cystine accumulation leads to disulfidptosis.
Although xCT-GPX4-GSH is considered to be the primary mechanism that prevents ferroptosis, inhibition of GPX4 or GPX4 knockout fails to trigger ferroptosis in certain cancer cell lines, suggesting alternative resistance mechanisms79, 91-93. Therefore, to identify genes complementing GPX4 loss, Doll et al. generated a cDNA expression library derived from MCF7 cells (a ferroptosis-resistant cell line) and, through screening, discovered that the pro-apoptotic gene AIFM2 (apoptosis inducing factor mitochondria-associated 2) is a novel anti-ferroptotic gene, which they renamed “ferroptosis-suppressor-protein 1” (FSP1)93. A back-to-back study using a synthetic lethality strategy with CRISPR-Cas9 also shown FSP1 to be a potent ferroptosis-resistance factor in U-2 OS osteosarcoma cells treated with the GPX4 inhibitor RSL392. Furthermore, the myristoylation of FSP1 and its subsequent plasma membrane localization were critical for reducing non-mitochondrial CoQ10, which is an antioxidant that prevents lipid peroxidation and ferroptosis92. These studies indicated that FSP1-CoQ10 is a novel anti-ferroptotic pathway that parallels to the canonical xCT-GPX4-GSH pathway. In addition, an FSP1-dependent noncanonical VK cycle has recently been reported to function as a ferroptosis suppressor, and FSP1 efficiently reduced VK to its hydroquinone VKH2 form, which function as a potent radical-trapping antioxidant to inhibit lipid peroxidation18. In addition to CoQ10 at the plasma membrane, mitochondrial CoQ10 is also reduced into CoQH2 in the mitochondrial inner membrane via the action of DHODH to attenuate ferroptosis17. In an RSL3-, imidazole ketone erastin (IKE)-, or GPX4 knockout-induced ferroptosis model, CRISPR activation screening revealed that GCH1, the rate-limiting enzyme for 6(R)-L-erythro-5,6,7,8-tetrahydrobiopterin (BH4) synthesis, is a gene that antagonizes ferroptotic cell death94. BH4 is a critical cofactor for nitric oxide synthases (NOS); under BH4-deficient conditions, superoxide is generated instead of NO, increasing the susceptibility of cells to ferroptosis95. Furthermore, it is reported that IKE-induced high BH4 levels led to CoQ10 synthesis, thus alleviating oxidative damage and preventing ferroptosis95. Interestingly, Wu et al. found that ferroptosis was non-cell-autonomously regulated by E-cadherin-mediated intercellular interactions, which repressed ferroptosis by activating intracellular NF2 (neurofibromin 2)-YAP (Yes1-associated transcriptional regulator) signaling96. In addition, many other mechanisms regulating ferroptosis have been revealed, such as the AMPK-mediated anti-ferroptotic effect of energy stress97 and glutamate-cysteine ligase catalytic subunit (GCLC)-conferred protection against ferroptosis mediated by its contribution to maintaining glutamate homeostasis86.
More importantly, epigenetic modifications are essential regulatory mechanisms for biological processes in the body, and recent studies have shown that ferroptosis is regulated by various epigenetic modifications, including DNA methylation, the RNA m6A modification and protein methylation3, 8, 98, 99. Research in this field is rapidly producing advancements, with new epigenetic mechanisms in ferroptosis being uncovered at an ever-increasing rate. Therefore, it is essential to review and discuss relevant studies.
DNA methylation in ferroptosis
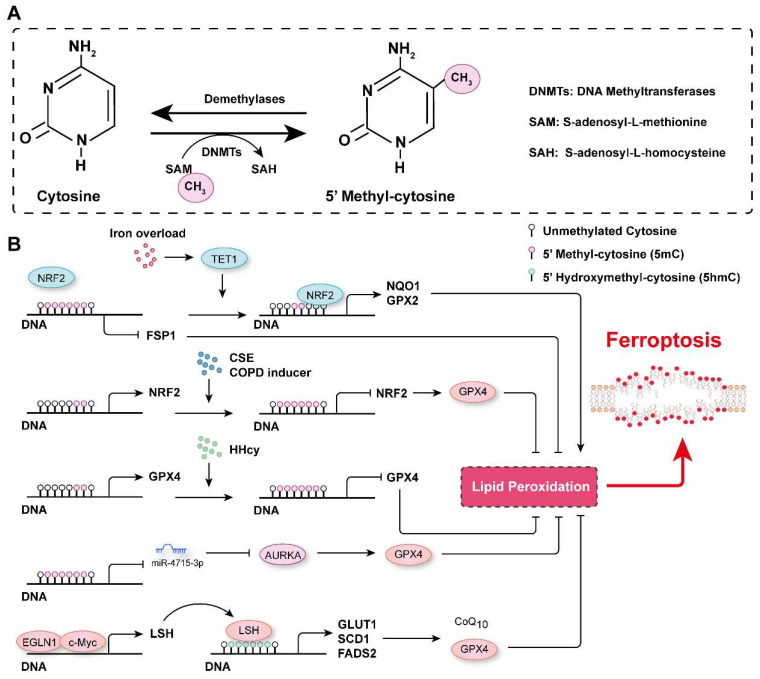
DNA methylation is an epigenetic modification that controls gene expression, typically occurring at the fifth position of the cytosine base in mammals to generate 5-methyl-cytosine (5mC). This modification is usually observed on cytosine-phosphate-guanine (CpG) dinucleotides, and approximately 60-90% of CpGs in the genome are methylated100. DNA methylation is dynamically regulated by DNA methyltransferases and demethylases (Figure 3A). DNA methyltransferase 3A (DNMT3A), DNMT3B and DNMT3C are critical for de novo DNA methylation, which is maintained by DNMT1 through DNA replication101, 102. In contrast, ten-eleven translocation (TET) enzymes actively remove methyl groups from DNA and successively oxidize 5mC to hydroxymethyl-cytosine (5hmC), formyl-cytosine (5fC) and carboxyl-cytosine (5caC)100, 103-105. DNA methylation marks have been reported to be involved in many biological processes and diseases106, 107, and their roles in ferroptosis regulation has recently been highlighted.
Figure 3.
DNA methylation in the regulation of ferroptosis. A. Schematic representation of DNA methylation. DNA is usually methylated on the fifth position of the cytosine base in mammals, generating 5-methyl-cytosine (5mC), which is catalyzed by DNMTs, and the methyl group is removed by demethylases. B. The mechanisms related to DNA methylation involved in the regulation of ferroptosis. CSE: Cigarette smoke extract; COPD: Chronic obstructive pulmonary disease; (HHcy): Hyperhomocysteinemia.
By analyzing the data in The Cancer Genome Atlas, Liu et al. revealed that DNA methylation and somatic copy number alterations (SCNAs) may contribute to the differential expression of most ferroptosis regulator genes (FRGs) in tumors108. They further established the ferroptosis potential index (FPI) and found that, compared with normal tissues, most cancer cells were characterized with a high FPI and that a high FPI predicted poor prognosis for several tumors108. Similarly, many ferroptosis-associated DNA methylation signatures have been developed to predict the prognosis for various tumors, such as glioblastoma multiforme (GBM), head and neck squamous cell carcinoma (HNSCC), cutaneous melanoma (CM), and lung squamous cell carcinoma (LUSC)109-112. DNA methylation of FRGs is involved in the regulation of their expression. For instance, analyzing the most recent TCGA multidimensional omics data revealed that multiple iron-related genes (e.g., scavenger receptor class A member 5 (SCARA5), erythroferrone (ERFE), lipocalin 2, transferrin receptor 2 (TFR2), solute carrier family 11 member 1 (SLC11A1), and cytochrome B reductase 1 (CYBRD1)) were dysregulated in different cancers, and this outcomes may be associated with aberrant DNA methylation112. Hypomethylation of the epigenome and increased TET1 expression have been observed in colonocytes subjected to continuous iron exposure113. Moreover, iron overload-induced DNA demethylation promotes the expression of nuclear factor erythroid 2-related factor 2 (NRF2) targets, such as NQO1 (NAD(P)H quinone dehydrogenase 1) and GPX2, which subsequently contribute to cellular lipid peroxidation and ferroptosis113. It has been reported that EGLN2 (Egl-9 family hypoxia inducible factor 2) is a candidate driver of iron chelation-mediated inhibition of cell death, and the hydroxylase activity of EGLNs is iron-dependent114, 115. In lung cancer cells, EGLN1 and c-Myc are recruited to the promoter of lymphoid-specific helicase (LSH) at two hypoxia inducible factor-1α (HIF-1α) binding sites, which directly facilitates the expression of LSH by inhibiting HIF-1α115. LSH, a DNA methylation modifier and a reader of 5-hmC, increases the 5-hmC levels at the promoters of the lipid metabolism-associated gene GLUT1 (glucose transporter type 1) and fatty acid desaturase genes SCD1 (stearoyl-CoA desaturase 1) and FADS2 (fatty acid desaturase 2) to activate their expression in H358 cells and PC9 cells115, 116. Activation of SCD1 increased antioxidant CoQ10, concomitantly increased the number of unsaturated fatty acyl chains in membrane phospholipids and decreased the number of long-chain saturated ceramides, which protected cells from ferroptosis37. In contrast, inhibition of SCD1/FADS2 directly downregulated the expression of GPX4 and reduced the ratio of GSH/GSSG, thereby accelerating iron-mediated lipid peroxidation, mitochondrial dysfunction and ferroptosis117. Phosphatidylethanolamine (PE)-linked arachidonic acid (AA) and adrenic acid (AdA) are substrates for lipid peroxidation118. Lee et al. demonstrated that FADS1 and elongation of very long-chain fatty acid protein 5 (ELOVL5) were required to maintain intracellular levels of AA and AdA, and the expression of FADS1 and ELOVL5 was found to be frequently inhibited by an increase in the DNA methylation marks on at their promoter/enhancer regions in intestinal-type gastric cancer cells (GCs), which resist ferroptosis118. In contrast, the expression of FADS1 and ELOVL5 was upregulated in mesenchymal-type GCs, which are sensitive to ferroptosis118.
GPX4 has been reported to be overexpressed in cancer tissues compared to normal tissues and to be associated with low levels of DNA methylation and enrichment of H3K4me3 and H3K27ac marks at its promoter, suggesting that epigenetic regulation may be involved in its aberrant overexpression22. Hyperhomocysteinemia (HHcy), a risk factor for cardiovascular diseases, neurological disorders, and musculoskeletal system dysfunction, has been reported to promote GPX4 methylation, leading to oxidative stress and ferroptosis119. In chronic obstructive pulmonary disease (COPD) patients and human bronchial epithelial (HBE) cells treated with cigarette smoke extract (CSE), GPX4 expression is decreased21. Furthermore, Nrf2 promoter hypermethylation is observed in both COPD patients and CSE-treated HBE cells, resulting in NRF2 downregulation, inhibited GPX4 expression and ferroptosis occurrence21. Gomaa et al. found increased DNA methylation levels on several CpG nucleotides upstream of miR-4715-3p in upper gastrointestinal adenocarcinoma (UGC) tissue samples, which binds to the 3' untranslated region (3' UTR) of Aurora kinase A (AURKA) to inhibit its expression120. Moreover, reconstitution of miR-4715-3p or inhibition of AURKA suppressed GPX4 expression to induce ferroptosis120. xCT has been reported to be involved in maintaining intracellular GSH levels, redox balance and anti-ferroptotic effects121. The stability of xCT is regulated by the MUC1-C (Mucin 1 C-terminal subunit)/CD44v (CD44 variant) complex, which directly interacts with xCT to promote its stability and control GSH levels122. Moreover, the expression of MUC1 is controlled by histone and DNA methylation on its promoter122. Additionally, the promoter of the Fsp1 gene has been shown to be hypermethylated in T- and B- acute lymphoblastic leukemia cell lines and patient samples, in which it suppressed FSP1 expression and promoted ferroptosis20. DNA methylation of the Fsp1 promoter prevented NRF2 from binding to its promoter and inhibited transcription20, 123. In addition, DNA methylation of many other genes has been reported to contribute to ferroptosis regulation98, 124-128. For example, embryonic lethal-abnormal vision-like protein 1 (ELAVL1), a well characterized RNA-binding protein that promotes target mRNA stability, has been found to bind to the 3'UTR of DNMT3B mRNA, thereby facilitating its expression during cerebral ischemia/reperfusion (I/R) injury. Increased DNMT3B levels accelerate the DNA methylation of the Pink1 (PTEN induced kinase 1) promoter, inhibiting PINK1 expression and promoting ferroptosis and brain damage129.
Studies have indicated that DNA methylation is critical for ferroptosis, as these marks regulate the expression of ferroptosis-related genes (Figure 3B). Generally, hypermethylation in the promoter or transcriptional start site or hypomethylation in the coding sequence of a gene suppresses the expression of that gene. However, it should be noted that the expression of a methylated gene can be increased when either the coding sequence is hypermethylated or the promoter or transcriptional start site is hypomethylated130. Thus, DNA methylation in coding regions needs to be further studied, especially when no difference in overall DNA methylation is identified, and the distribution of DNA methylation throughout the genome needs to characterized.
The RNA m6A modification in ferroptosis
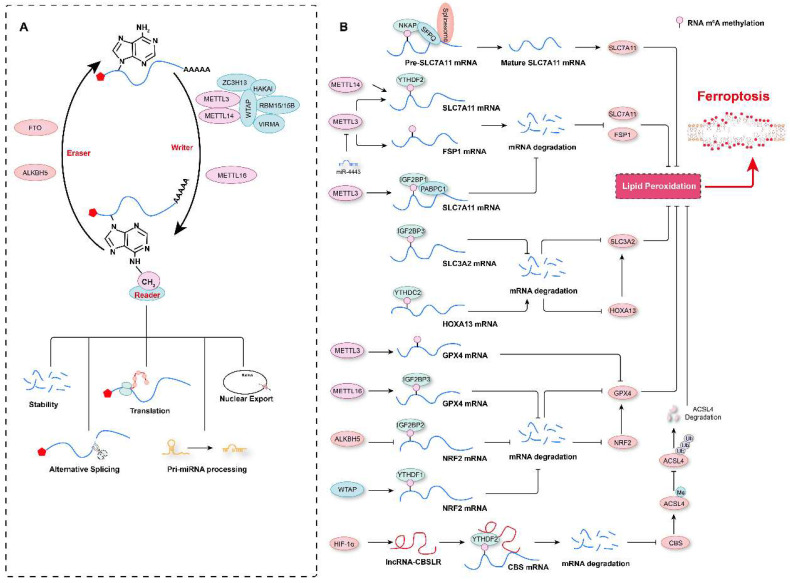
To date, more than 100 RNA modifications, including N6-methyladenosine (m6A), 5-methylcytosine (m5C), N1-methyladenosine (m1A), N3-methylcytosine (m3C), N7-methylguanosine (m7G), and pseudouridine (Ψ), have been identified131-133. Of these marks, m6A is one of the most abundant modifications of cellular RNA134. It was first discovered by two independent research groups in 1974 in poly(A) RNA fractions135, 136. RNA m6A marks are usually located in 3' UTRs, although there are also a few m6A sites located in regions surrounding start codons and 5' UTRs137-139. RNA m6A marks are deposited by a complex comprising methyltransferase-like 3 (METTL3), METTL14, Wilms' tumor 1-associated protein (WTAP), and KIAA1429, with METTL3 being the major subunit with the methyltransferase activity and acting as the “writer”134, 140, 141. In contrast, fat mass and obesity-associated protein (FTO) and AlkB homolog 5 (ALKBH5) have been found to have demethylase activity, functioning as “erasers” of the m6A mark142, 143. m6A-methylated RNA is recognized by “readers”, such as YT521-B homology (YTH) domain family proteins (YTHDF1, YTHDF2, YTHDF3, and YTHDC1) and heterogeneous nuclear ribonucleoprotein family proteins (HNRNPA2B1 and HNRNPC), which determine the fate of the marked RNA, such as degradation, translation or splicing144-147 (Figure 4A). Furthermore, the role of the RNA m6A modification in many biological processes and diseases has been elucidated in the past decade; these processes include autophagy, cell proliferation, stem-cell renewal and differentiation, and the diseases include tumorigenesis, and cardiovascular diseases139, 146, 148, 149. In the past three years, several studies have shown that RNA m6A marks are involved in the regulation of ferroptosis and thus affects the progression of diseases such as tumor growth and acute kidney injury133, 150, 151 and that m6A modification regulators used in combination with ferroptosis-related genes can be diagnostic or prognostic markers for tumors in humans130, 152-155.
Figure 4.
RNA m6A modification in the regulation of ferroptosis. A. Schematic representation of RNA m6A modification. RNA m6A marks are deposited by “writers”, and methyltransferase-like 3 (METTL3), METTL14, and METTL16 are the major methyltransferases. In contrast, an RNA m6A methyl group is removed by “erasers”, such as fat mass and obesity-associated protein (FTO) and AlkB homolog 5 (ALKBH5). m6A-modified RNA is recognized by “readers”, which determine the fate of the RNA, such as degradation, translation, alternative splicing, pri-miRNA processing, or nuclear export. B. The RNA m6A modification contributes to ferroptosis regulation by affecting the stability of mRNAs or their alternative splicing.
Multiple m6A modification regulators have been shown to be aberrantly expressed in various types of tumors, and a prediction model comprising these regulators and ferroptosis-associated genes has been shown to effectively reflect the progression and prognosis of these tumor patients23, 156-159. Recently, our research revealed that compared to aortic samples without aortic dissection (non-AD), higher levels of METTL3 and METTL14 protein, but lower levels of FTO were detected in aortic samples with Stanford type A aortic dissection (TAAD)3. Moreover, METTL3 protein levels were inversely correlated with the levels of ferroptosis modulators SLC7A11 and FSP1 in human aortas. METTL3 has also been shown to repress SLC7A11 and FSP1 expression by promoting decay of their mRNAs in human aortic smooth muscle cells (HASMCs). Furthermore, METTL3 accelerated IKE- and cystine deprivation-induced ferroptosis of HASMCs3. Similarly, in lipopolysaccharide (LPS)-induced cardiomyocytes, METTL3 deficiency inhibited LPS-induced ferroptosis by upregulating the expression of SLC7A11 in an m6A methylation-dependent manner160. METTL3 has been found to promote m6A modification of SLC7A11 mRNAs, which were then recognized by YTHDF2 to mediate their degradation160. Similar to METTL3, METTL14, another m6A methyltransferase, installs m6A modification on the 5'UTR of SLC7A11 mRNAs to facilitate their degradation in a YTHDF2-dependent manner, and hypoxia induces the downregulation of METTL14 by regulating HIF-1α levels, thus protecting hepatocellular carcinoma (HCC) cells from ferroptosis161. In contrast, in hepatoblastoma cells, IGF2 mRNA-binding protein 1 (IGF2BP1) has been identified as the m6A reader, and it was shown to compete with the BTG2/CCR4-NOT complex to bind PABPC1, thereby inhibiting SLC7A11 mRNA deadenylation and promoting its stability, resulting in increased SLC7A11 expression and the eventual inhibition of ferroptosis24. Intriguingly, the m6A demethylase FTO has been shown to inhibit SLC7A11 expression and thus trigger ferroptosis in an m6A-independent manner, attenuating the development of papillary thyroid carcinoma162. In addition to regulating SLC7A11 mRNA stability, the m6A modification has also been observed to participate in ferroptosis by affecting the mRNA splicing of SLC7A11163. For example, NF-κB-activating protein (NKAP) has been discovered to directly bind to m6A-methylated SLC7A11 mRNA and then recruit the splicing factor proline and glutamine-rich (SFPQ), which recognize the splice site and thus promotes SLC7A11 mRNA splicing and maturation, ultimately preventing the ferroptosis of glioblastoma cells163. These studies indicate that the m6A modification of SLC7A11 mRNA is critical for its expression regulation and ferroptosis. However, some results describing the impact of m6A on SLC7A11 expression are debated, and further investigation is needed. For example, more studies need to be conducted to explore the differences in m6A methylation sites in SLC7A11 mRNAs under different conditions, and these marks deposited on different sequence may lead to different fates of mRNAs after being recognized by different readers.
In addition to SLC7A11 mRNAs, the mRNAs of its partner SLC3A2 is also regulated by the m6A methylation. For example, in patients with lung adenocarcinoma (LUAD), the m6A reader insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) is highly expressed and binds to m6A-marked mRNAs encoding anti-ferroptosis regulators (e.g., SLC3A2, GPX4, ACSL3, and ferritin heavy chain 1 (FTH1)) to increase the stability of these mRNAs164. In contrast, Ma et al. demonstrated that SLC3A2 is not directly regulated by the m6A modification of its mRNA but is positively regulated by the transcription factor homeobox A13 (HOXA13), whose mRNAs are m6A-methylated on its 3'UTR, and this modification is recognized by YTHDC2, which causes the degradation of HOXA13 mRNAs in LUAD cells165. These results indicate that increasing YTHDC2 expression may be an alternative treatment option for LUAD because it facilitates ferroptosis via the downregulation of SLC3A2. Thus, the expression of SLC3A2 in LUAD cells may be regulated by in both an m6A methylation-dependent and -independent manner. GPX4 is another critical ferroptosis regulator166, 167. In samples obtained from breast cancer patients, METTL16 is overexpressed, which increases the rate of m6A modification on GPX4 mRNAs, increasing their stability and resulting in upregulation of GPX4. Enhanced GPX4 expression accelerates the proliferation and inhibits the ferroptosis of breast cancer cells168. However, in sepsis-associated acute lung injury (SI-ALI), neutrophil extracellular traps (NETs) activate the TLR9/MyD88/NF-κB pathway to upregulate METTL3 expression, which induces the m6A methylation of GPX4 mRNAs, inhibiting GPX4 expression and increasing the ferroptosis of alveolar epithelial cells169. It is crucial to elucidate the differences in GPX4 m6A marks on the regulation of GPX4 expression under different pathological conditions. Do different methylation sites or the activity of different readers lead to different fates of methylated mRNAs?
With research advances in this field, the mRNAs of multiple ferroptosis regulators have been shown to modified with m6A marks. For example, in non-small cell lung carcinoma (NSCLC) cells, exosomal miR-4443 targets METTL3 expression and negatively regulates METTL3-mediated m6A modifications on FSP1 and ferroptosis170. ALKBH5-mediated m6A demethylation at two m6A residues in the 3'UTR of NFE2L2/NRF2 mRNAs, which are recognized by IGF2BP2, contributes to reduced mRNA stability and decreased NFE2L2/NRF2 expression in hypopharyngeal squamous cell carcinoma cells171. WTAP can deposit m6A marks on the 3'UTR of NRF2 mRNA, and these marks are read by YTHDF1, enhancing the stability of NRF2 mRNA and inhibiting ferroptosis in bladder cancer cells172. In addition, autophagy is an important mechanism for regulating ferroptosis, and the m6A modification on the mRNAs of the autophagy-related gene BECN1 results in these m6A-marked mRNAs resistant to degradation after recognition by YTHDF1, contributing to dihydroartemisinin-induced hepatic stellate cell ferroptosis and conferring protection against liver fibrosis173. Hypoxia can also induce HIF-1α-mediated expression of lncRNA-CBSLR, which recruits YTHDF2 to read m6A-modified CBS (cystathionine β-synthase) mRNAs, leading to decreased mRNA stability and reduced CBS expression. Decreased CBS results in reduced methylation of the ACSL4 protein and facilitates polyubiquitination and degradation of ACSL4 via the ubiquitination-proteasome pathway, ultimately protecting gastric cancer against ferroptosis99.
Therefore, these aforementioned studies suggest that the RNA m6A modification is involved in ferroptosis by regulating the mRNA stability and protein expression of multiple ferroptosis-related genes (Figure 4B). However, there are still at least three scientific questions that must be answered. First, what are the mechanisms underlying the RNA m6A modifications involved in ferroptosis? Second, what are the mechanisms by which specific m6A modification sites are recognized by different readers, which determine different fates of mRNAs after ferroptosis inducers treatment? Third, does targeting m6A marks to regulate ferroptosis has potential as a treatment for related diseases?
Protein methylation in ferroptosis
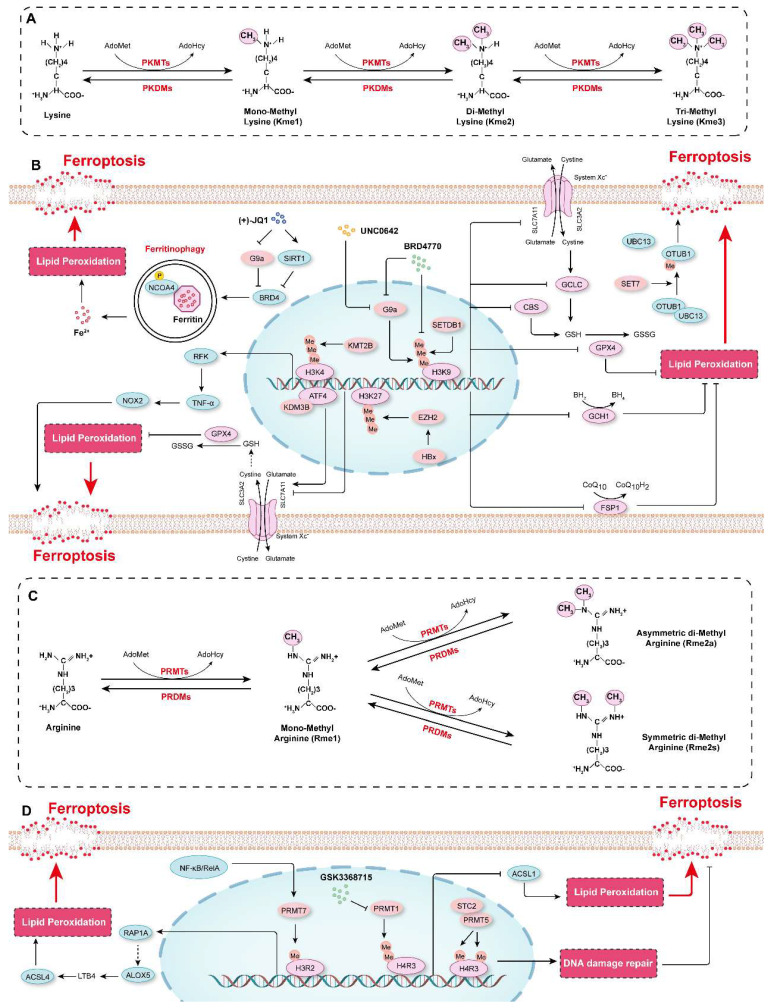
Protein methylation occurs mainly on lysine and arginine residues, and protein lysine methyltransferases (PKMTs or PLMTs) and protein arginine methyltransferases (PRMTs) are responsible for the addition of methyl groups to these residues174, 175. Lysine can be mono-, di-, or trimethylated (me1, me2 or me3, respectively), while arginine can be mono-, symmetrically dimethylated, or asymmetrically dimethylated (me1, me2s or me2a, respectively)176, 177 (Figure 5A and 5C). Histone methylation is the most common type of protein methylation, such as methylation of H3K27, H3K4, H3K9, H3K36, H4K20, and H4R3178-183. In recent years, a variety of non-histone proteins have also been shown to be methylated, such as P53 and AKT184-186. Conversely, methyl groups can be removed from different residues on proteins by demethylases187-190. Protein methylation has been implicated in many biological processes (e.g., programmed cell death, proliferation, and autophagy) and diseases (e.g., cardiovascular diseases, cancers, and mental health disorders)4, 181-183, 191, 192. Recently, the role of protein methylation in ferroptosis has been increasingly recognized and highlighted193.
Figure 5.
Protein methylation in the regulation of ferroptosis. A. Schematic diagram showing protein methylation on lysine residues. Lysine residues in protein can be methylated by lysine methyltransferases (PKMTs) with S-adenosyl-L-methionine (AdoMet) functioning as the primary methyl group donor. Lysine can be monomethylated (Kme1), dimethylated (Kme2) and trimethylated (Kme3), and these modifications are reversible, and marks can be erased by lysine demethylases (PKDMs). B. The mechanisms by which lysine methylation regulates ferroptosis. C. Schematic diagram showing protein methylation on arginine residues. Arginine residues in proteins can be monomethylated (Rme1), and symmetrically dimethylated (Rme2s) or asymmetrically dimethylated (Rme2a) by arginine methyltransferases (PRMTs), and they are demethylated by arginine demethylases (PRDMs). D. The molecular mechanisms by which arginine methylation regulates ferroptosis.
Our recent studies showed that compared to those in control cells, the protein levels of H3K9me1, H3K9me2, and H3K9me3 were increased in HASMCs treated with ferroptosis inducers (cystine deprivation, IKE, or RSL3). Further screening of the effects of several histone methyltransferase inhibitors on ferroptosis revealed that BRD4770, an inhibitor of H3K9me1/2/3, significantly inhibited ferroptosis in HASMCs, and its effect was comparable to that of Fer-1, a classical ferroptosis inhibitor. More importantly, BRD4770 delayed the progression of β-aminopropionitrile monofumarate (BAPN)-induced aortic dissection in mice by inhibiting the inflammatory response, lipid peroxidation, and ferroptosis4. BRD4770 had been previously reported to be an inhibitor of G9a194. Our findings thus suggest that G9a may be involved in the regulation of ferroptosis. Rothammer et al. showed that the level of H3K9me2 was significantly increased in humans with multiple sclerosis and in a murine experimental autoimmune encephalomyelitis model. G9a suppressed anti-ferroptotic genes (GPX4, CBS and GCLC), reduced intracellular GSH levels, triggering ferroptosis and resulting in neuronal damage, while targeting G9a by its inhibitor UNC0642 alleviated inflammation-induced neurodegeneration195. On the contrary, lysine demethylase 3B (KDM3B) increased the expression of SLC7A11 by interacting with activating transcription factor 4 (ATF4) to inhibit erastin-induced ferroptosis196. (+)-JQ1, an inhibitor of bromodomain-containing 4 (BRD4), suppressed the expression of the ferroptosis-associated genes GPX4, SLC7A11, and SLC3A2 by repressing the expression of G9a while simultaneously promoting the expression of histone deacetylase Sirtuin 1 (SIRT1). Subsequently, (+)-JQ1 promoted ferritinophagy to increase iron levels, which induced ferroptosis in cancer cells197. SET domain bifurcated 1 (SETDB1, also known as ESET or KMT1E) is another important methyltransferase that methylates H3K9198. SETDB1 deficiency clearly promoted the acquisition of the TGF-β-induced mesenchymal phenotype but reduced the expression of E-cadherin in human alveolar epithelial cells by catalyzing the deposition of H3K9me3 on Snai1, which inhibited the protein expression of Snai1, the main transcription factor that initiates the epithelial-mesenchymal transition (EMT). Overexpression of SETDB1 inhibited the EMT and caused ferroptosis, preventing pulmonary fibrosis199. These studies indicate that H3K9 methylation is a key epigenetic mechanism that regulates ferroptosis and that targeting H3K9 methylation regulators (e.g., with BRD4770) significantly inhibits ferroptosis and is expected to be a potential treatment for degenerative diseases characterized by cell loss (e.g., aortic dissection). On the other hand, enhancing H3K9 methylation can treat the diseases with excessive cell proliferation such as tumors by promoting ferroptosis.
H3K27 methylation is an important histone mark that suppresses gene expression and is primarily catalyzed by enhancer of zeste homolog 2 (EZH2)182, 200, 201. It has been reported that the hepatitis B virus X protein (HBx) facilitated D-GalN-induced hepatotoxicity and ferroptosis by inhibiting the expression of SLC7A11 through the EZH2-mediated H3K27me3 modification202. In contrast, Yu et al. demonstrated that EZH2 mitigated the ferroptosis of tongue squamous cell carcinoma cells by suppressing miR-125b-5p but upregulating SLC7A11 expression203. Additionally, GSK-J4, a dual inhibitor of histone lysine demethylase 6A/6B that prevents H3K27 demethylation, can suppress palmitic acid (PA)-induced hypersensitivity to ferroptosis by inhibiting ACSL4 expression and lipid peroxidation in cardiomyocytes204. KMT2B activated the transcription of riboflavin kinase (RFK) by enhancing the trimethylation of H3K4, which subsequently accelerated tumor necrosis factor-α (TNF-α)/NADPH oxidase 2 (NOX2) axis activation to promote ferroptosis during myocardial ischemia/reperfusion injury205.
In contrast, SET7 attenuated the inhibitory effect of OTUB1 (OTU domain-containing ubiquitin aldehyde-binding protein 1) on cystine starvation/erastin-induced ferroptosis by directly interacting with non-histone OTUB1 to catalyze its methylation on lysine 122. Interestingly, this effect is not achieved by affecting the deubiquitinase (DUB) activity of OTUB1 but by impairing its noncanonical activity, binding to the E2 enzyme UBC13206. Furthermore, menin-mixed-lineage leukemia (MLL) inhibitors, such as MI-463, induced ferroptosis in several cancer cell lines207.
In addition to lysine methylation, arginine methylation also plays a critical role in ferroptosis. For example, protein arginine methyltransferase 1 (PRMT1) has been reported to function as a suppressor of ferroptosis by increasing the level of asymmetrically dimethylated histone H4 on Arg 3 (H4R3me2a) to downregulate the expression of ACSL1 and then further decrease the lipid peroxidation208. Moreover, the combination of the Type I PRMT inhibitor GSK3368715 and ferroptosis inducers (e.g., RSL3) shows potential as a treatment of acute myeloid leukemia208. Stanniocalcin 2 (STC2) can interact with and activate PRMT5, which catalyzes symmetric dimethylation of histone H4 on Arg 3 (H4R3me2s) to induce radioresistance by activating DNA damage repair, but inhibits ferroptosis in esophageal squamous cell carcinoma209. The expression of PRMT7 was induced by the activation of NF-κB/RelA in macrophages in lung tissue characterized by COPD210. Elevated PRMT7 caused an increase in the levels of H3R2me1 at the regulatory elements of Ras-related protein Rap-1A (RAP1A), thereby promoting RAP1A expression and accelerating the adhesion and migration of monocytes. Furthermore, accumulation of monocyte-derived macrophages resulted in the overexpression of ALOX5 and the accumulation of its metabolite LTB4, which activates the expression of ACSL4 to accelerate ferroptosis and tissue injury in COPD210.
These studies have indicated that protein methylation, such as H3K9 and H3K27 methylation, is a critical epigenetic mechanism in the regulation of ferroptosis (Figure 5B and 5D). However, there are still few studies in this research field, and in-depth studies are needed to elucidate the relationship between protein methylation and ferroptosis and to develop strategies for targeting protein methylation to modulate ferroptosis and treat disease. Moreover, to date, there are few reports on the correlation between non-histone methylation and ferroptosis. For example, although the relationship between P53 and ferroptosis has been well established88, 211, 212, there is no evidence suggesting that its methylation is involved in the regulatory effect of P53 on ferroptosis.
Conclusions and perspectives
In the present review, we summarize the basis of ferroptosis and the evidence acquired to date to show how DNA, RNA, and protein methylation regulate ferroptosis. It is clear that methylation modification of all three types of molecules is essential for the regulation of ferroptosis and that these modified molecules exert influence mainly by regulating the expression of ferroptosis-associated proteins. There are at least six key unanswered scientific questions for which the answers are likely to drive advances in understanding the epigenetic regulation of ferroptosis in the near future. First, how do DNA, RNA and protein methylation mutually regulate and synergistically influence ferroptosis under certain conditions, and what is the spatiotemporal regulatory relationship among them? Second, what are the mechanisms by which DNA methylation in different regions during ferroptosis regulates related gene expression? Specifically, how methylated DNA in coding regions affects the expression of these genes should be investigated further. Third, what determines the specificity of the RNA m6A modification sites in ferroptosis, and how do RNA m6A modification sites and reader proteins jointly regulate the fate of related mRNAs in this context? Fourth, are methylated non-histone proteins, in particular those non-histones that have been linked to ferroptosis, involved in the regulation of ferroptosis? Fifth, how do other epigenetic modifications form a regulatory network with DNA, RNA, and protein methylation to regulate ferroptosis? Sixth, further exploration is needed to determine the potential of targeting DNA, RNA, or protein methylation to regulate ferroptosis and thereby treat and prevent disease. Moreover, whether combined inhibitors/inducers of ferroptosis and inhibitors/agonists of biomolecular methylation can function synergistically to mitigate related diseases needs to be explored in depth. Answers to these questions will not only advance our understanding of ferroptosis but will also provide important new insights into the biological functions related to DNA, RNA, and protein methylation.
Acknowledgments
We apologize to authors whose papers could not be cited due to space restrictions.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82170502).
Author contributions
DSJ supervised the general concept of this review. XW and XK wrote the manuscript. XF drew the diagrams. DSJ and XF participated in the revision and literature search. All authors contributed to the editing process and read and approved the final manuscript.
Abbreviations
- 5mC
5-methyl-cytosine
- 5hmC
Hydroxymethyl-cytosine
- 5fC
Formyl-cytosine
- 5caC
Carboxyl-cytosine
- ACSL4
Acyl-CoA synthetase long-chain family member 4
- ALOX12
Arachidonate 12-lipoxygenase, 12S type
- ALOX15
Arachidonic acid 15-lipoxygenase-1
- ATG3
Autophagy related gene 3
- AMPK
AMP-activated protein kinase
- AIFM2
Apoptosis inducing factor mitochondria-associated 2
- AA
Arachidonic acid
- AdA
Adrenic acid
- AURKA
Aurora kinase A
- ALKBH5
AlkB homolog 5
- ATF4
Activating transcription factor 4
- BH4
Tetrahydrobiopterin
- BAPN
β-aminopropionitrile monofumarate
- BRD4
Bromodomain containing 4
- CDDO
2-amino-5-chloro-N, 3-dimethylbenzamide
- CpG
Cytosine-phosphate-guanine
- CM
Cutaneous melanoma
- CYBRD1
Cytochrome B reductase 1
- COPD
Chronic obstructive pulmonary disease
- CSE
Cigarette smoke extract
- CBS
Cystathionine β-synthase
- DHODH
Dihydroorotate dehydrogenase
- DFO
Deferoxamine
- DMT1
Divalent metal transporter 1
- DNMT3A
DNA Methyltransferase 3A
- ER
Endoplasmic reticulum
- ERFE
Erythroferrone
- EGLN2
Egl-9 family hypoxia inducible factor 2
- ELOVL5
Elongation of very long chain fatty acid protein 5
- ELAVL1
Embryonic lethal-abnormal vision like protein 1
- EMT
Epithelial-mesenchymal transition
- EZH2
Enhancer of zeste homolog 2
- FPN
Ferroportin
- FRGs
Ferroptosis regulator genes
- FPI
Ferroptosis potential index
- FADS2
Fatty acid desaturase 2
- FTO
Fat mass and obesity-associated protein
- FTH1
Ferritin heavy chain 1
- FSP1
Ferroptosis-suppressor-protein 1
- Fer-1
Ferrostatin-1
- GPX4
Glutathione peroxidase 4
- GSH
Glutathione
- GCH1
GTP cyclohydrolase 1
- GCLC
Glutamatecysteine ligase catalytic subunit
- GBM
Glioblastoma multiforme
- GLUT1
Glucose transporter type 1
- GCs
Gastric cancer cells
- HHcy
Hyperhomocysteinemia
- HSCs
Hematopoietic stem cells
- HO-1
Heme oxygenase-1
- HERC2
HECT and RLD domain containing E3 ubiquitin protein ligase 2
- HSP90
Heat shock protein 90
- HSPA5
Heat shock protein family A member 5
- HNSCC
Head and neck squamous cell carcinoma
- HIF-1α
Hypoxia inducible factor-1α
- HBE
Human bronchial epithelial
- HNRNPA2B1
Heterogeneous nuclear ribonucleoprotein family protein A2/B1
- HASMCs
Human aortic smooth muscle cells
- HCC
Hepatocellular carcinoma
- HOXA13
Homeobox A13
- HBx
Hepatitis B virus X protein
- I/R
Ischemia/reperfusion
- IGF2BP1
IGF2 mRNA-binding protein 1
- IGF2BP3
Insulin-like growth factor 2 mRNA binding protein 3
- KDM3B
Lysine demethylase 3B
- LPCAT3
Lysophosphatidylcholine acyltransferase 3
- LOXs
Lipoxygenases
- LC3 II
Microtubule-associated protein 1 light chain 3 II
- LAMP2a
Lysosome-associated membrane protein 2a
- LUSC
Lung squamous cell carcinoma
- LSH
Lymphoid-specific helicase
- LPS
Lipopolysaccharide
- LUAD
Lung adenocarcinoma
- MYSM1
Myb like, SWIRM and MPN Domains 1
- MUFA
Monounsaturated fatty acyl
- MUC1-C
Mucin 1 C-terminal subunit
- m5C
5-methylcytosine
- m1A
N1-methyladenosine
- m3C
N3-methylcytosine
- m7G
N7-methylguanosine
- METTL3
Methyltransferase-like 3
- m6A
N6-Methyladenosine
- MLL
Menin-mixed-lineage leukemia
- NRF2
NFE2 like BZIP transcription factor 2
- NCOA4
Nuclear receptor coactivator 4
- NOXs
NADPH oxidases
- NOS
Nitric oxide synthases
- NF2
Neurofibromin 2
- NKAP
NF-κB activating protein
- NETs
Neutrophil extracellular traps
- NSCLC
Non-small cell lung carcinoma
- NOX2
NADPH oxidase 2
- OTUB1
OTU domain-containing ubiquitin aldehyde-binding protein 1
- PLOOHs
Phospholipid hydroperoxides
- PUFA
Polyunsaturated fatty acid
- PRX
Peroxiredoxin
- PE
Phosphatidylethanolamine
- PINK1
PTEN Induced Kinase 1
- PKMTs
Protein lysine methyltransferases
- PRMTs
Protein arginine methyltransferases
- PA
Palmitic acid
- PRMT1
Protein arginine methyltransferase 1
- ROS
Reactive oxygen species
- RFK
Riboflavin kinase
- RAP1A
Ras-related protein Rap-1A
- SCD1
Stearoyl-CoA desaturase 1
- SLC39A14
Solute carrier family 39 member 14
- SQSTM1
Sequestosome 1
- SLC7A11
Solute carrier family 7 member 11
- SCNA
Somatic copy number alterations
- SCARA5
Scavenger receptor class A member 5
- SLC11A1
Solute carrier family 11 member 1
- SCD1
Stearoyl-CoA desaturase 1
- SFPQ
Splicing factor proline and glutamine-rich
- SI-ALI
Sepsis-associated acute lung injury
- SIRT1
Histone deacetylase Sirtuin 1
- SETDB1
SET domain bifurcated 1
- STC2
Stanniocalcin 2
- Tf
Transferrin
- TRIM7
Tripartite motif-containing protein 7
- TRX
Thioredoxin
- TET
Ten-eleven translocation
- TFR2
Transferrin receptor 2
- TAAD
Stanford type A aortic dissection
- TNF-α
Tumor necrosis factor-α
- UGC
Upper gastrointestinal adenocarcinoma
- VK
Vitamin K
- WTAP
Wilms' tumor 1-associated protein
- xCT
System xc-
- YAP
Yes1 associated transcriptional regulator
- YTHDF1
YT521-B homology (YTH) domain family protein F1
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockwell BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N, Yi X, He Y, Huo B, Chen Y, Zhang Z. et al. Targeting Ferroptosis as a Novel Approach to Alleviate Aortic Dissection. Int J Biol Sci. 2022;18:4118–4134. doi: 10.7150/ijbs.72528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Yi X, Huo B, He Y, Guo X, Zhang Z. et al. BRD4770 functions as a novel ferroptosis inhibitor to protect against aortic dissection. Pharmacol Res. 2022;177:106122. doi: 10.1016/j.phrs.2022.106122. [DOI] [PubMed] [Google Scholar]
- 5.Fang X, Zhang J, Li Y, Song Y, Yu Y, Cai Z. et al. Malic Enzyme 1 as a Novel Anti-Ferroptotic Regulator in Hepatic Ischemia/Reperfusion Injury. Adv Sci (Weinh) 2023;10:e2205436. doi: 10.1002/advs.202205436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X, Wang H, Han D, Xie E, Yang X, Wei J. et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, An P, Xie E, Wu Q, Fang X, Gao H. et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66:449–465. doi: 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei X, Yi X, Zhu XH, Jiang DS. Posttranslational Modifications in Ferroptosis. Oxid Med Cell Longev. 2020;2020:8832043. doi: 10.1155/2020/8832043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–96. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 10.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–45. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N. et al. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–28. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 12.Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–48. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B. et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174. doi: 10.1186/s13045-022-01392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan M, Yin Y, Ma X, Zhang J, Pan W, Tan M. et al. Glutathione system enhancement for cardiac protection: pharmacological options against oxidative stress and ferroptosis. Cell Death Dis. 2023;14:131. doi: 10.1038/s41419-023-05645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Jia Y, Mahmut D, Deik AA, Jeanfavre S, Clish CB. et al. Human hematopoietic stem cell vulnerability to ferroptosis. Cell. 2023;186:732–747. doi: 10.1016/j.cell.2023.01.020. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–590. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishima E, Ito J, Wu Z, Nakamura T, Wahida A, Doll S. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature. 2022;608:778–783. doi: 10.1038/s41586-022-05022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Fang ZM, Yi X, Wei X, Jiang DS. The interaction between ferroptosis and inflammatory signaling pathways. Cell Death Dis. 2023;14:205. doi: 10.1038/s41419-023-05716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pontel LB, Bueno-Costa A, Morellato AE, Carvalho Santos J, Roue G, Esteller M. Acute lymphoblastic leukemia necessitates GSH-dependent ferroptosis defenses to overcome FSP1-epigenetic silencing. Redox Biol. 2022;55:102408. doi: 10.1016/j.redox.2022.102408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Fu C, Liu J, Sai X, Qin C, Di T. et al. Hypermethylation of the Nrf2 Promoter Induces Ferroptosis by Inhibiting the Nrf2-GPX4 Axis in COPD. Int J Chron Obstruct Pulmon Dis. 2021;16:3347–3362. doi: 10.2147/COPD.S340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Sui S, Wang L, Li H, Zhang L, Xu S. et al. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J Cell Physiol. 2020;235:3425–3437. doi: 10.1002/jcp.29232. [DOI] [PubMed] [Google Scholar]
- 23.Wu T, Qian TY, Lin RJ, Jin DD, Xu XB, Huang MX. et al. Construction and validation of a m6A RNA methylation and ferroptosis-related prognostic model for pancreatic cancer by integrated bioinformatics analysis. J Gastrointest Oncol. 2022;13:2553–2564. doi: 10.21037/jgo-22-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, He J, Sun G, Huang N, Bian Z, Xu C. et al. The N6-methyladenosine modification enhances ferroptosis resistance through inhibiting SLC7A11 mRNA deadenylation in hepatoblastoma. Clin Transl Med. 2022;12:e778. doi: 10.1002/ctm2.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N. et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stockwell BR, Jiang X, Gu W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020;30:478–490. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J. et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13:110. doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, He Y, Wei X, Jiang DS. Targeting regulated cell death in aortic aneurysm and dissection therapy. Pharmacol Res. 2022;176:106048. doi: 10.1016/j.phrs.2021.106048. [DOI] [PubMed] [Google Scholar]
- 30.von Krusenstiern AN, Robson RN, Qian N, Qiu B, Hu F, Reznik E. et al. Identification of essential sites of lipid peroxidation in ferroptosis. Nat Chem Biol. 2023;19:719–730. doi: 10.1038/s41589-022-01249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed A, Ichu TA, Milosevich N, Melillo B, Schafroth MA, Otsuka Y. et al. LPCAT3 Inhibitors Remodel the Polyunsaturated Phospholipid Content of Human Cells and Protect from Ferroptosis. ACS Chem Biol. 2022;17:1607–1618. doi: 10.1021/acschembio.2c00317. [DOI] [PubMed] [Google Scholar]
- 33.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JH, Lewin TM, Coleman RA. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J Biol Chem. 2001;276:24667–73. doi: 10.1074/jbc.M010793200. [DOI] [PubMed] [Google Scholar]
- 35.Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A. et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem Biol. 2019;26:420–432. doi: 10.1016/j.chembiol.2018.11.016. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966–75. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J. et al. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 2019;79:5355–5366. doi: 10.1158/0008-5472.CAN-19-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta. 2015;1851:308–30. doi: 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenzel SE, Tyurina YY, Zhao J, St Croix CM, Dar HH, Mao G. et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell. 2017;171:628–641. doi: 10.1016/j.cell.2017.09.044. e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu B, Kon N, Chen D, Li T, Liu T, Jiang L. et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21:579–591. doi: 10.1038/s41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma XH, Liu JH, Liu CY, Sun WY, Duan WJ, Wang G. et al. ALOX15-launched PUFA-phospholipids peroxidation increases the susceptibility of ferroptosis in ischemia-induced myocardial damage. Signal Transduct Target Ther. 2022;7:288. doi: 10.1038/s41392-022-01090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Xin W, Anderson GJ, Li R, Gao L, Chen S. et al. Double-edge sword roles of iron in driving energy production versus instigating ferroptosis. Cell Death Dis. 2022;13:40. doi: 10.1038/s41419-021-04490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang X, Ardehali H, Min J, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20:7–23. doi: 10.1038/s41569-022-00735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu XM, Chapman GB, Wang H, Durante W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation. 2002;105:79–84. doi: 10.1161/hc0102.101369. [DOI] [PubMed] [Google Scholar]
- 45.Soares MP, Hamza I. Macrophages and Iron Metabolism. Immunity. 2016;44:492–504. doi: 10.1016/j.immuni.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng Y, Zak O, Aisen P, Harrison SC, Walz T. Structure of the human transferrin receptor-transferrin complex. Cell. 2004;116:565–76. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 47.Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P. et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726–739. doi: 10.1182/blood.2019002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tripathi AK, Haldar S, Qian J, Beserra A, Suda S, Singh A. et al. Prion protein functions as a ferrireductase partner for ZIP14 and DMT1. Free Radic Biol Med. 2015;84:322–330. doi: 10.1016/j.freeradbiomed.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen J, Wilbon AS, Zhou M, Pan Y. Mechanism of Ca(2+) transport by ferroportin. Elife. 2023;12:e82947. doi: 10.7554/eLife.82947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S. et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Aschemeyer S, Qiao B, Stefanova D, Valore EV, Sek AC, Ruwe TA. et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. 2018;131:899–910. doi: 10.1182/blood-2017-05-786590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang DL, Wu J, Shah BN, Greutelaers KC, Ghosh MC, Ollivierre H. et al. Erythrocytic ferroportin reduces intracellular iron accumulation, hemolysis, and malaria risk. Science. 2018;359:1520–1523. doi: 10.1126/science.aal2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nekhai S, Xu M, Foster A, Kasvosve I, Diaz S, Machado RF. et al. Reduced sensitivity of the ferroportin Q248H mutant to physiological concentrations of hepcidin. Haematologica. 2013;98:455–63. doi: 10.3324/haematol.2012.066530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou H, Zhou YL, Mao JA, Tang LF, Xu J, Wang ZX. et al. NCOA4-mediated ferritinophagy is involved in ionizing radiation-induced ferroptosis of intestinal epithelial cells. Redox Biol. 2022;55:102413. doi: 10.1016/j.redox.2022.102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y, Zheng Y, Wang C, Liu Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018;9:753. doi: 10.1038/s41419-018-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song N, Zhang J, Zhai J, Hong J, Yuan C, Liang M. Ferritin: A Multifunctional Nanoplatform for Biological Detection, Imaging Diagnosis, and Drug Delivery. Acc Chem Res. 2021;54:3313–3325. doi: 10.1021/acs.accounts.1c00267. [DOI] [PubMed] [Google Scholar]
- 57.Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P. et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ Res. 2020;127:486–501. doi: 10.1161/CIRCRESAHA.120.316509. [DOI] [PubMed] [Google Scholar]
- 58.Wang P, Cui Y, Ren Q, Yan B, Zhao Y, Yu P. et al. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021;12:447. doi: 10.1038/s41419-021-03725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–9. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang M, Chen Z, Wu D, Chen L. Ferritinophagy/ferroptosis: Iron-related newcomers in human diseases. J Cell Physiol. 2018;233:9179–9190. doi: 10.1002/jcp.26954. [DOI] [PubMed] [Google Scholar]
- 61.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd. et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–8. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H, Liu Q, Shan X, Gao W, Chen Q. ATM orchestrates ferritinophagy and ferroptosis by phosphorylating NCOA4. Autophagy. 2023. pp. 1–16. [DOI] [PMC free article] [PubMed]
- 63.Li K, Chen B, Xu A, Shen J, Li K, Hao K. et al. TRIM7 modulates NCOA4-mediated ferritinophagy and ferroptosis in glioblastoma cells. Redox Biol. 2022;56:102451. doi: 10.1016/j.redox.2022.102451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu J, Lou C, Zhen C, Wang Y, Shang P, Lv H. Iron plays a role in sulfasalazine-induced ferroptosis with autophagic flux blockage in K7M2 osteosarcoma cells. Metallomics. 2022;14:mfac027. doi: 10.1093/mtomcs/mfac027. [DOI] [PubMed] [Google Scholar]
- 65.Lin PL, Tang HH, Wu SY, Shaw NS, Su CL. Saponin Formosanin C-induced Ferritinophagy and Ferroptosis in Human Hepatocellular Carcinoma Cells. Antioxidants (Basel) 2020;9:682. doi: 10.3390/antiox9080682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–32. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M. et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci U S A. 2019;116:2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu S, Zhang Q, Sun X, Zeh HJ 3rd, Lotze MT, Kang R. et al. HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 2017;77:2064–2077. doi: 10.1158/0008-5472.CAN-16-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J. et al. AMPK-Mediated BECN1 Phosphorylation Promotes Ferroptosis by Directly Blocking System Xc(-) Activity. Curr Biol. 2018;28:2388–2399. doi: 10.1016/j.cub.2018.05.094. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang M, Chen P, Liu J, Zhu S, Kroemer G, Klionsky DJ. et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. 2019;5:eaaw2238. doi: 10.1126/sciadv.aaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J, Yang M, Kang R, Klionsky DJ, Tang D. Autophagic degradation of the circadian clock regulator promotes ferroptosis. Autophagy. 2019;15:2033–2035. doi: 10.1080/15548627.2019.1659623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H. et al. Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun. 2019;508:997–1003. doi: 10.1016/j.bbrc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 73.Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89–100. doi: 10.1016/j.semcancer.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem Biol. 2020;27:420–435. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J, Kim J, Lee R, Lee E, Choi TG, Lee AS. et al. Therapeutic strategies for liver diseases based on redox control systems. Biomed Pharmacother. 2022;156:113764. doi: 10.1016/j.biopha.2022.113764. [DOI] [PubMed] [Google Scholar]
- 76.Cencioni C, Comunanza V, Middonti E, Vallariello E, Bussolino F. The role of redox system in metastasis formation. Angiogenesis. 2021;24:435–450. doi: 10.1007/s10456-021-09779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 78.Bogeski I, Gulaboski R, Kappl R, Mirceski V, Stefova M, Petreska J. et al. Calcium binding and transport by coenzyme Q. J Am Chem Soc. 2011;133:9293–303. doi: 10.1021/ja110190t. [DOI] [PubMed] [Google Scholar]
- 79.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou L, Chen J, Li R, Wei L, Xiong H, Wang C. et al. Metal-Polyphenol-Network Coated Prussian Blue Nanoparticles for Synergistic Ferroptosis and Apoptosis via Triggered GPX4 Inhibition and Concurrent In Situ Bleomycin Toxification. Small. 2021;17:e2103919. doi: 10.1002/smll.202103919. [DOI] [PubMed] [Google Scholar]
- 81.Wang Q, Bin C, Xue Q, Gao Q, Huang A, Wang K. et al. GSTZ1 sensitizes hepatocellular carcinoma cells to sorafenib-induced ferroptosis via inhibition of NRF2/GPX4 axis. Cell Death Dis. 2021;12:426. doi: 10.1038/s41419-021-03718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jia JN, Yin XX, Li Q, Guan QW, Yang N, Chen KN. et al. Neuroprotective Effects of the Anti-cancer Drug Lapatinib Against Epileptic Seizures via Suppressing Glutathione Peroxidase 4-Dependent Ferroptosis. Front Pharmacol. 2020;11:601572. doi: 10.3389/fphar.2020.601572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M. et al. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem Biol. 2015;10:1604–9. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rochette L, Dogon G, Rigal E, Zeller M, Cottin Y, Vergely C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int J Mol Sci. 2022;24:449. doi: 10.3390/ijms24010449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang YP, Mockabee-Macias A, Jiang C, Falzone A, Prieto-Farigua N, Stone E. et al. Non-canonical Glutamate-Cysteine Ligase Activity Protects against Ferroptosis. Cell Metab. 2021;33:174–189. doi: 10.1016/j.cmet.2020.12.007. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shin D, Lee J, You JH, Kim D, Roh JL. Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol. 2020;30:101418. doi: 10.1016/j.redox.2019.101418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu X, Olszewski K, Zhang Y, Lim EW, Shi J, Zhang X. et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat Cell Biol. 2020;22:476–486. doi: 10.1038/s41556-020-0496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X, Nie L, Zhang Y, Yan Y, Wang C, Colic M. et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat Cell Biol. 2023;25:404–414. doi: 10.1038/s41556-023-01091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W. et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10:1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 94.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Muller C, Zandkarimi F. et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B. et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–31. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR. et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22:225–234. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Logie E, Van Puyvelde B, Cuypers B, Schepers A, Berghmans H, Verdonck J. et al. Ferroptosis Induction in Multiple Myeloma Cells Triggers DNA Methylation and Histone Modification Changes Associated with Cellular Senescence. Int J Mol Sci. 2021;22:12234. doi: 10.3390/ijms222212234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang H, Hu Y, Weng M, Liu X, Wan P, Hu Y. et al. Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J Adv Res. 2022;37:91–106. doi: 10.1016/j.jare.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parry A, Rulands S, Reik W. Active turnover of DNA methylation during cell fate decisions. Nat Rev Genet. 2021;22:59–66. doi: 10.1038/s41576-020-00287-8. [DOI] [PubMed] [Google Scholar]
- 101.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 102.Papanicolau-Sengos A, Aldape K. DNA Methylation Profiling: An Emerging Paradigm for Cancer Diagnosis. Annu Rev Pathol. 2022;17:295–321. doi: 10.1146/annurev-pathol-042220-022304. [DOI] [PubMed] [Google Scholar]
- 103.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–33. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA. et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–3. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dor Y, Cedar H. Principles of DNA methylation and their implications for biology and medicine. Lancet. 2018;392:777–786. doi: 10.1016/S0140-6736(18)31268-6. [DOI] [PubMed] [Google Scholar]
- 107.Wu X, Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet. 2017;18:517–534. doi: 10.1038/nrg.2017.33. [DOI] [PubMed] [Google Scholar]
- 108.Liu Z, Zhao Q, Zuo ZX, Yuan SQ, Yu K, Zhang Q. et al. Systematic Analysis of the Aberrances and Functional Implications of Ferroptosis in Cancer. iScience. 2020;23:101302. doi: 10.1016/j.isci.2020.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhong H, Wang Y, Jia J, Yang H, Zhang H, Li T. et al. Ferroptosis related genes are regulated by methylation and predict the prognosis of glioblastoma patients. Transl Cancer Res. 2022;11:603–614. doi: 10.21037/tcr-21-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo W, Ma S, Zhang Y, Liu H, Li Y, Xu JT. et al. Genome-wide methylomic analyses identify prognostic epigenetic signature in lower grade glioma. J Cell Mol Med. 2022;26:449–461. doi: 10.1111/jcmm.17101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo W, Wang X, Wang Y, Zhu S, Zhu R, Zhu L. Identification and Validation of Ferroptosis-Related DNA Methylation Signature for Predicting the Prognosis and Guiding the Treatment in Cutaneous Melanoma. Int J Mol Sci. 2022;23:15677. doi: 10.3390/ijms232415677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang X, Meng Y, Liu C, Yang H, Zhou S. A Novel Prognosis Signature Based on Ferroptosis-Related Gene DNA Methylation Data for Lung Squamous Cell Carcinoma. J Oncol. 2022;2022:9103259. doi: 10.1155/2022/9103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Horniblow RD, Pathak P, Balacco DL, Acharjee A, Lles E, Gkoutos G. et al. Iron-mediated epigenetic activation of NRF2 targets. J Nutr Biochem. 2022;101:108929. doi: 10.1016/j.jnutbio.2021.108929. [DOI] [PubMed] [Google Scholar]
- 114.Siddiq A, Aminova LR, Troy CM, Suh K, Messer Z, Semenza GL. et al. Selective inhibition of hypoxia-inducible factor (HIF) prolyl-hydroxylase 1 mediates neuroprotection against normoxic oxidative death via HIF- and CREB-independent pathways. J Neurosci. 2009;29:8828–38. doi: 10.1523/JNEUROSCI.1779-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang Y, Mao C, Yang R, Yan B, Shi Y, Liu X. et al. EGLN1/c-Myc Induced Lymphoid-Specific Helicase Inhibits Ferroptosis through Lipid Metabolic Gene Expression Changes. Theranostics. 2017;7:3293–3305. doi: 10.7150/thno.19988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spruijt CG, Gnerlich F, Smits AH, Pfaffeneder T, Jansen PW, Bauer C. et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell. 2013;152:1146–59. doi: 10.1016/j.cell.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 117.Xuan Y, Wang H, Yung MM, Chen F, Chan WS, Chan YS. et al. SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells. Theranostics. 2022;12:3534–3552. doi: 10.7150/thno.70194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee JY, Nam M, Son HY, Hyun K, Jang SY, Kim JW. et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci U S A. 2020;117:32433–32442. doi: 10.1073/pnas.2006828117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang X, Huang Z, Xie Z, Chen Y, Zheng Z, Wei X. et al. Homocysteine induces oxidative stress and ferroptosis of nucleus pulposus via enhancing methylation of GPX4. Free Radic Biol Med. 2020;160:552–565. doi: 10.1016/j.freeradbiomed.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 120.Gomaa A, Peng D, Chen Z, Soutto M, Abouelezz K, Corvalan A. et al. Epigenetic regulation of AURKA by miR-4715-3p in upper gastrointestinal cancers. Sci Rep. 2019;9:16970. doi: 10.1038/s41598-019-53174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim DH, Kim WD, Kim SK, Moon DH, Lee SJ. TGF-beta1-mediated repression of SLC7A11 drives vulnerability to GPX4 inhibition in hepatocellular carcinoma cells. Cell Death Dis. 2020;11:406. doi: 10.1038/s41419-020-2618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hasegawa M, Takahashi H, Rajabi H, Alam M, Suzuki Y, Yin L. et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget. 2016;7:11756–69. doi: 10.18632/oncotarget.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koppula P, Lei G, Zhang Y, Yan Y, Mao C, Kondiparthi L. et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat Commun. 2022;13:2206. doi: 10.1038/s41467-022-29905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou F, Chen B. Prognostic significance of ferroptosis-related genes and their methylation in AML. Hematology. 2021;26:919–930. doi: 10.1080/16078454.2021.1996055. [DOI] [PubMed] [Google Scholar]
- 125.Gong Q, Guo Z, Sun W, Du X, Jiang Y, Liu F. CX3CL1 promotes cell sensitivity to ferroptosis and is associated with the tumor microenvironment in clear cell renal cell carcinoma. BMC Cancer. 2022;22:1184. doi: 10.1186/s12885-022-10302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang Y, Hu H, Chen L, Zhang H, Yang J. A new survival model based on ferroptosis-related genes (FRGS) for prognostic prediction in bladder cancer. Actas Urol Esp (Engl Ed) 2022;46:494–503. doi: 10.1016/j.acuroe.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 127.Candido S, Tomasello B, Lavoro A, Falzone L, Gattuso G, Russo A. et al. Bioinformatic analysis of the LCN2-SLC22A17-MMP9 network in cancer: The role of DNA methylation in the modulation of tumor microenvironment. Front Cell Dev Biol. 2022;10:945586. doi: 10.3389/fcell.2022.945586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yan J, Ye G, Shao Y. High expression of the ferroptosis-associated MGST1 gene in relation to poor outcome and maladjusted immune cell infiltration in uterine corpus endometrial carcinoma. J Clin Lab Anal. 2022;36:e24317. doi: 10.1002/jcla.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Du Y, Zhang R, Zhang G, Wu H, Zhan S, Bu N. Downregulation of ELAVL1 attenuates ferroptosis-induced neuronal impairment in rats with cerebral ischemia/reperfusion via reducing DNMT3B-dependent PINK1 methylation. Metab Brain Dis. 2022;37:2763–2775. doi: 10.1007/s11011-022-01080-8. [DOI] [PubMed] [Google Scholar]
- 130.Liao X, Chen J, Luo D, Luo B, Huang W, Xie W. Prognostic value of long non-coding RNA MALAT1 in hepatocellular carcinoma: A study based on multi-omics analysis and RT-PCR validation. Pathol Oncol Res. 2022;28:1610808. doi: 10.3389/pore.2022.1610808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang B, Wang JQ, Tan Y, Yuan R, Chen ZS, Zou C. RNA methylation and cancer treatment. Pharmacol Res. 2021;174:105937. doi: 10.1016/j.phrs.2021.105937. [DOI] [PubMed] [Google Scholar]
- 132.Zhang Q, Liu F, Chen W, Miao H, Liang H, Liao Z. et al. The role of RNA m(5)C modification in cancer metastasis. Int J Biol Sci. 2021;17:3369–3380. doi: 10.7150/ijbs.61439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheung JCT, Deng G, Wong N, Dong Y, Ng SSM. More than a duologue: In-depth insights into epitranscriptomics and ferroptosis. Front Cell Dev Biol. 2022;10:982606. doi: 10.3389/fcell.2022.982606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boulias K, Greer EL. Biological roles of adenine methylation in RNA. Nat Rev Genet. 2023;24:143–160. doi: 10.1038/s41576-022-00534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71:3971–5. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schafer KP. RNA synthesis and processing reactions in a subcellular system from mouse L cells. Hoppe Seylers Z Physiol Chem. 1982;363:33–43. doi: 10.1515/bchm2.1982.363.1.33. [DOI] [PubMed] [Google Scholar]
- 137.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635–46. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luo GZ, MacQueen A, Zheng G, Duan H, Dore LC, Lu Z. et al. Unique features of the m6A methylome in Arabidopsis thaliana. Nat Commun. 2014;5:5630. doi: 10.1038/ncomms6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen J, Wei X, Yi X, Jiang DS. RNA Modification by m(6)A Methylation in Cardiovascular Disease. Oxid Med Cell Longev. 2021;2021:8813909. doi: 10.1155/2021/8813909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guan Q, Lin H, Miao L, Guo H, Chen Y, Zhuo Z. et al. Functions, mechanisms, and therapeutic implications of METTL14 in human cancer. J Hematol Oncol. 2022;15:13. doi: 10.1186/s13045-022-01231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang W, Chen TQ, Fang K, Zeng ZC, Ye H, Chen YQ. N6-methyladenosine methyltransferases: functions, regulation, and clinical potential. J Hematol Oncol. 2021;14:117. doi: 10.1186/s13045-021-01129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qu J, Yan H, Hou Y, Cao W, Liu Y, Zhang E. et al. RNA demethylase ALKBH5 in cancer: from mechanisms to therapeutic potential. J Hematol Oncol. 2022;15:8. doi: 10.1186/s13045-022-01224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shen D, Wang B, Gao Y, Zhao L, Bi Y, Zhang J. et al. Detailed resume of RNA m(6)A demethylases. Acta Pharm Sin B. 2022;12:2193–2205. doi: 10.1016/j.apsb.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fang Z, Mei W, Qu C, Lu J, Shang L, Cao F. et al. Role of m6A writers, erasers and readers in cancer. Exp Hematol Oncol. 2022;11:45. doi: 10.1186/s40164-022-00298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. 2019;20:608–624. doi: 10.1038/s41580-019-0168-5. [DOI] [PubMed] [Google Scholar]
- 146.Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z. et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021;6:74. doi: 10.1038/s41392-020-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qin S, Mao Y, Chen X, Xiao J, Qin Y, Zhao L. The functional roles, cross-talk and clinical implications of m6A modification and circRNA in hepatocellular carcinoma. Int J Biol Sci. 2021;17:3059–3079. doi: 10.7150/ijbs.62767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen J, Fang Y, Xu Y, Sun H. Role of m6A modification in female infertility and reproductive system diseases. Int J Biol Sci. 2022;18:3592–3604. doi: 10.7150/ijbs.69771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fang ZM, Zhang SM, Luo H, Jiang DS, Huo B, Zhong X. et al. Methyltransferase-like 3 suppresses phenotypic switching of vascular smooth muscle cells by activating autophagosome formation. Cell Prolif. 2023;56:e13386. doi: 10.1111/cpr.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ni L, Bai R, Zhou Q, Yuan C, Zhou LT, Wu X. The Correlation between Ferroptosis and m6A Methylation in Patients with Acute Kidney Injury. Kidney Blood Press Res. 2022;47:523–533. doi: 10.1159/000524900. [DOI] [PubMed] [Google Scholar]
- 151.Liu B, Ao S, Tan F, Ma W, Liu H, Liang H. et al. Transcriptomic analysis and laboratory experiments reveal potential critical genes and regulatory mechanisms in sepsis-associated acute kidney injury. Ann Transl Med. 2022;10:737. doi: 10.21037/atm-22-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tang F, Chen L, Gao H, Xiao D, Li X. m(6)A: An Emerging Role in Programmed Cell Death. Front Cell Dev Biol. 2022;10:817112. doi: 10.3389/fcell.2022.817112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xie H, Shi M, Liu Y, Cheng C, Song L, Ding Z. et al. Identification of m6A- and ferroptosis-related lncRNA signature for predicting immune efficacy in hepatocellular carcinoma. Front Immunol. 2022;13:914977. doi: 10.3389/fimmu.2022.914977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zou J, Li Y, Liao N, Liu J, Zhang Q, Luo M. et al. Identification of key genes associated with polycystic ovary syndrome (PCOS) and ovarian cancer using an integrated bioinformatics analysis. J Ovarian Res. 2022;15:30. doi: 10.1186/s13048-022-00962-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu X, Sun Q, Cao Z, Liu W, Zhang H, Xue Z. et al. Identification of RNA N6-methyladenosine regulation in epilepsy: Significance of the cell death mode, glycometabolism, and drug reactivity. Front Genet. 2022;13:1042543. doi: 10.3389/fgene.2022.1042543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hou J, Lu Z, Cheng X, Dong R, Jiang Y, Wu G. et al. Ferroptosis-related long non-coding RNA signature predicts the prognosis of bladder cancer. BMC Cancer. 2022;22:719. doi: 10.1186/s12885-022-09805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li J, Tian X, Nie Y, He Y, Wu W, Lei X. et al. BTBD10 is a Prognostic Biomarker Correlated With Immune Infiltration in Hepatocellular Carcinoma. Front Mol Biosci. 2021;8:762541. doi: 10.3389/fmolb.2021.762541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang D, Zhang L, Zhang Y, Zhang Y, Xu S. A ferroptosis-associated lncRNAs signature predicts the prognosis of hepatocellular carcinoma. Medicine (Baltimore) 2022;101:e29546. doi: 10.1097/MD.0000000000029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhu R, Gao C, Feng Q, Guan H, Wu J, Samant H. et al. Ferroptosis-related genes with post-transcriptional regulation mechanisms in hepatocellular carcinoma determined by bioinformatics and experimental validation. Ann Transl Med. 2022;10:1390. doi: 10.21037/atm-22-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shen H, Xie K, Tian Y, Wang X. N6-methyladenosine writer METTL3 accelerates the sepsis-induced myocardial injury by regulating m6A-dependent ferroptosis. Apoptosis. 2023;28(3-4):514–524. doi: 10.1007/s10495-022-01808-y. [DOI] [PubMed] [Google Scholar]
- 161.Fan Z, Yang G, Zhang W, Liu Q, Liu G, Liu P. et al. Hypoxia blocks ferroptosis of hepatocellular carcinoma via suppression of METTL14 triggered YTHDF2-dependent silencing of SLC7A11. J Cell Mol Med. 2021;25:10197–10212. doi: 10.1111/jcmm.16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ji FH, Fu XH, Li GQ, He Q, Qiu XG. FTO Prevents Thyroid Cancer Progression by SLC7A11 m6A Methylation in a Ferroptosis-Dependent Manner. Front Endocrinol (Lausanne) 2022;13:857765. doi: 10.3389/fendo.2022.857765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun S, Gao T, Pang B, Su X, Guo C, Zhang R. et al. RNA binding protein NKAP protects glioblastoma cells from ferroptosis by promoting SLC7A11 mRNA splicing in an m(6)A-dependent manner. Cell Death Dis. 2022;13:73. doi: 10.1038/s41419-022-04524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu X, Cui J, Wang H, Ma L, Zhang X, Guo W. et al. IGF2BP3 is an essential N(6)-methyladenosine biotarget for suppressing ferroptosis in lung adenocarcinoma cells. Mater Today Bio. 2022;17:100503. doi: 10.1016/j.mtbio.2022.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma L, Zhang X, Yu K, Xu X, Chen T, Shi Y. et al. Targeting SLC3A2 subunit of system X(C)(-) is essential for m(6)A reader YTHDC2 to be an endogenous ferroptosis inducer in lung adenocarcinoma. Free Radic Biol Med. 2021;168:25–43. doi: 10.1016/j.freeradbiomed.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 166.Ding Y, Chen X, Liu C, Ge W, Wang Q, Hao X. et al. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J Hematol Oncol. 2021;14:19. doi: 10.1186/s13045-020-01016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chang MT, Tsai LC, Nakagawa-Goto K, Lee KH, Shyur LF. Phyto-sesquiterpene lactones DET and DETD-35 induce ferroptosis in vemurafenib sensitive and resistant melanoma via GPX4 inhibition and metabolic reprogramming. Pharmacol Res. 2022;178:106148. doi: 10.1016/j.phrs.2022.106148. [DOI] [PubMed] [Google Scholar]
- 168.Ye F, Wu J, Zhang F. METTL16 epigenetically enhances GPX4 expression via m6A modification to promote breast cancer progression by inhibiting ferroptosis. Biochem Biophys Res Commun. 2023;638:1–6. doi: 10.1016/j.bbrc.2022.10.065. [DOI] [PubMed] [Google Scholar]
- 169.Zhang H, Liu J, Zhou Y, Qu M, Wang Y, Guo K. et al. Neutrophil extracellular traps mediate m(6)A modification and regulates sepsis-associated acute lung injury by activating ferroptosis in alveolar epithelial cells. Int J Biol Sci. 2022;18:3337–3357. doi: 10.7150/ijbs.69141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Song Z, Jia G, Ma P, Cang S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021;276:119399. doi: 10.1016/j.lfs.2021.119399. [DOI] [PubMed] [Google Scholar]
- 171.Ye J, Chen X, Jiang X, Dong Z, Hu S, Xiao M. RNA demethylase ALKBH5 regulates hypopharyngeal squamous cell carcinoma ferroptosis by posttranscriptionally activating NFE2L2/NRF2 in an m(6) A-IGF2BP2-dependent manner. J Clin Lab Anal. 2022;36:e24514. doi: 10.1002/jcla.24514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang K, Wang G, Li G, Zhang W, Wang Y, Lin X. et al. m6A writer WTAP targets NRF2 to accelerate bladder cancer malignancy via m6A-dependent ferroptosis regulation. Apoptosis. 2023;28(3-4):627–638. doi: 10.1007/s10495-023-01817-5. [DOI] [PubMed] [Google Scholar]
- 173.Shen M, Guo M, Li Y, Wang Y, Qiu Y, Shao J. et al. m(6)A methylation is required for dihydroartemisinin to alleviate liver fibrosis by inducing ferroptosis in hepatic stellate cells. Free Radic Biol Med. 2022;182:246–259. doi: 10.1016/j.freeradbiomed.2022.02.028. [DOI] [PubMed] [Google Scholar]
- 174.Li R, Wei X, Jiang DS. Protein methylation functions as the posttranslational modification switch to regulate autophagy. Cell Mol Life Sci. 2019;76:3711–3722. doi: 10.1007/s00018-019-03161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yi X, Jiang XJ, Li XY, Jiang DS. Histone methyltransferases: novel targets for tumor and developmental defects. Am J Transl Res. 2015;7:2159–75. [PMC free article] [PubMed] [Google Scholar]
- 176.Michalak EM, Burr ML, Bannister AJ, Dawson MA. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat Rev Mol Cell Biol. 2019;20:573–589. doi: 10.1038/s41580-019-0143-1. [DOI] [PubMed] [Google Scholar]
- 177.Yi X, Jiang X, Li X, Jiang DS. Histone lysine methylation and congenital heart disease: From bench to bedside (Review) Int J Mol Med. 2017;40:953–964. doi: 10.3892/ijmm.2017.3115. [DOI] [PubMed] [Google Scholar]
- 178.Wei X, Yi X, Zhu XH, Jiang DS. Histone methylation and vascular biology. Clin Epigenetics. 2020;12:30. doi: 10.1186/s13148-020-00826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yi X, Zhou Y, Chen Y, Feng X, Liu C, Jiang DS. et al. The Expression Patterns and Roles of Lysyl Oxidases in Aortic Dissection. Front Cardiovasc Med. 2021;8:692856. doi: 10.3389/fcvm.2021.692856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jiang DS, Yi X, Li R, Su YS, Wang J, Chen ML. et al. The Histone Methyltransferase Mixed Lineage Leukemia (MLL) 3 May Play a Potential Role on Clinical Dilated Cardiomyopathy. Mol Med. 2017;23:196–203. doi: 10.2119/molmed.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.He Y, Yi X, Zhang Z, Luo H, Li R, Feng X. et al. JIB-04, a histone demethylase Jumonji C domain inhibitor, regulates phenotypic switching of vascular smooth muscle cells. Clin Epigenetics. 2022;14:101. doi: 10.1186/s13148-022-01321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li R, Yi X, Wei X, Huo B, Guo X, Cheng C. et al. EZH2 inhibits autophagic cell death of aortic vascular smooth muscle cells to affect aortic dissection. Cell Death Dis. 2018;9:180. doi: 10.1038/s41419-017-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen TQ, Hu N, Huo B, Masau JF, Yi X, Zhong XX. et al. EHMT2/G9a Inhibits Aortic Smooth Muscle Cell Death by Suppressing Autophagy Activation. Int J Biol Sci. 2020;16:1252–1263. doi: 10.7150/ijbs.38835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yi X, Jiang XJ, Fang ZM. Histone methyltransferase SMYD2: ubiquitous regulator of disease. Clin Epigenetics. 2019;11:112. doi: 10.1186/s13148-019-0711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT. et al. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–10. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- 186.Wang G, Long J, Gao Y, Zhang W, Han F, Xu C. et al. SETDB1-mediated methylation of Akt promotes its K63-linked ubiquitination and activation leading to tumorigenesis. Nat Cell Biol. 2019;21:214–225. doi: 10.1038/s41556-018-0266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem. 2010;79:155–79. doi: 10.1146/annurev.biochem.78.070907.103946. [DOI] [PubMed] [Google Scholar]
- 188.Manni W, Jianxin X, Weiqi H, Siyuan C, Huashan S. JMJD family proteins in cancer and inflammation. Signal Transduct Target Ther. 2022;7:304. doi: 10.1038/s41392-022-01145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang S, Liu M, Yao Y, Yu B, Liu H. Targeting LSD1 for acute myeloid leukemia (AML) treatment. Pharmacol Res. 2021;164:105335. doi: 10.1016/j.phrs.2020.105335. [DOI] [PubMed] [Google Scholar]
- 190.Zhang X, Wang X, Wu T, Yin W, Yan J, Sun Y. et al. Therapeutic potential of targeting LSD1/ KDM1A in cancers. Pharmacol Res. 2022;175:105958. doi: 10.1016/j.phrs.2021.105958. [DOI] [PubMed] [Google Scholar]
- 191.Hoogewerf PE, Hislop TG, Morrison BJ, Burns SD, Sizto R. Patient compliance with screening for fecal occult blood in family practice. CMAJ. 1987;137:195–8. [PMC free article] [PubMed] [Google Scholar]
- 192.Bhat KP, Umit Kaniskan H, Jin J, Gozani O. Epigenetics and beyond: targeting writers of protein lysine methylation to treat disease. Nat Rev Drug Discov. 2021;20:265–286. doi: 10.1038/s41573-020-00108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang M, Luo H, Yi X, Wei X, Jiang DS. The epigenetic regulatory mechanisms of ferroptosis and its implications for biological processes and diseases. MedComm (2020) 2023;4:e267. doi: 10.1002/mco2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yuan Y, Tang AJ, Castoreno AB, Kuo SY, Wang Q, Kuballa P. et al. Gossypol and an HMT G9a inhibitor act in synergy to induce cell death in pancreatic cancer cells. Cell Death Dis. 2013;4:e690. doi: 10.1038/cddis.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rothammer N, Woo MS, Bauer S, Binkle-Ladisch L, Di Liberto G, Egervari K. et al. G9a dictates neuronal vulnerability to inflammatory stress via transcriptional control of ferroptosis. Sci Adv. 2022;8:eabm5500. doi: 10.1126/sciadv.abm5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang Y, Zhao Y, Wang H, Zhang C, Wang M, Yang Y. et al. Histone demethylase KDM3B protects against ferroptosis by upregulating SLC7A11. FEBS Open Bio. 2020;10:637–643. doi: 10.1002/2211-5463.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sui S, Zhang J, Xu S, Wang Q, Wang P, Pang D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 2019;10:331. doi: 10.1038/s41419-019-1564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Griffin GK, Wu J, Iracheta-Vellve A, Patti JC, Hsu J, Davis T. et al. Epigenetic silencing by SETDB1 suppresses tumour intrinsic immunogenicity. Nature. 2021;595:309–314. doi: 10.1038/s41586-021-03520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu T, Xu P, Ke S, Dong H, Zhan M, Hu Q. et al. Histone methyltransferase SETDB1 inhibits TGF-beta-induced epithelial-mesenchymal transition in pulmonary fibrosis by regulating SNAI1 expression and the ferroptosis signaling pathway. Arch Biochem Biophys. 2022;715:109087. doi: 10.1016/j.abb.2021.109087. [DOI] [PubMed] [Google Scholar]
- 200.Huang X, Yan J, Zhang M, Wang Y, Chen Y, Fu X. et al. Targeting Epigenetic Crosstalk as a Therapeutic Strategy for EZH2-Aberrant Solid Tumors. Cell. 2018;175:186–199. doi: 10.1016/j.cell.2018.08.058. e19. [DOI] [PubMed] [Google Scholar]
- 201.Yoo KH, Hennighausen L. EZH2 methyltransferase and H3K27 methylation in breast cancer. Int J Biol Sci. 2012;8:59–65. doi: 10.7150/ijbs.8.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu GZ, Xu XW, Tao SH, Gao MJ, Hou ZH. HBx facilitates ferroptosis in acute liver failure via EZH2 mediated SLC7A11 suppression. J Biomed Sci. 2021;28:67. doi: 10.1186/s12929-021-00762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yu Y, MohamedAl-Sharani H, Zhang B. EZH2-mediated SLC7A11 upregulation via miR-125b-5p represses ferroptosis of TSCC. Oral Dis. 2023;29:880–891. doi: 10.1111/odi.14040. [DOI] [PubMed] [Google Scholar]
- 204.Xu K, Liu X, Wen B, Liu Y, Zhang W, Hu X. et al. GSK-J4, a Specific Histone Lysine Demethylase 6A Inhibitor, Ameliorates Lipotoxicity to Cardiomyocytes via Preserving H3K27 Methylation and Reducing Ferroptosis. Front Cardiovasc Med. 2022;9:907747. doi: 10.3389/fcvm.2022.907747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cao Y, Luo F, Peng J, Fang Z, Liu Q, Zhou S. KMT2B-dependent RFK transcription activates the TNF-alpha/NOX2 pathway and enhances ferroptosis caused by myocardial ischemia-reperfusion. J Mol Cell Cardiol. 2022;173:75–91. doi: 10.1016/j.yjmcc.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 206.Deng H, Jia S, Tang J, Rong F, Xu C, Chen X. et al. SET7 methylates the deubiquitinase OTUB1 at Lys (122) to impair its binding to E2 enzyme UBC13 and relieve its suppressive role on ferroptosis. J Biol Chem. 2023;299:103054. doi: 10.1016/j.jbc.2023.103054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kato I, Kasukabe T, Kumakura S. Menin-MLL inhibitors induce ferroptosis and enhance the anti-proliferative activity of auranofin in several types of cancer cells. Int J Oncol. 2020;57:1057–1071. doi: 10.3892/ijo.2020.5116. [DOI] [PubMed] [Google Scholar]
- 208.Zhou L, Jia X, Shang Y, Sun Y, Liu Z, Liu J, PRMT1 inhibition promotes ferroptosis sensitivity via ACSL1 upregulation in acute myeloid leukemia. Mol Carcinog. 2023. pp. 1–17. doi: 10.1002/mc.23550. [DOI] [PubMed]
- 209.Jiang K, Yin X, Zhang Q, Yin J, Tang Q, Xu M. et al. STC2 activates PRMT5 to induce radioresistance through DNA damage repair and ferroptosis pathways in esophageal squamous cell carcinoma. Redox Biol. 2023;60:102626. doi: 10.1016/j.redox.2023.102626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gunes Gunsel G, Conlon TM, Jeridi A, Kim R, Ertuz Z, Lang NJ. et al. The arginine methyltransferase PRMT7 promotes extravasation of monocytes resulting in tissue injury in COPD. Nat Commun. 2022;13:1303. doi: 10.1038/s41467-022-28809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ji H, Wang W, Li X, Han X, Zhang X, Wang J. et al. p53: A double-edged sword in tumor ferroptosis. Pharmacol Res. 2022;177:106013. doi: 10.1016/j.phrs.2021.106013. [DOI] [PubMed] [Google Scholar]
- 212.Yang Y, Ma Y, Li Q, Ling Y, Zhou Y, Chu K. et al. STAT6 inhibits ferroptosis and alleviates acute lung injury via regulating P53/SLC7A11 pathway. Cell Death Dis. 2022;13:530. doi: 10.1038/s41419-022-04971-x. [DOI] [PMC free article] [PubMed] [Google Scholar]