Abstract
Physiological studies of a mutant of Escherichia coli lacking the three lytic transglycosylases Slt70, MltA, and MltB revealed that interference with murein turnover can prevent AmpC β-lactamase induction. The triple mutant, although growing normally, shows a dramatically reduced rate of murein turnover. Despite the reduction in the formation of low-molecular-weight murein turnover products, neither the rate of murein synthesis nor the amount of murein per cell was increased. This might be explained by assuming that during growth in the absence of the major lytic transglycosylases native murein strands are excised by the action of endopeptidases and directly reused without further breakdown to muropeptides. The reduced rate of murein turnover could be correlated with lowered cefoxitin-induced expression of β-lactamase, present on a plasmid carrying the ampC and ampR genes from Enterobacter cloacae. Overproduction of MltB stimulated β-lactamase induction, whereas specific inhibition of Slt70 by bulgecin repressed ampC expression. Thus, specific inhibitors of lytic transglycosylases can increase the potency of penicillins and cephalosporins against bacteria inducing AmpC-like β-lactamases.
Growth of most bacteria depends on the enlargement and division of the exoskeleton, the murein sacculus that completely encloses the cell (33, 46). Insertion of new building blocks into the sacculus, a latticework made of glycan strands and peptides, is believed to require a concerted action of murein hydrolases and synthases (3, 16, 46). Hydrolases must cleave covalent bonds in the murein netting to allow additional murein subunits to be stitched in by synthases (39). Importantly, cleavage of bonds in the stress-bearing sacculus has to be controlled tightly in order to avoid the risk of bacteriolysis (38, 39, 43, 46).
In most gram-positive rods, as well as in gram-negative bacteria, growth is accompanied by a massive release of soluble muropeptides from the sacculus (8, 11, 12, 31). Such a turnover of murein takes place even during growth of the mostly single-layered murein of Escherichia coli. Its rate of about 40 to 50% per generation is comparable with that of gram-positive bacteria. The muropeptides released are marked by a 1,6-anhydro ring structure of the terminal muramic acid due to the action of lytic transglycosylases (LTs) (9, 18, 31). In contrast to the loss of the products to the medium in the case of gram-positive bacteria, the presence of an outer membrane in gram-negative bacteria leads to an accumulation of the turnover products in the periplasm (9, 11).
The 1,6-anhydro muropeptides that are retained in the periplasmic space are quite efficiently recycled for murein biosynthesis after being taken back into the cytoplasm (9, 10, 31, 34). Most of the muropeptides are imported into the cell by the AmpG transporter (27). To a minor extent, turnover products are cleaved in the periplasm by the amidase AmiA into the peptide and sugar parts (44). The released peptides are transported into the cytoplasm by the general oligopeptide permease Opp and by a specific murein peptide permease, Mpp (10, 35). The imported muropeptides are degraded by the cytoplasmic AmpD amidase that is specific for 1,6-anhydro turnover products (17, 21). The disaccharide can be cleaved by the β-glucosaminidase NagZ, but nothing is known about the fate of the released 1,6-anhydromuramic acid (12, 34). The peptides are directly linked to UDP-N-acetylmuramic acid (UDP-MurNAc) through the action of the specific ligase Mpl, giving rise to the formation of UDP-MurNAc-peptide intermediates (29), which are used for the synthesis of murein precursors. Recently it was shown that the presence of the l,d-carboxypeptidase LdcA prevents the formation of precursors with tetrapeptide substitutions (42). LdcA turned out to be an essential enzyme, since increased incorporation of disaccharide tetrapeptide subunits into the murein sacculus affects the overall cross-linkage, finally leading to autolysis.
The multilayered peptidoglycan of gram-positive bacteria has been shown to grow by an inside-to-outside mechanism (23). According to this strategy, new murein material is hooked underneath the stress-bearing layers before it is gradually forced to take over more and more of the stress due to the hydrolytic dissolution of the outermost layers of the murein shell by the action of murein hydrolases. Murein turnover is thus a consequence of the inside-to-outside growth strategy. Murein turnover in gram-negative bacteria may likewise be the result of the growth mechanism used. A hypothesis, called the three-for-one model (15, 16), claims that in E. coli three newly synthesized cross-linked murein strands (see Fig. 5) are first hooked underneath a given strand in the sacculus, called the docking strand, and become inserted into the stress-bearing layer upon the hydrolytic breakdown of the docking strand. The rate of murein turnover determined for E. coli is consistent with a replacement of every second murein strand by three new ones per generation (16).
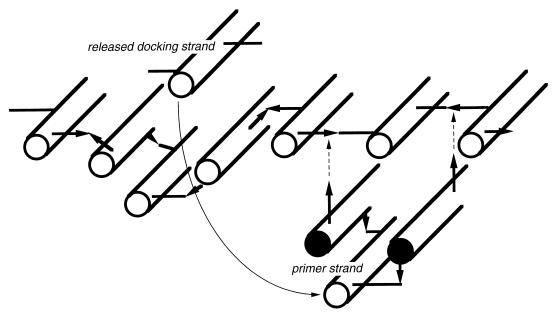
FIG. 5.
Schematic representation of a hypothetical growth mechanism as it may occur in multiple-LT-deletion mutants. The murein glycan strands are indicated by rods, and the peptide cross bridges are indicated by bars (acceptor peptide) and arrows (donor peptide). It is proposed that a docking strand released by the action of endopeptidases during the insertion of a murein triplet precursor is directly reused as a central primer strand for the formation of a new murein triplet.
The meaning of murein turnover in E. coli, that is, the release of muropeptides from the sacculus, is still a matter of debate. One suggestion is that turnover is the result of the growth strategy used. Indeed, murein turnover is an inherent feature of the three-for-one growth model (16). Another hypothesis claims that turnover represents a signalling device that senses the actual state of the cell-protecting exoskeleton (32, 34). Indeed, murein turnover products have been shown to function as signals for the induction of β-lactamases (19–21).
Here it is shown that murein turnover in a mutant lacking three of the major murein hydrolases, the LTs Slt70, MltA, and MltB, is reduced by about 70% compared to that in the isogenic wild-type strain without affecting growth. Although the rate of murein synthesis in the mutant remains unchanged, the amount of murein per cell is not significantly increased, as would be expected. This apparent contradiction may be explained by assuming that by the action of endopeptidases native murein strands are released and recycled without becoming part of the soluble turnover pool. Consequently, the inducibility of the cloned AmpC β-lactamase from Enterobacter cloacae, known to depend on soluble muropeptides, was greatly diminished in mutants lacking one or more of the LTs.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The LT deletion mutants were derived from E. coli MC1061 (4) and included MUF16 ΔsltY::kan (41), LT12 ΔmltA::cat, LT28 ΔsltY::kan ΔmltA::cat, LT60 Tetr ΔmltA::cat ΔsltY::kan, MUF33 ΔmltB::Tn10, MUF45 ΔmltA::cat ΔmltB::Tn10, MUF49 ΔsltY::kan ΔmltB::Tn10, and MUF61 ΔsltY::kan ΔmltA::cat ΔmltB::Tn10 (28). E. coli XL1-Blue (2) was used for plasmid amplification. The plasmids pAK1 (Ampr) carrying recA (28), pBP131 (sulfomethoxazol resistant) carrying ampC-ampR (25), and pWSK129 (Kanr) (45) have been described previously. The strains were grown aerobically in Luria broth (LB) supplemented with the required antibiotics in a shaking water bath at 37 or 30°C as indicated. Growth was monitored by optical density readings at 578 nm (OD578) in an Eppendorf (Hamburg, Germany) photometer.
Construction of inducible expression systems.
DNA ligations, restriction endonuclease digestions, agarose gel electrophoresis, and transformations were performed according to standard techniques (37). Amplification of plasmids was done in E. coli XL1-Blue, and transformations were performed following the method described by Hanahan et al. (13).
In order to have ampC-ampR on an inducible expression vector with a suitable resistance marker, these genes present on plasmid pBP131 were transferred onto the vector pWSK129. From pBP131, a 3.5-kb fragment covering ampC-ampR, originally taken from E. cloacae, could be obtained by digestion with SalI and BamHI. The fragment was ligated into pWSK129 digested with SalI and BamHI, yielding pJP1, which, in addition to the ampC-ampR region, carries a kanamycin cassette.
Plasmid pAU1 carries the mltB gene under the control of the tac promoter. It was constructed by removing the kanamycin resistance cassette of pMK360 (28) and replacing it with the chloramphenicol resistance cassette from pBCSK+ (Stratagene, Heidelberg, Germany). For that purpose, pMK360 was digested with NcoI and XhoI and ligated with the Camr-containing HhaI fragment from pBCSK+ after the ends were filled in with the Klenow DNA polymerase I fragment.
Determination of murein turnover.
Cells in the exponential growth phase (OD578, 0.345) were labeled by growth in the presence of 37 kBq of N-acetyl-d-[1-3H]glucosamine (GlcNAc) (259 GBq/mmol) per ml in LB medium without antibiotics at 30°C. After 20 min, when incorporation of the radiochemical had almost reached its maximum, reincorporation of the released radiolabel was suppressed by diluting the cultures 11-fold with prewarmed LB medium containing unlabeled GlcNAc at a final concentration of 300 μg/ml. At different time points, aliquots (0.5 ml) were withdrawn and added to 1 ml of boiling 8% sodium dodecyl sulfate (SDS). Samples were kept at 100°C for 30 min and filtered through 0.22-μm-pore-size GS cellulose-nitrate-and-acetate filters (Millipore) (25-mm diameter). The filters were washed once with 3 ml of 0.1 M LiCl and three times with 3 ml of water. The radioactivity on the dried filters was measured in the presence of 1.5 ml of Ultima Gold (Canberra Packard) scintillation cocktail in a TriCarb1500 (Canberra Packard) scintillation counter.
Rates of murein synthesis and murein content of cells.
The rates of murein synthesis of the wild type and triple mutant were compared by determining the incorporation of [3H]GlcNAc into murein during short periods of labeling, when the effect of turnover is negligible. Exponentially growing cells (OD578, 0.350) were labeled with [3H]GlcNAc (37 kBq/ml; 10 μg/ml) in LB medium without antibiotics at 30°C. The incorporated radioactivity in 0.5-ml aliquots taken after 5, 10, 15, 20, and 25 min was determined as described above. In contrast, the murein content of cells was determined by continuous labeling with [3H]GlcNAc (74 kBq/ml; 150 μg/ml) for more than four generation times, starting at an OD578 of 0.025. Again, the incorporation into 0.5-ml aliquots was determined as described above.
β-Lactamase induction.
To induce β-lactamase, cells (5 ml) from the exponential growth phase were diluted 1:2 with growth medium containing cefoxitin at the indicated concentrations (between 0.8 and 4 μg/ml) and incubated further at 37°C. Samples (1 ml) were removed at the indicated time points, and the cells were harvested by centrifugation (15 min; 12,000 × g; 4°C), washed once with 0.05 M sodium phosphate buffer, pH 7.0, and resuspended in 1 ml of the same buffer. The cells (250 μl) were added to 100 μl of glass beads (0.17- to 0.18-mm diameter) in a microcentrifuge tube. After the addition of DNase (10 μg/ml), the tube was vigorously shaken on a vortex shaker in the cold room for 30 min. The glass beads were removed by a short centrifugation step, and the supernatant was centrifuged for 20 min at 100,000 × g at 4°C. The protein concentration in the supernatant was determined by the method of Bradford (1), and the supernatant was used to measure β-lactamase activity.
β-Lactamase enzyme assay.
As a substrate for β-lactamase, the chromogenic β-lactam nitrocefin (30) was used. Hydrolysis of nitrocefin was followed by recording the change in the absorbance at 492 nm in an Eppendorf photometer at room temperature. Enzyme assay mixtures consisted of 50 μl of 1 mM nitrocefin, 3 to 5 μl of enzyme sample (10 μg of protein), and 0.05 M sodium phosphate buffer, pH 7.0, to produce a final volume of 1,000 μl. Under these conditions, the reaction was linear for at least 5 min.
Agar diffusion tests.
Agar plates were prepared from 20 ml of LB medium containing 0.7% agar and inoculated with 0.01 ml of an overnight culture at 40°C. Filter strips (0.5 cm wide) were soaked with solutions of the indicated concentrations of cefoxitin or bulgecin. The wet strips were applied to the surface of the agar plates at right angles to each other. Photographs were taken after overnight incubation of the plates at 37°C.
RESULTS
Murein turnover in the absence of Slt70, MltA, and MltB.
During growth of E. coli, about 40% of the total material is released from the murein sacculus per generation, as can be measured by following the loss of radiolabeled diaminopimelic acid (A2pm) from the sacculus in mutants that are defective in the major recycling pathway via AmpG (34). The turnover products were shown to be mainly the monomeric anhydro-muropeptides GlcNAc-β-1,4-(1,6-anhydro)MurNAc-l-Ala-d-Glu-m-A2pm-d-Ala and GlcNAc-β-1,4-(1,6-anhydro)MurNAc-l-Ala-d-Glu-m-A2pm. Thus, besides endopeptidases, primarily LTs are involved in murein turnover (22, 24). It was therefore of interest to study murein turnover in mutants lacking several of the LTs. To study turnover in strains that do recycle the murein turnover products, we used a label that incorporates into the glycan strands rather than into the peptides, since it has been shown that the amino sugars are recycled at only approximately half the rate of the peptide moiety of murein (12).
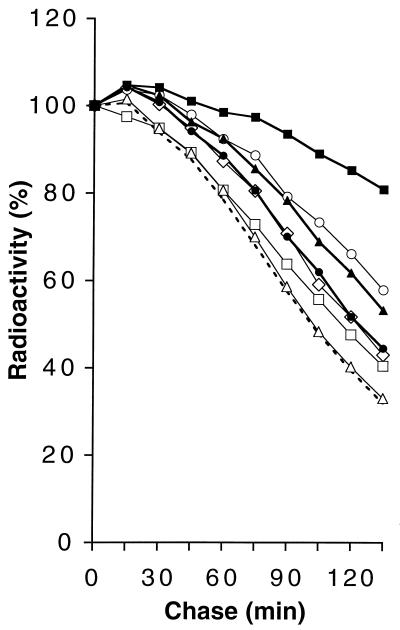
E. coli strains containing single or multiple deletions in the LT Slt70, MltA, and/or MltB as well as the corresponding wild-type strain were pulse labeled with [3H]GlcNAc, and the loss of radiolabel from the murein was determined for a chase period of more than three generations (generation time, 42 to 44 min). To our surprise, although growing with the same generation time as the wild type, most of the mutants showed substantially decreased turnover compared to the wild type (Fig. 1).
FIG. 1.
Murein turnover in mutants with deletions in LTs. Cells were pulse labeled with [3H]GlcNAc, and the loss of radiolabel from SDS-insoluble murein during a chase was determined as described in Materials and Methods. The curves are averages of four independent experiments. The average standard deviation was less than 2%. Dotted line, MC1061 (wild type); open squares, LT12 (ΔMltA); open diamonds, MUF33 (ΔMltB); open triangles, MUF16 (ΔSlt70); solid circles, LT60 (ΔSlt70 ΔMltA); solid triangles, MUF49 (ΔSlt70 ΔMltB); open circles, MUF45 (ΔMltA ΔMltB); solid squares, MUF61 (ΔSlt70 ΔMltA ΔMltB).
A small but significant decrease in murein turnover was observed for the MltA and the MltB deletions, which showed 13 and 16% less turnover than the wild type (Table 1). Deletions of two different LTs showed quite pronounced effects on murein turnover. The turnover in an Slt70-MltA mutant was decreased by 19%, that in an Slt70-MltB double mutant was decreased by 31%, and that in an MltA-MltB mutant was decreased by 38%. A triple mutant showed a reduction by 72% compared to the wild type.
TABLE 1.
β-Lactamase induction and murein turnover in LT deletion mutants of E. coli harboring pJP1 (ampC+ ampR+)
| Strain (ΔLT) | β-Lactamase activity (pmol/min × mg of protein)a
|
Murein turnover
|
|||
|---|---|---|---|---|---|
| Uninduced | Induced | Induction factorb | Muropeptides released (%)c | Decrease in turnover (%)d | |
| MC1061 (wild type) | 34 | 400 | 12 | 68 | 0 |
| MUF16 (ΔSlt70) | ND | ND | 67 | 2 | |
| LT12 (ΔMltA) | 42 | 330 | 8 | 60 | 13 |
| MUF33 (ΔMltB) | 30 | 180 | 6 | 57 | 16 |
| MUF45 (ΔMltA/MltB) | 26 | 75 | 3 | 42 | 38 |
| LT60 (ΔSlt70/MltA) | ND | ND | 56 | 19 | |
| MUF49 (ΔSlt70/MltB) | ND | ND | 47 | 31 | |
| MUF61 (ΔSlt70/MltA/MltB) | ND | ND | 19 | 72 | |
β-Lactamase activity was determined in crude cell extracts by following the hydrolysis of nitrocefin in a photometer at 492 nm as described in detail in Materials and Methods. The values are averages of four independent experiments. The average standard deviation was less than 2%. ND, not done.
Quotient of β-lactamase activity of uninduced and induced cells.
Decrease in radioactivity in SDS-insoluble material after growth for 135 min in the absence of label (chase). The cells had been prelabeled with [3H]GlcNAc as described in detail in Materials and Methods.
Relative to the turnover of the wild type (100%).
Rate of murein synthesis and amount of murein in the triple mutant.
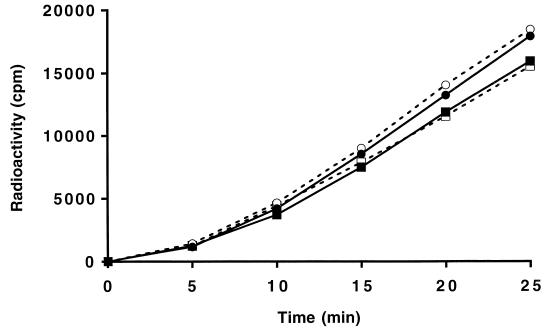
The rate of murein synthesis in the triple mutant was analyzed to find out whether the absence of the murein hydrolases, which are believed to be fundamentally involved in murein synthesis by functioning as spacemaker and pacemaker enzymes, has an effect on murein synthesis. Steady-state incorporation was reached after about 15 min, as shown in Fig. 2. Under these conditions, the rates of incorporation of labeled GlcNAc into SDS-insoluble material was found to be unchanged when the triple mutant was compared with the wild-type strain MC1061. The rate of incorporation of [3H]GlcNAc (determined in two independent experiments) was 7,300 cpm/min × 109 cells for MC1061 and 7,700 cpm/min × 109 cells for MUF61/pAK1.
FIG. 2.
Rates of murein synthesis in E. coli MUF61 and MC1061. The incorporation of [3H]GlcNAc into SDS-insoluble material was determined as described in Materials and Methods. Two independent experiments (circles and squares) are shown. The open symbols represent the wild-type E. coli MC1061, and the solid symbols represent the LT triple mutant MUF61.
In order to determine the amount of murein per cell, the strains were continuously labeled with [3H]GlcNAc (150 μg/ml; 74 kBq/ml) for more than four generation times. At identical cell densities, about 8% more radiolabel was found in the SDS-insoluble material of cultures of the triple mutant MUF61 than in that of cultures of the wild type, reflecting a slightly higher murein content of mutant cells.
Effect of murein turnover on β-lactamase induction.
Recently, it has been shown that murein turnover products accumulating in the cytoplasm can function as signals for the induction of AmpC-type β-lactamase (19–21). Therefore, it was interesting to study β-lactamase induction in mutants with a reduced rate of murein turnover. β-Lactamase activity in mutants lacking one or two of the LTs grown in the presence of cefoxitin was dramatically reduced compared to that in the wild-type strain (Table 1). A calculation of induction factors shows that these values correspond nicely to the degree of reduction in murein turnover determined for these mutants. Whereas single deletions reduced the induction level by 25 to 50%, a double deletion in MltA and MltB resulted in only marginal β-lactamase induction.
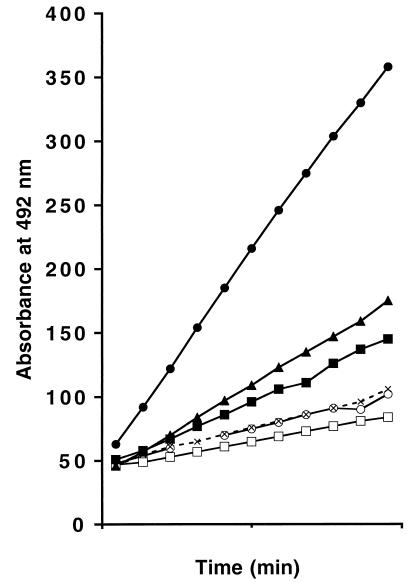
Because of the kanamycin resistance cassette present in both the sltY deletion mutant and the plasmid pWSK129 carrying ampC-ampR, the effect of a mutation in Slt70 on β-lactamase induction could not be studied directly. However, since a specific inhibitor of Slt70, bulgecin, is known (41), we could follow lactamase induction in the presence of this antibiotic. Similar experiments with E. cloacae have recently been reported by Pfeifle et al. (36). As was expected on the basis of the results with the LT mutant strains, cefoxitin-induced β-lactamase expression was significantly reduced in the presence of bulgecin (Fig. 3). On the other hand, overproduction of MltB from pAU1 greatly stimulated β-lactamase induction.
FIG. 3.
Dependency of β-lactamase induction on the activity of LTs. Expression of β-lactamase from pJP1 (ampC ampR) by cefoxitin (4 μg/ml) was determined in E. coli with different levels of active LTs. To vary the intracellular concentrations of active Slt70 and MltB, respectively, either the Slt70-specific inhibitor bulgecin was added (50 μg/ml) or MltB was overproduced by induction of expression from plasmid pAU1 by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) (10 μg/ml) as described in Materials and Methods. Hydrolysis of nitrocefin by crude cell extracts (10 μg of protein) was measured as described in Materials and Methods. The change in absorbance at 492 nm is shown. Solid circles, IPTG and cefoxitin; solid triangles, cefoxitin; solid squares, IPTG; crosses, bulgecin and cefoxitin; open circles, control; open squares, bulgecin.
Suppression of β-lactam resistance due to β-lactamase induction.
The observed dependency of β-lactamase induction on the presence of enzymatically active LTs prompted us to study the effect of specific inhibitors of LTs on β-lactamase induction. In order to prove the idea that LT inhibitors would increase the potency of β-lactams on strains carrying ampC-ampR, we performed classical agar diffusion tests with paper strips soaked with cefoxitin and the specific inhibitor of Slt70, bulgecin, respectively (Fig. 4). The two strips were applied at a right angle to each other to agar plates inoculated with E. coli MC1061 harboring pJP1 or, as a control, pWSK129. The effect of bulgecin on the potency of cefoxitin can be directly observed at the site where the two diffusion gradients cross each other. The zone of growth inhibition at the site where bulgecin acts together with cefoxitin has nearly the same width (21.9 cm) as the cefoxitin-specific inhibition zone (23.0 cm) observed with the wild-type strain. The combination of cefoxitin and bulgecin had no synergistic effect when tested with an Slt70 deletion mutant. The experiments nicely illustrate that the AmpC β-lactamase-inducing and therefore cefoxitin-resistant strain E. coli MC1061/pJP1 can be rendered sensitive to cefoxitin by the specific Slt70 inhibitor bulgecin.
FIG. 4.
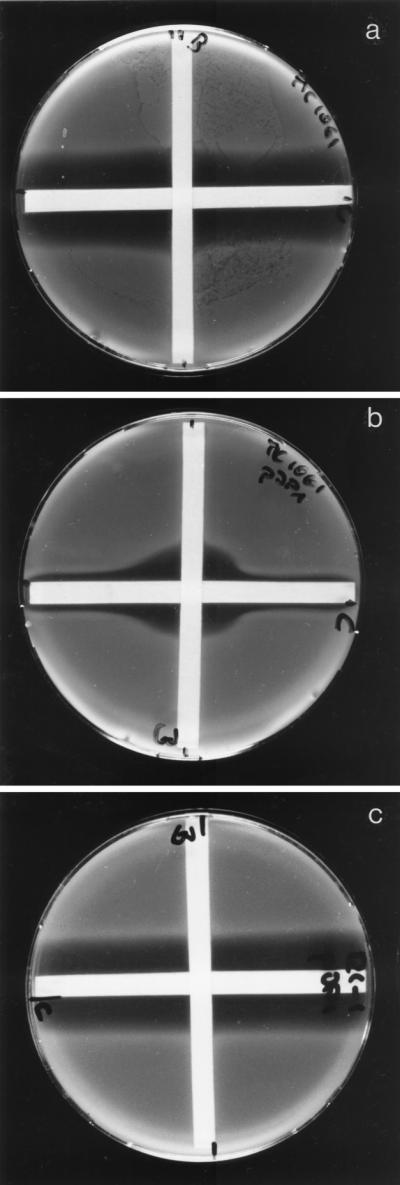
Resensitization of β-lactamase-inducing E. coli by the Slt70 inhibitor bulgecin. An agar diffusion test with a combination of bulgecin (horizontal strip soaked in 500 μg/ml) and cefoxitin (vertical strip soaked in 500 μg/ml) is shown. (a) MC1061/pWSK129; (b) MC1061/pJP1 (ampC ampR+); (c) 122-1 (slty mutant)/pJP1 (ampC ampR+).
DISCUSSION
The LTs Slt70, MltA, and MltB are shown here to be responsible for most of the murein turnover, when measured as the formation of low-molecular-weight murein degradation products. As expected, all three LTs that were investigated participate in this process. The residual turnover observed in the triple mutant is likely to be due to the presence of additional murein hydrolases. These include the LTs EmtA (26) and the recently identified MltC and MltD (6, 7) and the endopeptidases PBP4, PBP7, and MepA (14, 39), as well as the amidase AmiC (40). All of these enzymes are known to be capable of interacting with the growing murein sacculus.
A reduction in murein turnover without any change in the rate of murein synthesis, as is observed in the LT triple mutant, must result in a thickening of the murein. However, this is not the case, except for a slight increase in the total amount of murein by about 8%. Yet there is a possibility that the turnover material escaped detection. If material is released but is reused without ever becoming part of the pool of low-molecular-weight turnover products, none of it would be diverted into other metabolic pathways or diluted by unlabeled GlcNAc during the chase. From the absence of release of radiolabel we would falsely conclude that turnover did not take place.
Because residual lytic transglycosylase activity in the LT triple mutant is negligible, release of turnover material might be restricted to endopeptidase activity. Hence, it is likely that undegraded, native murein strands are released from the murein net. Such murein strands may be directly reused for the synthesis of nascent murein.
According to the three-for-one growth mechanism model (16), the murein sacculus is enlarged by the replacement of murein strands present in the stress-bearing sacculus by three new strands cross-linked to one another, a murein triplet (Fig. 5). The mode of formation of the murein triplets may differ during septum formation and cell elongation, since it has been shown by pulse-chase labeling experiments that nascent strands are cross-linked to preexisting murein during elongation, whereas newly synthesized murein strands are inserted side by side and cross-linked to one another during cell division (5, 33). Therefore, the three strands of a triplet seem to be synthesized simultaneously during septum formation. By contrast, it is assumed that during cell elongation the murein triplets are synthesized by complementing a preexisting, so-called primer strand with two new strands, one added from the left and one added from the right side to the primer strand in the middle of the triple pack (Fig. 5). It is tempting to speculate that in the LT triple mutant, where probably mostly undegraded murein strands are released by the action of endopeptidases during growth, the cell takes advantage of the accumulation of these strands and uses them as primer strands for the synthesis of new triple packs. Such a direct recycling of released docking strands would explain the apparently drastically reduced murein turnover. It has to be pointed out that release of old murein very likely takes place at a normal rate in the triple mutant but cannot be detected because the mechanism of turnover differs substantially from that of wild-type cells. The turnover material is probably represented by murein strands, which are formed and directly reused in the periplasm, rather than by muropeptides that are imported into the cytoplasm, as is the case in wild-type cells. The absence of the formation of low-molecular-weight turnover products, however, has another important implication, namely, for the induction of β-lactamases.
Quite ingeniously, murein turnover products can function as signalling compounds. Whether this is used to respond to general changes in the murein structure by adjusting the quantity and/or quality of the murein synthesized, as has been proposed (32), is not yet known. However, in the case of a disturbance of murein synthesis by β-lactam antibiotics, the cell indeed measures the intracellular concentration of certain murein turnover products to induce β-lactamase expression (19–21). Recently, it has been shown that the AmpR regulator specifically interacts with the 1,6-anhydro-muramyl-tripeptide in the periplasm (19).
In agreement with these reports, it is shown here that a reduction in the formation of low-molecular-weight murein turnover products and thereby in the formation of the β-lactamase-inducing compound(s) results in a severe block in inducible β-lactamase expression. This finding suggests a novel strategy to inhibit β-lactamase induction, which can be a major problem in β-lactam therapy. Inhibition of the LTs in Enterobacteriaceae may in certain cases increase the efficiency of penicillins. Indeed, we could show that a combination of the specific inhibitor of Slt70, bulgecin, with the β-lactam cefoxitin is a powerful strategy to lyse E. coli, even though the bacterium harbors a plasmid-encoded inducible β-lactamase.
ACKNOWLEDGMENTS
We thank Uli Schwarz for his support and interest and B. Wiedemann for supplying the pBP131 plasmid.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Bullock W O, Fernandez J M, Short J M. XL-1 Blue: a high efficiency plasmid transforming a recA Escherichia coli strain with β-galactosidase selection. BioTechniques. 1987;5:376. [Google Scholar]
- 3.Burman L G, Park J T. Molecular model for elongation of the murein sacculus of Escherichia coli. Proc Natl Acad Sci USA. 1984;81:1844–1848. doi: 10.1073/pnas.81.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadaban M J, Chou J, Cohen S N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980;143:971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jonge B L, Wientjes F B, Jurida I, Driehuis F, Wouters J T, Nanninga N. Peptidoglycan synthesis during the cell cycle of Escherichia coli: composition and mode of insertion. J Bacteriol. 1989;171:5783–5794. doi: 10.1128/jb.171.11.5783-5794.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dijkstra A J. Ph.D. thesis. Groningen, The Netherlands: University of Groningen; 1997. [Google Scholar]
- 7.Dijkstra A J, Keck W. Identification of new members of the lytic transglycosylase family in Haemophilus influenzae and Escherichia coli. Microb Drug Resist. 1996;2:141–145. doi: 10.1089/mdr.1996.2.141. [DOI] [PubMed] [Google Scholar]
- 8.Doyle R J, Koch A L. The functions of autolysins in the growth and division of Bacillus subtilis. Crit Rev Microbiol. 1987;15:169–222. doi: 10.3109/10408418709104457. [DOI] [PubMed] [Google Scholar]
- 9.Goodell E W. Recycling of murein by Escherichia coli. J Bacteriol. 1985;163:305–310. doi: 10.1128/jb.163.1.305-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodell E W, Higgins C F. Uptake of cell wall peptides by Salmonella typhimurium and Escherichia coli. J Bacteriol. 1987;169:3861–3865. doi: 10.1128/jb.169.8.3861-3865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodell E W, Schwarz U. Release of cell wall peptides into culture medium by exponentially growing Escherichia coli. J Bacteriol. 1985;162:391–397. doi: 10.1128/jb.162.1.391-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenway D L, Perkins H R. Turnover of the cell wall peptidoglycan during growth of Neisseria gonorrhoeae and Escherichia coli. Relative stability of newly synthesized material. J Gen Microbiol. 1985;131:253–263. doi: 10.1099/00221287-131-2-253. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Jessee J, Bloom F R. Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 1991;204:61–113. doi: 10.1016/0076-6879(91)04006-a. [DOI] [PubMed] [Google Scholar]
- 14.Höltje J-V. From growth to autolysis: the murein hydrolases in Escherichia coli. Arch Microbiol. 1995;164:243–254. doi: 10.1007/BF02529958. [DOI] [PubMed] [Google Scholar]
- 15.Höltje J-V. A hypothetical holoenzyme involved in the replication of the murein sacculus of Escherichia coli. Microbiology. 1996;142:1911–1918. doi: 10.1099/13500872-142-8-1911. [DOI] [PubMed] [Google Scholar]
- 16.Höltje J-V. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höltje J-V, Kopp U, Ursinus A, Wiedemann B. The negative regulator of β-lactamase induction AmpD is a N-acetyl-anhydromuramyl-L-amidase. FEMS Microbiol Lett. 1994;122:159–164. doi: 10.1111/j.1574-6968.1994.tb07159.x. [DOI] [PubMed] [Google Scholar]
- 18.Höltje J-V, Mirelman D, Sharon N, Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975;124:1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs C, Frere J-M, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in gram-negative bacteria. Cell. 1997;88:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs C, Huang L, Bartowsky E, Normark S, Park J T. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 1994;13:4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs C, Joris B, Jamin M, Klarsov K, van Beeumen J, Mengin-Lecreulx D, van Heijenoort J, Park J T, Normark S, Frere J M. AmpD, essential for both beta-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-l-alanine amidase. Mol Microbiol. 1995;15:553–559. doi: 10.1111/j.1365-2958.1995.tb02268.x. [DOI] [PubMed] [Google Scholar]
- 22.Kitano K, Tuomanen E, Tomasz A. Transglycosylase and endopeptidase participate in the degradation of murein during autolysis of Escherichia coli. J Bacteriol. 1986;167:759–765. doi: 10.1128/jb.167.3.759-765.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch A L, Doyle R J. Inside-to-outside growth and turnover of the wall of gram-positive rods. J Theor Biol. 1985;117:137–157. doi: 10.1016/s0022-5193(85)80169-7. [DOI] [PubMed] [Google Scholar]
- 24.Kohlrausch U, Höltje J-V. Analysis of murein and murein precursors during antibiotic-induced lysis of Escherichia coli. J Bacteriol. 1991;173:3425–3431. doi: 10.1128/jb.173.11.3425-3431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korfmann G, Wiedemann B. Genetic control of β-lactamase production in Enterobacter cloacae. Rev Infect Dis. 1988;10:793–799. doi: 10.1093/clinids/10.4.793. [DOI] [PubMed] [Google Scholar]
- 26.Kraft A R, Templin M F, Höltje J-V. Membrane-bound lytic transglycosylase in Escherichia coli. J Bacteriol. 1998;180:3441–3447. doi: 10.1128/jb.180.13.3441-3447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindquist S, Weston-Hafer K, Schmidt H, Pul C, Korfmann G, Erikson J, Sanders C, Martin H H, Normark S. AmpG, a signal transducer in chromosomal β-lactamase induction. Mol Microbiol. 1993;9:703–715. doi: 10.1111/j.1365-2958.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 28.Lommatzsch J, Templin M F, Kraft A R, Vollmer W, Höltje J-V. Outer membrane localization of murein hydrolases: MltA, a third lipoprotein lytic transglycosylase in Escherichia coli. J Bacteriol. 1997;179:5465–5470. doi: 10.1128/jb.179.17.5465-5470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mengin-Lecreulx D, van Heijenoort J, Park J T. Identification of the Mpl gene encoding UDP-N-acetylmuramate-l-alanyl-gamma-d-glutamyl-meso-diaminopimelate ligase in Escherichia coli and its role in recycling of cell wall peptidoglycan. J Bacteriol. 1996;178:5347–5352. doi: 10.1128/jb.178.18.5347-5352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Callaghan L H, Morris A, Kirby S M, Shingler A H. Novel method for detection of β-lactamase by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972;1:283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J T. Turnover and recycling of the murein sacculus in oligopeptide permease-negative strains of Escherichia coli: indirect evidence for an alternative permease system and for a monolayered sacculus. J Bacteriol. 1993;175:7–11. doi: 10.1128/jb.175.1.7-11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J T. Why does Escherichia coli recycle its cell wall peptides? Mol Microbiol. 1995;17:421–426. doi: 10.1111/j.1365-2958.1995.mmi_17030421.x. [DOI] [PubMed] [Google Scholar]
- 33.Park J T. The murein sacculus. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 48–57. [Google Scholar]
- 34.Park J T. The convergence of murein recycling research with β-lactamase research. Microb Drug Resist. 1996;2:105–112. doi: 10.1089/mdr.1996.2.105. [DOI] [PubMed] [Google Scholar]
- 35.Park J T, Raychaudhuri D, Li H S, Normark S, Mengin-Lecreulx D. MppA, a periplasmic binding protein essential for import of the bacterial cell wall peptide l-alanyl-gamma-d-glutamyl-meso-diaminopimelate. J Bacteriol. 1998;180:1215–1223. doi: 10.1128/jb.180.5.1215-1223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeifle D, Wiegand I, Wiedemann B. Presented at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy. Calif: San Francisco; 1999. [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Schwarz U, Asmus A, Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969;41:419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- 39.Shockman G D, Höltje J-V. Microbial peptidoglycan (murein) hydrolases. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1994. pp. 131–166. [Google Scholar]
- 40.Templin, M. F. Unpublished data.
- 41.Templin M F, Edwards D H, Höltje J-V. A murein hydrolase is the specific target of Bulgecin in Escherichia coli. J Biol Chem. 1992;267:20039–20043. [PubMed] [Google Scholar]
- 42.Templin M F, Ursinus A, Höltje J-V. A defect in cell wall recycling triggers autolysis during the stationary growth phase of Escherichia coli. EMBO J. 1999;18:4108–4117. doi: 10.1093/emboj/18.15.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomasz A, Höltje J-V. Murein hydrolases and the lytic and killing action of penicillin. In: Schlessinger D, editor. Microbiology—1977. Washington, D.C.: American Society for Microbiology; 1977. pp. 209–215. [Google Scholar]
- 44.van Heijenoort J, Parquet C, Flouret B, van Heijenoort Y. Envelope-bound N-acetylmuramyl-L-alanine amidase of Escherichia coli K 12. Purification and properties of the enzyme. Eur J Biochem. 1975;58:611–619. doi: 10.1111/j.1432-1033.1975.tb02412.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 46.Weidel W, Pelzer H. Bagshaped macromolecules—a new outlook on bacterial cell walls. Adv Enzymol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]