Abstract
Per- and polyfluoroalkyl substances (PFAS) represent a large chemical class lacking hazard, toxicokinetic, and exposure information. To accelerate PFAS hazard evaluation, new approach methodologies (NAMs) comprised of in vitro high-throughput toxicity screening, toxicokinetic data, and computational modeling are being employed in read across strategies to evaluate the larger PFAS landscape. A critical consideration to ensure robust evaluations is a parallel assessment of the quality of the screening stock solutions, where dimethyl sulfoxide (DMSO) is often the diluent of choice. Challenged by the lack of commercially available reference standards for many of the selected PFAS and reliance on mass spectrometry approaches for such an evaluation, we developed a high-throughput framework to evaluate the quality of screening stocks for 205 PFAS selected for these NAM efforts. Using mass spectrometry coupled with either liquid or gas chromatography, a quality scoring system was developed that incorporated observations during mass spectral examination to provide a simple pass or fail notation. Informational flags were used to further describe findings regarding parent analyte presence through accurate mass identification, evidence of contaminants and/or degradation, or further describe characteristics such as isomer presence. Across the PFAS-DMSO stocks tested, 148 unique PFAS received passing quality scores to allow for further in vitro testing whereas 57 received a failing score primarily due to detection issues or confounding effects of DMSO. Principle component analysis indicated vapor pressure and Henry’s Law Constant as top indicators for a failed quality score for those analyzed by gas chromatography. Three PFAS in the hexafluoropropylene oxide family failed due to degradation in DMSO. As the PFAS evaluated spanned over 20 different structural categories, additional commentary describes analytical observations across specific groups related to PFAS stock composition, detection, stability, and methodologic considerations that will be useful for informing future analytical assessment and downstream HTS efforts. The high-throughput stock quality scoring workflow presented holds value as a tool to evaluate chemical presence and quality efficiently and for informing data inclusion in PFAS or other NAM screening efforts.
Keywords: PFAS, chemical quality, mass spectrometry, in vitro toxicity testing, new approach methods, high-throughput screening
1. Introduction
Commencing production nearly 80 years ago, per- and polyfluoroalkyl substances (PFAS) are a diverse class of chemicals with global uses in consumer products and numerous industrial processes (Buck et al. 2011). As a class, these chemicals contain the strongly electronegative element fluorine attached to the carbon backbone (CnF2n+1−) which imparts chemical and thermal stability as well as hydrophobic and lipophobic characteristics. PFAS, as a result, are desirable for many applications, including stain repellants, food-contact paper, pesticide formulation, and aqueous film-forming foams (Gluge et al. 2020; Wang et al. 2017). Although these properties make PFAS desirable, many of these fluorinated chemicals are very stable, non-reactive, and resistant to degradation, thereby increasing concern for their occurrence and persistence in the environment with such widespread use (Evich et al. 2022).
PFAS human health, epidemiological, and experimental in vivo toxicity studies have largely been limited to legacy PFAS comprised of carboxylic acid and sulfonic acid moieties of varying carbon chain lengths (e.g., perfluorobutanoic acid (PFBA; or C4); through perfluorodecanoic acid (PFDA, or C10) (Fenton et al. 2021). Although in vivo vertebrate studies have provided cross-species evaluations of PFAS toxicokinetics and subchronic and chronic effects ranging from endocrine disruption, reproductive and developmental toxicity and cancer, studies have similarly been limited to these legacy PFAS; primarily perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) (Fenton et al. 2021; Lau et al. 2006; Slotkin et al. 2008; White et al. 2011). Moreover, concern exists as other PFAS entities emerge as potential commercial replacements for legacy PFAS discontinued from manufacturing (Lindstrom et al. 2011; Wang et al. 2017). Compared against the list of 4730 substances meeting the Organisation for Economic Co-operation and Development (OECD)-defined criteria for PFAS (OECD 2018, 2021), it is clear that knowledge on the exposure, properties and health impacts remain largely unknown for a majority of these PFAS. Efficient, multi-pronged evaluation strategies are required to ensure timely protection of human health and ecological species.
With the release of the PFAS Strategic Roadmap (USEPA 2021), the United States Environmental Protection Agency (USEPA) has presented a comprehensive approach that invests in research, development, and innovation to increase understanding of PFAS exposures, toxicities, human health, and ecological effects that incorporate the best available science (USEPA 2021). Within the Agency, efforts have been underway to characterize the effects of individual PFAS and define categories of PFAS to establish toxicity values that will increase scientific understanding across the universe of PFAS. Such efforts hinge on the establishment of a PFAS testing library that provides adequate coverage across multiple structural categories, enabling application of a read-across approach (Patlewicz et al. 2019; Patlewicz 2022). To achieve this, a library of chemicals was selected that spans the structural diversity of this class with considerations of available exposure and toxicity data. This approach makes use of new approach methodologies (NAMs) to evaluate these substances more rapidly to fill data gaps, namely by evaluating in vitro high-throughput toxicity screening (HTS) and in vitro toxicokinetic assays to prioritize further in vivo testing. Not only are PFAS challenging due to their diverse chemical landscape but ensuring confidence in generated data is paramount to applying this approach to inform health risk.
Development of a robust HTS program is equally dependent on establishment of best practices in assay design and execution as it is on the quality and stability of the test agent stock solutions evaluated in such assays. Well-characterized HTS assays may employ positive and negative controls to confirm assay performance, but they are mainly chemical agnostic, where endpoint and dose-response evaluation is the focus (Judson et al. 2009; Kavlock et al. 2009; Krewski et al. 2010; Villeneuve et al. 2019). Commonly, in vitro assays utilize dimethyl sulfoxide (DMSO) which possesses amphipathic properties to solubilize a range of polar and non-polar substances; however, solubility issues have been noted that can impact HTS findings (Di and Kerns 2006; Kavlock et al. 2009). Whereas the establishment of a diverse PFAS screening library is driven by the need to gap fill across this largely data-poor space, the ability to provide a comprehensive stock evaluation is limited. Of the PFAS selected for this effort, certified reference standards are only available for 36: approximately 35% of PFAS in this library. Moreover, many of the selected PFAS were only available from one commercial source, obviating the ability to perform a secondary source verification. While scrutiny of the stock solutions is still warranted, flexibility in applied approaches will be necessary to provide the needed evaluation (Cousins et al. 2020).
As application of nuclear magnetic resonance (NMR) spectroscopy to evaluate stock purity may be outside the scope in certain efforts due to resource limitations, lack of expertise or low sensitivity, mass spectroscopy approaches can be used as a surrogate to monitor for stock stability concentration and/or degradation (Gathungu et al. 2020). The pharmaceutical industry heavily relies on liquid chromatography-mass spectrometry (LC-MS) to characterize new chemical entities, observe interferences, and identify both known and unknown metabolites, as it provides sensitive and selective qualitative and quantitative results for acidic and basic chemicals (Tolonen et al. 2009; Youdim and Saunders 2010). Some research groups have even created multi-step processes to assess complex mixtures, where specific indices are used in ensuring quality and/or purity as reference materials are unavailable for comparison (Z Li et al. 2021; Onel et al. 2019). In parallel, LC-MS methods exist that evaluate dozens of PFAS simultaneously or monitor specific PFAS in environmental samples through validated methods (Brase et al. 2021; Gremmel et al. 2017; Shoemaker and Tettenhorst 2020). The diversity of this class of perfluorinated chemicals has been shown to require additional tools for characterization, including derivatization and gas chromatography-mass spectrometry (GC-MS) for chemicals lacking ionizable groups and using high-resolution mass spectrometry to characterize unknown species (Henderson et al. 2007; McCord and Strynar 2019; Strynar et al. 2015; Washington et al. 2014). Overall, the tools developed for screening and PFAS can be brought together to enable comprehensive evaluations of these emerging PFAS chemistries.
Herein we present an analytical framework that utilizes mass spectrometry approaches to evaluate PFAS stock solution quality and stability. This workflow utilizes low resolution analytical techniques to determine that the chemical of interest is present in each solubilized stock by scoring each solution with simple data analysis criteria related to the presented data (i.e., molecular weight match, fragmentation pattern analysis, and/or minimal chemical interferences). Whereas three failed due to degradation in DMSO, the majority failed due to high vapor pressure and/or low boiling points, implicating volatilization either during solution preparation or analysis. While it should be noted that lack of detection may not mean a chemical is absent or degraded in a particular stock but rather may indicate a lack of amenability for the methods used, volatile compounds are similarly not amenable to in vitro toxicity screens and were triaged from further analysis. In summary, this mass spectrometry workflow proved very useful in providing an efficient evaluation to use during NAM data review. Lessons learned from these assessments are discussed, particularly regarding the diversity and unpredictability of PFAS chemicals and the critical aspects of ensuring good solution quality for in vitro assessment.
2. Materials and Methods
2.1: PFAS Stock Solutions
The PFAS that underwent evaluation were selected using a range of criteria including structural diversity and availability of toxicity data to aid in the development of a NAMs-based read-across strategy for evaluating PFAS (Patlewicz et al. 2019; Patlewicz 2022). Procurement of PFAS for preparation of DMSO screening stocks was conducted by Evotec Inc. (Bradford, CT, USA) under EPA contract (# EP-D-12–034). PFAS were obtained in neat (i.e., undiluted, powder or liquid) form from commercial vendors with a target purity concentration of ≥95%, confirmed by review of vendor certificates of analysis (Table S1). This target was achievable for most of the PFAS; two with lower purity (perfluorobutyraldehyde (75%); perfluoro-1,3-dimethylcyclohexane (82.4%)), were included in the interest of evaluating as many PFAS as possible. Selected PFAS ranged in molecular weight from 148.076 to 726.231 g/mol (1,1,1,3,3-pentafluorobutane) and perfluorooctanesulfonamido ammonium iodide, respectively). If achievable without noted precipitation, the PFAS were solubilized at a concentration of 30 mM (mass per volume ranging from 4.44 to 21.792 mg/mL) in DMSO and/or EtOH. Due to solubility issues, some compounds were prepared at concentrations as low as 5 mM. All solutions received at USEPA were stored at −80°C until use. The PFAS stock solution combinations assessed are listed in Table S1. The full PFAS chemical testing library, comprised of 430 substances, can be viewed along with supporting structural, hazard and exposure information can be viewed on the USEPA CompTox Chemicals Dashboard (https://comptox.epa.gov/dashboard/chemicallists/EPAPFASINV).
2.2: Standards and Solvents
For use during the concentration verification experiments, unlabeled PFAS analytes were purchased from Wellington Laboratories (Guelph, Ontario, Canada), including PFAC-24PAR (a mixture of 24 PFAS) and perfluoro-2-methyl-3-oxahexanoic acid (HFPO-DA). Bis(1H,1H-perfluoropropyl)amine (96%) and 3-(Perfluoro-2-butyl)propane-1,2-diol (99%) were sourced from Apollo Scientific Ltd (Bredbury, Stockport, UK). Perfluoropentanamide was procured from Vitas M Chemical Ltd (Causeway Bay, Hong Kong, China) and 1H,1H-Heptafluorobutanol (98%) was obtained from Sigma Aldrich (St. Louis, MO, USA). Additionally, isotope-labeled perfluorinated analytes (MPFAC-24ES, M3HFPO-DA, 2-Perfluorobutyl-(1,1,2,2-2H4)-ethanol (MFBET), 2-Perfluorohexyl (1,1-2H2,1,2-13C2)ethanol (MFHET), and 2-Perfluorooctyl (1,1-2H2,1,2-13C2)) were purchased from Wellington Laboratories. LC-MS Optima™ grade acetonitrile, water, and methanol as well as pesticide-residue grade dichloromethane were sourced from Honeywell, Burdick & Jackson (Muskegon, MI, USA). DMSO and ethanol (190 proof) were of at least ACS reagent grade and acquired from Sigma Aldrich and Fisher Scientific (Hampton, NH, USA), respectively. Ammonium acetate and ammonia solution (25% w/v) were obtained from Sigma Aldrich while formic acid was acquired from Fisher Scientific (Hampton, NH, USA).
2.3: Stock Quality Scoring Workflow
2.3.1: Analytical Detection Technique Selection
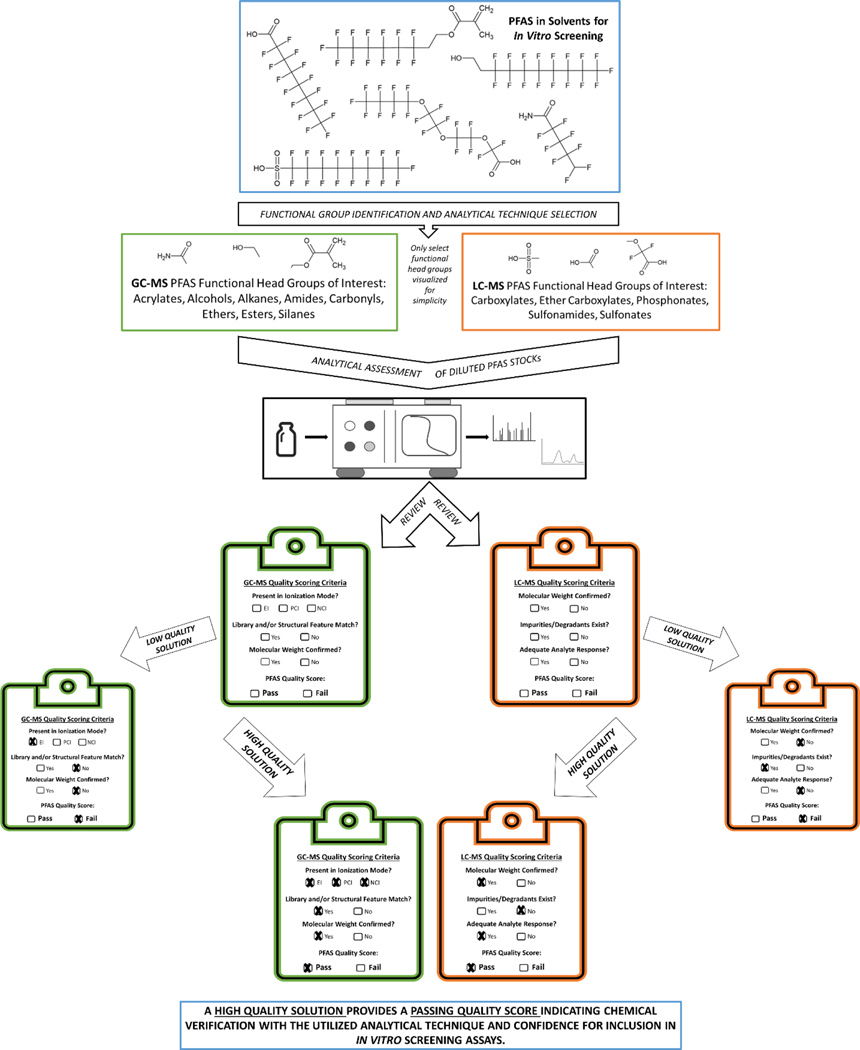
Figure 1 provides a workflow for this stock quality assessment. With the diverse number of PFAS known, the functional head group of each analyte was used to quickly determine the best analytical detection approach, either LC-MS or GC-MS. There is a wealth of available targeted PFAS detection methods used for environmental monitoring or developed in-house for other research and development purposes (Calafat et al. 2019; Gremmel et al. 2017; Henderson et al. 2007; USEPA 2020; Washington et al. 2014). Commonly, PFAS containing a carboxylate, sulfonate, ether carboxylate, or phosphonate functional head group are easily assessed by LC-MS, while alcohols, amides, and alkanes have improved detection capabilities by GC-MS (Figure 1, Table 1). There were only three PFAS for which this initial grouping strategy did not yield success: 2,2-difluoroethyl triflate, 1H, 1H, 7H-Perfluoroheptyl 4-methylbenzenesulfonate and 2-(trifluoromethoxy)ethyl trifluoromethanesulfonate: These were initially grouped to be evaluated by LC-MS. Subsequent evaluation by GC-MS yielded generation of successful quality scores for all but 2,2-Difluoroethyl triflate.
Figure 1.
PFAS Stock Solution Quality Assessment Workflow.
Table 1.
Representative PFAS groupings and Assigned Detection Methoda
| PFAS Group | Number per Group | Analytical Methodb | Examplesc |
|---|---|---|---|
| Per- and polyfluoroalkyl carboxylates | 28 | LC-MS | PFOA, 4H-PFBA, 5:3 FTCA |
| Alkanes | 27 | GC-MS | 1 -(Perfluorohexyl)octane |
| Per- and polyfluorinated alcohols and diols | 20 | GC-MS | 8:2 FTOH; Perfluoropinacol |
| Per- and polyfluoroalkyl sulfonates | 15 | LC-MS | 6:2 FTS; PFBS; PFOS |
| Amines and amides | 14 | GC-MS | Heptafluorobutyramide; 1H,1H-perflurooheptylamine |
| Acrylates, methacrylates and diacrylates | 13 | GC-MS | 8:2 Fluorotelomer acrylate |
| Ethers, esters and ethoxylates | 12 | GC-MS | 1H,1H-Heptafluorobutyl epoxide; tris(Trifluoroethoxy)methane; Methyl 2H,2H,3H,3H-perfluoroheptanoate |
| Perfluoroalkyl ether and polyether carboxylates | 8 | LC-MS | PFPE-7; PFPE-3 |
| Perfluoroalkanoyl chlorides | 7 | LC-MS | Hexafluoroglutaryl chloride |
| Per- and polyluoroalkyl sulfonamides | 7 | LC-MS | Perfluorohexanesulfonamide |
| Alkenes | 5 | GC-MS | 6H-Perfluorohex-1-ene |
| Silanes | 5 | GC-MS | Trichloro((perfluorohexyl)ethyl) silane |
| Sulfur-containing | 4 | GC-MS | 2-(Perfluorooctyl)ethanthiol |
Selected groups with 4 or more PFAS are listed. See Table S1 for the complete list of PFAS analyzed.
Analytical method initially selected for stock evaluation work.
Undefined abbreviations: 4H-PFBA, 4H-perfluorobutanoic acid; 5:3 FTCA, 5:3 fluorotelomer carboxylic acid; 8:2 FTOH, 8:2 fluorotelomer alcohol; 6:2 FTS, 6:2 fluorotelomer sulfonic acid; PFBS, Perfluorobutanesulfonic acid; PFPE-7, Perfluoro(4-methoxybutanoic) acid; PFPE-3, Perfluoro-3,6-dioxaheptanoic acid.
2.3.2: Liquid Chromatography-Mass Spectrometry (LC-MS)
To assess PFAS stock solutions amenable to LC-MS analysis, a Waters Corporation (Milford, MA, USA) ACQUITY I Class ultra-high-performance LC was used with modification of the Waters PFAS Solution Installation Kit (P/N 176004548). The chromatographic separation was carried out using a Waters CORTECS T3 reverse-phase column (3 mm x 100 mm, 2.7μm), a flow rate of 0.6 mL/min, and a binary mobile phase gradient with mobile phases A (95:5, 2.5 mM ammonium acetate: acetonitrile) and B (95:5, acetonitrile: 2.5 mM ammonium acetate). The gradient program was 6.5 min total and programmed as follows: 20% B (0.45 min), 20–50% B (0.15 min), 50–58% B (0.9 min), 58–66% B (0.75 min), 66–75% B (0.15 min), 75–80% B (1.2 min), 80–100% B (0.3 min), 100% B (1.74 min), 100–20% B (0.06 min), 20% B (0.8 min). 10 μL of each sample was injected.
Detection was performed with an interfaced Waters Xevo TQ-S micro triple quadrupole mass spectrometer operated in both positive (ESI+) and negative (ESI−) electrospray ionization modes. The source temperature was 150°C with desolvation temperature, desolvation gas flow, and cone gas flow at 500°C, 1000L/hr, 150 L/hr, respectively. MS full scan acquisitions (m/z 50–1000) in positive and negative modes were run simultaneously with multiple reaction monitoring (MRM) transitions (RADAR mode, (Waters 2015)), monitoring each PFAS unique transition while also any potential signals of interest in the full scan acquired. MRM transitions were previously optimized for PFAS analytes using available standards and/or PFAS stock solutions (Table S2).
2.3.3: Gas Chromatography-Mass Spectrometry (GC-MS)
Full scans were generated on a combination of Agilent Technologies (Palo Alto, CA) 6890/5973N GC-MS and 7890/7010B GC-MS-MS systems using a VF-624MS column (30m x 0.25mm, 1.4 μm film) with a helium flow rate of 1.2 mL/min, where one microliter of each sample was injected into a 150°C capillary inlet, operating in splitless mode (1 min, then 50 mL/min) fitted with a deactivated single-gooseneck liner. The temperature gradient used was as follows: 35°C for 2 min, then 5°C/min to 150°C, then 25°C/min to 280°C held for 5 min. The transfer line was maintained at 280°C. All solutions were run in electron impact (EI), and both positive (PCI) and negative (NCI) chemical ionization modes. Methane was used as the chemical ionization reagent with a flow of 20% and source temperature of 250°C in positive mode and 40% at 150°C in negative mode. Quadrupoles were maintained at 150°C in all modes and full scan data were collected in a range of m/z 40–550. When using the 7010B triple quadrupole, MS2 Scan mode was used, where all ions pass through Q1 and the collision cell and analyzed with Q2. Data were collected using Agilent ChemStation Version B.08 for the 6890/5973N system and MassHunter Acquisition Version 10.0 for the 7890B/7010B. All data were processed using Agilent MassHunter Qual version B.06 with NIST MS Search 2.0 Database and the NIST 17 mass spectra library (National Institute of Standards and Technology, Standard Reference Data Program, Gaithersburg, MD).
2.3.4: Preparation of PFAS Stock Solutions for Quality Assessment
Each PFAS stock solution was diluted to avoid saturating the detector from mM (ppm) to μM and nM (ppb) concentrations. Diluted solutions were prepared for each analytical instrument technique as described below.
For solutions assessed by LC-MS, an average PFAS molecular weight (MW) of 305 g/mol was used to convert the concentrated stocks in DMSO and EtOH to 104 ng/mL in acetonitrile to a final volume of 5 mL. These solutions were well mixed before a 1:100 dilution in LC-MS mobile phase was completed to provide a final at instrument concentration of approximately 105 pg/mL. A diluent blank prepared with the same dilution scheme was included to capture the solvent background signal and assess for interferences.
GC-MS analyzed PFAS stock solutions underwent a 1:1000 dilution by diluting 5 μL of the original DMSO stock in 5 mL dichloromethane for a final concentration of 30 μ.M (or 4 – 18μg/mL). No further dilution was required prior to GC-MS analysis.
2.3.5: Stock Quality Scoring Criteria
Quality scores were assigned for stock solutions to ensure each PFAS was present with the available analytical techniques performed. A stock solution could be assigned as either P (pass) or F (fail) (Table 2) based on specific criteria to the performed analytical technique. These scores erred on the side of passing a chemical for inclusion in subsequent testing due to the potential need for exposure dose verification. It is important to note that if a Fail decision was made due to lack of detection of the target analyte, it does not necessarily mean that the analyte was absent from the solution; rather the instrumentation and techniques utilized may have been unable to detect the chemical.
Table 2.
Scoring Descriptions for Quality Assessment of PFAS Stocks.
| Quality Score | Description |
|---|---|
| PASS | Chemical detected with utilized analytical instrumentation |
| FAIL | Chemical NOT detected and/or significant degradation evident |
To further provide interpretation of the quality score obtained or unique observations in the data analysis, identifier flags were used to indicate when caution is recommended for data interpretation. Often, these flags indicate characteristics or issues that may impact confidence in analyte presence, stability, and/or presence of contaminants, isomers, or transformation products. Flag identifiers were adapted from the ToxCast project (Richard et al. 2021) and are listed in Table 3. Visualizations of each flag identifier are shown in Figure S1.
Table 3.
Flag Identifiers for PFAS Stock Quality Assessment.
| Flag | Definition |
|---|---|
| E | Caution: suspected to be present but not confirmed |
| F | Caution: incorrect MW; biological activity unreliable |
| Fde | Caution: degradation evident, may be due to solvent or other unidentified source |
| Fns | Caution: no sample detected; biological activity unreliable |
| I | Isomers - two or more isomers detected |
| M | Defined mixture - two or more components present |
| P | Pseudo-parent or adduct monitored, no direct confirmation of parent analyte |
| W | Sample withdrawn |
| Z | MW confirmed, no purity information available |
2.3.5.1: LC-MS Quality Score Criteria:
Several criteria were used jointly to identify the presence of each PFAS by LC-MS. The main decisive factor used was a molecular weight match, which required knowledge of the ionization mode employed. Using MS full scan total ion chromatogram (TIC), an extracted ion chromatogram (XIC) was created and compared to the actual molecular weight while accounting for the ionization mode (e.g., [M+H]+ for ESI positive). The presence of a peak matching the parent compound suggested the presence of that chemical, where that retention time was noted to provide additional information for further confirmation.
Other measures used to further verify the presence of each PFAS included fragmentation pattern analysis (MS-MS), peak area percentage, and instrument response (e.g., signal attenuation). Using the retention time from the XIC, a mass spectrum was generated to examine what masses were present at that time. Through assessment of the chemical structure, observed fragmentation patterns could be confirmed, where a mass change of 50 m/z units (−CF2−) often indicates PFAS-like chemicals. The assessment of impurities or degradants was completed by using the MS TIC and determining if at least 85% of the total observed peak area was attributed to the retention time of interest from the XIC. Lastly, with comparison to a diluent-matched blank, the signal response could be examined to determine if any interferences and/or instrument background noise impacted peak shape and height.
Taken together, a high-quality sample was assigned as one where the compound was present by molecular match, when fragmentation patterns putatively confirmed structure, peak area percentage was greater than 85% of the total response, and the analyte of interest showed adequate instrument response. A mid-quality sample confirmed compound presence by MW match, but peak area was less than 85% of total response, and/or instrument attenuation was less than the threshold based on signal-to-noise or general peak height. A low-quality sample does not confirm the compound by MW match, exhibits poor instrument response, or may not be appropriate for LC-MS analysis. Ultimately, if the sample received high-quality or mid-quality score, a pass score was assigned. A sample was deemed fail if low-quality based on the assessed criteria.
2.3.5.2: GC-MS Quality Score Criteria:
Understanding the differences in ionization, detection, and instrument development between LC-MS and GC-MS, a modified approach was followed for PFAS stock assessment by GC-MS to determine solution quality. Chromatograms were evaluated for peak presence and co-occurrence across ionization modes (e.g., EI, NCI, PCI) to determine the quality score. Spectra were extracted, background subtracted, and evaluated to confirm chemical identity using NIST 17 (National Institute of Standards and Technology) database spectra for comparison when available. A high-quality passing score was assigned to solutions where the compound was detected in all three ionization modes, EI fragmentation was either confirmed by the library or matched key structural features, chemical ionization was confirmed by incorporation of ionization technique with molecular weight [M+1 (PCI), M-1 (NCI)], or common fluorinated alkyl fragments (EI) were observed. Potential degradation products or impurities were either not observed or were observed at insignificant levels in PFAS stocks receiving passing scores. Additionally, solutions were assigned passing scores where the identity was confirmed, one or more significant peaks related to the compound were observed, but the compound may not have been detected in all ionization modes. A failing score was assigned to solutions where the compound was not detected, or identity was unable to be confirmed regardless of ionization.
2.4: Concentration Check
To determine if the provided PFAS stock solutions in DMSO and EtOH were within 20% of the theoretical concentration, 25 unique analytes (10% of total number evaluated) were assessed by LC-MS or GC-MS. Detailed methodologic information is provided in Text S1. Briefly, DMSO and EtOH standards ranging from 5 to 30 mM were diluted in acetonitrile with matching labelled internal standards. A minimum of three technical replicates per stock solution were evaluated. Using a secondary standard for accurate concentration determination, a linear regression fit was applied to a calibration curve. Deviation from expected concentration was then computed for each PFAS stock to provide further knowledge on in vitro dose-effect calculations.
3. Results and Discussion
3.1: PFAS Stock Scoring Summary
PFAS stock solution preparation was conducted in multiple phases, with “DMSO1” representing the initial solubilization of 184 unique PFAS in DMSO. As reports emerged regarding potential degradation events associated with PFAS due in some instances to DMSO degradation (Liberatore et al. 2020), a second set of solubilizations (DMSO2) was undertaken to enable follow-up evaluation. This second set included many present in DMSO1 set but several additional PFAS to allow for substitution for any that may fail the quality check. A set solubilized in EtOH mirrored those included in DMSO2. In the end, a total of 471 PFAS-stock solutions in DMSO and EtOH, comprised of 205 unique PFAS were evaluated through this analytical stock quality workflow (Table 4). PFAS were assigned analysis by either LC-MS (75) or GC-MS (130) based on functional group presence, physicochemical properties. and knowledge of instrumentation performance as previously described. Two were withdrawn prior to analytical evaluation due to corrosivity concerns but remain included for tracking purposes. Out of the 205 unique PFAS in DMSO, 148 PFAS were confirmed to be present and of sufficiently high quality in the DMSO stock solutions for use in subsequent in vitro evaluations. It is important to note that failure to detect a specific analyte does not conclusively verify that the chemical is not present in the stock. Rather, given the detection strategies employed, it was not detected, and further instrumental assessment could be performed for verification and/or identification of the sample quality. A complete list of all quality scores and flag identifiers can be found in Table S1.
Table 4.
Quality Score Distribution for PFAS in DMSO and EtOH by LC-MS and GC-MS.
| Instrumentation Used in the Evaluation | |||||
|---|---|---|---|---|---|
| LC-MS | GC-MS | Total | |||
| PASS | FAIL | PASS | FAIL | ||
| DMSO1 | 63 | 3 | 62 | 56 | 184 |
| DMSO2 | 59 | 5 | 46 | 43 | 153 |
| Unique PFAS in DMSO | 72* | 3 | 76^ | 54 | 205 |
| EtOH | 49 | 2 | 43 | 40 | 134 |
| Total | 171 | 10 | 151 | 139 | 471 |
| TOTAT, Unique PFAS | 75 | 130 | |||
2 LC-able PFAS in DMSO showed a passing score in DMSO1, but not DMSO2
10 GC-able PFAS in DMSO showed a passing score in only one DMSO stock, not both
In summary, 54 of the 57 PFAS that failed were analyzed by GC-MS and primarily fell within the following structural categories: alkanes, alkenes, ethers, ketones, anhydrides. Specific scores and flags are provided in Table S1, Of those analyzed by LC-MS, the three that failed belonged to the hexafluoropropylene oxide class. Several unique trends were noticeable in stock quality, often easily differentiated by analytical technique and/or other properties related to structure, consumer use, and chemical interactions. These trends will be discussed below.
3.2: PFAS Concentration Verification
With the availability of certified standards for several well studied PFAS, a concentration check was possible for 25 unique analytes. The PFAS stock solutions were provided with the assumption that the concentration was as expected; typically, solutions in DMSO were 30 mM, whereas PFAS solubilized in EtOH were prepared at an expected concentration of 20 mM. Two sets of independently solubilized DMSO stock solutions were evaluated and are labeled as DMSO1 and DMSO2. Most PFAS evaluated were within 20% of the expected concentration (see Table 5 for a representative subset; Table S6 for the complete evaluation). Whereas many shorter chain-length carboxylic acid-containing PFAS showed reasonable agreement with target concentrations, a few PFAS carboxylates and sulfonates with at least an 8-carbon chain length were outside of this range. Poor DMSO solubility is implicated in lower than target concentrations for perfluorotridecanoic acid (13-carbon chain length); subsequent preparation of the DMSO2 stock at a 4-fold lower concentration yielded measured concentrations that fell within 13% of expected concentration. Stocks of perfluorotetradecanoic acid, well known to be insoluble in most commonly used solvents, were off by more than 50% in both DMSO preparations and by 37.6% in the EtOH preparation. Concentrations significantly deviating more than 20% from expected for a few other PFAS stocks were more difficult to explain and would require a more comprehensive evaluation that is beyond the scope of this effort. However, as studies across more diverse sets of PFAS evaluate sample stability and matrix effects, analyte-specific behaviors are emerging that implicate physicochemical properties (Taniyasu et al. 2022; Woudneh et al. 2019). Combined, our effort in conjunction with these studies, all underscore the importance of ongoing efforts to evaluate matrix-specific stability, quality, and solubility across these emerging PFAS. Fortunately, the use of sophisticated sample tracking and data pipelining in most HTS efforts allow for efficient flagging of problematic stocks during data review and evaluation (Filer et al. 2017; Richard et al. 2016).
Table 5.
Determined Concentration of Select PFAS in DMSO and EtOH Solutions.
| DTXSID | Common Name | Solvent Set | Expected Conc (mM) | Mean Measured Conc (mM)* | Deviation of Mean Measured vs Expected Conc(%) |
|---|---|---|---|---|---|
| DTXSID4059916 | Perfluorobutanoic acid (PFBA) | DMSO1 | 30 | 22.8 ± 1.1 | −23.9% |
| DMSO2 | 30 | 23.2 ± 1.2 | −22.7% | ||
| EtOH | 20 | 19.2 ± 0.6 | −3.8% | ||
| DTXSID6062599 | Perfluoropentanoic acid | DMSO1 | 20 | 17.50 ± 0.94 | −12.5% |
| DMSO2 | 30 | 24.69 ± 0.82 | −17.7% | ||
| EtOH | 20 | 19.63 ± 0.90 | −1.8% | ||
| DTXSID8031865 | Perfluorooctanoic acid (PFOA) | DMSO1 | 30 | 26.1 ± 0.6 | −13.0% |
| DMSO2 | 30 | 24.8 ± 1.0 | −17.5% | ||
| EtOH | 20 | 21.8 ± 0.8 | 8.9% | ||
| DTXSID90868151 | Perfluorotridecanoic acid | DMSO1 | 20 | 3.03 ± 0.47 | −84.80% |
| DMSO2 | 5 | 4.37 ± 0.66 | −12.60% | ||
| EtOH | 20 | 18.21 ± 1.57 | −8.90% | ||
| DTXSID3059921 | Perfluorotetradecanoic acid | DMSO1 | 10 | 4.55 ± 0.50 | −54.5% |
| DMSO2 | 5 | 7.53 ± 1.22 | 50.5% | ||
| EtOH | 20 | 12.48 ± 1.62 | −37.6% | ||
| DTXSID8059920 | Perfluoroheptanesulfonic acid | DMSO1 | 30 | 28.57 ± 2.18 | −4.8% |
| DMSO2 | 20 | 16.93 ± 1.52 | −15.4% | ||
| EtOH | 20 | 20.69 ± 2.40 | 3.4% | ||
| DTXSID6067331 | 6:2 Fluorotelomer sulfonic acid | DMSO1 | 30 | 21.08 ± 3.15 | −29.7% |
| DMSO2 | 30 | 30.61 ± 3.71 | 2.0% | ||
| EtOH | 20 | 24.81 ± 4.32 | 24.1% | ||
| DTXSID3031864 | Perfluorooctanesulfonic acid (PFOS) | DMSO1 | 30 | 11.8 ± 1.2 | −60.8% |
| DMSO2 | 30 | 18.6 ± 1.5 | −38.0% | ||
| EtOH | 20 | 16.1 ± 1.7 | −19.3% | ||
| DTXSID70366226 | Perfluoropentanamide | DMSO1 | 30 | 24.9 ± 2.5 | −20.5% |
| DMSO2 | 30 | 21.8 ± 0.6 | −37.6% | ||
| EtOH | 20 | 17.7 ± 1.1 | −13.2% | ||
| DTXSID1062122 | 4:2 Fluorotelomer alcohol | DMSO1 | 30 | 31.3 ± 2.5 | 4.2% |
| DMSO2 | 30 | 29.2 ± 1.2 | −2.8% | ||
| EtOH | 20 | 22.0 ± 1. 0 | 10.2% | ||
| DTXSID3038939 | Perfluorooctanesulfonamide | DMSO1 | 30 | 32.77 ± 1.30 | 9.2% |
| DMSO2 | 30 | 23.70 ± 1.04 | −21.0% | ||
| EtOH | 20 | 19.21 ± 1.09 | −4.0% |
Actual concentrations shown as mean ± standard deviation in mM notation. Three to ten technical replicates were analyzed per stock solution.
3.3: LC-MS Observations and Lessons Learned
3.3.1: LC-MS Method Development
The developed LC gradient method with a run time under 7 min was able to separate and detect 59 analytes, ultimately being able to independently examine 75 unique PFAS solubilized in DMSO and/or EtOH. Using MRM transitions (Table S2) allowed for sensitive detection of these analytes, as many eluted within a short window (Figure S2). Although method optimization could help improve the response or retention for specific PFAS (i.e., short chain dicarboxylic acid-bearing PFAS with early retention times), this method provided an appropriate foundation for the evaluation of each PFAS-stock solution. Below, we discuss several key findings from PFAS assessed by LC-MS that could be pivotal in decision making when it comes to conducting in vitro toxicity assays and interpreting generated results.
3.2.2: Legacy PFAS Assessment
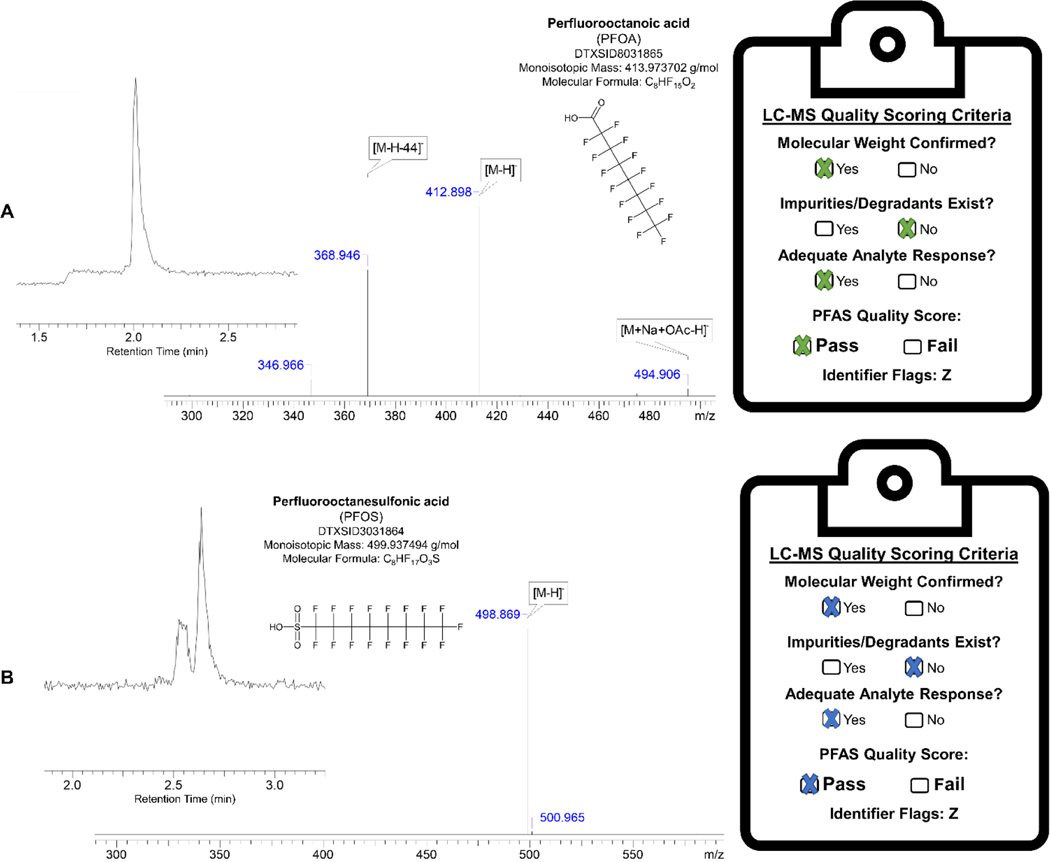
Historical long-chain PFAS consist of carboxylic or sulfonic acid functionality and are often referred to as legacy PFAS because of their prevalent use in society before 2000, where these chemicals gained regulatory attention due to human health and environmental impacts (Sun et al. 2016). For this assessment of PFAS in organic solvents, these legacy species ranged from 4 to 14 carbons in length bearing a carboxylate functional head group or up to 10 carbons with sulfonate functionality and were perfluorinated across the carbon backbone. This totaled 15 unique species, where all received passing quality scores in both DMSO and EtOH (Table S1). For instance, perfluorooctanoic acid (PFOA; Figure 2A) presented an easily detectable signal with confirmatory fragmentation as frequently reported in literature (Gremmel et al. 2017; Strynar and Lindstrom 2008). An identifier flag of Z was commonly assigned to substances with passing scores where the MW was confirmed but thorough purity checks requiring other sophisticated techniques like nuclear magnetic resonance (19F-NMR) were not performed in this body of work (Camdzic et al. 2021; Ellis et al. 2004). Perfluorooctanesulfonic acid (PFOS; Figure 2B) passed in both DMSO and EtOH, but presented as linear and branched isomers, being notated with an identifier flag of I. The presence of by-products (i.e., mixed linear and branched isomers) is commonly observed in PFAS produced through the synthetic route of electrochemical fluorination, while isomerically-pure PFAS is noted by telomerization, the new-age manufacturing approach (Benskin et al. 2010; Renner 2001). PFOS has been shown to have 70–75% of the chemical composition being the linear isomer. PFOS in DMSO (Figure 2B) includes about 35% branched isomers based on peak area percentage, within range of several noted manufacturers. Interestingly, other sulfonate-containing PFAS in DMSO and/or EtOH were not as predictable when it came to isomer composition (Table S1), suggesting that the synthetic production of some not possessing isomers were done by telomerization or other manufacturing processes (PFBS, PFHpS). Additionally, many salt forms (i.e., potassium, sodium, or ammonium) of these legacy analytes were examined, but were analytically perceived as the same ion as its legacy counterpart when put in an aqueous environment due to ion dissociation. All eight salt forms of legacy PFAS also received passing scores in DMSO and EtOH.
Figure 2.
LC-MS analysis of DMSO-prepared stocks of perfluorooctanoic acid (PFOA) [A] and perfluorooctanesulfonic acid (PFOS) [B]. Features of the MS ESI- TIC and mass spectrum were used to determine the presence of the chemical, ultimately indicating that both PFAS-stock solutions were of high quality and received a passing quality score.
3.3.3: Hexafluoropropylene Oxide (HFPO) Solvent Effects
One unique group of analytes examined are those belonging to the hexafluoropropylene oxide (HFPO) family, a set of chemicals designed to have shorter fluoroalkyl chains and to replace legacy PFAS of obvious toxicity concerns. Perfluoro-2-methyl-3-oxahexanoic acid (HFPO-DA) or its ammonium salt form GenX have gained attention as emerging PFAS of concern due to reported levels in water streams and subsequent toxicological assessment (Gaballah et al. 2020; Pan et al. 2018; Strynar et al. 2015). These chemicals as well as the trimer (Perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid, HFPO-TA,) and tetramer (Perfluoro-(2,5,8-trimethyl-3,6,9-trioxadodecanoic) acid, HFPO-TeA,) homologues were assessed in either DMSO, EtOH, and/or water in this work (Figure S3).
A unique feature of the HFPO family was that these species were detected as either decarboxylated species or fragments when examining the MS2 spectrum obtained by LC-MS (Figure S3). Although HFPO-DA can be detected as the full parent molecule, the signal intensity is much weaker than if observed as the decarboxylated fragment. As a result, each HFPO-containing PFAS received an identifier flag of P to note that the MW was not fully confirmed based on LC-MS assessment (Mullin et al. 2019). Improved sensitivity was also observed for HFPO-TA and HFPO-TeA accounting for this in-source effect. Additionally, adjustments to the method conditions may improve the response, especially if a universal method is desired for all PFAS species, including legacy chemicals and HFPO-containing species. One study showed that adjustments to the probe positioning, altering the energy applied, as well as increasing basicity, increased HFPO-DA for on-instrument sensitivity, allowing for incorporation with standard legacy PFAS for a more complete analysis method (Brase et al. 2021). Additionally, we found that lowering the desolvation gas temperature and flow rate improved the analyte response when these HFPO-containing PFAS were analyzed separately from other PFAS substances (data not shown). Other work has even suggested that UniSpray™, a novel ionization technique for LC-MS analysis, improves detectability of these less sensitive PFAS analytes, providing intensity gains more than four-times that of the traditional ESI source (Lubin et al. 2017; Organtini et al. 2020). These method alternations may be necessary as sample analysis is performed to meet quantitation and quality assurance standards but are not an endorsement to alter current USEPA methods if regulatory or environmental monitoring is being completed.
When HFPO-DA was solubilized in DMSO, no apparent signal was observed by LC-MS (Figure S4A). Former studies also have highlighted the apparent loss of HFPO-DA in several testing solvents for in vitro evaluation, including DMSO (Liberatore et al. 2020; C Zhang et al. 2021). Solvents like DMSO and acetone rapidly degrade HFPO-DA to Fluoroether E-1. HFPO-DA exhibited half-lives of 59 and 75 min, respectively, after solvent addition (Liberatore et al. 2020). In either EtOH or water, HFPO-DA was easily detected (Figure S4B and S4C, respectively), which also matches previous observations. HFPO-TA and HFPO-TeA, containing additional hexafluoropropylene units to HFPO-DA, exhibited the same phenomenon, indicating that DMSO solubilization is not appropriate, and a degradation product rapidly forms from this interaction (Bao et al. 2020; C Zhang et al. 2021). Of importance, stock solutions of any HFPO-containing PFAS should be methodically evaluated to ensure accurate toxicological evaluation.
3.3.4: Early Elution of Polar PFAS
A second set of PFAS that were manufactured as alternatives for long-chain legacy chemicals are short-chain and ultrashort-chain PFAS. Ultrashort-chain and short-chain PFAS have a carboxylic or sulfonic acid functional head group and are categorized by the number of CF2 moieties in their structure, with ultrashort-chain having 2–3 fully fluorinated carbons and short-chain compounds with 4–7 carbons (Ateia et al. 2019; Bjornsdotter et al. 2020; Brendel et al. 2018; Wang et al. 2017). These PFAS exhibit low MW, high polarity, and a charged-nature at environmental pH (Bjornsdotter et al. 2020); water remediation methods have failed to fully characterize this structural variant of PFAS compounds due to their mobile nature, where impacts have been observed in aquatic systems but remain uncertain in humans or co-eluting interferences in biological matrices and other materials masks accurate detection (Ateia et al. 2019; Bangma et al. 2021; Benskin et al. 2007; Brendel et al. 2018).
This assessed PFAS library contained several of these highly polar analytes (Table 6). Trifluoromethanesulfonic acid (TFMS) and trifluoroacetic acid (TFA) have low MW and are the shortest in chain length, but do not possess analytical assessment as they were removed from the library due to concerns of corrosivity. The remaining eight PFAS listed received passing quality scores, all eluting early in the LC gradient method. Interestingly, five of these polar PFAS appeared to not even be retained and exhibited poor peak resolution with the current reverse-phase LC method conditions (Figure S5), likely due to their physiochemical properties.
Table 6.
Select Properties of Assessed Polar PFAS Analytes
| Chemical Name | Molecular Formula | Average MW (g/mol) | Water Solubility (mol/L) | LogKow |
|---|---|---|---|---|
| Trifluoromethanesulfonic acid | CHF3O3S | 150.07 | 0.331 | 0.42 |
| Trifluoroacetic acid | C2HF3O2 | 114.02 | 8.744 | 0.65 |
| Tetrafluorosuccinic acid | C4H2F4O2 | 190.05 | 1.571 | 1.11 |
| Perfluoropropanoic acid (PFPrA) | C3HF5O2 | 164.03 | 0.147 | 1.42 |
| Hexafluoroglutaric acid | C5H2F6O2 | 240.06 | 0.145 | 1.90 |
| Perfluorobutane sulfonate (PFBS) | C4HF9O3S | 300.09 | 0.007 | 3.12 |
| Perfluorobutanoic acid (PFBA) | C4HF7O2 | 214.04 | 0.002 | 1.43 |
| Octafluoroadipic acid | C6H2F8O4 | 290.07 | 0.002 | 2.33 |
| Hexafluoroglutaryl chloride | C5Q2F6O2 | 276.94 | 0.005 | 2.96 |
| Perfluoro-3,6-dioxaoctane-1,8-dioic acid | C6H2F8O6 | 322.06 | 1.150 | 2.12 |
Water solubility and LogKow are predicted (Mansouri et al. 2019); other values were obtained from the US EPA CompTox Chemicals Dashboard (https://comptox.epa.gov/dashboard/).
Several analytical methods exist for examining ultrashort- and short-chain PFAS, ranging from GC coupled to electron capture detection to GC-MS with derivatization to LC-MS with variations to column chemistry to high-resolution MS (Ateia et al. 2019; Bjornsdotter et al. 2020; Janda et al. 2019; Taniyasu et al. 2008). Further investigation of how to better capture these PFAS with the available tools in our laboratory led us to examine hydrophilic interaction liquid chromatography (HILIC), a variant of normal phase LC to effectively separate i small polar compounds (Buszewski and Noga 2012). Utilizing an application note published by Waters (WA60096), a HILIC method was created for these polar PFAS, where the solvent pH was critical in effectively retaining the analytes for detection (Text S2, Figure S5). For instance, hexafluoroglutaric acid eluted within the dead volume on the CORTECS T3 column with reverse-phase chromatography (0.60 min), while the HILIC conditions increased confidence for quantitation with a retention time of 2.68 min. This method may still possess limitations for examining all ultrashort- and short-chain PFAS but provides one example of a more simplistic LC-MS method to improve retention and quantitation for future applications.
3.3.5: Fluorinated Alternatives Spectral Features
3.3.5.1: Sulfonamides as PFAS Mixtures
Perfluorooctane sulfonamides are a class of PFAS known as PFOS precursors, having a chemical structure of C8F17SO2NRR’ and are produced by electrochemical fluorination (W Zhang et al. 2021). This PFAS library examined seven unique chemicals belonging to this family, where over 85% of the unique stocks received passing quality scores with several identifier flags like I (linear and branched isomers), P (pseudo-parent monitored), and M (multiple components detected) (Table S1).
Interestingly, N-Ethylperfluorooctanesulfonamide (NEtFOSA) is also known as Sulfluramid, an insecticide having reported use since the late 1980s (Barbosa Machado Torres et al. 2021; Lofstedt Gilljam et al. 2016). This highly lipophilic PFAS compound exhibits transformation to PFOS by in vivo testing in earthworms and rodents, in vivo investigation of Artic animals, and sediment and groundwater testing (Letcher et al. 2014; Manning et al. 1991; Mejia Avendano and Liu 2015; Nascimento et al. 2018; Nguyen et al. 2016; Zhao et al. 2018). This transformation to PFOS has been suggested to occur through various intermediates, where NEtFOSA is also an intermediate from higher MW sulfonamides commonly used in food packaging (Scheme S1) (Evich et al. 2022; Fu et al. 2015; Mejia Avendano and Liu 2015).
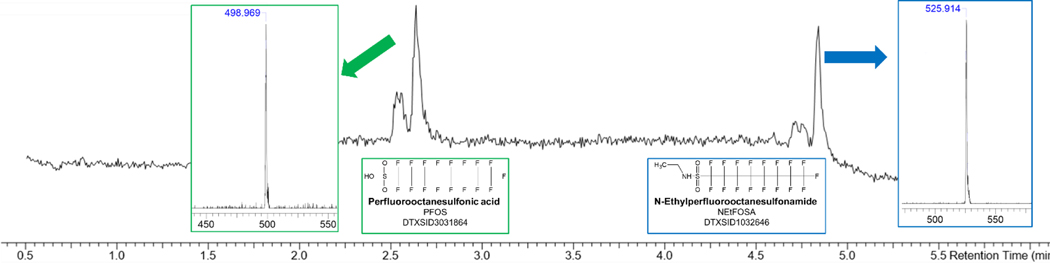
NEtFOSA was the only sulfonamide that visibly presented as a mixture of PFAS chemicals in our library. In DMSO (Figure 3), the stock solution presented as both NEtFOSA and PFOS, with only 32% of the peak area in the TIC being accounted by NEtFOSA. Other structurally similar PFAS did not present as a mixture in this study, which could be a result of the solvent-chemical interaction or vendor-specific synthesis. Work has also suggested that storage conditions play a critical role in the biodegradation of sulfonamide-containing PFAS, like NEtFOSA, particularly in environmental water samples (Woudneh et al. 2019). Our PFAS stocks in DMSO and EtOH were stored at −80°C, much lower than Woudneh’s recommendation, so future work would be required to characterize the impact of such cold storage temperature conditions on sample quality and potential transformation to other PFAS species, particularly for use in in vitro toxicological assessments.
Figure 3.
100 pg/μL N-Ethylperfluorooctanesulfonamide (NEtFOSA) in DMSO presented as a mixture of PFAS compounds. PFOS accounts for nearly two-thirds of the observed response, eluting near 2.6 min, while NEtFOSA is identified at 4.75 min.
3.3.5.2: Degradation of Acyl and Sulfonyl Halide PFAS
Evaluations of eight acyl and sulfonyl halides of PFAS within our library confirmed previous reports of analytical challenges posed by this particular group. Ranging from four to eight carbons in length (see Table S7), five acyl halides (four with Cl; one with F) and two sulfonyl halides (one with Cl; two with F) were screened. Exothermic hydrolysis of acyl chlorides (RC(O)Cl) in the presence of water or hydroxide to form a carboxylic acid and hydrogen chloride via three nucleophilic substitution mechanisms has been described (Scheme S2) (Douglas et al. 1993; Hall 1955; Jackson and Mabury 2013). Acyl fluorides (RC(O)F) likely can only undergo the addition-elimination pathway due to proposed bond breaking and formation of the related carboxylic acid (Bunton and Fendler 1966). Given the high likelihood of rapid hydrolysis under aqueous conditions, we examined these halide precursors by monitoring their carboxylate or sulfonate degradant by LC-MS. The flag of P was incorporated for these PFAS to denote that the parent molecule was not directly monitored by LC-MS. Compared to the carboxylic acid, acyl halides of the same chain length often had similar instrumental signal intensities based on a magnitude scale.
Sulfonyl fluorides (PFBS-F and pefluorooctanesulfonyl fluoride (POSF)) present analytical challenges that are sufficiently distinct from the acyl halides. Quantitation of POSF is particularly difficult due to the lack of a chromophore for UV or fluorescence detection, absence of peaks by GC or GC-MS analysis, and a lack of an ionizable functional group for LC-MS (Sun et al. 2011). Sun reported a method to determine POSF by LC-MS using conjugation with benzylamine, but this derivatization can be difficult and can provide an additional degree of uncertainty in analytical quantification efforts. In this evaluation, POSF and PFBS-F were less than 0.1% signal abundance to their sulfonic acid relative. As our assessment of these PFAS assumed complete transformation to their carboxylic and sulfonic acid relatives (in an aqueous environment), caution should still be applied for characterizing these analytes as sensitivity was low for most species and the interaction of these chemicals in an in vitro environment could further transform the original material.
Historically, sulfonyl and acyl halide species of PFAS have served as precursor species in the manufacture of PFOS and PFOA. In 2009, PFOS and its precursor POSF were placed on the Stockholm Convention Annex B list of persistent organic pollutants, restricting use in products ranging from carpet stain repellants to paper packaging to repel grease to fire-fighting foams (Paul et al. 2009; Wang et al. 2013). This subsequently limited the production and potential exposure to PFOS, yet manufacturers continued to produce alternative chemicals with similar properties. Concerns continue to exist on the impacts of these precursors, as greater quantities of such species were globally released with in vitro research indicating the ability of these sulfonyl fluorides to bind to human serum (Jin et al. 2019; Paul et al. 2009). The rates at which POSF degrades to PFOS in the environment has not been well characterized.
3.3.5.3: In-Source Fragmentation of Multi-Ether Carboxylic Acids
As legacy PFAS continue to be phased out, the production of diverse perfluoroethercarboxylic acids (PFECAs) has rapidly increased. This family of compounds differ only from their legacy PFAS ancestors by incorporating one or more ether linkages along the carbon-fluorine backbone, with expectations of being more degradable due to their increased hydrophilicity and easier elimination from biotic systems (Pan et al. 2018; Strynar et al. 2015; Wang et al. 2013). One well-known example from this family is HFPO-DA as discussed previously. Growing concern has emerged that the change in molecular configuration could be impactful to their toxicological effects and bioaccumulative potential (Y Li et al. 2021; Wang et al. 2020).
In our library, seven PFECAs besides those in the HFPO acid class were assessed. All PFECAs provided passing quality scores regardless of being solubilized in DMSO and EtOH, but four were monitored as pseudo-parents (identifier flag P) for quantitation purposes due to the poor sensitivity exhibited (Figure S6). These PFECAs contain multiple ether linkages and appeared to fragment in-source, while the three other mono-ether carboxylic acids remained as intact compounds by LC-MS, suggesting that the structure impacts ionization and fragmentation. A loss of 95 Da was observed, corresponding to -CF2COOH. The degradation of these multi ether PFECAs could be a result of altered bond dissociation energies, partially achieving one appeal of these replacement PFAS (Bentel et al. 2020). Additionally, these in-source artifacts could be a result of the specific instrumentation used or be pH or concentration dependent (McCord and Strynar 2019; Strynar et al. 2015; Washington et al. 2020; Zhang et al. 2019). It is suggested that the MRM transition of the fully intact structure be included alongside the pseudo-parent for additional confirmation. Given these PFECAs are known to fragment in source, detecting the pseudo-molecular ion in this case seems more suggestive of the parent being intact than to assume that the compound is degraded in solution. Regardless, further investigation of potential PFECA transformation in in vitro assay systems may be warranted to ensure appropriate interpretation of resulting bioactivity data.
3.4: GC-MS Observations and Lessons Learned
3.4.1: GC-MS Conditions
With the early retention of many of the PFAS that were selected for GC-MS analysis and the presence of a considerable amount of DMSO in the stock dilutions, using a DB-624 column with a 1.4 μm film was selected to enhance the separation of the analyte from the stock and dilution solvents. To further improve identification and detection, the choice was made to run each PFAS sample in EI, PCI, and NCI modes using the same chromatographic conditions and then compare results side-by-side to determine the quality score (a representative example is shown in Figure S7).
EI at 70 eV is the most common ionization method used in GC-MS for identification due to the structural information provided from positive ion fragments. These fragments and fragmentation patterns are representative of a compound’s chemical structure and are reproducible across GC-MS instruments, forming the basis of most mass spectral libraries (Dunn 2011). Since EI is a high energy technique, there are many cases where either low abundance of or no molecular ion is observed. Although decreasing the eV applied to the filament is possible, researchers generally rely on other softer ionization techniques where a pseudo-molecular ion is often observed in a [M+H]+ (PCI) or [M-H]− (NCI). PCI and NCI have been routinely used for qualitative and quantitative analysis of PFAS (Dufková et al. 2012; Jahnke et al. 2007; Whitehead et al. 2021) and thus were deemed valid confirmation techniques for our stock quality assessments. We used the frequency of detection among the three ionization modes as a factor in determining the quality score for each PFAS stock because the analyte was identified by all three techniques, resulting in a higher level of confidence due to the increased level of confirmation. Specifically, when parent ions were detected in chemical ionization modes, molecular weight of the stock being evaluated was considered and often confirmed.
3.4.2: Physiochemical Properties and Detection
It is well accepted that the more volatile and less polar a compound is, the more amenable it is for analysis by GC-MS when compared to LC-MS. Generally, esters, ketones, aldehydes, alcohols, ethers, and acrylates are preferential to GC-MS analysis, while organic acids and ionic species are analyzed by LC-MS as discussed in Section 3.3.
Several groups of PFAS assessed by GC-MS received failing quality scores. The diversity of PFAS functional head groups likely impacted the analytical detection capabilities and subsequent scoring values. Perfluorocycloalkanes, which have been historically used as atmospheric tracers (Lagomarsino 1996), were undetected in the stocks regardless of diluent. The 14 alkanes evaluated across 35 stocks have relatively low boiling points and likely eluted during the solvent delay. Techniques such as cryotrapping in the inlet have begun to become more routine in volatile and semi-volatile GC-MS analysis but such evaluations were beyond the scope of the current assessment. Likewise, the C4-C6 perfluorinated ethers were not detected, assumed due to co-elution with the solvent or degradation in the inlet. Recently, a few laboratories have utilized combinatorial GCxGC-MS analytical techniques as they have been shown to detect analytes less than C4 and cryo-based technique could be potentially applied to the failed GC-MS PFAS in this study as potential options for future investigation. Despite this indicating that the analytical QC approach was likely inadequate, the likelihood of these compounds volatilizing out of 37°C in vitro assay media is high, confounding meaningful bioactivity evaluations in conventional HTS assays. For the purposes of this evaluation, these stocks were flagged “Fns” to indicate the stock failed due to “no sample” detected, with additional annotations indicating compound volatility.
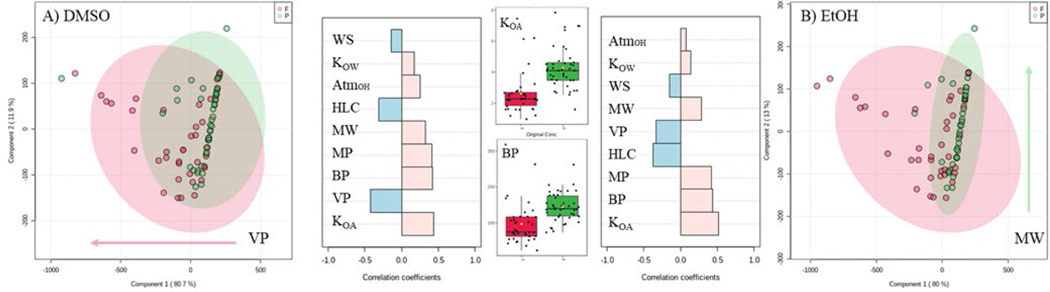
Due to the foundational principles of GC-MS analysis, (i.e. conversion of the analyte into its vapor phase), trends in the differences in the chemical properties of the analytes were investigated using multivariate statistical analyses. The Open (Quantitative) Structure-activity/property Relationship App (OPERA) was used to predict the physicochemical parameters of the GC-MS amenable compounds such as octanol-air partition coefficient (LogKOA), melting point, boiling point (BP), and vapor pressure (VP) (Mansouri et al. 2019). Using MetaboAnalyst 3.0 (www.metaboanalyst.ca), predicted properties for each PFAS were examined and compared to the binary pass/fail score system (Figure 4). As expected, vapor pressure was identified as significant in both PCA and partial least squares discriminant analysis (PLS-DA) with a variable importance in projection (VIP) >2.7 in the latter. Since the vapor pressure and boiling point of a compound is frequently associated with its polarity, it is understandable that this parameter was predictive of a passing score in the current study, and it should be noted that the closer the polarity of the stationary phase of the GC column, the more likely the analyte will be retained for chromatographic separation. Molecular weight was also positively associated with a passing score. Overall, higher LogKOA, MW and BP, but lower VP and Henry’s law constant were most associated with passing GC-MS scores.
Figure 4.
Score plots from the principal component analysis (PCA) using the physiochemical parameters of the GC-MS amenable compounds and classed by their binary pass/fail indicator for DMSO stocks (A) and EtOH stocks (B). Further correlation analysis was conducted with Metaboanalyst’s Pattern Hunter module setting the predefined profile as fail (negative correlation coefficients) and pass (positive correlation coefficients). Regardless of the solvent, the top indicators for failed quality scores were vapor pressure (VP) and Henry’s law constant (HLC), while pass criteria were more associated with the predicted octanol-air partition coefficient (KOA) and boiling point (BP). Other classifiers investigated include water solubility (WS), octanol-water partition coefficient (Kow), atmospheric hydroxylation rate (AtmoH), molecular weight (MW), and melting point (MP).
3.4.3: Impact of DMSO Co-Elution
One factor impacting GC detectability of the PFAS stocks was the presence of DMSO at a high concentration. DMSO has a density of 1.1 g/mL, so in each 1: 1000 diluted stocks, the concentration of DMSO and the PFAS analyte were 1.1 mg/mL and 4 to 18 μg/mL, respectively. The resulting DMSO peak was around 36 sec wide, spanning a temperature range of around 3°C in the current chromatographic method. While this is a relatively small temperature range, we observed co-elution with several analytes. When we were able to observe a peak co-eluting with DMSO, we were able to extract and identify ion chromatograms through background subtraction and confirm identities using chemical ionization data. Unfortunately, the detector of the mass spectrometer often was saturated, and the preservation of the compound’s expected fragments and even pseudo molecular ions were limited or reduced. While DMSO can be removed from solution using solid phase extraction (SPE), solvent exchange, or freeze drying, performing these techniques for volatile compounds introduces the risk of analyte loss. Limited attempts to remove DMSO from the stocks were performed by association with calcium chloride and solvent exchanging into methyl tert butyl ether. This practice reduced the peak area of DMSO over 90% and afforded better determination of select PFAS. As DMSO interference is likely to impact only a small subset, this more labor-intensive solvent exchange approach could be employed after the first-tier quality screening effort to provide further evaluation power in assessing the impacts of solvent diluent on analytes of interest.
3.4.4: Degradation Potential
DMSO has many positive properties for use in HTS assay, including low toxicity, low vapor pressure, water miscibility, and the ability to dissolve many chemicals, yet problems such as decomposition and precipitation have been noted (Waybright et al. 2009; Zitha-Bovens et al. 2009). One concern with the assessment of PFAS solubilized in DMSO and analyzed by GC-MS was the potential for degradation and may account for several determined failed quality scores.
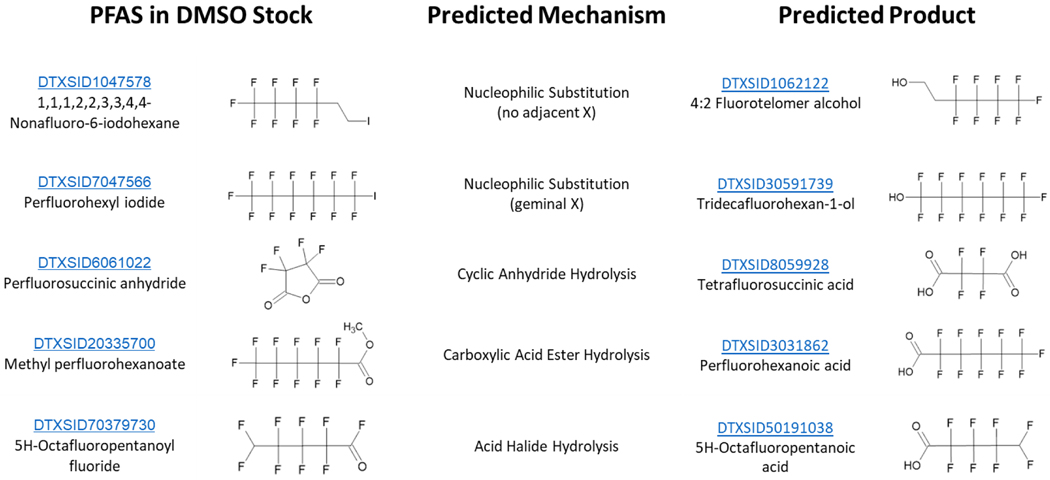
Predictions of hydrolysis pathways were generated using the CTS hydrolysis reaction library in the CTS: Chemical Transformation Simulator (https://qed.epa.gov/cts/) using a procedure described by Tebes-Stevens (Tebes-Stevens et al. 2017). When compared to PFAS with failing assessment scores, there were nine potential cases where, based on these predictions and observations in the GC-MS data, hydrolysis may have occurred. Several examples of these chemicals and their products that have the potential for hydrolysis are shown in Figure 5. This was not thoroughly investigated as part of this screening effort, but evidence of degradation was observed in each of those stocks. As a caution for the interpretation of in vitro assay results and an outlook for future investigations, controlled experiments as done by other researchers (Liberatore et al. 2020) will be needed to explore and accurately describe these mechanisms and degradation rates.
Figure 5.
Examples of potential PFAS hydrolysis products for chemicals assessed with failing assessment scores. Predictions were made using the CTS: Chemical Transformation Simulator.
4. Considerations for a Chemical Assessment Workflow for In Vitro Assays
The creation of an analytical chemistry workflow for in vitro HTS assays was completed to assess the quality of over 200 unique PFAS solubilized in solvents, totaling more than 460 unique solutions examined. Although the intent was to develop an efficient analytical evaluation for these specific chemical-solvent solutions, subsequent directed examinations may be required to inform data interpretation, depending on specific HTS study design and scope. We have developed several key takeaways for the scientific community to consider when undertaking either a large library of chemicals, diverse and unusual PFAS, and/or incorporation of multiple analytical techniques to guide in vitro toxicity data outcomes.
A key feature of this analytical approach is to determine the presence and general quality of each solubilized PFAS chemical, not purity. Chemical purity was assumed valid based on provided certificate of analysis documentation from third party chemical vendors. Based on our analysis, the diluent used for solubilization can greatly impact the stock quality utilized for in vitro assessments. A thorough and complete purity check would require the incorporation of additional chemical analyses, like nuclear magnetic resonance and high-resolution mass spectrometry, which would necessitate additional time for completion. This approach utilizing LC-MS and/or GC-MS was completed in a relatively rapid manner (on the order of weeks), from chemical receipt, to analysis, to interpretation of the acquired data with solution scoring. For this PFAS library, several scientific experts are completing a range of in vitro assays that focus on specific endpoints, including high-throughput transcriptomics, developmental neurotoxicity, and toxicokinetics, which will be incorporated to assess potential risk and prioritize subsequent toxicity testing (Patlewicz et al. 2019; Patlewicz 2022). The sophisticated in vitro approaches made assumptions that the chemical solution at hand was indeed what was listed. This analytical workflow provided the means to enable additional confidence on the presence of each chemical in solution, primarily DMSO, and provide a secondary source of information for in vitro data interpretation (Houck et al. 2021).
These results support the need to consider solubility, stability, and storage and handling early in the in vitro testing strategy. We recommend scientists not only confirm the chemical presence, but also ensure that the diluent of use for the in vitro assay does not confound the assay environment or the chemical itself. With DMSO being the preferred solvent to solubilize chemicals for such HTS assays, several PFAS exhibited poor stability in DMSO due to transformation to other like-chemicals, degradation, or instrumental detection interference. These observations are consistent with recently published efforts, further emphasizing the need to be considerate of such decisions (Liberatore et al. 2020; C Zhang et al. 2021). Use of water or protic solvents like ethanol may stabilize the parent structure, but case-by-case studies are necessary to further confirm the impact of solvent composition on chemicals, particularly PFAS, and the in vitro work at hand. Storage and handling of these compounds can also influence their stability in solution (Taniyasu et al. 2022; Woudneh et al. 2019), and solvent choice can promote or limit potential transformations, such as esterification (i.e., for PFCAs) or oxidation (i.e., for FTOHs).
The approach described has some limitations that bear additional articulation. As previously mentioned, lack of analyte detection using the approaches employed does not necessarily mean the analyte is not present, but rather not amenable to the detection strategies employed. For the purposes of informing stock use in HTS assay platforms, the association between failure to detect and high vapor pressure implies a lack of amenability for such assays due to likelihood of volatilization from in vitro systems. Additional consideration needs to be given to stock solution stability over time and after freeze-thaw cycles. Efforts that evaluated stock concentration did not extend to evaluating inter-day variations. The intent of this effort has been to provide an efficient, qualitative evaluation that could provide immediate value in HTS data interpretation while informing study design needs for any subsequent evaluations.
Exposure and dose verification are becoming paramount in HTS assays as the research community aims to transition away from whole animal or vertebrate testing strategies. Specifically, in the development and translation of NAMs for risk assessment, a toxicant’s dose response will ultimately best inform accurate hazard potential. In the current study, our data highlights that these verification techniques are especially necessary for PFAS since most of the compounds are presumed stable due to the strength of the carbon-fluorine bond and their designation as ‘forever chemicals’. Our aims were to develop a rapid and efficient testing strategy to classify procured stocks of these compounds by assessing parameters such as chemical presence, potential degradation or contamination, and variation of target concentration. Considerations in 1) analytical instrumentation and methodology challenges, 2) solubility, stability, and potential contamination of chemical standards, and 3) storage and handling conditions have been described and inform our workflow. Ultimately, the presented strategy specifically allows researchers to establish the presence of the solubilized PFAS prior to, during, or post-investigations in in vitro toxicity testing.
Supplementary Material
Acknowledgement and Disclaimers
The authors would like to thank Katherine Coutros (USEPA) for managing the Evotec contract, Michael Jobling and Rob Pinney of Evotec SE for the expert procurement of the PFAS chemical library, and Matt Phillips and Lucas Albrecht for their technical assistance. The authors would also like to acknowledge technical reviewers Brett Blackwell and Rogelio Tornero-Velez; and Ann Richard, Antony Willams, and Michael DeVito at USEPA and David Crizer at the National Toxicology Program for providing their subject matter expertise. The views expressed in this manuscript are those of the authors and do not necessarily reflect the statements, opinions, views, conclusions, or policies of the United States Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement for use.
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest or no known competing financial interests or personal relationships that could have appeared to influence the work reported in this publication.
References
- Ateia M, Maroli A, Tharayil N, Karanfil T. 2019. The overlooked short- and ultrashort-chain poly- and perfluorinated substances: A review. Chemosphere 220:866–882. [DOI] [PubMed] [Google Scholar]
- Bangma JT, Reiner J, Fry RC, Manuck T, McCord J, Strynar MJ. 2021. Identification of an analytical method interference for perfluorobutanoic acid in biological samples. Environmental Science & Technology Letters 8:1085–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Cagnetta G, Huang J, Yu G. 2020. Degradation of hexafluoropropylene oxide oligomer acids as pfoa alternatives in simulated nanofiltration concentrate: Effect of molecular structure. Chemical Engineering Journal 382. [Google Scholar]
- Barbosa Machado Torres F, Guida Y, Weber R, Machado Torres JP. 2021. Brazilian overview of per- and polyfluoroalkyl substances listed as persistent organic pollutants in the stockholm convention. Chemosphere: 132674. [DOI] [PubMed] [Google Scholar]
- Benskin JP, Bataineh M, Martin JW. 2007. Simultaneous characterization of perfluoroalkyl carboxylate, sulfonate, and sulfonamide isomers by liquid chromatography-tandem mass spectrometry. Anal Chem 79:6455–6464. [DOI] [PubMed] [Google Scholar]
- Benskin JP, De Silva AO, Martin JW. 2010. Isomer profiling of perfluorinated substances as a tool for source tracking: A review of early findings and future applications. Rev Environ Contam Toxicol 208:111–160. [DOI] [PubMed] [Google Scholar]
- Bentel MJ, Yu Y, Xu L, Kwon H, Li Z, Wong BM, et al. 2020. Degradation of perfluoroalkyl ether carboxylic acids with hydrated electrons: Structure-reactivity relationships and environmental implications. Environ Sci Technol 54:2489–2499. [DOI] [PubMed] [Google Scholar]
- Bjornsdotter MK, Yeung LWY, Karrman A, Ericson Jogsten I. 2020. Challenges in the analytical determination of ultra-short-chain perfluoroalkyl acids and implications for environmental and human health. Anal Bioanal Chem 412:4785–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brase RA, Mullin EJ, Spink DC. 2021. Legacy and emerging per- and polyfluoroalkyl substances: Analytical techniques, environmental fate, and health effects. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel S, Fetter E, Staude C, Vierke L, Biegel-Engler A. 2018. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under reach. Environ Sci Eur 30:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Environ Assess Manag 7:513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunton CA, Fendler JH. 1966. The hydrolysis of acetyl fluoride. J Org Chem 31:2307–2312. [Google Scholar]
- Buszewski B, Noga S. 2012. Hydrophilic interaction liquid chromatography (hilic)--a powerful separation technique. Anal Bioanal Chem 402:231–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC, Wong LY. 2019. Legacy and alternative per- and polyfluoroalkyl substances in the u.S. General population: Paired serum-urine data from the 2013–2014 national health and nutrition examination survey. Environ Int 131:105048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camdzic D, Dickman RA, Aga DS. 2021. Total and class-specific analysis of per- and polyfluoroalkyl substances in environmental samples using nuclear magnetic resonance spectroscopy. Journal of Hazardous Materials Letters 2. [Google Scholar]
- Cousins IT, DeWitt JC, Gluge J, Goldenman G, Herzke D, Lohmann R, et al. 2020. The high persistence of pfas is sufficient for their management as a chemical class. Environ Sci Process Impacts 22:2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di L, Kems EH. 2006. Biological assay challenges from compound solubility: Strategies for bioassay optimization. Drug Discov Today 11:446–451. [DOI] [PubMed] [Google Scholar]
- Douglas JE, Campbell G, Wigfield DC. 1993. Studies on the bal2 mechanism for ester hydrolysis. Can J Chem 71:1841–1844. [Google Scholar]
- Dufková V, Čabala R, Ševčík V. 2012. Determination of c5–c12 perfluoroalkyl carboxylic acids in river water samples in the czech republic by gc–ms after spe preconcentration. Chemosphere 87:463–469. [DOI] [PubMed] [Google Scholar]
- Dunn WB. 2011. Chapter two - mass spectrometry in systems biology: An introduction. In: Methods in enzymology, Vol. 500, (Jameson, Verma M, Westerhoff HV, eds):Academic Press, 15–35. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Denkenberger KA, Burrow TE, Mabury SA. 2004. The use of 19f nmr to interpret the structural properties of perfluorocarboxylate acids: A possible correlation with their environmental disposition. J Phys Chem A 108:10099–10106. [Google Scholar]
- Evich MG, Davis MJB, McCord JP, Acrey B, Awkerman JA, Knappe DRU, et al. 2022. Per- and polyfluoroalkyl substances in the environment. Science 375:eabg9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. 2021. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ Toxicol Chem 40:606–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer DL, Kothiya P, Setzer RW, Judson RS, Martin MT. 2017. Tcpl: The toxcast pipeline for high-throughput screening data. Bioinformatics 33:618–620. [DOI] [PubMed] [Google Scholar]
- Fu Z, Wang Y, Wang Z, Xie H, Chen J. 2015. Transformation pathways of isomeric perfluorooctanesulfonate precursors catalyzed by the active species of p450 enzymes: In silico investigation. Chem Res Toxicol 28:482–489. [DOI] [PubMed] [Google Scholar]
- Gaballah S, Swank A, Sobus JR, Howey XM, Schmid J, Catron T, et al. 2020. Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to genx and other pfas. Environ Health Perspect 128:47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathungu RM, Kautz R, Kristal BS, Bird SS, Vouros P. 2020. The integration of lc-ms and nmr for the analysis of low molecular weight trace analytes in complex matrices. Mass Spectrom Rev 39:35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluge J, Scheringer M, Cousins IT, DeWitt JC, Goldenman G, Herzke D, et al. 2020. An overview of the uses of per- and polyfluoroalkyl substances (pfas). Environ Sci Process Impacts 22:2345–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremmel C, Fromel T, Knepper TP. 2017. Hplc-ms/ms methods for the determination of 52 perfluoroalkyl and polyfluoroalkyl substances in aqueous samples. Anal Bioanal Chem 409:1643–1655. [DOI] [PubMed] [Google Scholar]
- Hall HK. 1955. Mechanisms of hydrolysis of carbonyl chlorides. J Am Chem Soc 77:5993–5996. [Google Scholar]
- Henderson WM, Weber EJ, Duirk SE, Washington JW, Smith MA. 2007. Quantification of fluorotelomer-based chemicals in mammalian matrices by monitoring perfluoroalkyl chain fragments with gc/ms. J Chromatogr B Analyt Technol Biomed Life Sci 846:155–161. [DOI] [PubMed] [Google Scholar]
- Houck KA, Patlewicz G, Richard AM, Williams AJ, Shobair MA, Smeltz M, et al. 2021. Bioactivity profiling of per- and polyfluoroalkyl substances (pfas) identifies potential toxicity pathways related to molecular structure. Toxicology 457:152789. [DOI] [PubMed] [Google Scholar]
- Jackson DA, Mabury SA. 2013. Polyfluorinated amides as a historical pfca source by electrochemical fluorination of alkyl sulfonyl fluorides. Environ Sci Technol 47:382–389. [DOI] [PubMed] [Google Scholar]
- Jahnke A, Ahrens L, Ebinghaus R, Temme C. 2007. Urban versus remote air concentrations of fluorotelomer alcohols and other polyfluorinated alkyl substances in germany. Environmental Science & Technology 41:745–752. [DOI] [PubMed] [Google Scholar]
- Janda J, Nodler K, Brauch HJ, Zwiener C, Lange FT. 2019. Robust trace analysis of polar (c2-c8) perfluorinated carboxylic acids by liquid chromatography-tandem mass spectrometry: Method development and application to surface water, groundwater and drinking water. Environ Sci Pollut Res Int 26:7326–7336. [DOI] [PubMed] [Google Scholar]
- Jin Z, Chi M, He Q, Pan Y, Sun C. 2019. Perfluoroalkane sulfonyl fluorides non-covalently bind to human serum albumin at sudlow’s sites. Toxicol Lett 301:17–23. [DOI] [PubMed] [Google Scholar]
- Judson R, Richard A, Dix DJ, Houck K, Martin M, Kavlock R, et al. 2009. The toxicity data landscape for environmental chemicals. Environ Health Perspect 117:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock RJ, Austin CP, Tice RR. 2009. Toxicity testing in the 21st century: Implications for human health risk assessment. Risk Anal 29:485–487; discussion 492–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewski D, Acosta D Jr., Andersen M, Anderson H, Bailar JC 3rd, Boekelheide K, et al. 2010. Toxicity testing in the 21st century: A vision and a strategy. J Toxicol Environ Health B Crit Rev 13:51–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagomarsino RJ. 1996. An improved gas chromatographic method for the determination of perfluorocarbon tracers in the atmosphere. Journal of Chromatographic Science 34:405–412. [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, et al. 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci 90:510–518. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Chu S, McKinney MA, Tomy GT, Sonne C, Dietz R. 2014. Comparative hepatic in vitro depletion and metabolite formation of major perfluorooctane sulfonate precursors in arctic polar bear, beluga whale, and ringed seal. Chemosphere 112:225–231. [DOI] [PubMed] [Google Scholar]
- Li Y, Yao J, Zhang J, Pan Y, Dai J, Ji C, et al. 2021. First report on the bioaccumulation and trophic transfer of perfluoroalkyl ether carboxylic acids in estuarine food web. Environ Sci Technol. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang X, Liao J, Fan X, Cheng Y. 2021. An ultra-robust fingerprinting method for quality assessment of traditional chinese medicine using multiple reaction monitoring mass spectrometry. J Pharm Anal 11:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore HK, Jackson SR, Strynar MJ, McCord JP. 2020. Solvent suitability for hfpo-da (“genx” parent acid) in toxicological studies. Environ Sci Technol Lett 7:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: Past, present, and future. Environ Sci Technol 45:7954–7961. [DOI] [PubMed] [Google Scholar]
- Lofstedt Gilljam J, Leonel J, Cousins IT, Benskin JP. 2016. Is ongoing sulfluramid use in south america a significant source of perfluorooctanesulfonate (pfos)? Production inventories, environmental fate, and local occurrence. Environ Sci Technol 50:653–659. [DOI] [PubMed] [Google Scholar]
- Lubin A, Bajic S, Cabooter D, Augustijns P, Cuyckens F. 2017. Atmospheric pressure ionization using a high voltage target compared to electrospray ionization. J Am Soc Mass Spectrom 28:286–293. [DOI] [PubMed] [Google Scholar]
- Manning RO, Bruckner JV, Misagel ME, Bowen JM. 1991. Metabolism and disposition of sulfluramid, a unique polyfluorinated insecticide, in the rat. Drug Metab Dispos 19:205–211. [PubMed] [Google Scholar]
- Mansouri K, Cariello NF, Korotcov A, Tkachenko V, Grulke CM, Sprankle CS, et al. 2019. Open-source qsar models for pka prediction using multiple machine learning approaches. J Cheminform 11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J, Strynar M. 2019. Identification of per- and polyfluoroalkyl substances in the cape fear river by high resolution mass spectrometry and nontargeted screening. Environ Sci Technol 53:4717–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia Avendano S, Liu J. 2015. Production of pfos from aerobic soil biotransformation of two perfluoroalkyl sulfonamide derivatives. Chemosphere 119:1084–1090. [DOI] [PubMed] [Google Scholar]
- Mullin L, Katz D, Riddell N, Plumb R, Burgess JA, Yeung LWY, et al. 2019. Analysis of hexafluoropropylene oxide-dimer acid (hfpo-da) by liquid chromatography-mass spectrometry (lc-ms): Review of current approaches and environmental levels. Trends Analyt Chem 118:828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento RA, Nunoo DBO, Bizkarguenaga E, Schultes L, Zabaleta I, Benskin JP, et al. 2018. Sulfluramid use in brazilian agriculture: A source of per- and polyfluoroalkyl substances (pfass) to the environment. Environ Pollut 242:1436–1443. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Reinhard M, Chen H, Gin KY. 2016. Fate and transport of perfluoro- and polyfluoroalkyl substances including perfluorooctane sulfonamides in a managed urban water body. Environ Sci Pollut Res Int 23:10382–10392. [DOI] [PubMed] [Google Scholar]
- OECD. 2018. Toward a new comprehensive global database of per and polyfluoroalkyl substances (pfass): Summary report on updating the oecd 2007 list of per and polyfluoroalkyl substances (pfass). OECD Environment, Health and Safety Publication Series on Risk Management; 39. [Google Scholar]
- OECD. 2021. Reconciling terminology of the universe of per- and polyfluoroalkyl substances: Recommendations and practical guidance. (Series on Risk Management). Paris, France:Environment Directorate. [Google Scholar]
- Onel M, Beykal B, Ferguson K, Chiu WA, McDonald TJ, Zhou L, et al. 2019. Grouping of complex substances using analytical chemistry data: A framework for quantitative evaluation and visualization. PLoS One 14:e0223517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organtini K, Oehrle S, Rosnack K. 2020. An alternative ionization technique for perfluorinated alkyl substnace (pfas) analysis: Evaluating unisray for water and soil samples. Waters Corporation Application Note; 720006760EN. [Google Scholar]
- Pan Y, Zhang H, Cui Q, Sheng N, Yeung LWY, Sun Y, et al. 2018. Worldwide distribution of novel perfluoroether carboxylic and sulfonic acids in surface water. Environ Sci Technol 52:7621–7629. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, Richard AM, Williams AJ, Grulke CM, Sams R, Lambert J, et al. 2019. A chemical category-based prioritization approach for selecting 75 per- and polyfluoroalkyl substances (pfas) for tiered toxicity and toxicokinetic testing. Environ Health Perspect 127:14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlewicz G, Richard, Williams, Judson, Thomas 2022. Towards reproducible structure-based chemical categories of pfas to inform and evaluate toxicity and toxicokinetic testing. Comput Toxicol 24:100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AG, Jones KC, Sweetman AJ. 2009. A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ Sci Technol 43:386–392. [DOI] [PubMed] [Google Scholar]
- Renner R. 2001. Growing concern over perfluorinated chemicals. Environ Sci Technol 35:154A–160A. [DOI] [PubMed] [Google Scholar]
- Richard AM, Judson RS, Houck KA, Grulke CM, Volarath P, Thillainadarajah I, et al. 2016. Toxcast chemical landscape: Paving the road to 21st century toxicology. Chem Res Toxicol 29:1225–1251. [DOI] [PubMed] [Google Scholar]
- Richard AM, Huang R, Waidyanatha S, Shinn P, Collins BJ, Thillainadarajah I, et al. 2021. The tox21 10k compound library: Collaborative chemistry advancing toxicology. Chem Res Toxicol 34:189–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JA, Tettenhorst DR. 2020. Method 537.1 determination of selected per- and polyfluorinated alkyl substances in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (lc/ms/ms). [Google Scholar]
- Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. 2008. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect 116:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E, et al. 2015. Identification of novel perfluoroalkyl ether carboxylic acids (pfecas) and sulfonic acids (pfesas) in natural waters using accurate mass time-of-flight mass spectrometry (tofms). Environ Sci Technol 49:11622–11630. [DOI] [PubMed] [Google Scholar]
- Strynar MJ, Lindstrom AB. 2008. Perfluorinated compounds in house dust from ohio and north carolina, USA. Environ Sci Technol 42:3751–3756. [DOI] [PubMed] [Google Scholar]
- Sun C, Sun H, Lai Y, Zhang J, Cai Z. 2011. Liquid chromatography/mass spectrometry method for determination of perfluorooctane sulfonyl fluoride upon derivatization with benzylamine. Anal Chem 83:5822–5826. [DOI] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, et al. 2016. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the cape fear river watershed of north carolina. Environmental Science & Technology Letters 3:415–419. [Google Scholar]
- Taniyasu S, Kannan K, Yeung LW, Kwok KY, Lam PK, Yamashita N. 2008. Analysis of trifluoroacetic acid and other short-chain perfluorinated acids (c2-c4) in precipitation by liquid chromatography-tandem mass spectrometry: Comparison to patterns of long-chain perfluorinated acids (c5-c18). Anal Chim Acta 619:221–230. [DOI] [PubMed] [Google Scholar]
- Taniyasu S, Yeung LWY, Lin H, Yamazaki E, Eun H, Lam PKS, et al. 2022. Quality assurance and quality control of solid phase extraction for pfas in water and novel analytical techniques for pfas analysis. Chemosphere 288:132440. [DOI] [PubMed] [Google Scholar]
- Tebes-Stevens C, Patel JM, Jones WJ, Weber EJ. 2017. Prediction of hydrolysis products of organic chemicals under environmental ph conditions. Environmental Science & Technology 51:5008–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolonen A, Turpeinen M, Pelkonen O. 2009. Liquid chromatography-mass spectrometry in in vitro drug metabolite screening. Drug Discov Today 14:120–133. [DOI] [PubMed] [Google Scholar]
- USEPA. 2020. Pfas methods and guidance for sampling and analyzing water and other environmental media. Technical Brief. [Google Scholar]
- USEPA. 2021. Pfas strategic roadmap: Epa’s commitments to action 2021–2024. [Google Scholar]
- Villeneuve DL, Coady K, Escher BI, Mihaich E, Murphy CA, Schlekat T, et al. 2019. High-throughput screening and environmental risk assessment: State of the science and emerging applications. Environ Toxicol Chem 38:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shi G, Yao J, Sheng N, Cui R, Su Z, et al. 2020. Perfluoropolyether carboxylic acids (novel alternatives to pfoa) impair zebrafish posterior swim bladder development via thyroid hormone disruption. Environ Int 134:105317. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbuhler K. 2013. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (pfcas), perfluoroalkane sulfonic acids (pfsas) and their potential precursors. Environ Int 60:242–248. [DOI] [PubMed] [Google Scholar]
- Wang Z, DeWitt JC, Higgins CP, Cousins IT. 2017. A never-ending story of per- and polyfluoroalkyl substances (pfass)? Environ Sci Technol 51:2508–2518. [DOI] [PubMed] [Google Scholar]
- Washington JW, Naile JE, Jenkins TM, Lynch DG. 2014. Characterizing fluorotelomer and polyfluoroalkyl substances in new and aged fluorotelomer-based polymers for degradation studies with gc/ms and lc/ms/ms. Environ Sci Technol 48:5762–5769. [DOI] [PubMed] [Google Scholar]
- Washington JW, Rosal CG, McCord JP, Strynar MJ, Lindstrom AB, Bergman EL, et al. 2020. Nontargeted mass-spectral detection of chloroperfluoropolyether carboxylates in new jersey soils. Science 368:1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters. 2015. Radar: Understanding sample complexity, improving quantitative data quality. [Google Scholar]
- Waybright TJ, Britt JR, McCloud TG. 2009. Overcoming problems of compound storage in dmso: Solvent and process alternatives. Journal of Biomolecular Screening 14:708–715. [DOI] [PubMed] [Google Scholar]
- White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE. 2011. Gestational and chronic low-dose pfoa exposures and mammary gland growth and differentiation in three generations of cd-1 mice. Environ Health Perspect 119:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead HD, Venier M, Wu Y, Eastman E, Urbanik S, Diamond ML, et al. 2021. Fluorinated compounds in north american cosmetics. Environmental Science & Technology Letters 8:538–544. [Google Scholar]
- Woudneh MB, Chandramouli B, Hamilton C, Grace R. 2019. Effect of sample storage on the quantitative determination of 29 pfas: Observation of analyte interconversions during storage. Environ Sci Technol 53:12576–12585. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Saunders KC. 2010. A review of lc-ms techniques and high-throughput approaches used to investigate drug metabolism by cytochrome p450s. J Chromatogr B Analyt Technol Biomed Life Sci 878:1326–1336. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hopkins ZR, McCord J, Strynar MJ, Knappe DRU. 2019. Fate of per- and polyfluoroalkyl ether acids in the total oxidizable precursor assay and implications for the analysis of impacted water. Environ Sci Technol Lett 6:662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McElroy AC, Liberatore HK, Alexander NLM, Knappe DRU. 2021. Stability of per- and polyfluoroalkyl substances in solvents relevant to environmental and toxicological analysis. Environ Sci Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Pang S, Lin Z, Mishra S, Bhatt P, Chen S. 2021. Biotransformation of perfluoroalkyl acid precursors from various environmental systems: Advances and perspectives. Environ Pollut 272:115908. [DOI] [PubMed] [Google Scholar]
- Zhao S, Wang B, Zhu L, Liang T, Chen M, Yang L, et al. 2018. Uptake, elimination and biotransformation of n-ethyl perfluorooctane sulfonamide (n-etfosa) by the earthworms (eisenia fetida) after in vivo and in vitro exposure. Environ Pollut 241:19–25. [DOI] [PubMed] [Google Scholar]
- Zitha-Bovens E, Maas P, Wife D, Tijhuis J, Hu Q-N, Kleinöder T, et al. 2009. Comdecom: Predicting the lifetime of screening compounds in dmso solution. Journal of Biomolecular Screening 14:557–565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.