Abstract
Background:
Early-life severe respiratory syncytial virus (RSV) infection has been associated with the onset of childhood wheezing illnesses. However, it is unknown whether being uninfected with RSV in infancy is associated with the development of childhood asthma.
Methods:
In a large, population-based, birth cohort of healthy, term infants (n=1,946), we ascertained RSV infection status (uninfected vs. infected) in the first year of life using a combination of passive and active surveillance with viral identification through molecular and serological techniques. Children were then followed prospectively for the primary outcome of 5-year current asthma. Statistical models were adjusted for child’s sex, race and ethnicity, ever breastfeeding, daycare attendance, exposure to secondhand smoking in utero or in early infancy, and maternal asthma.
Findings:
Of 1,946 eligible children enrolled, 1,741 (~89%) had available data to assess their RSV infection status in the first year of life. The proportion of children with RSV infection in infancy was 944/1,741 (54%, 95% confidence interval [CI]=52–57%). The proportion of 5-year current asthma was lower among RSV-uninfected infants (91/587 [15.50%]) than among RSV-infected infants (139/670 [20.75%]). Being uninfected with RSV in infancy was associated with ~25% lower risk of 5-year current asthma (adjusted RR=0.74, 95% CI=0.58–0.94, p=0.01). The estimated proportion of 5-year current asthma that could be attributed to preventing RSV infection in infancy was ~15% (95% CI=2.19–26.84).
Interpretation:
Among healthy, term infants, not being infected with RSV in the first year of life was associated with a substantially reduced risk of developing childhood asthma. These findings suggest a causal, age-dependent association between RSV infection in infancy and pediatric wheezing phenotypes. However, to definitively establish causality, the effect of interventions that prevent, delay, or decrease the severity of the initial RSV infection on childhood asthma will need to be studied.
Keywords: Asthma, bronchiolitis, children, epidemiology, infants, respiratory syncytial virus, wheeze
Introduction
Respiratory syncytial virus (RSV) is a ubiquitous, seasonal respiratory viral pathogen and a major cause of morbidity and mortality in infants worldwide.1 Sixty years of observational studies have consistently demonstrated an association between early-life RSV bronchiolitis and childhood asthma.2–5 However, in addition to only impacting a minority of all RSV-infected infants, RSV bronchiolitis is not a true exposure, as it just represents a severe clinical manifestation of RSV infection.6 Furthermore, we have shown that the relationship between RSV bronchiolitis in infancy and childhood asthma is likely confounded by the shared genetic susceptibility for early-life severe RSV infection and pediatric wheezing phenotypes.4 Therefore, findings from previous studies focused on early-life severe RSV infection as the exposure cannot support a causal effect of RSV infection in infancy on the onset of childhood asthma.
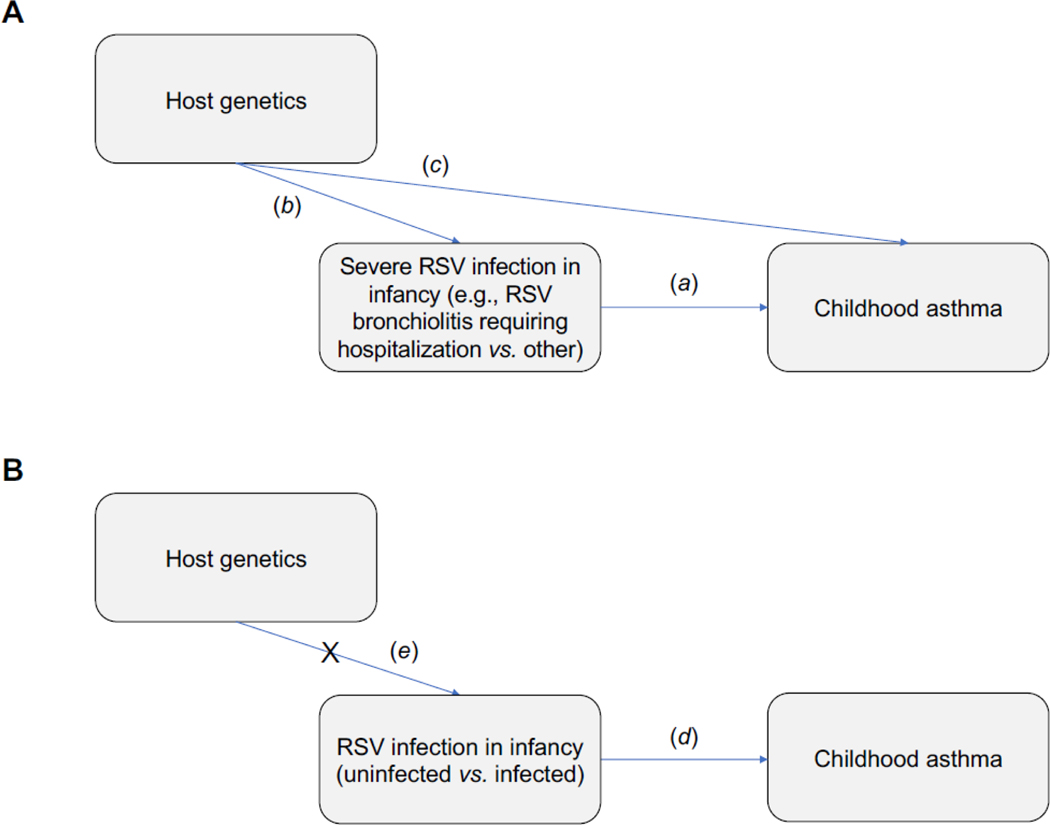
Understanding whether the prevention of RSV infection in infancy, a critical period of lung and immune development, can reduce the risk of childhood asthma is crucial to designing successful primary preventive strategies, preventing long-term childhood respiratory morbidity, and delineating health care policy measures. Since randomized controlled trials of RSV infection in the first year of life are obviously unethical, and current agents for RSV immunoprophylaxis decrease RSV severity but likely do not prevent RSV infection,7–9 we 1) conducted a population-based birth cohort designed to study RSV infection in infancy as an exposure, rather than only studying early-life severe RSV infection, 2) used a combination of passive and active surveillance with viral identification through molecular and serological techniques to ascertain RSV infection in infancy as a natural event, thereby overcoming the confounding effects of host genetics, and 3) examined the effect of being uninfected with RSV in infancy on the development of childhood asthma (Figure 1).
Figure 1:
Graphical representation showing the confounding effect of host genetics on the potential causal relationship between RSV infection in infancy and childhood asthma. (1A) Prior studies in this field have focused exclusively on severe RSV infection (usually defined as the presence vs. absence of RSV bronchiolitis requiring hospitalization) in infancy with childhood asthma (a) and have thus lacked a true control group of children without RSV infection in infancy. Furthermore, because both the severity of an RSV infection and the onset of childhood asthma may share a common genetic origin, or an RSV bronchiolitis requiring hospitalization in infancy may simply represent the first acute asthma exacerbation in an otherwise genetically predisposed child, these studies were highly susceptible to confounding by host genetics (b and c). (1B) In the current study, we examined the effect of being uninfected with RSV in infancy on the development of childhood asthma (d) and thus included a true control group of children not infected with RSV in the first year of life. In addition, because RSV is ubiquitous and nearly everyone is infected with it by age 2–3 years, being uninfected vs. infected with RSV in infancy is a natural event and less likely to be associated with host genetics (e). Hence, our study design is likely to mitigate confounding by shared genetic susceptibility. Definition of abbreviations: RSV = Respiratory syncytial virus.
Methods
Full details are available in the Supplementary Methods in the Supplementary Appendix.
Overview of the Study Population and Design
The Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure study (INSPIRE) is a large, population-based, birth cohort of healthy, term children specifically designed to test the main hypothesis that being uninfected with RSV in infancy decreases the risk of childhood asthma. We determined RSV infection status (i.e., uninfected vs. infected) in the first year of life among participating children. Eligible children were enrolled near birth and recruited from 11 participating pediatric practices across middle Tennessee. They were overall healthy, term, normal birthweight, and born between June and December of 2012 and 2013. Thus, by study design, children were ≤6 months of age at the beginning of their first RSV season (November to March in our region10,11). The catchment zone encompassed urban, suburban, and rural areas. The full eligibility criteria are shown in Table S1. Annual follow-up for the ascertainment of childhood asthma and recurrent wheeze was conducted. The Institutional Review Board of Vanderbilt University approved this study and one parent of each child provided informed consent for their participation. The detailed methods for INSPIRE have been previously reported.12
Determination of RSV Infection and Infection Severity in Infancy
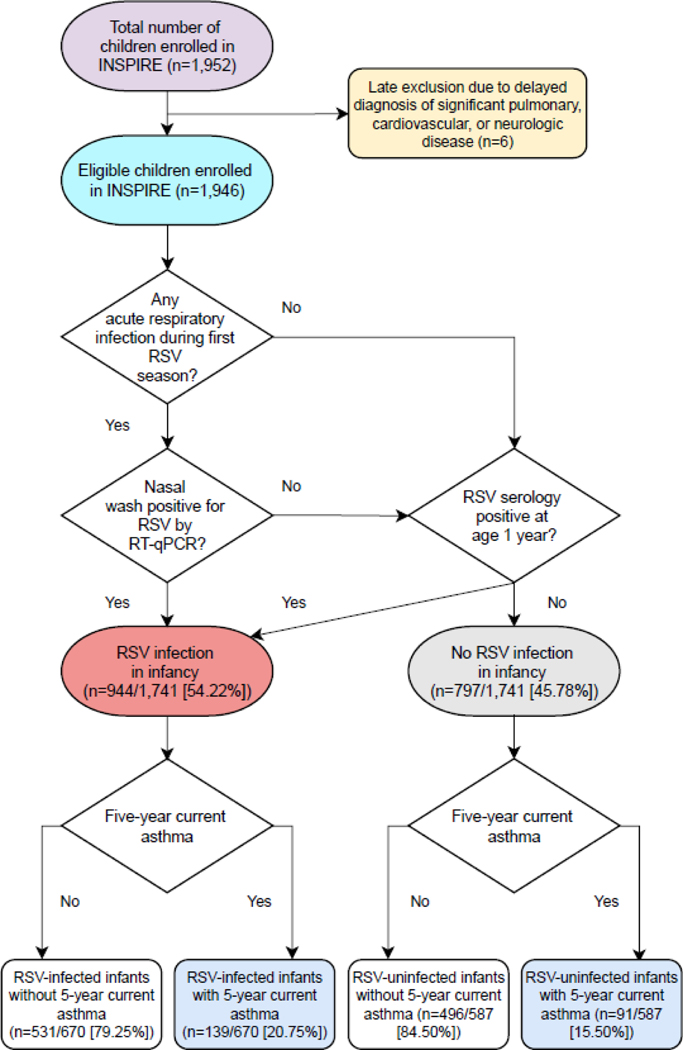
For the ascertainment of RSV infection in infancy, we first conducted intensive passive and active surveillance during each child’s first RSV season by 1) performing bi-weekly phone, email, and/or in person follow-up, 2) frequently educating and reminding parents to call us at the onset of any acute respiratory symptoms, and 3) approaching all children who were seen at one of the participating pediatric practices for an unscheduled visit. If a child met pre-specified criteria for an acute respiratory infection, we then conducted an in-person respiratory illness visit at which time we administered a parental questionnaire, performed a physical exam, collected a nasal wash, and —in those who required a health care encounter— completed a structured medical chart review. The nasal wash was used for the molecular detection of RSV by reverse transcription-quantitative PCR (RT-qPCR).13 In addition, we collected blood samples from all participating children at age 1 year and measured RSV serum antibody titers by an enzyme-linked immunosorbent assay using published protocols.14,15 Children were then classified as uninfected vs. infected with RSV in the first year of life using a hierarchical categorization with mutually exclusive group membership (Figure 2). To assess the severity of RSV infection among infants with an in-person respiratory illness visit, we used the Respiratory Severity Score (RSS), an ordinal scale that ranges from 0 to 12 with higher values indicating more severe disease.16
Figure 2:
Flow diagram of the hierarchical algorithm used for the classification of RSV infection in infancy. The number (%) of eligible children enrolled in the study with RSV infection in infancy and 5-year current asthma are also shown. Definition of abbreviations: INSPIRE = Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure Study, RSV = Respiratory syncytial virus, RT-qPCR = Reverse transcription-quantitative polymerase chain reaction.
Definitions of Outcomes
Our primary outcome was 5-year current asthma, which was defined as parental report of 1) physician-diagnosed asthma or use of asthma medications at any time point prior to age 5 years, and 2) any of the following during the 12 months prior to the 5-year visit: asthma symptoms, asthma-related systemic steroid use, or acute health care utilization for asthma.
Our secondary outcomes were 1) recurrent wheeze, which was ascertained annually between ages 1–4 years and defined as parental report of ≥2 episodes of wheeze since the prior birthday, and 2) 5-year current asthma inflammatory subtype (atopic vs. non-atopic), which was ascertained using the aforementioned definition of 5-year current asthma and two different definitions of atopy: a) evidence of aeroallergen sensitization by skin prick testing or blood specific IgE testing at age 3 years, or b) parental report of ever physician diagnosis of allergic rhinitis or atopic dermatitis at age 5 years.
Power Calculations
Power calculations were performed at the study design phase with a required initial sample size of 1,900 children and attrition proportion of ~25% at ages 4–6 years for a final sample size of 1500 children. We estimated that 60% of children would be infected with RSV in their first year of life giving 600 RSV-uninfected and 900 RSV-infected infants, respectively (2:3 unexposed to exposed ratio).17 The expected prevalence of childhood asthma in RSV-uninfected infants was 11%.18 Given these incidence ratios of exposure and outcome estimates, we calculated a minimum detectable risk ratio (RR) of childhood asthma in RSV-infected vs. RSV-uninfected infants of either 1.50 or 0.62 with 80% power and a type I error of 0.05.
Statistical Analyses
Descriptive statistics are presented as median (interquartile range [IQR]) for continuous variables and frequencies (%) for categorical variables. For initial group comparisons, we used Mann-Whitney U or chi-squared tests as appropriate. For our main analyses, modified Poisson regression, generalized estimating equations (GEE) with a Poisson random component and log link for repeated measures (using an independent working correlation), or multinomial logistic regression were used to estimate unadjusted and adjusted RRs or odds ratios (ORs) and corresponding 95% confidence intervals (CIs). We a priori selected covariates to be included in the adjusted models based on published literature and by creating a causal directed acyclic graph (Figure S1). These included the child’s sex, race and ethnicity, ever breastfeeding, daycare attendance in infancy, exposure to secondhand smoking in utero or in early infancy, and maternal asthma.19 Supplementary models were created by replacing these with other covariates (for example, daycare attendance in infancy with the presence of another child aged <6 years at home during infancy). The GEE models also included an interaction term between RSV infection in infancy and child’s age as a time-varying covariate.
In exploratory analyses of our primary outcome, we used separate models to test for multiplicative interactions and examine effect modifications of RSV infection in infancy on 5-year current asthma by child’s sex, race and ethnicity, daycare attendance in infancy, the presence of another child aged <6 years at home during infancy, and maternal asthma by including cross-product terms in the adjusted models.
Statistical significance was defined as p<0.05. Statistical analyses were performed using R version 4.0.1.20
Role of the Funding Source
The study sponsors had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors were not paid to write this article by a pharmaceutical company or other agency.
Results
Participant Characteristics and Follow-up
There were 1,946 eligible children enrolled in INSPIRE and their baseline characteristics are shown in Table 1. Of these, 1,220 (~63%) had one or more in-person respiratory illness visits and 1,709 (~88%) had a blood sample collected at age 1 year. There were 2,093 in-person respiratory illness visits completed and the median number of in-person respiratory illness visits per child was 1 (IQR=1–2). The 1-, 2-, 3-, 4-, and 5-year follow-up rates were 1,760/1,946 (~90%), 1,712/1,946 (~88%), 1,480/1,946 (~76%), 1,536/1,946 (~79%), and 1,371/1,946 (~71%), respectively. In comparison to children who did not complete their 5-year visit, those who completed it had a higher birth weight and were more likely to have private insurance, to have been breastfed in infancy, to have ever attended daycare in infancy, and less likely to have been exposed to secondhand smoking in utero or in early infancy (Table S2).
Table 1.
Baseline characteristics of eligible children enrolled in the study by RSV infection status in infancy. *†
| Baseline characteristic | All (n=1,946) | RSV infection in infancy (n=1,741)* | ||
|---|---|---|---|---|
|
| ||||
| No (n=797) | Yes (n=944) | p-value§ | ||
|
|
|
|
||
| Age at enrollment (days) | 55 (16–78) | 51 (15–75) | 60 (17–86) | 0.005 |
| Female sex | 926 (48%) | 391 (49%) | 436 (46%) | 0.23 |
| Race and ethnicity | 0.004 | |||
| Black non-Hispanic | 343 (18%) | 116 (15%) | 192 (20%) | |
| White non-Hispanic | 1,267 (65%) | 551 (69%) | 580 (61%) | |
| Hispanic | 170 (9%) | 65 (8%) | 91(10%) | |
| Other | 166 (9%) | 65 (8%) | 81 (9%) | |
| RSV season | 0.81 | |||
| 2012–2013 | 858 (44%) | 340 (43%) | 408 (43%) | |
| 2013–2014 | 1,088 (56%) | 457 (57%) | 536 (57%) | |
| Birth month | <0.001 | |||
| June | 270 (14%) | 90 (11%) | 161 (17%) | |
| July | 313 (16%) | 103 (13%) | 179 (19%) | |
| August | 329 (17%) | 141 (18%) | 153 (16%) | |
| September | 261 (13%) | 120 (15%) | 120 (13%) | |
| October | 269 (14%) | 123 (15%) | 106 (11%) | |
| November | 251 (13%) | 114 (14%) | 106 (11%) | |
| December | 253 (13%) | 106 (13%) | 119 (13%) | |
| Gestational age (weeks) | 39 (39–40) | 39 (39–40) | 39 (39–40) | 0.12 |
| Birth weight (grams) | 3,405 (3,120–3,740) | 3,405 (3,120–3,717) | 3,433 (3,121–3,749) | 0.28 |
| Birth by cesarean section | 611 (31%) | 233 (29%) | 325 (34%) | 0.02 |
| Ever breastfeeding | 1,528 (81%) | 653 (82%) | 730 (78%) | 0.07 |
| Daycare attendance in infancy | 590 (34%) | 234 (29%) | 344 (38%) | <0.001 |
| Presence of another child aged <6 years at home during infancy | 983 (51%) | 353 (44%) | 523 (55%) | <0.001 |
| Exposure to secondhand smoking in utero or in early infancy | 425 (22%) | 162 (20%) | 193 (20%) | 0.94 |
| Maternal asthma | 379 (19%) | 160 (20%) | 182 (19%) | 0.67 |
| Type of insurance | 0.13 | |||
| Federal or state | 1,055 (54%) | 402 (50%) | 521 (55%) | |
| Private | 867 (45%) | 384 (48%) | 411 (44%) | |
| Other or Unknown | 24 (2%) | 11 (1%) | 11 (1%) | |
Definition of abbreviations: RSV = Respiratory syncytial virus.
Data presented as median (interquartile range) for continuous variables or number (%) for categorical variables.
Data calculated for children with complete data.
Of the 1,946 eligible children enrolled in the study, 1,741 (88%) had available data to be classified into one of the two groups.
The p-values for the comparison between the groups using a Mann-Whitney U or chi-squared test are shown.
Epidemiology of RSV Infection in Infancy
Three hundred and sixty-one (~30%) of the 1,220 children with an in-person respiratory illness visit had at least one nasal wash positive for RSV by RT-qPCR. The rate of positive nasal washes for RSV by RT-qPCR tests peaked in January during the 2012–2013 RSV season and in December during the 2013–2014 RSV season (Figure S2). Among children with an in-person respiratory illness visit, the median age at the time of the first RSV infection was 20.29 weeks (IQR=11.57–26.14) and the median RSS was 3 (IQR=2–4).
Eight hundred ninety-seven (~52%) of the 1,709 children who had a blood sample collected at age 1 year had a positive RSV serology. In total, 1,741 (~89%) of the 1,946 eligible children enrolled had available data to assess their RSV infection status in the first year of life. The proportion of children with RSV infection in infancy was 944/1,741 (54%, 95% CI=52–57%) (Figure 2). Of the 944 children with RSV infection in infancy, 47 (~5%) had a nasal wash positive by RT-qPCR only, 583 (~62%) had a positive RSV serology at age 1 year only, and 314 (~33%) had both (Figure S3). In comparison to RSV-infected infants, RSV-uninfected infants were more likely to be White non-Hispanic, enrolled at a younger age, and born vaginally; to have not attended daycare; and to have not lived with another child aged <6 years (Table 1).
Primary Outcome
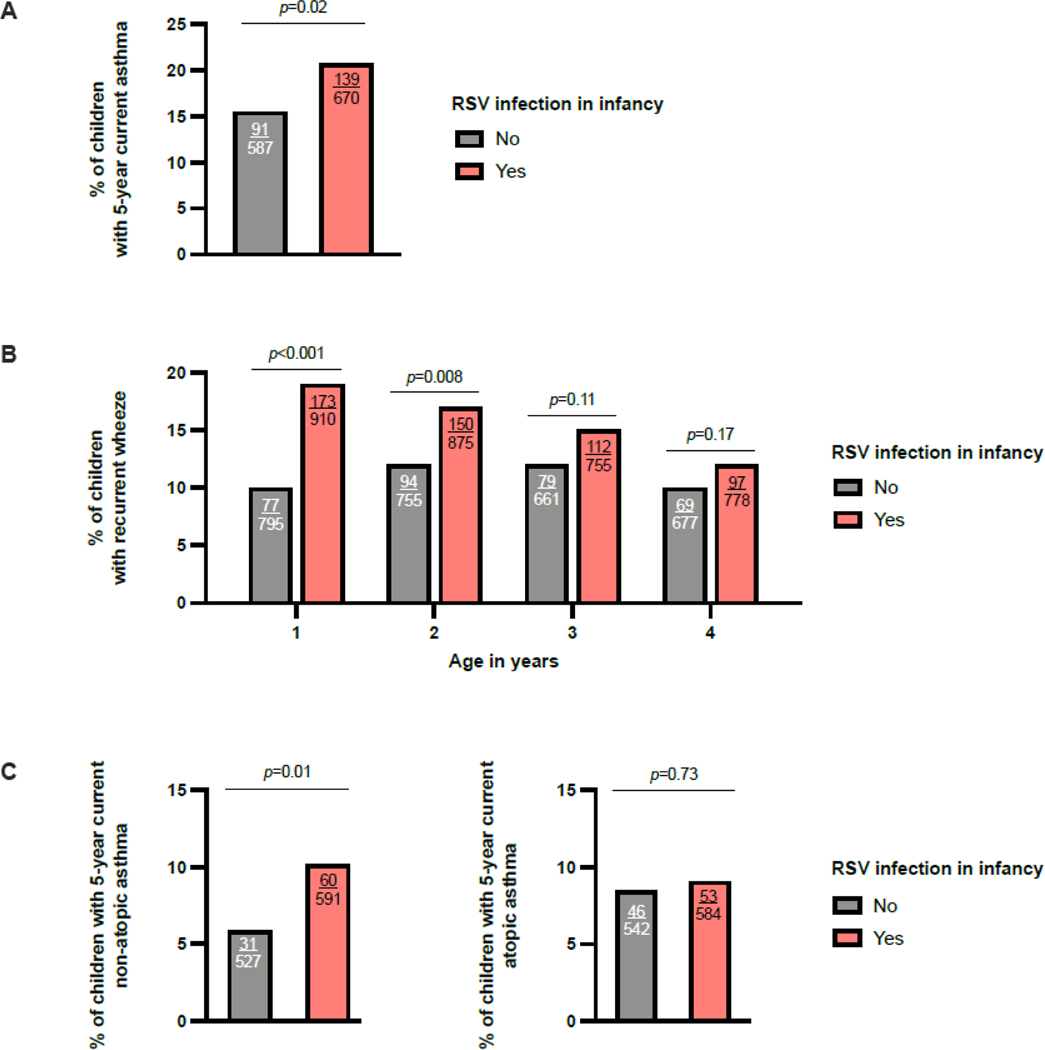
The number (%) of children with 5-year current asthma among those with available follow-up data was 238 (~18%). The proportion of 5-year current asthma was lower among RSV-uninfected infants (91/587 [15.50%]) than among RSV-infected infants (139/670 [20.75%]) (Figure 2 and Figure 3). Being uninfected with RSV in infancy was associated with ~25% lower risk of 5-year current asthma (adjusted RR=0.74, 95% CI=0.58–0.94, p=0.01) (Table 2 and Figure S4). The estimated proportion of 5-year current asthma that could be attributed to preventing RSV infection in infancy was ~15% (95% CI=2.19–26.84) (preventable fraction of 5-year current asthma for missing RSV infection in infancy = 0.15).
Figure 3:
The association of being uninfected with RSV in infancy with the primary and secondary outcomes. The bar plots show the % of children with 5-year current asthma (3A), recurrent wheeze (3B) at each of the measured timepoints, and 5-year current asthma inflammatory subtype ascertained using evidence of aeroallergen sensitization by skin prick testing or blood specific IgE testing at age 3 years (3C) in children infected and uninfected with RSV in infancy. The p-values shown for the comparison between groups were calculated using chi-squared tests. The number of children with each outcome and the total number of children for each group are shown inside the bars. Definition of abbreviations: RSV = Respiratory syncytial virus,
Table 2.
| Outcome | Unadjusted analyses | Adjusted analyses ‡ | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N° of children with outcome / total n° of children included in statistical analyses (%) | Estimate (95% CI) | p-value | N° of children with outcome / total n° of children included in statistical analyses (%) | Estimate (95% CI) | p-value | |
|
|
|
|
||||
| Primary outcome | ||||||
| Five-year current asthma | 230/1,257 (18.30%) | 0.75 (0.59–0.95) | 0.02 | 224/1,237 (18.11%) | 0.74 (0.58–0.94) | 0.01 |
| Secondary outcomes | ||||||
| Recurrent wheeze | ||||||
| One-year | 250/1,705 (14.66%) | 0.51 (0.40–0.65) | <0.001 | 239/1,687 (14.17%) | 0.54 (0.42–0.70) | <0.001 |
| Two-year | 244/1,630 (14.97%) | 0.73 (0.57–0.92) | 0.009 | 234/1,603 (14.60%) | 0.78 (0.61–0.99) | 0.04 |
| Three-year | 191/1,416 (13.49%) | 0.81 (0.62–1.05) | 0.11 | 185/1,393(13.81%) | 0.81 (0.61–1.06) | 0.12 |
| Four-year | 166/1,455 (11.41%) | 0.82 (0.61–1.09) | 0.18 | 162/1,434 (11.30%) | 0.85 (0.63–1.13) | 0.26 |
| 5-year current asthma inflammatory subtype - Definition 1§ | ||||||
| None | 1,027/1,217 (84.39%) | Reference | 1,013/1,197 (84.63%) | Reference | ||
| Non-atopic | 91/1,217 (7.48%) | 0.55 (0.35–0.87) | 0.01 | 89/1197 (7.44%) | 0.55 (0.35–0.86) | 0.01 |
| Atopic | 99/1,217 (8.13%) | 0.93 (0.61–1.41) | 0.73 | 95/1197 (7.94%) | 0.89 (0.58–1.36) | 0.59 |
| 5-year current asthma inflammatory subtype - Definition 2§ | ||||||
| None | 1,027/1,254 (81.90%) | Reference | 1,013/1,234 (82.09%) | Reference | ||
| Non-atopic | 86/1,254 (6.86%) | 0.84 (0.59–1.20) | 0.006 | 81/1,234 (6.56%) | 0.48 (0.30–0.78) | 0.003 |
| Atopic | 141/1,254 (11.24%) | 0.84 (0.59–1.20) | 0.34 | 140/1,234 (11.35%) | 0.83 (0.58–1.20) | 0.33 |
Definition of abbreviations: CI = Confidence interval, RSV = Respiratory syncytial virus.
For the outcomes of 5-year current asthma and recurrent wheeze at each of the measured time points, the estimates presented are risk ratios obtained from modified Poisson regression models. For the outcome of 5-year current asthma inflammatory subtype, the estimates presented are odds ratios from multinomial logistic regression models. For all models, the reference group included children infected with RSV in infancy.
The statistical analyses were conducted in children with complete data.
The adjusted models included child’s sex, race and ethnicity, ever breastfeeding, daycare attendance in infancy, exposure to secondhand smoking in utero or in early infancy, and maternal asthma as covariates.
For definition 1 of 5-year current asthma inflammatory subtype, atopy was ascertained using evidence of aeroallergen sensitization by skin prick testing or blood specific IgE testing at age 3 years. For definition 2 of 5-year current asthma inflammatory subtype, atopy was ascertained by parental report of ever physician-diagnosed allergic rhinitis or atopic dermatitis at age 5 years.
We found a nearly identical effect size in sensitivity analyses restricted to children who had RSV RT-qPCR testing when comparing those with a positive nasal wash for RSV by RT-qPCR to those with a negative nasal wash for RSV by RT-qPCR (adjusted RR=0.76, 05%CI=0.58–1.00, p=0.05) (Table S3). Similarly, we found the same direction of associations in sensitivity analyses examining the effect of RSV infection in infancy on the individual components of our definition of 5-year current asthma (Table S4).
In children with an in-person respiratory illness visit, there was a positive association of the severity of the RSV infection in infancy as measured by the RSS with the risk of 5-year current asthma (adjusted OR=1.24, 95% CI=1.05–1.45, p=0.009) (Table S5 and Figure S5).
There was no evidence of a modification of the effect of RSV infection in infancy on 5-year current asthma by the child’s sex, race and ethnicity, daycare attendance in infancy, or maternal asthma in exploratory analyses (Table S6). In supplementary adjusted analyses of the primary outcome, we obtained similar results in models including other covariates, such as the presence of another child aged <6 years at home during infancy (Table S7). Likewise, in exploratory analyses, the association of RSV infection in infancy on 5-year current asthma was not modified by this covariate (Table S8).
Secondary Outcomes
The number (%) of children with recurrent wheeze among those with available follow-up data was 256 (~15%) at age 1 year, 258 (~15%) at 2 years, 195 (~13%) at 3 years, and 177 (~12%) at 4 years. Of the 238 children with 5-year current asthma, 102 (~53%) had atopic asthma and 92 (~47%) had non-atopic asthma as defined by evidence of aeroallergen sensitization at age 3 years.
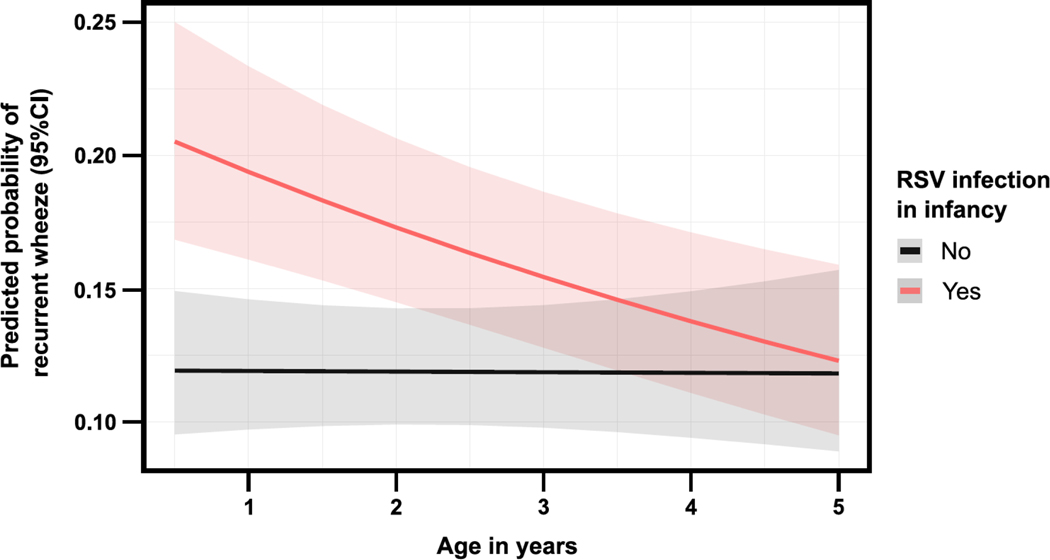
The proportion of recurrent wheeze was lower among RSV-uninfected infants at each of the measured time points between ages 1–4 years (Figure 3). In repeated outcome analyses, the effect of RSV infection in infancy on recurrent wheeze throughout the preschool years varied over time (p for interaction term=0.03) (Figure 4). In models stratified by child’s age, RSV-uninfected infants had a lower risk of recurrent wheeze annually, although this was only significant for 1- and 2-year recurrent wheeze (1-year recurrent wheeze: adjusted RR=0.54, 95% CI=0.42–0.70, p<0.001; 2-year recurrent wheeze: adjusted RR=0.78, 95% CI=0.61–0.99, p=0.04, 3-year recurrent wheeze: 0.81, 95% CI=0.61–1.06, p=0.12; and 4-year recurrent wheeze: 0.85, 95% CI=0.63–1.13, p=0.26) (Table 2).
Figure 4:
Predicted probability of recurrent wheeze over the first 4 years of life by RSV infection in infancy. The solid lines represent the predicted probability for each group. The adjacent shaded bands represent the corresponding lower and upper 95% CIs. The estimates were obtained from a generalized estimating equation regression model with a Poisson random component and log link for repeated measures (using an independent working correlation) and correcting covariance matrices using the Huber-White robust sandwich method. The model included the child’s sex, race and ethnicity, ever breastfeeding, daycare attendance in infancy, exposure to secondhand smoking in utero or in early infancy, and maternal asthma as covariates. Definition of abbreviations: CI = Confidence interval, RSV = Respiratory syncytial virus.
In comparison to RSV-infected infants, RSV-uninfected infants had a lower proportion of 5-year current non-atopic asthma, but not of 5-year current atopic asthma, as defined by evidence of aeroallergen sensitization at age 3 years (Figure 3). Using this definition of atopy, being uninfected with RSV in infancy was associated with ~45% lower odds of 5-year current nonatopic asthma (adjusted OR=0.55, 95% CI=0.35–0.86, p=0.01). There was no evidence of an effect of RSV infection in infancy on 5-year current atopic asthma (adjusted OR=0.89, 95% CI=0.58–1.36, p=0.6) (Table 2). We obtained similar results when ascertaining atopy by parental report of ever physician diagnosis of allergic rhinitis or atopic dermatitis at age 5 years (Table 2).
In supplementary adjusted analyses of the secondary outcomes, we obtained similar results in models including other covariates, such as the presence of another child aged <6 years at home during infancy (Table S7).
Discussion
In this large, population-based birth cohort of healthy, term infants, we demonstrate that RSV-uninfected infants have a substantially reduced risk of developing childhood asthma compared to RSV-infected infants. To our knowledge, this is the first study specifically designed to test the hypothesis that being uninfected with RSV in infancy decreases the risk of childhood asthma, as prior studies have focused exclusively on early-life severe RSV infection (usually defined as the presence vs. absence of RSV bronchiolitis requiring hospitalization) as the exposure.4,21,22 However, because —as previously noted— the latter is a clinical phenotype and not a true exposure, and the relationship between the severity of RSV disease in infancy and childhood wheezing illnesses is confounded by shared risks for both conditions (such as host genetics), early-life severe RSV infection cannot be used to support a causal association with asthma.4,23 In contrast, we have previously shown that there is no evidence that host genetics influences the risk of RSV infection in childhood.24 This is consistent with findings from prior studies showing that nearly all children are infected with RSV by age 2–3 years of life.17,25–27 Our unique study design and careful phenotyping of the study population allowed us to use observational data to examine the potential causal role of RSV infection in infancy in the development of childhood asthma by, in the absence of a randomized experiment, mitigating the confounding effect of shared genetic susceptibility on the severity of the RSV infection and the risk of childhood asthma. This adds to our prior study demonstrating that birth in relationship to RSV circulation is associated with the risk of childhood asthma, an association that is difficult to explain by non-causal mechanisms.10
Further, we found a severity-dependent effect of RSV infection in infancy across the entire spectrum of disease severity on the risk of childhood asthma, which supports a dose-response association, in which the risk was lower among those with the mildest RSV infections. While comparative clinical trials of RSV prevention products (such as monoclonal antibodies and vaccines) conceptually provide the highest level of evidence of causality, it is important to note that randomization to these agents is not necessarily an instrumental variable for RSV infection, unless there is evidence that they actually prevent RSV infection, as if they don’t, then an equal number of children in both arms of the clinical trial would still be expected to be infected with RSV.4 Two randomized clinical trials of RSV monoclonal antibodies have conducted long-term follow-up of childhood wheeze. One of these trials found that healthy preterm infants randomized to palivizumab had reduced wheezing days in their first year of life, any wheeze at ages 1 to 3 years, and recent wheeze at age 6 years compared to those randomized to placebo.28,29 In contrast, a clinical trial of motavizumab in Native American healthy infants demonstrated no effect on the rates of medically attended recurrent wheeze at ages 1 to 3 years, although the prevalence of recurrent wheeze in that study was substantially lower than reported in most other populations.30 A causal role of RSV infection in infancy on childhood asthma is also suggested by numerous in vitro and animal studies from our group and others that provide evidence for potential mechanisms through which early-life RSV infection may contribute to chronic airway diseases.31–38 The effect of RSV infection in infancy on respiratory health several years after the initial RSV infection suggests long-term reprogramming of the early-life immune response and airway epithelium. This is in line with our prior findings of persistent effects of RSV infection in infancy on T cell memory responses and airway epithelial development.35,36 Furthermore, as all children are infected with RSV by age 2–3 years,17,25–27 our results demonstrate an age-dependent effect of RSV infection on the onset of pediatric wheezing phenotypes. Biologic mechanisms are additionally supported by results from previous animal studies.39,40 Taken together with our present findings, these results support testing interventions that prevent, delay, or decrease the severity of the initial RSV infection to reduce the prevalence of childhood asthma at the population level.
The estimated proportion of 5-year current asthma that could be attributed to preventing RSV infection in infancy (i.e., the preventable fraction) was ~15%. Our findings fundamentally change how we have thought about the preventive potential of the relationship of early-life RSV infection and childhood asthma, which until now has been mainly based on preventing RSV bronchiolitis requiring hospitalization, a severe clinical manifestation of RSV infection that occurs in <3% of all RSV-infected infants.6 In contrast, we show that, although preventing early-life severe RSV infection could be beneficial, the majority of RSV infections during infancy are mild and thus it might be important to include strategies to prevent or delay the initial RSV infection during infancy to attain the maximum preventive potential for childhood asthma. Limited data suggests that currently available agents for RSV immunoprophylaxis do not prevent infection with RSV, rather decreasing RSV viral load and the severity of the RSV infection.7–9 However, newer prefusion RSV F-specific antibodies could be even more effective in preventing RSV infection and their impact on not only severe RSV infection, but long-term childhood respiratory morbidity should be studied. While other approaches might not have been considered in the past, the dramatic global reduction in the incidence of RSV infections resulting from public health measures to limit the transmission of severe acute respiratory syndrome coronavirus-2 during the early phase of the coronavirus disease 2019 pandemic (such as face masks, frequent hand washing, and physical distancing) demonstrates that potentially simple, non-pharmaceutical interventions during the age-dependent period when RSV infection has the greatest impact on childhood asthma could also be studied to determine if deferring the initial RSV infection would decrease childhood asthma risk.41
We note that the strength of the association of RSV infection in infancy with recurrent wheeze decreased over time. This has also been shown in other studies and could be explained by a decrease in incidence over time after the initial exposure is removed, a decreasing power to detect associations at later time points due to participant attrition, or an age-dependent effect of RSV infection in infancy on pediatric wheezing phenotypes that occurs early in life.42 Because RSV is ubiquitous and, in diverse populations that have been studied, a large percentage of children are infected with RSV in infancy,17,27,43–47 the proportion of recurrent wheeze that could be prevented by missing RSV infection in infancy is high, and even small reductions in the incidence of RSV infection in the first year of life could have a large public health impact worldwide.19 This remains true even if RSV infection in infancy is not associated with pediatric wheezing phenotypes beyond early childhood, as the burden of childhood wheezing illnesses throughout the preschool years is considerable.48
Our findings suggest that RSV infection in infancy is more likely associated with a non-atopic childhood asthma phenotype, although our sample sizes for these secondary analyses were smaller and, thus, these results should be interpreted with caution. Likewise, although our results were consistent when using two different definitions of atopy, there is also no standard non-invasive method to assess underlying atopic inflammation in childhood asthma. We have shown that RSV infection in infancy is associated with subsequent dampened type 1 (antiviral) memory T cell responses but does not appear to impact later type 2 (pro-allergic) immune responses, suggesting that early-life RSV infection may have a greater impact on the development of non-atopic pediatric wheezing phenotypes, such as viral-triggered wheeze.35 The results of other studies in children also suggest that early-life RSV infection predisposes to childhood asthma through atopy-independent pathways,49 although further research is needed to identify the precise mechanisms underlying these associations.
The epidemiology of RSV is continuously changing and updated estimates of the burden of disease are needed to prioritize research findings, inform public health policy, and adequately design and power experimental studies of early-life RSV immunoprophylaxis programs.50,51 Because the majority of early-life RSV infections are mild, do not require medical evaluation, and often do not necessitate specific diagnostic testing,52 there is a paucity of data on the epidemiology of this disease at the community level. The proportion of children with RSV infection in infancy in our study was ~54%. The few previous studies in the United States (all published before 1986) have shown estimates ranging between ~14–68%,17,43–45 while more recent studies in other countries have shown estimates ranging between ~32–56%.27,46,47 The variance between these estimates and ours may be explained by differences in the study populations and designs, regions’ climate, or year-to-year variation in the circulating RSV strains. Unlike all prior studies, we also used both active and passive surveillance with both molecular and serologic testing, whereas the majority of other studies only used serology and culture.
Our study has limitations. First, we cannot completely exclude misclassification of children categorized as uninfected with RSV in infancy. However, such misclassification would be expected to bias results towards the null. Furthermore, the epidemiological curve for RSV infections in our study (as assessed by RT-qPCR in nasal washes) coincided with the concurrent national trends,11 and the proportion of children with RSV infection in infancy is nearly identical to those reported in community-based serological surveys of children in Kenya and the United Kingdom.25,26 Likewise, although the number of children who only had evidence of RSV infection in infancy by RSV serology in our study was relatively high (~62%), this is consistent with recent reports suggesting that the rates of asymptomatic or pauci-symptomatic early-life RSV infections are higher than previously recognized.25,53 We have formerly shown that, although there is no universally accepted method for serologic assessment of RSV, the method we used performs well when compared to other laboratory assays.15 There is currently no standard definition of childhood asthma and there could also have been misclassification of the outcome. However, to prevent this, we required several criteria to ascertain childhood asthma for increased specificity.54 As with any observational study, the relationship between being infected with RSV in infancy and childhood asthma could have been confounded by unmeasured factors. Namely, being uninfected with RSV in infancy could be a marker of a lower risk of early-life infection with other respiratory viruses. However, we found no evidence of an effect of daycare attendance or young children in the household during infancy (markers of multiple other or more frequent early-life acute respiratory infections) on childhood asthma (data not shown), and the interactions between RSV infection and these covariates on childhood asthma were not significant, which makes confounding or effect modification by other respiratory viruses unlikely. Our statistical analyses also considered other parental, socioeconomic, and environmental factors that might be associated with a healthier lifestyle and potentially decreased exposure to other risk factors for childhood asthma. Due to the study’s eligibility criteria and participants’ sociodemographic characteristics, our results may not be generalizable to other populations. However, our study population is representative of the population from which the children were recruited. Last, among other assumptions, the estimation of the preventable fraction assumes a causal effect, which can never be definitively demonstrated with an observational study, and could have been impacted by some of the limitations inherent to this type of study design (such as residual confounding).55
In summary, our results demonstrate that children who are not infected with RSV in the first year of life have a substantially reduced risk of developing childhood asthma, an association that is age- and severity-dependent. Furthermore, our findings suggest that interventions that prevent, delay, or decrease the severity of the initial RSV infection could be studied as a strategy to reduce the prevalence of childhood asthma at the population level. It is important to recognize that while our findings suggest a causal association, because of the observational design, our study can never definitively establish causality. Instead, our results highlight the need for long-term follow-up of common respiratory outcomes among children participating in ongoing and future clinical trials of agents for RSV immunoprophylaxis. Given the sample sizes we have previously calculated,51,56 this would likely require many of the clinical trials to conduct this follow-up, ideally with standardized data collection and pooling of results, to inform the potential of RSV prevention products on long-term childhood respiratory morbidity.
Supplementary Material
Research in Context.
Evidence before this study
Respiratory syncytial virus (RSV) is a major cause of morbidity and mortality in young children worldwide. In addition to its well-established short-term effects, there is conflicting evidence suggesting that RSV infection in infancy (that is, in the first year of life) can have long-term effects on respiratory health and lead to the development of childhood asthma. However, prior studies in this field have exclusively focused on the association of early-life severe RSV infection (usually defined as the presence vs. absence of RSV bronchiolitis requiring hospitalization) with pediatric wheezing phenotypes. By mainly using a control group of children not hospitalized due to RSV bronchiolitis (a group that combines children with RSV bronchiolitis not requiring hospitalization, children with RSV upper respiratory infections, and children with no RSV infection), these studies have all been limited by misclassification of their comparator group. Furthermore, because the association of early-life severe RSV infection with the onset of childhood asthma is likely confounded by shared genetic susceptibility, these studies cannot support a causal association.
Added value of this study
To overcome most of the limitations of prior studies in this field, we 1) conducted a population-based birth cohort designed to study RSV infection in infancy as an exposure, rather than only studying early-life severe RSV infection, which is a clinical phenotype and not a true exposure, 2) used a combination of passive and active surveillance with viral identification through molecular and serological techniques to ascertain RSV infection in infancy as a natural event, thereby overcoming the confounding effects of host genetics, and 3) examined the effect of being uninfected with RSV in infancy on the development of childhood asthma. The proportion of children with RSV infection in infancy was ~54%. Being uninfected with RSV in infancy was associated with ~25% lower risk of 5-year current asthma. The estimated proportion of 5-year current asthma that could be attributed to preventing RSV infection in infancy was ~15%
Implications of all available evidence
Our results suggest a causal, age- and severity-dependent association between RSV infection in infancy and pediatric wheezing phenotypes. These findings are further supported by 1) our prior studies showing that birth in relationship to RSV circulation is associated with the risk of childhood asthma, an association that is difficult to explain by non-causal mechanisms, and 2) numerous in vitro and animal studies that provide evidence for potential mechanisms through which early-life RSV infection may contribute to chronic airway diseases. Our results also support the consideration of studies that prevent, delay, or decrease the severity of the initial RSV infection as strategies to reduce the prevalence of childhood asthma at the population level. While we present multi-level evidence of a robust relationship between RSV infection in infancy and childhood asthma, because this is an observational study, the results cannot definitively establish causality. Our findings highlight the need for long-term follow-up of common respiratory outcomes among children participating in ongoing and future clinical trials of RSV prevention products.
Acknowledgements:
We would like to thank Donald H. Arnold, Alyssa Bednarek, Andrew Bender, Steven M. Brunwasser, Sandra Alvarez-Macias, Sergejs Berdnikovs, Teresa M. Chipps, Alexandra S. Connolly, Kaitlin M. Costello, Suman R. Das, Marian T. Dorst, Roxanne Filardo-Collins, Rebecca Gammell, Kayla Goodman, Karin Han, Anca M. Ifrim, Ashudee Kirk, Emma K. Larkin, Jessica Levine, Zhouwen Liu, Christian E. Lynch, Megan Mccollum, Rendie McHenry, Kelsie McMurtry, Patricia A. Minton, Paul E. Moore, Dawn C. Newcomb, Barron L. Patterson, Fernando P. Polack, Sara Reiss, Theresa Rogers, Patty B. Russell, Meghan H. Shilts, Brittney M. Snyder, Stephanie D. Steen, ZhengZheng Tang, Kedir N. Turi, Shanda Vereen, Madison A. Wagener, Kimberly B. Woodward, and Pingsheng Wu from the Center for Asthma Research at Vanderbilt University Medical Center; Joy Laurienzo Panza from the National Institute of Allergy and Infectious Diseases; and all the INSPIRE participants and their families for their involvement in and dedication to this study.
Funding:
This study was funded by the National Institutes of Health of the United States.
Declaration of Funding Sources:
This work was supported in whole or in part with funds from the National Institute of Allergy and Infectious Diseases (under award numbers U19AI095227, UG3OD023282, and K24AI77930); the Vanderbilt Institute for Clinical and Translational Research (grant support from the National Center for Advancing Translational Sciences under award number UL1TR000445); the National Heart, Lung, and Blood Institute (under award number K23HL148638); and the Department of Pediatrics at Vanderbilt University Medical Center (grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award number K12HD087023). Dr. Gergen’s authorship does not constitute endorsement by the National Institute of Allergy and Infection Diseases, the National Institutes of Health, or any other Agency of the United States Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Abbreviations:
- CI
Confidence interval
- GEE
Generalized estimating equations
- INSPIRE
Infant Susceptibility to Pulmonary Infections and Asthma Following Respiratory Syncytial Virus Exposure study
- IQR
Interquartile range
- OR
Odds ratio
- RR
Adjusted risk ratio
- RT-qPCR
Reverse transcription-quantitative PCR
- RSS
Respiratory Severity Score
- RSV
Respiratory syncytial virus
Footnotes
Declaration of Interests:
LJA has served on respiratory syncytial virus (RSV) vaccine advisory boards for Bavarian Nordic, Novavax, Daiichi-Sankyo, ClearPath Development Company, ADVI, Pfizer, and Jansen Pharmaceuticals. Through Emory University, his laboratory currently receives funding from Pfizer for RSV surveillance studies in adults, from Advaccine Biopharmacueticals Suzhou Co. Ltd. for serologic studies of RSV vaccine recipients, and from Sciogen for animal studies on RSV vaccines. He is a co-inventor on several Centers for Disease Control and Prevention patents on the RSV G protein and its CX3C chemokine motif relative to immune therapy and vaccine development. He is also co-inventor on a patent filing for the use of RSV platform virus-like particles with the F and G proteins for vaccines. TVH has served on a Data Safety Monitoring Board for Pfizer and RSV vaccine advisory boards for Sanofi-Pasteur and Pfizer. The other authors do not have a commercial or other association that might pose a conflict of interest.
Notice of Prior Presentation:
The results of this study have not been previously presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing Statement
Fully de-identified individual participant data that underlie the results reported in this manuscript will be shared with other researchers beginning 12 months and ending 36 months following its publication, only for the purpose of conducting systematic reviews with meta-analyses, and upon approval by the corresponding author. For this, researchers requesting the de-identified individual participant data will need to have their study approved by an independent review committee (such as an institutional review board) and directly contact the corresponding author.
References
- 1.Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driscoll AJ, Arshad SH, Bont L, et al. Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? Critical review of the evidence and guidance for future studies from a World Health Organization-sponsored meeting. Vaccine 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartert TV, Wu P, Brunwasser SM. Respiratory syncytial virus and asthma: untying the Gordian knot. The Lancet Respiratory medicine 2021; 9(10): 1092–4. [DOI] [PubMed] [Google Scholar]
- 4.Brunwasser SM, Snyder BM, Driscoll AJ, et al. Assessing the strength of evidence for a causal effect of respiratory syncytial virus lower respiratory tract infections on subsequent wheezing illness: a systematic review and meta-analysis. The Lancet Respiratory medicine 2020; 8(8): 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wittig HJ, Glaser J. The relationship between bronchiolitis and childhood asthma; a follow-up study of 100 cases of bronchiolitis. J Allergy 1959; 30(1): 19–23. [DOI] [PubMed] [Google Scholar]
- 6.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360(6): 588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mejias A, Chavez-Bueno S, Rios AM, et al. Comparative effects of two neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibodies in the RSV murine model: time versus potency. Antimicrob Agents Chemother 2005; 49(11): 4700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mejias A, Chavez-Bueno S, Rios AM, et al. Anti-respiratory syncytial virus (RSV) neutralizing antibody decreases lung inflammation, airway obstruction, and airway hyperresponsiveness in a murine RSV model. Antimicrob Agents Chemother 2004; 48(5): 1811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu P, Escobar GJ, Gebretsadik T, et al. Effectiveness of Respiratory Syncytial Virus Immunoprophylaxis in Reducing Bronchiolitis Hospitalizations Among High-Risk Infants. Am J Epidemiol 2018; 187(7): 1490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med 2008; 178(11): 1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes AK, Prill MM, Iwane MK, Gerber SI. Respiratory syncytial virus—United States, July 2012–June 2014. Morbidity and Mortality Weekly Report 2014; 63(48): 1133. [PMC free article] [PubMed] [Google Scholar]
- 12.Larkin EK, Gebretsadik T, Moore ML, et al. Objectives, design and enrollment results from the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure Study (INSPIRE). BMC Pulm Med 2015; 15: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodani M, Yang G, Conklin LM, et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol 2011; 49(6): 2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadhao SJ, Anderson LJ. Detection of RSV Antibodies in Human Plasma by Enzyme Immunoassays. Methods Mol Biol 2016; 1442: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadhao SJ, Ha B, McCracken C, et al. Performance evaluation of antibody tests for detecting infant respiratory syncytial virus infection. J Med Virol 2021; 93(6): 3439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez H, Hartert TV, Gebretsadik T, Carroll KN, Larkin EK. A simple respiratory severity score that may be used in evaluation of acute respiratory infection. BMC research notes 2016; 9(1): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140(6): 543–6. [DOI] [PubMed] [Google Scholar]
- 18.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS data brief 2012; (94): 1–8. [PubMed] [Google Scholar]
- 19.Abreo A, Gebretsadik T, Stone CA, Hartert TV. The impact of modifiable risk factor reduction on childhood asthma development. Clin Transl Med 2018; 7(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 21.Regnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J 2013; 32(8): 820–6. [DOI] [PubMed] [Google Scholar]
- 22.Shi T, Ooi Y, Zaw EM, et al. Association Between Respiratory Syncytial Virus-Associated Acute Lower Respiratory Infection in Early Life and Recurrent Wheeze and Asthma in Later Childhood. J Infect Dis 2019. [DOI] [PubMed] [Google Scholar]
- 23.Larkin EK, Hartert TV. Genes associated with RSV lower respiratory tract infection and asthma: the application of genetic epidemiological methods to understand causality. Future Virol 2015; 10(7): 883–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawless D, McKennan CG, Das SR, et al. Viral genetic determinants of prolonged respiratory syncytial virus infection among infants in a healthy term birth cohort. J Infect Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zylbersztejn A, Pembrey L, Goldstein H, et al. Respiratory syncytial virus in young children: community cohort study integrating serological surveys, questionnaire and electronic health records, Born in Bradford cohort, England, 2008 to 2013. Euro Surveill 2021; 26(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyiro JU, Kombe IK, Sande CJ, et al. Defining the vaccination window for respiratory syncytial virus (RSV) using age-seroprevalence data for children in Kilifi, Kenya. PloS one 2017; 12(5): e0177803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andeweg SP, Schepp RM, van de Kassteele J, Mollema L, Berbers GAM, van Boven M. Population-based serology reveals risk factors for RSV infection in children younger than 5 years. Scientific reports 2021; 11(1): 8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368(19): 1791–9. [DOI] [PubMed] [Google Scholar]
- 29.Scheltema NM, Nibbelke EE, Pouw J, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. The Lancet Respiratory medicine 2018; 6(4): 257–64. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien KL, Chandran A, Weatherholtz R, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis 2015; 15(12): 1398–408. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamoorthy N, Khare A, Oriss TB, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med 2012; 18(10): 1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch JP, Werder RB, Curren BF, et al. Long-lived regulatory T cells generated during severe bronchiolitis in infancy influence later progression to asthma. Mucosal Immunol 2020; 13(4): 652–64. [DOI] [PubMed] [Google Scholar]
- 33.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med 2008; 14(6): 633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You D, Becnel D, Wang K, Ripple M, Daly M, Cormier SA. Exposure of neonates to respiratory syncytial virus is critical in determining subsequent airway response in adults. Respiratory research 2006; 7: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chirkova T, Rosas-Salazar C, Gebretsadik T, et al. Effect of Infant RSV Infection on Memory T Cell Responses at Age 2–3 Years. Frontiers in immunology 2022; 13: 826666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connelly AR, Jeong BM, Coden ME, et al. Metabolic Reprogramming of Nasal Airway Epithelial Cells Following Infant Respiratory Syncytial Virus Infection. Viruses 2021; 13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linfield DT, Gao N, Raduka A, Harford TJ, Piedimonte G, Rezaee F. RSV attenuates epithelial cell restitution by inhibiting actin cytoskeleton-dependent cell migration. Am J Physiol Lung Cell Mol Physiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harford TJ, Rezaee F, Gupta MK, Bokun V, Naga Prasad SV, Piedimonte G. Respiratory syncytial virus induces beta2-adrenergic receptor dysfunction in human airway smooth muscle cells. Sci Signal 2021; 14(685). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tasker L, Lindsay RW, Clarke BT, Cochrane DW, Hou S. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin Exp Immunol 2008; 153(2): 277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med 2002; 196(10): 1381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Mattia G, Nenna R, Mancino E, et al. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol 2021; 56(10): 3106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65(12): 1045–52. [DOI] [PubMed] [Google Scholar]
- 43.Cooney MK, Fox JP, Hall CE. The Seattle Virus Watch. VI. Observations of infections with and illness due to parainfluenza, mumps and respiratory syncytial viruses and Mycoplasma pneumoniae. Am J Epidemiol 1975; 101(6): 532–51. [DOI] [PubMed] [Google Scholar]
- 44.Monto AS, Lim SK. The Tecumseh study of respiratory illness. 3. Incidence and periodicity of respiratory syncytial virus and Mycoplasma pneumoniae infections. Am J Epidemiol 1971; 94(3): 290–301. [DOI] [PubMed] [Google Scholar]
- 45.Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas GR, Jr. Respiratory syncytial virus infections within families. N Engl J Med 1976; 294(8): 414–9. [DOI] [PubMed] [Google Scholar]
- 46.Munywoki PK, Koech DC, Agoti CN, Cane PA, Medley GF, Nokes DJ. Continuous Invasion by Respiratory Viruses Observed in Rural Households During a Respiratory Syncytial Virus Seasonal Outbreak in Coastal Kenya. Clin Infect Dis 2018; 67(10): 1559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broor S, Parveen S, Bharaj P, et al. A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PloS one 2007; 2(6): e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mallol J, Garcia-Marcos L, Sole D, Brand P, Group ES. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax 2010; 65(11): 1004–9. [DOI] [PubMed] [Google Scholar]
- 49.Simoes EA, Carbonell-Estrany X, Rieger CH, et al. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol 2010; 126(2): 256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Modjarrad K, Giersing B, Kaslow DC, Smith PG, Moorthy VS, WHO RSV Vaccine Consultation Expert Group. WHO consultation on Respiratory Syncytial Virus Vaccine Development Report from a World Health Organization Meeting held on 23–24 March 2015. Vaccine 2016; 34(2): 190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riddell CA, Bhat N, Bont LJ, et al. Informing randomized clinical trials of respiratory syncytial virus vaccination during pregnancy to prevent recurrent childhood wheezing: A sample size analysis. Vaccine 2018; 36(52): 8100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.American Academy of Pediatrics. Respiratory Syncytial Virus. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. Elk Grove, IL: American Academy of Pediatrics; 2018. p. 682–92. [Google Scholar]
- 53.Munywoki PK, Koech DC, Agoti CN, et al. Frequent Asymptomatic Respiratory Syncytial Virus Infections During an Epidemic in a Rural Kenyan Household Cohort. J Infect Dis 2015; 212(11): 1711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visness CM, Gebretsadik T, Jackson DJ, et al. Asthma as an outcome: Exploring multiple definitions of asthma across birth cohorts in the Environmental influences on Child Health Outcomes Children’s Respiratory and Environmental Workgroup. J Allergy Clin Immunol 2019; 144(3): 866–9 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khosravi A, Nazemipour M, Shinozaki T, Mansournia MA. Population attributable fraction in textbooks: Time to revise. Global Epidemiology 2021; 3: 100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Driscoll AJ, Ortiz JR, Hartert TV, Riddell CA. Recalibrating public health expectations of respiratory syncytial virus lower respiratory tract illness prevention on chronic respiratory disease. Vaccine 2021; 39(37): 5257–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This work was supported in whole or in part with funds from the National Institute of Allergy and Infectious Diseases (under award numbers U19AI095227, UG3OD023282, and K24AI77930); the Vanderbilt Institute for Clinical and Translational Research (grant support from the National Center for Advancing Translational Sciences under award number UL1TR000445); the National Heart, Lung, and Blood Institute (under award number K23HL148638); and the Department of Pediatrics at Vanderbilt University Medical Center (grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award number K12HD087023). Dr. Gergen’s authorship does not constitute endorsement by the National Institute of Allergy and Infection Diseases, the National Institutes of Health, or any other Agency of the United States Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Fully de-identified individual participant data that underlie the results reported in this manuscript will be shared with other researchers beginning 12 months and ending 36 months following its publication, only for the purpose of conducting systematic reviews with meta-analyses, and upon approval by the corresponding author. For this, researchers requesting the de-identified individual participant data will need to have their study approved by an independent review committee (such as an institutional review board) and directly contact the corresponding author.