ABSTRACT
Biofilm formation begins when bacteria contacting a surface induce cellular changes to become better adapted for surface growth. One of the first changes to occur for Pseudomonas aeruginosa after surface contact is an increase in the nucleotide second messenger 3′,5′-cyclic AMP (cAMP). It has been demonstrated that this increase in intracellular cAMP is dependent on functional type IV pili (T4P) relaying a signal to the Pil-Chp system, but the mechanism by which this signal is transduced remains poorly understood. Here, we investigate the role of the type IV pilus retraction motor PilT in sensing a surface and relaying that signal to cAMP production. We show that mutations in PilT, and in particular those impacting the ATPase activity of this motor protein, reduce surface-dependent cAMP production. We identify a novel interaction between PilT and PilJ, a member of the Pil-Chp system, and propose a new model whereby P. aeruginosa uses its PilT retraction motor to sense a surface and to relay that signal via PilJ to increased production of cAMP. We discuss these findings in light of current T4P-dependent surface sensing models for P. aeruginosa.
IMPORTANCE T4P are cellular appendages that allow P. aeruginosa to sense a surface, leading to the production of cAMP. This second messenger not only activates virulence pathways but leads to further surface adaptation and irreversible attachment of cells. Here, we demonstrate the importance of the retraction motor PilT in surface sensing. We also present a new surface sensing model in P. aeruginosa whereby the T4P retraction motor PilT senses and transmits the surface signal, likely via its ATPase domain and interaction with PilJ, to mediate production of the second messenger cAMP.
KEYWORDS: surface sensing, type IV pili, biofilm, PilT, motor, Pil-Chp, Pseudomonas aeruginosa
INTRODUCTION
Biofilm formation is initiated when free swimming, planktonic cells contact a surface. This contact serves as a signal that must be transmitted across the cell envelope into the cytoplasm to initiate appropriate physiological changes to adapt to the biofilm mode of growth (1). For many bacteria, this initial surface contact is mediated through motility appendages such as type IV pili (T4P) or flagella (2–8). Contact between these appendages and the surface creates forces that are not normally present in planktonic environments and can serve as a “surface signal” to the microbe (9).
Early work on Vibrio parahaemolyticus demonstrated that the signals encountered during surface contact could be mimicked by increasing the load on the flagellum either through changes in viscosity of the medium or by addition of antibodies specific to the flagellum (10, 11). Recent work in Caulobacter crescentus demonstrated that holdfast formation and DNA replication, which normally occurs during surface contact, could be stimulated by increasing the load on Tad pili during retraction. Furthermore, the baseline number of cells with a holdfast without prior pilus obstruction was higher in mutants that were unable to rotate their flagellum (2). Others have demonstrated that the flagellar motor itself is able to sense surface contact to trigger cyclic di-GMP (c-di-GMP [here cdG]) production leading to holdfast synthesis (12). Together, these data indicate that bacteria use their cellular appendages to help sense surface engagement and indicate that impeding the motion (i.e., retraction and/or rotation) of these appendages might serve as the proximal signal for surface engagement.
Pseudomonas aeruginosa also utilizes T4P as well as its polar flagellum to sense and traverse surfaces (3, 13–16). One of the first changes to occur for many organisms upon surface contact is an increase in the second messenger cdG (17). In P. aeruginosa PA14, this initial increase in cdG is produced by the diguanylate cyclase SadC and recent work from our lab and others has shown that SadC activity is regulated by both components of the flagellum and the T4P (3, 18). Prior to an increase in cdG level, P. aeruginosa PA14 increases the level of another second messenger, 3′,5′-cyclic AMP (cAMP) (15).
The surface-dependent increase of cAMP by P. aeruginosa PA14 depends on functional T4P, the Pil-Chp chemotaxis-like system, and the adenylate cyclase CyaB, and to a lesser extent, the adenylate cyclase CyaA. The methyl-accepting chemotaxis protein (MCP) PilJ relays a signal to the kinase ChpA (19). Activation of the system causes ChpA to phosphorylate the response regulator PilG; phosphorylation of PilG as well as FimV and FimL are required to then activate the adenylate cyclases CyaAB to produce cAMP from ATP (20–22). The transcription factor Vfr then binds cAMP and activates genes necessary for further surface adaptation as well as for virulence (23, 24).
Recent work by Yarrington, Limoli, and colleagues shows that the PilJ likely detects phenyl-soluble modulins via its periplasmic domain as a ligand to trigger signaling, a finding that strongly suggests that PilJ can function like a classic MCP (25). Others have recently uncovered the function of PilG and PilH in twitching motility (TM) and surface adaptation (26, 27). In contrast, how surface engagement by T4P triggers cAMP signaling in a PilJ-dependent manner is still an open question. A previous study showed that the ligand binding domain (LBD) of PilJ is not required for surface-dependent cAMP production, although the extent of cAMP induction is significantly reduced relative to the wild type (WT) (28). One model to explain T4P-mediated surface signaling includes interactions between PilA-PilJ via a mechanosensitive change in pilin conformation (4); we recently reported data at odds with this model (29).
P. aeruginosa utilizes the T4P as a cellular grappling hook that pulls the cell along a surface through rounds of pilus extension, surface binding, and pilus retraction (14). Functional pili are also required for sensitivity to infection by the phage DMS3 (30). Extension and retraction are powered by three hexameric ATPases: PilB, PilT, and PilU (31–33). In a recent study from our group, we found that pili on the outside of the cell actively engaging a surface are required for surface-dependent phenotypes, consistent with previous studies (15, 19, 23, 34, 35). Furthermore, we showed that the ability to retract pili with only enough force to allow phage infection was necessary for surface-dependent cAMP production. That is, the force required for twitching motility was not necessary for cAMP signaling (29).
While the PilT and PilU proteins both power retraction of T4P through ATP hydrolysis, these ATPases individually have unique roles in T4P dynamics and surface sensing. PilU is the accessory retraction motor for T4P in P. aeruginosa, whose function is dependent on the presence of PilT (36, 37). Both PilT and PilU hydrolyze ATP to power retraction, but only PilT can interact with the platform protein PilC to coordinate PilA disassembly from extended T4P (32, 37). Others have shown that PilC and PilU can interact via the bacterial adenylate cyclase two-hybrid (B2H) assay, but there is no known functional consequence of this interaction in terms of T4P motility or surface-dependent cAMP production (36, 38). While PilT alone is able to retract pili bound to phage (as judged by phage sensitivity assays), PilU in addition to PilT is required for T4P retraction that can pull the cell body along a surface to power twitching motility (TM) (14, 29, 30). Interestingly, deletion of pilU increases the amount of cAMP produced by P. aeruginosa when grown on a surface, and PilU is the only T4P protein that, when mutated, results in an increased level of this second messenger. To investigate how PilU affects levels of cAMP during surface contact, we generated strains lacking one or both retraction motors and measured the cAMP level via a transcriptional reporter. We found that like phage susceptibility, cAMP production was dependent on the presence of PilT and that overexpression of PilU decreased cAMP levels when grown on a surface.
Since the effects of PilU on cAMP are dependent on PilT, we next explored the role of PilT in surface signaling. We began by characterizing the effect of different PilT mutations on surface-dependent cAMP production during biofilm formation. We found that mutations in PilT affecting ATP binding and hydrolysis affected cAMP production. A B2H screen revealed a novel interaction between PilT and PilJ. We report here a strong relationship between the extent of PilT-PilJ interaction for PilT mutants that are defective in ATPase activity and the magnitude of cAMP signaling. For strains with all T4P proteins, we also find a strong relationship between twitching motility zone size and the extent of cAMP production. We also identify a mutation in PilT that disrupts its interaction with PilJ in a B2H assay in Escherichia coli that does not appear to perturb signaling in P. aeruginosa, suggesting a possible unappreciated level of complexity in PilT-PilJ signaling. Our data are consistent with a model in which PilT senses a surface through tension on the pilus fiber and relays this signal—likely to PilJ—to modulate cAMP production.
RESULTS
PilU levels significantly affect cAMP levels during surface attachment.
To quantify cAMP levels during surface adaptation, the previously reported PaQa cAMP-responsive transcriptional reporter (4) was integrated onto the chromosome of P. aeruginosa PA14. This reporter is composed of two fluorescent proteins, mKate2 and enhanced yellow fluorescent protein (EYFP), under the control of two different promoters, PrpoD and PPaQa, respectively. PPaQa has been shown to be regulated by Vfr in a cAMP dependent manner, and an increase in PPaQa-eyfp expression is correlated with an increase in cAMP (4). PrpoD-mKate2 is used to normalize the EYFP levels for microscopy and used to gate on cells containing the reporter for flow cytometry (4). The PaQa reporter was integrated onto the chromosome at a neutral site of the P. aeruginosa PA14 chromosome by using the mini-CTX1 system (4, 39). We validated this PaQa reporter using a mutant that is defective in cAMP production (ΔcyaAB) and a mutant lacking the phosphodiesterase that degrades cAMP (ΔcpdA), which leads to the accumulation of cAMP (see Fig. S1A and B in the supplemental material). After gating on single cells with PrpoD-mKate2 signal, the mean EYFP intensity was recorded and normalized to the WT signal. After 5 h of surface growth on M8 agar, cells were scraped up and analyzed on a flow cytometer, and as expected, the ΔcyaAB mutant showed reduced levels of the cAMP reporter compared to the WT, while the ΔcpdA mutant showed an increased signal (Fig. S1C).
To better understand the respective contributions of PilT and PilU to surface-mediated cAMP production, we used single and double mutants and a series of phenotypic assays. The absence of PilT phenocopies a ΔpilT ΔpilU double mutant in terms of TM, phage susceptibility, and cAMP response (Fig. 1A and B) (as reported previously by our group [29]). In contrast, a ΔpilU strain retains phage susceptibility due to the presence of PilT but shows an increase in surface-dependent cAMP response (Fig. 1A and B) (15, 29, 33, 36–38, 40). Given that PilU is the only T4P protein whose loss increases the level of surface-dependent cAMP and that this motor can only exert effects through PilT, we reasoned that PilT may be sensing the surface via forces occurring during retraction of surface-bound pili and relaying this signal to the Pil-Chp system, a model we probe in more detail below.
FIG 1.
PilU levels affect surface-dependent cAMP production and T4P-related phenotypes. (A) Images of phage sensitivity plates (top panel) and images of twitch zones stained with crystal violet (bottom panel). “+” indicates a phage-susceptible strain, which forms a clear plaque at the center of the plate where the lysate is spotted, and “–” indicates a phage-resistant strain. Below is the quantification of the twitch zone diameter for each strain. Data are from four biological replicates. (B) Quantification of the PaQa reporter as a surrogate for cAMP levels as measured by flow cytometry after 5 h of growth on agar. Data are from six biological replicates. (C) Quantification of the PaQa reporter as measured by flow cytometry after 5 h of growth for the WT and ΔpilU mutant expressing the pilU gene from a multicopy plasmid or carrying the empty vector (EV) control. Growth was on M8 agar supplemented with 1 mM or no IPTG and the appropriate antibiotics. (D) Quantification of the normalized PilU protein levels of the cells in panel C. Values were normalized to a cross-reacting band. Data are from three biological replicates. Bars and error bars in all panels are the mean and standard deviation, and statistical significance was determined by one-way analysis of variance (ANOVA), followed by Tukey's post hoc test, **, P ≤ 0.001; ***, P ≤ 0.0001; ****, P ≤ 0.00001; ns, not significant.
As mentioned above, PilT appears capable of retracting T4P under low loads like that of a phage bound to the pilus, but is unable to power twitching motility, which requires ATP hydrolysis from both PilT and PilU (36, 40). We believe this to mean that the PilT hexamer is able to undergo conformational changes necessary for ATP hydrolysis while bound to PilC, which in turn causes conformational changes that allow for the disassembly of PilA monomers into the inner membrane (IM) for pili not bound to a surface. When pili are bound to a surface, we propose the tension on each pilus resists the conformational changes in PilC that are necessary for disassembly and that the coordinated hydrolysis of ATP by both retraction motors are necessary to force PilC into the disassembly conformation that was achieved by just PilT for unbound pili. If PilT attempts to retract a T4P filament bound to a surface without PilU present, we hypothesize that the motor would stall and enter a force-induced conformational change, potentially due to improper ATP binding to, ATP hydrolysis of, and/or ADP release from the hexamer (32). This PilT signaling model makes several predictions. First, a ΔpilU strain should show increased cAMP, but only on a surface, a finding we have reported previously (15, 29, 39) and shown here (Fig. 1B). Also, overexpression of PilU (a condition that is the opposite of deleting the pilU gene) should suppress the cAMP response. We predict that by increasing levels of PilU, we will increase the frequency that this motor protein will assist in retraction events during surface contact and in turn lead to less stalled PilT and thus less cAMP.
To test this second prediction, we created the pilU expression plasmid pVLT31-PTAC-pilU and transformed this construct into the WT and ΔpilU mutant backgrounds with the PaQa reporter on the chromosome. cAMP was measured via flow cytometry with pilU expressed from a multicopy plasmid for surface-grown bacteria (Fig. 1C). Western blots were performed, confirming levels of PilU greater than the wild type in all the analyzed strains (Fig. 1D).
All strains harboring the pilU overexpression construct had cAMP levels significantly lower than those with the vector control even in the absence of inducer. In the WT background, excess PilU significantly reduced the amount of cAMP when grown on a surface and the addition of inducer modestly further reduced the level of cAMP. The trends observed in the WT background were also observed in the ΔpilU mutant background. Interestingly, the levels of cAMP production in the WT and ΔpilU backgrounds with the PilU expression construct were both significantly reduced from those of the WT but not significantly different from each other. This observed decrease in surface-dependent cAMP is consistent with a model of PilT acting as a signaling protein during surface contact.
To determine whether the effects of PilU overexpression on cAMP levels are dependent on the ability of this retraction motor to bind and hydrolyze ATP, a PilU-K136A mutant (this mutation is in the Walker A box [WA] of the ATPase domain) was generated using the pVLT31-pilU plasmid and transformed into the WT and ΔpilU backgrounds. After 5 h of surface growth, levels of cAMP were measured using flow cytometry. Interestingly, expression of PilU-WA in the WT and ΔpilU backgrounds significantly lowered cAMP to levels near that of WT pilU (Fig. S2A). Western blots confirmed the presence of stable PilU-WA protein for cells with and without inducer (Fig. S2B). To determine how pilU overexpression affects T4P dynamics, TM assays were performed in strains expressing pilU and pilU-WA. In the WT background, increased levels of PilU and PilU-WA significantly decreased TM. Increased levels of PilU may bias T4P dynamics to a retracted state and limit the ability of PilB to extend T4P as seen with excess PilT (41). While we observe the same result with excess PilU-WA, we interpret this to be due to the reduction of meaningful retraction events since PilU must now compete with PilU-WA within the cell for retracting PilT. Expression of pilU in the ΔpilU background rescued TM, and the size of the twitch zone increased with the addition of inducer. Complementing this background with pilU-WA did not rescue TM, again demonstrating the need for both retraction motors to have ATPase activity to power twitching, and confirming that the PilU-WA was nonfunctional (Fig. S2C). The fact that PilU-WA is able to affect cAMP during surface contact without affecting T4P dynamics in the ΔpilU background indicates that binding of PilT to PilU even in the absence of PilU ATPase activity can affect surface signal transduction. One possible explanation is that PilU and PilJ share a binding interface on PilT, and PilU-WA overexpression blocks signaling to PilJ without aiding in T4P retraction.
To determine whether the increase in cAMP production in the ΔpilU background was due to improper PilT ATP hydrolysis during retraction, we expressed the PilT-K136A variant from a multicopy plasmid in the WT background. PilT-K136A is unable to bind ATP, and we hypothesized that the incorporation of this monomer into the functional hexamer would lead to hexameric conformations similar to those that occur for the WT PilT during pilus retraction in the absence of PilU. We observed a modest and nonsignificant increase in cAMP in strains containing the PilT- K136A expression vector relative to the empty vector control for the WT background (Fig. S3, first 3 bars). While this negative result is difficult to interpret because we do not know if the mutant protein is indeed incorporated into the motor, we believe it does incorporate as others have demonstrated via B2H assay that the PilT-K136A allele can still interact with the WT allele (36), and we observed a significant decrease in TM compared to the WT when expressing PilT-K136A from a multicopy plasmid (Fig. S3B), indicating a dominant-negative effect for PilT-K136A. Together, these data suggest that expression of the PilT-K136A (WA) mutation does not lock the T4P motor in an altered signaling conformation.
Mutations in PilT change the dynamics of surface-dependent cAMP induction.
We previously showed that mutating critical residues in the Walker A and Walker B motifs of PilT, which abolish ATP binding and hydrolysis, respectively, also disrupts the surface-dependent cAMP response (29, 42). To investigate the role of PilT in surface sensing, these mutations, as well as other previously published mutations in the PilT protein of P. aeruginosa that affect PilT’s hexameric structure or retraction dynamics (32, 33, 41, 43), were inserted into the genome of P. aeruginosa PA14 at the gene’s native locus with the PaQa cAMP reporter on the chromosome at the neutral attB site. A list of mutations tested here with their characteristics, either previously published or determined in this report, is summarized in Table 1 and mapped onto the PilT protein structure (Fig. 2A).
TABLE 1.
Mutant alleles characterized in this study
| pilT allele | Phenotypea | Reference(s) |
|---|---|---|
| D31K | Decreased stability, increased twitching, decreased closed conformation, partial phage sensitivity | 32 |
| K58A | Increase in open conformation, increased twitching, increased OOCOOC hexamer conformation | 32 |
| R123D | Decreased stability of OOOOOO and CCCCCC hexameric conformations | 32 |
| K136A (WA) | Cannot bind ATP | 29, 36 |
| E204A (WB) | Cannot hydrolyze ATP | 36 |
| T216R | Eliminates OOOOOO hexameric conformation | 32 |
| H222A | Decreased twitching, phage sensitive, decreased retraction velocity, increase in falling off T4P complex | 33, 41 |
| H229A | No twitching, phage sensitive, decreased ATPase in vitro | 32, 33 |
OOCOOC indicates open, open, closed, open, open, closed for the conformation of the 6 hexamers of PilT. OOOOOO or CCCCCC indicates all open or all closed conformations, respectively, of the 6 hexamers of PilT.
FIG 2.
Characterization of PilT motor mutants. (A) Schematic showing the domain architecture of PilT. Numbers represent the residue number of the beginning and ending of each domain. Below is the three-dimensional (3D) structure of the P. aeruginosa PilT monomer and hexamer (PDB no. 3jVV). (B) Quantification of PilT protein levels via Western blot analysis. The PilT band intensity from whole cells were normalized to a cross-reacting band. Bars and errors bars represent the mean and standard error of the mean (SEM) from 3 biological replicates. Data were analyzed by one-way ANOVA, followed by Tukey’s posttest comparison. *, P < 0.05; **, P < 0.01. Here, and in panel C, strains that were able to twitch at >75% of the WT level are purple bars, strains that twitch at between 25 and 75% of the WT level are blue bars, and strains that twitch at <25% of the WT level are green bars. (C) Assays for T4P function of pilT mutant strains. Images of phage sensitivity plates (top panel) and images of twitch zones stained with crystal violet (bottom panel). “+” denotes a phage-susceptible strain, and “−” denotes a phage-resistant strain as described for Fig. 1. The graph shows the quantification of twitch zone diameters for each strain. Bars and errors bars represent the mean and standard deviation of 4 biological replicates. Data were analyzed by one-way ANOVA, followed by Tukey’s posttest comparison. ****, P < 0.0001.
For these mutants, we assessed protein stability, twitching motility, phage susceptibility, and levels of cAMP when grown on a surface. The stability of each allele was assessed via Western blotting and quantified by densitometry (Fig. 2B). As previously reported, alleles E204A and D31K showed decreased stability (or perhaps reduced antibody binding) (32). We also observed a significant decrease in the level of the protein with the PilT-H229A variant. The other alleles result in PilT levels that were reduced but not significantly different from the level of the WT. The discrepancy in protein stability for some alleles could be due to the fact that previous reports used inducible promoters to express the pilT gene on multicopy plasmids and/or perhaps due to the fact the experiments were performed in the PAO1 strain (32, 33, 41). Here, we produce PilT from a single copy with the gene’s native promoter to preserve endogenous regulation during surface sensing and, furthermore, to not perturb levels of PilU as the pilU gene is located directly downstream of the pilT gene.
To characterize the effects of these mutations on pilus retraction, we performed twitching motility (TM) and phage susceptibility assays (Fig. 2C). Twitching motility requires a fully functional PilT and PilU, while phage susceptibility requires only PilT (29, 44, 45). Despite the decrease in the level of the PilT proteins as measured by Western blotting, all of the indicated PilT variants phenocopied previously published results in terms of twitching motility and phage susceptibility. Mutations that prevent ATP binding or hydrolysis (K136A and E204A [indicated in green in Fig. 2B]) are unable to power TM but remain phage susceptible due to the presence of a functional PilU (29, 36). Mutating the first histidine in the His box reduced TM, while mutating the second histidine completely abolished TM (H222A and H229A [indicated in magenta and blue, respectively, in Fig. 2B]); however, both alleles maintained phage susceptibility (32, 33). Mutations in the N terminus of PilT and mutations that affect the overall hexameric structure of the PilT protein (D31K, K58A, R123D, and T216R [blue and magenta bars in Fig. 2B]) maintained phage susceptibility, but the D31K and R123D mutations exhibited reduced TM (32).
Next, to capture the full dynamics of cAMP signaling during surface attachment, the first 6 h of biofilm formation on the bottom of a glass bottom well was imaged using fluorescence microscopy. The average normalized fluorescent intensity per cell was plotted over time for cells harboring the PaQa reporter on the chromosome (2) (Fig. 3A; Fig. S4). All backgrounds initially start at the same level of intracellular cAMP (i.e., the lower levels associated with planktonic cells) and then begin to differ significantly for the measured cAMP level within the first 2 h. Throughout the time course, the ΔpilT (Fig. 3A) and Walker box mutants (WA, PilT-K136A; WB, PilT-E204A) (Fig. 3A; Fig. S4) maintained the lowest levels of cAMP. A strain carrying the PilT-D31K mutation (Fig. S4) exhibited the greatest level of cAMP among all tested strains early, and then the level decreased after 3 h of growth. The H229A allele maintained an intermediate level of cAMP relative to the other mutants. The remaining PilT alleles converged with the WT allele around h 3 and maintained this trajectory until the end of the experiment, although their cAMP levels differed from the WT level at most time points (Fig. 3A; Fig. S4).
FIG 3.
Measuring cAMP levels in strains carrying mutations in pilT. (A) Graph depicting the average PPaQaYFP/PrpoDmKate per cell of selected strains during the first 6 h of surface attachment in glass well dishes, as described in the text and Materials and Methods. Solid lines represent the mean fluorescence of YFP/mKate per cell, and the shaded region represents the 95% confidence interval. At least 3 biological replicates were performed for each strain. A corresponding plot for all the pilT alleles described in this article can be found in Fig. S4. (B) Cells were grown on an agar surface for 5 h as described in Materials and Methods and then analyzed by flow cytometer to quantify the amount of intracellular cAMP via the PaQa reporter. These values were then normalized by the WT value for that biological replicate. Bars and errors bars represent the mean and standard deviation from 6 biological replicates. Strains that were able to twitch at >75% of the WT level are purple bars, strains that twitch at between 25 and 75% of the WT level are blue bars, and strains that twitch at <25% of the WT level are green bars. Data were analyzed by one-way ANOVA, followed by Tukey’s posttest comparison. *, P < 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001.
We noted that every genetic background that was unable to perform TM showed reduced levels of cAMP relative to the WT (Fig. 3B), with the exception of the ΔpilU mutant as shown above; this mutant background showed an increase in cAMP (Fig. 1B) as previously reported (15, 29, 40). cAMP was lowest in backgrounds that lacked the pilT gene, followed by strains that expressed the Walker A (WA, PilT-K136A) and Walker B (WB, PilT-E204A) alleles of PilT. The PilT-D31K mutation resulted in a decrease in twitch zone and slight decrease in cAMP levels compared to those of the WT. In contrast, PilT-H229A and R213D showed a significant decrease in twitching and cAMP levels. PilT-K58A, H222A, and T216R mutants displayed TM and cAMP not significantly different from WT.
As a control, we measured cAMP levels in liquid-grown cultures in the absence of a surface, and all strains carrying these pilT alleles were not significantly different from the WT (Fig. S5A). As an additional control, we grew the ΔcpdA mutant (15) planktonically as well, and it displayed high levels of cAMP (Fig. S5A). We also showed that the cAMP measured in selected PilT alleles was PilJ dependent, supporting the known role of PilJ in cAMP signaling (Fig. S5B).
Together, these data show that mutations in various domains of the PilT motor alter cAMP signaling of bacteria grown on a surface, and these pilT alleles also impact pilus function by varying the extent of TM while retaining phage sensitivity.
Does PilT need to adopt both an ATP-bound (closed) state and ADP-bound (open) state to support surface-dependent signaling?
Our previous work demonstrated that only the retractive force necessary for phage infection is necessary but not sufficient for surface-dependent cAMP induction (29). This conclusion was reached based on the observation that a ΔpilU mutant is phage susceptible, TM negative, and has a cAMP level above that of the WT strain, while strains expressing the PilT-K136A (Walker A [WA]) and PilT-E204A (Walker B [WB]) mutations in a background with functional PilU are phage susceptible and TM negative but do not induce the cAMP response when grown on a surface (29).
While this observation could be due to a nuanced difference in the force threshold for cAMP induction versus phage infection, the lack of cAMP signaling in these PilT variants could also be due to the fact that these mutations limit the conformations that the PilT motor can adopt as a hexamer. That is, a fully functional PilT hexamer exists as a mixture of ATP- and ADP-bound states, and we hypothesized that a mixture of ATP-bound (closed) and ADP-bound (open) states of PilT might be necessary for cAMP signaling.
Given that the Walker A mutation prevents ATP binding and the Walker B mutation prevents hydrolysis of ATP to ADP (32, 42), based on previous studies (32), locking the hexamer in either a fully unbound or fully ATP-bound state, respectively, might interfere with surface signaling. Furthermore, given the ADP-bound state is structurally similar to the nucleotide free state, we reasoned that we may be able to observe the cAMP response if we expressed both Walker A and B mutants of PilT within the same cell.
To perform this experiment, we transformed a multicopy plasmid expressing the PilT-WA mutation (PBAD-pilT-K136A) in a background expressing the PilT-WB allele (PilT-E204A) integrated at its native locus with the PaQa reporter on the chromosome to measure the cAMP response. As shown in Fig. S3A (last 3 bars), these mutations had no impact on cAMP levels, indicating that locking the PilT motor in these particular mixed conformations does not alter cAMP signaling.
The retraction motor PilT binds to PilJ of the Pil-Chp system.
The data presented so far are consistent with a model wherein PilT is required for the surface-dependent cAMP response. To determine how PilT might be influencing cAMP production, using the bacterial adenylate cyclase two-hybrid system (B2H), we screened for interactions between PilT and members of the Pil-Chp system and found an interaction between PilT and the protein at the top of the Pil-Chp signaling system, PilJ (34) (Fig. 4A and B). As a control we assessed binding between PilJ, the other retraction motor, PilU, and the extension motor, PilB, but did not observe any such interaction (Fig. 4A and B). We also assessed the interactions between PilT and the other components of the Pil-Chp system as well as other proteins that are known to influence surface attachment through T4P (Fig. 5; Fig. S6). We only detected robust interaction between PilT and PilJ, as well as the previously reported interaction between PilU and PilT (38).
FIG 4.
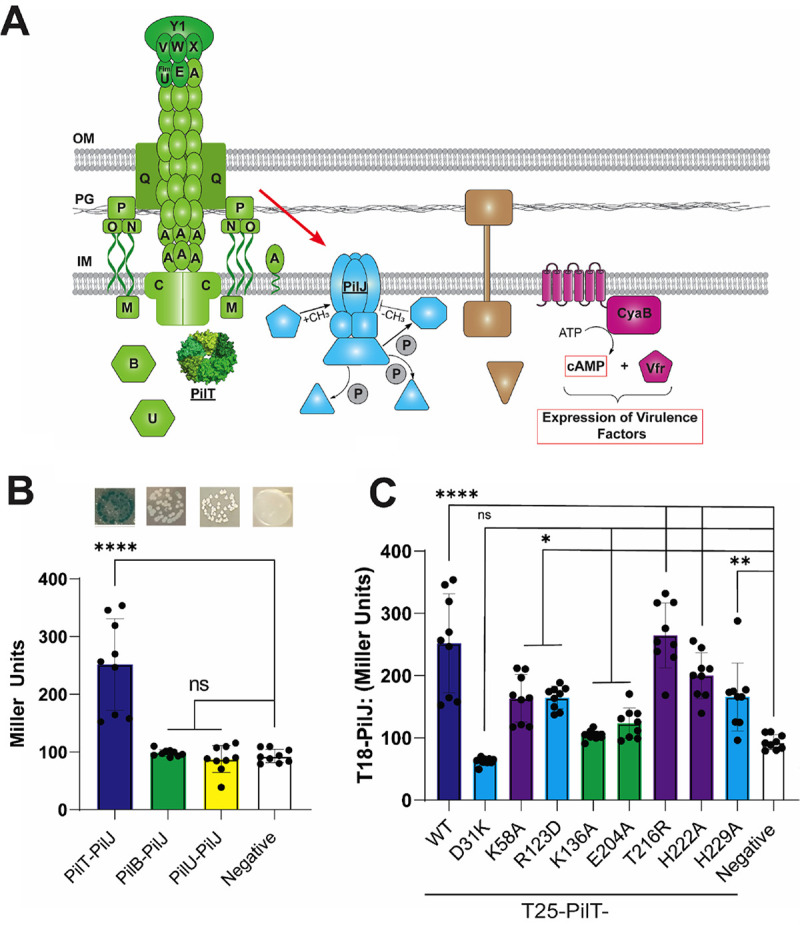
PilT interacts with PilJ. (A) Schematic depicting the components of the TFP and cAMP-signaling pathway; (B) Quantification of the B2H interaction between T4P motor proteins and PilJ in Miller units. Shown are images of B2H colonies plated on X-Gal plates (top of panel) and the interaction quantified (bottom of panel). Bars and errors bars represent the mean and standard deviation from 3 biological replicates. Data were analyzed by one-way ANOVA, followed by Tukey’s posttest comparison. ns, not significant; ****, P < 0.00001. (C) Quantification of the level of interaction between different PilT mutants and PilJ by using the B2H system. Strains that were able to twitch at .75% of the WT level are purple bars, strains that twitch at between 25 and 75% of the WT level are blue bars, and strains that twitch at .25% of the WT level are green bars. Bars and errors bars represent the mean and standard deviation from 3 biological replicates. Data were analyzed by one-way ANOVA, followed by Tukey’s posttest comparison. *, P < 0.05; **, P ≤ 0.01; ****, P < 0.00001.
FIG 5.
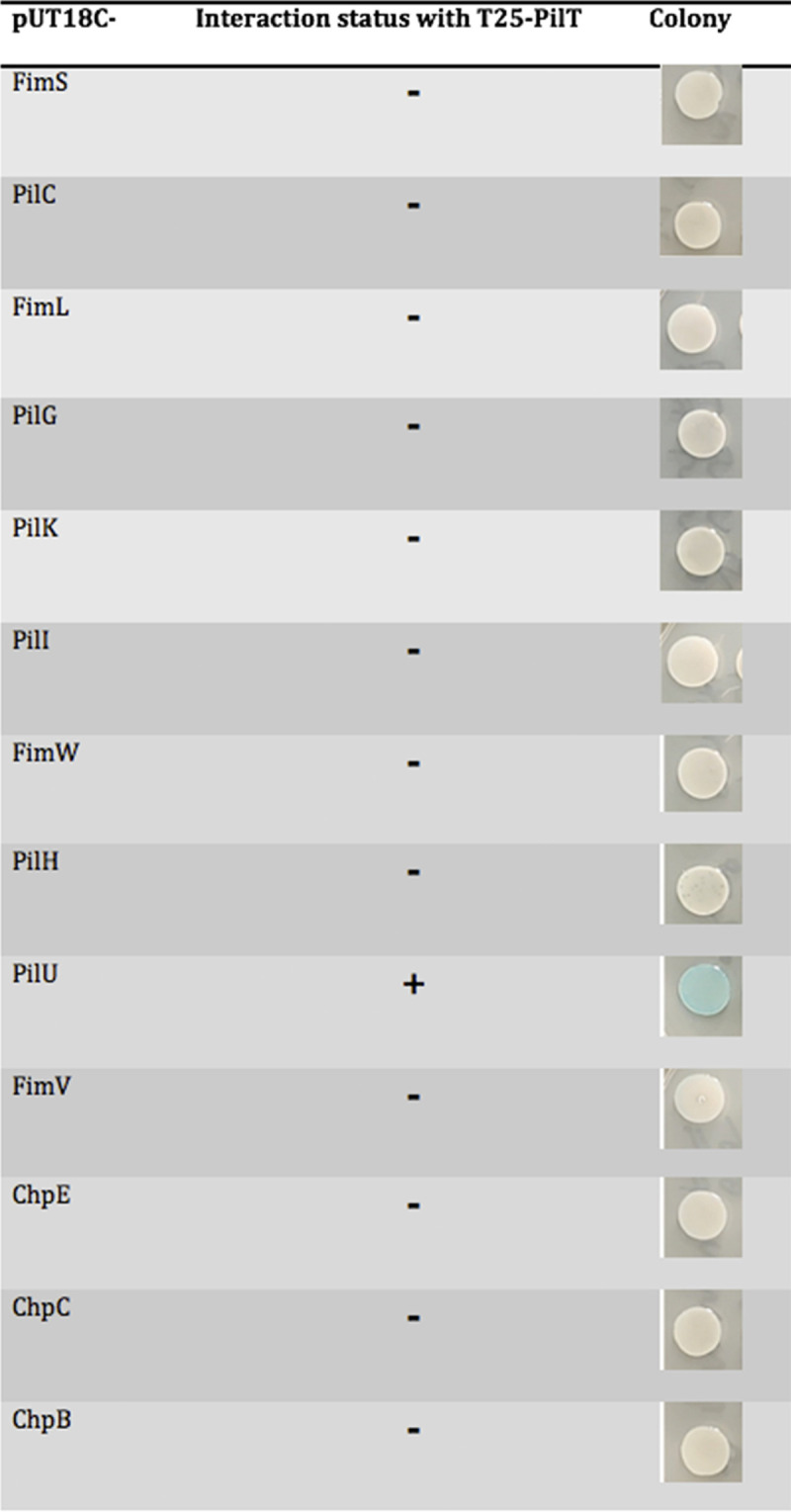
Interaction status with PilT by the B2H assay.
To characterize this interaction with the pilT alleles described above, these mutants were cloned into the B2H system, and the level of interaction with PilJ was measured via β-galactosidase activity. In general, PilT mutants that were able to perform TM and induce a surface-dependent cAMP response had higher levels of interaction with PilJ (Fig. 4C). We explore the consequences of the changes in PilJ-PilT interaction for the PilT variants below.
Associations between PilT-related phenotypes and cAMP signaling.
We next examined associations between cAMP signaling and other measured phenotypes of the PilT variants. We first plotted the diameter of the twitch zone for each PilT allele versus the level of cAMP for surface-grown cells as measured by flow cytometry. Analyzing these data with a linear model, we observed a highly significant, positive correlation between the twitch zone diameter and level of cAMP for strains with a functional PilU (Fig. 6A). While this link between the production of cAMP and twitching motility is well known, the direct relationship between the levels of cAMP and the extent of TM, to our knowledge, has not been previously reported.
FIG 6.
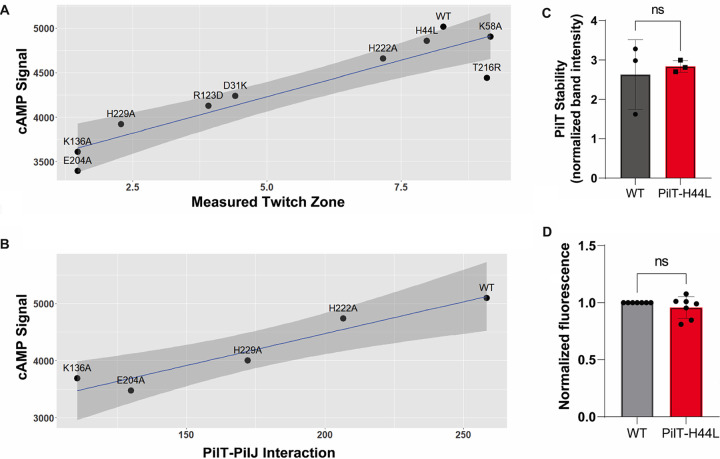
cAMP levels are positively associated with TM and the extent of PilT-PilJ interaction. (A) Linear model depicting the relationship between twitching motility and surface-induced cAMP production. The model was built using data from Fig. 2, 3, and 4 using all tested alleles of pilT (R2 = 0.8565; adjusted R2 = 0.8386; P = 0.0001232). (B) Linear model depicting the relationship between level of PilT-PilJ interaction as measured by the B2H system and surface-induced cAMP production. The model was built using data from the PilT-K136A, PilT-204A, PilT-H222A, and PilT-H229A strains and the WT strain (R2 = 0.9176; adjusted R2 = 0.8902; P = 0.01029). (C) Quantification of PilT level for the PilT-H44L mutant compared to the WT strain. PilT bands were normalized by a cross-reacting band. Bars and errors bars represent the mean and standard deviation from 3 biological replicates compared to the WT. Data are from 3 biological replicates and were analyzed by one-way ANOVA, followed by Tukey’s posttest comparison. ns, not significant. (D) Normalized fluorescence from the PaQa reporter for the WT and PilT-H44L strains. Values were normalized by the WT for each biological replicate. Bars and errors bars represent the mean and standard deviation from 7 biological replicates compared to the WT. Data were analyzed by one-way ANOVA, followed by Tukey’s posttest comparison. ns, not significant.
Importantly, using the data in Fig. 3 and 4, we also observed a positive, significant relationship between cAMP levels and level of interaction between PilJ and PilT mutants that are completely or partially defective in ATPase activity (Fig. 6B) (32).
As a control, using the B2H assay we also quantified the strength of interaction between the PilT mutants shown in Fig. 6 and PilU (Fig. S7A). These values were then used with cAMP data to build a linear model to evaluate the relationship between PilT-PilU interaction strength and cAMP production (Fig. S7B). We did not find any significant relationship between PilT-PilU interaction strength and cAMP levels when analyzing either all the PilT alleles or only those that impact ATPase activity, indicating that the relationship between PilT-ATPase variants and interaction strength with cAMP levels is specific to the PilT-PilJ interaction.
Together, these data indicate that the functional state of the ATPase domain of PilT alters the interaction of this motor protein with the PilJ. Furthermore, this association between interaction strength of these ATPase mutants with PilJ and cAMP signaling suggests a mechanism whereby PilT ATPase activity could be linked to PilJ-mediated cAMP signaling, a possibility we discuss further below.
Isolation of a mutation that disrupts PilJ-PilT interaction in E. coli using a B2H-based screen.
To attempt to further understand the interaction between PilT and PilJ, and to assess the impact of this interaction on the influence of the cAMP response in P. aeruginosa, using a B2H-based assay, we screened for mutants of PilT that were able to perform twitching motility but no longer able to interact with PilJ. A schematic describing the screening process can be found in Fig. S8. Briefly, random mutations were introduced into the pilT sequence using error-prone PCR, and then the mutant library was cloned into the B2H backbone. This pool was then cotransformed with the WT PilJ construct, and we picked white colonies indicating a loss of interaction with PilJ. The PilJ-noninteracting alleles were then pooled and screened for the ability to retract pili by cloning this population of mutant pilT alleles into an expression vector and transforming this pool of mutants into the ΔpilT background of P. aeruginosa. The transformants were then screened for twitching motility. The pilT alleles that were able to twitch and did not interact with PilJ, according to a plate-based B2H assay, were sequenced.
The extent of interaction of PilJ with candidate PilT alleles was then quantified using the B2H assay (Fig. S9A), the stability of the proteins assessed by Western blotting (Fig. S9B), and the mutations were mapped onto the PilT hexameric structure (Fig. S9C). The majority of these mutations mapped to a patch on the surface of the N-terminal domain of the PilT protein.
As mentioned above, these mutant variants of PilT were checked for their stability via Western blotting, and unfortunately, the only stable allele was PilT-H44L (Fig. 6C; Fig. S9B). The P. aeruginosa strain carrying the H44L showed levels of TM similar to the WT, and the strain expressing this allele is phage susceptible (Fig. S9D). The H44 residue maps to the N-terminal domain of PilT and should not impact ATPase activity. Although this allele does not interact with PilJ by B2H in E. coli, it appears to have phenotypes identical to the WT P. aeruginosa for twitching and phage susceptibility, and it produces cAMP levels that are not significantly different from those of the WT (Fig. 6D). These data suggest that the relationship between PilT and PilJ interaction and cAMP signaling may be complex or that, for this allele, the lack of interaction is E. coli specific, points we discuss below.
DISCUSSION
Here, we examine the contribution of the T4P retraction motor PilT to surface sensing and present a model whereby PilT transmits a surface signal to the Pil-Chp system to activate cAMP production upon surface contact. Data presented here and from previous studies indicate that PilT is involved in sensing a surface, promoting at least minimal T4P retraction activity (as judged by phage susceptibility), and that PilT’s role may extend beyond its function in TFP retraction. We show that mutations in PilT that affect its structure and/or T4P-related phenotypes also impact the surface-dependent cAMP response as measured through kinetic and endpoint assays. We also describe a novel interaction between PilT and PilJ of the Pil-Chp system. We then quantified the level of interaction between these PilT variants and PilJ by using a B2H assay. A linear model showed a positive, significant correlation between cAMP level and twitching motility and, importantly, cAMP level and the strength or PilJ-PilT interaction for variants of PilT with known deficiencies in ATPase activity. We interpret the latter finding to mean that the combination of ATP binding and hydrolysis is critical, not only for retraction activity, but also for PilT to bind and transmit a signal to PilJ. Alternatively, PilJ could be transmitting a signal to PilT that affects twitching motility, or alternatively, this interaction could also facilitate localization of PilT to the poles of the cell. However, we believe that the simplest interpretation of our data is that the flow of information is from PilT to PilJ since the interaction strength between the PilT ATPase mutants and PilJ MCP is positively correlated with cAMP level. We did not observe this correlation when examining mutant variants of PilT with full ATPase activity or for the interaction between PilT and PilU. Together, these data support a model whereby the retraction motor PilT participates in sensing the surface during T4P retraction and relays this signal to PilJ in a mechanism that incorporates the PilT ATPase activity.
Any model involving T4P retraction must incorporate both retraction motors PilT and PilU. We and others have shown that loss of PilU results in an increase in surface-dependent cAMP levels while retaining phage sensitivity (15, 29, 40). These data indicate that PilT can facilitate some level of T4P retraction even in the absence of PilU. We believe these data mean that in the absence of PilU, the PilT hexamer is able to undergo conformational changes necessary for ATP hydrolysis while bound to PilC, for unbound pili (or a pilus bound to phage), which in turn results in conformational changes in PilT and/or PilC that allow for the disassembly of PilA monomers into the IM and retraction of the pilus. Consistent with this idea, previous studies have shown that in the absence of PilU, PilT is still able to bind PilC and begin retraction in a manner similar to that of the wild type (46). Furthermore, the two retraction motors do not always localize to the same pole, indicating that PilT and PilU are not necessarily always bound to each other when engaging PilC (33, 47). Thus, even for the WT, there may be instances when PilT is attempting to retract a pilus in the absence of PilU.
In contrast to planktonic cells, when P. aeruginosa is bound to a surface, we propose that the tension on a pilus might prevent the typical conformational changes in PilC and/or the PilT motor that are necessary for disassembly of the pilus. For the WT with a bound T4P, this model proposes that the coordinated hydrolysis of ATP by both retraction motors PilT and PilU is necessary for PilC to attain the needed conformation for T4P disassembly. Indeed, the measured binding force of this pilus to a surface is at or above retraction force of the T4P of P. aeruginosa (48, 49), indicating that the retraction motors operate at the cusp of their ability to unbind a pilus from the surface. That is, it is difficult for P. aeruginosa to pull a pilus off the surface to which it is attached. Finally, we propose that during retraction, PilT may undergo a force-induced conformational change, perhaps through stalling of the motor (i.e., an incomplete cycle of ATP binding, ATP hydrolysis and/or ADP release) while attempting to retract a bound pilus, in turn transmitting a signal of surface engagement to the Pil-Chp system via the PilT-PilJ interaction. This conformational change may occur even when PilU is present for a surface-engaged cell with bound pili, but the observation that loss of PilU results in an increase in cAMP levels suggests that PilT is more likely to attain (or less likely to leave) a signaling conformation in the absence of its accessory motor, whether PilU is absent via mutation or for instances when PilU is not complexed with PilT in the WT. The fact that PilU-WA, which is unable to bind ATP and restore TM in a ΔpilU background, still reduces cAMP to levels similar to those of the functional PilU suggests that PilU and PilJ may share a binding interface on the N terminus of PilT.
Our model makes several predictions that have been confirmed in previous publications (15, 29, 40) and this study. First, we demonstrated that cAMP levels when grown on a surface are dependent on the presence of PilT and PilJ. Second, the absence of PilU leads to elevated cAMP levels, a finding made by others and confirmed here (15, 29, 40). Finally, our data show that excess PilU, has the opposite effect, reducing the surface-dependent cAMP response.
We also observed a strong, positive correlation between twitching ability as measured through twitch zone diameter and cAMP production for multiple mutants when analyzed with a linear model. This finding is consistent with previous studies that demonstrated that twitching motility requires cAMP production and that, at the single-cell level, oscillations of T4P activity and cAMP level are highly correlated (40). While cAMP production is dependent on T4P activity, this second messenger activates a positive-feedback loop whereby Vfr and cAMP positively regulate FimS-AlgR, which in turn leads to increased expression of minor pilins and the number of active T4P complexes per cell. This positive regulatory loop is part of the rapid surface adaptation response of P. aeruginosa, and we now know that part of this signaling cascade is initiated by the retraction motor PilT. Thus, PilT appears to play integrated roles in surface sensing and the control of TM in response to surface inputs.
To complement our candidate mutant approach for studying the PilT-PilJ interaction, we performed a genetic screen to identify PilT variants that could promote TM despite a defect in interaction with PilJ. This screen yielded two interesting findings. First, most of the mutations mapped to a surface-exposed region on the N terminus of PilT. The N terminus of PilT binds to the C terminus of the adjacent monomer to form the hexamer. This interface also undergoes the major conformational change during ATP binding and also makes contacts that facilitate open and closed motor conformations. Unfortunately, most of these PilT variants were unstable, and thus we could not unwind whether the lack of signaling was due to reduced levels of the PilT protein or the inability of these mutant proteins to interact with PilJ.
Using a B2H assay, we did isolate one allele of pilT that was able to perform TM but did not interact with PilJ in E. coli; for this allele (H44L), we still observed WT surface-dependent cAMP production in P. aeruginosa. This mutation was near the N-terminal region of PilT and not in proximity to any parts of the protein thought to contribute to its ATPase activity. Consistent with this idea, this allele was able to perform TM at a level similar to that of WT, indicating that this mutant variant does not have a defect in its ATPase activity. A similar phenotype was observed for the PilT-D31K allele, which retains ATPase activity (32). This PilT-D31K allele, which also maps to the N terminus of PilT, was able to perform TM at WT levels and induce cAMP production when on a surface, but had very low levels of interaction with PilJ when measured through the B2H assay in E. coli. Thus, it is possible that mutations at the N terminus of PilT can impact its ability to interact with PilJ in the B2H, but perhaps not impact PilT-PilJ interaction when this allele is expressed in P. aeruginosa in the presence of the rest of the T4P machinery or in the context of the hexamer. We believe it is important to acknowledge that while our findings here allow us to posit a model connecting T4P to surface sensing and cAMP signaling, we still lack key pieces of information to build a model that explains all of the current data. We look forward to interrogating our model further.
We believe our findings are consistent with previous studies, as T4P motors as signaling proteins is not unprecedented. For example, Myxococcus xanthus requires T4P for exopolysaccharide (EPS) production as well as a type of surface-based motility known as S-motility. Researchers performed a suppressor screen for EPS production in a T4P-deficient background. Mutations in the T4P assembly ATPase PilB were isolated that led to the production of EPS without S-motility. A Walker A mutation in PilB phenocopied this mutation and was dominant over the WT allele (50). Consistent with our studies of T4P/PilY1 regulating surface-dependent cdG signaling (3), these data link a T4P and motor function to second messenger signaling and may represent a more general strategy whereby T4P (and perhaps members of other pilus families) serve double duty as adhesins and signal transduction machinery.
Others have suggested that the surface signal is transmitted from the T4P pilin, PilA, to PilJ to activate cAMP production (4). Although we have previously shown no correlation between PilA-PilJ binding strength and cAMP production (29), this does not exclude a role for PilA signaling through a different mechanism. Regardless of the extent of signaling through PilA, the pilin remains a critical part of our motor signaling model, as the motor relies on the presence of a pilus fiber to extend and bind to the surface to create tension during PilT-mediated retraction. Overall, we have presented evidence for a new model of surface signal sensing and transduction through the T4P motor PilT, and we will continue to investigate the mechanism by which this signaling occurs.
MATERIALS AND METHODS
Strains and media.
Pseudomonas aeruginosa UCBPP PA14 was used as the WT strain. Mutations were made in this background using E. coli S17-1 λpir. E. coli BTH101 was used for bacterial adenylate cyclase two-hybrid assays. The strains used in this study are listed in Table S1 in the supplemental material. Bacterial strains were routinely cultured in 5 mL of lysogeny broth (LB) medium or plated on 1.5% agar with antibiotics when necessary. Tetracycline (Tet) was used at 15 μg/mL for E. coli and 120 μg/mL during P. aeruginosa selection and maintained with 75 μg/mL. Gentamicin (Gm) was used at 30 μg/mL for P. aeruginosa and 10 μg/mL for E. coli. Carbenicillin (Cb) was used at 250 μg/mL for P. aeruginosa and 100 μg/mL for E. coli. Kanamycin (Kan) was used at 50 μg/mL for E. coli. M8 minimal salts medium supplemented with MgSO4 (1 mM), glucose (0.2%), and Casamino Acids (0.5%) was used for all assays (51). Plasmids were induced with 0.2% arabinose for PBAD promoter induction, or 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to agar or liquid medium for PTAC promoter induction unless otherwise stated. β-Galactosidase activity from B2H assays was visualized using plates supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 40 μg/mL).
Construction of mutant strains and plasmids.
The plasmids used in this study are listed in Table S2, and the primers are listed in Table S3. Plasmids were constructed using Gibson assembly of purified PCR products. Chromosomal mutations were made using homologous recombination with the pMQ30 vector. Insertions at neutral sites in the P. aeruginosa genome were made using the mini-Tn7 vector (52, 53) and the mini-CTX1 vector (39). Resistance markers were removed using the pFLP2 plasmid, followed by sucrose counterselection (39). Point mutations were generated using QuikChange site-directed mutagenesis, followed by Gibson assembly. Expression vectors were generated using Gibson assembly of purified PCR products into pMQ72 or pVLT31 and then transformed into P. aeruginosa or E. coli by electroporation.
Twitching motility.
Twitching motility plates were made using M8 medium supplemented with Casamino Acids, MgSO4, glucose, and 1% agar. Plates were inoculated from liquid cultures using a sterile toothpick plunged through the agar to the bottom of the plate. Plates were incubated for 24 h at 37°C and then 24 h at room temperature. The agar was then removed, and the twitch zones were stained with 0.1% crystal violet. Images were obtained, and the twitch zone diameter was measured twice using a ruler.
Phage plaque assay.
Phage susceptibility assays were performed in 60- by 15-mm plates using M8 medium supplemented with Casamino Acids, MgSO4, glucose, and 1% agar. One milliliter of 0.5% M8 molten agar was then inoculated with 50 μL from a P. aeruginosa overnight culture. This mixture was poured over the solidified 1% M8 agar to form a bacterial lawn. After solidifying, 2 μL of phage DMS3vir lysate was pipetted onto the bacterial lawn and incubated for 24 h at 37°C.
Bacterial adenylate cyclase two-hybrid assays.
The B2H system from Euromedex (54) was used to assess protein-protein interactions in E. coli BTH101. Alleles of pilT and other T4P proteins were cloned into the pKT25 vector, and PilJ and other Pil-Chp proteins were cloned into the pUT18 and pUT18C vectors. A pair of pKT25 and pUT18/UT18C vectors were then cotransformed into E. coli BTH101. To visualize the interaction, transformants were plated on LB agar containing Cb, Kan, X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (40 g/mL) and IPTG (isopropyl-d-thiogalactopyranoside) (0.5 mM) and incubated at 30C until an interaction was observed through the transformation of X-Gal to a blue pigment or until the negative control began to produce a blue pigment. To quantify the level of interaction between proteins, transformants were plated on LB agar with Cb, Kan, and IPTG. After incubation at 30C, cells were harvested and β-galactosidase assays were performed as previously described (54).
Protein detection and quantification.
Strains were grown in M8 liquid medium supplemented with arabinose or IPTG and grown at 37°C for 6 h. Whole-cell lysates were prepared as previously described (55). Cultures were normalized to an optical density (OD) of 1, and an equal volume was resolved on either a 12% or 10% polyacrylamide gel. Proteins were then transferred to a nitrocellulose membrane and probed with either anti-PilT or anti-PilU antiserum. Detection of proteins was performed using fluorescence detection with IRDye-labeled fluorescent secondary antibodies and imaged using the Odyssey CLx Imager (LICOR Biosciences, Inc., Lincoln, NE). Quantification of protein bands was performed using Image Studio Lite software (LICOR Biosciences, Inc., Lincoln, NE). Protein levels were then normalized by a cross-reacting band.
Flow cytometry measurements.
Bacterial strains harboring the PaQa reporter on the chromosome were subcultured into liquid M8 medium supplemented with glucose, Casamino Acids, and MgSO4 and incubated at 37°C until an OD of 0.5 was reached (~3 h). Gm, Tet, IPTG, or arabinose was added to the liquid medium when indicated. Two hundred microliters of the culture was then spread onto M8 agar plates and allowed to incubate for 5 h at 37°C. Cells were then harvested from these plates, washed, diluted, and analyzed on a Beckman Coulter Cytoflex S. FlowJo software v.10.8.1 was used to gate on populations of single cells that had mKate fluorescence. The EYFP fluorescence from the PPaQa promoter was then measured on the gated population. A workflow of the gating strategy can be found in Fig. S10 in the supplemental material. For plots reporting the normalized fluorescent intensity, the average PPaQa-eyfp value for each gated mutant subpopulation was normalized by the wild-type value for that biological replicate.
Microscopy experiments using 8-well dishes.
Bacterial strains were subcultured in liquid M8 supplemented with glucose, MgSO4, and Casamino Acids after being cultured overnight in liquid LB at 37°C. After reaching an OD600 of ~0.5, cultures were diluted 1:100 into fresh, liquid M8 and then 300 μL was used to fill a glass bottom chamber (Cellvis 8 chambered cover glass system). The chamber was then mounted on a Nikon Ti Eclipse epifluorescence microscope in an environmental chamber set to 37°C. Images of at least 3 fields of view (FOV) were taken every 5 min for the first 8 h of surface attachment. Images were then analyzed using a python script, which can be found at https://github.com/GeiselBiofilm.
Statistical analysis.
Data visualization and statistical analysis were performed in GraphPad Prism 9 (v.9.2.0). Linear mixed models were built in R (v.4.0.2) and visualized using ggplot2 (v.3.3.2). The script used to perform the analysis can be found at https://github.com/GeiselBiofilm.
Data availability.
All code is available on GitHub at https://github.com/GeiselBiofilm.
ACKNOWLEDGMENTS
We thank Katie Forest and Lori Burrows for helpful discussions, Lori Burrows for providing antibodies, Tom Hampton for help with linear modeling, Zdenek Svindrych for help with microscopy, Ko-Wei Liu, Matthew James, Stacie Stutt, and Gary Ward for help with flow cytometry, and Sherry Kuchma, Shanice Webster, Berenike Maier, and Amruta Karbelkar for helpful discussions. We also thank Lori Burrows and Nathan Roberge for critical feedback on the manuscript. The initiation of this project was inspired by a conversation with Z. Yang during a seminar visit to VTU.
This work was funded by the NIH (RO1 R01AI143730 to GAO) and BioMT through NIH NIGMS grant P20-GM113132.
Footnotes
Supplemental material is available online only.
Contributor Information
G. A. O’Toole, Email: georgeo@dartmouth.edu.
Michael Y. Galperin, NCBI, NLM, National Institutes of Health
Ankur Dalia, Indiana University Bloomington.
Joshua Shrout, University of Notre Dame.
REFERENCES
- 1.O'Toole GA, Wong GC. 2016. Sensational biofilms: surface sensing in bacteria. Curr Opin Microbiol 30:139–146. doi: 10.1016/j.mib.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellison CK, Kan J, Dillard RS, Kysela DT, Ducret A, Berne C, Hampton CM, Ke Z, Wright ER, Biais N, Dalia AB, Brun YV. 2017. Obstruction of pilus retraction stimulates bacterial surface sensing. Science 358:535–538. doi: 10.1126/science.aan5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster SS, Lee CK, Schmidt WC, Wong GCL, O’Toole GA. 2021. Interaction between the type 4 pili machinery and a diguanylate cyclase fine-tune c-di-GMP levels during early biofilm formation. Proc Natl Acad Sci USA 118:e2105566118. doi: 10.1073/pnas.2105566118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Persat A, Inclan YF, Engel JN, Stone HA, Gitai Z. 2015. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 112:7563–7568. doi: 10.1073/pnas.1502025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Utada AS, Bennett RR, Fong JCN, Gibiansky ML, Yildiz FH, Golestanian R, Wong GCL. 2014. Vibrio cholerae use pili and flagella synergistically to effect motility switching and conditional surface attachment. Nat Commun 5:4913. doi: 10.1038/ncomms5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad JC, Gibiansky ML, Jin F, Gordon VD, Motto DA, Mathewson MA, Stopka WG, Zelasko DC, Shrout JD, Wong GC. 2011. Flagella and pili-mediated near-surface single-cell motility mechanisms in P. aeruginosa. Biophys J 100:1608–1616. doi: 10.1016/j.bpj.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schniederberend M, Williams JF, Shine E, Shen C, Jain R, Emonet T, Kazmierczak BI. 2019. Modulation of flagellar rotation in surface-attached bacteria: a pathway for rapid surface-sensing after flagellar attachment. PLoS Pathog 15:e1008149. doi: 10.1371/journal.ppat.1008149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibiansky ML, Conrad JC, Jin F, Gordon VD, Motto DA, Mathewson MA, Stopka WG, Zelasko DC, Shrout JD, Wong GCL. 2010. Bacteria use type IV pili to walk upright and detach from surfaces. Science 330:197. doi: 10.1126/science.1194238. [DOI] [PubMed] [Google Scholar]
- 9.Dufrêne YF, Persat A. 2020. Mechanomicrobiology: how bacteria sense and respond to forces. Nat Rev Microbiol 18:227–240. doi: 10.1038/s41579-019-0314-2. [DOI] [PubMed] [Google Scholar]
- 10.McCarter L, Hilmen M, Silverman M. 1988. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell 54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 11.McCarter L, Silverman M. 1990. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol Microbiol 4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 12.Hug I, Deshpande S, Sprecher KS, Pfohl T, Jenal U. 2017. Second messenger-mediated tactile response by a bacterial rotary motor. Science 358:531–534. doi: 10.1126/science.aan5353. [DOI] [PubMed] [Google Scholar]
- 13.Burrows LL. 2012. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu Rev Microbiol 66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 14.Skerker JM, Berg HC. 2001. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci USA 98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y, Zhao K, Baker AE, Kuchma SL, Coggan KA, Wolfgang MC, Wong GCL, O’Toole GA. 2015. A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. mBio 6:e02456-14. doi: 10.1128/mBio.02456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laventie B-J, Sangermani M, Estermann F, Manfredi P, Planes R, Hug I, Jaeger T, Meunier E, Broz P, Jenal U. 2019. A surface-induced asymmetric program promotes tissue colonization by Pseudomonas aeruginosa. Cell Host Microbe 25:140–152.e6. doi: 10.1016/j.chom.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Jenal U, Reinders A, Lori C. 2017. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol 15:271–284. doi: 10.1038/nrmicro.2016.190. [DOI] [PubMed] [Google Scholar]
- 18.Baker AE, Webster SS, Diepold A, Kuchma SL, Bordeleau E, Armitage JP, O’Toole GA. 2019. Flagellar stators stimulate c-di-GMP production by Pseudomonas aeruginosa. J Bacteriol 201:e00741-18. doi: 10.1128/JB.00741-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulcher NB, Holliday PM, Klem E, Cann MJ, Wolfgang MC. 2010. The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity. Mol Microbiol 76:889–904. doi: 10.1111/j.1365-2958.2010.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inclan YF, Huseby MJ, Engel JN. 2011. FimL regulates cAMP synthesis in Pseudomonas aeruginosa. PLoS One 6:e15867. doi: 10.1371/journal.pone.0015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inclan YF, Persat A, Greninger A, Von Dollen J, Johnson J, Krogan N, Gitai Z, Engel JN. 2016. A scaffold protein connects type IV pili with the Chp chemosensory system to mediate activation of virulence signaling in Pseudomonas aeruginosa. Mol Microbiol 101:590–605. doi: 10.1111/mmi.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silversmith RE, Wang B, Fulcher NB, Wolfgang MC, Bourret RB. 2016. Phosphoryl group flow within the Pseudomonas aeruginosa Pil-Chp chemosensory system. J Biol Chem 291:17677–17691. doi: 10.1074/jbc.M116.737528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfgang MC, Lee VT, Gilmore ME, Lory S. 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev Cell 4:253–263. doi: 10.1016/s1534-5807(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs EL, Brutinel ED, Jones AK, Fulcher NB, Urbanowski ML, Yahr TL, Wolfgang MC. 2010. The Pseudomonas aeruginosa Vfr regulator controls global virulence factor expression through cyclic AMP-dependent and -independent mechanisms. J Bacteriol 192:3553–3564. doi: 10.1128/JB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarrington KD, Shendruk TN, Limoli DH. 2022. Twitching cells use a chemoreceptor to detect bacterial competitors. bioRxiv https://www.biorxiv.org/content/10.1101/2022.11.28.518211v2.
- 26.Kühn MJ, Talà L, Inclan YF, Patino R, Pierrat X, Vos I, Al-Mayyah Z, Macmillan H, Negrete J, Engel JN, Persat A. 2021. Mechanotaxis directs Pseudomonas aeruginosa twitching motility. Proc Natl Acad Sci USA 118:e2101759118. doi: 10.1073/pnas.2101759118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kühn MJ, Macmillan H, Talà L, Inclan Y, Patino R, Pierrat X, Al-Mayyah Z, Engel JN, Persat A. 2023. Two antagonistic response regulators control Pseudomonas aeruginosa polarization during mechanotaxis. EMBO J 42:e112165. doi: 10.15252/embj.2022112165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansari VH, Potharla VY, Riddell GT, Bardy SL. 2016. Twitching motility and cAMP levels: signal transduction through a single methyl-accepting chemotaxis protein. FEMS Microbiol Lett 363:fnw119. doi: 10.1093/femsle/fnw119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuchma SL, O'Toole GA. 2022. Surface-induced camp signaling requires multiple features of the Pseudomonas aeruginosa type IV pili. J Bacteriol 204:e00186-22. doi: 10.1128/jb.00186-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budzik JM, Rosche WA, Rietsch A, O'Toole GA. 2004. Isolation and characterization of a generalized transducing phage for Pseudomonas aeruginosa strains PAO1 and PA14. J Bacteriol 186:3270–3273. doi: 10.1128/JB.186.10.3270-3273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCallum M, Tammam S, Khan A, Burrows LL, Howell PL. 2017. The molecular mechanism of the type IVa pilus motors. Nat Commun 8:15091. doi: 10.1038/ncomms15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCallum M, Benlekbir S, Nguyen S, Tammam S, Rubinstein JL, Burrows LL, Howell PL. 2019. Multiple conformations facilitate PilT function in the type IV pilus. Nat Commun 10:5198. doi: 10.1038/s41467-019-13070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang P, Sampaleanu LM, Ayers M, Pahuta M, Howell PL, Burrows LL. 2008. Functional role of conserved residues in the characteristic secretion NTPase motifs of the Pseudomonas aeruginosa type IV pilus motor proteins PilB, PilT and PilU. Microbiology (Reading) 154:114–126. doi: 10.1099/mic.0.2007/011320-0. [DOI] [PubMed] [Google Scholar]
- 34.Whitchurch CB, Leech AJ, Young MD, Kennedy D, Sargent JL, Bertrand JJ, Semmler ABT, Mellick AS, Martin PR, Alm RA, Hobbs M, Beatson SA, Huang B, Nguyen L, Commolli JC, Engel JN, Darzins A, Mattick JS. 2004. Characterization of a complex chemosensory signal transduction system which controls twitching motility in Pseudomonas aeruginosa. Mol Microbiol 52:873–893. doi: 10.1111/j.1365-2958.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 35.Bertrand JJ, West JT, Engel JN. 2010. Genetic analysis of the regulation of type IV pilus function by the Chp chemosensory system of Pseudomonas aeruginosa. J Bacteriol 192:994–1010. doi: 10.1128/JB.01390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chlebek JL, Hughes HQ, Ratkiewicz AS, Rayyan R, Wang JC, Herrin BE, Dalia TN, Biais N, Dalia AB. 2019. PilT and PilU are homohexameric ATPases that coordinate to retract type IVa pili. PLoS Genet 15:e1008448. doi: 10.1371/journal.pgen.1008448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams DW, Pereira JM, Stoudmann C, Stutzmann S, Blokesch M. 2019. The type IV pilus protein PilU functions as a PilT-dependent retraction ATPase. PLoS Genet 15:e1008393. doi: 10.1371/journal.pgen.1008393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCallum M, Tammam S, Little DJ, Robinson H, Koo J, Shah M, Calmettes C, Moraes TF, Burrows LL, Howell PL. 2016. PilN binding modulates the structure and binding partners of the Pseudomonas aeruginosa type IVa pilus protein PilM. J Biol Chem 291:11003–11015. doi: 10.1074/jbc.M116.718353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoang TT, Kutchma AJ, Becher A, Schweizer HP. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59–72. doi: 10.1006/plas.1999.1441. [DOI] [PubMed] [Google Scholar]
- 40.Lee CK, de Anda J, Baker AE, Bennett RR, Luo Y, Lee EY, Keefe JA, Helali JS, Ma J, Zhao K, Golestanian R, O'Toole GA, Wong GCL. 2018. Multigenerational memory and adaptive adhesion in early bacterial biofilm communities. Proc Natl Acad Sci USA 115:4471–4476. doi: 10.1073/pnas.1720071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch MD, Fei C, Wingreen NS, Shaevitz JW, Gitai Z. 2021. Competitive binding of independent extension and retraction motors explains the quantitative dynamics of type IV pili. Proc Natl Acad Sci USA 118:e2014926118. doi: 10.1073/pnas.2014926118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomsen ND, Berger JM. 2008. Structural frameworks for considering microbial protein- and nucleic acid-dependent motor ATPases. Mol Microbiol 69:1071–1090. doi: 10.1111/j.1365-2958.2008.06364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koch MD, Black ME, Han E, Shaevitz JW, Gitai Z. 2022. Pseudomonas aeruginosa distinguishes surfaces by stiffness using retraction of type IV pili. Proc Natl Acad Sci USA 119:e2119434119. doi: 10.1073/pnas.2119434119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley D. 1980. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can J Microbiol 26:146–154. doi: 10.1139/m80-022. [DOI] [PubMed] [Google Scholar]
- 45.Bradley D. 1972. Evidence for the retraction of Pseudomonas aeruginosa RNA phage pili. Biochem Biophys Res Commun 47:142–149. doi: 10.1016/s0006-291x(72)80021-4. [DOI] [PubMed] [Google Scholar]
- 46.Talà L, Fineberg A, Kukura P, Persat A. 2019. Pseudomonas aeruginosa orchestrates twitching motility by sequential control of type IV pili movements. Nat Microbiol 4:774–780. doi: 10.1038/s41564-019-0378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain R, Sliusarenko O, Kazmierczak BI. 2017. Interaction of the cyclic-di-GMP binding protein FimX and the type 4 pilus assembly ATPase promotes pilus assembly. PLoS Pathog 13:e1006594. doi: 10.1371/journal.ppat.1006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beaussart A, Baker AE, Kuchma SL, El-Kirat-Chatel S, O'Toole GA, Dufrêne YF. 2014. Nanoscale adhesion forces of Pseudomonas aeruginosa type IV pili. ACS Nano 8:10723–10733. doi: 10.1021/nn5044383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribbe J, Baker AE, Euler S, O'Toole GA, Maier B. 2017. Role of cyclic di-GMP and exopolysaccharide in type IV pilus dynamics. J Bacteriol 199:e00859-16. doi: 10.1128/JB.00859-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Black WP, Wang L, Jing X, Saldaña RC, Li F, Scharf BE, Schubot FD, Yang Z. 2017. The type IV pilus assembly ATPase PilB functions as a signaling protein to regulate exopolysaccharide production in Myxococcus xanthus. Sci Rep 7:7263. doi: 10.1038/s41598-017-07594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Köhler T, Curty LK, Barja F, Delden C, Pechère J-C. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol 182:5990–5996. doi: 10.1128/JB.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi K-H, Schweizer HP. 2006. mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc 1:153–161. doi: 10.1038/nprot.2006.24. [DOI] [PubMed] [Google Scholar]
- 53.Choi K-H, Gaynor JB, White KG, Lopez C, Bosio CM, Karkhoff-Schweizer RR, Schweizer HP. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat Methods 2:443–448. doi: 10.1038/nmeth765. [DOI] [PubMed] [Google Scholar]
- 54.Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci USA 95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuchma SL, Griffin EF, O'Toole GA. 2012. Minor pilins of the type IV pilus system participate in the negative regulation of swarming motility. J Bacteriol 194:5388–5403. doi: 10.1128/JB.00899-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S10. Download jb.00179-23-s0001.pdf, PDF file, 1.8 MB (1.8MB, pdf)
Tables S1 to S3. Download jb.00179-23-s0002.pdf, PDF file, 0.1 MB (110.8KB, pdf)
Data Availability Statement
All code is available on GitHub at https://github.com/GeiselBiofilm.